Abstract
Cyclooxygenase-2 (COX-2) is the rate-limiting enzyme in prostanoid biosynthesis and is involved in tumor progression. We investigated expression of COX-1 and COX-2 in cell lines and tumors from ovarian carcinomas. Expression of COX-2 mRNA and protein was detectable in three of five ovarian carcinoma cell lines and was inducible by interleukin-1β or phorbolester in a subset of cell lines. Prostaglandin E2 (PGE2) production could be inhibited by the selective COX-2 inhibitor NS-398. In malignant ascites of ovarian carcinomas significantly increased levels of PGE2 were found compared to other carcinomas or nonmalignant ascites (P = 0.03). We investigated expression of COX-2 by immunohistochemistry in 117 ovarian surface epithelial tumors. Expression of COX-2 was detected in 42% of 86 ovarian carcinomas and in 37% of 19 low malignant potential tumors, but not in 12 cystadenomas or 2 normal ovaries. Expression of COX-1 was detected by immunohistochemistry in 75% of 75 invasive ovarian carcinomas and in 75% of 16 low malignant potential tumors, whereas 2 samples from normal ovaries and 8 cystadenomas were positive for COX-1. In univariate survival analysis of invasive carcinomas, expression of COX-2 was associated with a significantly reduced median survival time (log rank test, P = 0.04). For patients younger than 60 years of age, this association was even more significant (P < 0.004). In contrast, expression of COX-1 was no prognostic parameter (P = 0.89). There was no significant correlation between COX-2 or COX-1 expression and other clinicopathological markers. In multivariate analysis expression of COX-2 was an independent prognostic factor for poor survival (relative risk, 2.74; 95% CI, 1.38 to 5.47). Our data indicate that COX-2 expression is an independent prognostic factor in ovarian carcinoma. Based on the results of this study, it would be interesting to investigate whether ovarian carcinoma patients with tumors positive for COX-2 would benefit from treatment with selective COX-2 inhibitors.
Ovarian carcinoma is the fifth most common cancer of females in the United States and has the highest mortality rate among gynecological malignancies. 1 Patients with tumors at stage III have a 5-year survival rate of only 28%, 1 and unfortunately 60% of patients are diagnosed with already advanced disease. The prognosis of patients with ovarian carcinoma mainly depends on the stage of disease and to some extent on patient age, histological type, and grade. The identification of additional prognostic parameters particularly for patients with advanced disease would be very helpful for planning of treatment.
Cyclooxygenases (COXs) are involved in control of inflammatory reactions and catalyze the rate-limiting step in the biosynthesis of prostaglandins, the conversion of arachidonic acid to prostaglandin H2. There are two COX isoenzymes encoded by different genes: COX-1 is expressed constitutively in many cell types and is regarded as a housekeeping gene, whereas COX-2 is highly inducible by inflammatory stimuli. 2 Cyclooxygenases are the targets for nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin or sulindac. Epidemiological studies show that NSAIDs reduce the incidence and mortality of colorectal carcinoma and several other types of cancer. 3-6 Furthermore, in animal experiments inhibition of COX-2 reduced the incidence of colon carcinoma in rats treated with chemical carcinogens 7 as well as in APC knockout mice. 8 COX-2 is expressed in other carcinomas as well, such as gastric or pancreatic adenocarcinomas, 9 hepatocellular carcinomas, 10 adenocarcinomas of the lung, 11 and squamous carcinomas of the head and neck. 12
Cyclooxygenases, especially COX-2, are important for normal ovarian function. COX-2 (−/−) female mice show defective ovulation and are infertile, 13,14 whereas COX-1 (−/−) mice are fertile. 15 Despite the importance of cyclooxygenases in ovarian physiology, the impact of COX-1 and COX-2 expression on prognosis of malignant ovarian tumors has not been investigated so far. In the present study we investigated the expression and regulation of cyclooxygenases (COX-1 and COX-2) in five ovarian carcinoma cell lines as well as in human primary ovarian carcinomas.
Materials and Methods
Cell Lines
The human ovarian carcinoma cell lines OVCAR-3, 16 SKOV-3, 17 and CAOV-3 17 have been isolated from ovarian adenocarcinomas and were obtained from the American Type Culture Collection (ATCC, Rockville, MD). OAW-42 18 has been established from ascites of a patient with a serous cystadenocarcinoma of the ovary, and was from ECACC, Salisbury, UK. The cell line ES-2 19 has been isolated from a poorly differentiated ovarian clear-cell carcinoma and was from ATCC. Cell lines were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum.
Polymerase Chain Reaction
Confluent monolayers of cells were incubated in medium without serum for 24 hours and subsequently stimulated with recombinant human interleukin (IL)-1β (R&D Systems, Minneapolis, MN) or phorbol ester (TPA; Sigma, St. Louis, MO) for 6 hours. Total RNA was prepared with RNeasy Kit (Qiagen, Hilden, Germany). Tissue from ovarian carcinomas was dissected by a senior pathologist in the operating room from surgical specimens sent for frozen section analysis and was immediately frozen in liquid nitrogen and stored at −80°C until analysis. Tissue samples were homogenized, total RNA was prepared with RNeasy Kit, and residual DNA was digested with DNase. For polymerase chain reaction (PCR) analysis of RNA, cDNA was made by reverse transcription and PCR reactions were performed. Cycling conditions were 35 cycles of denaturation, annealing, and extension (94°C for 45 seconds, 54°C for 45 seconds, and 72°C for 120 seconds). The primers used were human COX-1 sense 5′-TGCCCAGCTCCTGGCCCGCCGCTT-3′ and antisense 5′-GTGCATCAACACAGGCGCCTCTTC-3′ (generating a 303-bp band), human COX-2 sense 5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′ and antisense 5′-AGATCATCTCTGCCTGAGTATCTT-3′(generating a 304-bp band), 20 GAPDH sense 5′-ACCACAGTCCATGCCATCAC-3′ and antisense 5′-TCCACCACCCTGTTGCTGTA-3′ (generating a 452-bp band).
Immunoblotting
Cells grown to confluency in 60-mm Petri dishes were incubated in medium without serum for 24 hours and subsequently stimulated with 10 ng/ml of IL-1β or 10 nmol/L of TPA for 24 hours. Cells were lysed in 100 μl of 62.5 mmol/L Tris-HCl (pH 6.8) containing 2% sodium dodecyl sulfate, 10% glycerol, 50 mmol/L dithiothreitol, and 0.1% bromophenol blue. One hundred μg of protein/sample were loaded on a 10% polyacrylamide gel. Proteins were blotted onto nitrocellulose membranes (Biometra, Göttingen, Germany), washed in phosphate-buffered saline (PBS), and incubated in blocking buffer [1× Tris-buffered saline, 0.1% Tween-20, 5% I-block (Tropix, Bedford, MA)] for 1 hour at 21°C. Membranes were washed three times with PBS/0.1% Tween-20 and incubated overnight at 4°C with a monoclonal anti-COX-1 (Cayman Chemical, Ann Arbor, MI) or anti-COX-2 antibody (Cayman Chemical) diluted 1:1000 in blocking buffer, followed by incubation with alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (Tropix, Bedford, MA). Bands were visualized using the CDP star RTU luminescence system (Tropix).
To evaluate the specificity of the COX-2 antibody for the bands of different sizes, blocking experiments were performed using the COX-2 blocking peptide (Cayman Chemical). According to the manufacturer’s instructions, we preincubated the COX-2 antibody for 1 hour in the presence of the blocking peptide (10 μg/ml) before immunoblotting.
