Abstract
Retinal neovascularization occurs in a variety of diseases including diabetic retinopathy, the most common cause of blindness in the developed world. There is accordingly considerable incentive to develop drugs that target the aberrant angiogenesis associated with these conditions. Previous studies have shown that a number of anti-angiogenic agents can inhibit retinal neovascularization in a well-characterized murine model of ischemia-induced proliferative retinopathy. Combretastatin-A4 (CA-4) is an anti-vascular tubulin-binding agent currently undergoing clinical evaluation for the treatment of solid tumors. We have recently shown that CA-4 is not tumor-specific but elicits anti-vascular effects in nonneoplastic angiogenic vessels. In this study we have examined the capacity of CA-4 to inhibit retinal neovascularization in vivo. CA-4 caused a dose-dependent inhibition of neovascularization with no apparent side effects. The absence of vascular abnormalities or remnants of disrupted neovessels in retinas of CA-4-treated mice suggests an anti-angiogenic mechanism in this model, in contrast to the anti-vascular effects observed against established tumor vessels. Importantly, histological and immunohistochemical analyses indicated that CA-4 permitted the development of normal retinal vasculature while inhibiting aberrant neovascularization. These data are consistent with CA-4 eliciting tissue-dependent anti-angiogenic effects and suggest that CA-4 has potential in the treatment of nonneoplastic diseases with an angiogenic component.
The recognition that angiogenesis, the sprouting of neovessels from pre-existing vasculature, is a prerequisite for tumor growth beyond a threshold size 1 has led recently to the development of many anti-angiogenic agents for cancer therapy. 2 Such agents may also prove useful in the treatment of other diseases with an angiogenic component, which include rheumatoid arthritis, atherosclerosis, and retinopathy. 3 Furthermore, the characterization of the effects of these agents in nonneoplastic tissues may identify diseases against which they have potential therapeutic effects. It is probable that tissue-specific differences in the regulation of angiogenesis will result in differential efficacy of individual anti-angiogenic agents in the treatment of different pathological conditions.
Pathological angiogenesis is the underlying cause of several retinopathies that collectively are the major cause of blindness in the developed world. 4 In the normal eye the inner retinal layers are supplied by the retinal vasculature that is formed by three main capillary beds. The superficial retinal capillary bed is comprised of arterioles running along the inner surface of the retina, beneath the inner limiting membrane that is the interface between the retina and the avascular vitreous. This capillary bed develops through vasculogenesis, the generation of functional blood vessels from endothelial precursor cells. Capillaries branch from the peripheral retinal vasculature by sprouting angiogenesis and penetrate through the inner two-thirds of the retina to form the intermediate and deep retinal capillary beds. The choroidal circulation 5 supplies the outer retinal layers. Retinopathy arises from ischemia-induced pathological retinal angiogenesis in which aberrant neovascular growth results in the development of neovessels that protrude beyond the retinal inner limiting membrane into the vitreous, causing severe loss of vision and frequently leading to retinal detachment. Photocoagulation is the most common therapeutic strategy for the treatment of retinopathy but it causes deleterious side effects including loss of peripheral and night vision. There is significant interest therefore in the characterization of drugs that may be of therapeutic benefit in proliferative retinopathies.
A well-characterized murine model of oxygen-induced proliferative retinopathy closely simulates retinopathy of prematurity and exhibits characteristics common to a variety of other ischemia-induced retinopathies including diabetic retinopathy. 6 In this model neonatal mice are exposed to hyperoxic conditions that lead to the regression of the developing retinal vasculature. A return to the relative hypoxia of ambient atmospheric conditions results in ischemia and induces extensive neovascularization leading to the formation of numerous neovessels. These vessels breach the inner limiting membrane into the vitreous in a manner closely resembling retinopathy of prematurity that also shares several characteristics with diabetic retinopathy. A variety of anti-angiogenic agents have been shown to inhibit proliferative retinopathy in this model with varying degrees of efficacy. The predominance of vascular endothelial growth factor as the proangiogenic stimulus for retinal neovascularization 7-9 has focused attention on the use of vascular endothelial growth factor antagonists, some of which have been shown to be highly effective inhibitors of proliferative retinopathy achieving almost complete inhibition of neovascularization. However, other vascular endothelial growth factor antagonists are less effective, which may reflect the limitations of individual experimental systems or the activity of additional proangiogenic signals. 10,11 Inadequate inhibition of angiogenesis by endogenous anti-angiogenic factors is also likely to be important in the progression of pathological angiogenesis and such factors offer potential for therapeutic exploitation. For example, recent reports have described the importance of the naturally occurring ocular angiogenesis inhibitor pigment epithelial-derived factor in the regulation of retinal angiogenesis and its efficacy at inhibiting retinopathy. 12
Cis combretastatin-A4 (CA-4) is a tubulin-binding agent originally isolated from the South African shrub Combretum caffrum. The water-soluble phosphate prodrug (CA-4P) is rapidly hydrolyzed by endogenous phosphatases in vivo to yield the parent drug. CA-4 elicits acute anti-vascular effects in established tumor blood vessels, causing tumor necrosis secondary to hemorrhage, and has recently completed phase I clinical trials in the United Kingdom and United States for the treatment of solid tumors. 13 The extent to which the tubulin-binding activity of CA-4 accounts for its mechanism of action in vivo remains to be elucidated. Using the hyperplastic thyroid as a model of pathological angiogenesis, we have recently shown that CA-4 is not a tumor-specific anti-vascular agent because it causes disruption of neovessels in nonneoplastic tissue. 14 In the hyperplastic thyroid this anti-vascular activity was manifested as the formation of multiple microthrombi, indicating that that the anti-vascular effects elicited by CA-4 are tissue-specific. These data suggested that CA-4 should be evaluated for the treatment of a variety of angioproliferative diseases in addition to its current potential as an anti-cancer agent. In this study we have investigated the effects of CA-4 on proliferative retinopathy using the murine model described.
