Abstract
Autosomal-dominant polycystic kidney disease, one of the most frequent human genetic disorders, is genetically heterogeneous. Most cases result from mutations of PKD1 or PKD2 encoding polycystin-1 or polycystin-2, respectively. Polycystin-1 is a large transmembrane protein containing several domains involved in cell-cell and/or cell-matrix interactions. Polycystin-2 is transmembrane glycoprotein sharing homology with some families of cation channels. Despite a large number of reports, the tissue distribution of these two proteins, especially of polycystin-1, is still debated. We investigated the expression pattern of PKD1 and PKD2 transcripts and proteins during human embryogenesis and kidney development, using Northern blot analysis, in situ hybridization, and immunohistochemical methods. For each gene, the expression pattern of transcripts and protein was concordant. In human 5- to 6-week-old embryos, both genes are widely expressed, mainly in neural tissue, cardiomyocytes, endodermal derivatives, and mesonephros. At this age, PKD2 but not PKD1 expression is observed in the ureteric bud and the uninduced metanephros. Thereafter, PKD2 is diffusely expressed at all stages of nephron development, whereas high PKD1 expression first appears in differentiated proximal tubules. Proximal tubule expression of both genes decreases from weeks 20 to 24 onwards. PKD1 transcripts, later restricted to distal tubules in fetal nephrogenesis, are no longer detected in adult kidneys, which nevertheless maintain a faint expression of polycystin-1, whereas persistent expression of PKD2 transcripts and protein is observed throughout nephrogenesis. Overall, contrary to previous observations, we found profound differences in the spatiotemporal expression of PKD1 and PKD2 during nephrogenesis, PKD2 being expressed earlier and more diffusely than PKD1. These data suggest that polycystins could interact with different partners, at least during kidney development.
Autosomal-dominant polycystic kidney disease (ADPKD) is one of the most frequent human genetic disorders with an estimated incidence of 1 to 500 to 1 to 1000 in all ethnic groups worldwide. 1 It is characterized by the progressive development of large renal cysts and a number of extrarenal features including hepatic cysts, cardiac valvular anomalies, intracranial aneurysms, and colonic diverticulae. It accounts for 10% of all cases of end-stage renal disease. 1 ADPKD is genetically heterogeneous. More than 95% of cases are because of mutations in two recently identified genes, PKD1 2-4 and PKD2. 5 In rare instances, affected families do not show genetic linkage with either of these two genes, suggesting the existence of at least one additional unidentified causative gene. 6,7
The vast majority of ADPKD cases (85%) are linked to PKD1. The PKD1 gene encodes a large protein (≥450 kd), polycystin-1, thought to be involved in cell-cell or cell-matrix interactions. 2-4 The extracellular domain of polycystin-1 contains a region of homology with a sea urchin protein (the receptor for egg jelly, REJ) that is implicated in ion movements leading to conformational changes before fertilization. 8 The cytoplasmic tail of polycystin-1 may bind heterotrimeric G proteins in vitro, 9 activate the transcription factor AP-1, 10 and have a role in modulating Wnt signaling. 11 Recently, using MDCK cells expressing the human PKD1 gene, it has been shown that polycystin-1 down-regulates proliferation and induces resistance to apoptosis. 12
The PKD2 gene is mutated in more than 10% of ADPKD cases. It encodes polycystin-2, which shares similarity with polycystin-1, voltage-activated calcium, and transient receptor potential (TRP) channel subunits. 5 Interaction between polycystin-2 and the TRP protein TRPC1 has been shown in vitro. 13 Polycystin-2 is predicted to form homomultimers and/or heteromultimers, especially with polycystin-1 via coiled-coil domains in their cytoplasmic regions, 14,15 suggesting that these two proteins interact in a common signaling pathway. Furthermore, it was recently shown that co-assembly of polycystin-1 and polycystin-2 in CHO cells produces a unique channel activity. 16
In ADPKD kidneys, cysts originate from only a small number of nephrons. Studies of renal 17,18 and hepatic cysts 19 in PKD1 as well as in PKD2 20,21 patients, suggest a two-hit molecular mechanism for their focal occurrence, making ADPKD a recessive disease at the cellular level. More recently, it has been reported that somatic mutations may affect PKD2 in ADPKD1 cysts, or PKD1 in ADPKD2 cysts, leading to a trans-heterozygous status. 22,23
Previous reports have shown that PKD1 and PKD2 transcripts are widely expressed in most tissues. 5,24 However, the localization of transcripts in human kidneys is unknown and the distribution of the proteins, especially of polycystin-1 is still debated. 25 The knowledge of their precise distribution could help to clarify their role and the mechanism of cyst formation. Here we report the expression patterns of human PKD1 and PKD2 transcripts and their encoded proteins using Northern blot analysis, in situ hybridization, and immunohistochemical methods. The study was performed on normal embryos, and normal fetal and postnatal kidneys.
Materials and Methods
Patients and Tissue Samples
Ten normal fetal kidneys (10 to 38 weeks) and fetal extrarenal tissues from two fetuses (16 and 19 week) were obtained at autopsy after spontaneous abortion or termination of pregnancy for medical reasons (Table 1) ▶ . Two morphologically normal intact embryos (5 and 6 weeks) were obtained after legal abortion by Mifepristone (RU486) performed at the Hôpital Broussais (Paris, France). Written maternal consent was obtained after information about the research project was given and the abortion had been performed. The entire procedure was approved by INSERM and the ethics committee. Normal mature kidneys not used for transplantation and the tumor-free pole of a kidney removed for polar carcinoma were also used for the study. Specimens were immediately snap-frozen in liquid nitrogen and stored at −80°C until use, or fixed in 4% paraformaldehyde before embedding. Embryos were fixed in 4% paraformaldehyde in phosphate-buffered saline solution, microdissected from the whole trophoblast, dehydrated, and embedded in paraplast before sectioning. Normal kidney specimens were also snap-frozen for Northern blot analysis.
Table 1.
