Abstract
We found that up-regulation of major histocompatibility complex (MHC) class I expression accompanies, but is not required for, appearance of spontaneous myopathy in SJL/J mice. In some neuromuscular diseases, MHC class I expression is markedly up-regulated in muscles, though the consequences of this up-regulation for pathology are not clear. To study MHC class I in myopathy, we compared muscles of SJL/J mice to muscles of SJL/J mice that were also MHC class I-deficient due to targeted mutation in the β-2-microglobulin gene (SJL/J B2m (−/−) mice). SJL/J mice show spontaneous myopathy and have a mutation in the dysferlin gene, a gene which is also mutated in human limb-girdle muscular dystrophy type 2B (LGMD2B). Muscles of eight-month-old SJL/J mice had higher levels of MHC class I expression than muscles of either C57BL/6J (wild-type) or SJL/J B2m (−/−) mice. In contrast, the percentage of abnormal muscle fibers was similar in SJL/J and SJL/J B2m (−/−) muscles. Invading Mac-1+ cells were most abundant in SJL/J B2m (−/−) muscles, moderately abundant in SJL/J muscles, and rare in C57BL/6J muscles. Thus, MHC class I was markedly up-regulated in SJL/J muscles, but this high level of MHC class I was not necessary for the appearance of myopathy.
Though MHC class I proteins are detectable on most types of cells, these proteins are not normally detectable on skeletal muscle fibers in healthy adults. During the course of some neuromuscular diseases, however, MHC class I proteins are markedly up-regulated on skeletal muscle fibers. 1-3 In particular, MHC class I proteins are up-regulated during those neuromuscular diseases in which significant inflammation is found. This finding raises the possibility that antigen presentation by the MHC class I complex on the surface of skeletal muscle cells is required for progression of myopathy in some diseases. Though induced expression of the MHC class I protein, H-2Kb, in normal adult muscle causes an inflammatory myopathy in transgenic mice, 4 the consequences of the MHC class I up-regulation that is often seen in diseased muscle is not well understood. In this work, we use mouse mutants to examine the relationship between up-regulation of MHC class I and the progression of muscle disease.
Mice of the SJL/J strain spontaneously develop myopathy with inflammation and macrophage invasion. 5-7 This myopathy appears to be due to a mutation in the dysferlin gene of SJL/J mice. 8,9 The dysferlin gene is also mutated in the human disease, limb-girdle muscular dystrophy type 2B (LGMD2B), suggesting that SJL/J mice might be used as a model for the human disease. 10,11 By eight months of age, muscles in 100% of SJL/J mice show extensive abnormalities, such as centrally nucleate fibers, necrotic fibers, hypertrophic fibers, atrophic fibers, and infiltrating cells. 7 Most of the muscle-infiltrating cells are macrophages, with smaller numbers of CD4+ and CD8+ cells. 12 SJL/J mice also have abnormalities that appear not to be related to the dysferlin mutation, including suspectibility to lymphoma, experimental myositis, and vascular leakage, 6,7,13,14 perhaps complicating the use of these mice as a LGMD 2B model.
To analyze how MHC class I functions in a myopathy with inflammation, we have compared muscles from three types of mice: C57BL/6J (controls); SJL/J (dysferlin-deficient with normal MHC class I); and SJL/J: β-2-microglobulin (−/−) (both dysferlin- and MHC class I-deficient). Because β-2-microglobulin (B2m) is required for proper assembly of MHC class I proteins on the cell surface, functional MHC class I proteins are nearly eliminated in B2m (−/−) mice. 15 Mice with a targeted mutation in the B2m gene have been used successfully to analyze the role of MHC class I proteins in a number of biological processes. 15-18 Our results show that MHC class I expression is markedly up-regulated in myopathic muscles of SJL/J mice. However, the extent of myopathy in 8- to 9-month-old mice was not affected when this up-regulation of MHC class I was eliminated in SJL/JB2m (−/−) muscles.
Materials and Methods
Mice
C57BL/6J mice, as well as mice of the SJL/J and SJL.129P2(B6)-B2mtm1Unc (termed SJL/J B2m (−/−) in this paper) strains were obtained from the Jackson Laboratory (Bar Harbor, ME). The B2mtm1Unc mutant strain 19 was developed by targeted mutation of the β-2-microglobulin gene in the 129-derived E14TG2a ES cell line. The SJL.129P2(B6)-B2mtm1Unc strain was produced in the laboratory of Dr. Derry Roopenian at The Jackson Laboratory by back-crossing the B2mtm1Unc mutation 10 times to SJL/J inbred mice. Genotypes of the B2m (+/+) and (−/−) mice were confirmed using a polymerase chain reaction-based protocol supplied by the Jackson Laboratory. All mice were females and were analyzed at 8 to 9 months of age. At this age, all SJL/J mice exhibit significant myopathology. 7 SJL/J mice are of the H-2s haplotype and C57BL/6J mice are of the H-2b haplotype.