Materials and Methods
Drug Treatment of Mice with Proliferative Retinopathy
Proliferative retinopathy was induced in neonatal C57BL/6J mice as previously described. 6 Seven-day-old mice (P7) and nursing dams were placed in an airtight chamber and exposed to 75% O2 (±2%, flow rate 1 L/min) for 5 days, when mice were removed from the chamber and returned to ambient conditions. After 24 hours, daily treatment with 0.78, 1.56, 3.125, 6.25, or 12.0 mg/kg CA-4P (intraperitoneally, total injection volume of 20 μl, synthesized as previously described 15-17 ) was commenced. At P17, mice were killed and eyes were enucleated, fixed in neutral-buffered formalin for 24 hours and processed for paraffin sectioning. To visualize the retinal vasculature, mice were perfused via the left ventricle with 700 μl of fluorescein isothiocyanate-conjugated dextran (Mr 2,000,000, 50 mg/ml; Sigma, Poole, UK). Mice were killed and eyes were enucleated and fixed in neutral-buffered formalin (NBF) for 24 hours. Retinal flat mounts were prepared and viewed by confocal microscopy (Leica, Heidelberg, Germany). Animal studies were performed in accordance with institutional procedures.
Quantitation of Retinal Neovascularization
For histological analysis, retinal sections were stained using hematoxylin and eosin. To aid quantitation of nuclei penetrating beyond the inner limiting membrane, coded sections were stained using 4,6-diamidino-2-phenylindole (Molecular Probes, Leiden, The Netherlands) as previously described. 18 The number of nuclei crossing the inner limiting membrane were counted independently by two investigators in a minimum of 12 sections per eye from at least three levels 50 μm apart. Statistical analysis was performed using Student’s t-test. Values are expressed as the mean ± SD. Values of P < 0.01 were considered statistically significant.
Volume Fraction of Endothelial Cells Below the Inner Limiting Membrane
The volume fraction (Vv) of endothelial cells below the inner limiting membrane was estimated by stereological analysis at a magnification of ×1250. A square lattice with 10-μm intervals was overlaid on the section image. Vv was estimated from:
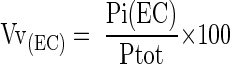 |
where Pi(EC) = points landing on endothelial cells and Ptot = total points on reference area. 19 Variance in group data (n = 4) was tested initially by analysis of variance that revealed statistically significant differences between groups (P < 0.032). Analysis for homogenous means between groups was tested using a post hoc Duncan’s multiple range test.
Histochemical Analysis
To assess vascular development and to determine whether endothelial cells penetrated beyond the inner limiting membrane, sections were stained using the endothelial-specific Griffonia simplicifolia lectin B4. Paraffin sections were cleared through xylene and ethanol washes, permeabilized in ice-cold acetone for 10 minutes, and endogenous peroxidase activity quenched by using 3% H2O2 in methanol for 15 minutes. Biotinylated lectin B4 (Sigma) was applied for 1 hour at 37°C and detected using ExtrAvidin:HRP and metal-enhanced diaminobenzidine (Sigma).
Results
Inhibition of Neovascularization by CA-4
The murine model of ischemia-induced proliferative retinopathy was used to determine whether CA-4 inhibits pathological angiogenesis. To test the effects of CA-4 on retinal neovascularization, mice were exposed to hyperoxia, returned to ambient conditions, and daily intraperitoneal administration of CA-4P was commenced after 24 hours. Histological analysis of retinas from all mice that did not receive CA-4P revealed extensive retinopathy with multiple neovascular tufts extending into the vitreous. Erythrocytes were frequently detectable in these neovessels (Figure 1A) ▶ . In contrast, the retinas of normal P17 mice had no cellular protrusions beyond the inner limiting membrane (Figure 1B) ▶ . A dose-dependent inhibition of neovascularization was observed in retinas of mice treated with CA-4P. In mice treated with 3.125-mg/kg/24 hours CA-4P, vascular tufts were rarely observed and the predominating morphology of the retinal surface appeared normal (Figure 1C) ▶ . No remnants of disrupted neovessels or microthrombi associated with the inner limiting membrane were evident and histological analysis indicated that the normal underlying retinal architecture was maintained. Similar qualitative analysis showed that lower doses of CA-4P were less effective in preventing the formation of neovascular tufts (Figure 1D) ▶ .