PGE2 Enzyme-Linked Immunosorbent Assay (ELISA)
Cells (1 × 105)/well in 12-well plates were stimulated with IL-1β (5 ng/ml) or TPA (10 nmol/L) with or without 10 μmol/L of NS–398 (Alexis) in Dulbecco’s modified Eagle’s medium and 10% fetal calf serum. After 24 hours supernatants were harvested and centrifuged at 5000 rpm for 10 minutes before blocking the cyclooxygenase by addition of 10 μg/ml of indomethacin (Sigma). Samples were stored at −80°C. Samples of ascitic fluid were centrifuged at 900 rpm and stored at −80°C until analysis.
Concentration of PGE2 in cell culture supernatants and ascitic fluid was determined using a specific ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The concentration of PGE2 was estimated from the absorbance of the calculated standard curve. The results were expressed as pg/ml.
Study Population
Immunohistochemical examination was performed retrospectively on tissue samples taken for routine diagnostic purposes. For determination of expression of COX-2 in benign and malignant ovarian tumors, 119 patients with ovarian lesions who were diagnosed at the Institute of Pathology, Charité Hospital, Berlin, and the Institute of Pathology, RWTH, Aachen, between 1989 and 2000 were included in the study. The cases were selected based on the availability of tissue and were not stratified for known preoperative or pathological prognostic factors. The tissue specimens included 86 invasive ovarian carcinomas, 19 tumors of low malignant potential (LMP) (borderline tumors, atypical proliferating tumors), 12 benign cystadenomas, as well as 2 samples of normal ovaries. COX-1 expression was determined in 101 cases (75 invasive ovarian carcinomas, 16 LMP tumors, 8 cystadenomas, 2 normal ovaries). For further statistical evaluation and survival analysis, only the patients with invasive ovarian carcinomas were included. The duration of follow-up ranged from 0.30 to 121.7 months (mean, 32.5 months).
Histopathological Examination
Tissue samples were fixed in 4% neutral buffered formaldehyde and embedded in paraffin. Routine hematoxylin and eosin sections were performed for histopathological evaluation. The stage of tumors was assessed according to the International Federation of Gynecology and Obstetrics staging system. All cases were re-evaluated for histological type and grade by the same pathologist (SH). For grading of tumors the Silverberg grading system composed of architectural, nuclear, and mitotic features was used. 21
Immunohistochemistry
Immunohistochemical staining was performed according to standard procedures. We used the mouse anti-human COX-2 monoclonal antibody from Cayman Chemical Company, which has been widely used for immunohistochemical staining of COX-2 and has been evaluated by blocking experiments with the specific peptide. 22 For investigation of COX-1, the mouse anti-COX-1 monoclonal antibody was used (Cayman Chemical). Briefly, slides were boiled in citrate buffer in a pressure cooker for 5 minutes and incubated with the monoclonal COX-1 (1:200) or COX-2 antibody (1:1000) overnight at 4°C, followed by incubation with a biotinylated anti-mouse secondary antibody and the multilink biotin-streptavidin-amplified detection system (Biogenex, San Ramon, CA). Staining was visualized using a fast-red chromogen system (Immunotech, Hamburg, Germany). The intensity of the COX-1 or COX-2 immunostaining in tumor cells was evaluated independently by two pathologists (SH and CD), who were blinded to patient outcome, and scored as COX-1- or COX-2-negative or -positive. Tumors were scored as positive for COX if there was either a diffuse staining or a focal expression in several clusters of cells. Cases with a minimal expression of COX in few single cells were scored as negative. For preliminary analysis, we evaluated the cases with a particularly strong expression of COX-2 as a separate group. We did not detect any differences between cases with strong and moderate expression of COX-2. For this reason both groups were combined and subsequent statistical analysis was performed comparing positive and negative cases.
Statistical Analysis
The statistical significance of the correlation between expression of COX-1 or COX-2 and several clinicopathological parameters was assessed by Fisher’s exact test. The probability of overall survival as a function of time was determined by the Kaplan-Meier method. Different survival curves were compared by the log rank test. Multivariate survival analysis was performed using the Cox regression model. Generally, P values <0.05 were considered as significant. For the statistical evaluation the SPSS software Version 10.0 was used.
Results
Expression of COX-2 mRNA and Protein in Ovarian Carcinoma Cell Lines
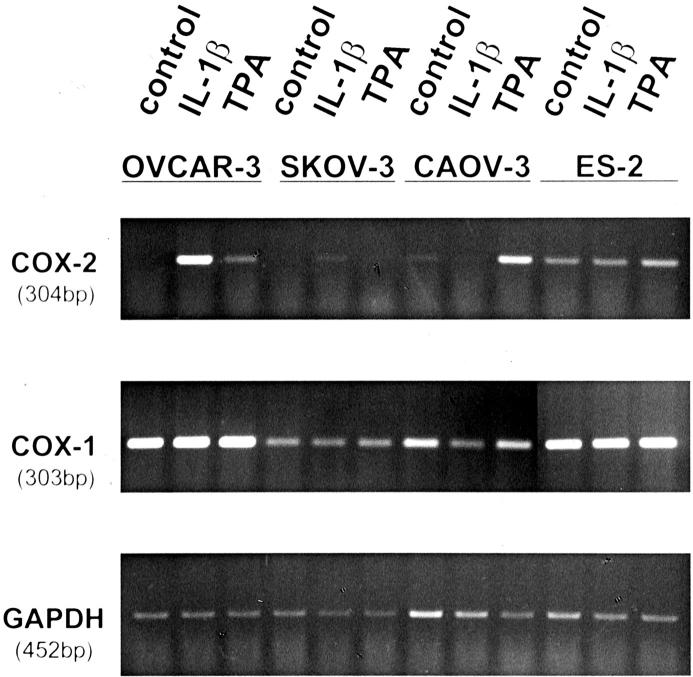
We determined expression of COX-2 mRNA by reverse transcriptase (RT)-PCR in five ovarian carcinoma cell lines (OVCAR-3, SKOV-3, CAOV-3, ES-2, and OAW-42). Cells were incubated with IL-1β (10 mg/ml) or the phorbol ester TPA (10 nmol/L). As shown in Figure 1 ▶ , expression of COX-2 mRNA was induced by IL-1β and TPA in OVCAR-3 cells and by TPA in CAOV-3 cells. The cell line ES-2 showed a constitutive expression of COX-2. Neither SKOV-3 (Figure 1) ▶ nor OAW-42 (not shown) expressed COX-2 mRNA. The expression of COX-1 mRNA was detected in all cell lines and was not changed by IL-1β or TPA.
Figure 1.
Expression of COX-1 and COX-2 mRNA in ovarian carcinoma cell lines. Human ovarian carcinoma cell lines were stimulated with IL-1β or TPA for 6 hours. Expression of COX-1, COX-2, and GAPDH mRNA was investigated by RT-PCR. One of three independent experiments is shown.
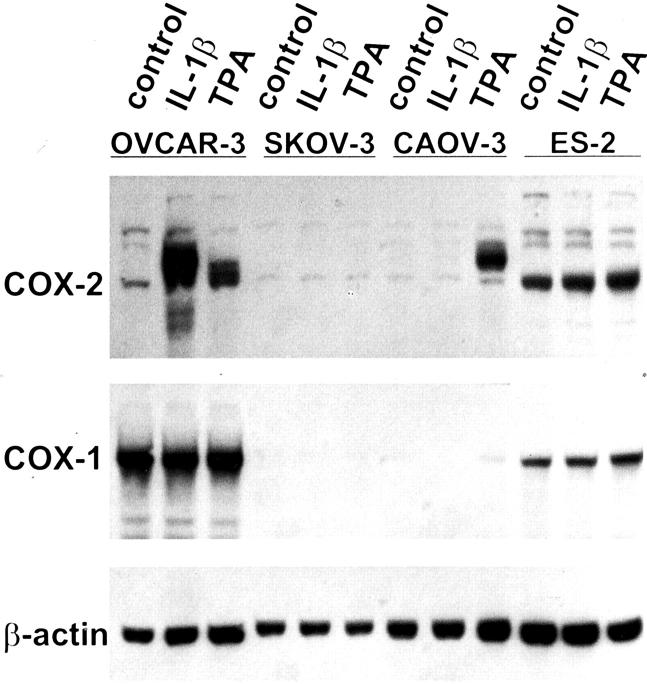
In Western blot analysis, similar results were obtained. Expression of the COX-2 protein with a size of ∼72 kd was induced in OVCAR-3 cells by IL-1β and TPA and in CAOV-3 cells by TPA (Figure 2) ▶ . As shown in mRNA analysis, ES-2 cells had a constitutive expression of COX-2 protein. Interestingly, the apparent molecular weight of the COX-2 protein was slightly lower in ES-2 cells (∼60 kd), suggesting a different glycosylation of the protein. To demonstrate the specificity of the various bands we performed blocking experiments with a specific COX-2 peptide. The bands of different sizes in COX-2 Western blots of different cell lines disappeared after preincubation of the antibody with a COX-2 peptide (data not shown).