Antibodies
MHC class I expression was analyzed using a monoclonal antibody produced by M1/42.3.9.8.HLK cells (TIB-126, obtained from American Type Culture Collection, Manassas, VA). This M1/42 mAb, which is a rat IgG2a, reacts with an epitope that is common to all haplotypes of the H-2 protein components of MHC class I molecules. The Mac-1 α-chain (also known as CD11b and integrin αM chain) was analyzed using biotinylated mAb M1/70, a rat IgG2b that is specific for the mouse Mac-1 α-chain at a dilution of 1:250 (Pharmingen, San Diego CA). Expression of β-2-microglobulin was analyzed with a goat anti-B2m serum (Santa Cruz Biotechnology, Santa Cruz, CA) used at a 1:100 dilution. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was detected with a mouse mAb (Research Diagnostics Inc., Flanders, NJ) used at 1:4000 dilution. Binding of primary antibodies was detected with an appropriate secondary antibody system: biotinylated anti-rat secondary antibody used at a 1:200 dilution and coupled with avidin-peroxidase detection (Vector Elite system, Vector Laboratories, Burlingame, CA) with diaminobenzidine as substrate; or HRP-conjugated donkey anti-goat IgG (1:1500 dilution) or goat anti-mouse IgG (1:2000 dilution) (Jackson Immunoresearch, West Grove, PA) used with chemiluminescent substrate (ECL substrate, Amersham, Piscataway, NJ) for immunoblots. Preparation of tissue homogenates and immunoblotting of proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were carried out as described previously. 20 Before SDS-PAGE, tissue homogenates were analyzed by Bradford protein assay, and equal amounts of total protein (60 μg) were analyzed in each lane. After proteins were transferred to polyvinylidene difluoride (PVDF) membranes, protein loading was demonstrated by immunoblotting for GAPDH in muscle samples, or by Ponceau S staining of the most abundant ∼13-kd band for spleen samples (spleen samples did not show reaction with the anti-GAPDH antibody).
Histology
Muscles were dissected, frozen immediately in 2-methylbutane chilled by liquid nitrogen, and 10- to12-μm cryostat sections were prepared from matching regions of muscles for comparison of different genotypes. Sections were stained with hematoxylin and eosin (H&E) or by immunohistochemistry as described previously. 21,22 Abnormal fibers and Mac-1-positive cells were counted by microscopy using 10× or 20× objectives. For each count, the abnormal fibers or Mac-1-positive cells were counted in multiple, adjacent 10× or 20× fields until the entire area of each muscle section was counted, with at least three complete sections observed for each point. The average number ± SD per field was calculated and converted to average ± SD per mm2. Mean differences were assessed for statistical significance by the appropriate analysis of variance test; unpaired, two-tailed t-test; or non-parametric Mann-Whitney test using the InStat computer program (v2.03, GraphPad Software, San Diego, CA).
Results
MHC Class I and B2m Expression
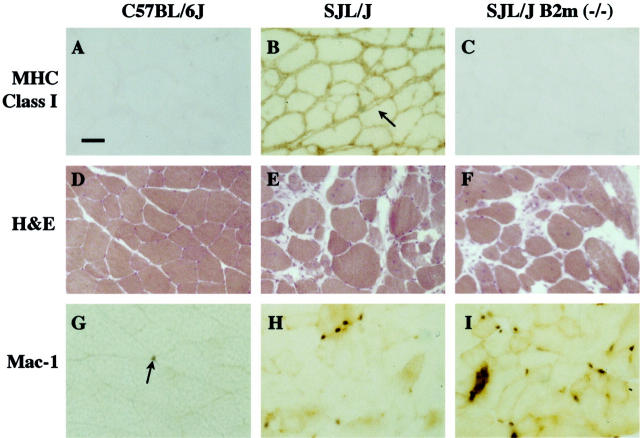
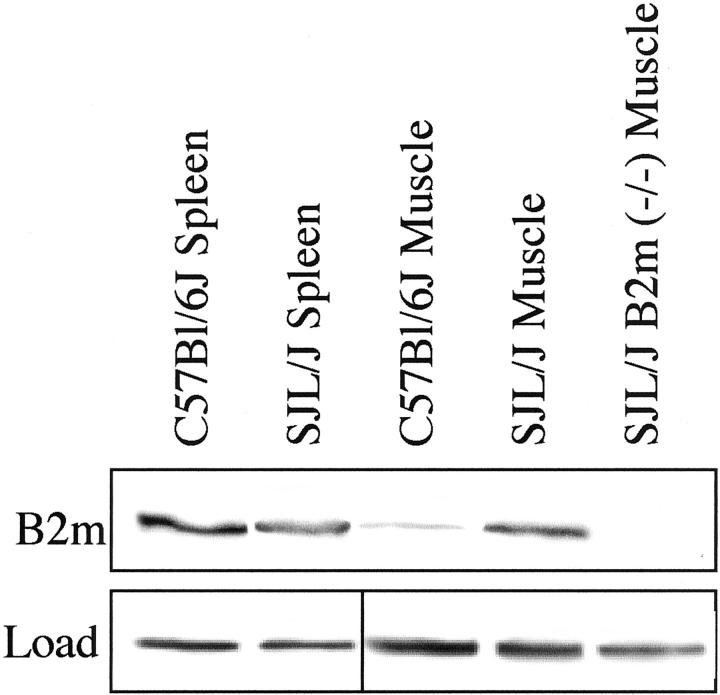
Skeletal muscles in SJL/J mice showed a marked up-regulation of MHC class I and β-2-microglobulin expression. Individual muscles (quadriceps, tibialis anterior, gastrocnemius/soleus complex) were dissected from 8- to 9-month-old mice of the C57BL/6J, SJL/J, and SJL/J B2m (−/−) genotypes and examined by immunohistology with a mAb that reacts with an epitope common to all MHC class I proteins (Figure 1, A–C) ▶ or by immunoblotting with an antibody specific for β-2-microglobulin (Figure 2) ▶ .
