Abstract
Laminin-5 is an extracellular matrix protein that plays a key role in cell migration and tumor invasion. Cox-2 is an induced isoform of cyclooxygenases that plays an important role in carcinogenesis, suppression of apoptosis, angiogenesis, and metastasis of colon cancer. We report frequent co-expression of cox-2 and laminin-5 at the invasive front of early-stage lung adenocarcinomas. We investigated the expression of cox-2 and laminin-5 immunohistochemically in 102 cases of small-sized lung adenocarcinoma (maximum dimension, 2 cm or less). Cox-2 and laminin-5 were expressed in 97 (95.1%) and 82 (80.4%) cases, respectively. Both were preferentially localized in cancer cells at the cancer-stroma interface, although cox-2 tended to show a diffuse staining pattern in some cases. A comparison of their staining patterns revealed a striking similarity in their distribution in 24 cases, and a partial overlap between their localization in another 20 cases. Moreover, an overall correlation was found between the expression levels of cox-2 and laminin-5 (P = 0.018). To gain insight into the mechanisms that regulate the expression of these proteins, we additionally studied their expression in 58 cases of stage I lung adenocarcinoma, in which p53 status was determined by immunohistochemistry, polymerase chain reaction-single strand conformation polymorphism analysis, and direct sequencing. The results showed that tumors with mutant p53 tended to express more cox-2 than those with wild-type p53 (P = 0.080). Also, tumors that overexpressed p53 had higher levels of cox-2 and laminin-5 than those without p53 overexpression (P = 0.032 and 0.047, respectively). Further immunohistochemical analysis showed that tumors that overexpressed both epidermal growth factor receptor (EGFR) and erbB-2 had higher levels of cox-2 and laminin-5 than those without concomitant overexpression of these proteins (P = 0.014 and P = 0.018, respectively). To see whether EGFR signaling is involved in cox-2 and laminin-5 expression, we further conducted in vitro analyses using six lung adenocarcinoma cell lines (A549, HLC-1, ABC-1, LC-2/ad, VMRC-LCD, and L27). Western blot analyses showed that cox-2 mRNA levels, and to a lesser extent laminin-5 γ2 mRNA levels, correlated with the expression levels of erbB-2 and the phosphorylated form of MAPK/ERK-1/2 protein. The addition of transforming growth factor-α increased both cox-2 and laminin-5 γ2 mRNA levels in A549, ABC-1, and L27 with different kinetics; the induction of cox-2 occurred earlier than that of laminin-5 γ2. Finally, the migration of ABC-1 cells was inhibited by MAP kinase kinase inhibitor PD98059 and a selective cox-2 inhibitor NS-398. In contrast, the migration of A549 cells was inhibited by PD98059, but much less effectively by NS-398. These results suggest that co-stimulatory mechanisms may exist that increase the expression of cox-2 and laminin-5 at the invasive front of lung adenocarcinomas and that EGFR signaling could be one of the mechanisms. Further investigations are warranted concerning the role of cox-2 and laminin-5 in cancer cell invasion and the significance of p53 and EGFR signaling in the regulation of cox-2 and laminin-5 expression.
Cyclooxygenases are rate-limiting enzymes that catalyze the conversion of arachidonic acid to prostaglandins. 1-4 Cyclooxygenases exist in two isoforms: a constitutively expressed isoform, cox-1, and an induced isoform, cox-2. The expression of cox-2 is induced by cytokines and growth factors. Numerous clinical and experimental studies have suggested that cox-2 plays an important role in carcinogenesis, suppression of apoptosis, angiogenesis, and metastasis of colon cancer. 1-4 Overexpression of cox-2 is frequently observed in colon adenoma and carcinoma. 5-7 Inhibition of cox-2 reduced colon adenoma formation in experimental animals 8,9 and patients with familial adenomatous polyposis. 10 Specific inhibitors of cox-2 reduced tumor growth in vivo 11 and induced apoptosis in tumor cells both in vitro and in vivo. 12-14 Moreover, introduction of cox-2 cDNA increased the production of metalloproteinases and a variety of angiogenic factors 15,16 and enhanced the metastatic potential of colon cancer cells. 15
Overexpression of cox-2 has been documented in various other cancers, 17-22 including lung cancer. 23-25 Among the four histological types of lung cancer, cox-2 is most frequently expressed in adenocarcinoma. 23,24 In patients with stage I lung adenocarcinoma, overexpression of cox-2 is associated with poor prognosis. 25 Cox-2 inhibitors inhibited proliferation and induced apoptosis in various lung cancer cell lines. 26 Although these studies suggested the involvement of cox-2 in invasion and metastasis of lung adenocarcinoma, the underlying mechanism for the overexpression of cox-2 in these tumors is currently unclear. Results of culture studies suggest that cox-2 is induced by epidermal growth factor receptor (EGFR) signaling, 27,28 interleukin (IL)-1, 29,30 tumor necrosis factor-α, 31 and the activated H-ras oncogene. 32 A recent study by Subbaramaiah and colleagues 33 suggests a potential role of p53 in suppressing the expression of cox-2. However, it is currently unclear which of these factors are actually involved in the up-regulation of cox-2 in primary cancers.
We have recently reported that the laminin-5 γ2 chain is frequently overexpressed at the invasive front of small-sized lung adenocarcinomas (maximum dimension, 2 cm or less), and that overexpression of the laminin-5 γ2 chain is associated with poor patient prognosis. 34 Laminin-5 consists of three subunits, the α3, β3, and γ2 chains, the latter two being unique to this isoform. Laminin-5 is an extracellular matrix protein that plays a key role in cell migration and tumor cell invasion. 35-38 Several previous studies have shown that laminin-5 is frequently expressed at the invasive front of several cancers, including colorectal, 39-41 gastric, 42 pancreatic, 43 breast adenocarcinomas, 39 uterine cervical 39,40,44 and oral 45-47 squamous cell carcinomas, and malignant melanoma. 39,40 However, the regulatory mechanism for the overexpression of laminin-5 in cancer is currently unclear.
In this study, we report that cox-2 and laminin-5 are frequently co-localized at the invasive front of early-stage lung adenocarcinomas. We also present data showing that overexpression of cox-2 and laminin-5 is associated with p53 abnormalities and concomitant overexpression of EGFR and erbB-2. Finally, the results of our in vitro experiments also support the hypothesis that EGFR signaling is involved in the aberrant expression of cox-2 and laminin-5 in lung adenocarcinomas.
Materials and Methods
Patients and Tumors
We analyzed two groups of early-stage lung adenocarcinomas. First, we investigated the expression of cox-2 and laminin-5 immunohistochemically in 102 cases of small-sized lung adenocarcinoma (maximum dimension, 2 cm or less) that were resected at the National Cancer Center Hospital between 1984 and 1991. We recently reported the expression of laminin-5 and its prognostic significance in these small-sized adenocarcinomas. 34 The clinicopathological features of these patients and tumors are detailed in that report. Second, we examined the expression of cox-2 and laminin-5 immunohistochemically in 58 cases of stage I lung adenocarcinoma resected at the same hospital between 1985 and 1994, and analyzed its relationships with p53 abnormalities and the expression of EGFR and erbB-2. The p53 status of these adenocarcinomas was extensively characterized throughout the coding regions (exons 2 to 11), along with that of other non-small cell carcinomas (squamous cell, adenosquamous, and large-cell carcinomas), by polymerase chain reaction-single strand conformation polymorphism analysis, and direct sequencing. 48 Most of these 58 tumors were resected after 1990 and exceeded 2 cm in maximum dimension; therefore, there was no overlap between the two groups of tumors. Additionally, the distributions of laminin-5 α3, β3, and γ2 chains were studied in 20 cases of lung adenocarcinoma resected at Tokyo University Hospital between 2000 and 2001. Histological typing of the tumors was based on the new World Health Organization classification. 49 Disease stage was determined according to the TNM classification of the International Union Against Cancer. 50 The tumor tissues were routinely fixed in 10% formalin and embedded in paraffin.
Immunohistochemistry
Tissue blocks were cut into 4-μm-thick sections and deparaffinized through graded xylene and ethanol series. The sections were then washed in phosphate-buffered saline (pH 7.4), and treated with 0.3% hydrogen peroxide in methanol for 30 minutes. For antigen retrieval, sections were heated for 10 minutes at 120°C by autoclave treatment (for cox-2, laminin-5, p53, and erbB-2) or digested with 0.1% protease type XXVII (Sigma, St. Louis, MO, USA) for 20 minutes at room temperature (for EGFR). After incubation for 10 minutes with 10% normal swine serum to block nonspecific binding of the antibodies, the sections were incubated with rabbit polyclonal anti-cox-2 antibody (at a concentration of 2 μg/ml; IBL, Gunma, Japan), mouse monoclonal anti-laminin-5 γ2 chain antibody 46 (at a concentration of 1 μg/ml), mouse monoclonal anti-p53 antibody (at a dilution of ×1/100, clone DO-7; Novocastra, Newcastle-upon-Tyne, UK), mouse monoclonal anti-EGFR (at a dilution of ×1/10, clone 31G7; Zymed), or rabbit polyclonal anti-c-erbB-2 (at a concentration of 0.25 μg/ml; Nichirei, Tokyo, Japan). After overnight incubation with the primary antibody at 4°C, the sections were reacted with biotinylated secondary antibody for 45 minutes. Subsequently, the sections were allowed to react for 30 minutes with avidin-biotin-peroxidase complex (ABC) by using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) and subjected to the peroxidase reaction with 0.02% 3,3′-diaminobenzidine tetrahydrochloride as a chromogen in Tris-HCl buffer (pH 7.6) containing 0.007% hydrogen peroxide. No significant staining was observed in the negative controls, which were prepared by using the same class of mouse immunoglobulin at the same concentration.
