Abstract
Conflicting reports have led to the description of nitric oxide as a “double-edged sword” in animal models of autoimmunity. In this study we show that tissue damage within the eye during experimental autoimmune uveoretinitis correlates with peroxynitrite formation in infiltrating monocytes/macrophages within the outer retina together with extensive photoreceptor apoptosis and apoptosis of Fas+ T cells within the retina. Inducible nitric oxide synthase (NOS2) expression was primarily restricted to infiltrating monocytes/macrophages in the outer retina and photoreceptor rod outer segments (target tissue), but despite showing evidence of lipid peroxidation, myeloid cells remained resistant to apoptosis. The protective effect of the NOS inhibitor NG-nitro-l-arginine methyl ester could be attributed to dramatically reduced photoreceptor apoptosis, absence of nitrotyrosine formation, and reduced NOS2 protein expression. However, inhibition of NOS by NG-nitro-l-arginine methyl ester was accompanied by a sparing of CD3+ and CD2+ T cells despite continued expression of Fas and Fas ligand, thus compromising functional inactivation of T cells in the target tissue. These data suggests that in addition to contributing to tissue damage in the retina through generation of reactive oxygen species, nitric oxide also seems to be required for activation-induced cell death and elimination of T cells in the retina.
Immune privilege is now viewed as an active process that protects vulnerable organs from damaging inflammation. 1-3 For instance, in the eye, in addition to the blood retina barrier, locally produced immunosuppressive cytokines, 4,5 restricted distribution of classical antigen-presenting cells, 6 and elimination of activated T cells through strategic expression FasL on uveal and retinal tissue cells 1,3 have all been demonstrated. Despite these protective mechanisms, inflammation of the uveal tract and retina is not uncommon, and in the absence of an infectious etiology is presumed to be autoimmune in origin. The condition is often refractory to treatment and may require powerful T-cell suppressants such as cyclosporin A for control. Severe visual impairment occurs in >30% of cases implying a need to target additional cells and mediators of the immune system therapeutically. 7
Experimental models of autoimmune uveoretinitis (EAU) induced by immunization with soluble retinal antigens (REs) closely resemble human disease, 8 and are mediated by CD4+ T cells and activated macrophages. 9-11 Monocytes/macrophages within the outer retina that are associated with tissue damage have been identified as a major source of nitric oxide synthase (NOS2) during inflammation. 12,13 This tissue is the location of the eliciting retinal autoantigens, and the site of infiltrating T cells and major histocompatibility complex class II+ antigen-presenting cells. However, there are conflicting reports of the effects NOS inhibitors and NOS2 gene inactivation have on the incidence and severity of disease in models of autoimmunity such as experimental autoimmune uvcoretintis (EAU) and experimental autoimmune encephalomyelitis (EAE) that target sites of immune privilege. 12,14-19
Nitric oxide (NO), when produced in large quantities, usually by macrophages and neutrophils, provides an important component of the innate immune system, particularly in the elimination of microorganisms and parasites. 20 This can be achieved through reaction with superoxide anion to yield peroxynitrite, which can produce the toxic hydroxyl radical or promote oxidative injury via formation of peroxynitrous acid. 21 More subtle effects of NO include direct nitrosylation of target proteins, inactivation of membrane ion channels, and disruption of signaling proteins. 22,23 Low levels of NO have also been implicated in lymphocyte activation and proliferation, increasing TNF-α production, nuclear factor-κB binding activity, and enhancing tyrosine kinase p56 activity. 24 Conversely, higher levels can suppress antigen-presenting cell activity and T-cell proliferation by inhibiting the actions of thiol- and heme-containing enzymes and thus mitochondrial respiration. 25 This has led to the hypothesis that NOS2 may have different functions in different cells and that tissue-specific expression may be beneficial and contribute to the resolution of inflammation through mechanisms such as activation-induced cell death (AICD). 26 In particular, various models of organ-specific autoimmune disease have indicated that although NOS2-dependent tissue destruction occurs, protective functions linked to the presence of interferon (IFN)-γ have been demonstrated, possibly involving suppression of T-cell proliferation and Th1 cytokine production. 27 What then is the role of NOS2 in inflammation affecting sites of immune privilege such as the eye, and can modulation of expression be used to restore immunological homeostasis and reduce tissue damage?
In this histological study, we show that although tissue damage in the inner retina occurs via generation of peroxynitrite anion by monocytes/macrophages, leading to apoptosis of photoreceptors but not the macrophages, NOS also seems to be required for apoptosis of Fas+ T cells infiltrating the tissue. Apoptosis of activated T cells is a recognized mechanism for restoring immunological homeostasis and as elevated Fas and Fas-ligand expression in the retina is a feature of EAU, may play a role in down-regulating EAU. 28,29 Thus prevention of membrane nitrosylation, coupled with strategies to trigger apoptosis in infiltrating macrophages while maintaining T-cell AICD may provide a therapeutic advantage in sites of immune privilege vulnerable to damaging inflammatory reactions.
Materials and Methods
Induction and Treatment of EAU
Groups of six, 8- to 10-week-old male, Lewis rats from an inbred strain, housed and treated in accordance with United Kingdom Home Office Regulations, were used in this study. Animals were weighed and selected to give groups an approximately equal average weight. Extract of soluble RE (6.4 mg/ml) was prepared as previously described 30 by hypertonic lysis of freshly dissected bovine retinas in the dark, and contained S-antigen and interphoto-receptor retinoid binding protein (IRBP) as confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. EAU was induced by intradermal immunization in one hind footpad with 0.1 ml of RE emulsified with an equal volume of complete Freund’s adjuvant (CFA), containing 0.5 mg/ml of Mycobacterium tuberculosis H37RA (Difco, West Molesey, UK). This regime reliably induces a moderate to severe uveoretinitis 10 to 11 days after immunization without the use of Bordetella pertussis toxin. 12 Control animals received 0.1 ml of phosphate-buffered saline (PBS) in CFA. Immediately after immunization, test animals were treated with 50 mg/kg of NG-nitro-l-arginine methyl ester (L-NAME) per day (Sigma, Poole, Dorset UK) dissolved in the drinking water as previously described. 12 Control animals received normal drinking water. The weight and fluid intake of the rats was monitored before the start of experiments to determine initial dose of L-NAME. Weight and fluid intake was then monitored throughout all experiments and dosages adjusted accordingly.
Clinical and Histopathological Grading of Disease
Ocular examination was performed daily from 7 days after immunization using slit lamp biomicroscopy. The day of onset and course of disease were noted and were graded 0 to 4 according to the degree of cellular inflammatory activity within the anterior chamber of the eye. 30 Groups of animals were killed by CO2 asphyxiation on days 9, 10, 11 (early disease stage), or 12. Eyes were removed and either snap-frozen for cryosectioning or fixed in neutral-buffered formalin for paraffin embedding. Tissue sections taken from eyes of untreated immunized control animals and those treated with L-NAME were sampled at each time point, stained with hematoxylin and eosin, and examined. At least three sections from each eye were scored in a masked manner using a semiquantitative scoring system that has been described 30 and combines the extent of the inflammatory infiltrate and structural changes or tissue damage in the anterior and posterior chambers of the eye. Results are expressed as mean ± 1 SD. Comparison of the clinical and histological assessment of disease in treated and untreated animals were analyzed using one-way analysis of variance, and P values equal to or less than 0.05 were considered significant. As inflammatory cell infiltration does not always result in tissue damage, the data from each component was also scored independently. Infiltrative scores were measured on a scale of 0 to 7 and structural changes or tissue damage on a scale of 0 to 5.
Ex Vivo NO Production by Splenic Macrophages
Spleens were removed from naïve or immunized control and immunized L-NAME-treated rats at day 10 after immunization and macrophages isolated from single cell suspensions by density gradient centrifugation and adherence to plastic. After 4 hours of incubation in flasks at 37°C in RPMI 5%/fetal calf serum (Myoclone Super Plus, endotoxin-free; Life Technologies, Paisley UK) nonadherent cells were removed by washing with warm medium and further purified on nylon wool columns to yield T cells. Macrophages were then harvested from the flasks with cold medium and a cell scraper. Macrophages were seeded at 5 × 10 6 per ml in 24-well plates in culture medium alone or with L-NMMA (0.5 μmol/L, Sigma). Cytokines (500 ng/ml tumor necrosis factor-α, 100 U/ml IFN-γ; R&D Systems, Abingdon, UK), lipopolysaccharide (1 μg/ml, Sigma), were added and NO production measured after 72 hours by the Greiss reaction as described. 12 All results are expressed as mean ± 1 SD. Results from each treatment group were compared using Student’s t-test. P values equal to or less than 0.05 were considered significant.
