Abstract
Angiogenesis is an essential component of endometrial repair and regeneration following menses. Perturbation of this process is associated with menorrhagia, a common gynecological disorder that results in excessive menstrual bleeding. Angiopoietin-1 (Ang-1) promotes vascular maturation via the Tie-2 receptor, while angiopoietin-2 (Ang-2) is its natural antagonist that destabilizes vessels and initiates neovascularization in the presence of vascular endothelial growth factor. To test the hypothesis that menorrhagia arises as a result of poor signal for vascular maturation, we have examined the expression of Ang-1, Ang-2, and Tie-2 in endometrium throughout the menstrual cycle from 30 normal women and 28 patients with menorrhagia. Ribonuclease protection assay and Western blot analysis showed Ang-2 expression was consistently higher than Ang-1 in normal endometrium throughout the cycle. However, with menorrhagia Ang-1 mRNA and protein were not detected or down-regulated, while Ang-2 was observed at similar levels in both normal and menorrhagic endometrium resulting in a greater than a 50% decrease in the ratio of Ang-1 to Ang-2 protein. In situ hybridization and immunohistochemical studies supported these findings and revealed cyclical changes in the expression of Ang-1 and Ang-2. These results suggest that the angiopoietin/Tie-2 system promotes vascular remodeling in endometrium and loss of normal Ang-1 expression may contribute to the excessive blood loss observed in menorrhagia.
During menstruation, shedding of the functional layer of the endometrium occurs followed by intense vasoconstriction of the remaining basal arteriolar fragments, which prevents excessive blood loss until the damaged surrounding tissues and blood vessels are repaired and regenerated. 1 Excessive menstrual bleeding (>80 ml) is a common gynecological problem in women of reproductive age, accounting for over 20% of outpatient clinic visits, which may lead to iron deficiency anemia and require hysterectomy. Although commonly associated with the presence of conditions such as fibroids and carcinoma, the finding that approximately 50% of menorrhagia cases occur in the absence of any uterine pathology 2 suggests a defect in the cellular processes of menstruation. The mechanisms controlling menstruation are regulated by local factors within the endometrium that include vasoregulators, 3,4 angiogenic growth factors, 3,5,6 and matrix metalloproteinases. 7
A large number of angiogenic mediators may contribute to the repair and regeneration of the endometrium although none have been clearly demonstrated to play a role in vivo. 6 The angiopoietin family of growth factors are ligands for the largely endothelial-restricted Tie-2 receptor tyrosine kinase which is essential for vascular development. 8-12 Mice deficient in tie-2 die in utero by embryological day E10.5 indicating that Tie-2 is required for the remodeling and maintenance of the primary vascular network during development. 9,13-15 Ang-1 binds and induces autophosphorylation of Tie-2, 10 promoting endothelial cell migration, 16 sprouting, 17,18 and survival 19,20 in vitro; and increases new vessel growth, branching, maturation, and integrity in vivo. 21,22 Ang-1 secreted by perivascular cells is thought to promote the production of smooth muscle cell mitogens such as platelet-derived growth factor and heparin-binding epidermal growth factor in the endothelium. 23 In contrast, Ang-2 acts as a natural antagonist of Tie-2 signaling leading to vessel destabilization and neovascularization in the presence of vascular endothelial cell growth factor (VEGF), or vascular regression in its absence. 23 Supporting their opposing activities, disruption of the ang-1 11 or over-expression of ang-2 12 in transgenic mice results in death in utero due to a broad failure of vascular morphogenesis with a similar phenotype to tie-2 (−/−) knock-out mice. 9,13-15 Thus the angiopoietins appear to play a key role in coordinating new blood vessel growth sending permissive signals via Tie-2 to either promote vascular maturation/integrity, or destabilize vessels leading to angiogenesis or vascular regression depending on the molecular context in which they act. 23
It has been suggested that disturbances of the normal processes of angiogenesis occurring in the endometrium during menstruation may result in menorrhagia. 5,24,25 Although there is little direct evidence to support this hypothesis, increased endothelial cell turnover and multiple abnormalities in endometrial microvascular morphology have been noted in patients with menorrhagia. 24,25 Moreover, any perturbation in the development of endometrial spiral arterioles (through which the majority of menstrual blood loss occurs) might result in inadequate vasoconstriction and repair leading to menorrhagia. In this study we examined the expression and distribution of Ang-1, Ang-2, and Tie-2 at a mRNA and protein level in both normal and menorrhagic endometrium during the proliferative and secretory phases of the menstrual cycle. We found that the normal pattern of Ang-1 expression is down-regulated in the endometrium of women with menorrhagia. Our observations indicate that Ang-1 may be involved in endometrial regeneration by stabilizing newly formed blood vessels and that loss of normal Ang-1 expression may lead to inadequate vascular remodeling/maturation and excessive blood loss observed in menorrhagia.
