Abstract
Most spontaneously developing hyperplastic and neoplastic lesions of the pituitary occur in the anterior pituitary. Targeted disruption of various cell-cycle proteins, including Rb, p27kip1 (p27), and p18INK4c (p18), is associated with intermediate lobe pituitary hyperplasia. To develop a model of anterior pituitary proliferation to study the pathogenesis of pituitary tumors, we crossed the glycoprotein hormone α-subunit (αSU)-null mice that develop thyroid-stimulating hormone (TSH) cell hyperplasia with p18-null mice. The resulting offsprings developed accelerated enlargement of the anterior lobe with predominantly TSH cell hyperplasia. Immunohistochemical and histological analyses of these mice along with p27/p18 double-null mice, p18-null mice, and p27-null mice showed evidence of TSH, adrenocorticotropic hormone, prolactin, and luteinizing hormone hyperplasia. To determine whether there were alterations of p27 and the target proteins implicated in the ubiquitin degradation of p27 and other cyclin-dependent kinase inhibitors, we examined expression of SKP 2, Grb 2, and Jab 1 in the pituitaries of null mice. In the αSU-null mice there were decreased levels of SKP 2 and elevated levels of Grb 2 expression by Western blot analysis. Immunohistochemical analysis of the pituitary showed elevated Grb 2 in αSU-null and p18/αSU double-null mice. Jab 1 levels were not different from controls in the pituitary. These results show that 1) the p18/αSU double-null mice represent a good model to study the rapid development of anterior pituitary hyperplasia, and 2) various proteins important in p27 and other cyclin-dependent kinase inhibitor protein degradation are altered in the pituitary of αSU-null and p18/αSU double-null mouse models.
The molecular changes leading to the pathogenesis of anterior pituitary tumors are primarily unknown. Recent studies with targeted disruption of cell-cycle genes such as retinoblastoma (Rb), p27kip1 (p27), and 18INK4C (p18) have provided some insights into the role of cell-cycle proteins in the development of pituitary tumors. 1-8 Most of these hyperplastic pituitaries in Rb-, p27-, and p18-null mice develop in the intermediate lobe, so these are not good models to study anterior pituitary tumor development, which is where most of these tumors develop spontaneously in rodents and humans. Single knockout of the α-subunit gene 9 and transgenic mice expressing the growth hormone-releasing hormone with a metalloproteinase-driven promotor crossed with p27-null mice 10 have also been used to study anterior pituitary hyperplasia. 9,10
The levels of p27 proteins are decreased in many human cancers compared to normal tissues and have prognostic significance, suggesting that p27 may be a tumor suppressor gene. However, there are few mutations in the p27 gene and the mRNA levels are relatively unchanged compared to the decreased levels of p27 protein in tumors. 11,12 These observations suggest that the proteins regulating posttranslational degradation of p27 may be potential targets to explain the mechanism of down-regulation of p27 and other cyclin-dependent kinase inhibitory (CDKI) cell-cycle genes during tumor development.
Although it has been shown that the ubiquitin-proteasome system 13,14 regulated short-lived CKDI proteins such as p27, the role of various proteins in the degradation are currently being investigated. 15 In studies with p27, the jun-activated protein Jab 1, 16 various F-box proteins including SKP 2 17-20 and the signal-transducing adaptor protein Grb 2 21,22 have been shown to have a regulatory roles in p27 degradation.
In this study, we targeted pituitary hyperplasia to the anterior pituitary of p18-deficient mice by creating double-null animals with loss of the p18 and α-subunit genes. These mice as well as p27-null and p18/p27 double-null mice were used to examine expression of some of the major proteins that play a role in p27 and other CDKI ubiquitin-mediated degradation of CDKIs.
Materials and Methods
Mice
The p27 mice with a C57BL/6 background (a gift from Dr. M. L. Fero and J. L. Roberts, Fred Hutchinson Cancer Center, Seattle, WA), the p18 mice with a C57BL/6 background (a gift from Drs. D. S. Franklin and Y. Xiang, University of North Carolina, Chapel Hill, NC), and the α-subunit of glycoprotein hormones (αSU) mice had a background of C57BL/6J (a gift from Dr. S. A. Camper, University of Michigan, Ann Arbor, MI) were all maintained in a specialized mouse barrier facility at the Mayo Clinic.
