Abstract
Risk factors for vulvar squamous cell carcinoma (SCC) are human papilloma virus (HPV) infections and lichen sclerosus (LS). The significance of monoclonal γ-T-cell receptor (γ-TCR) rearrangement in the lymphoid infiltrate of LS and the consequence for vulvar carcinogenesis is unknown. One hundred sixty-one biopsies of vulvar LS and SCC, with and without LS, were examined for monoclonal γ-TCR rearrangement and HPV16 expression, and for the expression of B- and T-cell markers and fascin. Monoclonal γ-TCR rearrangement was identified in 8 of 17 patients with LS and 11 of 21 patients with SCC arising in LS with only occasional HPV16 DNA detection. None of the 19 SCC without LS showed monoclonal γ-TCR rearrangement, but 14 of 19 patients had strong HPV16 detection. The lichenoid infiltrate of LS with germline configuration consisted predominantly of T cells (CD8 > CD4), along with numerous B cells. However, in biopsies with monoclonally rearranged γ-TCR, CD4-positive T cells dominated along with B cells and fascin-positive cells in the lichenoid infiltrate and in deeply located lymphocyte aggregates (LAs). These LAs additionally contained fascin-positive dendritic cells with only individual CD8, CD57, and granzyme-positive cells. LAs in biopsies with germline configuration demonstrated numerous T cells (CD8 >CD4), but only single peripheral B cells, CD57, and fascin-positive lymphocytes. Our data suggest that monoclonal γ-TCR rearrangement is characteristic for and limited to LS and SCC arising in LS, raising the question for a LS-associated antigen. We interpret B cells, CD4-positive T cells, and fascin-expressing dendritic cells within LS as a cellular immune response to antigen or proliferating T-cell clones. The resulting local immune dysregulation in LS may provide a permissive environment for the development of a SCC.
Lichen sclerosus (LS) is a skin disease predominantly restricted to genital skin. The vast majority of LS occurs in vulvar skin of postmenopausal women, but LS can also be seen in younger women and even in prepubertal girls. 1 Well-developed LS is characterized histologically by atrophic epidermis, destruction of the dermoepidermal junction with basal keratinocyte vacuolization, basement membrane homogenization, dermal edema and sclerosis, rarefaction of vessels, and a band-like lymphocytic infiltrate with a predominant T-cell phenotype. A recent study on vulvar and penile LS reports a monoclonally rearranged γ-T-cell receptor (γ-TCR) within the lymphoid infiltrate in ∼50% of the examined biopsies. 2 This has been an unexpected finding in so-called “chronic dermatitis,” the preferred term for LS in the dermatological literature. The classification and etiology of LS is an ongoing topic of controversy. 3-5 LS may truly represent a benign chronic inflammatory skin lesion; however, vulvar LS carries an increased risk for development of vulvar squamous cell carcinoma (SCC). 6 Patients with vulvar SCC have been divided epidemiologically into two subgroups: 1) younger women with human papilloma virus (HPV) infections, and 2) elderly women without HPV risk factors but with a longstanding history of LS. 7 The etiology of LS and SCC arising in LS is not well understood. 8 At present the significance of monoclonal T cells in the lymphoid infiltrate of LS is unclear. Furthermore, the consequence of this finding for the carcinogenesis of vulvar SCC arising in LS is completely unknown. We investigated if and to what extent monoclonal γ-TCR rearrangement can be observed in tumor-infiltrating lymphocytes of vulvar SCC, especially in SCC arising in LS. For this purpose, we used tissue samples of three patient groups that we analyzed in a retrospective study: patients with LS only, patients with SCC arising in LS (SCC/LS), and patients with vulvar SCC without LS. All biopsies were further examined for the presence of HPV16 DNA for correlation of our data with the known main etiological risk factors of vulvar SCC. In addition, we analyzedthe immunophenotypical profile of the lymphoid infiltrate in tissues with and without monoclonal γ-TCR rearrangement.
Materials and Methods
Patient and Sample Selection
Three patient groups were investigated. The first group contained 50 biopsies from 22 patients with LS with an average age of 62 years (range, 35 to 87 years). The second group consisted of 71 samples of 21 patients with surgical excisions of vulvar SCC/LS with an average age of 69 years (range, 45 to 87 years) and the third group consisted of 40 biopsies of 19 patients with an average age of 69 years (range, 37 to 87 years) with surgically resected SCC unassociated with LS (for summary see Tables 2 to 4 ▶ ▶ ▶ ). Representative formalin-fixed, paraffin-embedded material obtained during the last 6 years from the archives of the Institute of Pathology and the Department of Obstetrics and Gynecology of the University of Graz, Austria, were used for this investigation (histological criteria according to Carlson and colleagues 4 ). All available biopsies of the patients with LS only were investigated. Multiple biopsies represent concomitant biopsies. Within the SCC/LS patient group, separate tissue blocks of the SCC and the surrounding LS obtained from the excision specimen were analyzed. Two separate tissue blocks of carcinoma from the excision specimens of SCC without LS were analyzed. All analyzed samples contained representative and comparable lymphohistiocytic infiltrates.
Table 2.