Materials and Methods
Reagents
Rabbit polyclonal antibodies against human Ang-1, Ang-2, and Tie-2 cDNA constructs were kindly provided by Dr. George Yancopoulos, (Regeneron Pharmaceuticals, Tarrytown, NY). Rabbit anti-Tie-2 polyclonal antibodies (sc-324) were purchased from Santa Cruz Biotechnology, Inc. (Autogenbioclear, Wilts, UK). All other reagents were obtained from Sigma (Poole, Dorset, UK) unless stated otherwise.
Tissue Collection
Endometrium was obtained from patients 25 to 48 years of age under ethical approval from the South Birmingham Ethical Committee. Normal endometrium was obtained from 30 patients undergoing hysterectomy for benign conditions or dilatation and curettage for laparoscopic sterilization. Endometrium was collected by Pipelle biopsy from 28 patients who were referred from their general practitioners complaining of menorrhagia as described previously. 5 These patients had regular menstrual cycles, had not had recent pregnancies, or received any hormonal treatments for the 3 months preceding endometrial biopsy. Tissues were rinsed in sterile saline and immediately snap frozen in liquid nitrogen. Endometrial samples were dated from the last menstrual period and the stage of cycle confirmed by independent histological analysis using the Fox and Buckley criteria 26 as described previously. 5
Ribonuclease Protection Assay
Total RNA was isolated from snap-frozen endometrial tissue following homogenization by the method of Chomczynski and Sacchi. 27 Ang-1 (570 bases), Ang-2 (388 bases), and Tie-2 riboprobes were transcribed from cDNA templates in the presence of [α-32P]-UTP (Amersham Pharmacia Biotech, High Wycombe, Bucks, UK) as described previously. 28 Ang-1 and Ang-2 (1.0 to 5.0 × 10 5 cpm) probes and a 127 base β-actin (1.0 to 5.0 × 10 4 cpm) control probe (Ambion, Austin, TX) were combined with 10 μg of total RNA and co-precipitated. Ribonuclease protection assays were then performed using the RPA II kit according to the manufacturer’s instructions (Ambion) and the protected fragments resolved by 6% denaturing polyacrylamide gel electrophoresis (PAGE). The Ang-1, Ang-2, and Tie-2 protected fragments were quantified from autoradiographs using the UVP Gelbase densitometry software and the results normalized to β-actin or 28s RNA respectively.
Western Blotting
Endometrial tissue lysates were prepared and subjected to Western blot analysis as described previously. 5,29 Briefly, samples (50 μg) were separated by 8% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (SDS-PAGE) and semidry electroblotted onto Hybond-C nitrocellulose (Amersham, Pharmacia Biotech). The blots were blocked in 1% (w/v) milk protein and incubated with a 1:1000 dilution of either anti-Ang-1, Ang-2, or Tie-2 polyclonal antibodies overnight at 4°C. Antibody binding was detected using a goat anti-rabbit-peroxidase conjugate and enhanced-chemiluminescence (ECL detection kit; Amersham Pharmacia Biotech), and quantified by laser densitometry.
In Situ Hybridization
Plasmids containing human Ang-1, Ang-2, and Tie-2 cDNAs were linearized and used as a templates to generate digoxigenin-11-UTP-labeled riboprobes using the RNA color kit (Amersham Pharmacia Biotech) and in situ hybridization performed as described previously. 28 Briefly, sections were hybridized with digoxigenin-labeled riboprobes at 55°C overnight. The sections were incubated with sheep anti-digoxigenin antibody for 3 hours and probe-binding detected using 5-bromo-4-chloro-3 indoyl phosphate/nitro blue tetrazolium (BCIP/NBT) at 4°C for 24 to 48 hours.
Immunohistochemistry
Immunohistochemistry was performed as described previously. 30 Serial 3-μm formalin-fixed, wax-embedded sections were incubated with a 1:100 dilution of either anti-Ang-1, Ang-2, or Tie-2 polyclonal antibodies for 1 hour. Antibody binding was detected using biotinylated goat anti-rabbit secondary antibody, streptavidin-biotin-peroxidase complex (ABC Kit; DAKO Ltd., Bucks, UK), and diaminobenzidine, and the sections were then counterstained with hematoxylin. In control sections the primary antibody was replaced with non-immune rabbit immunoglobulin or omitted.