We additionally used five commercially available antibodies against each subunit of laminin-5, ie, α3, β3, and γ2 chains. Immunohistochemistry was performed after antigen retrieval by autoclave treatment as described above for other antibodies. The sources and dilutions used for these five antibodies are summarized in Table 1 ▶ .
Table 1.
Sources of Antibodies to Laminin-5 Subunits Used in this Study
| Laminin subunit | Source | Species | Dilutions |
|---|---|---|---|
| Laminin α3 | Chemicon | Mouse monoclonal (clone P3H9-2) | ×1 /200 |
| Laminin α3 | Chemicon | Mouse monoclonal (clone P3E4) | ×1 /200 |
| Laminin β3 | Transduction Laboratories | Mouse monoclonal (clone 17) | ×1 /4,000 |
| Laminin β3 | Santa Cruz | Goat polyclonal | ×1 /50 |
| Laminin γ2 | Chemicon | Mouse monoclonal (clone D4B5) | ×1 /50 |
| Laminin γ2 | Pathology Division, NCCRI | Mouse monoclonal (clone 1-97)53 | 1 μg/ml |
NCCRI, National Cancer Center Research Institute.
Immunohistochemical Evaluation
Tumor cells were often heterogeneous with respect to cox-2 and laminin-5 expression, even within the same tumor (see below). Therefore, the expression of cox-2 and laminin-5 was graded by using the following method. 34 First, sections were scanned at low magnification to identify the area showing the highest level of expression. Then, that area was viewed with a ×10 objective, and the expression levels were graded on a scale of 0 to 2+ as follows: 0, either no positive cancer cells present or only a few scattered positive cancer cells; 1+, cluster(s) of positive cancer cells present, but accounting for less than 30% of the tumor cells within the visual field; 2+, cluster(s) of positive cancer cells that accounted for more than 30% of the tumor cells within the visual field. The grading for cox-2 was independently performed without previous knowledge of the grading for laminin-5, and vice versa. Overexpression of EGFR and erbB-2 was judged positive when most cancer cells (>50%) showed clear membranous staining. Only membranous staining was evaluated; cytoplasmic staining was not taken into account in evaluating EGFR or erbB-2 staining. Overexpression of p53 was evaluated as positive when more than 20% of the tumor cells showed nuclear staining.
Cell Lines and Culture
Lung adenocarcinoma cell lines A549, VMRC-LCD, and ABC-1 were obtained from the Japanese Cancer Research Resources Bank (Osaka, Japan), and HLC-1 and LC-2/ad from the RIKEN Cell Bank (Tsukuba, Japan). Lung adenocarcinoma cell line L27 was established in our laboratory. All cell lines were maintained in culture with Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum, glutamine, and antibiotics, in a humidified atmosphere with 5% CO2 and 95% air. To investigate the effect of transforming growth factor (TGF)-α on the expression of cox-2 and laminin-5 γ2 mRNA, preconfluent culture was washed and the culture media were replaced with serum-free Dulbecco’s modified Eagle medium containing 0.2% bovine serum albumin. After culture of the cells in the serum-free media for 18 hours, recombinant human TGF-α was added to a final concentration of 50 ng/ml, and cells were lysed for RNA extraction at 1, 3, 8, and 24 hours after addition of TGF-α.
RNA Extraction
Total RNA was isolated using an RNeasy kit (Qiagen, Hilden, Germany). All samples were treated with RNase-free DNase (Qiagen) during the isolation, following the manufacturer’s protocol. The purity and concentration of RNA were determined by spectrometry at 260 nm.
Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Real-time RT-PCR was performed by using the SYBR green system as described previously. 51 Briefly, 2 μg of total RNA was reverse transcribed by using random hexanucleotide primers and SuperScript II reverse transcriptase (Life Technologies, Inc., Gaithersburg, MD). To prevent the reamplification of carryover PCR products, cDNAs were first treated with AmpErase uracil-N-glycosylase (Perkin-Elmer Biosystems, Valencia, CA, USA). The PCRs were performed by using the SYBR Green Core Reagents kit (Perkin-Elmer). The PCR amplification was performed by using a 96-well optical tray and caps in a final reaction volume of 50 μl. We used the PCR cycle parameters as recommended by the manufacturer’s protocol. Real-time detection of the amplified cDNA was performed by using a Gene Amp 7700 Sequence Detection System (Perkin-Elmer).
The following oligonucleotides were used for the PCR: forward cox-2 primer, 5′-TGCATTCTTTGCCCAGCACT-3′; reverse cox-2 primer, 5′-AAGGCGCAGTTTACGCTGTCT-3′; forward laminin γ2 chain primer, 5′-TGGATGAGTTCAAGCGTACACA-3′; reverse laminin γ2 chain primer, 5′-GCTTTTAGCAAGATTGGCACG-3′. These primers were designed by using the computer program Primer Express (Perkin-Elmer) following the manufacturer’s instructions. Primers were chosen from sequences of different exons. Sequence specificity of the primers was confirmed by homology searches through databases at NCBI by using the computer program BLASTN. Primers were purchased from Greiner Japan (Tokyo). To normalize the data, 18S rRNA was quantitated by real-time RT-PCR using the TaqMan Ribosomal RNA Control Reagents kit. After normalization, the results were expressed in arbitrary units. Negative controls lacking template RNA were always included in each experiment.
Western Blot Analysis
Cell lysates were obtained as follows: for total cell lysates, cells were lysed in a lysis buffer consisting of 50 mmol/L Tris-HCl (pH 6.8) and 2% sodium dodecyl sulfate with a cocktail of proteinase inhibitors. After sonication, lysates were boiled for 5 minutes and cleared by centrifugation. For phosphoprotein analysis, cells were lysed in a cold buffer containing 1% deoxycholate, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 50 mmol/L NaF, 1 mmol/L sodium orthovanadate, and a cocktail of proteinase inhibitors under constant shaking for 30 minutes, and then cleared by centrifugation. Protein concentrations were determined by the DC Protein Assay kit (BioRad, Hercules, CA, USA). For Western blotting, equal amounts of protein samples were size-separated on premade 5 to 12.5% gradient polyacrylamide gels (Biocraft, Tokyo, Japan) and electroblotted onto nitrocellulose membranes. Nonspecific binding was blocked by immersion of the membranes for 20 minutes in a solution containing 5% skim milk and 0.1% Tween 20. The membranes were then incubated for 2 hours at room temperature with sheep polyclonal anti-EGFR antibody (at a dilution of ×1/1000; Upstate Biotechnology, Lake Placid, NY), rabbit polyclonal anti-c-erbB-2 antibody (at a concentration of 0.025 μg/ml; Nichirei), rabbit polyclonal anti-ERK1/2 (p42/44 MAP kinase) antibody (at a dilution of ×1/1000; New England Biolabs, Beverly, MA), or rabbit polyclonal phospho-ERK1/2 (Thr202/Tyr204) antibody (at a dilution of ×1/1000; QCB, Camarillo, CA). After washing, the membranes were incubated for 1 hour at room temperature with peroxidase-linked secondary antibody. The antigen was detected using enhanced chemiluminescence Western blotting detection reagents (Amersham, Arlington Heights, IL) following the manufacturer’s instructions.
Cell Migration Assay
Cell migration assay was performed using Biocoat cell culture inserts with 8-μm porosity polyethylene teraphthalate filters (BD Biosciences, NJ). Briefly, confluent tumor cells were trypsinized and plated onto the upper chamber and allowed to attach to the membrane for 1 hour. Then TGF-α (50 ng/ml) was added into both upper and lower chambers with or without 50 μmol/L PD98059 (Calbiochem, Darmstadt, Germany) or 100 μmol/L NS-398 (Cayman Chemical, Ann Arbor, MI). Preliminary experiments showed that no cytotoxicity occurred at these concentrations as inspected under the microscope or by the trypan blue exclusion test. Control culture received only vehicle (0.2% dimethyl sulfoxide). Cells were allowed to migrate for 24 hours (A549 cells) or 48 hours (ABC-1 cells). Then, the upper surface of the membrane was wiped to remove nonmigratory cells. Cells that had migrated to the undersurface of the membrane were fixed with methanol, stained with Giemsa solution, and counted. To determine the number of migrated cells for individual wells, cells in five randomly chosen fields were viewed at ×400 magnification with an eyepiece equipped with a grid square, and the number of cells within the largest square was counted, and the means were calculated. The results for each culture condition were expressed as mean ± SE of four individual wells.
Statistics
The correlation between cox-2 and laminin-5 expression was determined by Spearman’s rank coefficient. The differences in cox-2 and laminin-5 expression according to the presence or absence of p53 abnormalities or EGFR/erbB-2 overexpression were examined by the Mann-Whitney U test. The results were considered significant if the P value was <0.05. All tests were two-sided. With regard to the induction of mRNA levels of cox-2 and laminin-5 γ2 by TGF-α in cultured cells, the ratios of TGF-α-treated levels versus control levels were calculated for each time point, and the results are expressed as mean ± SE. The differences were considered significant if 1.0 did not lie within the 95% confidence interval of the treated-to-control ratio. Statistical calculations were performed with the StatView computer program (Abacus Concepts, Berkeley, CA).