Lichen Sclerosus
| Patient no. | Age at diagnosis | Specimen number | Diagnosis* | γ-TCR rearrangement | HPV16 |
|---|---|---|---|---|---|
| 22 1 | 67 years | 39435/99 | LS early, lichenoid infiltrate, no LA | ;*>;0>† | ;*>;0>† |
| 39435/99 | LS early, lichenoid infiltrate, no LA | ;*>;0>† | ;*>;0>† | ||
| 2 | 62 years | 6009/00 | LS, hypertrophic, scant lichenoid infiltrate | − | − |
| 3 | 68 years | 109943/99 | LS, lichenoid infiltrate | ;*>;0>† | ;*>;0>† |
| 109943/99 | LS, lichenoid infiltrate | ;*>;0>† | ;*>;0>† | ||
| 4 | 62 years | 7822/00 | LS, lichenoid infiltrate | ;*>;0>† | − |
| 5 | 66 years | 117349/99 | LS, scant lichenoid infiltrate | − | ;*>;0>† |
| 117349/99 | LS, scant lichenoid infiltrate | − | ;*>;0>† | ||
| 6 | 64 years | 8693/99 | LS, hypertrophic, scant lichenoid infiltrate, no LA | − | + |
| 8694/99 | LS, hypertrophic, scant lichenoid infiltrate, no LA | − | ++ | ||
| 98280/00 | LS, hypertrophic, scant lichenoid infiltrate, no LA | − | +++ | ||
| 98280/00 | LS, hypertrophic, scant lichenoid infiltrate, no LA | − | +++ | ||
| 98280/00 | LS, hypertrophic, scant lichenoid infiltrate, no LA | − | ++ | ||
| 7 | 66 years | 118700/99 | LS, dense lichenoid infiltrate | + | − |
| 8 | 66 years | 120030/00 | LS, hypertrophic, dense lichenoid infiltrate | +++ | − |
| 120031/00 | LS, hypertrophic, dense lichenoid infiltrate | − | + | ||
| 120032/00 | LS, scant lichenoid infiltrate | − | − | ||
| 9 | 58 years | 77667/99 | LS, lichenoid infiltrate, no LA | + | + |
| 77668/99 | LS, lichenoid infiltrate, no LA | + | + | ||
| 10 | 59 years | 67714/01 | LS, atrophic, scant infiltrate | − | − |
| 11 | 85 years | 89087/98 | LS, atrophic, scant infiltrate | − | − |
| 89087/98 | LS, atrophic, scant infiltrate | − | + | ||
| 89087/98 | LS, atrophic, scant infiltrate | − | + | ||
| 89087/98 | LS, atrophic, scant infiltrate | − | − | ||
| 89087/98 | LS, atrophic, scant infiltrate | − | − | ||
| 89088/98 | LS, atrophic, scant infiltrate | − | − | ||
| 89088/98 | LS, atrophic, scant infiltrate | − | − | ||
| 89089/98 | LS, atrophic, scant infiltrate | +++ | − | ||
| 12 | 60 years | 123682/00 | LS, dense lichenoid infiltrate | − | − |
| 123682/00 | LS scant lichenoid infiltrate, LA | + | + | ||
| 13 | 35 years | 61892/01 | LS, dense lichenoid infiltrate | ;*>;0>† | ;*>;0>† |
| 61893/01 | LS, dense lichenoid infiltrate | ;*>;0>† | ;*>;0>† | ||
| 14 | 86 years | 38349/98 | LS, atrophic, scant infiltrate | − | − |
| 38350/98 | LS, no lichenoid infiltrate, LA | + | + | ||
| 38350/98 | LS, atrophic, scant infiltrate | − | − | ||
| 111742/98 | LS, hypertrophic, scant lichenoid infiltrate, no LA | − | + | ||
| 111742/98 | LS, hypertrophic, scant lichenoid infiltrate, no LA | − | + | ||
| 15 | 36 years | 43056/01 | LS, atrophic, scant lichenoid infiltrate | − | − |
| 16 | 70 years | 95974/00 | LS, atrophic, scant infiltrate | + | + |
| 95975/00 | LS, atrophic, scant infiltrate | + | +++ | ||
| 17 | 76 years | 75547/00 | LS, atrophic, scant infiltrate | ;*>;0>† | ++ |
| 75548/00 | LS, atrophic, scant infiltrate | ;*>;0>† | − | ||
| 18 | 87 years | 34633/00 | LS, dense lichenoid infiltrate | ;*>;0>† | + |
| 34634/00 | LS, dense lichenoid infiltrate | ;*>;0>† | ++ | ||
| 19 | 60 years | 3423/01 | LS, atrophic, scant infiltrate | − | − |
| 3424/01 | LS, atrophic, scant infiltrate | − | − | ||
| 20 | 61 years | 81076/01 | LS, hypertrophic, lichenoid infiltrate | + | − |
| 21 | 89 years | 30665/99 | LS atrophic, no lichenoid infiltrate, LA | +++ | − |
| 30666/99 | LS, atrophic, no infiltrate, LA | − | − | ||
| 22 | 61 years | 24409/00 | LS, hypertrophic, scant lichenoid infiltrate | − | − |
* Each lane represents a different paraffin-embedded tissue block.
† Poor DNA-quality.
+, One of three separately obtained and analyzed samples; ++, two of three separately obtained and analyzed samples; +++, three of three separately obtained and analyzed samples.
−, Negative; +, positive. LS, lichen sclerosus; LA, lymphocytic aggregate.
Table 3.
SCC with Lichen Sclerosus
| Patient no. | Age at diagnosis | Specimen number | Diagnosis* | γ-TCR rearrangement | HPV 16 |
|---|---|---|---|---|---|
| 23 | 62 years | 99604/98 | SCC | + | − |
| 99604/98 | SCC | − | − | ||
| 99604/98 | LS | + | − | ||
| 111985/98 | LS | +++ | − | ||
| 9377/01 | Recurrent SCC | − | − | ||
| 9377/01 | Recurrent SCC | +++ | − | ||
| 24 | 61 years | 35428/97 | LS (vag) | + | + |
| 35428/97 | LS (anal) | + | +++ | ||
| 25559/97 | SCC | + | +++ | ||
| 25 | 75 years | 3598/98 | SCC | + | − |
| 3598/98 | LS | + | − | ||
| 26 | 45 years | 13737/01 | LS | − | − |
| 13737/01 | LS | + | − | ||
| 13737/01 | SCC | − | − | ||
| 13737/01 | SCC | − | − | ||
| 27 | 74 years | 73873/97 | LS | † | † |
| 73873/97 | SCC | † | † | ||
| 73873/97 | SCC | † | † | ||
| 28 | 58 years | 78201/93 | SCC | − | − |
| 78201/93 | LS | − | − | ||
| 29 | 81 years | 76857/01 | LS | − | − |
| 76857/01 | LS | − | − | ||
| 76857/01 | SCC | − | − | ||
| 76857/01 | SCC | − | + | ||
| 30 | 75 years | 65352/95 | SCC | − | − |
| 118700/99 | LS | + | − | ||
| 118717/99 | Recurrent SCC | − | − | ||
| 108845/99 | Recurrent SCC | − | |||
| 31 | 77 years | 67013/98 | SCC | + | + |
| 67013/98 | SCC | − | − | ||
| 67013/98 | LS | + | − | ||
| 32 | 56 years | 60045/96 | SCC | + | − |
| 60045/96 | LS | ++ | + | ||
| 60045/96 | LS | − | − | ||
| 33 | 80 years | 131820/00 | SCC | − | − |
| 131820/00 | SCC | − | − | ||
| 131820/00 | LS | − | − | ||
| 34 | 87 years | 29185/99 | SCC | − | + |
| 29185/99 | SCC | ++ | − | ||
| 29185/99 | LS | +++ | − | ||
| 35 | 74 years | 77327/96 | SCC | − | − |
| 77327/96 | SCC | − | ++ | ||
| 77327/96 | LS | − | + | ||
| 77327/96 | LS | − | + | ||
| 36 | 73 years | 40416/00 | SCC | + | − |
| 40416/00 | SCC | − | − | ||
| 40416/00 | LS | − | − | ||
| 40416/00 | LS | + | − | ||
| 37 | 86 years | 40089/01 | SCC | +++ | − |
| 40090/01 | LS | − | + | ||
| 40090/01 | LS | − | − | ||
| 38 | 75 years | 21082/95 | SCC | + | − |
| 21082/95 | SCC | + | − | ||
| 21082/95 | LS | − | − | ||
| 21082/95 | LS | − | − | ||
| 39 | 78 years | 104327/99 | SCC | − | − |
| 104327/99 | SCC in-situ | − | − | ||
| 104327/99 | SCC | − | − | ||
| 40 | 62 years | 55290/97 | SCC | − | + |
| 55290/97 | SCC | − | +++ | ||
| 55290/97 | LS hypertrophic | − | + | ||
| 55290/97 | LS hypertrophic | − | +++ | ||
| 41 | 51 years | 67601/99 | SCC | − | +++ |
| 67601/99 | SCC | − | − | ||
| 67601/99 | LS | − | − | ||
| 42 | 76 years | 69181/01 | SCC | − | + |
| 69181/01 | LS | − | − | ||
| 43 | 80 years | 26194/00 | SCC | − | − |
| 26194/00 | SCC | − | − | ||
| 26194/00 | LS | − | − | ||
| 26194/00 | SCC | − | − |
See Table 2 ▶ legend for footnotes.