Figure 2.
Expression of COX-1 and COX-2 protein in ovarian carcinoma cell lines. Human ovarian carcinoma cell lines were stimulated with IL-1β or TPA for 24 hours. Expression of COX-1, COX-2, and β-actin was investigated by immunoblotting. One of three independent experiments is shown.
SKOV-3 (Figure 1) ▶ as well as OAW-42 (not shown) did not express COX-2 protein. Although COX-1 was expressed constitutively on the mRNA level in all cell lines, only OVCAR-3 and ES-2 showed an expression of COX-1 protein.
PGE2 Production of Ovarian Carcinoma Cell Lines
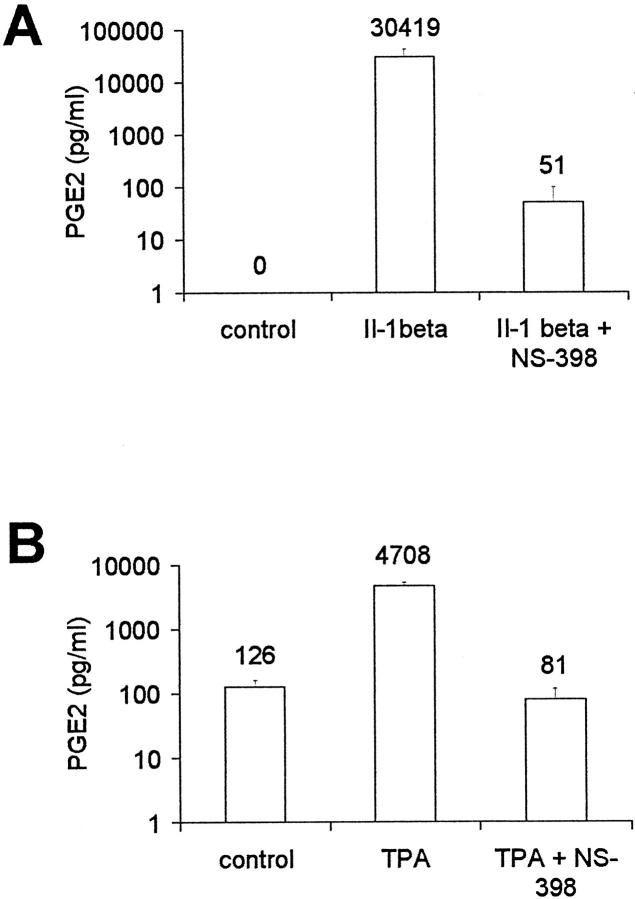
Using specific ELISA, we measured production of PGE2 in ovarian carcinoma cells. Parallel to the induction of COX-2 mRNA and protein, we found an increase of PGE2 in supernatant of OVCAR-3 cells stimulated with IL-1β (Figure 3A) ▶ as well as of CAOV-3 cells treated with TPA (Figure 3B) ▶ . Inhibition of COX-2 by the specific inhibitor NS-398 at concentrations of 50 μmol/L reduced PGE2 levels. The other cell lines, including ES-2, did not produce PGE2, even after stimulation with IL-1β or TPA.
Figure 3.
Production of PGE2 in ovarian carcinoma cell lines. A: OVCAR-3 cells were stimulated with IL-1β for 24 hours with or without the addition of NS-398 (50 μmol/L). B: CAOV-3 cells were stimulated with TPA for 24 hours with or without NS-398. PGE2 in supernatant was measured by specific ELISA. Mean and SD from three independent experiments is shown.
Expression of COX-2 mRNA in Ovarian Carcinomas
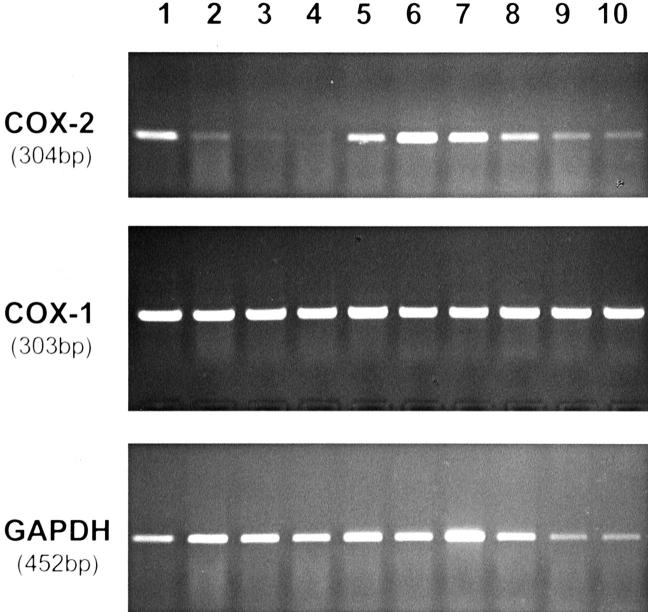
An expression of COX-2 mRNA was detected by RT-PCR in seven of eight ovarian carcinomas as well as in one LMP tumor (Figure 4) ▶ . One G1 serous-papillary carcinoma was negative for COX-2, whereas one G2 clear-cell ovarian carcinoma and one G3 serous papillary ovarian carcinoma showed a very weak expression of COX-2. Additionally, we investigated one sample of a malignant mixed Mullerian tumor that was negative for COX-2. All cases expressed COX-1 mRNA (Figure 4) ▶ . For six cases, COX-2 expression was also investigated by immunohistochemistry. Three of the cases showed identical results in RT-PCR and immunohistochemistry. In the remaining three cases COX-2 mRNA expression was detected by RT-PCR, but tumors were negative for COX-2 protein by immunohistochemistry. This may be explained by the increased sensitivity of RT-PCR. On the other hand, part of the COX-2 signal in RT-PCR may be contributed by inflammatory cells in the tumor stroma.
Figure 4.
Expression of COX-1 and COX-2 mRNA in 10 cases of ovarian tumors. RNA from samples of ovarian carcinoma tissue was isolated and expression of COX-1, COX-2, and GAPDH mRNA was investigated by RT-PCR. Histological diagnoses: 1, serous papillary ovarian carcinoma, G3; 2, clear cell ovarian carcinoma, G2; 3, malignant mixed Mullerian tumor; 4, serous papillary ovarian carcinoma, G1; 5, serous papillary ovarian carcinoma, G3; 6, endometrioid ovarian carcinoma, G3; 7, endometrioid ovarian carcinoma, G2; 8, serous papillary ovarian carcinoma, G3; 9, serous LMP tumor; 10, serous papillary ovarian carcinoma, G3.