Figure 1.
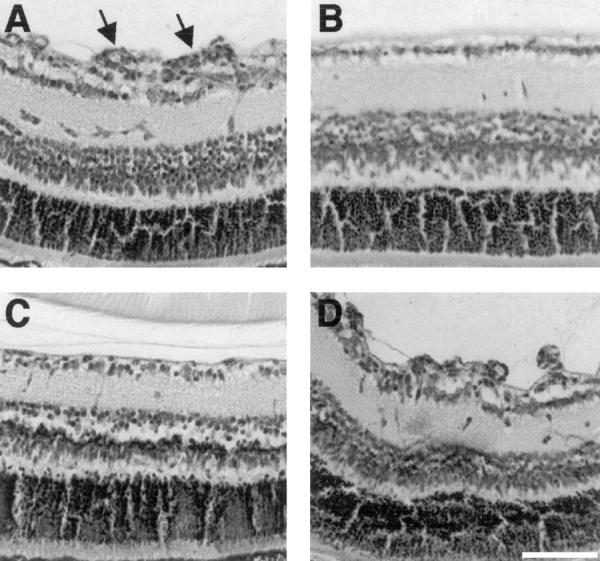
CA-4 blocks ischemia-induced retinal neovascularization in a dose-dependent manner in mice. P7 mice were exposed to hyperoxia for 7 days, returned to ambient conditions, and daily intraperitoneal administration of CA-4P was commenced after 24 hours and continued until P17 when mice were killed. Retinal sections were stained using H&E. A: Untreated retinopathy showing multiple neovascular tufts (arrows) extending beyond the inner limiting membrane. B: Normal nonischemic retina showing clear delineation of retina and vitreous by the inner limiting membrane. C: P17 ischemic mouse treated with CA-4P (3.125 mg/kg/24 hours) showing similarity to normal retina with no neovascular tufts. D: P17 ischemic mouse treated with CA-4P (0.78 mg/kg/24 hours) showing no evidence for inhibition of neovascular tuft formation. Scale bar, 100 μm.
Administration of 3.125-mg/kg/24 hours CA-4P or lower doses caused no evident side effects during 5 days of treatment. Comparisons were made by Student’s unpaired t-test and there was no significant difference (P > 0.3) in body mass between different groups at the termination of the experiment [mass, g ± SD: 8.02 ± 0.14 (untreated); 7.95 ± 0.53 (0.78 mg/kg CA-4P); 8.21 ± 0.40 (1.56 mg/kg CA-4P); 7.88 ± 0.52 (3.125 mg/kg CA-4P)]. Higher doses of CA-4P (6.25 and 12.0 mg/kg/24 hours) caused significant toxicity and were fatal in four of six mice for each group within 24 hours of commencing treatment.
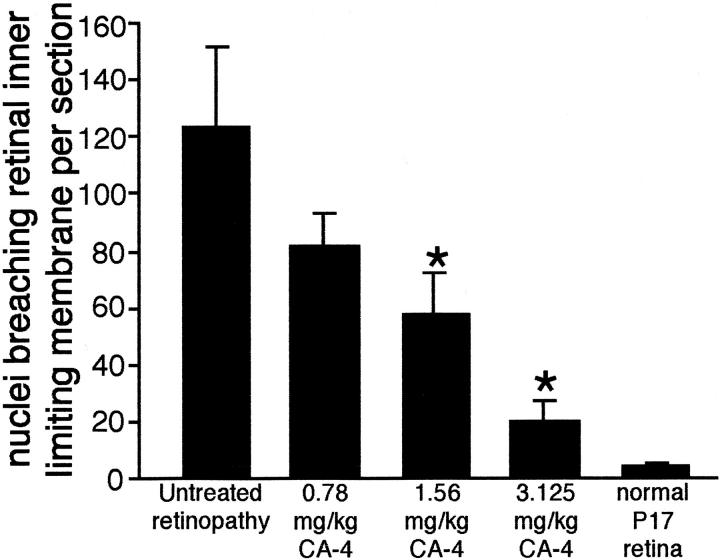
To quantify retinopathy, nuclei penetrating the inner limiting membrane were counted in representative sections. Consistent with the histological data, this quantitative analysis revealed an 84% decrease in nuclei penetrating the inner limiting membrane in mice treated with 3.125 mg/kg of CA-4P (n = 5, ±5.9%) compared with untreated mice. The quantitative data for the titration of CA-4P (Figure 2) ▶ were consistent with the histological data demonstrating a dose-dependent effect.