Results
Co-Localization of Cox-2 and Laminin-5
During the course of our studies on lung cancer, we found that cox-2 was often expressed at the cancer-stromal interface in small-sized lung adenocarcinoma. Because this pattern of expression was similar to that of the laminin-5 γ2 chain, 34 we examined a series of small-sized lung adenocarcinomas for the expression of cox-2 and laminin-5. Overall, cox-2 was expressed in 97 of 102 cases (95.1%), and laminin-5 in 82 of 102 cases (80.3%). Cox-2 and laminin-5 were frequently co-localized in the cytoplasm of cancer cells at the cancer-stromal interface or at the invasive front of the tumors (Figure 1, A and B) ▶ . Strong staining was typically observed in cancer cells that invaded the fibrous stroma in a scattered manner. In some cases, tumor cells near the necrotic area stained positive for both laminin-5 and cox-2 23 (Figure 1, C and D) ▶ . A comparison of cox-2 and laminin-5 staining revealed a striking similarity in the distribution of these two proteins in 24 of 102 cases, and a partial overlap between their distribution in another 20 cases. In the remaining cases, discrepancies in distribution occurred owing to a somewhat diffuse staining pattern of cox-2, and to relatively strong cox-2 staining in some bronchioloalveolar carcinomas (Figure 1, E and F) ▶ or in some cancer cells that showed bronchioloalveolar carcinoma-like spread along the alveolar structure. Both cox-2 and laminin-5 were localized mainly within the cytoplasm of cancer cells; however, stromal cells, including fibroblasts, endothelial cells, and macrophages, were occasionally stained for cox-2. The expression levels of cox-2 and laminin-5 were then graded on a scale of 0 to 2+ based on the area that showed the highest expression of these proteins (see Materials and Methods). As shown in Table 2 ▶ , a positive correlation was found between the expression levels of cox-2 and laminin-5 (P = 0.018).
Figure 1.
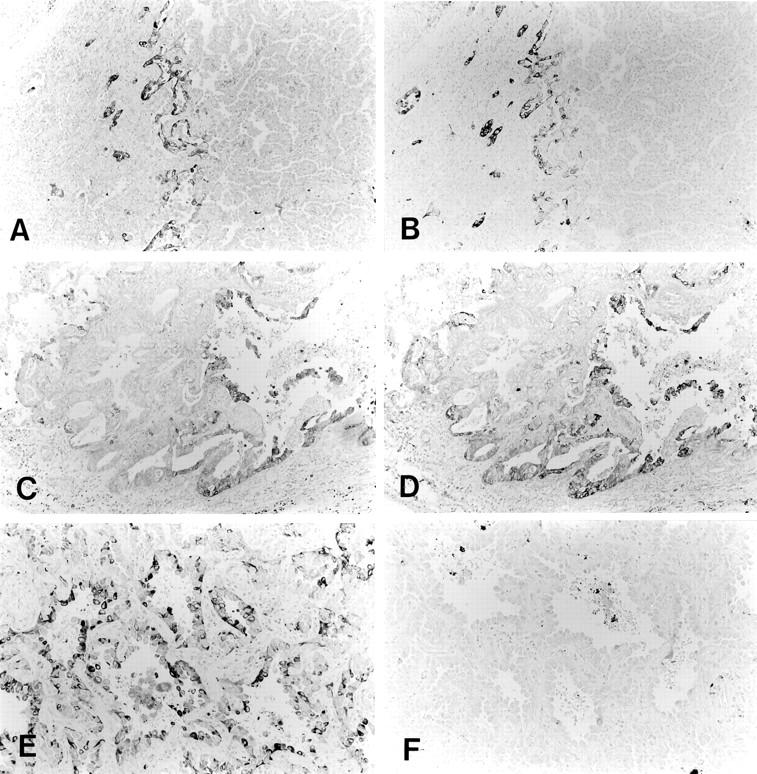
Expression and localization of cox-2 and laminin-5. Cox-2 and laminin-5 were frequently co-localized in the cytoplasm of cancer cells at the cancer-stroma interface. A: Cox-2. B: Laminin-5. In some cases, cox-2 and laminin-5 were co-localized in cancer cells near the necrotic area. C: Cox-2. D: Laminin-5. In other cases, discrepancy in distributions occurred owing to a somewhat diffuse staining pattern of cox-2, and to relatively strong cox-2 staining in some bronchioloalveolar carcinomas. E: Cox-2. F: Laminin-5.
Table 2.
Correlation between Levels of Cox-2 and Laminin-5 Expression in Lung Adenocarcinomas
| Laminin-5 | Cox-2 | ||
|---|---|---|---|
| − | + | 2+ | |
| − | 2 | 12 | 4 |
| + | 2 | 42 | 16 |
| 2+ | 0 | 13 | 11 |
Relationships of p53 Status with Expression of Cox-2 and Laminin-5
The genetic mechanisms underlying the overexpression of cox-2 and laminin-5 in cancer are not clearly understood. However, a recent study by Subbaramaiah and colleagues 33 suggested a potential role of p53 in suppressing the expression of cox-2. Therefore, we studied the relationships between p53 status and the expression of cox-2 and laminin-5 in 58 cases of stage I lung adenocarcinomas. We determined the p53 status of these cases by PCR-single strand conformation polymorphism analysis and direct sequencing. We also immunohistochemically studied the overexpression of p53. Overall, p53 mutation was found in 15 of 58 cases (25.9%), and p53 overexpression in 20 of 58 cases (34.5%). Data concerning p53 mutation and other immunohistochemical results are shown in Table 3 ▶ . Three tumors with p53 mutation were negative for p53 overexpression, whereas eight tumors without p53 mutation overexpressed p53. The concordance rate of p53 mutation and overexpression was 81.0%. The relationships between p53 status and the expression of cox-2 and laminin-5 are shown in Table 4 ▶ . Tumors with mutant p53 showed a tendency for higher expression levels of cox-2 than those with wild-type p53 (P = 0.080) (Table 4 ▶ A). Also, tumors that overexpressed p53 had higher expression levels of cox-2 and laminin-5 than those without p53 overexpression (P = 0.032 and P = 0.047, respectively) (Table 4 ▶ B).
Table 3.
Summary of 58 Cases of Stage I Lung Adenocarcinoma
| Case | Age | Sex | Size | WHO | Diff. | Laminin-5 | Cox-2 | EGFR | ErbB-2 | p53 | Exon | Codon | Nucleotide change | Amino acid change | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IHC | Mutation | ||||||||||||||
| N141T | 64 | M | 7 | pap | M | 1+ | 2+ | − | + | + | + | 5 | 141 | TGC to TAC | Lys to Tyr |
| A011T | 67 | M | 4.3 | mix | W | 2+ | 1+ | − | + | + | + | 6 | 220 | TAT to TGT | Tyr to Cys |
| Q181T | 69 | M | 2.7 | sol | P | 2+ | 2+ | + | + | + | + | 7 | 242 | CTT to CGT | Leu to Arg |
| Q311T | 63 | M | 2.5 | mix | M | 1+ | 1+ | − | + | + | + | 8 | 283 | CGC to CCC | Arg to Pro |
| Q491T | 59 | F | 4.6 | aci | M | 2+ | 1+ | − | − | + | + | 6 | 194 | CTT to CGT | Leu to Arg |
| Q191T | 56 | M | 3.3 | mix | M | 2+ | 2+ | + | − | + | + | 7 | 250 | CCC to CTC | Pro to Leu |
| Q691T | 45 | F | 1.8 | mix | W | 1+ | 1+ | − | − | + | + | 5 | 157 | GTC to TTC | Val to Phe |
| 5 | 143 | GTG to xTG | frameshift | ||||||||||||
| Q701T | 57 | M | 2.5 | sol | P | 2+ | 2+ | + | − | + | + | 5 | 158 | CGC to CTC | Arg to Leu |
| Q721T | 49 | M | 2.2 | mix | W | 1+ | 2+ | + | + | + | + | 8 | 281 | GAC to CAC | Asp to His |
| Q771T | 60 | M | 1.9 | mix | W | − | 1+ | − | − | + | + | 8 | 282 | CGG to TGG | Arg to Trp |
| Q861T | 49 | F | 1.9 | mix | W | 1+ | 2+ | + | − | + | + | 6 | 213 | CGA to CAA | Arg to Gln |
| Q591T | 66 | M | 16 | mix | W | 2+ | 2+ | − | − | + | + | 7 | 247 | AAC to ATC | Asn to Ile |
| Q391T | 72 | M | 2 | pap | M | 2+ | 1+ | − | + | − | + | 8 | 298 | GAG to TAG | Glu to stop |
| Q351T | 73 | F | 3.5 | mix | W | 1+ | 2+ | + | + | − | + | 4 | 64 | CCC to xCC | frameshift |
| Q801T | 72 | M | 5.5 | mix | M | 2+ | 2+ | + | + | − | + | 9 | 317 | CAG to xAG | frameshift |
| Q121T | 56 | M | 3.1 | mix | M | 2+ | 2+ | + | + | + | − | ||||
| Q571T | 64 | M | 4 | pap | M | 2+ | 1+ | + | + | + | − | ||||
| Q551T | 52 | F | 1.6 | pap | M | 2+ | 2+ | − | − | + | − | ||||
| Q411T | 67 | M | 5 | sol | P | 2+ | 2+ | + | − | + | − | ||||