Immunohistochemistry and Detection of Apoptosis in Tissue Sections
Serial sections were cut from formalin-fixed and paraffin-embedded eyes for indirect immunoperoxidase, dual immunofluorescence, and terminal dUTP nick-end labeling (TUNEL) methods using the following antibodies: NOS2 (clone 6, 1:100; Transduction Laboratories, Affiniti Research Products, Exeter, UK), Bcl2 (rabbit polyclonal, 1:40; Calbiochem, CN Biosciences UK, Nottingham, UK), BAX (rabbit polyclonal, 1:20; Calbiochem), Fas (rabbit polyclonal,1:20; Calbiochem), Fas ligand (N20, rabbit polyclonal, 1:20; Santa Cruz, Autogenbioclear UK Ltd., Calne, UK), and inducible Hsp70 (rabbit polyclonal, 1:150; ImmunoKontact, AMS Biotechnology Europe Ltd. Abingdon, UK). Leukocyte markers were mouse monoclonal for monocytes/macrophages (ED1, 1:50), tissue macrophages (ED2, 1:50) CD2 (OX34, 1:20), and CD3 (IF4, 1:100) from Serotec, Oxford, UK. Paraffin-embedded sections were treated with proteinase K and for indirect immunoperoxidase, horseradish peroxidase-labeled kits appropriate for mouse monoclonal or rabbit polyclonal antibodies were used according to the manufacturer’s instructions (Vector Laboratories, Peterborough, UK). Nitrotyrosine was detected using mouse monoclonal anti-nitrotyrosine (1:20; Upstate Biotechnology, Lake Placid, NY) with overnight incubation. Secondary antibodies were biotinylated rabbit anti-mouse Ig (DAKO Ltd., High Wycome, UK) or biotinylated swine anti-rabbit Ig (DAKO) followed by streptavidin fluorescein isothiocyanate (FITC) or Texas Red (Amersham, Little Chalfont, UK).
Dual immunofluorescence was performed as previously described. 12 Clone OX 34 (IgG2a anti-CD2) was used in dual-immunofluorescence experiments in preference to clone IF4 (IgM anti-CD3) that gave unacceptably high background fluorescence. Serial 10-μm cryostat sections were cut onto poly-l-lysine-coated slides and air-dried overnight for use with Cox-2/PGHS antibody (mouse monoclonal clone 33, 1:25; Transduction Labs) and transforming growth factor (TGF)-β1 antibody (rabbit polyclonal 1:100; Santa Cruz), in a standard avidin-biotin (alkaline phosphatase complex, APAAP) technique.
Apoptotic cells in the sections were detected using the fluorescence-labeled (FITC) terminal deoxynucleotidyl transferase (TdT) method exactly according to the instructions of the kit manufacturer (Apoptag; Oncor Appligene, Chester-le-Street, UK). For dual labeling, the propidium iodide counterstain was omitted and the sections rinsed in PBS before further incubation with ED1, OX34 (CD2), nitrotyrosine, IHsp70, Fas, Fas ligand, Bax, or Bcl2 antibody. After washing, the appropriate secondary biotinylated rabbit anti-mouse Ig or swine anti-rabbit Ig (both 1:150; DAKO Ltd., Ely, UK) was applied for 30 minutes followed by streptavidin/Texas Red (1:50) conjugate for a further 30 minutes. For triple labeling, OX34 antibody was directly conjugated using a Cy5 mAb-labeling kit (Amersham, Little Chalfont, Bucks, UK) and incubated for 30 minutes on dual-labeled specimens that had been blocked with normal mouse serum. After extensive washing sections were mounted in Citifluor (Agar Scientific, Stanstead, UK) and dual-photographic exposures taken with appropriate filters using an Olympus BH2-RFC microscope, or examined using an MRC 1024 confocal microscope to demonstrate co-localization of signal.
Negative controls included omission of primary or secondary antibodies, addition of preimmune or normal serum Ig in place of polyclonal antibodies, or substitution of irrelevant primary antibodies of the same species isotype for monoclonal antibodies. For NOS2, nitrotyrosine, iHsp70, and TUNEL techniques, control sections from various inflammatory tissues known to be positive were included as positive controls.
Quantification of Positively Stained Cells
Sections of retina from animals taken at days 10 and 11 were examined and lesions selected that displayed equivalent inflammatory infiltrates affecting the full thickness of the retina, including a subretinal exudate (SRE). Apoptosis and nitrotyrosine staining was evaluated from fluorescently labeled sections. Other markers including CD3 and CD2 (T cells), Fas and Fas ligand, and protein products NOS2, iHSP70, Bcl2, and Bax were evaluated using horseradish peroxidase-stained sections using a light microscope. In a separate experiment, the percentage of ED1 (monocytes/macrophages and ED2 (tissue macrophages) within the tissues were calculated by computer-assisted densitometric scanning of individually stained serial sections at time points from day 10 to day 18. Areas were analyzed using one of three methods, and separate data acquired from the inner retina [ganglion cell layer, inner nuclear layer (INL)], outer retina [outer nuclear layer (ONL), photoreceptors including the rod outer segments], or SRE. 1) In fluorescently labeled sections, the total numbers of apoptotic cells present in a minimum of six fields (×20 objective) in retina, SRE, and choroid were counted. 2) For evaluation of membrane markers on inflammatory cells the percentage of positively stained mononuclear cells were counted in a minimum of six fields (×40 objective) per lesion. 3) For evaluation of protein or enzyme expression we used the Aphelion Active X image analysis program from ADCIS (ADCIS SA, Herouville-Saint-Clair, France). The program was adapted using Visual basic to allow analysis of immunostaining within user-defined regions of the image. An average value (percentage of tissue positively stained per ×20 field) for each section was obtained from four to six fields. For each technique a minimum of six sections from separate animals were examined in each treatment group. All results are expressed as mean ± 1 SD and were analyzed by the Mann-Whitney U test or using one-way analysis of variance for groups of three or more values. P values equal to or less than 0.05 were considered significant.
Results
L-NAME Reduces Inflammation and Retinal Damage in Early EAU Independent of Macrophage Priming for NO Generation
In our model, L-NAME administered orally in drinking water (50 mg/kg) is well tolerated and reduces EAU disease severity throughout a period of 21 days. 12 In the experiments described here, fluid intake was found to be equal between the groups, and although the average weight of L-NAME-treated animals (n = 6) was slightly reduced compared with controls at day 9 (typically 231.7 ± 26.4 g versus 253.2 ± 21.2 g) no other differences in general health were observed.
Nonspecific inhibitors of NOS such as L-NAME given in drinking water may have effects other than NOS inhibition, 26 and in some models can actually induce compensatory expression of NOS2. 31 This effect is attributed to the dose of inhibitor used and has led to the idea that the toxicity of NOS2 is restricted to certain areas or cells within inflammatory lesions in a number of diseases, and that NOS2 may also have beneficial effects in inflammation. To assess the systemic effects of L-NAME treatment on macrophage function we examined NO generation by macrophages isolated from treated rats. Figure 1a ▶ shows that splenic macrophages from naïve rats produced basal levels of 17 ± 3.1 μmol/L nitrite ex-vivo, increasing to 33 ± 3.2 μmol/L nitrite in the presence of an NOS2-stimulating cytokine cocktail containing lipopolysaccharide, tumor necrosis factor-α, and IFN-γ. 32 This was reduced to 11.9 ± 1.3 μmol/L nitrite (P < 0.05) with the addition of 0.5 μmol/L L-NMMA. In contrast, macrophages isolated from animals 10 days after immunization with RE spontaneously produced much higher basal levels nitrite levels (194.3 ± 70.2 μmol/L) even when treated with L-NAME in vivo (130.4 ± 29.4 μmol/L). These cells were not responsive to further stimulation with the cytokine/lipopolysaccharide cocktail. Nitrite levels were reduced to 78.5 ± 17.5 μmol/L in macrophages from L-NAME-treated animals compared with 153.2 ± 40.4 μmol/L by macrophages from control-immunized animals (P < 0.05). This was attributed to NO toxicity and macrophage death in culture because phase dark necrotic cells could be observed in the cultures before harvesting of supernatants (data not shown). This toxicity may have been because of the in vivo microenvironment before isolation or to the additional in vitro stimulation. Although addition of 0.5 mmol/L of L-NMMA reduced nitrite production by macrophages from untreated RE-immunized rats, no significant reduction in spontaneous or cytokine-stimulated macrophages from L-NAME-treated RE-immunized rats was observed. This data indicates that treatment with L-NAME in vivo did not prevent priming of macrophages for NO production, and that priming in immunized animals was maximal and irreversible. To assess the possible impact of in vivo macrophage cell death on overall macrophage numbers and NO production in the inflamed retina, we analyzed the numbers of infiltrating macrophages in the choroid and retina during the course of disease. Figure 1b ▶ shows that although numbers of ED2 tissue-resident macrophages remained constant throughout the disease, the numbers of ED1 macrophages increased as the disease progressed.