Figure 1.
A–C: MHC class I expression is up-regulated in muscles of SJL/J mice compared to muscles of SJL/J B2m (−/−) and C57BL/6J mice. A: Immunohistology of C57BL/6J gastrocnemius muscle showed little staining, a result consistent with the very low expression of MHC class I proteins on normal muscle fibers. B: In contrast, SJL/J muscle showed dark staining apparently on the surface of muscle fibers (eg, arrow), as well as staining on cells in between, and sometimes within, muscle fibers. C: In contrast, SJL/J B2m (−/−) muscle showed very little staining, a result expected for tissues lacking β-2-microglobulin. Scale bar, 40 μm. D–F: Muscles from SJL/J and SJL/J B2m (−/−) mice showed similar extents of myopathy. D: H&E staining showed normal fibers in wild-type C57BL/6J gastrocnemius muscle. E and F: In contrast, abnormal fibers were abundant and found at similar percentages in SJL/J (E) and SJL/J B2 m (−/−) (F) muscles as described in Table 1 ▶ . G and H: Mac-1-positive cells were most abundant in myopathic muscles of SJL/J B2m (−/−) mice, moderately abundant in SJL/muscles, and rare in the normal muscles of C57BL/6J mice. G: Immunohistochemistry showed that Mac-1-postive cells (arrow) were rare in the gastrocnemius muscle of C57BL/6J wild-type mice. H and I: In contrast, Mac-1-positive cells were abundant in SJL/J (H) and SJL/J B2m (−/−) (I) muscles, being most abundant in the B2m (−/−) muscles as described in Table 2 ▶ .
Figure 2.
β-2-microglobulin levels were up-regulated in SJL/J muscle. Hindlimb muscles from mice of the indicated genotypes were analyzed by immunoblotting with antibody specific for β-2-microglobulin (Upper panel, B2m). Spleens from C57BL/6J and SJL/J mice had about equal levels of β-2-microglobulin. In contrast, β-2-microglobulin was much more abundant in SJL/J muscle than in C57BL/6J muscle, and was absent from SJL/J B2m (−/−) muscle, a result that was consistent with the MHC class I immunohistochemistry analyses in Figure 1 ▶ . Each lane had 60 μg of cell lysate protein. The loading controls (Lower panel, Load) for the spleen samples show Ponceau S staining of an abundant ∼13-kd protein band, whereas loading controls for the muscle samples show immunoblotting for GAPDH.
Muscle fibers from control C57BL/6J mice showed little staining for MHC class I proteins, a result that is consistent with the usually undetectable level of class I proteins on healthy, adult muscle fibers (Figure 1A) ▶ . Light staining was detected on non-muscle cells. In contrast, SJL/J mice showed extensive staining for MHC class I proteins in muscles (Figure 1B) ▶ . This staining was continuous around entire muscle fibers in most regions of the muscles (Figure 1B) ▶ , but limited to a subset of fibers in other regions (not shown). Though the staining often appeared to fill the space between myofibers, there were areas in which staining on adjacent myofibers could be resolved into two distinct lines (Figure 1B ▶ , arrow), suggesting that staining in these regions may have been associated with myofiber surfaces. In addition, these SJL/J muscles also contained class I-positive mononucleate cells that were located in between fibers and sometimes within apparently dying fibers. Finally, muscles from SJL/J B2m (−/−) mice showed little or no staining for MHC class I expression, consistent with the lack of β-2-microglobulin and subsequent large reduction of functional MHC class I proteins (Figure 1C) ▶ .
Consistent with the increased MHC class I expression, SJL/J muscle also had increased expression of β-2-microglobulin. Immunoblotting showed that β-2-microglobulin was much more abundant in SJL/J muscles than in C57BL/6J muscles, even though spleens from mice of these two genotypes showed similar levels of β-2-microglobulin (Figure 2) ▶ . As expected, the SJL/J B2m (−/−) muscles showed no detectable β-2-microglobulin.