Table 4.
SCC Without Lichen Sclerosus
| Patient no. | Age at diagnosis | Specimen number | Diagnosis* | γ-TCR rearrangement | HPV 16 |
|---|---|---|---|---|---|
| 44 | 58 years | 13858/98 | SCC | − | +++ |
| 13858/98 | SCC | − | +++ | ||
| 45 | 84 years | 84068/98 | SCC | − | +++ |
| 84068/98 | SCC | − | +++ | ||
| 46 | 86 years | 77640/01 | biopsy, SCC | − | ++ |
| 47 | 70 years | 104792/95 | SCC | − | + |
| 104792/95 | SCC | − | − | ||
| 48 | 60 years | 20760/95 | SCC | − | +++ |
| 20760/95 | SCC | − | +++ | ||
| 49 | 53 years | 713/01 | SCC in-situ | − | +++ |
| 713/01 | SCC in-situ | − | +++ | ||
| 50 | 67 years | 90036/98 | SCC | − | − |
| 90036/98 | SCC | − | − | ||
| 90036/98 | SCC | − | − | ||
| 51 | 69 years | 90920/95 | SCC | − | ++ |
| 90920/95 | SCC | − | ++ | ||
| 90920/95 | SCC | − | ++ | ||
| 52 | 87 years | 68542/99 | SCC | − | − |
| 68542/99 | SCC | − | − | ||
| 53 | 45 years | 42751/94 | SCC | − | +++ |
| 42751/94 | SCC | − | +++ | ||
| 54 | 85 years | 107315/00 | SCC | − | +++ |
| 107315/00 | SCC | − | +++ | ||
| 55 | 77 years | 10558/00 | SCC | − | − |
| 10558/00 | SCC | − | − | ||
| 56 | 78 years | 80764/96 | SCC | − | +++ |
| 80764/96 | SCC | − | +++ | ||
| 57 | 79 years | 2353/99 | SCC | − | +++ |
| 2353/99 | SCC | − | +++ | ||
| 58 | 85 years | 64296/95 | SCC | − | − |
| 64296/95 | SCC | − | − | ||
| 64296/95 | SCC | − | − | ||
| 59 | 85 years | 94169/96 | SCC | − | − |
| 94170/96 | SCC | − | − | ||
| 60 | 45 years | 18880/99 | SCC | − | +++ |
| 18881/99 | SCC | − | + | ||
| 61 | 71 years | 10911/99 | SCC | − | +++ |
| 10911/99 | SCC | − | +++ | ||
| 62 | 37 years | 93613/97 | SCC | − | + |
| 93613/97 | SCC | − | − |
See Table 2 ▶ legend for footnotes.
Polymerase Chain Reaction (PCR) Analysis
Genomic DNA for detection of γ-TCR rearrangement and HPV16 DNA was prepared from formalin-fixed and paraffin-embedded tissue specimens essentially as described. 9 For each paraffin block we analyzed three separate samples. A 50-μm section of the tissue block was cut and immediately transferred to an Eppendorf tube that was immediately sealed. The next 50-μm section was harvested for the second sample, and a third section was obtained for the third sample. Between sections, the knife was cleaned.
γ-TCR Rearrangement Detection
The T-cell receptor γ gene was analyzed according to McCarty and colleagues 10 with minor modifications. DNA amplification was performed in the same buffer as above except that MgCl2 was 1.5 mmol/L and 1 U of Taq polymerase was used. Primer concentration was 0.5 μmol/L for primers A and B and 1 μmol/L for primer C (denaturation, 94°C for 60 seconds; annealing, 55°C for 90 seconds; extension, 72°C for 110 seconds). Forty cycles were performed with an annealing temperature of 55°C. All three separately obtained PCR products of each individual paraffin block were analyzed on 6% polyacrylamide vertical gel electrophoresis. A single band in one of three analyzed probes was interpreted as positive for γ-TCR rearrangement. Specimens were not enriched for lymphocytic infiltrates.
Detection of HPV16 DNA
A 119-bp stretch was amplified in a 50-μl reaction using primers (5′ TCA AAA GCC ACT GTC TCC TG 3′, 5′ CGT GTT CTT GAT GAT CTG CAA 3′) 0.5 μmol/L (each), 1× buffer containing Tris-Cl, KCl, (NH4)2SO4, 1.5 mmol/L MgCl2, pH 8.7, 0.8 mmol/L dNTP. Taq polymerase (2.5 U; Qiagen Hot Star) were added after an initial denaturation for 10 minutes at 95°C. Forty cycles of denaturation (95°C for 60 seconds; annealing, 55°C for 60 seconds; extension, 72°C for 120 seconds) were performed followed by a 10-minute terminal extension at 72°C. All three separately obtained PCR products harvested from each paraffin tissue block were analyzed on 3% agarose gels containing ethidium bromide (NuSieve, FMC/SeaKem, FMC 2:1). A single band in one of three lanes was interpreted as positive.