Figure 1.
Pathogenic role of macrophages in EAU. a: Nitrite production by macrophages ex vivo. Macrophages isolated from the spleens of normal RE-immunized, or RE-immunized and L-NAME-treated rats were cultured for 72 hours in medium alone, or in a cytokine cocktail containing 500 ng/ml tumor necrosis factor-α, 100 U/ml IFN-γ, and 1 μg/ml lipopolysaccharide, with or without 0.5 mmol/L L-NMMA. NO production was then measured as nitrite in the culture medium by the Greiss reaction and the results expressed as means ± 1 SD of quadruplicate cultures, and are representative of at least three experiments. *, P < 0.05 versus macrophages from untreated rats stimulated with the cytokine cocktail. b: Numbers of ED1, but not ED2, macrophages within the uveitic eye correlate with disease severity. The percentage of cells within the choroid and retina of uveitic eyes was calculated by computer-assisted densitometric scanning of individual sections. Values shown represent mean ± 1 SD of serial sections from three separate experiments in which each group contained at least four rats.
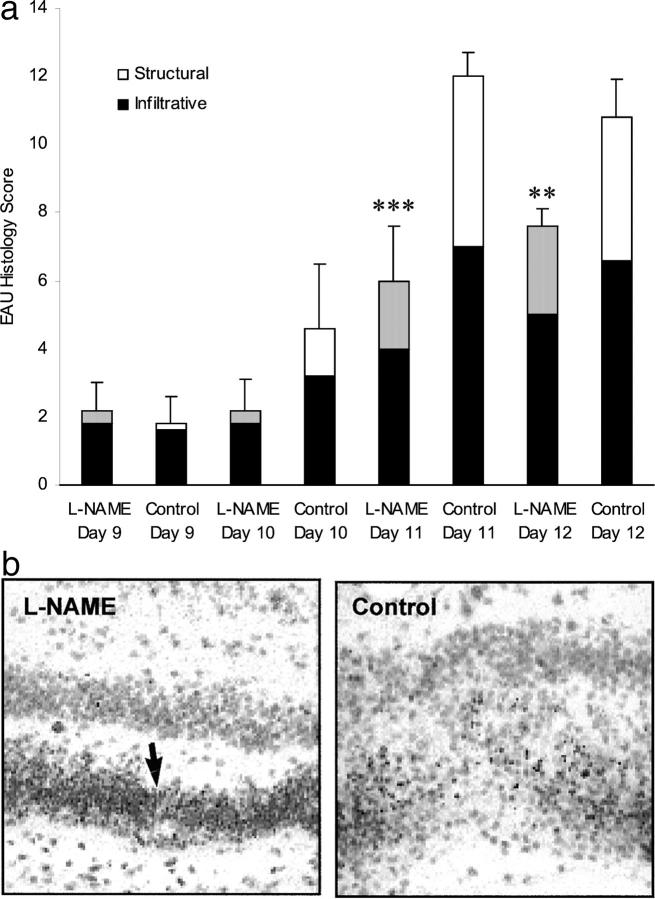
Inhibition of NO by L-NAME has been reported to affect the hyperpermeability of microvascular cells to protein 33 but if significant breakdown of the barrier to inflammatory cells in the L-NAME group was occurring in our model of EAU we would expect an earlier onset of inflammation in the treated groups, but this was not observed. In fact, in animals treated with L-NAME both the severity and the duration of EAU was reduced. 12 In this study, using a scoring method that quantifies both inflammatory cell infiltrates and tissue damage, 30 significant protection in the L-NAME group at day 11 (P < 0.001) and day 12 (P < 0.01) was again observed (Figure 2a) ▶ . Histological evaluation of posterior chamber inflammation showed an infiltrate that was predominantly mononuclear, with focal lesions within the choroid and retina, and SREs in both groups, consistent with our previous observations in this model. 12,30 It became apparent during scoring that both the distribution of mononuclear cells within the lesions and the extent of photoreceptor disruption differed between the groups (Figure 2b) ▶ . Further qualitative analysis of these differences was undertaken using immunostaining techniques (Figures 3 and 4) ▶ ▶ and where feasible the differences were quantified (Table 1) ▶ .
Figure 2.
L-NAME reduces retinal inflammation in RE-immunized rats. a: Histological scores in rats immunized with RE and treated with 50 mg/kg of L-NAME or plain water as control. Solid bar represents infiltrative score, reflecting the numbers of inflammatory cells within the retina and uveal tract, dotted bar represents structural damage including rod outer segment and neural cell loss. Results are expressed as means ± 1 SD of the cumulative infiltrative and structural scores, n = 6, and are representative of two experiments. Histological scores were reduced (***, P < 0.001 and **, P < 0.01) in L-NAME versus control-treated groups at day 11 and day 12, respectively. b: Photoreceptor damage is reduced in L-NAME-treated rats compared with control at day 10 despite equivalent inflammatory cell infiltrate. Nuclei of photoreceptors (arrows) are still present in L-NAME group. Photoreceptor cell bodies are lysed in the control eye. H&E-stained sections; original magnifications, ×150.
Figure 3.

L-NAME reduces NOS2 expression, macrophage nitrotyrosine formation, and apoptosis within the retina in EAU. a: Section of the normal retina showing ganglion cell layer (G), INL, ONL, photoreceptor rod outer segments (ROS), choroid (C), and the RPE layer (arrows). b: TUNEL (FITC)-stained normal retina from FCA-immunized rat (control) with propidium iodide counterstaining of nonapoptotic nuclei (red). c: Bcl2-stained section of normal control retina. d: Bax-stained section of normal control retina. e: TUNEL (FITC)-stained section from RE-immunized rat showing many green apoptotic nuclei in the retina, no counterstain. f: Bax staining of retina from RE-immunized rat showing positive staining throughout the inflamed retina including the SRE. g: NOS2 staining in retina from RE-immunized rat. h: CD3+ T cells in the inner retina of RE-immunized rat. i: TUNEL (FITC)-stained section from L-NAME-treated rat showing reduced apoptotic cells in the retina, no counterstain. j: Bax staining is unaffected in RE-immunized rats treated with L-NAME. k: NOS2 expression is profoundly reduced in the retina of L-NAME-treated RE-immunized rats. l: CD3+ T cells are found throughout all layers of the retina in L-NAME-treated rats. m: Nitrotyrosine staining of cells in the SRE of RE-immunized rats not treated with L-NAME. n: Fas-ligand staining of ciliary body of L-NAME-treated rat. o: Diffuse Fas-ligand staining of retina and SRE of L-NAME-treated rat, showing stronger positively stained infiltrating cells (arrows). p: Fas ligand-positive mononuclear cells in SRE of L-NAME-treated rat. Sections a to d are of normal retinas taken at day 10 from control rats immunized with fetal calf serum alone; sections e to p were taken from inflamed eyes of RE-immunized rats and given water (control group), or RE-immunized and L-NAME-treated rats displaying equivalent clinical scores (score 2 to 3) at day 10 or 11. All sections are from formalin-fixed and wax-embedded eyes and with the exception of b, e, and i that are TUNEL (FITC) stained, are stained using the indirect immunoperoxidase method with hematoxylin counterstain. Photomicrographs are representative of similar sections prepared and stained from at least six eyes. Original magnifications: ×130 (a, b, d–l, and n); ×200 (c, m, and p).