| Q461T | 66 | F | 2.9 | pap | M | 2+ | 1+ | + | + | + | − | ||||
| Q511T | 76 | M | 2.5 | mix | W | 1+ | 1+ | − | − | + | − | ||||
| Q611T | 55 | F | 2.5 | mix | W | 2+ | 2+ | + | + | + | − | ||||
| Q621T | 65 | M | 2.5 | mix | W | 2+ | 2+ | + | + | + | − | ||||
| Q761T | 72 | F | 2.8 | mix | W | 1+ | − | − | − | + | − | ||||
| 1041T | 38 | F | 7 | pap | M | 2+ | 1+ | − | − | − | − | ||||
| 1151T | 57 | F | 3.3 | mix | W | 1+ | 1+ | − | + | − | − | ||||
| 2071T | 55 | M | 2.5 | mix | W | − | − | − | − | − | − | ||||
| Q131T | 60 | M | 5.4 | aci | M | 2+ | 2+ | − | − | − | − | ||||
| Q151T | 70 | M | 7 | mix | M | 1+ | 2+ | + | + | − | − | ||||
| Q141T | 75 | M | 3.8 | aci | W | 2+ | 1+ | − | + | − | − | ||||
| Q171T | 79 | M | 3.7 | sol | P | 2+ | 2+ | − | − | − | − | ||||
| Q561T | 62 | M | 2.2 | mix | W | 1+ | 1+ | − | − | − | − | (Polymorphism) | |||
| Q421T | 50 | F | 1.6 | pap | M | 1+ | 1+ | − | − | − | − | ||||
| Q291T | 71 | F | 2 | bac | W | 1+ | 1+ | − | − | − | − | ||||
| Q321T | 62 | M | 2.2 | mix | M | 2+ | 2+ | − | − | − | − | ||||
| Q261T | 63 | M | 3.2 | mix | W | 2+ | 2+ | − | − | − | − | ||||
| Q251T | 67 | M | 3.8 | mix | W | 1+ | 1+ | − | + | − | − | (Silent) | |||
| Q281T | 59 | F | 2.6 | mix | W | 1+ | − | − | − | − | − | (Silent) | |||
| Q361T | 52 | M | 4 | bac | W | − | 1+ | − | − | − | − | ||||
| Q371T | 65 | F | 1.3 | mix | M | 1+ | 1+ | − | − | − | − | ||||
| Q301T | 59 | F | 1.9 | mix | W | 1+ | 2+ | − | − | − | − | ||||
| Q381T | 50 | F | 1.9 | pap | M | 2+ | 2+ | − | + | − | − | ||||
| Q451T | 73 | F | 4 | mix | W | 2+ | 1+ | − | + | − | − | ||||
| Q501T | 78 | F | 3.2 | mix | M | 2+ | 1+ | − | + | − | − | ||||
| Q481T | 58 | F | 2.5 | mix | W | 1+ | 1+ | − | − | − | − | ||||
| Q441T | 55 | F | 2.3 | mix | M | 1+ | 1+ | − | − | − | − | ||||
| Q631T | 57 | M | 2.5 | mix | M | 1+ | 1+ | − | − | − | − | ||||
| Q651T | 63 | M | 3.5 | mix | W | 1+ | 1+ | − | − | − | − | ||||
| Q661T | 70 | M | 2.1 | pap | M | 2+ | 2+ | + | − | − | − | ||||
| Q671T | 55 | F | 3.2 | mix | W | 1+ | 1+ | + | − | − | − | ||||
| Q581T | 73 | M | 3.8 | aci | W | 1+ | 2+ | + | − | − | − | ||||
| Q821T | 54 | M | 3.2 | mix | W | 1+ | 1+ | + | − | − | − | ||||
| Q841T | 57 | M | 2.4 | mix | M | 1+ | 1+ | − | − | − | − | ||||
| Q851T | 70 | F | 3.2 | mix | W | 1+ | 1+ | − | − | − | − | ||||
| Q911T | 65 | M | 2.8 | aci | M | 2+ | 2+ | + | + | − | − | ||||
| Q931T | 65 | M | 2.5 | sol | P | 2+ | 2+ | + | − | − | − | ||||
| Q941T | 74 | M | 2.8 | pap | M | 1+ | − | − | − | − | − | ||||
| Q951T | 62 | M | 2.7 | sol | P | 2+ | − | + | + | − | − | ||||
| Q961T | 71 | M | 3.8 | mix | P | 2+ | 2+ | + | + | − | − | ||||
WHO, World Health Organization histological classification; pap, papillary adenocarcinoma; aci, acinar adenocarcinoma; sol, solid adenocarcinoma with mucin; mix, adenocarcinoma with mixed subtypes; Diff, differentiation; W, well; M, moderately; P, poorly differentiated.
P53 IHC, p53 overexpression by immunohistochemistry. X in nucleotide change indicates one base deletion. Q691T had two distinct p53 mutations. Q561T had a nucleotide change that was interpreted as a polymorphism. Q251T and Q281T, which had a silent mutation, were included in the group negative for p53 mutation.
Table 4.
Expression Levels of Cox-2 and Laminin-5 According to the Presence or Absence of p53 Mutation or Overexpression
| Cox-2 | Laminin-5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | 2+ | − | + | 2+ | ||||||
| A | |||||||||||
| p53 mutation | − | 5 | 22 | 16 | 3 | 20 | 20 | ||||
| + | 0 | 6 | 9 | 0 | 7 | 8 | |||||
| P = 0.0802 | P = 0.5153 | ||||||||||
| B | Cox-2 | Laminin-5 | |||||||||
| − | + | 2+ | − | + | 2+ | ||||||
| p53 overexpression | − | 5 | 20 | 13 | 3 | 20 | 15 | ||||
| + | 0 | 8 | 12 | 0 | 7 | 13 | |||||
| P = 0.0316 | P = 0.0474 | ||||||||||
Relationships of EGFR/erbB-2 versus Cox-2/Laminin-5 Expression
Previous in vitro studies suggested that cox-2 could be induced by tumor necrosis factor-α, 31 IL-1β, 29,30 and EGFR signaling, 27,28 whereas the expression of laminin-5 could be stimulated by epidermal growth factor (EGF) and phorbol myristate acetate. 52 Because EGFR seemed to be a common upstream regulator of cox-2 and laminin-5, we immunohistochemically studied the expression of EGFR and its heterodimeric partner erbB-2 in the 58 cases of stage I lung adenocarcinoma. The results showed that the expression levels of cox-2 and laminin-5 were higher in tumors that overexpressed both EGFR and erbB-2 than in those without concomitant overexpression of these proteins (P = 0.014 and P = 0.019, respectively) (Table 5) ▶ .
Table 5.
Expression Levels of Cox-2 and Laminin-5 According to the Presence or Absence of Concomitant Overexpression of EGFR and ErbB-2
| Overexpression of EGFR and erbB-2 | Cox-2 | Laminin-5 | ||||
|---|---|---|---|---|---|---|
| − | + | 2+ | − | + | 2+ | |
| − | 4 | 26 | 15 | 3 | 24 | 18 |
| + | 1 | 2 | 10 | 0 | 3 | 10 |
| P = 0.0139 | P = 0.0180 | |||||
Expression Analyses of Cox-2, Laminin-5, EGFR, and ErbB-2 in Lung Adenocarcinoma Cell Lines
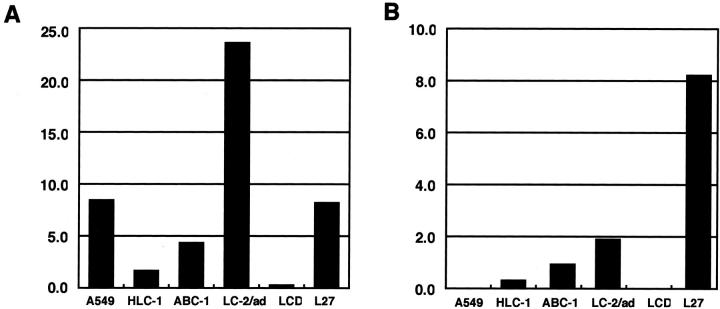
Next, we investigated whether similar relationships occurred in lung adenocarcinoma cell lines. We analyzed six cell lines: A549, HLC-1, ABC-1, LC-2/ad, VMRC-LCD, and L27. The results are shown in Figures 2 and 3 ▶ ▶ . Quantitative RT-PCR analysis showed that three cell lines (ABC-1, LC-2/ad, and L27) expressed mRNAs of cox-2 and laminin-5 γ2 chain at relatively high levels, and that two lines (HLC-1 and VMRC-LCD) expressed them at low levels (Figure 2, A and B) ▶ . Cell line A549 had a high level of cox-2 mRNA but a low level of laminin-5 γ2 chain mRNA. Western blot analysis showed that both EGFR and erbB-2 were expressed at variable levels in all cell lines except VMRC-LCD (Figure 3, A and B) ▶ . A comparison between Figures 2 and 3 ▶ ▶ shows that mRNA levels of cox-2, and to a lesser extent laminin-5 γ2, correlated well not only with erbB-2 but also with the phosphorylated form of MAPK/ERK-1/2 (Figure 3, B and C) ▶ , one of the major downstream molecules in the EGFR signaling pathway. The levels of total ERK-1/2 were similar in all cell lines examined (Figure 3D) ▶ .