Production of PGE2 in Ascitic Fluid of Patients with Ovarian Carcinomas
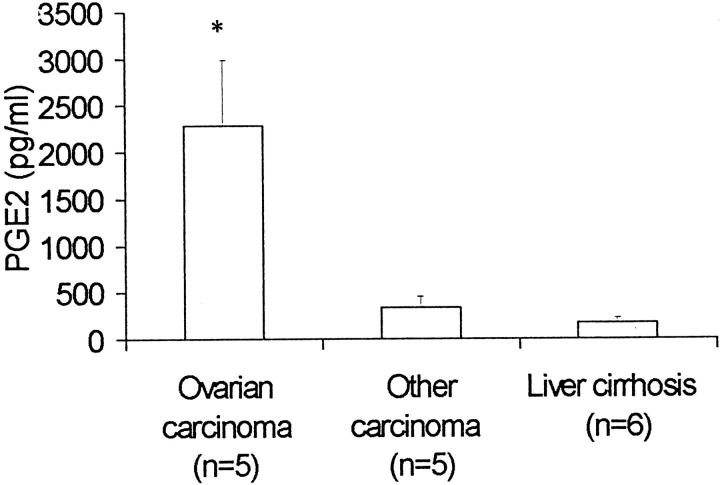
We measured levels of PGE2 in samples of ascitic fluid from patients with ovarian carcinomas (n = 5), other malignancies (n = 5), as well as liver cirrhosis (n = 6). Samples of patients with ovarian carcinomas showed significantly increased levels of PGE2 (mean plus SEM: 2287 ± 705 pg/ml) compared to ascitic fluid of patients with other carcinomas (337 ± 116 pg/ml; P = 0.03, Student’s t-test) or liver cirrhosis (172 ± 58 pg/ml; P = 0.03) (Figure 5) ▶ .
Figure 5.
Production of PGE2 in ascitic fluid. PGE2 in ascitic fluid was measured by specific ELISA in five cases of ovarian carcinoma,, five non-ovarian malignancies, and six cases of nonmalignant ascites (liver cirrhosis). Mean and SEM is shown; *, P = 0.03, Student’s t-test.
Clinical and Pathological Characteristics of Patients with Ovarian Lesions
Samples from a total of 119 patients were investigated for COX-2 immunoreactivity. The mean age of patients at surgery was 59.2 years (range, 28 to 85 years). Eighty-six patients (72.3%) had invasive ovarian carcinomas, 19 patients (16%) had tumors of low malignant potential (LMP tumors, borderline tumors, atypical proliferating tumors), 12 patients (10.1%) had benign ovarian cysts, and 2 patients (1.7%) had normal ovaries. Of the 19 LMP tumors, 14 were serous, 3 mucinous, 1 mixed serous-mucinous, and 1 transitional. Of the 86 invasive carcinomas, 48 (55.8%) were serous carcinomas, 6 (7%) mucinous carcinomas, 12 (14%) endometrioid carcinomas, 3 (3.5%) clear cell carcinomas, 3 (3.5%) transitional cell carcinomas, and 14 (16.3%) undifferentiated carcinomas. Of the patients with invasive carcinomas, 17 (19.8%) were in FIGO stage I, 9 (10.5%) in stage II, 56 (65.1%) in stage III, and 4 (4.7%) in stage IV. From 48 patients, lymph nodes were examined. Twenty-one (43.8%) of these patients were pN0 and 27 (56.3%) were pN1. Four patients (4.7%) had distant metastases at the time of diagnosis. Forty-two patients (48.8%) with invasive carcinomas died during the mean follow-up period of 32.5 months. The mean (median) survival time was 55.4 (41.2) months with a range of 43.6 to 67.3 (26.6 to 55.8) months. For determination of COX-1 immunoreactivity, a total of 101 cases were investigated. The percentage of different tumor types and tumor stages was similar to the samples investigated for COX-2.
COX-2 Immunostaining in Primary Ovarian Carcinomas, LMP Tumors, and Adenomas
Expression of COX-1 and COX-2 in normal ovaries and different ovarian lesions is shown in Figure 6 ▶ and Table 1 ▶ . Normal ovarian surface epithelium (2 cases) as well as benign adenomas (12 cases) did not show any expression of COX-2. LMP tumors were positive for COX-2 in 7 (36.8%) of 19 cases. An expression of COX-2 was observed in 36 (41.9%) of 86 invasive ovarian carcinomas. COX-2 immunoreactivity was a granular cytoplasmatic staining.
Figure 6.
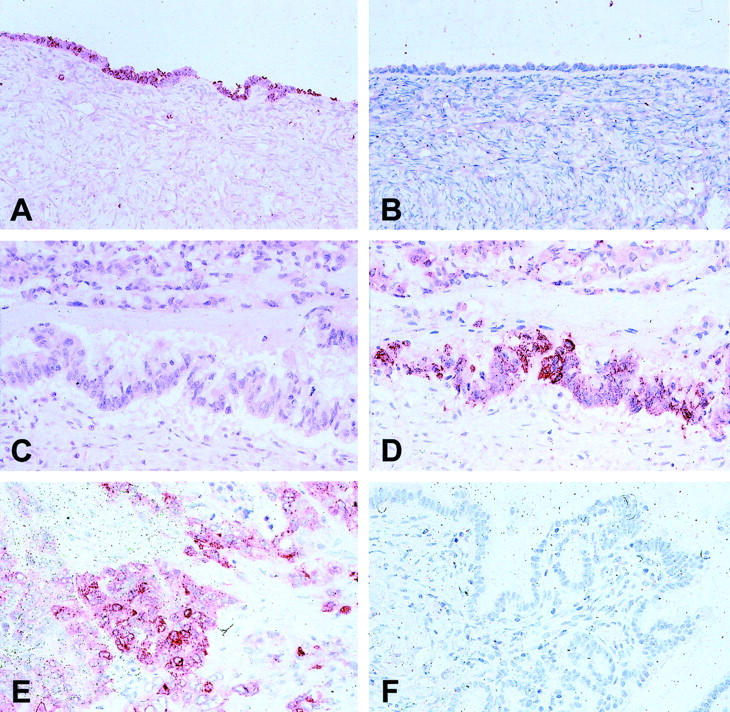
Expression of COX-1 and COX-2 in normal ovaries, LMP tumors, as well as ovarian carcinomas investigated by immunohistochemistry. Normal ovarian surface epithelium was positive for COX-1 (A), but negative for COX-2 (B). A mucinous ovarian carcinoma negative for COX-1 (C), but positive for COX-2 (D). Positive cytoplasmatic staining of COX-2 in an undifferentiated invasive ovarian carcinoma (E). LMP tumor negative for COX-2 (F).
Table 1.
Expression of COX-1 and COX-2 in Normal Ovaries and Benign and Malignant Ovarian Tumors
| Invasive carcinomas | LMP-tumors | Cystadenomas | Normal ovaries | |
|---|---|---|---|---|
| COX-2 expression, n | 86 (100%) | 19 (100%) | 12 (100%) | 2 (100%) |
| Negative | 50 (58.1%) | 12 (63.2%) | 12 (100%) | 2 (100%) |
| Positive | 36 (41.9%) | 7 (36.8%) | 0 (0%) | 0 (0%) |
| COX-1 expression, n | 75 (100%) | 16 (100%) | 8 (100%) | 2 (100%) |
| Negative | 19 (25.3%) | 4 (25.0%) | 0 (0%) | 0 (0%) |
| Positive | 56 (74.7%) | 12 (75%) | 8 (100%) | 2 (100%) |
In contrast to COX-2, COX-1 was expressed in normal ovarian surface epithelium (2 of 2 cases) and in benign adenomas (8 of 8 cases). Twelve (75%) of 16 cases of LMP tumors and 56 (72%) of invasive ovarian carcinomas were positive for COX-1. In univariate analysis, no significant correlation was observed between expression of COX-1 and COX-2 (Table 2) ▶ .