Figure 4.

Nitrotyrosine is formed by ED1+ monocytes/macrophages in EAU, and the cells undergoing apoptosis in the retina are photoreceptors and Fas+ CD2+ T cells. a–d: Dual-immunofluorescence staining of cells for nitrotyrosine (Texas Red) and ED1 (FITC) shows that nitrotyrosine is produced by monocytes/macrophages, and its formation is primarily prevented by L-NAME. a: SRE of RE-immunized and L-NAME-treated rats. b: SRE of untreated RE-immunized rats. c: Section of retina from control RE-immunized rat showing that the majority of ED1+ cells in the INL and ONL of the retina also stain for nitrotyrosine, but ED1+ cells in the vitreous (V) are nitrotyrosine-negative. d: Section of retina from RE-immunized, L-NAME-treated rat showing that very few ED1+ cells are also nitrotyrosine-positive, these being within a retinal vessel (arrows) or in the rod outer segments. Apparent fluorescence of the rod outer segments and RPE is an artifact caused by autofluorescence detected in control sections (data not shown). e: Dual immunofluorescence for ED1+ cells (Texas Red) and TUNEL (FITC) in retina of untreated RE-immunized rat. f: Dual immunofluorescence for CD2+ T cells (Texas Red) and TUNEL (FITC) in retina and choroid of untreated RE-immunized rat. Dual immunofluorescence for CD2+ T cells (Texas Red) and TUNEL (FITC) in the SRE of an untreated RE-immunized rat. h and i: Serial sections of the same area of choroid and SRE shown in f, and are stained for Bax (h), and Bcl2 (i), by the indirect immunoperoxidase method with hematoxylin counterstain. The RPE layer in both is indicated by arrows. j: TGF-β1 labeled using the APAAP method with hematoxylin counterstain. k: Dual immunofluorescence for Fas (Texas Red) and TUNEL (FITC) in photoreceptors of an untreated RE-immunized rat. l and m: Confocal images of triple immunofluorescence-labeled sections from control (l) and L-NAME-treated (m) rats; stained for ED1 (FITC), Fas ligand (Texas Red), and CD2 (Cy5 blue). Dual-stained macrophages are orange or show red signal on their cell surface and internal green fluorescence, whereas dual-stained T cells are pink. n: Dual immunofluorescence for Bax (Texas Red) and TUNEL (FITC) in photoreceptors of an untreated RE-immunized rat. Inducible Hsp 70 stained by indirect immunoperoxidase of: untreated RE-immunized rat retina with some weak expression in and cells in SRE (o, arrows), compared with strong expression in rod outer segments of L-NAME-treated rat (p). Confocal analysis of ED1 (FITC)- and iHsp 70 (Texas red)-stained sections of retina from untreated RE-immunized rat (q), and L-NAME-treated rat (r), showing few dual-stained cells (arrows). With the exception of i, which is a frozen section, all sections are from formalin-fixed and paraffin-embedded eyes selected as described in the legend to Figure 3 ▶ . Photomicrographs are representative of similar sections prepared and stained from at least six eyes. Original magnifications: ×200 (a, h–j); × 300 (b, g, k, and n); ×150 (c–f, o, and p); ×60 (l, m, q, and r; confocal microscope digital images).
Table 1.
Quantification of Staining in Sections Taken at Day 10 to 11 after Immunization
| Subretinal exudate | Retina | |||||
|---|---|---|---|---|---|---|
| Control | L-NAME | P value* | Control | L-NAME | P value | |
| Apoptotic cells† | 48.9 ± 23.9 | 1.5 ± 2.2 | <0.001 | 292.2 ± 75.1 | 36 ± 29.6 | <0.001 |
| Nitrotyrosine+ve† | 60 ± 32.04 | 4 ± 6.2 | <0.001 | 71.9 ± 32.2 | 6.0 ± 4.7 | <0.001 |
| NOS2 staining‡ | 41.72 ± 30.9 | 0.94 ± 1.20 | <0.01 | 42.6 ± 27.1 | 0.16 ± 0.27 | <0.01 |
| Bax staining‡ | 12.6 ± 6 | 18.4 ± 4.8 | ns | 29.6 ± 9.5 | 35.04 ± 10.6 | ns |
| Bcl2 staining‡ | 0 | 0 | ;*>;0>§ | 36.5 ± 9.4 | 36.1 ± 16.8 | ns |
| Fas-1 staining‡ | 10.1 ± 8.8 | 15.1 ± 7.6 | ns | 14.1 ± 6 | 19.5 ± 3.9 | ns |
| IHSP70 staining‡ | 0.91 ± 0.6 | 56.9 ± 22.3 | <0.01 | 4.12 ± 4 | 44.9 ± 8.4 | <0.01 |
*Mann Whitney U: n = 6.
†Numbers of cells stained positively per ×20 field.
‡Percentage of tissue stained per ×20 field.
§Staining absent or too weak to be detected by image analysis.
ns, Not significant.
L-NAME Significantly Reduces NOS2 Expression, Macrophage Nitrotyrosine Formation, and Apoptosis within the Retina in EAU
The target tissue in RE-induced EAU is the photoreceptor and rod outer segment layers of the outer retina. Immunocytochemical analysis showed that apoptotic cells were not present in the normal retina, and correspondingly there was strong Bcl2 expression by cells in the ONL and rod outer segments without Bax protein expression (Figure 3 ▶ ; a to d). In EAU many apoptotic cells were seen in the retina at day 10 in positive control rats immunized with RE, with Bax-positive cells present throughout the tissue (Figure 3, e and f) ▶ , In the normal retina NOS2 is not expressed, and in EAU is expressed only during the early phases of the inflammation. 12 By day 10 many NOS2-positive mononuclear cells were present, particularly in the ONL, rod outer segments, and SRE, and large infiltrates of CD3+ T cells were identified, particularly in the INL of the retina around vessels (Figure 3, g and h) ▶ . In contrast, in the RE-immunized, L-NAME-treated group, far fewer apoptotic cells were observed (P > 0.0001; Table 1 ▶ ) despite equivalent Bax expression (Table 1 ▶ ; Figure 3, i and j ▶ ). NOS2 expression was also much reduced (P < 0.01, Table 1 ▶ ) with only a few cells staining in the rod outer segments and ONL (Figure 3k) ▶ . Abundant CD3+ T cells were now found throughout all layers of the retina (Figure 3l) ▶ . Nitrotyrosine staining to detect free radical production and lipid peroxidation as an end product of NO production was strong in mononuclear cells within the retina and SRE of untreated animals (Figure 3m) ▶ , but was primarily absent in the L-NAME group (P = 0.0006; Table 1 ▶ ). In both groups Fas ligand was expressed by ciliary body epithelium (Figure 3n) ▶ with diffuse expression in the retina (Figure 3, n and o) ▶ . Fas ligand was also expressed by a proportion of infiltrating mononuclear cells (Figure 3p) ▶ , but no significant difference between the groups was found (Table 1) ▶ . NOS inhibitors have been reported to increase PGE2 synthesis, 34 but very little positive staining for COX-2 protein was found, and when present was primarily restricted to corneal epithelial cells in the L-NAME group (data not shown). No staining for NOS2 or COX-2 protein could be found in the retinal pigment epithelium (RPE) or choroid of either group at any of the three time points examined. The cells of the outer retina remained strongly Bcl2-positive even in diseased retinas whereas cells in the inner retina and choroid were only weakly positive or negative in all sections. No significant differences in expression of Bax or Bcl2 in the two groups were found (Table 1) ▶ .