Figure 2.
Levels of cox-2 (A) and laminin-5 γ2 (B) mRNAs in lung adenocarcinoma cell lines. Levels of mRNAs were determined by real-time quantitative RT-PCR analysis. After normalization for 18S rRNA, data were expressed in arbitrary units. The results are the mean of duplicate measurements. Three cell lines (ABC-1, LC-2/ad, and L27) expressed cox-2 and laminin-5 γ2 chain mRNA at relatively high levels. Two lines (HLC-1 and VMRC-LCD) expressed them at low levels. Line A549 had a relatively high level of cox-2 mRNA, but a very low level of laminin-5 γ2 chain mRNA. LCD indicates VMRC-LCD.
Figure 3.
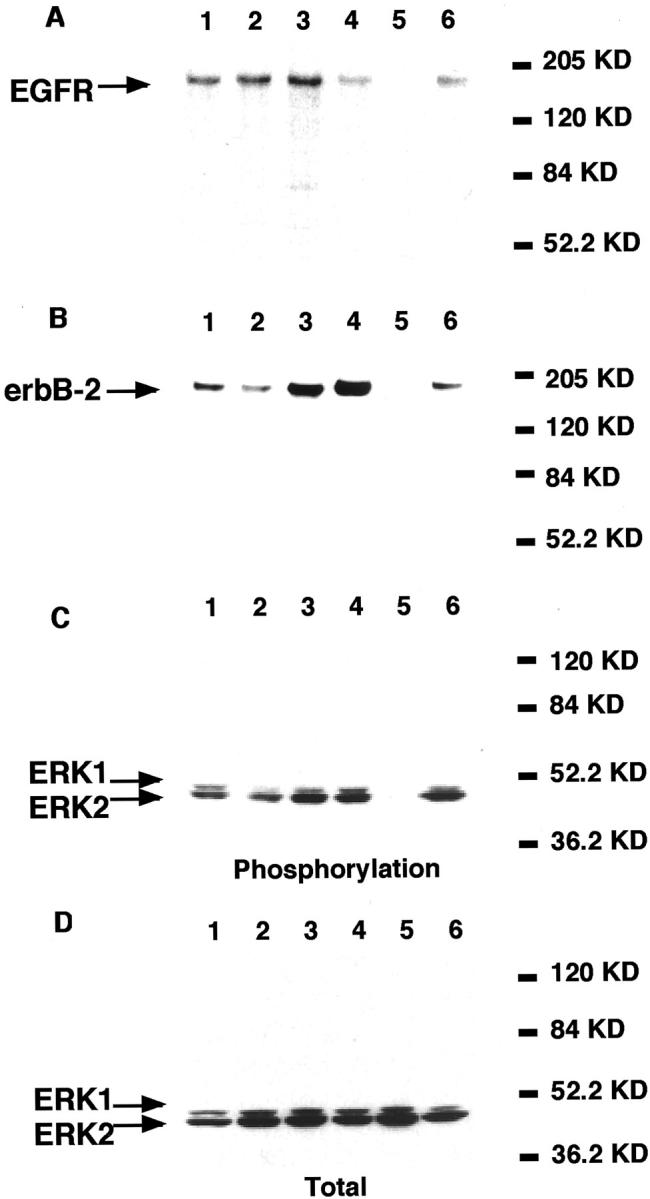
Expression of EGFR (A), erbB-2 (B), phosphorylated form of ERK-1/2 (C), and total ERK-1/2 protein (D) in lung adenocarcinoma cell lines (Western blotting). Lane 1, A549; lane 2, HLC-1; lane 3, ABC-1; lane 4, LC-2/ad; lane 5, VMRC-LCD; lane 6, L27. Both EGFR and erbB-2 were expressed at variable levels in all cell lines except VMRD-LCD (A and B). mRNA levels of cox-2 and to a lesser extent laminin-5 γ2 correlated well not only with erbB-2 but also with the phosphorylated form of MAPK/ERK-1/2 (B and C; see Figure 2 ▶ ). The levels of total ERK-1/2 were similar in all cell lines examined (D).
Effect of TGF-α on Cox-2 and Laminin-5 γ2 Chain mRNAs
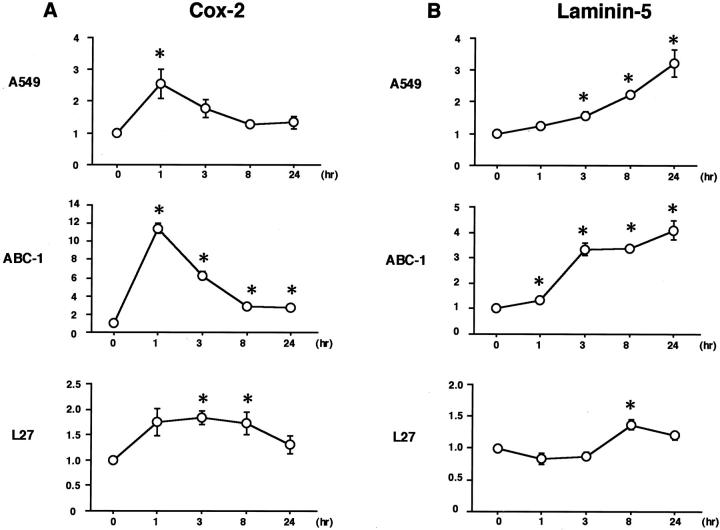
Next, we looked to see whether the addition of TGF-α, one of the ligands for EGFR, could stimulate the expression of cox-2 and laminin-5 γ2 mRNAs. Four cells lines—A549, ABC-1, L27, and LC-2/ad—were exposed to 50 ng/ml of TGF-α for up to 24 hours. Total RNA was isolated at 1, 3, 8, and 24 hours, and subjected to real-time RT-PCR analysis. Results are shown in Figure 4, A and B ▶ . Treatment with TGF-α increased cox-2 mRNA levels 2.6-fold in A549, 11.4-fold in ABC-1, and 1.8-fold in L27. Laminin-5 γ2 mRNA levels were also stimulated 3.2-fold in A549, 4.1-fold in ABC-1, and 1.4-fold in L27. A comparison of Figure 4, A and B ▶ , shows that the induction kinetics differed for cox-2 and laminin-5; cox-2 mRNA levels peaked early at 1 hour, whereas laminin-5 γ2 was induced gradually for up to 24 hours. Treatment with TGF-α did not induce any significant change in mRNA levels of cox-2 or laminin-5 γ2 chain in LC-2/ad (data not shown).
Figure 4.
Lung adenocarcinoma cell lines A549, ABC-1, and L27 were exposed to 50 ng/ml of TGF-α for up to 24 hours. Total RNA was isolated at 1, 3, 8, and 24 hours, and subjected to real-time RT-PCR analysis. Treatment with TGF-α increased cox-2 mRNA levels 2.6-fold in A549, 11.4-fold in ABC-1, and 1.8-fold in L27 (A). Laminin-5 γ2 mRNA levels were also stimulated 3.2-fold in A549, 4.1-fold in ABC-1, and 1.4-fold in L27 (B). Cox-2 mRNA levels peaked early at 1 hour, whereas laminin-5 γ2 was induced gradually for up to 24 hours. Error bars indicate SE of quadruplicate measurements. *, P < 0.05.
Expression of Laminin-5 α3, β3, and γ2 Chains in Lung Adenocarcinomas
Although the induction of laminin-5 at the invasive front is consistent with the hypothesis that laminin-5 contributes to the invasion of cancer cells, the predominantly cytoplasmic localization of laminin-5 γ2 chain raises some doubt as to whether laminin-5 is ever secreted and deposited as an extracellular matrix, and whether our laminin-5 γ2 chain antibody may not recognize matrix-deposited form of laminin-5. Also, Koshikawa and colleagues 42 recently reported that laminin-5 γ2 chain was strongly expressed at the invasive margin of cancer cells without detectable signal for laminin-5 β3 or α3. To address these issues, we used commercially available antibodies against laminin α3, β3, and γ2 chains, and compared the staining patterns of these antibodies to those of our laminin-5 γ2 antibody. Thus, in total, we tested six antibodies, ie, two antibodies for each chain of laminin-5. The results are shown in Figure 5 ▶ ; A to C. Laminin α3 chain was weakly positive in most cases of lung adenocarcinoma cells. In comparison to laminin γ2 chain, however, the staining pattern of α3 chain was diffuse and localization at the invasive front was not evident in most cases. Poorly differentiated adenocarcinomas tended to be negative for laminin α3 chain. In contrast, laminin β3 chain showed a staining pattern identical to that of γ2 chain. Although laminin-5 γ2 chain was mainly localized in the cytoplasm of invading cancer cells, we did observe staining of basement membrane for laminin γ2 chain, especially when 3,3′-diaminobenzidine tetrahydrochloride reaction was extended (Figure 5, D and E) ▶ . These results were the same for each pair of antibodies.
Figure 5.