Immunohistochemistry
All 161 biopsies were examined with antibodies to CD3, CD4, CD8, CD20, CD21, CD57, perforin, granzyme B, TIA, and fascin (Table 1) ▶ using the streptavidin-biotin complex method, with previous trypsinization or microwave treatment as required. For control purposes, tissues known to contain the respective antigens were included (positive controls). Replacement of the primary antibody by normal serum always led to negative results (negative controls).
Table 1.
List of Antibodies
| Antibody | Source | Type | Comment |
|---|---|---|---|
| CD20 | Dakopatts | Monoclonal | B cells |
| CD21 | Dakopatts | Monoclonal | FDC, some B cells |
| CD3 | Dakopatts | Polyclonal | Peripheral T cells |
| MIB-1 | Dianova | Monoclonal | Proliferating cells |
| CD8 | Dakopatts | Monoclonal | Cytotoxic/suppressor T cells |
| CD4 | Ylem | Monoclonal | Helper/inducer T cells |
| CD57 | Dakopatts | Monoclonal | Natural killer cells |
| TIA-1 | Immunotech | Monoclonal | Cells with cytolytic potential |
| Perforin | Ancell | Monoclonal | Natural killer cells, cytotoxic T cells |
| Granzyme B | Alexis | Monoclonal | Natural killer cells, activated cytotoxic T cells |
| Fascin Ab-1 | NeoMarkers | Monoclonal | Actin-bundling protein |
Results
Patients with LS
A total of 50 biopsies in 22 patients with LS were analyzed (Table 2) ▶ . Histologically, early lesions of LS had a dense lichenoid infiltrate (Figure 1a) ▶ with numerous intraepidermal lymphoid cells and hyperplastic epidermis. Approximately 50% of the analyzed samples were of longstanding LS with an atrophic or hypertrophic epidermis with papillary edema and sclerosis with a scant or no lichenoid infiltrate. These biopsies, however, often showed lymphocytic aggregates (LA), either around blood vessels or hair appendages in the deep submucosa of deeply extending biopsies (Figure 2, a and b) ▶ . Analysis of γ-TCR rearrangement was technically impossible in 10 biopsies of five patients because of poor DNA quality. A single biopsy only was available in four patients, one of which showed monoclonal γ-TCR rearrangement. Eight of 13 patients with multiple biopsies showed monoclonal T cells in at least one of their analyzed biopsies (Figure 3a ▶ , patient no. 12). Intralesional and interlesional heterogeneity was pronounced, best illustrated in a patient with only one of six analyzed paraffin blocks demonstrating monoclonally rearranged γ-TCR (patient no. 11). Only two patients (patients no. 9 and no. 16) had monoclonal γ-TCR rearrangement in all analyzed samples of their biopsies. HPV16 DNA was demonstrated in 8 of 22 patients in 19 of 50 biopsies, but only in 29 of the 150 separately analyzed samples. However, not a single one of these biopsies displayed morphological evidence of HPV infection.
Figure 1.
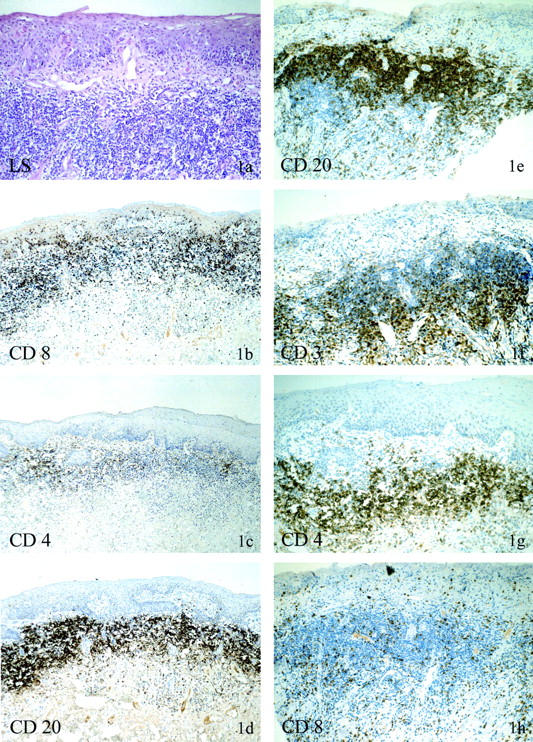
Immunohistochemical phenotype of LS with dense lichenoid infiltrate with and without monoclonal γ-TCR rearrangement. a–d: Patient no. 12 (123682/00), LS without monoclonal γ-TCR rearrangement. The H&E stain shows vulvar skin with parakeratosis, focal basement membrane thickening with dermal sclerosis, and ectatic venules. The lymphocytic infiltrate is predominantly dermal, dense, and band-like (a) with scant interface infiltrate. The majority of the lymphocytes are CD8-positive. Note the intraepidermal presence of CD8-positive lymphocytes (b). CD4-positive lymphocytes are sprinkled with the dermal infiltrate or occur in small clusters or individually (c). Within the dense dermal infiltrates are numerous CD20-positive B cells (d). e–h: Patient no. 34 (29185/99), LS with monoclonal γ-TCR rearrangement. CD20-positive B cells within the dermal infiltrate are also a feature of LS with monoclonal γ-TCR rearrangement (e). The B cells are located in the superficial aspects of the submucosa, whereas the T-cell population (CD3, f) is localized in the deeper aspects, creating a zonation effect. Within the infiltrate CD4-positive T cells (g) dominate over CD8-positive T cells (h), quite in contrast to the infiltrate without monoclonal γ-TCR rearrangement.
Figure 2.