Nitrotyrosine Is Formed by ED1+ Monocytes/Macrophages in EAU, but the Cells Undergoing Apoptosis Are Photoreceptors and Fas+ CD2+ T Cells
Dual immunofluorescence studies revealed that the cells positive for nitrotyrosine were ED1+ monocytes/macrophages in the SRE and retina (Figure 4, b and c) ▶ , and although equivalent infiltrates of ED1+ cells were present in the L-NAME treatment group, very little nitrotyrosine staining was detected (Figure 4, a and d ▶ ; Table 1 ▶ ). Nitrotyrosine is a toxic NO reaction product and evidence of NO formation in vivo, it was therefore surprising that very few of the cells undergoing apoptosis were ED1+ (Figure 4e) ▶ . Further investigation revealed that in addition to the photoreceptors, CD2+ T cells present in the ONL and rod outer segments, but not the SRE and choroid were also undergoing apoptosis (Figure 4, f and g) ▶ . Anti-CD2 antibody (clone OX34; IgG2a) was used here as anti-CD3 (clone IF4; IgM) gave unacceptably high background in fluorescence studies. The absence of apoptosis in the choroid and SRE adjacent to the RPE where infiltrating mononuclear cells were strongly Bax-positive and where there was little Bcl2 staining (Figure 4, h and i) ▶ , suggests other protective mechanisms could be operating out/with the target tissue. In the outer retina and rod outer segments, the majority of apoptotic cells were CD2+, and dual immunofluorescence showed that they were also Fas + (Figure 4k) ▶ . Triple immunofluorescence showed that many CD2+ T cells also expressed Fas ligand, particularly in the control group although no difference in Fas-ligand expression by ED1+ macrophages was observed. The diffuse nature of Fas-ligand staining within the retina made accurate cell counts difficult, but image analysis of overall percentage of tissue stained for Fas ligand did not reveal significant differences between the groups (Table 1) ▶ . Although some apoptotic cells were also Bax-positive, the majority of photoreceptors undergoing apoptosis were both Fas- and Bax-negative (Figure 4n) ▶ .
IHSP70 Is Expressed in the Rod Outer Segments and SRE Cells in L-NAME-Treated Animals
What was preventing apoptosis of monocytes and Bax-positive cells within the choroid? TGF-β1 is reported to regulate apoptosis 35 via an increased sensitivity or loss of resistance to apoptosis in the absence of TGF-β1. Cells expressing TGF-β1 could be found in inflammatory infiltrates in the anterior chamber of most animals with EAU, but staining in the retina was restricted to the RPE in some inflamed eyes particularly in the control group (Figure 4j) ▶ . In the remainder of the posterior chamber, staining for TGF-β1 was found only in large granular cells (possibly mast cells) in the choroid and ciliary body of normal and inflamed eyes from both L-NAME-treated or control groups.
Expression of the inducible 70-kd heat shock protein (iHsp70) has also been shown to prevent apoptosis in myeloid cells. 36 We hypothesized that induction of iHsp70 might protect ED1 monocytes or indeed other cells from apoptotic cell death in the retina. Inducible iHsp70 could not be detected in eyes from normal or negative control animals immunized with FCA only (data not shown). In positive-control animals immunized with RE some diffuse expression was found in the cytoplasm of the rod outer segments and faint expression was seen in a very few cells in the SRE (Figure 4m) ▶ . This expression was markedly up-regulated in L-NAME-treated animals, with strong expression, both in the rod outer segments, cytoplasm, and inflammatory cells particularly in the outer retina and SRE (Figure 4n ▶ ; Table 1 ▶ , P > 0.01). No expression was found in the anterior chamber, ciliary body, choroid, or RPE. Confocal analysis revealed that although a few ED1+ cells expressed inducible iHsp70, the majority of positive cells were either photoreceptors or other mononuclear cells within the inflammatory infiltrate of L-NAME-treated animals (Figure 4, o and p) ▶ .
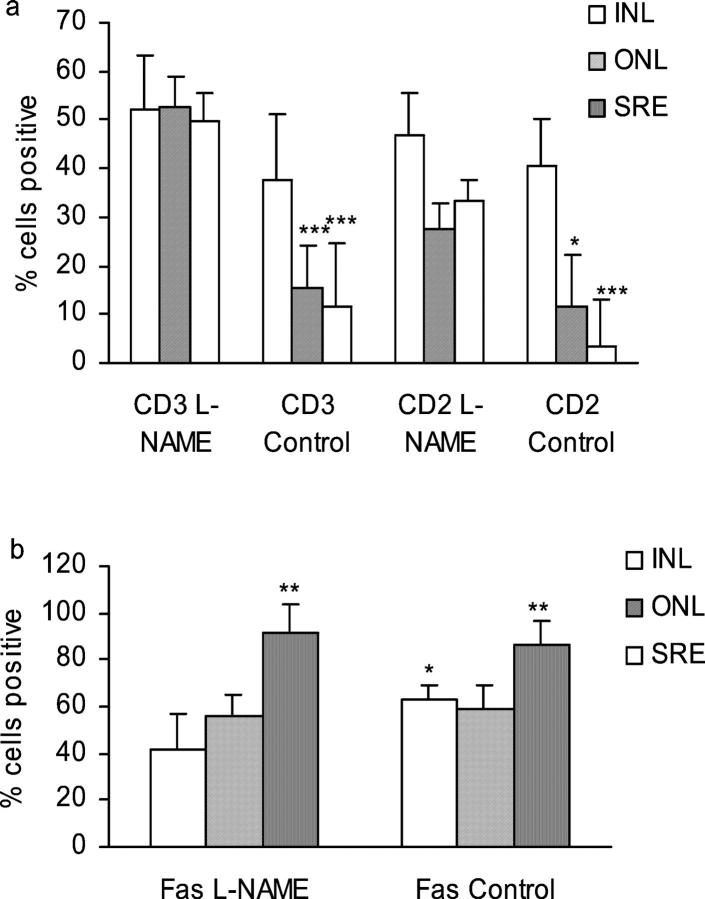
Treatment with L-NAME Significantly Increases the Percentage of CD2+ and CD3+ T Cells in the Target Organ in EAU Despite Continued High Expression of Fas
The overall numbers of T cells (CD2+ or CD3+) as a percentage of mononuclear cells in the inner retina (including ganglion cell layer and INL) was the same in all animals with EAU. Treatment with L-NAME significantly increased the T cell infiltrate in the outer retina, including ONL and rod outer segments that constitute the target organ in EAU and in the SRE (Figure 5a) ▶ . Both CD2+ and CD3+ cells were scored as CD2 may be down-regulated in the early stages of apoptosis. Although fewer CD2+ cells were present, particularly in the L-NAME group, no significant difference between numbers of CD2+ or CD3+ cells were found in any of the tissues. The reduction in CD3+ T cells in both the ONL and the SRE of control rats compared to treated rats was highly significant (P < 0.001). The reduction in CD2+ T cells in the ONL was less marked compared with control, individual variation within the group reducing significance to P < 0.05, but reduction in the SRE was again highly significant (P < 0.001). Figure 5b ▶ shows that levels of Fas were reduced in the inner retina of L-NAME-treated animals (P < 0.05) but no differences were found in the outer retina or SRE. In both groups levels were significantly higher in the SRE compared with the INL with up to 100% of inflammatory cells positive (P < 0.01). Reduction in T cell numbers correlated with increased expression of Fas in the SRE of control animals and it was notable that T cells in the SRE of L-NAME-treated animals were spared despite equivalent high Fas expression. This may also be linked to the possible reduction in Fas-ligand expression by CD2+ T cells in L-NAME-treated animals but the overall trend was for a slight increase in Fas-ligand expression in the retina of these animals, but these changes were not significant.
Figure 5.
Treatment with L-NAME significantly increases the percentage of CD2+ and CD3+ T cells in the target organ in EAU despite continued high expression of Fas and Fas ligand. Sections from inflamed eyes of RE-immunized and water-treated (control), or RE-immunized and L-NAME-treated rats displaying equivalent clinical scores (score 2 to 3) at day 10 or 11 were stained by the indirect immunoperoxidase method for the presence of CD2+ and CD3+ T cells (a) or Fas and Fas ligand (b) and scored as a percentage of infiltrating mononuclear cells in the INL, ONL, or SRE as described in Materials and Methods. Results expressed as means ± 1 SD from a minimum of six sections from separate animals in each treatment group. Both CD3+ and CD2+ T-cell numbers in the INL and SRE were significantly reduced in the untreated control group compared to the same regions in L-NAME-treated groups (***, P < 0.001; *, P < 0.05), but no significant differences between percentages of CD2+ and CD3+ cells were noted. Fas expression was significantly raised in the SRE in both groups compared with ONL and INL (P < 0.05). L-NAME treatment slightly reduced the percentage of Fas+ cells in the ONL compared with ONL of untreated rats with EAU (P < 0.05).