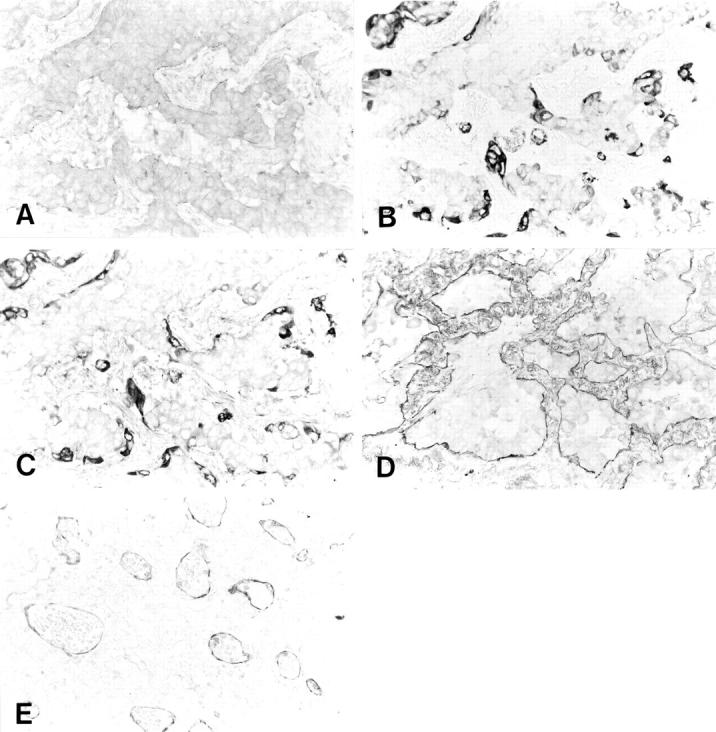
Expression of laminin α3, β3, and γ2 chain in lung adenocarcinomas. A: Laminin α3 chain was weakly positive in most lung adenocarcinoma cells. In comparison to laminin β3 (B) or γ2 chain (C), the staining pattern of the α3 chain was diffuse and localization at the invasive front was not evident. In contrast, the laminin β3 chain (B) showed a staining pattern identical to that of γ2 chain (C). Although the laminin-5 γ2 chain was mainly localized in the cytoplasm of invading cancer cells, we did observe staining of basement membrane for the laminin γ2 chain. Basement membranes of well-differentiated adenocarcinoma (D) and capillaries (E) stained positive for laminin-5.
The Effect of Cox-2 and MAPK Kinase Inhibitors on Tumor Cell Migration
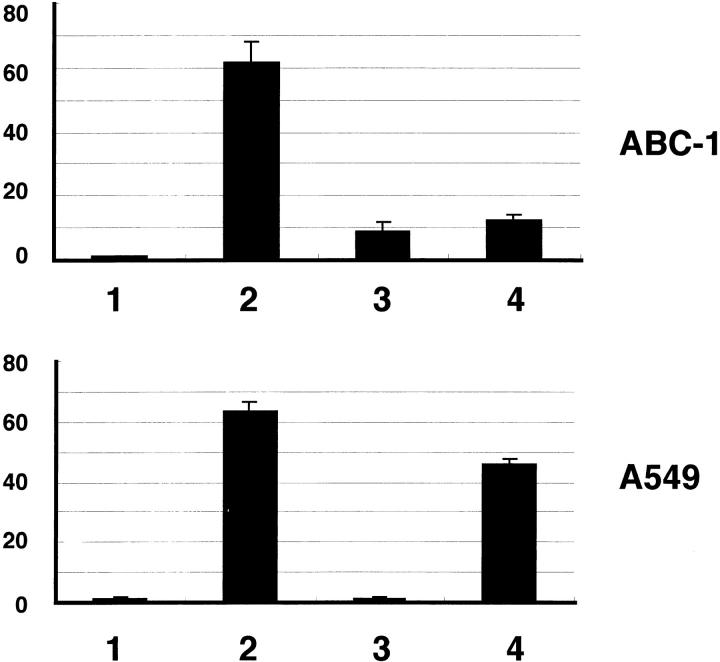
Finally, we investigated the roles of MAPK kinase cascade and cox-2 in tumor cell migration that occur after stimulation with TGF-α. For this purpose we used a pharmacological inhibitor of MAPK kinase, PD98059, and a selective cox-2 inhibitor, NS-398. The results are shown in Figure 6 ▶ . In ABC-1 cells, both PD98059 and NS-398 strongly inhibited cell migration. In contrast, A549 cells showed different responses to these inhibitors; PD98059 strongly inhibited the migration of A549 cells, but NS-398 was much less effective.
Figure 6.
The effect of cox-2 and MAPK kinase inhibitors on migration of lung adenocarcinoma cells. Cell migration assay was performed using cell culture inserts. The number of cells that had migrated to the undersurface of the membranes was counted as stated in Materials and Methods. In ABC-1 cells, both PD98059 (50 μmol/L) and NS-398 (100 μmol/L) strongly inhibited cell migration. In contrast, A549 cells showed different responses to these inhibitors; PD98059 strongly inhibited the migration of A549 cells, but NS-398 was much less effective. 1, control (+0.2% DMSO); 2, TGF-α (50 ng/ml) (+0.2% DMSO); 3, PD98059 (50 μmol/L); 4, NS-398 (100 μmol/L). Results are given as mean ± SE of four wells.
Discussion
We have shown that cox-2 and laminin-5 γ2 chain are frequently co-localized at the cancer-stromal interface and at the invasive front of tumors. We often observed strong expression of these two proteins in cancer cells that invaded the fibrous stroma in a scattered manner. Expression levels of these proteins were also strongly correlated. In recent years, cox-2 has been the subject of intensive investigation in cancer research. Those studies collectively suggest that cox-2 plays an important role in carcinogenesis, tumor angiogenesis, and metastasis of colon cancer. 1-4 Cox-2 is frequently overexpressed in various types of cancer, including lung cancer. 17-25 It has been shown that high levels of cox-2 protein expression correlate with poor prognosis of patients with stage I lung adenocarcinoma. 25 However, the precise role of cox-2 in the development and progression of cancer is not fully understood. Our results provide a link between cox-2 and laminin-5, a molecule that plays an important role in cell migration and cancer invasion. The frequent co-localization of cox-2 and laminin-5 points to the existence of a mechanism that regulates tumor cell invasion, angiogenesis, and metastasis in a coordinated manner.
Previous in vitro studies have shown that the expression of cox-2 is induced by tumor necrosis factor-α, 31 IL-1β, 29,30 and EGFR signaling, 27,28 whereas the expression of laminin-5 can be stimulated by EGF and phorbol myristate acetate. 52 Recent data from our laboratory also show that in squamous cell carcinoma cell lines, the expression levels of laminin-5 correlate with gene amplification of EGFR. 53 Thus, EGFR signaling would be a common upstream regulator of cox-2 and laminin-5. The results of the present study are consistent with this hypothesis; lung adenocarcinomas that overexpressed EGFR and erbB-2, a heterodimeric partner of the EGFR family, had higher levels of cox-2 and laminin-5 than those without concomitant overexpression of these proteins. It has been shown that erbB-2 potentiates EGFR signaling. 54 Also, treatment with TGF-α increased the expression levels of mRNA of cox-2 and laminin-5 γ2 chain.
Although EGFR signaling seems to be involved in the induction of cox-2 and laminin-5, the induction kinetics were different. Also, the magnitude of response to TGF-α was different in different cell lines tested. These observations suggest that the expression of cox-2 and laminin-5 is not regulated in the same manner. Clearly, further investigations are required to elucidate the regulatory mechanisms for the expression of these molecules. In this regard, another candidate molecule likely to be involved in the regulation of cox-2 and laminin-5 is nuclear factor (NF)-κB. NF-κB was initially isolated as a transcription factor regulating immunoglobulin gene expression in B lymphocytes. 55 Studies show that NF-κB plays a key role in inflammation, tissue remodeling, and possibly cancer. 56 NF-κB is involved in the gene regulation of urokinase-type plasminogen activator, 57 vascular endothelial growth factor, 58 and IL-8. 58,59 Indeed, a putative NF-κB binding site, as well as two AP-1 sites, can be identified in the promoter sequence of the human laminin-5 γ2 gene (data not shown). We are currently investigating whether IL-1β and tumor necrosis factor-α stimulate the expression of cox-2 and laminin-5 in a coordinated manner in lung adenocarcinoma cell lines, and if so, whether inhibitors of NF-κB attenuate this stimulating effect.
Another explanation for the co-expression of cox-2 and laminin-5 in lung adenocarcinoma may be that prostaglandins produced through the action of cox-2 up-regulate laminin-5. This hypothesis could be tested by investigating whether the addition of PGE2 or other prostaglandins to culture media stimulates laminin-5 expression in lung adenocarcinoma cell lines.
Previous studies showed that introduction of cox-2 cDNA resulted in a clone of cells expressing high levels of angiogenic factors, including fibroblast growth factors 1 and 2, vascular endothelial growth factor, and platelet-derived growth factor. 15,16 With regard to cell migration, Tsujii and colleagues 16 also showed that cox-2 over-expression in colon cancer cells promoted the motility of co-cultured endothelial cells. Several recent studies also indicate that cox-2 is involved in migration of cancer cells, 60-64 consistent with the our results obtained with NS-398. However, our data indicate that the role of cox-2 in cell migration is cell-dependent; cox-2 inhibitor inhibited the migration of ABC-1 cells, but was much less effective toward A549 cells. The basis of such cell-specific effect is unknown, and this issue certainly deserves further investigations. The relative contribution of laminin-5 and cox-2 in cancer cell invasion remains unclear, too. Blocking antibody that specifically inhibits motility-promoting function of laminin-5 would be required to address this issue.