Results
Quantitation of Ang-1, Ang-2, and Tie-2 mRNA in Normal and Menorrhagic Endometrium
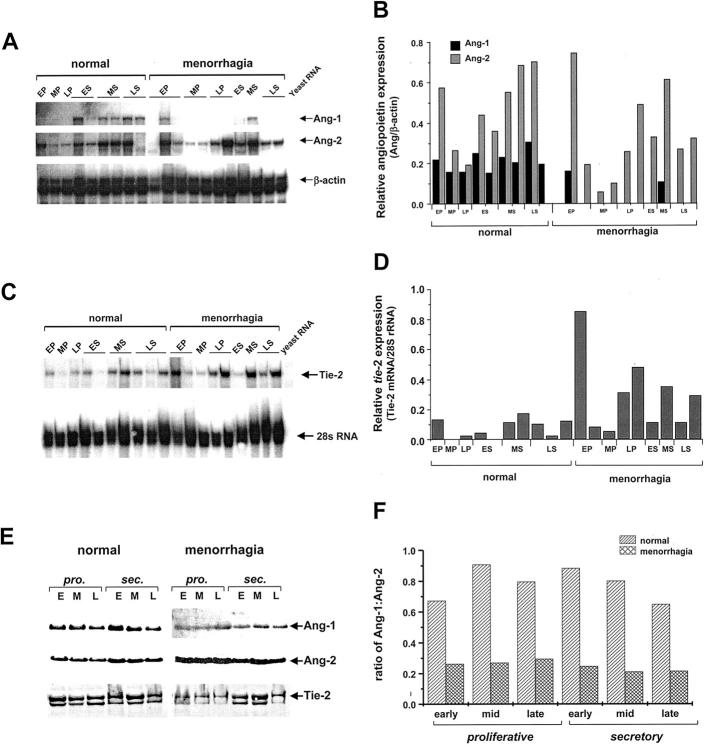
Ribonuclease protection analysis was used to quantify total ang-1, ang-2, and tie-2 expression in endometrial samples isolated from normal and menorrhagic endometrium. The levels of Ang-1, Ang-2, and Tie-2 mRNA detected in endometrial tissue relative to β-actin mRNA or 28s rRNA controls, respectively, and typical results from these assays are shown in Figure 1, A–D ▶ . Although there were variations in ang-1 and ang-2 expression between tissues isolated from different individuals, levels of Ang-1 mRNA were found to be consistently lower than Ang-2 mRNA detected in normal cyclic endometrium (Figure 1, A and B) ▶ . Tie-2 mRNA was detected in the majority of endometrial samples analyzed and similarly showed interindividual variation (Figure 1, C and D) ▶ . In contrast to normal endometrium, total ang-1 expression was broadly down-regulated in samples of menorrhagic endometrium from both the proliferative and secretory phases of the menstrual cycle, while Ang-2 mRNA was detected at similar levels to those observed in normal endometrium. Conversely, there was generally elevated Tie-2 mRNA expression in endometrial samples from menorrhagia patients (Figure 1, C and D) ▶ .
Figure 1.
Quantitative analysis of Ang-1, Ang-2, and Tie-2 expression in normal and menorrhagic endometrium. A: A representative RNase protection autoradiograph showing the relative expression of Ang-1 and Ang-2 mRNA expression in normal 9 and menorrhagic 11 endometrium samples. Endometrial samples were collected at the early proliferative (EP), mid-proliferative (MP), late proliferative (LP), early secretory (ES), mid-secretory (MS) and late secretory (LS) phases of the menstrual cycle. Total RNA was extracted from endometrial tissue and analyzed by RNase protection for the presence of Ang-1, Ang-2, and β-actin (control) mRNA. B: Densitometric analysis was performed on the autoradiographs and the densities of the Ang-1 and Ang-2 protected fragments normalized to the internal β-actin control fragments and represented graphically as relative density units. C:Tie-2 expression in endometrium was determined by RNase protection and the protected Tie-2 fragments normalized to 28s rRNA. D: The relative levels of Tie-2 mRNA in normal 9 and menorrhagic 11 endometrium samples is represented graphically as relative densitometric units. E: Ang-1, Ang-2, and Tie-2 protein expression was examined in endometrial samples by Western blot analysis. Protein extracts (50 μg) pooled from endometrial tissue from 5 individuals taken at early (E), mid- (M), and late (L) proliferative (pro.) and secretary (sec.) phases of the menstrual cycle were subjected to Western blotting and enhanced chemiluminescent detection with antibodies against Ang-1, Ang-2, and Tie-2. F: Densitometric analysis of Ang-1 and Ang-2 expression was performed on the luminographs and the results expressed as the ratio of Ang-1:Ang-2 for normal and menorrhagic endometrium throughout the cycle.
Quantitation of Ang-1, Ang-2, and Tie-2 Protein in Normal and Menorrhagic Endometrium
Endometrial tissue was pooled from five normal or menorrhagic women for each phase of the menstrual cycle and 50 μg of total protein subjected to Western blot analysis for Ang-1, Ang-2, and Tie-2. In normal endometrium, Ang-1 and Ang-2 were detected as ∼70 kd bands in all phases of the cycle examined with maximal levels detected in early secretory endometrium (Figure 1, E and F) ▶ . Consistent with the observed decrease in Ang-1 transcripts (Figure 1, A and B) ▶ , Ang-1 protein was significantly reduced in menorrhagic compared with normal endometrium in both the proliferative (P < 0.05) and secretory (P < 0.01) phases of the cycle (Figure 1, E and F) ▶ . Whereas an increase of total Ang-2 protein in proliferative endometrium was evident in menorrhagia compared with normal cyclic endometrium (Figure 1E) ▶ . Moreover, the overall ratio of Ang-1 to Ang-2 was markedly reduced in menorrhagic endometrial tissue in all phases of the cycle (Figure 1F) ▶ .