Figure 2.
Quantitation of neovascularization in retinas of mice with proliferative retinopathy treated with CA-4P or untreated. Retinal sections were stained using 4,6-diamidino-2-phenylindole and nuclei penetrating beyond the inner limiting membrane were quantitated as described in Materials and Methods. All groups were comprised of five mice. Error bars represent SD. *, P < 0.01, t-test.
The use of some anti-angiogenic agents in this model has been reported to inhibit development of the normal retinal vasculature. 20,21 To assess the effects of CA-4P administration on the development of the normal retinal vasculature, endothelial cells of retinal sections were stained using G. simplicifolia lectin B4. In retinal sections where retinopathy was evident, lectin staining confirmed the presence of endothelial cells in the vascular tufts penetrating the inner limiting membrane (Figure 3A) ▶ . In retinas from all treatment groups in which CA-4P administration had inhibited proliferative retinopathy, lectin staining identified a normal pattern of retinal vasculature with endothelial cells forming the superficial capillary bed within the inner limiting membrane on the surface of the retina and branches forming the intermediate and deep capillary beds (Figure 3 ▶ ; A to D). No qualitative differences were detected between retinas from different treatment groups, indicating that CA-4P administration did not affect development of the normal retinal vasculature as assessed histologically. To confirm that CA-4 did not affect normal vascular development in the retina, stereological analysis was performed to estimate the volume fraction of endothelial cells beneath the inner limiting membrane. As previously described 21 samples from untreated mice with retinopathy had a significantly increased volume fraction of endothelial cells, as compared with age-matched wild-type controls [Vv ± SE: 5.47 ± 1.9 (untreated); 1.9 ± 0.2 (wild-type) n = 4]. In retinas of mice in which retinopathy had been inhibited by treatment with 3.125-mg/kg/24 hours CA-4-P, the corresponding volume fraction of endothelial cells was 2.23 ± 0.23. Statistical analysis (see Materials and Methods) revealed no significant difference between wild-type and CA-4P-treated animals. These data confirm that CA-4 did not inhibit the development of the normal retinal vasculature.
Figure 3.
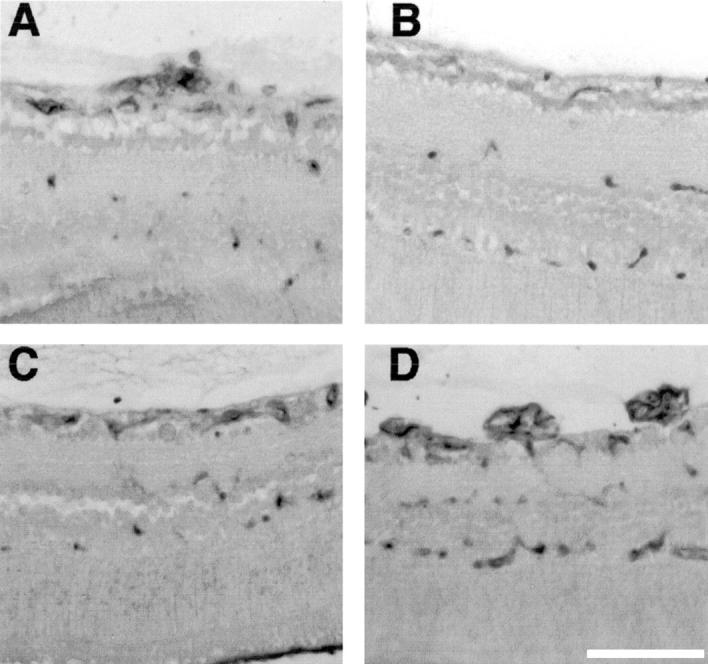
Histochemical analysis of retinal capillary beds in mice with proliferative retinopathy treated with CA-4P or untreated. P7 mice were treated as described in Figure 1 ▶ . Sections were stained using Griffonia simplicifolia lectin B4 detected by diaminobenzidine. A: Untreated retinopathy showing retinal capillaries and endothelial neovascular tufts breaching inner limiting membrane. B: Nonischemia retina showing normal retinal capillary beds. C: P17 ischemic mouse treated with CA-4P (3.125 mg/kg/24 hours) showing normal development of retinal capillary beds concurrent with inhibition of neovascularization. D: P17 ischemic mouse treated with CA-4P (0.78 mg/kg/24 hours) showing no evident inhibition of neovascular tuft formation and reduced central perfusion.