Myopathology
In contrast to the marked differences in MHC class I expression in the muscles of SJL/J and SJL/J B2m (−/−) mice, the muscles of these two genotypes showed no consistent differences in muscle pathology (Figure 1, D–F ▶ , Table 1 ▶ ). Individual muscles (quadriceps, tibialis anterior, gastrocnemius/soleus complex) were dissected from 8- to 9-month-old mice of the C57BL/6J, SJL/J, and SJL/J B2m (−/−) genotypes. Muscles were sectioned, H&E stained, and examined to determine the relative numbers of normal and abnormal muscle fibers. Muscle fibers were considered to be abnormal if they were (1) centrally nucleate or small and basophilic (indicating regeneration), (2) atrophic (<20 μm diameter) or hypertrophic (>100 μm diameter, (3) apparently necrotic with infiltrating cells, or (4) apparently replaced by fat or collagen (cf. ref. 7 ).
Table 1.
Effect of Genotype on Number of Abnormal Muscle Fibers in 8 to 9-Month-Old Mice
| Muscle | Genotype | Mouse | Fibers/mm2 ± SD (n)* | % Abnormal | |
|---|---|---|---|---|---|
| Abnormal† | Normal | ||||
| Tibialis anterior | SJL/J | 1 | 170.0 ± 30.2 (8) | 229.0 ± 51.1 (8) | 43% |
| 2 | 108.5 ± 28.3 (8) | 343.5 ± 41.5 (8) | 24% | ||
| 3 | 252.8 ± 36.9 (6) | 142.3 ± 20.0 (6) | 64% | ||
| SJL/J B2m (−/−) | 1 | 281.2 ± 56.0 (5) | 137.7 ± 42.0 (5) | 67% | |
| 2 | 364.0 ± 87.2 (5) | 104.0 ± 18.1 (5) | 77% | ||
| Gastrocnemius/Soleus | SJL/J | 1 | 86.0 ± 29.2 (8) | 359.0 ± 30.1 (8) | 19% |
| 2 | 101.0 ± 26.0 (8) | 370.6 ± 69.5 (8) | 21% | ||
| 3 | 152.0 ± 20.0 (6) | 257.5 ± 54.3 (6) | 37% | ||
| SJL/J B2m (−/−) | 1 | 119.1 ± 28.4 (9) | 313.4 ± 58.8 (9) | 28% | |
| 2 | 116.8 ± 24.4 (5) | 313.5 ± 35.4 (5) | 27% | ||
| Quadriceps | SJL/J | 1 | 216.5 ± 63.3 (8) | 228.0 ± 28.3 (8) | 49% |
| 2 | 249.5 ± 50.9 (8) | 238.5 ± 41.5 (8) | 51% | ||
| 3 | 172.7 ± 46.4 (10) | 228.3 ± 63.3 (10) | 43% | ||
| SJL/J B2m (−/−) | 1 | 181.6 ± 57.6 (9) | 259.2 ± 118.5 (9) | 41% | |
| 2 | 296.0 ± 15.2 (5) | 156.0 ± 93.3 (5) | 65% | ||
*Number of sections examined.
†Criteria for distinguishing abnormal from normal fibers are listed in Materials and Methods.
SJL/J muscles with or without β-2-microglobulin showed extensive muscle pathology when compared to normal muscles. In C57BL/6J muscles, abnormal fibers were very rare (<0.5%) as expected for healthy, normal muscles (Figure 1D) ▶ . In contrast, abnormal fibers were abundant in both SJL/J (Figure 1E) ▶ and SJL/J B2m (−/−) muscles (Figure 1F) ▶ . On examination of multiple individual muscles, we sometimes found differences in the percentages of abnormal fibers between the SJL/J and SJL/J B2m (−/−) muscles, but these percentage differences were neither consistently in one direction nor statistically significant (Table 1) ▶ . Muscles of both genotypes showed the same distribution of abnormalities, with >90% of the abnormal fibers classified as central nucleate (not shown). Myopathy appeared to have progressed to different extents in different muscles. About one-third (19 to 37%) of the fibers were abnormal in the gastrocnemius/soleus muscles, whereas about one-half or more of the fibers were abnormal in most samples of the quadriceps (41 to 65%) and tibialis anterior muscles (24 to 77%) (Table 1) ▶ .
Infiltrating Cells
Mac-1-positive cells are the predominant infiltrating cells in myopathic SJL/J muscles. 7,12 To examine how infiltrating cell numbers might be affected by MHC class I expression in SJL/J myopathy, we used immunohistology with an anti-Mac-1 mAb to determine the number of Mac-1-positive cells in muscles of the C57BL/6J, SJL/J, and SJL/J B2m (−/−) genotypes (Figure 1, G–I) ▶ .
