Abstract
Cationic antimicrobial protein of 37 kd (CAP37), originally isolated from human neutrophils, is an important multifunctional inflammatory mediator. Here we describe its localization within the vascular endothelium associated with atherosclerotic plaques. Evidence from in vitro immunocytochemical, Northern blot, and reverse transcriptase-polymerase chain reaction analysis indicates that CAP37 is induced in endothelial cells in response to inflammatory mediators. Endothelial-derived CAP37 shows sequence identity with an extensive region of neutrophil-derived CAP37. This is the first demonstration of endogenous endothelial CAP37, confirmed by sequence analysis. We suggest that, because of its induction and location in the endothelium and its known monocyte- and endothelial-activating capabilities, CAP37 has potential to modulate monocyte/endothelial dynamics at the vessel wall in inflammation.
Cationic antimicrobial protein of 37 kd (CAP37) was originally isolated from granule extracts of human polymorphonuclear leukocytes (PMNs) in 1984. 1 The amino acid sequence of PMN-CAP37 revealed its relation to members of the serine protease family that have a conserved catalytic active site consisting of his-57, asp-102, and ser-195 in the charge relay system. 2 Of these sites, the conserved histidine and serine of the catalytic triad have been replaced with serine and glycine residues, respectively, rendering CAP37 ineffective as a serine protease. 2,3 However, CAP37 has been demonstrated to have a diverse and exciting repertoire of functions. It was first analyzed regarding its bactericidal properties against gram-negative bacteria including, but not limited to, Salmonella typhimurium, Escherichia coli, and Pseudomonas aeruginosa 4 and its ability to bind to and neutralize lipopolysaccharide (LPS). 5 Subsequently we showed CAP37 to be a potent chemoattractant for monocytes. 6 Additionally, regarding its effects on the monocyte, CAP37 has been reported to stimulate their survival and thrombospondin secretion, 7 also to enhance the LPS-stimulated release of prostaglandin E2, 8 interleukin (IL)-6, 9 and tumor necrosis factor (TNF)-α. 8-10 To add even further to its extensive range of known functions, CAP37 has been demonstrated to stimulate the reversible contraction of fibroblasts and endothelial cells 7 and to activate endothelial cell protein kinase C. 11 Recently, CAP37 released from stimulated PMNs was reported to be taken up and sequestered in nearby endothelial mitochondria and has been suggested to protect against apoptosis. 12
We have shown the presence of CAP37 in the endothelium of Alzheimer’s brain microvessels 13 and have shown it to be induced in rat brain endothelial cells in response to stimulation with inflammatory molecules TNF-α, IL-1α, and LPS. 13 Here, we report the presence of CAP37 in endothelium associated with atherosclerotic plaques, and in and around foam cells, and cholesterol clefts in complex plaques. There is strong evidence that both Alzheimer’s disease and atherosclerosis are inflammatory-modulated diseases 14,15 in which inflammation and associated mediators can exacerbate or augment the disease. We believe that the association of CAP37 in both these diseases lends credence to our hypothesis that it is an important mediator of inflammation leading to the exacerbation or augmentation of the chronic inflammatory responses observed in Alzheimer’s disease and atherosclerosis.
The aims of this study were to demonstrate the presence of CAP37 in atherosclerotic lesions, show its induction in endothelial cell culture, and confirm that it is CAP37 by nucleotide sequence analysis. We demonstrate that LPS induces CAP37 protein and mRNA expression in vitro in a time-dependent manner. Furthermore, isolated mRNA from activated human umbilical vein endothelial cells (HUVECs) shows sequence identity with an extensive region of PMN-CAP37. This is the first demonstration of endogenous endothelial-CAP37 (E-CAP37) as confirmed by sequence analysis and suggests that, because of its induction and location in the endothelium and its known monocyte- and endothelial-activating capabilities, CAP37 has the potential to modulate monocyte/endothelial dynamics at the vessel wall.
Materials and Methods
Human Tissues
Sections of atherosclerotic vessels (carotid, iliac, coronary and femoral arteries, and aorta) were obtained from the Department of Pathology, University of Oklahoma archival tissue bank.
Cell Culture
Rat aorta endothelial cells (RAECs) were isolated and maintained in Dulbecco’s modified Eagle’s medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), as previously described. 16 Cells were used within the first 15 passages.
Human endothelial cells were isolated from umbilical cords (HUVECs) (University Hospital, Oklahoma City, OK), according to methods modified from Jaffe and colleagues. 17 Umbilical veins were washed, flushed with phosphate-buffered saline, and the lumen filled with 0.125% trypsin/Dulbecco’s modified Eagle’s medium for 15 minutes. The cell suspension was centrifuged for 5 minutes at 250 × g, and the pellet resuspended in endothelial growth media (EGM; Clonetics, San Diego, CA) supplemented with bovine brain extract (Clonetics). The cells were passaged at a 1 to 4 split ratio and were used in the first six passages.
Human lung microvessel endothelial cells (HMVEC-Ls) were purchased from Clonetics and maintained in Endothelial Growth Media-2 (Clonetics). Cells were used within passages 4 to 11.