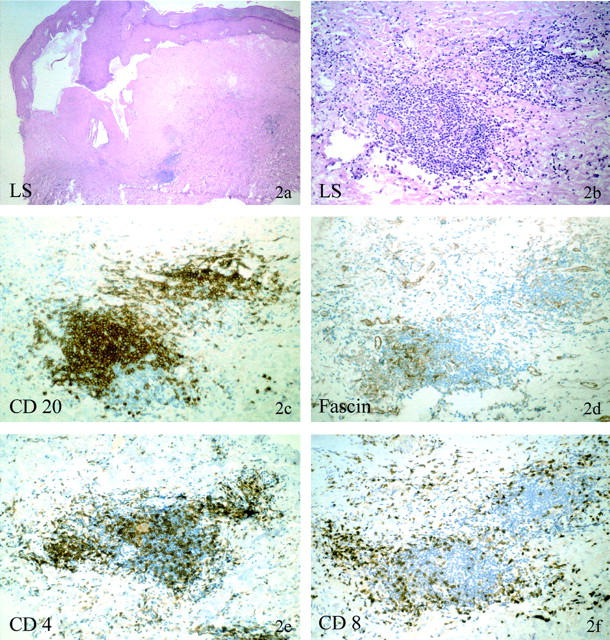
Immunohistochemical phenotype of deep dermal/submucosal lymphocytic aggregates in atrophic LS with monoclonal γ-TCR rearrangement. Patient no. 21 (30665/99). The H&E stain shows a late stage of LS with massive hyperkeratosis and focal acanthotic epidermis. The basement membrane is massively thickened, the papillary dermis highly sclerotic and avascular with prominent clefting along the dermoepidermal junction. No lichenoid dermal or interface infiltrate is present; however, beneath the sclerosis several lymphocytic cell aggregates are present (a and b). These aggregates are composed of predominately CD20-positive B cells (c), fascin-positive dendritic cells (d), and CD4-positive T cells (e). CD4-positive T cells dominate over CD8-positive T cells (f), which are not found within the B-cell fraction but at the periphery of these lymphocytic aggregates.
Figure 3.
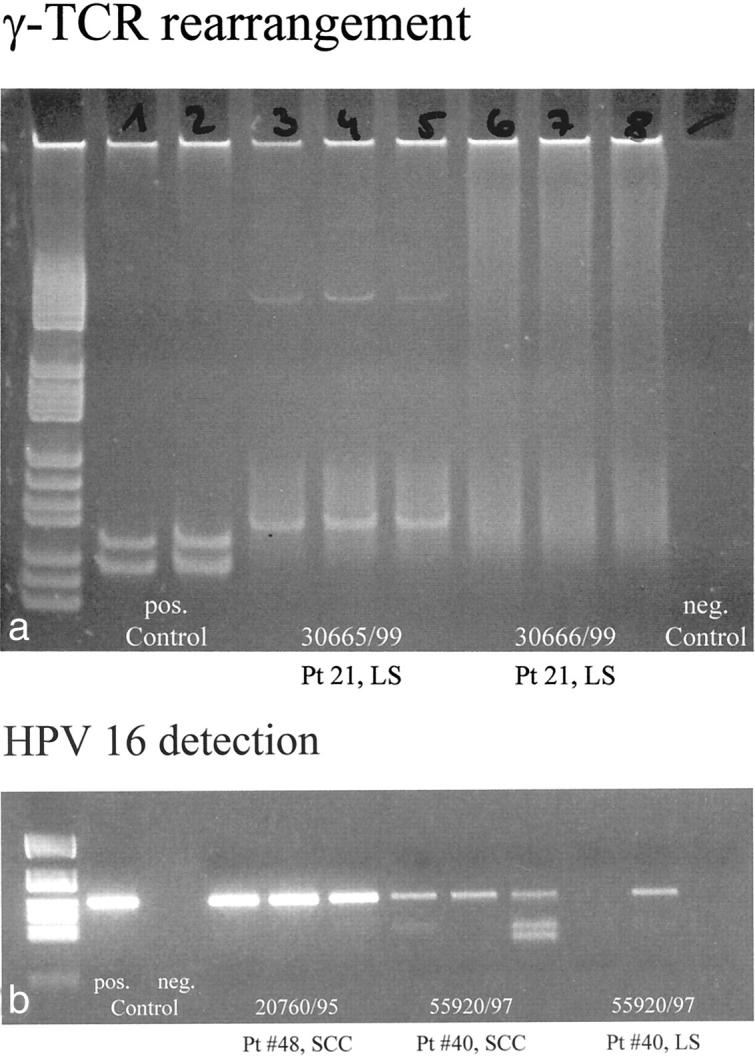
PCR analysis of the lymphoid infiltrate of LS for γ-TCR rearrangement. a: Gel electrophoretic analysis with positive controls in lanes 1 and 2, and negative controls in lane 9. Lanes 3 to 5 represent PCR products of three different areas of a biopsy of LS (patient no. 21, 30665/99) of an 89-year-old female that show a single band in all three lanes corresponding to monoclonal rearrangement of the γ-TCR. The same analysis (lanes 6 to 8) of a different lesion biopsied in the same patient (patient no. 21, 30666/99) at the same time shows no bands, indicating the absence of monoclonal γ-TCR rearrangement. PCR analysis of LS for the presence of HPV16 DNA. b: The detection of HPV16 DNA on an ethidium bromide gel with positive and negative controls in lanes 1 and 2. Lanes 3 to 5 are a typical example of detection of strong bands of HPV16 DNA in all three samples obtained from a single paraffin block (patient no. 48, SCC without LS). In another patient with SCC arising in LS (patient no. 40), weaker bands of HPV16 DNA are visualized in all three samples of the analyzed block containing SCC in lanes 6 to 8. Lanes 9 to 11 illustrate the typical finding of HPV16 DNA in LS as a weak band in just one of three samples of an analyzed biopsy of LS (patient no. 40).
On hematoxylin and eosin-stained sections, no differences between biopsies with and without monoclonally rearranged γ-TCR were recognizable. Immunohistochemically, the intraepidermal lymphocytes of all biopsies were CD3-, CD8-, CD57-, TIA-, and granzyme B-positive. CD4-, CD20-, fascin-, and perforin-positive lymphocytes were not identified within the epidermis. The lichenoid dermal band-like lymphohistiocytic infiltrate of LS with germline configuration was uniformly CD3-positive with a CD8 predominance over CD4 (Figure 1 ▶ ; a to c, patient no.12). A unique finding was the demonstration of numerous B cells within the band-like lichenoid infiltrate in biopsies with a well-developed lichenoid infiltrate (Figure 1d) ▶ . In biopsies with monoclonally rearranged γ-TCR and a dense lichenoid infiltrate, however, CD20-positive B cells were bordered by a broad band-like infiltrate of CD3-positive T cells (Figure 1, e and f ▶ , patient no. 34) creating a zonation effect. In these infiltrates, CD4 expression dominated over CD8 (Figure 1, g and h) ▶ . Granzyme B- and TIA-positive T cells were numerous. Perforin expression was not observed. CD57-positive cells were located in the epidermis and dermis alike. Fascin-positive dendritic cells were not identified within the lichenoid infiltrate.