Mac-1-positive cells were quite rare in healthy C57BL/6J muscles (Figure 1G ▶ and Table 2 ▶ ). In myopathic SJL/J muscles, in contrast, Mac-1-positive cells were much more abundant, reaching levels that were ∼3- to 5-fold higher than in normal muscles (Figure 1H ▶ and Table 2 ▶ ). However, the highest abundance of Mac-1-positive cells was found in SJL/J B2m (−/−) muscles, where the density of Mac-1-psoitive cells was ∼10- to 13-fold greater than in C57BL/6J muscles and ∼2 to 4 × higher than in SJL/J muscles (Figure 1I ▶ , Table 2 ▶ ). Statistical analysis by analysis of variance showed that, with the sole exception of the quadriceps sample from SJL/J mouse no. 3 (Table 2) ▶ , individual muscles of the same genotype had similar abundances of Mac-1-positive cells, whereas individual muscles of different genotypes had significantly different abundances of Mac-1-positive cells (P < 0.01).
Table 2.
Effect of Genotype on Number of Mac-1-Positive Cells in Skeletal Muscles of 8-Month-Old Mice
| Muscle | Genotype | Mouse | Mac-1+ cells/mm2 ± SD (n)* |
|---|---|---|---|
| Tibialis anterior | SJL/J | 1 | 20.5 ± 9.4 (15) |
| 2 | 14.8 ± 8.3 (18) | ||
| 3 | 14.5 ± 13.5 (25) | ||
| SJL/J B2m (−/−) | 1 | 55.7 ± 13.5 (15) | |
| 2 | 48.2 ± 18.6 (17) | ||
| C57BL/6J | 1 | 4.1 ± 2.8 (22) | |
| 2 | 4.1 ± 2.4 (23) | ||
| Gastrocnemius/soleus | SJL/J | 1 | 13.0 ± 5.4 (22) |
| 2 | 16.5 ± 9.5 (22) | ||
| 3 | 15.8 ± 13.1 (18) | ||
| SJL/J B2m (−/−) | 1 | 45.3 ± 10.3 (15) | |
| 2 | 41.6 ± 23.5 (19) | ||
| C57BL/6J | 1 | 4.4 ± 2.0 (20) | |
| 2 | 4.5 ± 2.6 (23) | ||
| Quadriceps | SJL/J | 1 | 25.3 ± 19.0 (30) |
| 2 | 23.5 ± 20.5 (33) | ||
| 3 | 37.1 ± 28.6 (21) | ||
| SJL/J B2m (−/−) | 1 | 53.8 ± 17.5 (20) | |
| 2 | 39.8 ± 19.3 (21) | ||
| C57BL/6J | 1 | 4.0 ± 4.3 (20) | |
| 2 | 4.6 ± 6.5 (25) |
*Number of 1 mm2 fields examined.
Discussion
Though MHC class I expression was markedly up-regulated in myopathic muscles of dysferlin-deficient SJL/J mice, elimination of this up-regulation by genetic deletion of β-2-microglobulin did not alter the extent of myopathy seen in adult mice. Thus, high level expression of functional MHC class I proteins does not appear to play a necessary role in the initiation of the spontaneous myopathy seen in SJL/J mice.
All SJL/J mice spontaneously develop a myopathy that is well established by eight months of age. 7 This spontaneous myopathy is accompanied by up-regulation of MHC class I expression on muscle fibers that begins at 4 to 6 weeks of age 12 and is nearly complete by eight months of age (this work). Because up-regulation of MHC class I proteins is found in many neuromuscular diseases, it has been suggested that MHC class I may play a key role in regulating inflammation and disease progression in some neuromuscular diseases. However, despite the fact that induced expression of MHC class I in adult muscle is sufficient to initiate inflammatory myositis, 4 class I up-regulation does not appear to necessary for either experimentally induced autoimmune myasthenia gravis 16 or dysferlin-deficiency myopathy in SJL/J mice (this work).
The use of B2m (−/−) mice for analysis of MHC class I function in muscle disease has certain limitations. First, SJL/J B2m (−/−) mice lack functional MHC class I not just on muscle fibers, where it is up-regulated compared to healthy muscle, but also on all other cell types that normally express these proteins. It is possible, though unlikely, that muscle-specific depletion of MHC class I, unlike complete depletion, would change the outcome of myopathy in SJL/J mice.
Second, B2m (−/−) mice may have a very low level (<5% of wild-type) of functional class I protein of the H-2D group (eg, H-2Ds for SJL/J mice or H-2Db for C57BL/6J mice), though this low level of functional protein may be confined to particular T cell subgroups. 15 It is clear, however, that muscles in the SJL/J B2m (−/−) mice have greatly reduced MHC class I protein levels compared to the very high levels found in muscles in SJL/J mice. Thus, we conclude that this marked up-regulation of MHC class I in muscles is not required for spontaneous myopathy in SJL/J mice.