To visualize the retinal vasculature, mice were perfused with fluorescently labeled high-molecular weight dextran and retinal flat mounts prepared. Analysis of the retinal vasculature of normal age-matched mice revealed vessels radiating from the optic verve branching to perfuse the entire retina (Figure 4B) ▶ . Consistent with the histological data, perfusion analysis of retinas from untreated mice confirmed retinopathy, manifested as a characteristic reduction in central perfusion and the appearance of multiple vascular tufts (Figure 4A) ▶ . A similar extent of retinopathy was apparent in mice treated with 0.78 mg/kg (Figure 4D) ▶ and 1.56 mg/kg of CA-4P (not shown). Qualitative analysis of retinas from mice treated with 3.125 mg/kg of CA-4P revealed a pattern of staining closely resembling that seen in normal retinas (Figure 4C) ▶ . There was no evidence of vascular tufts and, although marginally reduced by comparison with normal retina, there was almost complete perfusion of the vascular tree. These observations are consistent with the histological data indicating a significant inhibition of neovascularization by CA-4 at this dose.
Figure 4.
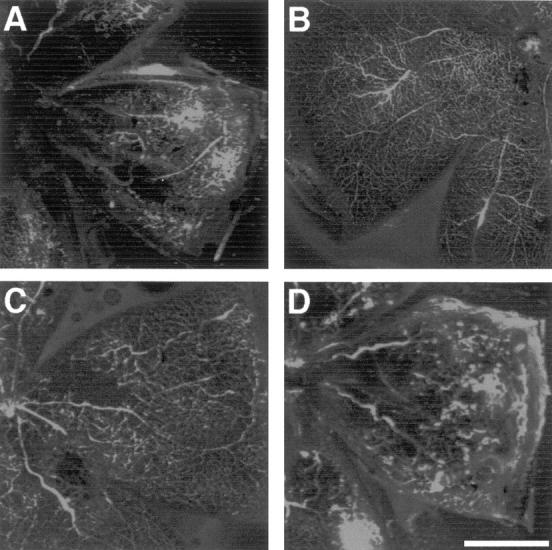
Perfusion analysis of retinas of mice with proliferative retinopathy treated with CA-4P or untreated. P7 mice were treated as described in Figure 1 ▶ . At P17 mice were perfused via the left ventricle with fluorescein isothiocyanate-conjugated dextran, eyes were enucleated, fixed, and flat mounts prepared and viewed by confocal microscopy. Images are maximum intensity projections of 16 sections each with 4 acquisitions. A: Untreated retinopathy showing neovascular tufts and absence of central perfusion. B: Nonischemic retina showing normal retinal perfusion. C: P17 ischemic mouse treated with CA-4P (3.125 mg/kg/24 hours) showing similarity to normal retina with no neovascular tufts (see text). D: P17 ischemic mouse treated with CA-4P (0.78 mg/kg/24 hours) showing no evident inhibition of neovascular tuft formation and reduced central perfusion. Scale bar, 1 mm.
Discussion
Ischemia-induced development of irregular neovessels underlies the pathology of a variety of retinopathies that cause loss of vision in premature infants and diabetic patients. Although retinal photocoagulation is a beneficial therapy in many patients, it is frequently insufficient to inhibit continued retinal neovascularization and causes side effects that can lead to a deterioration in night and peripheral vision and exacerbate existing macular pathologies. 20 Therefore, the development of drugs that can inhibit proliferative retinopathy without causing damage to the normal retinal vasculature is highly desirable. In this study we have shown that systemic treatment with CA-4P potently inhibits proliferative retinopathy in a murine model without causing detectable side effects as assessed by animal weight, and histological and immunohistochemical analyses.
It has been previously reported that CA-4 displays a wide therapeutic window and elicits anti-vascular effects in tumor vessels at one-tenth of the maximum-tolerated dose. This is in contrast to similar agents eg, colchicine, the clinical development of which was limited by a narrow therapeutic window. However, it is important to differentiate between the toxicity profiles of CA-4 after acute (single dose) and prolonged treatment regimes. Thus the maximum-tolerated single dose of CA-4P in mice has been defined as >1000 mg/kg 22,23 and several reports 24,25 have described anti-tumor effects after the administration of 100 mg/kg, ie, at <1/10th maximum-tolerated dose. In contrast, our own unpublished work and other studies 26 have shown that systemic toxicity results from repeated intraperitoneal injections of 25 and 50 mg/kg/12 hours in adult mice. The most dramatic effect of these treatments is extensive damage to liver vasculature leading to death within ∼5 days (Griggs J, Brindle JM, Metcalfe JC, Smith GA, Hesketh TR, unpublished data). However, administration of 12.5-mg/kg/12 hours CA-4P causes substantial inhibition of lung metastasis and may be continued for at least 6 weeks with no adverse effects (Griggs J, Brindle KM, Metcalfe JC, Smith GA, Hesketh TR, unpublished data). 27 Thus, the therapeutic window of CA-4 is reduced significantly in treatment regimes involving continuous or repeated administration. In the present study this is reflected by the highly efficacious inhibition of retinal neovascularization achieved without apparent side effects by 3.125-mg/kg/24 hours CA-4P in contrast to 6.5 mg/kg/24 hours that is usually toxic within 24 hours. Although these observations emphasize the importance of toxicity data during the preclinical development of anti-angiogenic agents, 28,29 it is encouraging that recent data from phase I clinical trials indicates that CA-4 elicits anti-vascular effects in human primary tumors, similar to those observed in animal models, at doses below those eliciting dose-limiting toxicity. 30-35
Previous reports have described the anti-vascular activity of CA-4 in established tumor vessels, in which it causes hemorrhage and vessel collapse. 36,37 In a model of nonneoplastic neovascularization CA-4 caused vascular disruption manifested as the formation of multiple microthrombi, and no hemorrhage was observed. 14 Although the mechanism of action giving rise to this effect remains to be elucidated, it has been proposed that CA-4, which binds to the colchicine binding site of tubulin, elicits a shape change in the endothelial cells of tumor vessels that compromises the integrity of the lumenal monolayer. 38 Neither of the previously described consequences of vascular disruption by CA-4, ie, hemorrhage or microthrombus formation, was detected in retinas in which CA-4 had inhibited proliferative retinopathy, suggesting that CA-4 affects neovascularization in this model system in a manner distinct from its effects on neovasculature in either tumors or hyperplastic tissue. The data in this study are consistent with an anti-angiogenic mechanism of action for CA-4 in this model. Thus, the nature of the disruption of neovessels and angiogenic processes by CA-4 seems to depend on the specific tissue environment.