Recently, Koshikawa and colleagues 42 reported that laminin-5 γ2 chain was strongly expressed at the invasive margin of cancer cells without significant signal for laminin-5 β3 or α3. These authors also demonstrated the secretion of the laminin γ2 monomer, as well as the laminin-5 heterotrimer, by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. They speculated that the monomeric form of the γ2 chain may have a function distinct from the laminin-5 trimer. In contrast to their results, we observed identical staining pattern for the laminin β3 and γ2 chains. Sordat and colleagues 41 also found co-expression of the laminin β3 and γ2 chains in colorectal cancers. We do not know the reason for these discrepancies, but it may be because of the use of different antibodies and/or different types of cancer specimens investigated. Certainly, these issues need to be addressed by further investigations.
It has been reported that laminin-5 may perform two opposite functions, ie, promoting cell migration and assembly of hemidesmosomes. Giannelli and colleagues 65 reported that the cleavage of the laminin γ2 chain by MMP-2 elicits cell migration on laminin-5. Conversely, after cleavage of the laminin α3 chain by plasmin, laminin-5 impedes cell motility and promotes hemidesmosome assembly. 66 Thus, different functions of laminin-5 could be explained by differential processing of the subunits that comprise laminin-5. More recently, Koshikawa and colleagues 67 found that MT1-MMP, which cleaves laminin γ2 chain more efficiently than MMP-2, plays essential roles in cell migration on laminin-5. These authors found that cell migration on laminin-5 was significantly reduced by metalloproteinase inhibitors and MT1-MMP antisense oligonucleotides. 67 Interestingly, they found co-localization of MT1-MMP and laminin-5 in breast and colon cancer tissues. Whether cox-2 is directly involved in induction of MMPs 16 and/or processing of laminin-5 needs to be explored in the future.
With regard to the negative regulation of cox-2 and laminin-5, it has recently been shown that wild-type p53 suppresses promoter activities of cox-2 and the expression of cox-2 protein. 33 This observation prompted us to examine whether expression levels of cox-2 or laminin-5 are associated with the p53 status of tumor cells. Our results showed that p53 abnormalities, including p53 mutation and overexpression, were associated with overexpression of cox-2 and laminin-5. p53 mutation in tumor cells is associated with poor prognosis of patients in various cancers. 68 The reason for this association is not clear, but it is possible that overexpression of cox-2 or laminin-5 could be at least partially responsible for the poor prognosis of patients with tumors bearing a p53 mutation or overexpression. In this regard, it is noteworthy that wild-type p53 has a suppressive effect on the expression of other genes involved in inflammation and tissue remodeling, including vascular endothelial growth factor, 69 the inducible isoform of nitric oxide synthase, 70 and IL-6. 71
In summary, we have shown frequent co-localization of cox-2 and laminin-5 at the invasive front of early-stage lung adenocarcinomas, and provided data that support the hypothesis that p53 abnormalities and EGFR signaling are involved in the aberrant expression of these proteins. The data point to the existence of a mechanism that co-regulates the expression of these proteins at the invasive front of cancer, probably facilitating tumor angiogenesis and invasion in a coordinated manner. Further investigations are warranted to elucidate the role of p53 and EGFR signaling in the regulation of cox-2 and laminin-5.
Acknowledgments
We thank Y. Ono, Y. Nakanishi, and Y. Ino for anti-laminin-5 antibody; Y. Yamauchi and A. Harada for technical assistance; and M. Suzuki for secretarial work.
Footnotes
Address reprint requests to Setsuo Hirohashi, M.D., Pathology Division, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan. E-mail: shirohas@gan2.ncc.go.jp.
Supported by a Grant-in-Aid for the Second Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health and Welfare of Japan and Grants-in-Aid from the Ministry for Health and Welfare and from the Ministry of Education, Science, Sports, and Culture of Japan.
Present address of T. N.: Department of Human Pathology, Faculty of Medicine, the University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan.
References
- 1.Taketo MM: Cyclooxygenase-2 inhibitors in tumorigenesis (part I). J Natl Cancer Inst 1998, 90:1529-1536 [DOI] [PubMed] [Google Scholar]
- 2.Taketo MM: Cyclooxygenase-2 inhibitors in tumorigenesis (part II). J Natl Cancer Inst 1998, 90:1609-1620 [DOI] [PubMed] [Google Scholar]
- 3.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE: Cyclooxygenase in biology and disease. FASEB J 1998, 12:1063-1073 [PubMed] [Google Scholar]
- 4.Williams CS, Mann M, DuBois RN: The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18:7908-7916 [DOI] [PubMed] [Google Scholar]
- 5.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN: Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994, 107:1183-1188 [DOI] [PubMed] [Google Scholar]
- 6.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T: Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res 1995, 55:3785-3789 [PubMed] [Google Scholar]
- 7.Kargman SL, O’Neill GP, Vickers PJ, Evans JF, Mancini JA, Jothy S: Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res 1995, 55:2556-2559 [PubMed] [Google Scholar]
- 8.Reddy BS, Hirose Y, Lubet R, Steele V, Kelloff G, Paulson S, Seibert K, Rao CV: Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res 2000, 60:293-297 [PubMed] [Google Scholar]
- 9.Kawamori T, Rao CV, Seibert K, Reddy BS: Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res 1998, 58:409-412 [PubMed] [Google Scholar]
- 10.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B: The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000, 342:1946-1952 [DOI] [PubMed] [Google Scholar]
- 11.Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN: Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest 1997, 99:2254-2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu XH, Yao S, Kirschenbaum A, Levine AC: NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res 1998, 58:4245-4249 [PubMed] [Google Scholar]
- 13.Elder DJ, Halton DE, Hague A, Paraskeva C: Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res 1997, 3:1679-1683 [PubMed] [Google Scholar]
- 14.Sawaoka H, Kawano S, Tsuji S, Tsujii M, Gunawan ES, Takei Y, Nagano K, Hori M: Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol 1998, 274:G1061-G1067 [DOI] [PubMed] [Google Scholar]
- 15.Tsujii M, Kawano S, DuBois RN: Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 1997, 94:3336-3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN: Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998, 93:705-716 [DOI] [PubMed] [Google Scholar]
- 17.Hwang D, Scollard D, Byrne J, Levine E: Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst 1998, 90:455-460 [DOI] [PubMed] [Google Scholar]
- 18.Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M: Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res 1997, 57:1276-1280 [PubMed] [Google Scholar]
- 19.Mohammed SI, Knapp DW, Bostwick DG, Foster RS, Khan KN, Masferrer JL, Woerner BM, Snyder PW, Koki AT: Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res 1999, 59:5647-5650 [PubMed] [Google Scholar]
- 20.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K: Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res 1999, 59:198-204 [PubMed] [Google Scholar]
- 21.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey III TJ: Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res 1999, 59:987–990 [PubMed]
- 22.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, Masferrer JL, Dannenberg AJ: Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res 1999, 59:991-994 [PubMed] [Google Scholar]
- 23.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A: Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res 1998, 58:4997-5001 [PubMed] [Google Scholar]
- 24.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T: Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res 1998, 58:3761-3764 [PubMed] [Google Scholar]
- 25.Achiwa H, Yatabe Y, Hida T, Kuroishi T, Kozaki K, Nakamura S, Ogawa M, Sugiura T, Mitsudomi T, Takahashi T: Prognostic significance of elevated cyclooxygenase 2 expression in primary, resected lung adenocarcinomas. Clin Cancer Res 1999, 5:1001-1005 [PubMed] [Google Scholar]
- 26.Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, Sugiura T, Ogawa M, Takahashi T: Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res 2000, 6:2006-2011 [PubMed] [Google Scholar]
- 27.Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, Chinery R, Kirkland SC, DuBois RN, Jetton TL, Morrow JD: Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci USA 1997, 94:657-662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuura H, Sakaue M, Subbaramaiah K, Kamitani H, Eling TE, Dannenberg AJ, Tanabe T, Inoue H, Arata J, Jetten AM: Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells. Role of mitogen-activated protein kinases. J Biol Chem 1999, 274:29138-29148 [DOI] [PubMed] [Google Scholar]