Tie-2 was detected as two bands, at ∼135 kd and ∼140 kd, which probably correspond to cytoplasmic precussor and fully mature forms 31 of the receptor respectively as previously reported with this antibody 32 (Figure 1E) ▶ . Tie-2 was expressed throughout the proliferative and secretory phases of the cycle. In contrast to the observed general increase in Tie-2 mRNA levels the relative level of Tie-2 receptor detected in menorrhagic endometrium was reduced in the proliferative and late secretory phases of the cycle in comparison with normal endometrium (Figure 1E) ▶ .
Localization of Ang-1, Ang-2, and Tie-2 mRNA in Endometrium during the Menstrual Cycle
In situ hybridization was used to determine the distribution of mRNA in human endometrium. Ang-1, Ang-2, and Tie-2 expression showed cyclical changes during the menstrual cycle (Figure 2) ▶ . Ang-1 mRNA was most abundant in the endometrial stroma and glandular epithelium of the early and mid-proliferative menstrual phases (Figure 2A) ▶ and decreased in the late proliferative phase of the cycle. Although we did not detect significant Ang-1 mRNA in the blood vessels during the proliferative phases it was predominantly expressed in the stroma surrounding the blood vessels (Figure 2B) ▶ . In addition to the endometrial stroma, ang-1 expression was also present at lower levels in the glandular epithelium during the secretory phases (Figure 2B) ▶ .
Figure 2.
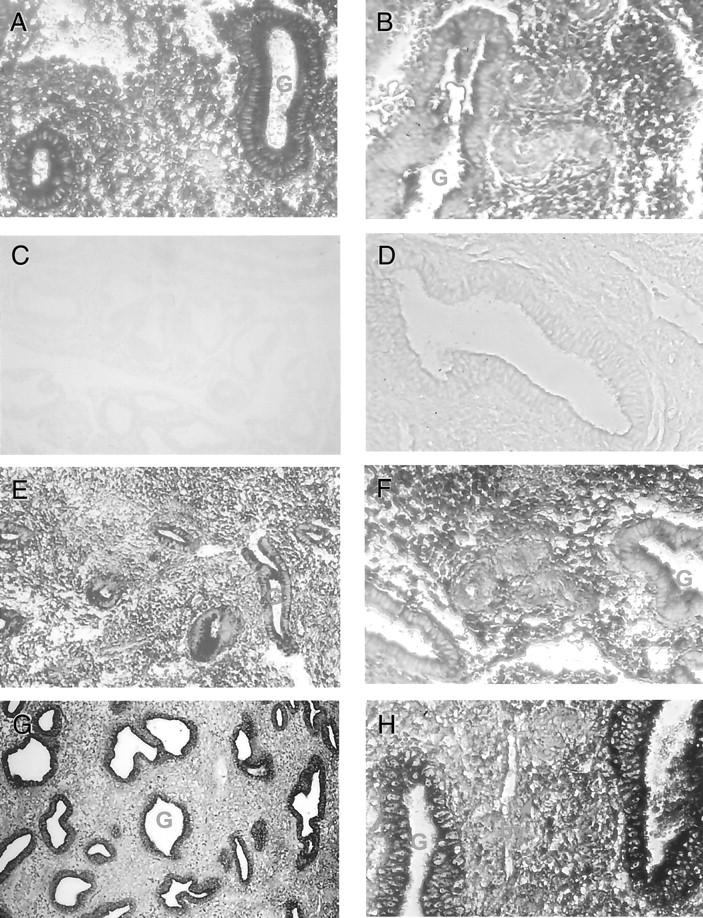
Localization of Ang-1, Ang-2, and Tie-2 transcripts in human endometrium from 30 normal (A, B, D–H) and 28 menorrhagic (C) individuals. In situ hybridization was performed on endometrial tissue sections using digoxigenin-labeled antisense riboprobes for Ang-1, Ang-2, and Tie-2. A: Strong Ang-1 hybridization signal was detected in the stroma (S) and glandular epithelium (G) of early proliferative phase endometrium (magnification, ×200). B: Ang-1 was highly expressed in the stroma surrounding blood vessels (bv) in secretory phase normal endometrium (magnification, ×100). C: Weak/absent Ang-1 hybridization signal in secretory phase menorrhagic endometrium (magnification, ×100). D: No hybridization was observed on control sections hybridized with sense Ang-1 riboprobe (magnification, ×200). E: Detection of Ang-2 mRNA in the glandular epithelium and stroma in proliferative phase endometrium (magnification, ×100). F: Moderate expression of ang-2 in the stroma, glandular epithelium, and blood vessels of mid-secretory endometrium (magnification, ×200). G: Strong expression of tie-2 was present in glandular epithelium and blood vessels in late secretory (magnification, ×100) and H, mid-secretory endometrium (magnification, ×200).