Clinical Details of Embryos and Fetuses from whom Tissue Was Obtained
| Gestational age, weeks | Postmortem diagnosis |
|---|---|
| 5 | Normal (spontaneous abortion) |
| 6 | Normal (spontaneous abortion) |
| 10 | Normal (spontaneous abortion) |
| 12 | Normal (spontaneous abortion) |
| 12 | Trisomy 21 (medical abortion) |
| 16 | Normal (spontaneous abortion) |
| 19 | Normal (spontaneous abortion) |
| 19 | Osteogenesis imperfecta |
| 24 | Normal (spontaneous abortion) |
| 31 | Hydrothorax |
| 36 | Normal (spontaneous abortion) |
| 38 | Spina bifida |
Northern Blot Analysis
DNA probes corresponding to a gel-purified 368-bp XhoI/BamHI-digested fragment and 850-bp EcoRI/NotI-digested fragments of pLig2-1 and XF-75, respectively, were synthesized using the random primers DNA-labeling system (Life Technologies, Inc., Gaithersburg, MD) with [α-32P]dCTP (Amersham Pharmacia Biotech, Buckinghamshire, UK). Total RNA was isolated with the RNeasy Maxi Kit (Qiagen, Hilden, Germany) from fetal and adult kidneys. These filters and Human Multiple Tissue Northern Blots (no. 7756-1 and no. 7760-1) (Clontech, Palo Alto, CA) were hybridized at 68°C with cDNA probes made as described above, according to the manufacturer’s instructions (Clontech). The cDNA used as template for probe synthesis was verified by sequencing before utilization for Northern blot analysis and in situ hybridization.
In Situ Hybridization
A portion of PKD1 cDNA corresponding to bp 11409 to 11771, located in the specific portion of the gene, 4,5 was amplified by polymerase chain reaction and subcloned into the vector pCRII (Invitrogen, San Diego, CA). The resulting construct is designated pLig2-1. For in situ hybridization, pLig2-1 was linearized by XhoI or BamHI digestion for the anti-sense and sense riboprobes, respectively. The 368-bp anti-sense and sense riboprobes were synthesized using Sp6 and T7 RNA polymerase (Boehringer-Mannheim, Mannheim, Germany), respectively, with [35S]UTP (35S-UTPαS, Amersham) according to the manufacturer’s instructions. A portion of PKD2 cDNA, corresponding to bp 2125 to 2973, designated XF-75, was subcloned into the vector pBluescript II KS (Stratagene, La Jolla, CA) linearized by EcoRV or NotI for anti-sense and sense probes, respectively. The corresponding 850-bp riboprobes were synthesized as detailed above using T7 and T3 RNA polymerase (Boehringer-Mannheim) for the anti-sense and sense probes, respectively. Six-μm-thick cryostat or paraffin-embedded sections were produced. Before hybridization, the paraffin-embedded tissues were pretreated by microwave heating in a sodium-citrate buffer (0.01 mol/L, pH 6) to enhance the hybridization signal as previously described. 26 In situ hybridization was performed according to a protocol previously reported. 27
Immunohistochemistry
Antibodies and Western Blot Analysis
The rabbit anti-polycystin-1 serum against the cytoplasmic C-terminal tail of polycystin-1, has been generated using a fusion protein encoding the last 215 amino acids of the protein (amino acids 4088 to 4302) and described by Boletta and colleagues. 12 Affinity purification of the antibodies was performed by immobilizing the immunizing antigen to resin, followed by affinity chromatography according to the manufacturer’s instructions (AminoLink Plus kit; Pierce, Rockford, IL). HEK cells were transiently transfected with the human full-length PKD1 cDNA. Transfected and untransfected cells were lysed in lysis buffer (250 mmol/L sucrose, 20 mmol/L imidazole, and 1 mmol/L ethylenediaminetetraacetic acid, pH 7.4, 0.5% Triton X-100) containing a protease inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany) by incubation at 4°C for 1 hour and centrifuged for 15 minutes at 14,000 rpm in a table microcentrifuge. The cleared total lysates were analyzed on Western blot similarly as described by Boletta and colleagues. 12 Antibodies were diluted in 2% bovine serum albumin in Tris-buffered saline-T. The dilution for affinity-purified antibody was 1:200. It was 1:100 for the affinity-depleted serum and 1:1000 for the unpurified serum.
Two rabbit anti-polycystin-2 antibodies have been used. One was generated using the human sequence from amino acids 724 to 968. It was affinity purified and its specificity was confirmed by Western blot and immunoprecipitation of endogenous polycystin-2 from MDCK cells. The second anti-polycystin-2 antibody, YCC2, a gift from S. Somlo (Renal Division, Department of Internal Medicine, Yale School of Medicine, New Haven, CT), was generated against a fusion protein corresponding to amino acids 687to 962 of human polycystin-2. Membrane proteins from fetal (30 gestational weeks) and adult human kidneys were prepared as previously described. 28 Western blot analysis using YCC2 polycystin-2 antibody (dilution, 1:4000) and the preabsorbed antisera as a negative control, was performed according to a protocol detailed by Cai and colleagues. 28
The specificity and characterization of both polycystin-1 and polycystin-2 antibodies have already been detailed in a large number of reports 12,16,20,28-30 . They have been used for immunohistochemistry and Western blot analysis and no signal was detected using the corresponding preimmune or depleted serum, on tissue sections or Western blot analysis.
Immunoperoxidase Staining
Immunostaining was performed on 6-μm-thick sections of paraffin-embedded or paraplast-embedded tissues using a standard immunoperoxidase protocol (Universal Immunostaining Streptavidin-Peroxidase Kit; Coulter-Immunotech, Marseille, France), after pretreatment by microwave heating in a 10 mmol/L citrate buffer, pH 6, as previously described. 31 Antibodies to polycystin-1 were diluted to 1:500. Those to polycystin-2 were diluted to 1:50 to 1:100 for the first one and 1:200 for YCC2. The labeling patterns obtained with the two anti-polycystin-2 antibodies were similar. The staining with YCC2 was stronger thus the micrographs shown here were obtained using this antibody. Rabbit IgG (secondary antibody), preimmune sera, or antibodies preincubated with the corresponding polycystin-1 or polycystin-2 fusion protein were used as controls.
Results
Northern Blot Analysis
The expression profile of PKD1 and PKD2 mRNAs was studied using multiple-tissue Northern blots. In adult tissues, the highest expression of PKD1 was seen in heart and brain; it was weaker in skeletal muscle and pancreas and very faint in the kidney (Figure 1A) ▶ . In fetal tissues, PKD1 mRNAs were mainly expressed in brain and kidney (Figure 1A) ▶ . The weak level of the signal obtained by Northern blot using Human Multiple Tissue Northern Blots (Clontech), may be because of the large size of the transcripts. We also performed Northern blot using RNA extracted from total fetal and adult kidneys and the hybridization obtained with the same PKD1 probe was consistently higher (data not shown). PKD1 transcript expression has been studied in different tissues using RNase protection assay. 24 This assay has a higher sensitivity than Northern blot and was used by these authors because of the size of the PKD1 transcripts.