Finally, B2m (−/−) mice have NK cell deficiency, decreased levels of serum Ig, few CD8+ cytotoxic T cells, and under some circumstances a compensatory increase in CD4+ cytotoxic T cells. 15 Evidently, these changes in immune system function do not inhibit the development of myopathy in SJL/J mice. Because CD8+ cytotoxic T cells appear to promote myopathy in dystrophin-deficient mice, 23-25 it is noteworthy that the severe (though not complete) depletion of these cells in SJL/JB2m (−/−) mice has no effect on the SJL/J, dysferlin-deficient myopathy. A further study is needed to determine whether complete elimination of CD8+ cytotoxic cells will ameliorate SJL/J myopathy.
In addition to exhibiting spontaneous myopathy at several months of age, the SJL/J strain is also uniquely susceptible to developing a rapid, apparently autoimmune myopathy in response to injection of syngeneic muscle proteins during the first 4 to 6 weeks of life. 26-29 This autoimmune myositis can be induced in normal mice that are dysferlin-positive by transfer of T cells or IgG from immunized SJL/J mice. 29 Whether the susceptibility to experimentally induced myositis in SJL/J mice is dependent in some manner on the presence of the dysferlin mutation remains to be determined. Further studies are also needed to determine whether this susceptibility may require the MHC class I up-regulation that begins on or near a small percentage of SJL/J muscle fibers as soon as 4 to 6 weeks after birth 12 and progresses until MHC class I is expressed around a large majority of muscle fibers by 8 to 9 months after birth (this work).
After injury or during some diseases, Mac-1-positive cells (largely macrophages) enter skeletal muscles in large numbers. Macrophages appear to regulate the removal of dead fibers, as well as the speed at which abnormal fibers are repaired or replaced by activated satellite cell myoblasts. 30-33 Macrophages are also the predominant infiltrating cell in SJL/J muscles, 12 and the abundance of infiltrating cells may correlate with the severity of myopathy. Because SJL/J myopathy appeared unaffected by the B2m (−/−) locus, it was surprising to find that the level of Mac-1-positive cells was higher in SJL/J B2m (−/−) muscles than the already high levels found in SJL/J muscles. The different Mac-1-positive cell numbers might indicate that disease progression may differ between SJL/J and SJL/J B2m (−/−) muscles, though our limited study at one time point was not designed to test this possibility.
The mechanism underlying the different numbers of Mac-1-positive cells remains to be determined, though it is possible that, because Mac-1 is expressed on other cell types such as granulocytes, the infiltrate in the B2m (−/−) cells may be composed of different proportions of macrophages, granulocytes (cf. ref. 24 ), and possibly additional Mac-1-positive cells. Another possibility is that Mac-1-positive cells may undergo less apoptosis and thus survive longer and at higher levels in B2m (−/−) muscles. A role for apoptosis in the survival of infiltrating cells is suggested by the finding that inhibition of the apoptosis regulator FasL improves survival of phagocytic cells during muscle regeneration. 33 Because damaged muscle is a source of several inflammatory signals (reviewed in 34), it is also possible that B2m (−/−) and wild-type muscles produce different levels of inflammatory signals such as intercellular adhesion molecule (ICAM)-1 (which interacts with the Mac-1 protein), nitric oxide, or inflammatory cytokines.
The spontaneous SJL/J myopathy was initially thought to be a model for autoimmune myositis. With the finding that SJL/J mice are homozygous for an inactivating mutation in the dysferlin gene, however, it was suggested that SJL/J mice are a model for the human disease limb-girdle muscular dystrophy 2B in which this gene is also inactivated. 10 In contrast to SJL/J mice, however, a recent study found that MHC class I expression was not up-regulated in the muscles of four LGMD 2B patients carrying a particular dysferlin splicing mutation, though muscles of two patients had an inflammatory infiltrate. 35 In dysferlin-deficient patients, the severity of disease varies with both the specific dysferlin mutation and additional modifying factors that are currently not well understood. 36-38 Whether up-regulation of MHC class I expression might accompany particular dysferlin mutations or vary with stage of the human disease remains to be determined. It will be useful to use breeding experiments to determine whether the MHC class I up-regulation seen in SJL/J muscle is independent of the dysferlin mutation, as might be predicted from the limited human studies. In the meantime, SJL/J mice should be used with caution as a model of human LGMD 2B.
Acknowledgments
We thank Michael Lin and Cliff Swap for help with experiments. We thank G. Pavlath (Emory University School of Medicine) for reagents and advice.
Footnotes
Address reprint requests to Dr. Jeffrey B. Miller, Boston Biomedical Research Institute, 64 Grove Street, Watertown, MA 02478. E-mail: miller@bbri.org.
Supported by grants from National Institute of Arthritis and Musculoskeletal and Skin Diseases and the W. R. Hearst Fund of Harvard Medical School (to J. D.) and by grants from USDA (National Research Initiative Competitive Grants Program), National Institute of Dental and Craniofacial Research, National Heart, Lung, and Blood Institute, and the Muscular Dystrophy Association (to J. B. M.).