Discussion
In this study, using a nonspecific NOS inhibitor, we show that although inhibition of NOS2 prevents peroxynitrite formation and protects photoreceptor cells from apoptosis in EAU, apoptosis of T cells in the target tissue is also compromised despite continued expression of Fas and Fas ligand. The mechanisms are unclear, but induction of iHsp70 in the presence of NOS inhibitors may play a role in the survival of both photoreceptors and potentially autoreactive effector T cells in the absence of superoxide (O2−).
Apoptosis can be induced by a variety of environmental, physical, or chemical stresses. Although alternative pathways must also exist, two major pathways to cell death by apoptosis can be identified and separated into either the mitochondria-mediated pathway with formation of the apoptosome and activation of caspase 9 or the CD95-CD95L (Fas or membrane death receptor)-mediated pathway that activates caspase 8. 37 Both pathways then converge at the level of caspase 3 and are subject to regulation at multiple points. The Fas pathway is generally considered to be independent of the mitochondrion, but caspase 8 can cleave Bid, a proapoptotic member of the Bcl2 family, allowing its translocation to the mitochondrion. The mitochondrial pathway is regulated by proteins of the Bcl2 family, whereas the Fas pathway can be regulated by co-stimulatory signals and cytokines. Heat shock proteins are also important regulators, and induction of iHsp70 in particular has been found to protect cells from apoptosis by preventing formation of the apoptosome and inhibiting caspase 3 processing. 38 The data presented here suggests that in EAU NO may contribute to AICD via the Fas or death receptor pathway, and to photoreceptor apoptosis through the mitochondrial pathway, but this interpretation requires further investigation.
Inducible NOS and NO have both regulatory and effector functions in the immune system, and in organ-specific autoimmunity in particular, may have pathogenic or protective functions giving rise to the concept of NO as a “double-edged sword” in autoimmunity. 20,27,39,40 This concept is supported by the conflicting data emerging from studies in animal models using specific NOS inhibitors or inactivation or deletion of the NOS2 gene. 12,16-19,14 The precise mechanisms are unclear but it is suggested that tissue-specific expression of NOS is important for regulation of immune responses in the periphery, with a balance between toxic and homeostatic functions and production of large and small quantities of NO, respectively. 26 The effectiveness of nonspecific inhibitors of NOS administered orally has been questioned. In particular, dose rate and route of administration seems to be crucial. 19,41 Access of inhibitors to peripheral tissues may be incomplete, but in some models a 60% reduction in inflammation despite only 15% reduction in NOS2 activity has been reported 31 suggesting that comparatively small reductions in NOS are physiologically relevant. Alternatively, other, as yet unappreciated effects of the treatment may influence inflammation. In our model, L-arginine exacerbates EAU, whereas L-NAME, given in drinking water at 50 mg/kg is well tolerated, reduces urinary nitrite indicating access to the systemic circulation, shows no obvious effects on retinal blood vessel permeability to leukocytes, and affords some protection from photoreceptor loss 12 (Figure 2) ▶ . Immunohistochemistry indicated that L-NAME was also very effective in reducing NOS2 protein expression in vivo (Figure 3) ▶ . The capacity of macrophages isolated from L-NAME-treated animals to release very large quantities of NO without any further stimulation in vitro, as demonstrated in this study, was therefore unexpected (Figure 1a) ▶ . The macrophages appeared to be maximally primed, as further cytokine stimulation had little effect. The reduction in NO observed was attributed to increased cell death in these cultures suggesting that other factors, possibly cytokines or growth factors, were present in the inflammatory lesions that could provide survival signals to the macrophages in vivo. Alternatively, necrotic changes to the cells in vivo were affecting cell viability in vitro. As ED1+ve macrophage numbers within the retina remained high until resolution of inflammation at days 16 to 18 (Figure 1b) ▶ it seems unlikely that macrophage death by necrosis in vivo during early and peak disease has a significant impact on disease progression. Despite highly restricted expression of the enzyme during the early and acute phases of the disease and effective suppression of NOS2 protein by L-NAME, our previous studies found that NOS2 mRNA expression persisted in the ocular tissues throughout the time course studied (9 to 21 days). This peaked on day 12, and coincided with peak expression of IFN-γ mRNA in the retina in untreated animals. As macrophages first exposed to IFN-γ are subsequently refractory to functional modulation by other cytokines 42 we can hypothesize that in our EAU model, monocytes/macrophages are primed for NO release despite the presence of NOS inhibitors. The possibility then arises, that under the influence of locally produced IFN-γ, small quantities of NO may continue to be produced in the lesions despite the absence of NOS2 enzyme, and underlines the difficulties of controlling NO release by targeting the NOS genes alone.
In this study we have focused on the earliest stages of EAU (days 9 to 11) with detailed histological examination of tissue from normal (adjuvant-only immunized) and inflamed eyes. L-NAME treatment given from the time of immunization profoundly reduced both NOS2 enzyme expression and nitrotyrosine deposition in monocytes as an end product of peroxynitrite formation in the outer retina. Oxidative damage and peroxynitrite concentrated in the photoreceptors in EAU together with early and restricted expression of NOS2 in Lewis rat EAU by extravasated ED1+ mononuclear cells has also been observed by Zhang and colleagues 13 and Wu and colleagues. 43 We now show that inhibition of NOS2 expression and reduction of peroxynitrite formation also reduces apoptosis within the retina. A previous study of apoptosis in EAU in Lewis rats has also shown that apoptosis in the retina peaked 2 days after disease onset, 28 coinciding with peak expression of Fas-ligand expression by ciliary body epithelium, other tissue-resident cells being Fas ligandlow. 44 Fas ligand is normally expressed only by activated inflammatory cells, principally T cells and macrophages, and mediates AICD primarily in CD4+ T cells. 45 In the eye and other sites of immune privilege, however, Fas ligand may be expressed on tissue resident cells. 1,46 As RE-specific cells are known to traffic to the retina where they apoptose, 47,48 our finding that Fas ligand-positive and apoptotic CD2+ T cells showed the same highly restricted tissue distribution as Fas+ apoptotic cells in the rod outer segments and SRE of untreated animals would suggest that AICD is occurring in EAU.
Nitrotyrosine formation indicated that large quantities of both NO and superoxide (O2−) were generated and reacted to form peroxynitrite (ONOO−) that is highly toxic, particularly to neuronal cells such as photoreceptors. Apoptosis as a result of NO-mediated cytotoxicity is mainly exerted at the level of the mitochondrion, and is regulated by members of the Bcl2 family. 37,49 Bcl2 cannot prevent apoptosis under all circumstances. Our study, in part, confirmed this as Bcl2 expression in normal retina remained at apparently high levels throughout retinal inflammation in treated as well as untreated rats, and yet severe apoptotic damage to the photoreceptors was observed. Stable expression of Bax protein in rat EAU has been observed before, 50 and in this study we could observe no obvious alterations in balance between proapoptotic Bax and anti-apoptotic Bcl2 that control cytochrome c release from the mitochondrion. Indeed we showed that apoptosis was apparently inhibited, despite continued Bax expression in treated animals, suggesting that regulation by other regulatory proteins of the Bcl family or other mechanisms downstream of the mitochondrion may be involved. One notion is that protection in the treated group may be attributed to induction of Hsp 70. Inducible Hsp70 protects against multiple apoptotic stimuli including DNA damage and growth factor withdrawal by inhibiting caspase processing. 51 Activity may occur at more than one point downstream of cytochrome c release from the mitochondrion, but upstream of caspase 3 via regulation of the apoptosome. 38
Previous studies have shown that T cell apoptosis driven by cytokine deprivation correlates with reduction in Bcl2 expression relative to Bax. 52-54 Using only histological methods we could distinguish no differences in staining intensity or proportion of cells positive in either group suggesting that alterations in cytokine availability were not involved in T cell apoptosis. More sensitive and quantitative molecular or biochemical approaches will be required to confirm this hypothesis. In our study, the most dramatic effect of NOS inhibition by L-NAME was the sparing of T cells within the outer retina and rod outer segments that is the target tissue in EAU. This was despite continued high expression of Fas on all inflammatory cells. Up to 100% of cells were Fas+ in both groups, so many Fas+ T cells in the L-NAME-treated retinas were apparently resistant to AICD despite the presence of 10 to 15% Fas ligand-positive cells. How does inhibition of NOS protect T cells from Fas-driven apoptosis? NO mediated apoptosis may be regulated by COX-2 55 and macrophage derived TGF-β1 can protect T cells from AICD 35,56 but no histological evidence for significant quantities of either was found in this study. The answer may be related to quantitative levels of NO and the redox state of the cells. 57,58 High levels of NO up-regulate expression of Fas and stimulate the release of the soluble form of Fas ligand. This would explain the efficient elimination of activated T cells in the outer retina of untreated rats, and inhibitors of NO have been shown to protect T cells from AICD directly through posttranslational or transcriptional modification of Fas ligand. 59 Alternatively, low levels of NO may induce protective stress protein responses such as induction of Hsp70 in T cells as observed in this study.