Immunohistochemistry
Immunohistochemistry on human atherosclerotic tissue sections was performed using our published methodology. 13 The antiserum used was previously characterized by enzyme-linked immunosorbent assay and shown to be specific for CAP37. 13 Briefly, 5-μm sections were incubated at 37°C with the primary anti-CAP37 antiserum (1:500 to 1:2000), followed by biotinylated goat anti-rabbit antiserum (Vectastain Elite, Vector Laboratories, Burlingame, CA), and then incubated in ABC reagent (Vectastain Elite). Color was developed with diaminobenzidine (Research Genetics, Huntsville, AL) for 2 to 6 minutes. Sections were counterstained with hematoxylin. Antibody controls included normal rabbit serum and immunoadsorbed rabbit anti-CAP37 antiserum, as we have described. 13
For immunocytochemical analysis of rat aorta cells the media was removed and replaced with serum-free Dulbecco’s modified Eagle’s medium overnight before start of the experiment. RAECs were incubated with 10 μg/ml of Salmonella minnesota LPS (Sigma, St. Louis, MO) for 0.5, 2, 4, 6, and 24 hours. Untreated cells at each time point were included as a control. The adherent cells on the LAB TEK slides were immunostained for CAP37 as described above 13 except the RAEC slides were fixed in ice-cold 100% methanol and the primary rabbit anti-human CAP37 antiserum was used at a 1:10 dilution.
For immunocytochemical analysis of surface-expressed and cell-associated CAP37 in human endothelial cells, the media was replaced with serum-free endothelial cell basal medium (EBM) (Clonetics) 6 hours before the start of the experiment. HUVECs were incubated in the absence or presence of 10 ng/ml of TNF-α for 10 and 18 hours. Samples were either fixed only or fixed and permeabilized essentially as described by Gräbner and colleagues. 18 Cells were stained as above using 5% normal donkey serum (Jackson, West Grove, PA) to block nonspecific binding, rabbit anti-human CAP37 (1:750) at room temperature, biotin-sp-donkey F(ab′)2 anti-rabbit IgG, (1:500, Jackson) and peroxidase-conjugated streptavidin (2 μg/ml, Jackson) for amplification of signal. Staining using normal rabbit serum was included as a control.
Northern Blot Analysis
Total cellular RNA was isolated from RAECs. 19 Thirty μg of total RNA per well were run on a 1% agarose/formaldehyde gel at 80 mA for 1.5 hours. The RNA was transferred to nylon membrane overnight in standard saline citrate (3 mol/L sodium chloride, 0.3 mol/L sodium citrate) transfer buffer and crosslinked to the membrane with a UV crosslinker. CAP37 mRNA was detected by hybridizing a 32P-labeled CAP37 cDNA probe (6.5 μg of probe at 50 μCi/blot), prepared with the Prime-it II Random Primer kit (Stratagene, La Jolla, CA), by incubating with the membrane at 60°C overnight. After a low [2× standard saline citrate buffer, 0.1% sodium dodecyl sulfate (SDS), room temperature] and high (0.1× standard saline citrate buffer, 0.1% SDS, 60°C) stringency wash the membrane was exposed to autoradiograph film at −80°C. To demonstrate the integrity and relative amounts of sample RNA, total cellular RNA (5 μg) was run on a 1% agarose/formaldehyde gel and visualized by ethidium bromide staining.
Western Blot Analysis
Human umbilical vein endothelial cells were grown to confluency, serum-starved for 6 hours before start of the experiment, and treated for 12 hours with 10 ng/ml of TNF-α (Boehringer-Mannheim, Indianapolis, IN). Cells were lysed in 1% SDS/TE [1% SDS (Fisher, Fair Lawn, NJ); TE, 1 mol/L Tris, 0.5 mol/L ethylenediaminetetraacetic acid, pH 8 (Fisher)] and 50 μg of lysate were loaded per lane onto a 12.5% SDS-polyacrylamide gel electrophoresis gel and transferred to nitrocellulose membrane (Schleicher and Schuell, Keene, NH) for Western analysis. 6 Briefly, blots were probed for CAP37 using a monospecific polyclonal rabbit antisera against human CAP37 (1:1000) and alkaline-phosphatase-conjugated donkey anti-rabbit IgG at 1:1000 (Jackson), and color developed with Nitro BT/5-bromo-4-chloro-3-indolyl phosphate ρ-toluidine salt (Fisher). An identical blot was probed with normal rabbit serum to show specificity of the reaction. Twenty μg of PMN extract was included as a positive control for CAP37.