References
- 1.Karpati G, Pouliot Y, Carpenter S: Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol 1988, 23:64-72 [DOI] [PubMed] [Google Scholar]
- 2.Emslie-Smith AM, Arahata K, Engel AG: Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum Pathol 1989, 20:224-231 [DOI] [PubMed] [Google Scholar]
- 3.McDouall RM, Dunn MJ, Dubowitz V: Expression of class I and class II MHC antigens in neuromuscular diseases. J Neurol Sci 1989, 89:213-226 [DOI] [PubMed] [Google Scholar]
- 4.Nagaraju K, Raben N, Loeffler L, Parker T, Rochon PJ, Lee E, Danning C, Wada R, Thompson C, Bahtiyar G, Craft J, Hooft Van Huijsduijnen R, Plotz P: From the cover: conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc Natl Acad Sci USA 2000, 97:9209–9214 [DOI] [PMC free article] [PubMed]
- 5.Hohlfeld R, Muller W, Toyka KV: Necrotizing myopathy in SJL mice. Muscle Nerve 1988, 11:184-185 [PubMed] [Google Scholar]
- 6.Tamir S, deRojas-Walker T, Gal A, Weller AH, Li X, Fox JG, Wogan GN, Tannenbaum SR: Nitric oxide production in relation to spontaneous B-cell lymphoma and myositis in SJL mice. Cancer Res 1995, 55:4391-4397 [PubMed] [Google Scholar]
- 7.Weller AH, Magliato SA, Bell KP, Rosenberg NL: Spontaneous myopathy in the SJL/J mouse: pathology and strength loss. Muscle Nerve 1997, 20:72-82 [DOI] [PubMed] [Google Scholar]
- 8.Bittner RE, Anderson LVB, Burkhardt E, Bashir R, Valiadake E, Ivanova S, Raffelsberger T, Maerk I, Hoger H, Jung M, Karbasiyan M, Storch M, Lassmann H, Moss JA, Davison K, Harrison R, Bushby KMD, Reis A: Dysferlin mutation in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat Genet 1999, 23:141-142 [DOI] [PubMed] [Google Scholar]
- 9.Vafiadaki E, Reis A, Keers S, Harrison R, Anderson LV, Raffelsberger T, Ivanova S, Hoger H, Bittner RE, Bushby K, Bashir R: Cloning of the mouse dysferlin gene and genomic characterization of the SJL-Dysf mutation. Neuroreport 2001, 12:625-629 [DOI] [PubMed] [Google Scholar]
- 10.Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, M, Richard I, Marchand S, Bourg N, Argov Z, Sadeh M, Mahjneh I, Marconi G, Passos-Bueno MR, Moreira ED, Zatz M, Beckmann JS, Bushby K: A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet 1998, 20:37–42 [DOI] [PubMed]
- 11.Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, Serrano C, Urtizberea JA, Hentati F, Hamida MB, Bohlega S, Culper EJ, Amato AA, Bossie K, Oeltjen J, Bejaoui K, McKenna-Yasek D, Hosler BA, Schurr E, Arahata K, de Jong PJ, Brown RH: Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet 1998, 20:31-36 [DOI] [PubMed] [Google Scholar]
- 12.Kampman MT, Benestad SL, Fladby T, Maehlen J: Denervation enhances spontaneous inflammatory myopathy in SJL mice. Muscle Nerve 1999, 22:883-888 [DOI] [PubMed] [Google Scholar]
- 13.Roberts P, McGeachie JK, Grounds MD: The host environment determines strain-specific differences in the timing of skeletal muscle regeneration: cross-transplantation studies between SJL/J and BALB/c mice. J Anat 1997, 191:585-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harmon KJ, Couper LL, Lindner V: Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol 2000, 156:1741-1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raulet DH: MHC class I-deficient mice. Adv Immunol 1994, 55:381-421 [DOI] [PubMed] [Google Scholar]
- 16.Shenoy M, Kaul R, Goluszko E, David C, Christadoss P: Effect of MHC class I and CD8 cell deficiency on experimental autoimmune myasthenia gravis pathogenesis. J Immunol 1994, 153:5330-5335 [PubMed] [Google Scholar]
- 17.Christianson GJ, Blankenburg RL, Duffy TM, Panka D, Roths JB, Marshak-Rothstein A, Roopenian DC: beta2-Microglobulin dependence of the lupus-like autoimmune syndrome of MRL-lpr mice. J Immunol 1996, 156:4932-4939 [PubMed] [Google Scholar]
- 18.Kay TW, Parker JL, Stephens LA, Thomas HE, Allison J: RIP-beta 2-microglobulin transgene expression restores insulitis, but not diabetes, in beta 2-microglobulin null non-obese diabetic mice. J Immunol 1996, 157:3688-3693 [PubMed] [Google Scholar]