Ang-2 mRNA was detected in normal endometrial tissue throughout the menstrual cycle. In the proliferative phases moderate staining was detected in the glands and stroma (Figure 2E) ▶ . In secretory endometrium, Ang-2 mRNA was also observed in the glands and stroma with increased expression in mid- to late secretory phases of the cycle (Figure 2F) ▶ .
Tie-2 expression was detected mainly in the blood vessels and glandular epithelium of normal endometrium. In the early proliferative phase (Figure 2G) ▶ of the cycle, intense tie-2 expression was observed in the glandular epithelium which decreased in mid- and late proliferative phases. Moderate Tie-2 mRNA hybridization signal was observed in the glands during the early secretory phase of the cycle. While in the early and mid-secretory phases tie-2 was strongly expressed in endothelium and glandular epithelium (Figure 2) ▶ , reduced tie-2 hybridization signal was detected in the late secretory phase of the cycle.
In menorrhagic endometrium ang-1 expression was notably reduced or absent from the proliferative and early secretory phases and absent in the majority of specimens examined from mid- and late secretory phases of the menstrual cycle (Figure 2C) ▶ . In contrast there were no consistent observable differences in the overall distribution and expression of Tie-2 and Ang-2 mRNA in the samples of menorrhagic endometrium examined. No hybridization was detected in control sections hybridized with sense riboprobes to Ang-1, Ang-2, and Tie-2 (Figure 2D) ▶ .
Immunolocalization of Ang-1, Ang-2, and Tie-2 during the Menstrual Cycle
We examined the distribution of Ang-1, Ang-2, and Tie-2 receptor in normal endometrium during the menstrual cycle and compared this with staining in endometrial samples from menorrhagic women (Figure 3) ▶ . Perinuclear staining for Ang-1 was detected in the stromal cells throughout the menstrual cycle. Ang-1 was also detected in the glandular epithelium and blood vessels and, in particular, the endothelium of small venules (Figure 3A) ▶ . In the secretory phase there was strong expression of Ang-1 in the perivascular stroma with low level expression in the glandular epithelium (Figure 3B) ▶ . Intense Ang-2 expression was detected in the glandular epithelium and weaker staining of the blood vessels and stroma in proliferative endometrium (Figure 3F) ▶ . Ang-2 exhibited a similar biphasic pattern of staining to that observed for Ang-1 with reduced Ang-2 staining of the decidualized stroma surrounding the spiral arterioles during the secretory phase.
Figure 3.
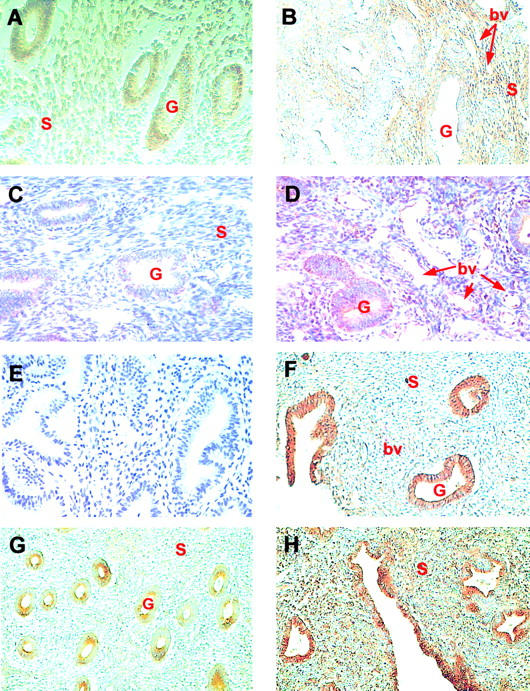
Immunohistochemical localization of Ang-1, Ang-2, and Tie-2 in 30 normal (A, B, F–H) and 28 menorrhagic (C–E) endometrium specimens taken throughout the menstrual cycle. A: In proliferative endometrium, Ang-1 staining was detected in the glandular epithelium (G), stroma (S) and blood vessels (magnification, ×200). B: Intense Ang-1 staining was observed in the stroma surrounding the blood vessels (bv) although it was very low or absent in the glandular epithelium of normal secretory endometrium (magnification, ×100). C and D: The presence of low levels of Ang-1 in glandular epithelium and endothelium in the secretory menorrhagic endometrium of two patients (magnification, ×200). E: Control section demonstrating a lack of background staining in sections in which the primary anti-Ang-1 antibody was replaced with rabbit serum. F: Strong Ang-2 immunoreactivity was detected in the glandular epithelium of proliferative endometrium (magnification, ×100). G: Moderate Tie-2 staining was detected in glandular epithelium with weak staining of some blood vessels in proliferative endometrium (magnification, ×100). H: In mid-secretory endometrium strong Tie-2 staining was present in the glandular and squamous epithelium and blood vessels with some staining of stroma (magnification, ×100).