- 29.Ridley SH, Dean JL, Sarsfield SJ, Brook M, Clark AR, Saklatvala J: A p38 MAP kinase inhibitor regulates stability of interleukin-1-induced cyclooxygenase-2 mRNA. FEBS Lett 1998, 439:75-80 [DOI] [PubMed] [Google Scholar]
- 30.Newton R, Stevens DA, Hart LA, Lindsay M, Adcock IM, Barnes PJ: Superinduction of COX-2 mRNA by cycloheximide and interleukin-1beta involves increased transcription and correlates with increased NF-kappaB and JNK activation. FEBS Lett 1997, 418:135-138 [DOI] [PubMed] [Google Scholar]
- 31.Chen CC, Sun YT, Chen JJ, Chiu KT: TNF-alpha-induced cyclooxygenase-2 expression in human lung epithelial cells: involvement of the phospholipase C-gamma 2, protein kinase C-alpha, tyrosine kinase, NF-kappa B-inducing kinase, and I-kappa B kinase 1/2 pathway. J Immunol 2000, 165:2719-2728 [DOI] [PubMed] [Google Scholar]
- 32.Sheng H, Williams CS, Shao J, Liang P, DuBois RN, Beauchamp RD: Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J Biol Chem 1998, 273:22120-22127 [DOI] [PubMed] [Google Scholar]
- 33.Subbaramaiah K, Altorki N, Chung WJ, Mestre JR, Sampat A, Dannenberg AJ: Inhibition of cyclooxygenase-2 gene expression by p53. J Biol Chem 1999, 274:10911-10915 [DOI] [PubMed] [Google Scholar]
- 34.Moriya Y, Niki T, Yamada T, Matsuno Y, Kondo H, Hirohashi S: Increased expression of laminin-5 and its prognostic significance in small-sized lung adenocarcinoma: an immunohistochemical analysis of 102 cases. Cancer 2001, 91:1129-1141 [DOI] [PubMed] [Google Scholar]
- 35.Zhang K, Kramer RH: Laminin 5 deposition promotes keratinocyte motility. Exp Cell Res 1996, 227:309-322 [DOI] [PubMed] [Google Scholar]
- 36.Tani T, Lumme A, Linnala A, Kivilaakso E, Kiviluoto T, Burgeson RE, Kangas L, Leivo I, Virtanen I: Pancreatic carcinomas deposit laminin-5, preferably adhere to laminin-5, and migrate on the newly deposited basement membrane. Am J Pathol 1997, 151:1289-1302 [PMC free article] [PubMed] [Google Scholar]
- 37.Kikkawa Y, Umeda M, Miyazaki K: Marked stimulation of cell adhesion and motility by ladsin, a laminin-like scatter factor. J Biochem (Tokyo) 1994, 116:862–869 [DOI] [PubMed]
- 38.Verrando P, Lissitzky JC, Sarret Y, Winberg JO, Gedde-Dahl Jr T, Schmitt D, Bruckner-Tuderman L: Keratinocytes from junctional epidermolysis bullosa do adhere and migrate on the basement membrane protein nicein through alpha 3 beta 1 integrin. Lab Invest 1994, 71:567–574 [PubMed]
- 39.Pyke C, Romer J, Kallunki P, Lund LR, Ralfkiaer E, Dano K, Tryggvason K: The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol 1994, 145:782-791 [PMC free article] [PubMed] [Google Scholar]
- 40.Pyke C, Salo S, Ralfkiaer E, Romer J, Dano K, Tryggvason K: Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res 1995, 55:4132-4139 [PubMed] [Google Scholar]
- 41.Sordat I, Bosman FT, Dorta G, Rousselle P, Aberdam D, Blum AL, Sordat B: Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J Pathol 1998, 185:44-52 [DOI] [PubMed] [Google Scholar]
- 42.Koshikawa N, Moriyama K, Takamura H, Mizushima H, Nagashima Y, Yanoma S, Miyazaki K: Overexpression of laminin gamma2 chain monomer in invading gastric carcinoma cells. Cancer Res 1999, 59:5596-5601 [PubMed] [Google Scholar]
- 43.Soini Y, Maatta M, Salo S, Tryggvason K, Autio-Harmainen H: Expression of the laminin gamma 2 chain in pancreatic adenocarcinoma. J Pathol 1996, 180:290-294 [DOI] [PubMed] [Google Scholar]
- 44.Skyldberg B, Salo S, Eriksson E, Aspenblad U, Moberger B, Tryggvason K, Auer G: Laminin-5 as a marker of invasiveness in cervical lesions. J Natl Cancer Inst 1999, 91:1882-1887 [DOI] [PubMed] [Google Scholar]
- 45.Kosmehl H, Berndt A, Strassburger S, Borsi L, Rousselle P, Mandel U, Hyckel P, Zardi L, Katenkamp D: Distribution of laminin and fibronectin isoforms in oral mucosa and oral squamous cell carcinoma. Br J Cancer 1999, 81:1071-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ono Y, Nakanishi Y, Ino Y, Niki T, Yamada T, Yoshimura K, Saikawa M, Nakajima T, Hirohashi S: Clinicopathologic significance of laminin-5 gamma2 chain expression in squamous cell carcinoma of the tongue: immunohistochemical analysis of 67 lesions. Cancer 1999, 85:2315-2321 [PubMed] [Google Scholar]
- 47.Kainulainen T, Autio-Harmainen H, Oikarinen A, Salo S, Tryggvason K, Salo T: Altered distribution and synthesis of laminin-5 (kalinin) in oral lichen planus, epithelial dysplasias and squamous cell carcinomas. Br J Dermatol 1997, 136:331-336 [PubMed] [Google Scholar]
- 48.Tomizawa Y, Kohno T, Fujita T, Kiyama M, Saito R, Noguchi M, Matsuno Y, Hirohashi S, Yamaguchi N, Nakajima T, Yokota J: Correlation between the status of the p53 gene and survival in patients with stage I non-small cell lung carcinoma. Oncogene 1999, 18:1007-1014 [DOI] [PubMed] [Google Scholar]
- 49.Travis W, Colby T, Corrin B, Shimosato Y, Brambilla E. World Health Organization: Histological Typing of Lung and Pleural Tumours. Berlin, Springer, 1999
- 50.Sobin L, Wittekind L: TNM classification of malignant tumours. New York, John Wiley & Sons, Inc. 1997, ,
- 51.Kanai Y, Ushijima S, Nakanishi Y, Hirohashi S: Reduced mRNA expression of the DNA demethylase, MBD2, in human colorectal and stomach cancers. Biochem Biophys Res Commun 1999, 264:962-966 [DOI] [PubMed] [Google Scholar]
- 52.Mizushima H, Miyagi Y, Kikkawa Y, Yamanaka N, Yasumitsu H, Misugi K, Miyazaki K: Differential expression of laminin-5/ladsin subunits in human tissues and cancer cell lines and their induction by tumor promoter and growth factors. J Biochem (Tokyo) 1996, 120:1196–1202 [DOI] [PubMed]
- 53.Ono Y, Nakanishi Y, Gotoh M, Sakamoto M, Hirohashi S: Epidermal growth factor receptor gene amplification is correlated with laminin-5 γ2 chain expression in oral squamous cell carcinoma cell lines. Cancer Lett 2002, 175:197-204 [DOI] [PubMed] [Google Scholar]
- 54.Olayioye MA, Neve RM, Lane HA, Hynes NE: The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 2000, 19:3159-3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sen R, Baltimore D: Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46:705-716 [DOI] [PubMed] [Google Scholar]
- 56.Rayet B, Gelinas C: Aberrant rel/nfkb genes and activity in human cancer. Oncogene 1999, 18:6938-6947 [DOI] [PubMed] [Google Scholar]
- 57.Novak U, Cocks BG, Hamilton JA: A labile repressor acts through the NFkappaB-like binding sites of the human urokinase gene. Nucleic Acids Res 1991, 19:3389-3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang S, Robinson JB, Deguzman A, Bucana CD, Fidler IJ: Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res 2000, 60:5334-5339 [PubMed] [Google Scholar]
- 59.Kunsch C, Rosen CA: NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol 1993, 13:6137-6146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, Tarnawski AS: Inhibition of angiogenesis by anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med 1999, 5:1418-1423 [DOI] [PubMed] [Google Scholar]
- 61.Attiga FA, Fernandez PM, Weeraratna AT, Manyak MJ, Patierno SR: Inhibitors of prostaglandin synthesis inhibit human prostate tumor cell invasiveness and reduce the release of matrix metalloproteinases. Cancer Res 2000, 60:4629-4637 [PubMed] [Google Scholar]
- 62.Rozic JG, Chakraborty C, Lala PK: Cyclooxygenase inhibitors retard murine mammary tumor progression by educing tumor cell migration, invasiveness and angiogenesis. Int J Cancer 2001, 93:497-506 [DOI] [PubMed] [Google Scholar]
- 63.Dohadwala M, Luo J, Zhu L, Lin Y, Dougherty GJ, Sharma S, Huang M, Pold M, Batra RK, Dubinett SM: Non-small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem 2001, 276:20809-20812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dormond O, Foletti A, Paroz C, Ruegg C: NSAIDS inhibit αVβ3 integrin-mediated and cdc42/Rac-dependent endothelial-cell spreading, migration and angiogenesis. Nat Med 2001, 7:1041-1047 [DOI] [PubMed] [Google Scholar]
- 65.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V: Induction of cell migration by matrix metalloproteinase-2 cleavage of laminin-5. Science 1997, 277:225-228 [DOI] [PubMed] [Google Scholar]
- 66.Goldfinger LE, Stack MS, Jones JCR: Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol 1998, 141:255-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quararnta V: Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol 2000, 148:615-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peller S: Clinical implications of p53: effect on prognosis, tumor progression and chemotherapy response. Semin Cancer Biol 1998, 8:379-387 [DOI] [PubMed] [Google Scholar]
- 69.Mukhopadhyay D, Tsiokas L, Sukhatme VP: Wild-type p53 and v-Src exert opposing influences on human vascular endothelial growth factor gene expression. Cancer Res 1995, 55:6161-6165 [PubMed] [Google Scholar]
- 70.Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, Felley-Bosco E, Wang XW, Geller DA, Tzeng E, Billiar TR, Harris CC: Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci USA 1996, 93:2442-2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santhanam U, Ray A, Sehgal PB: Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc Natl Acad Sci USA 1991, 88:7605-7609 [DOI] [PMC free article] [PubMed] [Google Scholar]