Figure 1.
Northern blot analysis of human adult (left; 1) (Clontech MTN 7760-1) and fetal (right; 2) (Clontech MTN 7756-1) poly (A)+ RNA, by hybridization with PKD1 (A) and PKD2 (B) probes. β-actin-specific probe was used as a loading control (C).
The PKD2 transcripts were detected in most fetal and adult tissues. In adult tissues, the highest expression was observed in heart, kidney, and pancreas (Figure 1B) ▶ . A lower band was also detected in liver at ≈3 kb. In fetal tissues, a strong expression was noted in lung and kidney (Figure 1B) ▶ .
Western Blot Analysis
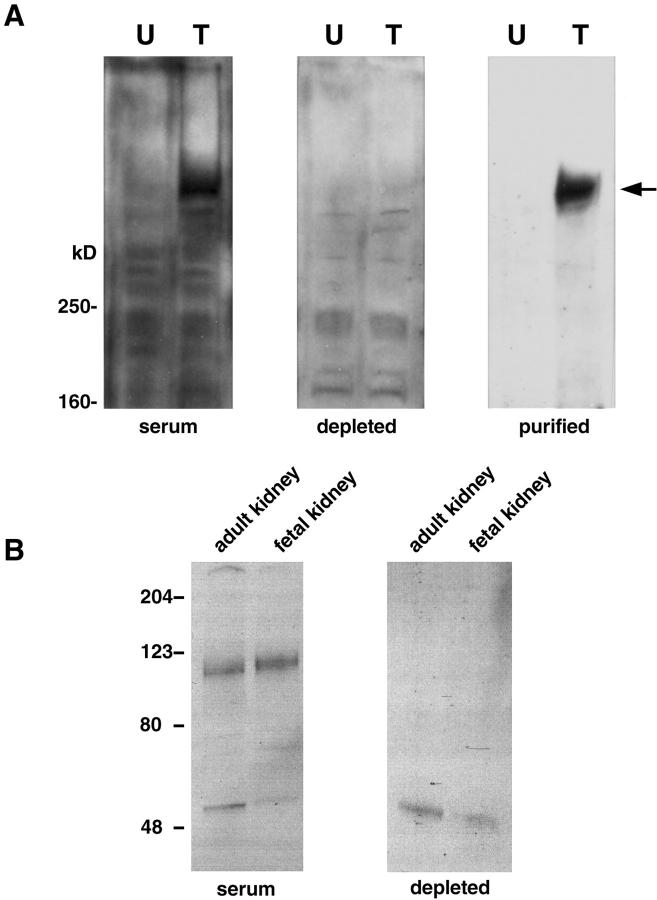
A polycystin-1 signal was difficult to detect in fetal and adult kidney preparations with our antibody. This could be explained by technical difficulties because of the size of the protein and the faint level of expression in late stages of development, as was the case of our tissue samples. However a band corresponding to the reported size (520 kd) was easily detected in transfected cells and not seen when the preimmune or the preabsorbed sera was used, nor was it seen in the wild-type cells (Figure 2A) ▶ . These results are in accordance with previous reports. 12,16 As previously reported, the 110-kd signal was detected both in fetal and adult kidneys after incubation with the polycystin-2 antibody but abolished using antisera preabsorbed with the corresponding fusion protein (Figure 2B) ▶ .
Figure 2.
Western blot analysis. A: Polycystin-1. The expression construct for the full-length human polycystin-1 was transiently expressed in HEK cells (T). Untransfected HEK cells served as the negative control for protein expression (U). The total protein lysates were subjected to Western blot analysis using either unpurified serum (serum), polycystin-1-specific antibody-depleted serum (depleted), or affinity-purified polycystin-1-specific antibody (purified). The protein marker is given on left. An arrow on right indicates the position of the full-length recombinant polycystin-1 protein (520 kd). B: Polycystin-2. A 110-kd signal was detected both in fetal and adult kidneys after incubation with the YCC2 polycystin-2 antibody but abolished using antisera preabsorbed with the corresponding fusion protein.
In Situ Expression of PKD1 and PKD2 mRNAs and Protein during Embryogenesis and Nephrogenesis
In situ hybridization and immunohistochemistry studies were performed on: 1) whole human embryo sections at 5 and 6 weeks of gestation; 2) fetal tissues (16 to 19 weeks); 3) fetal (10 to 38 weeks); and 4) postnatal (1 to 50 years) kidneys. In the human embryo three sets of kidneys differentiate successively from the intermediate mesoderm located on both sides of the body axis. The pronephros is a rudimentary and transitory structure that disappears after 4 weeks of embryonic development. The mesonephros begins to differentiate at 4 weeks, extends caudally along the mesonephric (or Wolffian) duct, is fully developed around the second month, and regresses at the end of the 4th month. The metanephros, the permanent kidney, begins to develop at 5 weeks when the ureteric bud, a diverticulum from the lower end of the Wolffian duct, reaches the caudal end of the nephric cord, or metanephric mesenchyma, and begins to branch. Differentiation of the first nephron units begins at 8 weeks and successive generations of nephrons develop up to 34 to 36 weeks of gestation, after the growth and branching of the ureteric bud. As such, mature and developing structures (vesicles, S-shaped bodies) co-exist during fetal life. The most mature nephrons are situated in the deep cortex, at the juxtamedullary junction whereas the immature structures are located in the superficial cortex.
Distribution of PKD1 mRNAs and Protein during Normal Kidney Development
In the 5- to 6-week embryos, PKD1 mRNAs were strongly expressed in mesonephric tubules (Figure 3, A and B) ▶ and absent in the primitive metanephros consisting of the uninduced mesenchyma and the first ureteric bud branches (Figure 3, C and D) ▶ . At 10 weeks, a strong hybridization signal had appeared in the first set of differentiated proximal tubules (Figure 3 ▶ ; E to H). No significant signal was detected in the ureteric bud branches or the blastema (mesenchyma). Between 10 to 24 weeks, the differentiated proximal tubules continued to express high levels of PKD1 mRNAs. Thereafter the expression decreased progressively. At week 15, discrete PKD1 expression was also seen in the distal part of the nephrons and the ureteric bud branches (Figure 3, J and K) ▶ . It persisted at the same moderate level during fetal life. Hybridization signals were no longer detected in mature kidneys. No signal was seen in glomeruli, renal interstitium, or arteries of fetal or postnatal kidneys. Results are summarized in Table 2 ▶ . No specific signal was detected with the sense probes (Figure 3, I and L) ▶ .