Treatment with L-NAME profoundly reduced NOS2 expression, and might be predicted to inhibit the constitutive isoforms NOS1 and NOS3 with equal efficiency, however persistent expression of NOS2 mRNA observed in our previous study, priming of monocytes/macrophages to release large quantities of NO despite L-NAME treatment shown here, and evidence of incomplete penetrance of oral L-NAME reported by others 31 does not preclude continued NO production in vivo in treated animals. Prevention or reduction of O2− formation and so prevention of ONOO− formation may be of more therapeutic relevance than simply reducing excessive NO production.
In conclusion, our study shows that NO seems to modulate both of the major pathways to apoptosis. The mitochondrial-dependent pathway leading to destruction of photoreceptors and the Fas or death receptor pathway leading to T cell apoptosis. Dysregulation of T cell apoptosis clearly contributes to the chronicity of inflammation and defects in the Fas-Fas-ligand pathway of T cell apoptosis have been suggested as a contributing factor in autoimmune disease particularly through defects in tolerance induction. 46 Not only are excess activated T cells killed, their removal by local phagocytic cells generates interleukin-10, and supports immune deviation through development of Th2 cells and control of delayed type hypersensitivity (DTH). 60 Therefore, strategies to promote T cell apoptosis while regulating monocyte toxicity to prevent photoreceptor damage because of local production of reactive oxygen species may hold the key to nonantigen-specific strategies to control uveoretinal inflammation.
Acknowledgments
We thank Zoe Fletcher and Rosemary Dawson for excellent technical assistance, Dr. Rob van t’Hof for image analysis advice, and Dr. Mike Rogers for critical reading of the manuscript.
Footnotes
Address reprint requests to Janet Liversidge, Ph.D., University of Aberdeen Medical School, Department of Ophthalmology, Polwarth Building, Foresterhill, Aberdeen, UK AB25 2ZD. E-mail: j.liversidge@abdn.ac.uk.
Supported by a grant from The Wellcome Trust.
References
- 1.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA: Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995, 270:1189-1192 [DOI] [PubMed] [Google Scholar]
- 2.Streilein JW, Ksander BR, Taylor AW: Immune deviation in relation to ocular immune privilege. J Immunol 1997, 158:3557-3560 [PubMed] [Google Scholar]
- 3.Pender MP: Activation-induced apoptosis of autoreactive and alloreactive T lymphocytes in the target organ as a major mechanism of tolerance. Immunol Cell Biol 1999, 77:216-223 [DOI] [PubMed] [Google Scholar]
- 4.Liversidge J, McKay D, Mullen G, Forrester JV: Retinal pigment epithelial cells modulate lymphocyte function at the blood-retina barrier by autocrine PGE2 and membrane-bound mechanisms. Cell Immunol 1993, 149:315-330 [DOI] [PubMed] [Google Scholar]
- 5.Holtkamp GM, de Vos AF, Kijlstra A, Peek R: Expression of multiple forms of IL-1 receptor antagonist (IL-1ra) by human retinal pigment epithelial cells: identification of a new IL-1ra exon. Eur J Immunol 1999, 29:215-224 [DOI] [PubMed] [Google Scholar]
- 6.Forrester JV, McMenamin PG, Holthouse I, Lumsden L, Liversidge J: Localization and characterization of major histocompatibility complex class II-positive cells in the posterior segment of the eye: implications for induction of autoimmune uveoretinitis. Invest Ophthalmol Vis Sci 1994, 35:64-77 [PubMed] [Google Scholar]
- 7.Dick AD: Immune mechanisms of uveitis: insights into disease pathogenesis and treatment. Int Ophthalmol Clin 2000, 40:1-18 [DOI] [PubMed] [Google Scholar]
- 8.Nussenblatt RB, Gery I: Experimental autoimmune uveitis and its relationship to clinical ocular inflammatory disease. J Autoimmun 1996, 9:575-585 [DOI] [PubMed] [Google Scholar]
- 9.Caspi RR, Sun B, Agarwal RK, Silver PB, Rizzo LV, Chan CC, Wiggert B, Wilder RL: T cell mechanisms in experimental autoimmune uveoretinitis: susceptibility is a function of the cytokine response profile. Eye 1997, 11:209-212 [DOI] [PubMed] [Google Scholar]
- 10.Dick AD, Duncan L, Hale G, Waldmann H, Isaacs J: Neutralizing TNF-alpha activity modulates T-cell phenotype and function in experimental autoimmune uveoretinitis. J Autoimmun 1998, 11:255-264 [DOI] [PubMed] [Google Scholar]
- 11.Forrester JV, Huitinga I, Lumsden L, Dijkstra CD: Marrow-derived activated macrophages are required during the effector phase of experimental autoimmune uveoretinitis in rats. Curr Eye Res 1998, 17:426-437 [DOI] [PubMed] [Google Scholar]
- 12.Hoey S, Grabowski PS, Ralston SH, Forrester JV, Liversidge J: Nitric oxide accelerates the onset and increases the severity of experimental autoimmune uveoretinitis through an IFN-gamma-dependent mechanism. J Immunol 1997, 159:5132-5142 [PubMed] [Google Scholar]
- 13.Zhang J, Wu LY, Wu GS, Rao NA: Differential expression of nitric oxide synthase in experimental uveoretinitis. Invest Ophthalmol Vis Sci 1999, 40:1899-1905 [PubMed] [Google Scholar]
- 14.Thillaye-Goldenberg B, Goureau O, Naud MC, de Kozak Y: Delayed onset and decreased severity of experimental autoimmune uveoretinitis in mice lacking nitric oxide synthase type 2. J Neuroimmunol 2000, 110:31-44 [DOI] [PubMed] [Google Scholar]
- 15.Ding M, Zhang M, Wong JL, Voskuhl RR, Ellison GW: Antisense blockade of inducible nitric oxide synthase in glial cells derived from adult SJL mice. Neurosci Lett 1996, 220:89-92 [DOI] [PubMed] [Google Scholar]
- 16.Silver PB, Tarrant TK, Chan CC, Wiggert B, Caspi RR: Mice deficient in inducible nitric oxide synthase are susceptible to experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci 1999, 40:1280-1284 [PubMed] [Google Scholar]
- 17.Fenyk-Melody JE, Garrison AE, Brunnert SR, Weidner JR, Shen F, Shelton BA, Mudgett JS: Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol 1998, 160:2940-2946 [PubMed] [Google Scholar]
- 18.O’Brien NC, Charlton B, Cowden WB, Willenborg DO: Nitric oxide plays a critical role in the recovery of Lewis rats from experimental autoimmune encephalomyelitis and the maintenance of resistance to reinduction. J Immunol 1999, 163:6841-6847 [PubMed] [Google Scholar]
- 19.Kolb H, Kolb-Bachofen V: Nitric oxide in autoimmune disease: cytotoxic or regulatory mediator? Immunol Today 1998, 19:556-561 [DOI] [PubMed] [Google Scholar]
- 20.Bogdan C, Rollinghoff M, Diefenbach A: Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol 2000, 12:64-76 [DOI] [PubMed] [Google Scholar]
- 21.Pryor WA, Squadrito GL: The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol 1995, 268:L699-L722 [DOI] [PubMed] [Google Scholar]
- 22.Hausladen A, Privalle CT, Keng T, DeAngelo J, Stamler JS: Nitrosative stress: activation of the transcription factor OxyR. Cell 1996, 86:719-729 [DOI] [PubMed] [Google Scholar]
- 23.Lander HM, Ogiste JS, Pearce SF, Levi R, Novogrodsky A: Nitric oxide-stimulated guanine nucleotide exchange on p21ras. J Biol Chem 1995, 270:7017-7020 [DOI] [PubMed] [Google Scholar]