Flow Cytometry
Human umbilical vein endothelial cells that were serum-starved for 6 hours were incubated in the absence or presence of 10 ng/ml of TNF-α for 10 hours and 18 hours. Permeabilized and nonpermeabilized cells were fixed and stained essentially as described by Gräbner and colleagues. 18 The cells were first blocked with 4% normal donkey serum (Jackson), then incubated at 4°C with rabbit anti-human CAP37 antisera (1:300), and followed by biotin-sp-donkey F(ab′)2 anti-rabbit IgG, (1:200, Jackson) at 4°C. For detection the cells were incubated with fluorescein dichloro triazinyl amino fluorescein (DTAF)-conjugated streptavidin (Jackson) at 2 μg/ml at 4°C. Cells were analyzed by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA). Unstained cells and cells stained with normal rabbit serum were included as controls.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Endothelial cells from umbilical cords from four donors were incubated for 1 to 24 hours at 37°C with 10 ng/ml of TNF-α (Boehringer-Mannheim). The supernatant was aspirated and the cells homogenized with Trizol (Life Technologies, Inc., Gaithersburg, MD) according to the manufacturer’s instructions for total RNA isolation.
cDNA was prepared using Superscript II reverse transcriptase and oligo (dt)12–18 (Life Technologies, Inc.) essentially according to the manufacturer’s protocol with an additional 30-minute incubation at 50°C before termination of the reaction. cDNA was amplified in the polymerase chain reaction with primers designed for a 468-bp internal fragment (5′-GTGCTGGGTGCCTATGACCTGAGG-3′, 5′-AAGAGCGCCACTCGGGTGAAGAA-3′) flanking exons and introns of HL60-CAP37. The PCR reaction mix (1.5 mmol/L MgCl2, 0.3 mmol/L dNTPs, 0.3 U Taq polymerase (Life Technologies, Inc.), 0.4 μmol/L primer mix, and cDNA in a 25-μl total volume) was amplified for 30 cycles on a Biometra T Gradient thermocycler followed by visualization on a 1% agarose gel with 0.5 μg/ml ethidium bromide. RNA samples with no reverse transcriptase were included in the PCR reaction to demonstrate lack of genomic DNA contamination.
Cloning and Sequencing of E-CAP37
RT-PCR was performed essentially as above using primers designed for an internal (5′-CAGAATCAAGGCAGGCACTTCTGC-3′, 5′-GAGAACACCATCGATCCAGTCTCG-3′) 597-bp fragment of CAP37. The products were excised and eluted from the agarose gel with Gene Clean II (Bio101, Vista, CA) and ligated into a pCR2.1 vector (The Original TA Cloning Kit; Invitrogen, Carlsbad, CA), and cloned in INVαF’ E. coli (One Shot Chemically Competent E. coli, Invitrogen) according to the manufacturer’s protocol. Plasmids from transformants were sequenced by the Oklahoma Medical Research Foundation Sequencing Facility in both forward and reverse directions using the T7 and M13 reverse primers for four clones from three separate induction experiments. The resulting sequences were aligned using Pôle Bio-Informatique Lyonnais, Network Protein Sequence @nalysis 20 for DNA and the consensus sequence blasted against the HL60-CAP37 cDNA sequence.
Results
CAP37 Is Present in Atherosclerotic Plaques
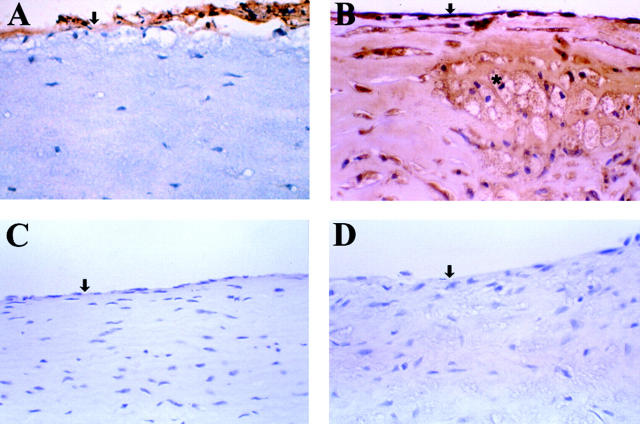
Tissue sections from human atherosclerotic lesions showed strong staining for CAP37 in the endothelium associated with the plaque area (Figure 1A) ▶ . CAP37 was also detected in and around foam cells and cholesterol clefts in plaques with advanced disease (Figure 1B) ▶ . Normal endothelium away from the injured endothelium associated with the plaque did not stain for CAP37 (Figure 1C) ▶ . Antibody controls using immunoadsorbed antisera to CAP37 13 showed no staining for CAP37 (Figure 1D) ▶ indicating the specificity of the reaction obtained in Figure 1 ▶ ; A, B, and C.
Figure 1.
Localization of CAP37 in formalin-fixed, paraffin-embedded carotid artery A: Immunohistochemistry performed on atherosclerotic lesion present in the carotid artery using antisera to human CAP37 and the Vectastain Elite technique as described in the text. Strong staining (brown) indicated the presence of CAP37 in the endothelium. B: Detection of CAP37 in advanced atherosclerotic plaque indicating strong positive staining in endothelium and foam cells. C: Normal vessel stained with antisera to CAP37 indicating an absence of CAP37 in normal endothelium. D: Atherosclerotic lesion stained using an immunoadsorbed antisera to CAP37 that shows no staining. This lack of staining in D indicates the specificity of the antisera for CAP37 used in these assays. Sections were counterstained with hematoxylin. ↓, endothelium; *, foam cell. Original magnifications: ×400.