Table 2.
Relationship of COX-2 Expression and Various Clinicopathological Factors as Well as Between COX-2 Expression and COX-1 Expression in All Patients with Invasive Ovarian Carcinomas
| Characteristic | All cases | COX-2 negative | COX-2 positive | Significance |
|---|---|---|---|---|
| All carcinomas | 86 (100%) | 50 (58.1%) | 36 (41.9%) | |
| Histological type | n.s. | |||
| Serous | 48 (100%) | 29 (60.4%) | 19 (39.6%) | |
| Undifferentiated | 14 (100%) | 8 (57.1%) | 6 (42.9%) | |
| Nonserous | 24 (100%) | 13 (54.2%) | 11 (45.8%) | |
| pT | n.s. | |||
| pT1 | 19 (100%) | 14 (73.7%) | 5 (26.3%) | |
| pT2 | 11 (100%) | 8 (72.7%) | 3 (27.3%) | |
| pT3 | 56 (100%) | 28 (50%) | 28 (50%) | |
| pN | n.s. | |||
| pN0 | 21 (100%) | 14 (66.7%) | 7 (33.3%) | |
| pN1 | 27 (100%) | 16 (59.3%) | 11 (40.7%) | |
| pM | n.s. | |||
| pMX | 82 (100%) | 48 (58.5%) | 34 (41.5%) | |
| pM1 | 4 (100%) | 2 (50%) | 2 (50%) | |
| FIGO Stage | n.s. | |||
| I | 17 (100%) | 12 (70.6%) | 5 (29.4%) | |
| II | 9 (100%) | 7 (77.8%) | 2 (22.2%) | |
| III | 56 (100%) | 29 (51.8%) | 27 (48.2%) | |
| IV | 4 (100%) | 2 (50%) | 2 (50%) | |
| Histological grade (Silverberg) | n.s. | |||
| G1 | 22 (100%) | 12 (54.5%) | 10 (45.5%) | |
| G2 | 33 (100%) | 19 (57.6%) | 14 (42.4%) | |
| G3 | 32 (100%) | 19 (61.3%) | 12 (38.7%) | |
| Age at surgery (years) | n.s. | |||
| ≤ 60 | 46 (100%) | 26 (56.5%) | 20 (43.5%) | |
| > 60 | 40 (100%) | 24 (60%) | 16 (40%) | |
| COX-1 expression (n = 75) | n.s. | |||
| Negative | 19 (100%) | 11 (57.9%) | 8 (42.1%) | |
| Positive | 56 (100%) | 33 (58.9%) | 23 (41.1%) |
In univariate analysis we investigated correlations between expression of COX-2 and various clinicopathological factors (Table 2) ▶ . No significant correlations were observed between COX-2 expression and histological type, tumor stage, lymph node involvement, metastasis, FIGO stage, histological grade, and age at diagnosis. Similarly, no significant correlations were observed between expression of COX-1 and the clinicopathological factors (data not shown).
COX-2 Immunostaining and Patient Survival
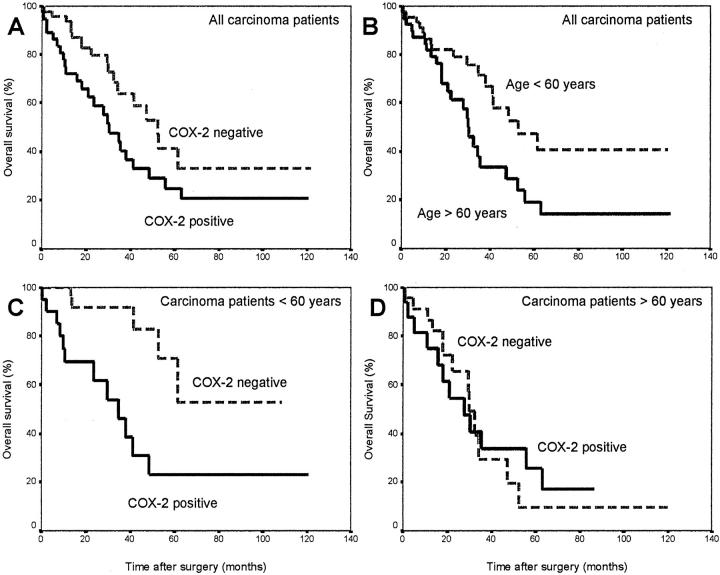
We compared the survival among all patients with invasive ovarian carcinoma in univariate analysis according to the expression status for COX-2. The median survival time of the 50 patients with tumors negative for COX-2 was 52.47 months, whereas that of the 36 patients with tumors positive for COX-2 was 30.40 months (log rank test, P = 0.04) (Table 3 ▶ , Figure 7A ▶ ). In contrast to COX-2, expression of COX-1 was not a significant prognostic parameter in ovarian carcinomas (P = 0.89) (Table 3) ▶ . Other significant prognostic markers in univariate analysis were histological diagnosis (P < 0.002), FIGO stage (P < 0.002), metastasis (P = 0.0002), histological grade (P < 0.003), and age at diagnosis (P < 0.02) (Table 3) ▶ .
Table 3.
Univariate Survival Analysis (Kaplan-Meier): Median Survival Time of All Patients with Invasive Ovarian Carcinomas According to Clinicopathological Factors and COX-1 or COX-2 Expression
| Characteristic | No. of cases | Median survival time (months) | Standard error | Log rank |
|---|---|---|---|---|
| COX-2 expression | 0.0414 | |||
| Negative | 50 | 52.47 | 6.68 | |
| Positive | 36 | 30.40 | 5.70 | |
| COX-1 expression | 0.8932 | |||
| Negative | 19 | 37.90 | 11.61 | |
| Positive | 56 | 47.47 | 9.90 | |
| Histological type | 0.0010 | |||
| Serous | 48 | 47.47 | 11.29 | |
| Undifferentiated | 14 | 17.83 | 5.71 | |
| Nonserous | 24 | 48.70 | 10.30 | |
| pT | 0.4040 | |||
| pT1 | 19 | 55.77 | 23.42 | |
| pT2 | 11 | 52.47 | 22.40 | |
| pT3 | 56 | 35.53 | 5.34 | |
| pN | 0.2742 | |||
| pN0 | 21 | 61.60 | 11.87 | |
| pN1 | 27 | Not reached | — | |
| pM | 0.0002 | |||
| pMX | 82 | 41.53 | 7.36 | |
| pM1 | 4 | 0.80 | 5.28 | |
| FIGO Stage | 0.0016 | |||
| I | 17 | 55.77 | — | |
| II | 9 | 52.47 | 29.67 | |
| III | 56 | 37.90 | 5.47 | |
| IV | 4 | 0.80 | 5.28 | |
| Histological grade (Silverberg) | 0.0029 | |||
| G1 | 22 | Not reached | — | |
| G2 | 33 | 34.50 | — | |
| G3 | 31 | 32.70 | — | |
| Age at surgery (years) | 0.0147 | |||
| ≤ 60 | 46 | 52.77 | 11.78 | |
| > 60 | 40 | 30.10 | 2.85 |
Figure 7.
Univariate survival analysis (Kaplan-Meier) of all 86 patients with invasive ovarian carcinomas. A: Patients with tumors negative for COX-2 have an increased median survival time (52.47 months, n = 50) compared to patients with tumors positive for COX-2 (30.40 months, n = 36) (log rank test; P = 0.04). B: Patients <60 years of age have a longer median survival time (52.77) compared to patients >60 years of age (30.10 months, P < 0.02). C: For patients younger than 60 years of age with tumors negative for COX-2 the median survival time is not reached during the follow-up period of 110 months, whereas patients in the same age group with tumors positive for COX-2 have a median survival time of 34.63 months (P < 0.004). D: In contrast, for patients older than 60 years of age there are no differences in median survival time between patients with tumors negative for COX-2 (30.10 months) and tumors positive for COX-2 (36.13 months, P = 0.97).