Immunostaining for Tie-2 was present in the endothelium of blood vessels and also in the glandular epithelium throughout the menstrual cycle. Generally the distribution of Tie-2 produced an inverse pattern of staining for the Ang-1 with pronounced staining of the endothelium and epithelium (Figure 3G) ▶ . There was clear membranous staining of the glands which became concentrated in the apical portions of the glandular epithelial cells during the secretory phase (Figure 3H) ▶ .
In menorrhagia Ang-1 staining was markedly reduced compared with normal endometrium. Weak staining for Ang-1 was observed in the apical portions of the glandular and squamous epithelium with reduced stromal staining during the secretory phase of the menstrual cycle compared with normal endometrium (Figure 3B) ▶ (Figure 3, C and D) ▶ . Ang-1 was also detected on the endothelium of blood vessels during the secretory phase of the cycle (Figure 3B) ▶ .
Discussion
Normal menstruation is characterized by rapid regression of endometrial thickness, followed by intense vasoconstriction of the spiral arterioles and subsequent bleeding from focal points. A rapid and highly coordinated vascular response is required for endometrial repair and regeneration following menstruation. In this study, we have examined the expression Ang-1, Ang-2, and their cognate receptor, Tie-2, in normal and menorrhagic endometrium during the menstrual cycle. In endometrium of patients suffering from menorrhagia there was a marked down-regulation of both Ang-1 mRNA and protein while the expression of Ang-2 increased or remained at similar levels resulting in a large decrease in the ratio of Ang-1 to Ang-2.
Tie-2 and its ligands were expressed in a coordinated manner in endometrial tissue throughout the menstrual cycle with marked differences in the cellular distribution of Ang-1 and Ang-2. Consistent with its largely endothelial-restricted expression and recent studies of endometrium, 33,34 Tie-2 was detected in the endometrial blood vessels. However, we also observed Tie-2 mRNA and protein in the glandular epithelium of the endometrium. Similarly, Tie-2 expression was recently reported in the epithelium of the thyroid. 32 Tie-2 and its activating ligand, Ang-1, were highly expressed in the blood vessels and associated stroma of early proliferative phase endometrium, where the levels of Ang-2 were relatively low. The expression of Ang-1 mRNA predominantly in the stroma surrounding blood vessels and detection of Ang-1 protein on the endothelium of these blood vessels supports the paracrine mode of action of this ligand-receptor system. 10,11,21,23 Moreover, it suggests that they play a role in the neovascularization of early proliferative endometrium, which coincides with the initial distinct phase of angiogenesis in human endometrium. 35 Many angiogenic factors implicated in endometrial development such as basic fibroblast growth factor (bFGF), VEGF, and platelet activating factor are reported to be highly expressed during the late proliferative stage. 6 However, we found that Ang-1, Ang-2, and Tie-2 decreased in the late proliferative phase of the cycle. It is suggested that the major mechanism of new vessel growth in the endometrium is intussuception and/or vessel elongation rather than classical sprouting angiogenesis. 6 The critical involvement of the Tie-2 receptor in the process of intussusception has been demonstrated in tie-2(−/−) knock-out mice. 14
The angiopoietins are thought to coordinate angiogenesis by providing permissive signals that result in the stabilization of newly formed blood vessels by mediating perivascular cell (pericyte and smooth muscle cell) migration and the formation of basement membranes. 11,23 That Ang-2 mRNA is only readily detected in the uterus, ovary, and placenta, tissues that are undergoing vascular remodeling in the adult, highlights its importance in blood vessel development. 12 In agreement with Li and colleagues 33 we found Ang-2 mRNA expression to be consistently greater than Ang-1 throughout the cycle in normal endometrium. However, we detected Ang-2 mRNA and protein in the glandular epithelium, blood vessels, and associated stroma, whereas Li and colleagues 33 only detected Ang-2 mRNA in natural killer cells.