Figure 3.
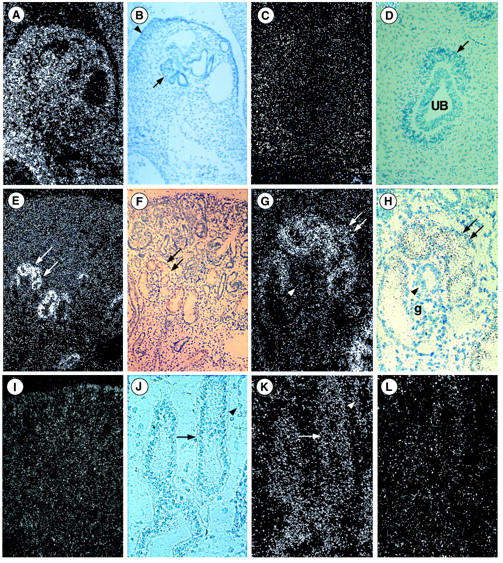
PKD1 expression in human fetal and postnatal kidneys. A–L: Light and dark views of in situ hybridization with anti-sense (A–H, J, and K) and sense (I and L) probes. A and B: Strong PKD1 expression is seen in mesonephrons (arrow) and the genital ridge (arrowhead) of a 6-week embryo. C and D: In 5-week embryo, absence of PKD1 expression in the ureteric bud (UB) or the uninduced mesenchyme (arrow). E and F: Dark- and light-field views of 10-week fetal kidney showing strong labeling of proximal tubules (arrows). G and H: At higher magnification the glomerulus (G) and the distal tubule (arrowhead) are unlabeled whereas the proximal tubule (arrows) is labeled from its glomerular origin. I: With the sense probe the background predominates in the superficial cortex; the proximal tubes are unlabeled. J and K: Light- and dark-field views of 19-week fetal kidney showing the labeling of medullary collecting ducts (arrow) and of Henle loops (arrowhead). L: With the sense probe very discrete labeling of the same structure is seen. Original magnifications: ×40 (E, F, and I); ×55 (A and B); ×90 (C and D); ×110 (G, H, J, K, and L).
Table 2.
Renal Expression of PKD1 and PKD2 Transcripts during Nephrogenesis by in Situ Hybridization
| PKD1 | PKD2 | |
|---|---|---|
| Mesonephros (5 to 6 GW) | ++ | ++ |
| Metanephros (5 to 6 GW) | Not detected | ++ |
| 10 GW fetal kidney | PT +++ | Developing nephrons++ |
| CD++ | ||
| Arteries++ | ||
| 24 GW fetal kidney | PT+++ | PT+ |
| DT+ | DT++ | |
| CD+ | CD++ | |
| Arteries++ | ||
| 38 GW fetal kidney | PT− | DT++ |
| DT+ | CD++ | |
| CD+ | Arteries ++ | |
| Adult kidney | Not detected | DT++ |
| CD++ | ||
| Arteries++ |
Level of expression: high (+++), moderate (++), and faint (+).
Proximal tubule (PT); distal tubule (DT); collecting duct (CD), gestational week (GW).
Polycystin-1 was expressed in the Wolffian duct and the mesonephric tubules of the 5- to 6-week embryos (Figure 4A) ▶ , with a positive gradient of expression with differentiation. No expression was detected in the metanephros at that time. At 10 weeks, polycystin-1 labeling was observed in proximal tubules. The expression increased rapidly with maturation (Figure 4B) ▶ , persisted at the same level to approximately week 24, then declined progressively to persist at a very low level in adult kidneys. Faint labeling of distal tubules and collecting ducts was detected at 15 weeks. It persisted at a low level in collecting ducts whereas it increased progressively with maturation in the ascending limb of Henle loops and distal tubules (Figure 4C) ▶ . The labeling of the distal tubules and collecting ducts persisted in adult kidneys (Figure 4D) ▶ . No significant labeling was detected in the blastema, early nephronic structures, glomeruli, vessels, or interstitium. No signal was seen with the preimmune serum or the serum absorbed with the polycystin-1 fusion protein (Figure 4, I and J) ▶ .
Figure 4.
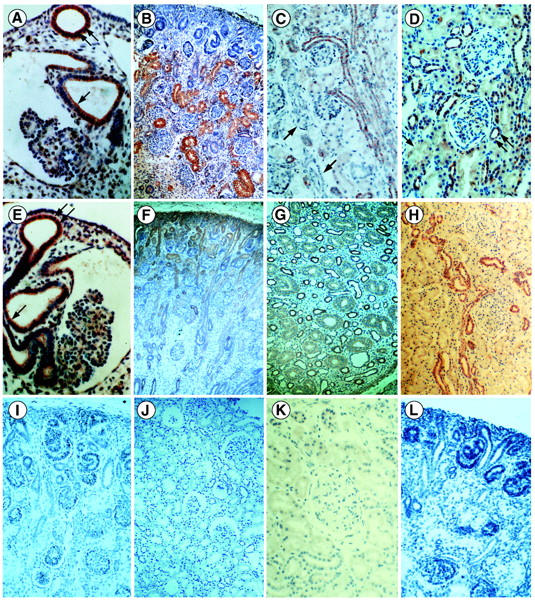
Polycystin-1 (A–D) and polycystin-2 (E–H) expression in human fetal and postnatal kidneys. A: In a 5-week embryo there is strong expression of polycystin-1 in the Wolffian duct (double arrow) and the mesonephric tubules (arrow). B: In 15-week fetal kidney, polycystin-1 is strongly expressed by proximal tubules. C: In a 38-week fetus, expression of polycystin-1 is faint in proximal tubules and prominent in distal tubules and collecting ducts. No labeling is seen in glomeruli or arteries (arrow). D: In mature kidney, distal tubules are labeled (double arrow) whereas proximal tubules are barely visible (arrow). E: Polycystin-2 is expressed by the mesonephric tubules and the Wolffian duct in a 6-week embryo. F: In a 12-week fetus, polycystin-2 is strongly expressed in the blastema and the tubules. G: In the medulla of 24-week fetus, Henle loops express strongly polycystin-2 and the expression is faint in collecting ducts. H: In adult kidney, persistence of strong expression of polycystin-2 by distal tubules and collecting ducts. No significant labeling is observed with polycystin-1 preimmune serum (I) or absorbed antibodies (J) nor with polycystin-2 preimmune serum (K) or absorbed antibodies (L). Original magnifications: ×35 (F and H); ×55 (B, C, D, I, and J); ×80 (L); ×120 (G and K); ×140 (A and E).