In addition, we investigated the influence of COX-2 expression on survival in patients of different age groups at the time of diagnosis. Figure 7B ▶ shows the different survival curves for patients younger than age 60 (median survival, 52.77 months) and patients older than age 60 (median survival, 30.10 months). Comparing the survival of patients in these two groups according to their COX-2 expression, we found that expression of COX-2 is especially valuable as a prognostic factor for patients younger than 60 years. As shown in Figure 7C ▶ , for patients younger than age 60 with tumors negative for COX-2 the median survival time is not reached during the follow-up period of 110 months, whereas patients in the same age group with tumors positive for COX-2 have a median survival time of 34.63 months (P < 0.004). Patients younger than age 60 with tumors positive for COX-2 have a 5-year survival rate of only 25%, whereas patients in the same age group with tumors negative for COX-2 have a 5-year survival rate of ∼55%. In contrast, for patients older than age 60 there are no differences in median survival time between patients with tumors negative for COX-2 (30.10 months) and tumors positive for COX-2 (36.13 months, P = 0.97) (Figure 7D) ▶ .
We used a multivariate regression analysis based on the Cox proportional hazard model to test the independent value of each parameter predicting overall survival. The estimated prognostic value of each variable in relation to overall survival among the 86 patients studied is expressed as a P value. We used COX-2 expression as well as the other prognostic markers of ovarian carcinomas that were significant in univariate analysis. The variables used in Cox regression analysis are shown in Table 4 ▶ . Expression of COX-2 was an independent prognostic factor for poor survival (relative risk, 2.74; 95% CI, 1.38 to 5.47). Other independent prognostic factors associated with poor prognosis were grade, FIGO stage, age at diagnosis >60 years, and undifferentiated histological type.
Table 4.
Multivariate Survival Analysis (Cox Regression Model)
| Beta | Standard error | Wald | df | Relative risk | 95% CI of Relative risk | P value | |
|---|---|---|---|---|---|---|---|
| COX-2 Expression | 0.004 | ||||||
| Negative | 1.00 | ||||||
| Positive | 1.009 | 0.352 | 8.197 | 1 | 2.74 | 1.38–5.47 | 0.004 |
| Histological type | 9.920 | 2 | 0.007 | ||||
| Serous | 1.00 | ||||||
| Nonserous | −0.515 | 0.482 | 1.142 | 1 | 0.60 | 0.23–1.54 | 0.285 |
| Undifferentiated | 1.356 | 0.474 | 8.198 | 1 | 3.88 | 1.53–9.82 | 0.004 |
| FIGO stage | 9.380 | 3 | 0.025 | ||||
| I | 1.00 | ||||||
| II | −0.142 | 0.677 | 0.044 | 1 | 0.87 | 0.23–3.27 | 0.834 |
| III | −0.421 | 0.563 | 0.559 | 1 | 0.66 | 0.22–1.99 | 0.455 |
| IV | 1.738 | 0.794 | 4.789 | 1 | 5.68 | 1.20–26.95 | 0.029 |
| Grade (Silverberg) | 10.347 | 2 | 0.006 | ||||
| G1 | 1.00 | ||||||
| G2 | 1.818 | 0.567 | 10.291 | 1 | 6.16 | 2.03–18.70 | 0.001 |
| G3 | 1.560 | 0.600 | 6.748 | 1 | 4.76 | 1.47–15.43 | 0.009 |
| Age | 0.020 | ||||||
| < 60 years | 1.00 | ||||||
| > 60 years | 0.755 | 0.326 | 5.371 | 1 | 2.13 | 1.12–4.03 | 0.020 |
Discussion
In this study, we systematically evaluated expression of COX-1 and COX-2 mRNA and protein as well as PGE2 production in ovarian carcinoma in vitro and in vivo. We show that COX-2 was expressed in 36 (42%) of 86 cases of primary ovarian carcinomas, whereas COX-1 was expressed in 65 (75%) of 75 primary ovarian carcinomas. Similar results were obtained on the mRNA level, where we found an expression of COX-2 mRNA by RT-PCR in seven of eight cases of ovarian carcinomas, whereas all cases were positive for COX-1. In cell culture, an expression of COX-2 mRNA and protein was observed in three out of five ovarian carcinoma cell lines. The production of PGE2 of the ovarian carcinoma cell lines can be inhibited by the specific COX-2 inhibitor NS-398 and is thus mediated by the COX-2 isoform. Taken together, our data indicate that COX-2 is expressed by a subset of ovarian carcinomas as well as ovarian carcinoma cell lines. In our experiments we obtained consistent results on expression and activity of COX-2 using in vitro and in vivo investigations and different methodological approaches.
Two previous studies have failed to detect expression of COX-2 in ovarian tissues. Ristimäki and colleagues 23 found 12 cases of mucinous ovarian carcinomas that were negative for COX-2 mRNA by Northern blot. In their study no immunohistochemistry was performed on the ovarian carcinoma tissue. Because COX-2 is expressed only in a subset of tumors, this subset may have been missed because of the lower number of cases studied. In an immunohistological study, Dore and colleagues 24 investigated 16 cases of ovarian carcinomas and found an expression of COX-1, but not of COX-2. These discrepancies may depend on the use of different antibodies or staining procedures. The antibody used in our study has been evaluated before using blocking experiments. 22 Recently, two additional studies have shown an expression of COX-2 in ovarian carcinomas, consistent with our results. Klimp and colleagues 25 found an expression of COX-2 in 15 of 18 ovarian carcinomas and in 10 of 15 borderline tumors. Similarly, Matsumoto and colleagues 26 found an expression of COX-2 in 79% of 28 ovarian carcinomas and in 67% of 21 borderline tumors. In these previous studies, no survival analysis was performed. To our knowledge, this is the first study showing expression of COX-2 in ovarian carcinoma cell lines and this is the first study showing that COX-2 is an independent prognostic factor in ovarian carcinomas.
In addition to the expression of COX-2 in tumor tissue of ovarian carcinomas, we found significantly increased levels of PGE2 in ascites samples of patients with ovarian cancer. This indicates that PGE2 is present in vivo in the microenvironment of ovarian carcinomas. The production of PGE2 in ascitic fluid may be partly from COX-2 activity in ovarian carcinoma cells, but peritoneal macrophages may be additional sources of PGE2. Because the majority of ovarian carcinomas are positive for COX-1, it could also be possible that COX-1 activity contributes to the PGE2 in ascitic fluid. However, our experiments with ovarian carcinoma cell lines using the specific COX-2 inhibitor NS-398 suggest that the COX-2 isoform is the main source of PGE2 in ovarian carcinoma cells. Further experiments are needed to fully characterize the source of elevated levels of PGE2 in ascitic fluid from ovarian carcinoma patients. We have not been able to compare the PGE2 production in ascitic fluid with the expression of COX-1 and COX-2 in the corresponding tumors, because no material from these tumors was available for immunohistochemistry. Although we could only measure a comparably small set of samples in the present study, elevated levels of PGE2 have been described previously in primary tumors, metastases, and ascitic fluid of patients with ovarian carcinomas. 27 The level of PGE2 in ascites might be relevant for patients’ response to therapy, because tumors without response to chemotherapy were found to contain higher levels of PGE2 and other prostaglandins than tumors responding to chemotherapy. 28 Thus, it may be interesting to investigate whether COX-2 expression may be a predictive factor for response to chemotherapy as well.