A variety of other anti-angiogenic agents have been tested in this model, including antagonists of vascular endothelial growth factor and integrin signaling 10,11,39,40 that inhibit proliferative retinopathy with varying degrees of efficacy. Recently, the endogenous retinal anti-angiogenic factor pigment epithelial-derived factor has been shown to be a potent inhibitor of neovascularization in this model. 12 The tyrosine kinase inhibitors CGP 441251 and PTK787 were also effective in the inhibition of neovessel formation but in addition inhibited the development of the normal retinal intermediate and deep capillary beds. This activity precludes their use in the treatment of retinopathy of prematurity, although they may have potential for the treatment of diabetic retinopathy. 20,21 In contrast, the immunohistochemical data and stereological analyses described in this report indicate that CA-4 at concentrations that essentially inhibit pathological angiogenesis does not interfere with the development of the normal retinal capillary beds.
CA-4 represents a class of agents distinct from the anti-angiogenic agents directed against one or more of the processes involved in angiogenesis previously tested in this model. CA-4 was initially characterized by its capacity to disrupt established neovasculature in tumors, causing necrosis secondary to hemorrhage, without affecting normal vasculature. However, inhibition of proliferative retinopathy and our previous findings that CA-4 causes thrombosis in the vasculature of hyperplastic tissue without causing hemorrhagic necrosis suggest that CA-4 may have more than one mechanism of action and may elicit anti-vascular effects in a tissue- and context-specific manner. It is thus important to distinguish between the anti-vascular effects observed in established tumors and the anti-angiogenic effects apparent from its capacity to inhibit retinal neovascularization. However, the current data suggest that CA-4 exerts an anti-angiogenic effect if administered concurrently with neovessel development in the retina. Previous studies of CA-4 have focused on its anti-tumor activity and there are few data that relate to mechanism. The in vitro selectivity of CA-4P for endothelial cells has been ascribed to the elevated surface membrane activity of alkaline phosphatase in these cells compared with, for example, fibroblasts. 41 CA-4 induces apoptosis in proliferating endothelial cells in vitro but causes cell-cycle arrest in cells derived from a vascular hemangioendothelioma and the question of whether CA-4 causes apoptosis therefore remains unresolved. 24,26,42 Tumors having higher nitric oxide synthase activity have been shown to be more resistant to CA-4P, suggesting that neutrophil infiltration, which is increased by CA-4P, may be inhibited by the action of nitric oxide. 43 Mechanistic data are limited partially because the properties of tumor vessels that render them differentially susceptible to the anti-vascular effects of CA-4 are unknown, as are the molecular target(s) of this agent in proliferating endothelial cells of other angiogenic vascular beds. Other than its capacity to bind to tubulin, little is known of the mode of action of CA-4, despite its progression into clinical trials. This lack of mechanistic information emphasizes the significance of the current study characterizing the in vivo effects of CA-4 on an angiogenic vascular bed. The results identify a further model system for the study of the anti-vascular action of CA-4 and suggest that there are diseases in addition to cancers against which CA-4 may have clinical potential.
Footnotes
Address reprint requests to Robin Hesketh, Department of Biochemistry, University of Cambridge, Tennis Court Rd., Cambridge, CB2 1QW, United Kingdom. E-mail: t.r.hesketh@bioc.cam.ac.uk.