- 24.Togashi H, Sasaki M, Frohman E, Taira E, Ratan RR, Dawson TM, Dawson VL: Neuronal (type I) nitric oxide synthase regulates nuclear factor kappaB activity and immunologic (type II) nitric oxide synthase expression. Proc Natl Acad Sci USA 1997, 94:2676-2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clancy RM, Amin AR, Abramson SB: The role of nitric oxide in inflammation and immunity. Arthritis Rheum 1998, 41:1141-1151 [DOI] [PubMed] [Google Scholar]
- 26.Kubes P: Inducible nitric oxide synthase: a little bit of good in all of us. Gut 2000, 47:6-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogdan C: The multiplex function of nitric oxide in (auto)immunity. J Exp Med 1998, 187:1361-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe T, Nakajima A, Satoh N, Sakuragi S: Fas expression and apoptosis in rats with experimental autoimmune uveoretinitis. Jpn J Ophthalmol 2001, 45:240-246 [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Sun B, Matteson DM, O’Brien TP, Chan CC: Cytokines and apoptotic molecules in experimental melanin-protein induced uveitis (EMIU) and experimental autoimmune uveoretinitis (EAU). Autoimmunity 1999, 30:171-182 [DOI] [PubMed] [Google Scholar]
- 30.Dick AD, Cheng YF, Liversidge J, Forrester JV: Immunomodulation of experimental autoimmune uveoretinitis: a model of tolerance induction with retinal antigens. Eye 1994, 8:52-59 [DOI] [PubMed] [Google Scholar]
- 31.Miller MJ, Thompson JH, Liu X, Eloby-Childress S, Sadowska-Krowicka H, Zhang XJ, Clark DA: Failure of L-NAME to cause inhibition of nitric oxide synthesis: role of inducible nitric oxide synthase. Inflamm Res 1996, 45:272-276 [DOI] [PubMed] [Google Scholar]
- 32.Liversidge J, Grabowski P, Ralston S, Benjamin N, Forrester JV: Rat retinal pigment epithelial cells express an inducible form of nitric oxide synthase and produce nitric oxide in response to inflammatory cytokines and activated T cells. Immunology 1994, 83:404-409 [PMC free article] [PubMed] [Google Scholar]
- 33.Kubes P: Nitric oxide-induced microvascular permeability alterations: a regulatory role for cGMP. Am J Physiol 1993, 265:H1909-H1915 [DOI] [PubMed] [Google Scholar]
- 34.Habib A, Bernard C, Lebret M, Creminon C, Esposito B, Tedgui A, Maclouf J: Regulation of the expression of cyclooxygenase-2 by nitric oxide in rat peritoneal macrophages. J Immunol 1997, 158:3845-3851 [PubMed] [Google Scholar]
- 35.Wahl SH, Orenstein JM, Chen W: TGF-β influences the life and death decisions of T lymphocytes. Cytokine and Growth Factor Reviews 2000, 11:71-79 [DOI] [PubMed] [Google Scholar]
- 36.Kwak HJ, Jun CD, Pae HO, Yoo JC, Park YC, Choi BM, Na YG, Park RK, Chung HT, Chung HY, Park WY, Seo JS: The role of inducible 70-kDa heat shock protein in cell cycle control, differentiation, and apoptotic cell death of the human myeloid leukemic HL-60 cells. Cell Immunol 1998, 187:1-12 [DOI] [PubMed] [Google Scholar]
- 37.Hengartner MO: The biochemistry of apoptosis. Nature 2000, 407:770-776 [DOI] [PubMed] [Google Scholar]
- 38.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR: Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2000, 2:469-475 [DOI] [PubMed] [Google Scholar]
- 39.Nathan C: Inducible nitric oxide synthase: what difference does it make? J Clin Invest 1997, 100:2417-2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacMicking J, Xie QW, Nathan C: Nitric oxide and macrophage function. Annu Rev Immunol 1997, 15:323-350 [DOI] [PubMed] [Google Scholar]
- 41.Paul-Clark MJ, Gilroy DW, Willis D, Willoughby DA, Tomlinson A: Nitric oxide synthase inhibitors have opposite effects on acute inflammation depending on their route of administration. J Immunol 2001, 166:1169-1177 [DOI] [PubMed] [Google Scholar]
- 42.Erwig LP, Kluth DC, Walsh GM, Rees AJ: Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol 1998, 161:1983-1988 [PubMed] [Google Scholar]
- 43.Wu GS, Zhang J, Rao NA: Peroxynitrite and oxidative damage in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci 1997, 38:1333-1339 [PubMed] [Google Scholar]
- 44.Sueda J, Hikita N, Mochizuki M, Jimi A, Kojiro M: Kinetics of apoptotic cells in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci 2000, 41:799-804 [PubMed] [Google Scholar]
- 45.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH: Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 1995, 373:438-441 [DOI] [PubMed] [Google Scholar]
- 46.Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA: CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity 1996, 5:7-16 [DOI] [PubMed] [Google Scholar]
- 47.Dick AD, McMenamin PG, Korner H, Scallon BJ, Ghrayeb J, Forrester JV, Sedgwick JD: Inhibition of tumor necrosis factor activity minimizes target organ damage in experimental autoimmune uveoretinitis despite quantitatively normal activated T cell traffic to the retina. Eur J Immunol 1996, 26:1018-1025 [DOI] [PubMed] [Google Scholar]
- 48.Prendergast RA, Iliff CE, Coskuncan NM, Caspi RR, Sartani G, Tarrant TK, Lutty GA, McLeod DS: T cell traffic and the inflammatory response in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci 1998, 39:754-762 [PubMed] [Google Scholar]
- 49.Gross A, McDonnell JM, Korsmeyer SJ: BCL-2 family members and the mitochondria in apoptosis. Genes Dev 1999, 13:1899-1911 [DOI] [PubMed] [Google Scholar]
- 50.Li Q, Sun B, Matteson DM, O’Brien TP, Chan CC: Cytokines and apoptotic molecules in experimental melanin-protein induced uveitis (EMIU) and experimental autoimmune uveoretinitis (EAU). Autoimmunity 1999, 30:171-182 [DOI] [PubMed] [Google Scholar]
- 51.Samali A, Orrenius S: Heat shock proteins: regulators of stress response and apoptosis. Cell Stress Chaperones 1998, 3:228-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oltvai ZN, Milliman CL, Korsmeyer SJ: Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74:609-619 [DOI] [PubMed] [Google Scholar]
- 53.Nunez G, Merino R, Grillot D, Gonzalez-Garcia M: Bcl-2 and Bcl-x: regulatory switches for lymphoid death and survival. Immunol Today 1994, 15:582-588 [DOI] [PubMed] [Google Scholar]
- 54.Akbar AN, Borthwick NJ, Wickremasinghe RG, Panayoitidis P, Pilling D, Bofill M, Krajewski S, Reed JC, Salmon M: Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur J Immunol 1996, 26:294-299 [DOI] [PubMed] [Google Scholar]
- 55.von Knethen A, Brune B: Cyclooxygenase-2: an essential regulator of NO-mediated apoptosis. FASEB J 1997, 11:887-895 [PubMed] [Google Scholar]
- 56.Cerwenka A, Kovar H, Majdic O, Holter W: Fas- and activation-induced apoptosis are reduced in human T cells preactivated in the presence of TGF-beta 1. J Immunol 1996, 156:459-464 [PubMed] [Google Scholar]
- 57.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS: A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 1993, 364:626-632 [DOI] [PubMed] [Google Scholar]
- 58.Stamler JS, Singel DJ, Loscalzo J: Biochemistry of nitric oxide and its redox-activated forms. Science 1992, 258:1898-1902 [DOI] [PubMed] [Google Scholar]
- 59.Williams MS, Noguchi S, Henkart PA, Osawa Y: Nitric oxide synthase plays a signaling role in TCR-triggered apoptotic death. J Immunol 1998, 161:6526-6531 [PubMed] [Google Scholar]
- 60.Gao Y, Herndon JM, Zhang H, Griffith TS, Ferguson TA: Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med 1998, 188:887-896 [DOI] [PMC free article] [PubMed] [Google Scholar]