CAP37 Is Induced in Cultured Endothelial Cells
Because CAP37 was detected in the endothelium of atherosclerotic plaques but not in normal endothelium, we hypothesized that CAP37 was induced in response to injurious and/or inflammatory mediators. To explore this possibility we obtained endothelial cells from various vascular beds including rat aorta (RAECs), umbilical vein (HUVECs), and human lung microvessel (HMVEC-Ls) and treated these cell cultures with LPS and cytokines including TNF-α and IL-1. We used immunocytochemical, Northern blot analysis, and RT-PCR to detect CAP37. The immunocytochemical data presented here (Figure 2) ▶ were obtained from RAECs. CAP37 protein was detected in LPS-treated endothelial cells as early as 2 hours. Maximum staining was obtained between 4 hours (Figure 2A) ▶ and 6 hours. Staining was reduced, but still evident at 24 hours. Untreated cells (Figure 2B) ▶ did not stain at any of the time points with anti-CAP37 antiserum. Antibody controls using normal rabbit serum showed virtually no background staining. Similar studies using HUVECs and HMVEC-Ls indicated expression of CAP37 in response to LPS (data not shown).
Figure 2.
Induction of CAP37 protein in RAECs. A: Immunocytochemistry of RAECs stimulated with 10 μg/ml of LPS for 4 hours and stained with antisera to CAP37 using the Vectastain Elite ABC technique indicating strong staining (brown) for CAP37. B: RAECs incubated with media alone and stained with antisera to CAP37 shows no positive reaction, indicating that E-CAP37 is not constitutively expressed in RAECs. Sections counterstained with hematoxylin. Original magnifications: ×400 (A); ×200 (B).
To further corroborate our immunocytochemical data we isolated total cellular RNA from unstimulated RAECs and RAECs stimulated with 10 μg/ml of S. minnesota LPS throughout a time course spanning 30 minutes to 24 hours and performed Northern blot analysis to identify CAP37 mRNA. Using a 32P-labeled CAP37 cDNA probe, we detected CAP37 mRNA at 30 minutes in LPS-stimulated cultures. CAP37 mRNA was also present in 2-, 4-, and 6-hour stimulated cultures but was not detected at 24 hours (Figure 3) ▶ . We did not detect CAP37 mRNA in unstimulated cultures at any time point. An HL-60 cell line (abundant in CAP37 mRNA) 21 was used as a positive control.
Figure 3.
Northern blot analysis of CAP37 mRNA in RAECs. Rat aorta endothelial cells were stimulated for 0 (lane 3), 0.5 (lane 4), 2 (lane 5), 4 (lane 6), 6 (lane 7), and 24 hours (lane 8) with S. minnesota LPS and the Northern blot performed on total RNA from each time point using the 32P-labeled CAP37 cDNA probe as described in text. An HL-60 cell line (lane 1) used as a positive control indicated presence of CAP37 mRNA (1000 bp). 18S and 28S rRNA (bottom) of total cellular RNA demonstrating the integrity and relative amounts of RNA. Lane 2 is empty.
In addition to the above studies with LPS-treated RAECs we demonstrated the induction of CAP37 in HUVECs in response to the inflammatory cytokine TNF-α. We performed these studies by incubating HUVECs in the absence or presence of 10 ng/ml of TNF-α. CAP37 mRNA induction was assessed by RT-PCR. Kinetic studies performed throughout a 24-hour time period indicated that CAP37 mRNA significantly increased after TNF-α stimulation. Initial up-regulated expression was observed as early as 1 hour and in general persisted for a 6-hour time period (Figure 4) ▶ . In certain experiments, up-regulated expression of CAP37 was observed as late as 12 hours. In contrast to the Northern blot data using RAECs, we observed constitutive CAP37 mRNA expression in HUVECs using RT-PCR. RT-PCR was performed with primers designed to flank exons and introns of CAP37 so that any genomic DNA contamination would be readily apparent (appearing as a PCR product much larger than that obtained from cDNA). In addition, the control PCR reactions using RNA samples containing no reverse transcriptase did not detect genomic DNA contamination.
Figure 4.
RT-PCR analysis of HUVECs for CAP37 mRNA. Human umbilical vein endothelial cells were incubated with 10 ng/ml of TNF-α or left untreated (unt) for the indicated times and CAP37 mRNA expression (top, 468 bp) determined by RT-PCR. cDNA integrity was assessed with β-actin primers (bottom, 267 bp). This is a representative figure of five independent experiments.
Endothelial-Derived CAP37 Has Nucleotide Sequence Identity to PMN-CAP37
Final confirmation that we were in fact dealing with CAP37 was obtained from sequence data. We cloned an extensive region of E-CAP37 and compared the cDNA sequence to the known HL60-CAP37 21 sequence. This comparison demonstrated complete identity with the known amino acid sequence of PMN-CAP37 between residues 19 through 217.