Distribution of PKD2 mRNAs and Protein during Kidney Development and in Mature Kidney
Strong expression of PKD2 transcripts was seen at 5 to 6 weeks in the mesonephros as well as in the uninduced metanephric mesenchyma and the ureteric bud (Figure 5 ▶ ; A to C). From 10 weeks, all developing structures of the superficial cortex (condensates, vesicles, and S-shaped bodies), as well as the ureteric bud branches, expressed PKD2 transcripts (Figure 5, E and I) ▶ . Expression by the uninduced blastema was also present but weaker. In the underlying parenchyma, all tubular segments expressed the PKD2 transcripts but the strongest expression was observed in distal tubules and ascending limbs of Henle loops (Figure 5 ▶ ; E, F, I, and J). Smooth muscle cells of large intrarenal arteries (future interlobar and arcuate arteries) showed clear positivity (Figure 5, G and K) ▶ . Up to 36 weeks, the level of PKD2 expression remained high in the differentiating structures of the outer cortex, in distal tubules, cortical collecting ducts, and arterial vessels. In contrast, PKD2 expression decreased rapidly and disappeared nearly totally in proximal tubules as soon as they differentiated. In adult kidneys, persistent expression was only observed in the ascending loop of Henle, distal tubules, collecting ducts, and blood vessels (Figure 5, H and L) ▶ . No expression was detected in the interstitium at any stage of development. No expression was detected with the sense probe (Figure 5D) ▶ . Results are summarized in Table 2 ▶ .
Figure 5.

PKD2 expression in human fetal and postnatal kidneys. A–L: Light and dark views of in situ hybridization with anti-sense (A–C and E–L) or sense (D) probes: A: Strong hybridization signal is seen in the mesonephros of a 5-week embryo. B and C: At the same age, the ureteric bud and the metanephric blastema strongly express PKD2. D: No significant signal is observed in the metanephros of the 5-week embryo hybridized with PKD2 sense probes. E and I: Light- and dark-field views of 19-week fetal kidney showing the strong labeling of developing nephrons, the moderate labeling of ureteric bud branches, and the faint labeling of the blastema. F and J: Light- and dark-field views of the medulla of a 12-week fetus showing PKD2 expression in collecting ducts (arrow) and Henle loops (arrowhead). G and K: Light- and dark-field views of a 10-week fetus showing PKD2 transcripts in large intrarenal arteries. H and L: Light- and dark-field views of the medulla of adult kidney showing PKD2 expression in collecting ducts (arrow) and Henle loops (arrowhead). Original magnifications: ×45 (C); ×65 (B and D); ×100 (E and I); ×110 (F, H, J, and L); ×130 (A, G, and K).
In the mesonephros of 5- to 6-week embryos, polycystin-2 was observed in the Wolffian duct and mesonephric tubules but not in glomeruli (Figure 4E) ▶ . It was also seen, in contrast to what was observed for polycystin-1, in the ureteric bud and the uninduced metanephric blastema (Figure 6J) ▶ . From 10 weeks, labeling was observed in the ureteric bud branches and in the developing nephrons from the vesicle stage (Figure 4F) ▶ . The muscular layer of large arteries was also strongly positive. During development (10 to 38 weeks), expression of polycystin-2 progressively decreased in proximal tubules, was low in the inner medulla portion of collecting ducts, and persisted at a high level in ascending loop of Henle (Figure 4G) ▶ , distal tubules, cortical collecting ducts, and large arteries. The same distribution including the entire collecting system was seen in mature kidneys (Figure 4H) ▶ . No expression was noted in the interstitium. No signal was observed with preimmune serum or after competitive inhibition with the fusion protein (Figure 4, K and L) ▶ .
Figure 6.

PKD1 and PKD2 expression in human embryos. A–C: In situ hybridization. Dark-field view of sagittal sections of a 5-week embryo using PKD1 (A) or PKD2 (B) anti-sense, or PKD2 sense (C) probes. PKD1 signals are prominent in the neural tube and the neural ganglia (arrowhead). PKD2 expression is more intense and is also seen in the primitive metanephros (arrow). Nonspecific signal is seen in the liver with the PKD2 control (sense) probe. D–F: Sagittal sections of a 5-week embryo. Immunohistochemistry using anti-polycystin-1 (D), anti-polycystin-2 (E) antibodies and anti-polycystin-1 preimmune serum (F). Both proteins are detected in cardiomyocytes, and epithelial and endodermal derivatives; polycystin-2 expression is prominent in neural structures; the primitive metanephros expresses only polycystin-2 (arrow). No significant labeling is seen with the preimmune serum. G: In situ hybridization using PKD1 anti-sense probes showing a strong labeling of the anterior horn of the spinal cord (arrowhead) and the spinal ganglia (arrow) of a 6-week embryo. H: A faint unspecific signal is seen with the sense probe. I: Strong hybridization signal with the PKD1 anti-sense probe in the neural ganglia of a 15-week fetus. J and K: Immunolabeling with anti-polycystin-2 antibodies. J: In the 6-week embryo, the anterior horn of the spinal cord and the spinal ganglia are labeled (arrow) as well as the ureteric buds and the metanephric mesenchyme (double arrow). K: Strong staining of the pyramidal cells of the anterior horn of the spinal cord in 15-week fetus. L: Strong expression of PKD2 transcripts in the aorta of a 15-week fetus. Original magnifications: ×10 (D–F); ×13 (A–C); ×22 (J); ×35 (L); ×56 (G and I); ×70 (H); ×110 (K).