- 19.Koller BH, Marrack P, Kappler JW, Smithies O: Normal development of mice deficient in beta-2-microglobulin, MHC class I proteins, and CD8+ T cells. Science 1990, 248:1227-1229 [DOI] [PubMed] [Google Scholar]
- 20.Dominov JA, Dunn JJ, Miller JB: Bcl-2 expression identifies an early stage of myogenesis and promotes clonal expansion of muscle cells. J Cell Biol 1998, 142:537-544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Z, Miller JB: MRF4 can substitute for myogenin during early myogenesis. Dev Dyn 1997, 209:233-241 [DOI] [PubMed] [Google Scholar]
- 22.Dominov JA, Houlihan-Kawamoto CA, Swap CJ, Miller JB: Pro- and anti-apoptotic members of the Bcl-2 family in skeletal muscle: a role for Bcl-2 in later stages of myogenesis. Dev Dyn 2001, 220:18-26 [DOI] [PubMed] [Google Scholar]
- 23.Spencer MJ, Walsh CM, Dorshkind KA, Rodriguez EM, Tidball JG: Myonuclear apoptosis in dystrophic mdx muscle occurs by perforin-mediated cytotoxicity. J Clin Invest 1999, 99:2745-2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai B, Spencer MJ, Nakamura G, Tseng-Ong L, Tidball JG: Eosinophilia of dystrophin-deficient muscle is promoted by perforin-mediated cytotoxicity by T cell effectors. Am J Pathol 2000, 156:1789-1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG: Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol 2001, 98:235-243 [DOI] [PubMed] [Google Scholar]
- 26.Hart MN, Linthicum DS, Waldschmidt MM, Tassell SK, Schelper RL, Robinson RA: Experimental autoimmune inflammatory myopathy. J Neuropathol Exp Neurol 1987, 46:511-521 [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg NL: Experimental models of inflammatory myopathies. Ballieres Clin Neurol 1987, 2:693-715 [PubMed] [Google Scholar]
- 28.Rosenberg NL, Bell KP, Kotzin BL: Susceptibility to induction of experimental myositis is not under primary control of the H-2 complex. Neurology 1989, 39:131-132 [Google Scholar]
- 29.Matsubara S, Okumura S: Experimental autoimmune myositis in SJL/J mice produced by immunization with syngeneic myosin B fraction: transfer by both immunoglobulin G and T cells. J Neurol Sci 1996, 144:171-175 [DOI] [PubMed] [Google Scholar]
- 30.Robertson TA, Maley MA, Grounds MD, Papadimitriou JM: The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res 1993, 207:321-331 [DOI] [PubMed] [Google Scholar]
- 31.Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E: Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord 1999, 9:72-80 [DOI] [PubMed] [Google Scholar]
- 32.Merly F, Lescaudron L, Rouaud T, Crossin F, Gardahaut MF: Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve 1999, 22:724-732 [DOI] [PubMed] [Google Scholar]
- 33.Sandri M, Sandri C, Brun B, Giuisato E, Cantini M, Rossin K, Destro C, Arslan P, Carraro U: Inhibition of fasL sustains phagocytic cells and delays myogenesis in regenerating muscle fibers. J Leukoc Biol 2001, 69:482-489 [PubMed] [Google Scholar]
- 34.Best TM, Hunter KD: Muscle injury and repair. Phys Med Rehabil Clin North Am 2000, 11:251-266 [PubMed] [Google Scholar]
- 35.McNally EM, Ly CT, Rosenmann H, Mitrani Rosenbaum S, Jiang W, Anderson LV, Soffer D, Argov Z: Splicing mutation in dysferlin produces limb-girdle muscular dystrophy with inflammation. Am J Med Genet 2000, 91:305–312 [DOI] [PubMed]
- 36.Weiler T, Bashir R, Anderson LV, Davison K, Moss JA, Britton S, Nylen E, Keers S, Vafiadaki E, Greenberg CR, Bushby CR, Wrogemann K: Identical mutation in patients with limb girdle muscular dystrophy type 2B or Miyoshi myopathy suggests a role for modifier gene(s). Hum Mol Genet 1999, 8:871-877 [DOI] [PubMed] [Google Scholar]
- 37.Illarioshkin SN, Ivanova-Smolenskaya IA, Greenberg CR, Nylen E, Sukhorukov VS, Poleshchuk VV, Markova ED, Wrogemann K: Identical dysferlin mutation in limb-girdle muscular dystrophy type 2B and distal myopathy. Neurology 2000, 55:1931-1933 [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa M, Matsuzaki T, Suehara M, Kanzato N, Takashima H, Higuchi II, Matsumura T, Goto K, Arahata K, Osame M: Phenotypic variation in a large Japanese family with Miyoshi myopathy with nonsense mutation in exon 19 of dysferlin gene. J Neurol Sci 2001, 184:15-19 [DOI] [PubMed] [Google Scholar]