Endothelial CAP37 Is Mainly Cell-Associated
To determine whether the induced form of CAP37 was cell associated or released we undertook a series of experiments that included immunocytochemistry, flow cytometry, enzyme-linked immunosorbent assay, and Western blot analysis. In the immunocytochemical studies, we treated HUVECs with TNF-α and compared the staining pattern for CAP37 in fixed cells with permeabilized cells. Figure 5A ▶ indicates that there is virtually no CAP37 detected when cells are fixed but not permeabilized indicating that there is very minimal, if any, cell surface-expressed CAP37. This is true regardless of whether cells are treated with TNF-α (Figure 5A) ▶ or remain untreated (Figure 5B) ▶ . On the other hand, when TNF-α-treated cells are permeabilized we observe dramatic staining for CAP37 indicating that the major component of endothelial CAP37 is cell associated (Figure 5C) ▶ . The staining is punctate throughout the cytoplasm with visible perinuclear localization. Untreated cells indicate a minimal amount of intracellular staining (Figure 5D) ▶ in comparison to the treated cells. Antibody controls using normal rabbit serum show absence of staining in TNF-α-treated cells (Figure 5E) ▶ .
Figure 5.
Immunocytochemical assessment of surface-bound and cell-associated CAP37 in HUVECs. A: HUVECs incubated with TNF-α for 10 hours, fixed (but not permeabilized), and stained with antisera to CAP37 indicating no staining for CAP37 on the outer surface of the cell. B: Untreated HUVECs, fixed but not permeabilized and stained with antisera to CAP37 indicating lack of staining. C: HUVECs incubated with 10 ng/ml of TNF-α for 10 hours, permeabilized, and stained with antisera to CAP37 indicating strong cytoplasmic and perinuclear staining (brown) for CAP37. D: HUVECs incubated with media alone for 10 hours, permeabilized, and stained with antisera to CAP37 indicating light intracellular staining. E: HUVECs incubated with media alone for 10 hours, permeabilized, and stained with normal rabbit serum indicating no staining. Original magnifications: ×1000.
Flow cytometry confirmed the studies described in Figure 5 ▶ . TNF-α-treated cells that were permeabilized indicated up to a fivefold increase of CAP37 expression over untreated cells (Figure 6) ▶ . Once again, the data indicated that there is a low level of constitutive expression of CAP37 protein in HUVECs. No detectable surface expression of CAP37 was observed in nonpermeabilized cells using flow cytometry irrespective of whether cells were treated or untreated (data not shown). To determine whether CAP37 is released from treated endothelial cells, we used enzyme-linked immunosorbent assay to analyze supernatants from TNF-α-treated HUVEC cultures. Levels of released CAP37 from treated HUVECs were twofold higher than untreated cells (data not shown). It was clear that the amount and proportion of CAP37 released from HUVECs was in general much less than the amount and proportion of CAP37 released from PMNs. 6 Almost 90% of total CAP37 is released from PMNs after phagocytosis. 6
Figure 6.
Flow cytometric analysis of cell-associated CAP37 in HUVECs. HUVECs were incubated 18 hours in the absence (unt) or presence of 10 ng/ml of TNF-α, permeabilized, and labeled with antisera to human CAP37 or normal serum control. Cells were permeabilized to determine intracellular levels of CAP37. A representative histogram from two independent experiments. The shift because of fluorescein isothiocyanate staining indicates increased expression of CAP37 in TNF-α stimulated cells. Also indicated is a low level of constitutive CAP37 expression (unt).
Western blot analysis of HUVEC lysates and supernatants from TNF-α-treated cells was performed to provide information regarding the molecular mass and processing of the various CAP37 species. Figure 7 ▶ indicates an extremely interesting finding. The released form of CAP37 from HUVECs seems to have a molecular mass closely correlating with the major form of PMN-derived protein, and is clearly a single species. However, there seem to be two forms of the cell-associated form of endothelial CAP37, one that migrates with a molecular mass of ∼26 kd and another stronger band at ∼33 kd. Because of the differential glycosylation of PMN-derived CAP37, the protein migrates as a smear on SDS-polyacrylamide gel electrophoresis with a range of molecular mass between 24 to 37 kd. Normal rabbit serum, used as a control antibody to probe an identical blot, showed no reaction with HUVEC lysate, supernatant, or PMN extract indicating the specificity of the antiserum used (data not shown).
Figure 7.
Western blot analysis of HUVECs for CAP37 protein. Human umbilical vein endothelial cells were incubated with TNF-α. Fifty μg of total protein was loaded into each lane. CAP37 protein expression, both cell-associated (lysate) and released (sup), was determined using rabbit antisera to human CAP37. PMN extract (20 μg) was included as a positive control for CAP37 staining.
Discussion
This study describes two novel and important observations about the inflammatory mediator CAP37. Firstly, we have convincingly demonstrated its presence in atherosclerotic lesions. Secondly, we have demonstrated the presence of an induced form of CAP37 in endothelial cells in response to cytokines and injurious mediators such as LPS and TNF-α. This is the first demonstration of endogenous endothelial CAP37. Thus, to confirm that the expressed protein we were dealing with was unequivocally CAP37 or an isoform of CAP37 we undertook its sequencing.