Distribution of PKD1/PKD2 mRNAs and Proteins in Extrarenal Tissues during Embryogenesis
In the 5- to 6-week embryos, the expression of PKD1 mRNAs was moderate and diffuse, the hybridization signal being prominent in the neural tube, especially in the anterior horn of the spinal cord and in the neural ganglia (Figure 6, A and G) ▶ . It was also visible in the skin, the bronchial epithelia and the surrounding mesenchyma, the digestive tract, the vertebral cartilaginous primordia, the genital ridges, and the heart. At 16 weeks the neural expression continues to be predominant (Figure 6I) ▶ . No PKD1 transcripts were detected in arterial walls. Polycystin-1 was mainly found in the same neural embryonic structures and the cardiomyocytes (Figure 6D) ▶ . Labeling persisted at 16 weeks and was also observed in pancreas, liver, adrenals, and testis.
PKD2 transcripts were diffusely expressed in a large variety of tissues in the 5- to 6-week human embryo, especially in the neural tube, the neural ganglia, the liver, and the heart (Figure 6B) ▶ . At 16 weeks PKD2 expression was also seen in the aorta (Figure 6L) ▶ . Polycystin-2 was found in the same structures (Figure 6E) ▶ , especially in neural tissue, with strong labeling of the anterior horn of the spinal cord in the 6-week embryo (Figure 6J) ▶ and in the 16-week fetus (Figure 6K) ▶ . With the sense probes a background signal was observed in the liver whereas no significant signals were detected in other tissues (Figure 6C) ▶ . No labeling was observed with preimmune serum, but a weak background was seen in the liver (Figure 6F) ▶ .
Discussion
Numerous studies of polycystin-1 expression pattern throughout renal development in humans, 24,25,32-37 mice, 38 or rat 39 have been reported, but they produced conflicting results. If staining of normal tubular epithelium is usually described, the exact pattern of polycystin-1 distribution varies, according to the different studies, from exclusive staining of the collecting system, to diffuse tubular expression of the protein, or predominant expression in proximal or distal convoluted tubules, or in parietal or even visceral glomerular epithelial cells. Arteries are usually described as polycystin-1-negative but vascular expression of the protein has also been reported. Moreover, in a recent paper, Nauta and colleagues 25 reported their critical experience in the immunohistochemical detection of polycystin-1 in human kidneys. They immunized 14 rabbits against polycystin-1 fragments derived from different parts of the C-terminal domain of the protein. By immunochemistry, immunoprecipitation, and Western blot analysis, most of the polyclonal antibodies recognized a 134-kd polycystin-1 fragment overexpressed in COS cells. The pattern of immunostaining of human kidneys was similar to that reported by most authors. However this pattern was demonstrated to be aspecific and to result from unrelated immunoreactivity. The authors concluded that the true localization of polycystin-1 remained to be determined. The distribution of polycystin-2 has also been described in fetal and postnatal kidneys. 28,30,40,41 Staining was usually observed in the ascending loop of Henle and the distal convoluted tubule, with delayed and low expression in the collecting system 28-30 but a different pattern has also been reported. 40
To overcome these difficulties, probably partly because of differences in antibody specificity, we analyzed by in situ hybridization the expression of both transcripts during embryogenesis and in renal development. This approach has not been reported previously in humans. In parallel, we analyzed by immunohistochemistry the distribution of polycystin-1 and polycystin-2, in an attempt to correlate proteins and transcripts distribution. For each gene, the expression pattern of transcripts and protein was concordant.
During human development, both transcripts are widely expressed at 5 weeks of gestation, the earliest stage studied. The expression was seen in cardiomyocytes, endodermal derivatives, and liver. It was prominent in neural tissue, especially the anterior horn of the spinal cord and the neural ganglia. This distribution persists in the 16-to 19-week fetuses, the latest stage examined. As expected from the pattern of expression, cardiac involvement, hepatic cysts, and colonic diverticulae may be observed in ADPKD patients. However, neurological symptoms are consistently absent in ADPKD patients despite the early and strong expression of both genes in neural tissue. This is not an unusual situation. Indeed, dissociation between a widespread gene expression and restricted clinical phenotype has been observed for example in nephronophthisis, 42 another hereditary renal disease. The arterial expression of the genes was carefully screened as one severe manifestation of both forms of ADPKD is the development of intracranial aneurysms. 43 An early and strong expression of PKD2 transcripts and protein was found in large renal and extrarenal arteries, including the aorta of a 16-week fetus, and persisted at the same level in adult renal arteries. This confirms previous immunohistochemical studies. 28,30,40,44 Vascular expression of Pkd1 transcripts has also been observed in mice, 45,46 and one model of Pkd1 knockout mice exhibits rupture of blood vessels leading to embryonic lethality. 47 However, no arterial PKD1 transcripts or protein could be detected in our study.
In human 5- to 6-week embryos, PKD1 and PKD2 are co-expressed in mesonephric tubules whereas PKD2 only is expressed in the metanephros. Later on, PKD2 continues to be widely expressed in the metanephros, at all stages of nephron development and in all segments of differentiated tubules. In contrast, PKD1 transcripts and protein are absent in the blastema and the immature nephrons whereas they abruptly arise in differentiated proximal tubules of the first row of developed nephrons. Findings are different in mice that show Pkd1 expression throughout the condensing metanephric mesenchyme, a distribution suggesting a role of polycystin-1 in the early processes of condensation and transdifferentiation, 45 a hypothesis not supported by our observations. Moreover, the initial and intense expression of PKD1 in proximal tubules is in agreement with the development of lesions in Pkd1 knockout mice: in the latter, nephrogenesis occurs normally and first cystic dilations develop secondarily from proximal tubules. 48 The expression of both genes in proximal tubules decreases progressively during fetal life to become practically undetectable in postnatal kidneys. PKD1 transcripts, secondarily seen in distal tubules and collecting ducts, are no longer detected in adult kidneys that maintain a faint expression of polycystin-1. In contrast, persistent expression of PKD2 transcripts and protein is observed. Generally, contrary to previous observations at the protein level in humans, 40 and at the RNA level in mice, 46,47 we found differences in the spatiotemporal expression of PKD1 and PKD2 during nephrogenesis, PKD2 being expressed earlier and more diffusely than PKD1. Differences in the developmental expression of polycystin-1 and polycystin-2 were also observed by Foggensteiner and colleagues. 41 These data suggest that polycystins could interact with different partners at least during kidney development.