J. G. is the recipient of a Biotechnology and Biological Sciences Research Council studentship.
References
- 1.Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86:353-364 [DOI] [PubMed] [Google Scholar]
- 2.Rosen L: Antiangiogenic strategies and agents in clinical trials. The Oncologist 2000, 5:20-27 [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK: Angiogenesis in cancer and other diseases. Nature 2000, 407:249-257 [DOI] [PubMed] [Google Scholar]
- 4.Kahn HA, Hiller R: Blindness caused by diabetic retinopathy. Am J Ophthalmol 1974, 78:58-67 [DOI] [PubMed] [Google Scholar]
- 5.Campochiaro PA: Retinal and choroidal neovascularization. J Cell Physiol 2000, 184:301-310 [DOI] [PubMed] [Google Scholar]
- 6.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA: Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994, 35:101-111 [PubMed] [Google Scholar]
- 7.Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, Yeo KT: Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994, 118:445-450 [DOI] [PubMed] [Google Scholar]
- 8.Miller JW, Adamis AP, Shima DT, Damore PA, Moulton RS, Oreilly MS, Folkman J, Dvorak HF, Brown LF, Berse B, Yeo TK, Yeo KT: Vascular endothelial growth-factor vascular-permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol 1994, 145:574-584 [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE: Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA 1995, 92:905-909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE: Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci USA 1996, 93:4851-4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE: Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 1995, 92:10457-10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stellmach VV, Crawford SE, Zhou W, Bouck N: Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci USA 2001, 98:2593-2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griggs J, Metcalfe JC, Hesketh TR: Targeting tumour vasculature: the development of combretastatin A4. Lancet Oncol 2001, 2:82-87 [DOI] [PubMed] [Google Scholar]
- 14.Griggs J, Hesketh R, Smith GA, Brindle KM, Metcalfe JC, Thomas GA, Williams ED: Combretastatin-A4 disrupts neovascular development in non-neoplastic tissue. Br J Cancer 2001, 84:832-835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedford SB, Quarterman CP, Rathbone DL, Slack JA, Griffin RJ, Stevens MFG: Synthesis of water-soluble prodrugs of the cytotoxic agent combretastatin A4. Bioorg Med Chem Lett 1996, 6:157-160 [Google Scholar]
- 16.Orsini F, Pelizzoni F, Bellini B, Miglierini G: Synthesis of biologically active polyphenolic glycosides (combretastatin and resveratrol series). Carbohydr Res 1997, 301:95-109 [DOI] [PubMed] [Google Scholar]
- 17.Pettit GR, Temple C, Narayanan VL, Varma R, Simpson MJ, Boyd MR, Rener GA, Bansal N: Antineoplastic agents-322—synthesis of combretastatin A-4 prodrugs. Anticancer Drug Des 1995, 10:299-309 [PubMed] [Google Scholar]
- 18.Majka S, McGuire P, Colombo S, Das A: The balance between proteinases and inhibitors in a murine model of proliferative retinopathy. Invest Ophthalmol Vis Sci 2001, 42:210-215 [PubMed] [Google Scholar]
- 19.Weibel ER: Stereological Methods. 1979. Academic Press, London
- 20.Seo MS, Kwak N, Ozaki H, Yamada H, Okamoto N, Yamada E, Fabbro D, Hofmann F, Wood JM, Campochiaro PA: Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am J Pathol 1999, 154:1743-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozaki H, Seo MS, Ozaki K, Yamada H, Yamada E, Okamoto N, Hofmann F, Wood JM, Campochiaro PA: Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol 2000, 156:697-707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaplin DJ, Hill SA, Dougherty GJ, Prise VE, Tozer GM, Pettit R: Combretastatin A-4 phosphate an agent that displays potent and selective toxicity toward tumour neovasculature. Hum Gene Ther 1999, 10:840 [Google Scholar]
- 23.Maxwell RJ, Nielsen FU, Breidahl T, StodkildeJorgensen H, Horsman MR: Effects of combretastatin on murine tumours monitored by P-31 MRS, H-1 MRS and H-1 MRI. Int J Radiat Oncol Biol Phys 1998, 42:891-894 [DOI] [PubMed] [Google Scholar]
- 24.Bohle AS, Leuschner I, Kalthoff H, Henne-Bruns D: Combretastatin A-4 prodrug: a potent inhibitor of malignant hemangioendothelioma cell proliferation. Int J Cancer 2000, 87:838-843 [DOI] [PubMed] [Google Scholar]
- 25.Tozer GM, Prise VE, Wilson J, Locke RJ, Vojnovic B, Stratford MRL, Dennis MF, Chaplin DJ: Combretastatin A-4 phosphate as a tumor vascular-targeting agent: early effects in tumors and normal tissues. Cancer Res 1999, 59:1626-1634 [PubMed] [Google Scholar]