Biopsies of longstanding LS with atrophic epidermis and basement membrane homogenization showed only scant or no lichenoid infiltrate, but deeply located submucosal LA (Figure 2, a and b) ▶ . Differences between concomitant biopsies of atrophic LS of the same patient with and without monoclonally rearranged γ-TCR were observed within these LAs. In biopsies with monoclonally rearranged γ-TCR, these LAs were predominantly positive for CD20 (Figure 2c ▶ , patient no.21). Numerous fascin-positive dendritic cells created an irregular meshwork within the B-cell population (Figure 2d) ▶ . Fascin also cross-reacted with endothelial cells. Numerous CD4-positive T cells were found within the B-cell aggregates (Figure 2e) ▶ with dominance over CD8-positive T cells, which were identified only in the peripheral zones of the LAs (Figure 2f) ▶ along with CD57, and granzyme-positive cells. In contrast, LA in biopsies with germline configuration of the same patient (Figure 4, a and b) ▶ , had only single peripherally located B cells (Figure 4c) ▶ , CD57, and fascin-positive lymphocytes. The majority of lymphocytes within these LAs were T cells with the typical CD8 dominance over CD4 (Figure 4, d and e) ▶ . Granzyme B and TIA were expressed, but perforin staining was absent.
Figure 4.
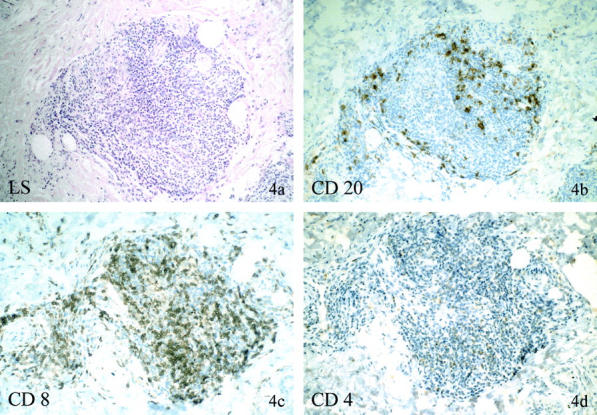
Immunohistochemical phenotype of deep dermal/submucosal lymphocytic aggregates in atrophic LS without monoclonal γ-TCR rearrangement. Patient no. 21 (30666/99) is the same patient as in Figure 2, a ▶ separate plaque of LS with histological identical findings as in Figure 2a ▶ . The lymphocytic cell aggregates (a) in this biopsy without γ-TCR rearrangement show only individual peripherally located CD20-positive B cells (b), but numerous CD8-positive T cells (c), and minor amounts of CD4-positive T cells (d), maintaining the usual CD8 dominance over CD4 of biopsies with germline mutations.
Patients with SCC Arising with LS
A total of 71 separate samples were analyzed in 21 patients with SCC arising in LS (Table 3) ▶ . All patients had a highly differentiated, keratinized SCC with a prominent exophytic component. Invasion was mostly superficial. The adjacent LS was predominantly of the classical type with epidermal atrophy, basal keratinocyte destruction, basement membrane homogenization and dermal edema with rarefaction of vessels, and a band-like lymphocytic infiltrate and occasional LA. Poor DNA quality did not allow analysis in one patient. A monoclonal γ-TCR rearrangement was observed in 11 of the remaining 20 patients, who showed monoclonal γ-TCR rearrangement in at least one of the analyzed samples. The intersample and intrasample heterogeneity was again pronounced (for details, see Table 3 ▶ ). Recurrent SCC, available in two patients (3 and 4 years after primary excision, respectively) also demonstrated monoclonal γ-TCR rearrangement with intralesional heterogeneity. Nine of 20 patients had no monoclonal γ-TCR rearrangement in all analyzed samples of SCC and histologically classical LS. HPV16 DNA was detected in a single biopsy only in 11 of 21 patients, mostly as a weak band in one of three lanes without morphological evidence of HPV infection (Figure 3b ▶ ; patient no. 34). Strong HPV16 DNA detection was observed in only two patients in all four analyzed biopsies of SCC and LS. The lymphoid infiltrate in LS with and without monoclonal γ-TCR rearrangement was of identical immunophenotype as described above. The tumor-infiltrating lymphocytes of the invasive SCC were a mixture of B cells and T cells.
Patients with SCC Unrelated to LS
None of the 40 samples of 19 patients with SCC unrelated to LS showed a monoclonal γ-TCR rearrangement (Table 4) ▶ . Eighteen patients had poorly differentiated, widely invasive SCC with scant keratinization, significant nuclear pleomorphism, high mitotic activity, and tumor-infiltrating lymphocytes. One patient had extensive SCC in situ with prominent HPV-related cell changes with a dense band-like lymphocytic lichenoid infiltrate, but no lymphocytic aggregates. Mostly strong bands of HPV16 DNA were detected in 14 of 19 patients (Figure 2b ▶ , patient no. 48). In five patients, all analyzed biopsies were negative for HPV16 DNA. The tumor-infiltrating lymphocytes were identified at the invasion front of the SCC, underscoring the cancerous tissue. Occasionally a lymphoid infiltrate was identified lateral to the invasive SCC underscoring the adjacent SCC in situ component or intraepithelial neoplasia. The infiltrate consisted of a mixture of CD4- and CD8-positive T lymphocytes and with interspersed individual CD20-positive B cells. T cells with perforin-containing cytotoxic granula were not identified in any of the examined biopsies.