In our immunohistochemical investigations expression of COX-2 was increased in ovarian carcinomas and LMP tumors compared to normal ovarian surface epithelium and cystadenomas. In invasive ovarian carcinomas, two subgroups could be identified based on the positive or negative expression of COX-2. We investigated survival time of patients of these two groups and found that expression of COX-2 was a predictor of short survival times in univariate and multivariate analysis. Other independent prognostic factors associated with poor prognosis were grade, FIGO stage, age at diagnosis, and histological type. It should be pointed out that because of the relatively small number of patients in some of the various subgroups the statistical power of the analysis may be insufficient to detect weaker prognostic factors or factors that are significant only in certain subgroups of tumors.
Comparing COX-2 expression in patients of different age groups, we found that COX-2 expression in tumor tissue is a highly significant prognostic factor for patients younger than age 60, but not for patients older than age 60. This might indicate that in younger patients hormonal influences on ovarian carcinoma cells act together with an expression of COX-2 to worsen the prognosis. It has been shown that estrogens increase COX-2 in rat myometrium, 29 rat mammary glands, 30 and human umbilical vein endothelial cells. 31 On the other hand, estrogen decreased COX-2 expression in bovine endometrial cells. 32 Thus, the regulation of COX-2 expression by estrogens seems to be dependent on the cell type and has not been studied in ovarian carcinoma cells.
Several epidemiological studies have investigated the role of regular NSAID-intake on prevention of ovarian cancer. Cramer and colleagues 33 found a modest but nonsignificant inverse association with aspirin use for at least 6 months and ovarian cancer, whereas Tavani and colleagues 34 found no association. In contrast, Rosenberg and colleagues 35 found that use of NSAIDs 4 or more days per week for at least 5 years significantly reduced the risk of ovarian cancer (odds ratio, 0.5). As a conclusion, long-term use of comparably high doses of NSAIDs could have a protective effect against ovarian carcinoma. Based on the results of our study it would be interesting to investigate if the protection by NSAIDs might be more pronounced in patients younger than 60 years.
The cellular mechanisms responsible for the worse prognosis of tumors with an increased expression of COX-2 are not clear, so far. Several functions of inducible cyclooxygenase (COX-2) have been described in the biology of various carcinomas: increased cell proliferation, 36 inhibition of apoptosis, 37 stimulation of angiogenesis, 38 as well as inhibition of immunosurveillance. 39 There are only few studies on the impact of the level of COX-2 expression in tumor tissue on the prognosis of the patients and studies using multivariate analysis have not been performed. Khuri and colleagues 40 showed in univariate analysis that COX-2 expression was a marker of poor prognosis in stage I non-small cell lung cancer. For colon carcinoma, COX-2 expression was a prognostic factor in univariate analysis and correlated with tumor neovascularization. 41 In ovarian carcinomas, several studies have shown that microvessel density is not an independent prognostic indicator. 42-44 Therefore, we did not measure microvessel density in the present study. Similarly, it has been shown that the apoptotic index is no independent prognostic indicator in ovarian carcinomas. 45,46 However, in some studies apoptosis-related proteins such as p53, bcl-2, or bax have been shown to affect prognosis of ovarian carcinomas. 46,47 Thus, it will be very interesting to investigate the correlation between COX-2 expression and different factors involved in apoptotic or necrotic cell death.
Studies on the function of COX-2 in other types of tumors support a role for COX-2 in tumor invasion. For example, COX-2 expression in gastric carcinoma was correlated with tumor invasion into lymphatic vessels as well as metastasis into lymph nodes. 48 Similar results have been shown for pulmonary adenocarcinomas, where COX-2 expression was enhanced in metastases as compared to primary tumors. 49 In colon carcinoma cell lines, transfection with COX-2 resulted in increased Matrigel invasion. 50 Thus, it might be possible that ovarian carcinomas with a higher expression of COX-2 show an increased metastatic potential and thus a poorer prognosis compared to tumors negative for COX-2.
The determination of the status of COX-2 expression, in combination with other clinicopathological factors, may improve the prognostic evaluation of ovarian carcinoma patients and enhance the ability to prospectively identify individuals who are at risk for poor survival. However large-scale prospective and retrospective studies are needed to establish whether COX-2 expression is indeed of practical utility as a prognostic predictor. The development of new specific inhibitors of COX-2 leads to new concepts of primary and secondary chemoprevention of cancer. 51 Based on the results of this study, it would be interesting whether ovarian carcinoma patients with tumors positive for COX-2 would benefit from treatment with selective COX-2 inhibitors.
Footnotes
Address reprint requests to Prof. Dr. Steffen Hauptmann, Institute of Pathology, Charité Hospital, Campus Mitte Schumannstr. 20/21, D-10117, Berlin, Germany. E-mail: steffen.hauptmann@charite.de.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA: Cancer statistics. Ca Cancer J Clin 2000, 50:7-33 [DOI] [PubMed] [Google Scholar]
- 2.O’Banion MK, Winn VD, Young DA: cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci USA 1992, 89:4888-4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thun MJ, Namboodiri MM, Heath Jr CW: Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 1991, 325:1593–1596 [DOI] [PubMed]
- 4.Schreinemachers DM, Everson RB: Aspirin use and lung, colon and breast cancer incidence in a prospective study. Epidemiology 1994, 5:138-146 [DOI] [PubMed] [Google Scholar]
- 5.Taketo MM: Cyclooxygenase inhibitors in tumorigenesis (part I). J Natl Cancer Inst 1998, 90:1529-1536 [DOI] [PubMed] [Google Scholar]
- 6.Taketo MM: Cyclooxygenase inhibitors in tumorigenesis (part II). J Natl Cancer Inst 1998, 90:1609-1615 [DOI] [PubMed] [Google Scholar]
- 7.Kawamori T, Rao CV, Seibert K, Reddy BS: Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res 1998, 58:409-412 [PubMed] [Google Scholar]
- 8.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM: Suppression of intestinal polyposis in Apc knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 1996, 87:803-809 [DOI] [PubMed] [Google Scholar]
- 9.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey III TJ: Cyclooxygenase-2 expression is upregulated in human pancreatic cancer. Cancer Res 1999, 59:987–990 [PubMed]
- 10.Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R, Nakashima Y, Nakashima O, Kojiro M, Kurohiji T, Sata M: Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology 1999, 29:688-696 [DOI] [PubMed] [Google Scholar]
- 11.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimäki A: Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res 1998, 58:4997-5001 [PubMed] [Google Scholar]
- 12.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, Masferrer JL, Dannenberg AJ: Cyclooxygenase-2 expression is upregulated in squamous cell carcinoma of the head and neck. Cancer Res 1999, 59:991-994 [PubMed] [Google Scholar]
- 13.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM: Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 1995, 378:406-409 [DOI] [PubMed] [Google Scholar]