The observed increase in Ang-1 expression in the perivascular stroma during the early secretory phase indicates that the angiopoietin/Tie-2 system is important for endometrial vessel development during the post ovulatory phase when the spiral arterioles are developing. 6,36 Human endometrium expresses angiogenic factors, including bFGF and VEGF, 3,5,6 that are reported to up-regulate Tie-2 expression in cultured endothelial cells. 37 The fundamental role of angiogenesis in the development of the endometrium was demonstrated with the angiogenesis inhibitor, AGM-1470, which suppressed endometrial maturation in mice. 38 Normal endometrial angiogenesis is perturbed in menorrhagia 24,25 and there is an increase in the number of small venules in the deep endometrium and inner myometrium in women with menorrhagia. 25 In the endometrium of women with menorrhagia, we observed decreased levels of Ang-1 and Tie-2 receptor that may contribute to disturbed endometrial vascular remodeling in this condition. Endothelial cell proliferation is reported to be significantly greater in menorrhagia without significant change in the overall endothelial cell density 24 implying a continual process vascular remodeling in menorrhagic endometrium throughout the menstrual cycle. This is consistent with our findings of a greater than 50% decrease in the ratio of Ang-1 to Ang-2 in menorrhagia and the proposed function of Ang-2 as a promoter of vessel destabilization. 12,23
In menorrhagia, the rate of blood loss increases significantly and menstrual bleeding persists on average 24 hours longer suggesting that repair of the endometrial blood vessels is compromised. 2,36 The majority of menstrual blood loss occurs through the spiral arterioles that develop from endometrial arterioles during the secretory phase of the cycle. 6,36 There appears to be no significant difference in the overall number of spiral arterioles in menorrhagic compared with normal endometrium. 36 However, recent evidence suggests that the phenotype of the smooth muscle cells in the spiral arterioles is altered in idiopathic menorrhagia without observable difference in the overall number of smooth muscle cells associated with these vessels. The proliferation of spiral arteriole smooth muscle cells is significantly reduced in the mid- to late secretory phases in menorrhagic endometrium. 36 It is therefore possible that the reported change in smooth muscle cell phenotype is a direct result of the altered balance of Ang-1 and Ang-2 levels leading to destabilization of the spiral arterioles in menorrhagia. It is clear, however, that the demonstration of ang-1 gene expression in the human endometrium and its consistent down-regulation in menorrhagia establishes this family of angiogenic growth factors as important regulators of uterine function.
Acknowledgments
We thank the clinical and laboratory staff of Birmingham Women’s Health Care National Health Service Trust for their help with tissue collection. We are extremely grateful to Dr. George Yancopoulos and colleagues at Regeneron Pharmaceuticals, Tarrytown, NY, for the Tie-2 and Ang-1/–2 cDNAs and antibodies.
Footnotes
Address reprint requests to Professor Asif S. Ahmed, Department of Reproductive and Vascular Biology, The Medical School, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK. E-mail: a.s.ahmed@bham.ac.uk.
Supported by grants from the British Heart Foundation (RG/98/0003, Medical Research Council (G96/02173), and the Wellcome Trust.
References
- 1.Christiaens GCML, Sixma JJ, Haspels AA: Morphology of haemostasis in menstrual endometrium. Br J Obstet Gynaecol 1980, 87:425-432 [DOI] [PubMed] [Google Scholar]
- 2.Haynes PJ, Anderson ABM, Turnball AC: Patterns of menstrual blood loss in menorrhagia. Res Clin Forums 1979, 1:73-78 [Google Scholar]
- 3.Li XF, Gregory J, Ahmed A: Immunolocalization of vascular endothelial growth factor in human endometrium. Growth Factors 1994, 11:277-282 [DOI] [PubMed] [Google Scholar]
- 4.Cameron IT, Davenport AP: Endothelins in reproduction. Reprod Med Rev 1992, 1:99-113 [Google Scholar]
- 5.Sangha RK, Li XF, Shams M, Ahmed A: Fibroblast growth factor receptor-1 is a critical component for endometrial remodeling: localization and expression of basic fibroblast growth factor and FGF-R in human endometrium during the menstrual cycle and decreased FGF-R1 expression in menorrhagia. Lab Invest 1997, 77:389-402 [PubMed] [Google Scholar]
- 6.Weston G, Rogers PAW: Endometrial angiogenesis. Bailliere’s Clin Obstet Gynaecol 2000, 14:919–936 [DOI] [PubMed]
- 7.Rodgers WH, Matrisian LM, Gludice LC, Dsupin B, Cannon P, Svitek C, Gorstein F, Osteen G: Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest 1994, 94:946-953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler SF, Bird TA, Schneringer JA, Schooley KA, Baum PR: Molecular cloning and characterization of a novel receptor protein tyrosine kinase from human placenta. Oncogene 1993, 8:663-670 [PubMed] [Google Scholar]
- 9.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML: Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 1994, 8:1897-1909 [DOI] [PubMed] [Google Scholar]
- 10.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD: Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 1996, 87:1161-1169 [DOI] [PubMed] [Google Scholar]