- 26.Malcontenti-Wilson C, Muralidharan V, Skinner S, Christophi C, Sherris D, O’Brien PE: Combretastatin A4 prodrug study of effect on the growth and the microvasculature of colorectal liver metastases in a murine model. Clin Cancer Res 2001, 7:1052-1060 [PubMed] [Google Scholar]
- 27.Griggs J, Brindle KM, Metcalfe JC, Hill SA, Smith GA, Beauregard DA, Hesketh R: Potent anti-metastatic activity of combretastatin-A4. Int J Oncol 2001, 19:821-825 [DOI] [PubMed] [Google Scholar]
- 28.Tozer GM, Prise VE, Wilson I, Vojnovic B, Chaplin DJ: Therapeutic targeting of the tumour vasculature using the tubulin-binding agent combretastatin A4-P. J Vasc Res 1999, 36:18 [Google Scholar]
- 29.Li LY, Rojiani A, Siemann DW: Targeting the tumor vasculature with combretastatin A-4 disodium phosphate: effects on radiation therapy. Int J Radiat Oncol Biol Phys 1998, 42:899-903 [DOI] [PubMed] [Google Scholar]
- 30.Stratford M, Folkes L, Galbraith S, Price P, Anderson H, Robbins A, Sena L, Rustin G: Phase I pharmacokinetic and toxicity study of weekly intravenous combretastatin A4 phosphate (CA4P). Clin Cancer Res 2000, 6:280 [Google Scholar]
- 31.Stevenson JP, Gallagher M, Sun W, Algazy K, Vaugn DJ, Haller DG, Hiller K, Halloran L, O’Dwyer PJ: Phase I/pharmacokinetic trials of the endothelial toxin combretastatin A4P (CA4P) administered as an IV bolus on a daily x 5 schedule every 21 days. Proceedings of the 91st Annual Meeting of the American Association of Cancer Research. Am Assoc Cancer Res 2000, 4:3469 [Google Scholar]
- 32.Remick SC, Dowlati A, Robertson K, Spiro T, Connell C, Levitan N, Stratford M: Phase I pharmacokinetic study of single dose intravenous (IV) combretastatin A4 prodrug (CA4P) in patients (pts) with advanced cancer. Clin Cancer Res 1999, 5:16 [Google Scholar]
- 33.Remick S, Robertson K, Dowlati A, Cooney M, Jessberger J, Lewin J, Stambler B, Taylor A, Petras W, Stratford M, Young S, Sherris D: A phase I pharmacokinetic (PK) study of single dose intravenous (IV) combretastatin A4 phosphate (CA4P) in patients (PTS) with advanced cancer: final report. Clin Cancer Res 2001, 7:393 [Google Scholar]
- 34.Anderson H, Jap J, Price P: Measurement of tumour and normal (NT) perfusion by positron emission tomography (PET) in the evaluation of antivascular therapy: results in the phase I study of combretastatin A4 phosphate (CA4P). Program Proceedings. Am Soc Clin Oncol 2000, 19:695 [Google Scholar]
- 35.Galbraith SM, Taylor NJ, Maxwell RJ, Lodge M, Tozer GM, Baddeley H, Wilson I, Prise VE, Rustin GJS: Combretastatin A4 phosphate (CA4P) targets vasculature in animal and human tumours. Br J Cancer 2000, 83(Suppl 1):12 [Google Scholar]
- 36.Dark GG, Hill SA, Prise VE, Tozer GM, Pettit GR, Chaplin DJ: Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res 1997, 57:1829-1834 [PubMed] [Google Scholar]
- 37.Grosios K, Holwell SE, McGown AT, Pettit GR, Bibby MC: In vivo and in vitro evaluation of combretastatin A-4 and its sodium phosphate prodrug. Br J Cancer 1999, 81:1318-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galbraith SM, Lee F, Vojnovic B, Tozer GM, Chaplin D: Quantitative analysis of endothelial cell shape change after treatment with combretastatin A4 phosphate. Clin Cancer Res 1999, 5:399 [Google Scholar]
- 39.Luna J, Tobe T, Mousa SA, Reilly TM, Campochiaro PA: Antagonists of integrin alpha v beta 3 inhibit retinal neovascularization in a murine model. Lab Invest 1996, 75:563-573 [PubMed] [Google Scholar]
- 40.Hammes HP, Brownlee M, Jonczyk A, Sutter A, Preissner KT: Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med 1996, 2:529-533 [DOI] [PubMed] [Google Scholar]
- 41.Boehle AS, Sipos B, Kliche U, Kalthoff H, Dohrmann P: Combretastatin A-4 prodrug inhibits growth of human non-small cell lung cancer in a murine xenotransplant model. Ann Thorac Surg 2001, 71:1657-1665 [DOI] [PubMed] [Google Scholar]
- 42.Iyer S, Chaplin DJ, Rosenthal DS, Boulares AH, Li LY, Smulson ME: Induction of apoptosis in proliferating human endothelial cells by the tumor-specific antiangiogenesis agent combretastatin A-4. Cancer Res 1998, 58:4510-4514 [PubMed] [Google Scholar]
- 43.Parkins CS, Holder AL, Hill SA, Chaplin DJ, Tozer GM: Determinants of anti-vascular action by combretastatin A-4 phosphate: role of nitric oxide. Br J Cancer 2000, 83:811-816 [DOI] [PMC free article] [PubMed] [Google Scholar]