- 14.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK: Multiple female reproductive failures in cyclooxygenase 2 deficient mice. Cell 1997, 91:197–208 [DOI] [PubMed]
- 15.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD: Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 1995, 83:483-492 [DOI] [PubMed] [Google Scholar]
- 16.Hamilton TC, Young RC, McKoy WM, Grotzinger KR, Green JA, Chu EW, Whang-Peng J, Rogan AM, Green WR, Ozols RF: Characterization of a human ovarian carcinoma cell line (NIH: OVCAR-3) with androgen and estrogen receptors. Cancer Res 1983, 43:5379-5389 [PubMed] [Google Scholar]
- 17.Fogh J: Human tumor cells in vitro. 1975:pp 115-159 Plenum Press, New York
- 18.Wilson AP: Characterization of a cell line derived from the ascites of a patient with papillary serous cystadenocarcinoma of the ovary. J Natl Cancer Inst 1984, 72:513-521 [PubMed] [Google Scholar]
- 19.Lau DH, Lewis AD, Ehsan MN, Sikic BI: Multifactorial mechanisms associated with broad cross-resistance of ovarian carcinoma cells selected by cyanomorpholino doxorubicin. Cancer Res 1991, 51:5181-5187 [PubMed] [Google Scholar]
- 20.Yoshimura R, Sano H, Masuda C, Kawamura M, Tsubouchi Y, Chargui J, Yoshimura N, Hla T, Wada S: Expression of cyclooxygenase-2 in prostate carcinoma. Cancer 2000, 89:589-566 [PubMed] [Google Scholar]
- 21.Shimizu Y, Kamoi S, Amada S, Hasumi K, Akiyama F, Silverberg SG: Toward the development of a universal grading system for ovarian epithelial carcinoma. Gynecol Oncol 1998, 70:2-12 [DOI] [PubMed] [Google Scholar]
- 22.Ristimäki A, Nieminen O, Saukkonnen K, Hotakainen K, Nordling S, Haglund C: Expression of Cyclooxygenase-2 in human transitional carcinoma of the urinary bladder. Am J Pathol 2001, 158:849-853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ristimäki A, Honkanen N, Jankala H, Sipponen P, Harkonen M: Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res 1997, 57:1276-1280 [PubMed] [Google Scholar]
- 24.Dore M, Cote LC, Mitchell A, Sirois J: Expression of prostaglandin G/H synthase type 1, but not type 2, in human ovarian adenocarcinomas. J Histochem Cytochem 1998, 46:77-84 [DOI] [PubMed] [Google Scholar]
- 25.Klimp AH, Hollema H, Kempinga C, van der Zee AG, de Vries EG, Daemen T: Expression of cyclooxygenase-2 and inducible nitric oxide synthase in human ovarian tumors and tumor-associated macrophages. Cancer Res 2001, 61:7305-7309 [PubMed] [Google Scholar]
- 26.Matsumoto Y, Ishiko O, Deguchi M, Nakagawa E, Ogita S: Cyclooxygenase-2 expression in normal ovaries and epithelial ovarian neoplasms. Int J Mol Med 2001, 8:31-36 [DOI] [PubMed] [Google Scholar]
- 27.Kushlinskii NE, Podistov YI, Laktionov KP, Karseladze AI, Babkina IV, Kerimova GI: Prostaglandins E in primary tumor, metastases and ascitic fluid of patients with ovarian cancer. Biull Eksp Biol Med 1997, 123:83-86 [PubMed] [Google Scholar]
- 28.Bauknecht T, Siegel A, Meerpohl HG, Zahradnik HP: Formation of prostaglandins by ovarian carcinomas. Prostaglandins 1985, 29:665-672 [DOI] [PubMed] [Google Scholar]
- 29.Engstrom T: The regulation by ovarian steroids of prostaglandin synthesis and prostaglandin-induced contractility in non-pregnant rat myometrium. Modulating effects of isoproterenol. J Endocrinol 2001, 169:33-41 [DOI] [PubMed] [Google Scholar]
- 30.Badawi AF, Archer MC: Effect of hormonal status on the expression of the cyclooxygenase 1 and 2 genes and prostaglandin synthesis in rat mammary glands. Prostaglandins Lipid Mediat 1998, 56:167-181 [DOI] [PubMed] [Google Scholar]
- 31.Akaresereenont P, Techatraisak K, Thaworn A, Chotewuttakorn S: The induction of cyclooxygenase-2 by 17beta-estradiol in endothelial cells is mediated through protein kinase C. Inflammation Res 2000, 49:460-465 [DOI] [PubMed] [Google Scholar]
- 32.Xiao CW, Liu JM, Sirois J, Goff AK: Regulation of cyclooxygenase-2 and prostaglandin F synthase gene expression by steroid hormones and interferon-tau in bovine endometrial cells. Endocrinology 1998, 139:2239-2299 [DOI] [PubMed] [Google Scholar]
- 33.Cramer DW, Harlow BL, Titus-Ernsthoff L, Bohlke K, Welch WR, Greenberg ER: Over-the counter analgesics and ovarian cancer. Lancet 1998, 351:104-107 [DOI] [PubMed] [Google Scholar]
- 34.Tavani A, Gallus S, La Veccia C, Conti E, Montella M, Franceschi S: Aspirin and ovarian cancer: an Italian case-control study. Ann Oncol 2000, 11:1171-1173 [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg L, Palmer JR, Rao RS, Coogan PF, Strom BL, Zauber AG, Stolley PD, Shapiro S: A case-control study of analgesic use and ovarian cancer. Cancer Epidemiol Biomarkers Prev 2000, 9:933-937 [PubMed] [Google Scholar]
- 36.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN: Modulation of apoptosis and bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 1998, 58:362-366 [PubMed] [Google Scholar]
- 37.Tsujii M, DuBois RN: Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 1995, 93:705-716 [DOI] [PubMed] [Google Scholar]
- 38.Tsujii M, Kawano S, Tsujii S, Sawaoka H, Hori M, DuBois RN: Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998, 93:705-716 [DOI] [PubMed] [Google Scholar]
- 39.Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, Wollman J, Herschman H, Dubinett SM: Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: upregulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res 1998, 58:1208-1216 [PubMed] [Google Scholar]
- 40.Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman SM, Feng L, Hong WK, Xu XC: Cyclooxygense-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res 2001, 7:861-867 [PubMed] [Google Scholar]
- 41.Masunaga R, Kohno H, Dhar DK, Ohno S, Shibakita M, Kinugasa S, Yoshimura H, Tachibana M, Kubota H, Nagasue N: Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res 2000, 10:4064-4068 [PubMed] [Google Scholar]
- 42.Obermair A, Wasicky R, Kaider A, Preyer O, Losch A, Leodolter S, Kolbl H: Prognostic significance of tumor angiogenesis in epithelial ovarian cancer. Cancer Lett 1999, 138:175-182 [DOI] [PubMed] [Google Scholar]
- 43.Nakyama K, Kanzaki A, Takebayashi Y, Toi M, Banso H, Nabei T, Miyazaki K, Fukumoto M: Different features of angiogenesis between ovarian and breast carcinoma. Cancer Lett 2001, 170:161-167 [DOI] [PubMed] [Google Scholar]
- 44.Terai Y, Ueda M, Kumagai K, Ueki K, Ueki M: Tumor angiogenesis and thymidine phosphorylase expression in ovarian carcinomas including serous surface papillary adenocarcinoma of the peritoneum. Int J Gynecol Pathol 2000, 19:354-360 [DOI] [PubMed] [Google Scholar]
- 45.Yamasaki F, Tokunaga O, Sugimori H: Apoptotic index in ovarian carcinoma: correlation with clinicopathologic factors and prognosis. Gynecol Oncol 1997, 66:439-448 [DOI] [PubMed] [Google Scholar]
- 46.Sengupta PS, McGown AT, Bajaj V, Blackhall F, Swindell R, Bromley M, Shanks JH, Ward T, Buckley CH, Reynolds K, Slade RJ, Jayson GC: p53 and related proteins in epithelial ovarian cancer. Eur J Cancer 2000, 36:2317-2328 [DOI] [PubMed] [Google Scholar]
- 47.Baekelandt M, Holm R, Nesland JM, Trope CG, Kristensen GB: Expression of apoptosis-related proteins is an independent determinant of patient prognosis in advanced ovarian cancer. J Clin Oncol 2000, 18:3775-3781 [DOI] [PubMed] [Google Scholar]
- 48.Murata H, Kawano S, Tsujii S, Tsujii M, Sawaoka H, Kimura Y, Shiozaki H, Hori M: Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol 1999, 94:451-455 [DOI] [PubMed] [Google Scholar]
- 49.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T: Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res 1998, 58:3761-3764 [PubMed] [Google Scholar]
- 50.Tsujii M, Kawano S, Du Bois RN: Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 1997, 94:3336-3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong WK, Sporn MB: Recent advances in chemoprevention of cancer. Science 1994, 278:1073-1077 [DOI] [PubMed] [Google Scholar]