Discussion
Monoclonal γ-TCR-rearrangement within the lymphoid infiltrate occurs not only in biopsies of vulvar LS but also in vulvar SCC arising in LS, sharply contrasting the complete lack of monoclonally rearranged γ-TCR in SCC unassociated with LS. We observed a pronounced intralesional heterogeneity when multiple concomitant biopsies of a single lesion were analyzed. Repeated PCR analysis with identical results, however, excluded the detection of pseudo-monoclonality. The sensitivity of the PCR method allows detection of at least 1% of clonal T cells on a T-cell background, even within low-density inflammatory infiltrates. On one hand, the heterogeneity may be a result of inclusion of atrophic and longstanding LS, which often had a rather scant lichenoid infiltrate, that probably was not sufficient for detection of monoclonal T cells. On the other hand, we know that the observed monoclonal γ-TCR rearrangement is a true focal event, although we cannot give a clear estimate of the extent of clonal outgrowth based on the used methods. In this study, the majority of biopsies/excisions had several different tissue blocks analyzed, and of each of these blocks three separately harvested individual tissue samples were analyzed electrophoretically. Therefore, we analyzed up to nine different areas within a single lesion of LS, SCC arising in LS, and SCC without LS. In the majority of analyzed blocks, the monoclonal γ-TCR rearrangement was detected in only one of the analyzed three probes. We therefore believe that such focal detection of monoclonal γ-TCR rearrangement is in support of a single clone buried and proliferating within a mixed-cell infiltrate as opposed to a primary lymphoproliferative disorder. Despite the heterogeneity, monoclonal γ-TCR rearrangement seems to be specific to LS and SCC arising in LS, although it is presently unclear at which time point in the evolution of LS and SCC arising in LS the T-cell clones emerge. Clonal T cells in skin diseases are rare. They occur in cutaneous lymphomas, eg, mycosis fungoides (inclusive parapsoriasis en plaque), and in lymphomatous skin lesions such as pityriasis lichenoides varioliformis acuta and lymphomatoid papulosis, 11-13 but among the classic inflammatory skin diseases, such as psoriasis, lichen planus, allergic and contact dermatitis, clonal T cells have been demonstrated only exceptionally. 2 Restricted TCR usage may reflect prolonged exposure of the host immune system to a local putative LS-associated antigen. Antigen-driven selection of cytotoxic T cells suggests a relationship to autoimmunediseases. The putative antigen seems unrelated tomalignant tumor cells as SCC without LS show no monoclonal γ-TCR rearrangement and SCC arising in LS demonstrates a high degree of intralesional heterogeneity. We found only one previous publication reporting a clonal expansion of T cells in carcinomas (six mucosal oropharyngeal SCCs); this study, however, postulated an immune reaction against tumor cell antigens. 14
Interestingly, on a light microscopic level, biopsies with and without monoclonal γ-TCR rearrangement were indistinguishable. The classical histology of basal keratinocyte destruction, basement membrane homogenization, and dermal edema with rarefaction of vessels can be explained through the action of TIA and granzyme B, which were identified in all biopsies. Perforin, another granular component of cytotoxic and natural killer T cells, capable of inducing epidermal injury 15 was not demonstrated. We are the first to report the presence of significant numbers of B cells in the lichenoid infiltrate (either band-like or in clusters) and in deeply located LA of vulvar LS and SCC. This supports an earlier description of occasional small numbers of B cells in LA of pediatric penile LS, 16 but contradicts Scrimin and colleagues, 17 who could not find any B cells in the lymphoid infiltrate of LS. These obvious discrepancies may be related to biopsy techniques, eg, only very superficial biopsies were analyzed. Alternatively, differences/problems with immunohistochemical techniques and antibody choices may account for the reported lack of B cells in LS. Biopsies with germline mutations showed the usual CD8 dominance over CD4 in both the lichenoid infiltrate and LA. In biopsies with monoclonally rearranged γ-TCR, however, we observed the reversal of the usual CD8 dominance, with CD4-positive T cells being the predominant phenotype. Interestingly, the CD4-positive T cells were closely associated with the B cells. Another unusual finding was the presence of fascin-positive dendritic cells. Fascin-expressing dendritic cells formed an irregular mesh work in the lymphocytic aggregates within the B cell and CD4-positive T-cell fraction in LS and SCC/LS. Fascin, a 55-kd actin-bundling protein associated with cell motility, is involved in antigen presentation 18 and has been demonstrated in human follicular dendritic cells, which are the antigen-presenting cells of germinal centers. 19 A principal feature of dendritic cells located within the peripheral tissues is antigen capture of foreign antigen and subsequent initiation of immune responses. It is generally accepted that CD4-positive T cells play a major role in the initial activation of resting naive B cells, and that B-cell activation occurs within the T-cell areas of secondary lymphoid tissues. Antigen-specific-activated CD4-positive cells stimulate antigen-specific naive B cells to proliferate and differentiate into germinal center founder cells. It has also recently been suggested that interdigitating dendritic cells act as a matrix on which antigen-specific T cells and B cells interact efficiently 20 and that they are directly involved in regulating T-cell-mediated humoral immune responses in humans. 21 In summary, monoclonally rearranged γ-TCR and the typical immunohistochemical profile (CD20, CD4 dominance, fascin-positive dendritic cells) in biopsies of LS and SCC arising in LS are best interpreted as a local antigen-dependent immune reaction, raising again the question for a LS-associated antigen.
HPV16 is certainly the major risk factor for SCC unassociated with LS. For SCC with LS, HPV16 seems to play a minor role as carcinogen; the occasional demonstration of weak bands of HPV16 DNA in individual samples of a biopsy is of uncertain clinical significance and may reflect HPV exposure in the distant past without HPV-related oncogenesis. Inadequate local host response secondary to proliferation of selected T-cell clones in LS may facilitate the development of the typically highly differentiated keratinized SCC in longstanding LS. In the absence of HPV16 oncogenes as well as monoclonal γ-TCR rearrangement in 10 of our 42 patients with vulvar SCC (six SCCs arising in LS, and four SCCs unrelated to LS), however, genetic alterations independent of HPV16 DNA or immunosuppression need to be further investigated.
In summary, our data show that monoclonal γ-TCR rearrangement of the lymphoid infiltrate is a characteristic feature of vulvar LS and vulvar SCC arising in LS, but not of SCC unassociated with LS. T-cell clones in such a high percentage of patients raise the question for a LS-associated antigen. The autoimmune hypothesis is further supported by the occurrence of B cells, CD4 dominance over CD8, and fascin-positive dendritic cells within LA, which represent a specific local immune reaction to an antigen or to proliferating T-cell clones. The resulting immune dysregulation may create a permissive environment for the development of a SCC in LS. It remains unclear what or which antigen drives the immune system toward the observed immune reaction and to what extent monoclonal γ-TCR rearrangement contributes to the carcinogenesis of vulvar SCC arising in LS.
Acknowledgments
We thank the technical staff of the Hematopathology Laboratory, the staff of the Diagnostic Molecular Laboratory of the Institute of Pathology, the Histological Laboratory of the Department of Gynecology and Obstetrics for excellent technical support, and Mr. R. Staber and Ms. K. Wagner for photographic help.