Sequence analysis demonstrated homology between E-CAP37 and PMN-CAP37, with a complete match of 199 amino acids between residues 19 to 217. This homologous region includes coding sequence for the domains of PMN-CAP37 reported to have bactericidal 22 and endotoxin-neutralizing 5 activity. The region reported to activate protein kinase C 11 in endothelial cells is also included within this region. Mature PMN-CAP37 is a 222-amino acid molecule 3 with a calculated molecular mass of ∼24 kd. Molecular masses ranging from 37 to 24 kd have been observed on SDS-polyacrylamide gel electrophoresis because of its differential glycosylation. 3 Based on the calculated molecular mass of endothelial CAP37 observed on our Western blots one would expect to find differences/extensions at the amino- and/or carboxy-terminus end of the molecule. We are currently performing 3′RACE and 5′RACE to determine the complete sequence of inducible endothelial CAP37 to define any upstream sequences that might have regulatory control. Although the entire sequence is currently unavailable, the extent of sequencing obtained so far strongly suggests that we are unequivocally dealing with CAP37. It is not unusual for inducible and constitutively expressed forms of the same molecule to have variations in size and amino acid sequence. This has been well documented for IL-12. 23,24
In addition to the differences in molecular mass between inducible E-CAP37 and PMN-derived CAP37, our data would suggest differences relating to the processing of endothelial CAP37. Previous findings from our laboratory indicate that PMN-derived CAP37 is easily released from the granules of the PMNs on activation, with almost 90% of total CAP37 detected in supernatant fluids. 6 On the other hand, E-CAP37 seems to have a distinct cell-associated and released form. The cell-associated protein migrated as a higher kd band whereas the released protein migrated equivalently to the PMN-derived protein. The two isoforms of IL-1 also demonstrate a differential pattern of extracellular release, IL-1β is easily released, whereas IL-1α is not. 25 We were unable to detect surface expression of CAP37 on endothelial cells and therefore do not believe that there is a membrane-anchoring component associated with the induced form. It is possible that the higher molecular weight band may indicate a glycoform of the protein, as glycosylation can be dependent on cell type. 26
To explore the identity of the mediators involved in the induction of CAP37 in endothelium we undertook a series of in vitro studies. In Figure 2 ▶ , we demonstrated immunohistochemically, that CAP37 is induced in endothelial cells in response to the injurious mediator, LPS. Kinetic studies showed that CAP37 protein was induced in RAECs in vitro by LPS as early as 30 minutes, peaked at 4 to 6 hours, and subsided by 24 hours. Corroborative studies using Northern blot analysis demonstrated the expression of CAP37 mRNA to follow a similar time course in which expression is no longer detected at 24 hours of LPS stimulation. The antiserum used for these experiments was raised against human CAP37, 13 and the probes used for the Northern blot analysis were based on the human CAP37 sequence 21 indicating that there is significant conservation of CAP37 across species. 6,11,27 Our studies were performed using RAECs because we believed that endothelial cells derived from the aorta would be the most appropriate site for studies dealing with atherosclerosis. It is important to note that this induction of CAP37 in endothelial cells does not seem to be limited to the aorta. Other studies from our laboratory indicate that TNF-α and IL-1α can induce CAP37 in cultured endothelial cells from rat cerebral microvessel endothelial cells 13 and as described in Figures 4 through 7 ▶ ▶ ▶ can also be induced in HUVECs and human lung microvessel endothelial cells (not shown). What is interesting is that PMN-CAP37 is entirely constitutive and cannot be induced. In fact, mature PMNs lack mRNA for CAP37. 21 In endothelial cells the constitutive expression of basal mRNA and protein levels seemed to vary. As seen in our figures with rat aorta we found no constitutive levels even at the mRNA or protein level whereas our study with HUVECs and human lung microvessels indicated some constitutive expression. This may reflect the species from which the cells are obtained, because rat cerebral vessel showed no constitutive expression either. 13 It might quite simply be a matter of sensitivity and that probes and antibodies based on the human protein are unable to detect low levels of rat mRNA or protein for CAP37. A third possibility is that the rat cells unlike the human cells are not primary cultures.