Acknowledgments
We thank Y. Deris for technical assistance and V. Kalaztsis for critical reading of the manuscript.
Footnotes
Address reprint requests to Dr. Marie Claire Gubler, INSERM U423, Tour Lavoisier, Hôpital Necker Enfants Malades, 149, rue de Sèvres, 75743 Paris Cedex 15, France. E-mail: gubler@necker.fr.
Supported by the Institut National de la Santé et de la Recherche Scientifique, the Association Claude Bernard, the Fondation pour la Recherche Médicale, the Association pour l’Utilisation du Rein Artificiel and the Assistance Publique des Hôpitaux de Paris, the Belgian agencies FNRS and FRSM, the Fondation Alphonse et Jean Forton, the Association pour l’Information et la Recherche sur les Maladies Rénales Génétiques, and a grant from the Fondation pour la Recherche Médicale, the INSERM, and a bilateral grant from the CGRI and INSERM (to V. C.).
Part of this work was presented in abstract form at the 32nd American Society of Nephrology meeting, Miami, October 1999.
References
- 1.Gabow PA: Autosomal dominant polycystic kidney disease. N Engl Med 1993, 329:332-342 [DOI] [PubMed] [Google Scholar]
- 2.: European Polycystic Kidney Disease Consortium: The polycystic kidney disease 1 gene encodes a 14 kb transcripts and lies within a duplicated region on chromosome 16. Cell 1994, 77:881-894 [DOI] [PubMed] [Google Scholar]
- 3.: American PKD1 Consortium: Analysis of the genomic sequence for the polycystic kidney disease gene (PKD1) predicts the presence of a leucine-rich repeat. Hum Mol Genet 1995, 4:575-582 [DOI] [PubMed] [Google Scholar]
- 4.: International Polycystic Kidney Disease Consortium: Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell 1995, 81:289-298 [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJM, Somlo S: PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 1996, 272:1339-1342 [DOI] [PubMed] [Google Scholar]
- 6.Daoust MC, Reynolds DM, Bichet DG, Somlo S: Evidence for a third genetic locus for autosomal dominant polycystic kidney disease. Genomics 1995, 25:733-736 [DOI] [PubMed] [Google Scholar]
- 7.De Almeida S, de Almeida E, Peters DJM, Pinto JR, Tavora I, Lavinha J, Breuning MH, Prata MM: Autosomal dominant polycystic kidney disease: evidence for the existence of a third genetic locus in a Portuguese family. Hum Genet 1995, 96:83-88 [DOI] [PubMed] [Google Scholar]
- 8.Hugues J, Ward CJ, Aspinwall R, Butler R, Harris PC: Identification of a human homologue of the sea urchin receptor for egg jelly: a polycystic kidney disease-like protein. Hum Mol Genet 1999, 8:543-549 [DOI] [PubMed] [Google Scholar]
- 9.Parnell SC, Magenheimer BS, Maser RL, Rankin CA, Smine A, Okamoto T, Calvet JP: The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun 1998, 251:625-631 [DOI] [PubMed] [Google Scholar]
- 10.Arnould T, Kim E, Tsiokas L, Jochimsen F, Grüning W, Chang JD, Walz G: The polycystic kidney disease 1 gene product mediates protein kinase C α-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J Biol Chem 1998, 273:6013-6018 [DOI] [PubMed] [Google Scholar]
- 11.Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Grüning W, Sokol SY, Drummond I, Walz G: The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem 1999, 274:4947-4953 [DOI] [PubMed] [Google Scholar]
- 12.Boletta A, Qian F, Onuchic LF, Bhunia AK, Phakdeekitcharoen B, Hanaoka K, Guggino W, Monaco L, Germino GG: Polycystin-1, the gene product of PKD1 induces resistance to apoptosis and spontaneous tubulogenesis in MDCK cells. Mol Cell 2000, 6:1267-1273 [DOI] [PubMed] [Google Scholar]
- 13.Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP: Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci USA 1999, 96:3934-3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG: PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 1997, 16:179-183 [DOI] [PubMed] [Google Scholar]
- 15.Tsiokas L, Kim K, Arnould T, Sukhtame VP, Walz G: Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA 1997, 94:6965-6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG: Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 2000, 408:990-994 [DOI] [PubMed] [Google Scholar]
- 17.Qian F, Watnick TJ, Onuchic LF, Germino GG: The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type 1. Cell 1996, 87:979-987 [DOI] [PubMed] [Google Scholar]
- 18.Brasier JL, Henske EP: Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J Clin Invest 1997, 99:194-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watnick TJ, Torres VE, Gandolph MA, Qian F, Onuchic LF, Klinger KW, Landes G, Germino GG: Somatic mutation in individual liver cysts supports a two-hit model of cystogenesis in autosomal dominant polycystic kidney disease. Mol Cell 1998, 2:247-251 [DOI] [PubMed] [Google Scholar]
- 20.Wu G, D’Agati VD, Cai Y, Markowitz GS, Park JH, Reynolds DM, Maeda Y, Le TC, Hou Jr J, Kucherlapati R, Edelmann W, Somlo S: Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 1998, 93:177–188 [DOI] [PubMed]
- 21.Koptides M, Hadjimichael C, Koupepidou P, Pierides A, Constantinou Deltas C: Germinal and somatic mutation in the PKD2 gene of renal cysts in autosomal dominant polycystic kidney disease. Hum Mol Genet 1999, 8:509–513 [DOI] [PubMed]
- 22.Koptides M, Mean R, Demetriou K, Pierides A, Constantinou Deltas C: Genetic evidence for a trans-heterozygous model for cystogenesis in autosomal dominant polycystic kidney disease. Hum Mol Genet 2000, 9:447–452 [DOI] [PubMed]
- 23.Watnick T, He N, Wang K, Liang Y, Parfrey P, Hefferton D, St. George-Hyslop P, Germino G, Pei Y: Mutations of PKD1 in ADPKD2 cysts suggest a pathogenic effect of trans-heterozygous mutations. Nat Genet 2000, 25:143-144 [DOI] [PubMed] [Google Scholar]