Footnotes
Address reprint requests to Sigrid Regauer, M.D., Institute of Pathology, University of Graz, Auenbruggerplatz 25, 8036 Graz, Austria. E-mail: sigrid.regauer@@kfunigraz.ac.at.
Supported by the Austrian Cancer Aid/Styria (project number 04/2001).
References
- 1.Powell J, Wojnarowska F: Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol 2001, 44:803-806 [DOI] [PubMed] [Google Scholar]
- 2.Lukowsky A, Muche JM, Sterry W, Audring H: Detection of expanded T cell clones in skin biopsy samples of patients with lichen sclerosus et atrophicus by T cell receptor-gamma polymerase chain reaction assays. J Invest Dermatol 2000, 115:254-259 [DOI] [PubMed] [Google Scholar]
- 3.Meyrick Thomas RH, Ridley CM, McGibbon DH, Black MM: Lichen sclerosus et atrophicus and autoimmunity—a study of 350 women. Br J Dermatol 1988, 118:41–46 [DOI] [PubMed]
- 4.Carlson JA, Lamb P, Malfetano J, Ambros RA, Mihm MC: Clinicopathologic comparison of vulvar and extragenital lichen sclerosus: histologic variants, evolving lesions, and etiology of 141 cases. Mod Pathol 1998, 11:844-854 [PubMed] [Google Scholar]
- 5.Schempp C, Bocklage H, Lange R, Kolmel HW, Rfanos CE, Gollnick H: Further evidence for Borrelia burgdorferi infection in morphea and lichen sclerosus et atrophicus confirmed by DNA amplification. J Invest Dermatol 1993, 100:717-720 [DOI] [PubMed] [Google Scholar]
- 6.Carli P, Cattaneo A, Magnis AD, Biggeri A, Taddei G, Giannotti B: Squamous cell carcinoma arising in vulval lichen sclerosus: a longitudinal cohort study. Eur J Cancer Prevention 1995, 4:491-495 [DOI] [PubMed] [Google Scholar]
- 7.Crum CP, McLachlin CM, Tate JE, Mutter GL: Pathobiology of vulvar squamous neoplasia. Curr Opin Obstet Gynecol 1997, 9:63-69 [PubMed] [Google Scholar]
- 8.Scurry J: Does lichen sclerosus play a central role in the pathogenesis of human papillomavirus negative vulvar squamous cell carcinoma? The itch-scratch-lichen sclerosus hypothesis. Int J Gynecol Cancer 1999, 9:89-97 [DOI] [PubMed] [Google Scholar]
- 9.Popper H, Winter E, Hoefler G: DNA sequences of mycobacterium tuberculosis in formalin fixed and paraffin embedded tissue in tuberculosis and sarcoidosis detected by PCR. Am J Clin Pathol 1994, 101:738-741 [DOI] [PubMed] [Google Scholar]
- 10.McCarty KP, Sloane J, Kabarowski J, Matutes E, Wiedemann L: A simplified method of detection of clonal rearrangements of the T-cell receptor-gamma chain gene. Diagn Mol Pathol 1992, 1:173-179 [PubMed] [Google Scholar]
- 11.Staib G, Sterry W: Use of polymerase chain reaction in the detection of clones in lymphoproliferative diseases of the skin. Recent Results Cancer Res 1995, 139:239-247 [DOI] [PubMed] [Google Scholar]
- 12.Bergman R: How useful are T-cell receptor gene rearrangement studies as an adjunct to the histopathologic diagnosis of mycosis fungoides? Am J Dermatopathol 1999, 21:498-502 [DOI] [PubMed] [Google Scholar]
- 13.Muche JM, Lukowsky A, Asadullah K, Gellrich S, Sterry W: Demonstration of frequent occurrence of clonal T cells in the peripheral blood of patients with primary cutaneous T-cell lymphoma. Blood 1997, 90:1636-1642 [PubMed] [Google Scholar]
- 14.Caignard A, Dietrich PY, Morand V, Lim A, Pannetier C, Leridant AM, Hercend T, Even J, Kourilsky P, Triebel F: Evidence for T-cell clonal expansion in a patient with squamous cell carcinoma of the head and neck. Cancer Res 1994, 54:1292-1297 [PubMed] [Google Scholar]
- 15.Tapinos NI, Polihronis M, Tzioufas AG, Moutsopoulus HM: Sjögren’s syndrome. Autoimmune epithelitis. Adv Exp Med Biol 1999, 455:127-134 [PubMed] [Google Scholar]
- 16.Hinchliffe SA, Ciftci AO, Khine MM, Rickwood AM, Ashwood J, McGill F, Clapham EM, Velzen DV: Composition of the inflammatory infiltrate in pediatric penile lichen sclerosus et atrophicus (balanitis xerotica obliterans): a prospective, comparative immunophenotyping study. Pediatr Pathol 1994, 14:223-233 [DOI] [PubMed] [Google Scholar]
- 17.Scrimin F, Rustja S, Radillo O, Volpe C, Abrami R, Guaschino S: Vulvar lichen sclerosus: an immunologic study. Obstet Gynecol 2000, 95:147-150 [DOI] [PubMed] [Google Scholar]
- 18.Al-Alwan MM, Rowden G, Lee TD, West KA: Fascin is involved in the antigen presentation activity of mature dendritic cells. J Immunol 2001, 166:338-345 [DOI] [PubMed] [Google Scholar]
- 19.Pinkus GS, Pinkus JL, Langhoff E, Matsumura F, Yamshiro S, Mosialos G, Said JW: Fascin, a sensitive new marker for Reed-Sternberg cells of Hodgkin’s disease. Evidence for a dendritic or B-cell derivation? Am J Pathol 1997, 150:543-562 [PMC free article] [PubMed] [Google Scholar]
- 20.Kushnir N, Wykes M: Dendritic cells, B cells and the regulation of antibody synthesis. Immunol Rev 1999, 172:325-334 [DOI] [PubMed] [Google Scholar]
- 21.Feyette J, Dubois B, Vandenabeele S, Bridon JM, Vanbervliet B, Durand I, Bandereau J, Caux C, Briere F: Human dendritic cells skew switching of CD40-activated naive B-cells towards IgA1 and IgA2. J Exp Med 1997, 185:1909-1918 [DOI] [PMC free article] [PubMed] [Google Scholar]