The major issues of what alters permeability and adhesiveness of the endothelial lining and which mediators are involved in monocyte recruitment in chronic inflammatory diseases have yet to be fully elucidated. In this example of atherosclerosis we speculate that CAP37 (either platelet, PMN, or endothelial derived) is responsible for endothelial cell contraction and permeability 7,28 as well as monocyte migration into the intima. Our immunohistochemical data on atherosclerotic lesions demonstrate that the expression of CAP37 protein is not confined solely to the endothelium but is also detected throughout the cholesterol clefts, foam cells, and proliferating smooth muscle cells in the subintimal area of advanced lesions. The source of CAP37 in these advanced lesions remains somewhat equivocal. Our data would strongly suggest that the CAP37 expressed in the endothelium is endogenous E-CAP37. We also believe that the CAP37 in the smooth muscle cells is of endogenous origin, because ongoing studies in our laboratory indicate that CAP37 is expressed in proliferating smooth muscle cells (unpublished data). However, in addition to these endogenous sources of CAP37 there may be a component of the CAP37 associated with atherosclerotic plaques that is entirely exogenous. It is known that platelets contain CAP37, 27 and platelets are intimately associated with atherosclerotic plaques. 14 It is possible that some of this strong staining for CAP37 is of platelet origin or might be exogenous CAP37 taken up by endothelial cells. The levels of PMN-CAP37 contributing to the staining are probably low, because PMNs are seldom associated with atherosclerotic plaques. 14 Our model would suggest that after injury to the endothelium, platelets and/or PMNs will adhere to it because of up-regulation of various adhesion molecules, and on activation will release CAP37. In addition, CAP37 is induced in endothelial cells in response to inflammatory cytokines. The presence of exogenous and endogenous CAP37 sets up a chemotactic gradient across the endothelium that ensures recruitment and migration of monocytes. CAP37 could also contribute to endothelial contraction 7 further influencing the transmigration of leukocytes across the endothelium.
Additional support of our hypothesis that E-CAP37 is involved in inflammatory-mediated disease comes from our studies in Alzheimer’s disease. 13 It is now well established that brain endothelial cells demonstrate an inflammatory phenotype in Alzheimer’s disease. We have demonstrated CAP37 in the blood vessels of Alzheimer’s disease brains and not in controls. 13,29 We have shown its induction in rat cerebral resistance vessels after stimulation with cytokines or injurious/immunomodulatory agents such as LPS and β-amyloid, 13 a significant component of Alzheimer’s disease lesions. It would seem that CAP37 is a biologically significant molecule whose expression in the vasculature may modulate cell migration and activation with important consequences on the progression of disease.
Acknowledgments
We thank Theresa Rush for help with the preparation of this manuscript; Dr. Terence Dunn for providing the tissues; Dr. Stan Lightfoot for help in reviewing the histological specimens; Dr. Ken Jackson, Molecular Biology Resource Facility, Warren Medical Research Institute, University of Oklahoma Health Sciences Center for primer production; the Flow Cytometry and Confocal Microscopy laboratory for assistance in obtaining flow cytometric data; and Dr. Donald J. Capra, Oklahoma Medical Research Foundation Sequencing Facility for sequence analysis.
Footnotes
Address reprint requests to H. Anne Pereira, Ph.D., Department of Pathology, University of Oklahoma Health Sciences Center, P.O. Box 26901, BMSB 434, Oklahoma City, OK, 73190. E-mail: anne-pereira@ouhsc.edu.
Supported by the Oklahoma Center for Advancement of Science and Technology, Public Health Service grant AI-28018-06 from the National Institute of Allergy and Infectious Disease, the American Heart Association, Established Investigator grant 9740193N, and an American Heart Association Heartland Affiliate Predoctoral award.
Current address of P. K.:US Oncology, Molecular/Cell Processing Center, 9000 Harry Hines Blvd., Suite 537, Dallas, TX, 75235.
Current address of S. C.-R.: University of Oklahoma College of Dentistry, 1001 Stanton L. Young Blvd., DCS 3044, Oklahoma City, OK, 73117.
References
- 1.Shafer WM, Martin LE, Spitznagel JK: Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun 1984, 45:29-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira HA, Spitznagel JK, Pohl J, Wilson DE, Morgan J, Palings I, Larrick JW: CAP37, a 37kD human neutrophil granule cationic protein shares homology with inflammatory proteinases. Life Sci 1990, 46:189-196 [DOI] [PubMed] [Google Scholar]
- 3.Pohl J, Pereira HA, Martin NM, Spitznagel JK: Amino acid sequence of CAP37, a human neutrophil granule-derived antibacterial and monocyte-specific chemotactic glycoprotein structurally similar to neutrophil elastase. FEBS Lett 1990, 272:200-204 [DOI] [PubMed] [Google Scholar]