- 24.Ward CJ, Turley H, Ong ACM, Comley M, Biddolph S, Chetty R, Ratcliffe PJ, Gatter K, Harris PC: Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult and polycystic kidney. Proc Natl Acad Sci USA 1996, 93:1524-1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nauta J, Goedbloed MA, van den Ouweland AMW, Nellist M, Hoogeveen AT: Immunological detection of polycystin-1 in human kidney. Histochem Cell Biol 2000, 113:303-311 [DOI] [PubMed] [Google Scholar]
- 26.Sibony M, Commo F, Callard P, Gasc JM: Enhancement of mRNA in situ hybridization signal by microwave heating. Lab Invest 1995, 73:586-591 [PubMed] [Google Scholar]
- 27.Heidet L, Cai Y, Sado Y, Ninomiya Y, Thorner P, Guicharnaud L, Boye E, Chauvet V, Cohen Solal L, Beziau A, Garcia Torres R, Antignac C, Gubler MC: Diffuse leiomyomatosis associated with X-linked Alport syndrome: extracellular matrix study using immunohistochemistry and in situ hybridization. Lab Invest 1997, 76:1–11 [PubMed]
- 28.Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S: Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem 1999, 40:28557-28565 [DOI] [PubMed] [Google Scholar]
- 29.Markowitz GS, Cai Y, Li L, Wu G, Ward LC, Somlo S, D’Agati VD: Polycystin-2 expression is developmentally regulated. Am J Physiol 1999, 46:F17-F25 [DOI] [PubMed] [Google Scholar]
- 30.Obermüller N, Gallacher AR, Cai Y, Gassler N, Gretz N, Somlo S, Witzgall R: The rat Pkd2 protein assumes distinct subcellular distributions in different organs. Am J Physiol 1999, 277:F914-F925 [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Jeanpierre C, Dressler GR, Lacoste M, Niaudet P, Gubler MC: WT1 and PAX-2 podocyte expression in Denys-Drash syndrome and isolated diffuse mesangial sclerosis. Am J Pathol 1999, 154:181-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffin MD, Torres VE, Grande JP, Kumar R: Immunolocalization of polycystin in human tissues and cultured cells. Proc Assoc Am Physicians 1996, 108:185-197 [PubMed] [Google Scholar]
- 33.Peters DJM, Spruit L, Klingel R, Prins F, Baelde HJJ, Giordano PC, Bernini LF, de Heer E, Breuning MH, Bruijin JA: Adult, fetal and polycystic kidney expression of polycystin, the polycystic kidney disease-1 gene product. Lab Invest 1996, 75:221-230 [PubMed] [Google Scholar]
- 34.Geng L, Segal Y, Peissel B, Deng N, Pei Y, Carone F, Rennke HG, Glücksmann-Kuis AM, Schneider MC, Ericsson M, Reeders ST, Zhou J: Identification and localization of polycystin, the PKD1 gene product. J Clin Invest 1996, 98:2674-2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Adelsberg J, Chamberlain S, D’Agato V: Polycystin expression is temporally and spatially regulated during renal development. Am J Physiol 1997, 272:F602-F609 [DOI] [PubMed] [Google Scholar]
- 36.Ibraghimov-Beskrovnaya O, Dackowski WR, Foggensteiner L, Coleman N, Thiru S, Petry LR, Burn TC, Connors TD, Van Raay T, Bradley J, Qiang F, Onuchic LF, Watnick TJ, Piontek K, Hakim RM, Landes GM, Germino GG, Sandford R, Klinger KW: Polycystin: in vitro synthesis, in vivo tissue expression and subcellular localization identifies a large membrane-associated protein. Proc Natl Acad Sci USA 1997, 94:6397-6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin MD, Torres VC, Grande JP, Kumar R: Vascular expression of polycystin. J Am Soc Nephrol 1997, 8:616-626 [DOI] [PubMed] [Google Scholar]
- 38.Geng L, Segal Y, Pavlova A, Barros EJG, Löhning C, Lu W, Nigam SK, Frischauf AM, Reeders ST, Zhou J: Distribution and developmentally regulated expression of murine polycystin. Am J Physiol 1997, 272:F451-F459 [DOI] [PubMed] [Google Scholar]
- 39.Palsson R, Sharma CP, Kim K, McLaughlin M, Brown D, Arnaout MA: Characterization and cell distribution of polycystin, the product of autosomal dominant polycystic kidney disease gene 1. Mol Med 1996, 2:702-711 [PMC free article] [PubMed] [Google Scholar]
- 40.Ong ACM, Ward CJ, Butler RJ, Biddolph S, Bowker C, Torra R, Harris PC: Coordinate expression of the autosomal dominant polycystic kidney disease proteins, polycystin-2 and polycystin-1, in normal and cystic tissue. Am J Pathol 1999, 154:1721-1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foggensteiner L, Bevan AP, Thomas R, Coleman N, Boulter C, Bradley J, Ibraghimov-Beskrovnaya O, Klinger K, Sandford R: Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol 2000, 11:814-827 [DOI] [PubMed] [Google Scholar]
- 42.Otto E, Kispert A, Schätzle S, Lescher B, Rensing C, Hildebrandt F: Nephrocystin: gene expression and sequence conservation between human, mouse, and Caenorhabditis elegans. J Am Soc Nephrol 2000, 11:270-282 [DOI] [PubMed] [Google Scholar]
- 43.Chapman AB, Rubinstein D, Hugues R, Stears JC, Earnest MP, Johnson AM, Gabow PA, Kaehny WD: Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Engl J Med 1992, 327:916-920 [DOI] [PubMed] [Google Scholar]
- 44.Torres VE, Cai Y, Chen X, Wu GQ, Geng L, Cleghorn KA, Johnson CM, Somlo S: Vascular expression of polycystin-2. J Am Soc Nephrol 2001, 12:1-9 [DOI] [PubMed] [Google Scholar]
- 45.Guillaume R, D’Agati V, Daoust M, Trudel M: Murine Pkd1 is a developmentally regulated gene from morula to adulthood: role in tissue condensation and patterning. Dev Dyn 1999, 214:337-348 [DOI] [PubMed] [Google Scholar]
- 46.Guillaume R, Trudel M: Distinct and common developmental expression patterns of the murine Pkd2 and Pkd1 genes. Mech Dev 2000, 93:179-183 [DOI] [PubMed] [Google Scholar]
- 47.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA: Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci USA 2000, 97:1731-1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, Zhou J: Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet 1997, 17:179-181 [DOI] [PubMed] [Google Scholar]