- 11.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davies S, Sato TN, Yancopoulos GD: Requisite role of angiopoietin-1, a ligand for the Tie-2 receptor, during embryonic angiogenesis. Cell 1996, 87:1171-1180 [DOI] [PubMed] [Google Scholar]
- 12.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davies S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277:55-60 [DOI] [PubMed] [Google Scholar]
- 13.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchhotz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y: Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1996, 376:70-74 [DOI] [PubMed] [Google Scholar]
- 14.Patan S: TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc Res 1998, 56:1-21 [DOI] [PubMed] [Google Scholar]
- 15.Jones N, Voskas D, Master Z, Sarao R, Jones J, Dumont DJ: Rescue of the early vascular defects in Tek/Tie-2 null mice reveals an essential survival function. EMBO Rep 2001, 2:438-445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM: Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie-2. J Biol Chem 1998, 273:18514-18521 [DOI] [PubMed] [Google Scholar]
- 17.Koblizek TL, Weiss C, Yancopoulos GD, Deutsch U, Risau W: Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol 1998, 8:529-532 [DOI] [PubMed] [Google Scholar]
- 18.Kim I, Kim HG, Moon S-O, Chae SW, So J-N, Koh KN, Ahn BC, Koh GY: Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res 2000, 86:952-959 [DOI] [PubMed] [Google Scholar]
- 19.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, Li F, Altieri DC, Sessa WC: Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem 2000, 275:9102-9105 [DOI] [PubMed] [Google Scholar]
- 20.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY: Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Circ Res 2000, 86:24-29 [DOI] [PubMed] [Google Scholar]
- 21.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD: Increased vascularization in mice overexpressing angiopoietin-1. Science 1998, 282:468-471 [DOI] [PubMed] [Google Scholar]
- 22.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM: Leakage-resistant blood vessels in mice transgenically over-expressing angiopoietin-1. Science 1999, 286:2511-2514 [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D: Signaling vascular morphogenesis and maintenance. Science 1997, 277:48-50 [DOI] [PubMed] [Google Scholar]
- 24.Kooy J, Taylor NH, Healy DL, Rogers PA: Endothelial cell proliferation in the endometrium of women with menorrhagia and in women following endometrial ablation. Hum Reprod 1996, 11:1067-1072 [DOI] [PubMed] [Google Scholar]
- 25.Hickey M, Fraser IS: Clinical implications of disturbances of uterine vascular morphology and function. Bailliere’s Clin Obstet Gynaecol 2000, 14:937–951 [DOI] [PubMed]
- 26.Fox H, Buckley CH: Endometrium. Gresham GA eds. Atlas of Gynaecological Pathology. 1983, :pp 61-63 MTP Press Limited, London [Google Scholar]
- 27.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 28.Dunk C, Shams M, Nijjar S, Rhaman M, Qiu Y, Bussolati B, Ahmed A: Angiopoietin-1 and angiopoietin-2 activate trophoblast Tie-2 to promote growth and migration during placental development. Am J Pathol 2000, 56:2185-2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khaliq A, Li XF, Shams M, Sisi P, Acevedo CA, Whittle MJ, Weich H, Ahmed A: Localization of placenta growth factor (PIGF) in human term placenta. Growth Factors 1996, 13:243-250 [DOI] [PubMed] [Google Scholar]
- 30.Ahmed A, Li XF, Dunk C, Whittle MJ, Rushton DI, Rollason T: Colocalisation of vascular endothelial growth factor and its Flt-1 receptor in human placenta. Growth Factors 1995, 12:235-243 [DOI] [PubMed] [Google Scholar]
- 31.Sato A, Iwama A, Takakura N, Nishio H, Yancopoulos GD, Suda T: Characterization of TEK receptor tyrosine kinase and its ligands, angiopoietins, in human hematopoietic progenitor cells. Int Immunol 1998, 10:1217-1227 [DOI] [PubMed] [Google Scholar]
- 32.Ramsden JD, Cocks HC, Shams M, Nijjar S, Watkinson JC, Sheppard MC, Ahmed A, Eggo MC: Tie-2 is expressed on thyroid follicular cells, is increased in goiter, and is regulated by thyrotropin through cyclic adenosine 3′,5′-monophosphate. J Clin Endocrinol Metab 2001, 86:2709-2716 [DOI] [PubMed] [Google Scholar]
- 33.Li XF, Charnock-Jones DS, Zhang E, Hiby S, Malik H, Day K, Licence D, Bowen JM, Gardner L, King A, Loke YW, Smith SK: Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J Clin Endocrinol Metab 2001, 86:1823-1834 [DOI] [PubMed] [Google Scholar]
- 34.Krikun G, Schatz F, Finlay T, Kadner S, Mesia A, Gerrets R, Lockwood CJ: Expression of angiopoietin-2 by human endometrial endothelial cells: regulation by hypoxia and inflammation. Biochem Biophys Res Commun 2000, 275:159-163 [DOI] [PubMed] [Google Scholar]
- 35.Goodgers AM, Rogers PAW: Endometrial cell proliferation during the menstrual cycle. Hum Reprod 1994, 9:399-405 [DOI] [PubMed] [Google Scholar]
- 36.Abberton KM, Taylor NH, Healy DL, Rogers PAW: Vascular smooth muscle cell proliferation in arterioles of the human endometrium. Hum Reprod 1999, 14:1072-1079 [DOI] [PubMed] [Google Scholar]
- 37.Mandriota SJ, Pepper MS: Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res 1998, 83:852-859 [DOI] [PubMed] [Google Scholar]
- 38.Klauber N, Rohan RM, Flynn E, D’Amato RJ: Critical components of the female reproductive pathway are suppressed by the angiogenesis inhibitor AGM-1470. Nat Med 1997, 8:442-446 [DOI] [PubMed] [Google Scholar]