- 4.Shafer WM, Martin LE, Spitznagel JK: Late intraphagosomal hydrogen ion concentration favors the in vitro antimicrobial capacity of a 37-kilodalton cationic granule protein of human neutrophil granules. Infect Immun 1986, 53:651-655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brackett DJ, Lerner MR, Lacquement MA, He R, Pereira HA: A synthetic lipopolysaccharide-binding peptide based on the neutrophil-derived protein CAP37 prevents endotoxin-induced responses in conscious rats. Infect Immun 1997, 65:2803-2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira HA, Shafer WM, Pohl J, Martin LE, Spitznagel JK: CAP37, a human neutrophil-derived chemotactic factor with monocyte specific activity. J Clin Invest 1990, 85:1468-1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Østergaard E, Flodgaard H: A neutrophil-derived proteolytic inactive elastase homologue (hHBP) mediates reversible contraction of fibroblasts and endothelial cell monolayers and stimulates monocyte survival and thrombospondin secretion. J Leukoc Biol 1992, 51:316-323 [DOI] [PubMed] [Google Scholar]
- 8.Heinzelmann M, Mercer-Jones MA, Flodgaard H, Miller FN: Heparin-binding protein (CAP37) is internalized in monocytes and increases LPS-induced monocyte activation. J Immunol 1998, 160:5530-5536 [PubMed] [Google Scholar]
- 9.Rasmussen PB, Bjørn S, Hastrup S, Nielsen PF, Norris K, Thim L, Wiberg FC, Flodgaard H: Characterization of recombinant human HBP/CAP37/azurocidin, a pleiotropic mediator of inflammation-enhancing LPS-induced cytokine release from monocytes. FEBS Lett 1996, 390:109-112 [DOI] [PubMed] [Google Scholar]
- 10.Heinzelmann M, Platz A, Flodgaard H, Polk Jr HC, Miller FN: Endocytosis of heparin-binding protein (CAP37) is essential for the enhancement of lipopolysaccharide-induced TNF-α production in human monocytes. J Immunol 1999, 162:4240–4245 [PubMed]
- 11.Pereira HA, Moore P, Grammas P: CAP37, a neutrophil granule-derived protein stimulates protein kinase C activity in endothelial cells. J Leukoc Biol 1996, 60:415-422 [DOI] [PubMed] [Google Scholar]
- 12.Olofsson AM, Vestberg M, Herwald H, Rygaard J, David G, Arfors K-E, Linde V, Flodgaard H, Dedio J, Müller-Esterl W, Lundgren-Åkerlund E: Heparin-binding protein targeted to mitochondrial compartments protects endothelial cells from apoptosis. J Clin Invest 1999, 104:885-894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira HA, Kumar P, Grammas P: Expression of CAP37, a novel inflammatory mediator, in Alzheimer’s disease. Neurobiol Aging 1996, 17:753-759 [PubMed] [Google Scholar]
- 14.Ross R: Atherosclerosis—an inflammatory disease. N Engl J Med 1999, 340:115-126 [DOI] [PubMed] [Google Scholar]
- 15.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WST, Hampel H, Hull M, Landreth G, Lue L-F, Mrak R, Mackenzie IR, McGeer PL, O’Banion K, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T: Inflammation and Alzheimer’s disease. Neurobiol Aging 2000, 21:383-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diglio CA, Grammas P, Giacomelli F, Wiener J: Angiogenesis in rat aorta ring explant cultures. Lab Invest 1989, 60:523-531 [PubMed] [Google Scholar]
- 17.Jaffe AE, Nachman RL, Becker CG, Minick CR: Culture of human endothelial cells derived from umbilical veins. J Clin Invest 1973, 52:2745-2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gräbner R, Till U, Heller R: Flow cytometric determination of E-selectin, vascular cell adhesion molecule-1, and intercellular cell adhesion molecule-1 in formaldehyde-fixed endothelial monolayers. Cytometry 2000, 40:238-244 [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 20.Corpet F: Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 1988, 16:10881-10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan JG, Sukiennicki T, Pereira HA, Spitznagel JK, Guerra ME, Larrick JW: Cloning of the cDNA for the serine protease homolog CAP37/azurocidin, a microbicidal and chemotactic protein from human granulocytes. J Immunol 1991, 147:3210-3214 [PubMed] [Google Scholar]
- 22.Pereira HA, Erdem I, Pohl J, Spitznagel JK: Synthetic bactericidal peptide based on CAP37: a 37-kDa human neutrophil granule-associated cationic antimicrobial protein chemotactic for monocytes. Proc Natl Acad Sci USA 1993, 90:4733-4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enk CD, Mahanty S, Blauvelt A, Katz SI: UVB induces IL-12 transcription in human keratinocytes in vivo and in vitro. Photochem Photobiol 1996, 63:854-859 [DOI] [PubMed] [Google Scholar]
- 24.Walter MJ, Kajiwara N, Karanja P, Castro M, Holtzman MJ: Interleukin 12 p40 production by barrier epithelial cells during airway inflammation. J Exp Med 2001, 193:339-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lonnemann G, Endres S, Van der Meer JWM, Cannon JG, Koch KM, Dinarello CA: Differences in the synthesis and kinetics of release of interleukin 1 alpha, interleukin 1 beta and tumor necrosis factor from human mononuclear cells. Eur J Immunol 1989, 19:1531-1536 [DOI] [PubMed] [Google Scholar]
- 26.Sears P, Wong C-H: Enzyme action in glycoprotein synthesis. Cell Mol Life Sci 1998, 54:223-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flodgaard H, Østergaard E, Bayne S, Svendsen A, Thomsen J, Engels M, Wollmer A: Covalent structure of two novel neutrophile leucocyte-derived proteins of porcine and human origin: neutrophil elastase homologues with strong monocyte and fibroblast chemotactic activities. Eur J Biochem 1991, 197:535–547 [DOI] [PubMed]
- 28.Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors K-E, Flodgaard H, Lindbom L: Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med 2001, 7:1123-1127 [DOI] [PubMed] [Google Scholar]
- 29.Grammas P: A damaged microcirculation contributes to neuronal cell death in Alzheimer’s disease. Neurobiol Aging 2000, 21:199-205 [DOI] [PubMed] [Google Scholar]