F2 mice heterozygous for p27, p18, or αSU were generated from F1 mice in each group. Mice were genotyped and the resulting F1 mice were intercrossed to created double-null animals. The p18/αSU double-null mice were derived from crossing p18-null mice with αSU heterozygous mice. The p27/p18 double-null mice were derived from crossing p27 and p18 heterozygous mice. All animals were mainly B6 in their genetic background. Genotyping was done by polymerase chain reaction (PCR).
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
PCR Genotyping
Tail snips were taken from all mice at 4 weeks of age and genomic DNA extracted for genotyping. The PCR reactions contained 1.25 U of Taq polymerase, 1× PCR buffer, 1.5 of mmol/L magnesium chloride (Promega, Madison, WI), 100 ng of each primer, and 1 [μ]l of genomic DNA in a total volume of 25 [μ]l unless otherwise specified. All PCR products were resolved on a 2% agarose gel stained with ethidium bromide.
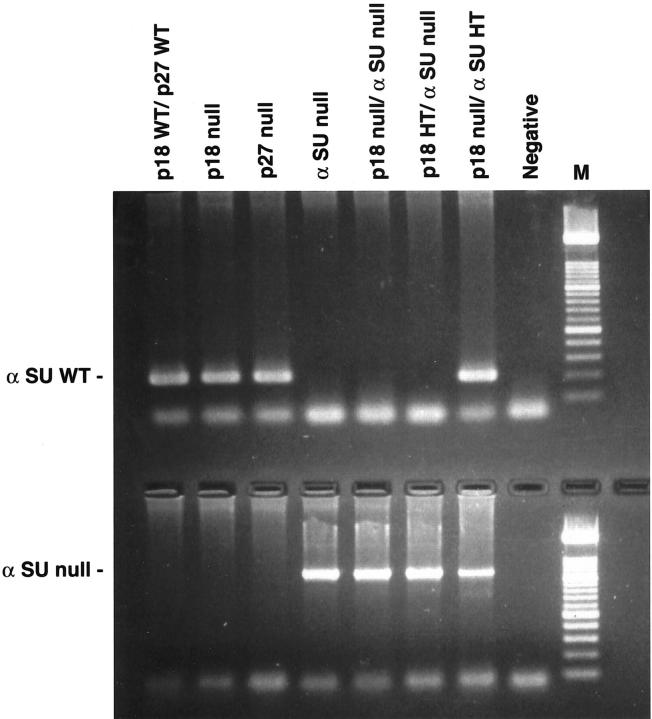
Two sets of primers were used to amplify the intact α-subunit wild-type allele and the disrupted nonfunctional allele. The wild-type sense primer (5′-GCA TAT CCC ACT CCC GCC AGG) located within exon 3 was used in conjunction with an antisense primer (5′-CAA TTA AAG AGG ATA AAT GCA GGT GTC GCC) within intron 3 resulting in a 220-bp product. Detection of the disrupted or null allele used a sense primer (5′-TGC CTT TTG TAT TAT CAG GGT ACC TAG ACT) and an antisense primer located within the neo gene (5′-CGC CTT CTA TCG CCT TCT TGA CGA GTT CTT) resulting in a 1.1-kb product. 23 Twice the amount of Taq polymerase was used for the null allele PCR (2.5 U). The α-subunit-null mice and the p18/αSU double-null mice required genetic sexing because of hypogonadism and hypothyroidism. Males were identified through PCR detection of the sry gene on the Y chromosome. 24 The sense primer (5′-GTC TAG AGA GCA TGG ACGG GCC) and antisense primer (5′-ACA GGT GTG CAG CTC TAC TCC) amplified a 381-bp product in males only. 25 PCR for the wild-type and null alleles of the α-subunit gene as well as the sex-determining PCR shared the same PCR profile: 95°C for 5 minutes; 30 cycles at 94°C for 1 minute, 58°C for 1 minute, and 72°C for 2 minutes followed by 10 minutes of final extension at 72°C.
Detection of p27 wild-type allele and p27-null allele used one common sense primer (5′-TGG AAC CCT GTG CCA TCT CTA T). The antisense primers used were specific for the p27 wild-type allele (5′-GAG CAG ACG CCC AAG AAG C) and the neo-disrupted allele (5′-CCT TCT ATG GCC TTC TTG ACG). PCR was performed for 40 cycles with an annealing temperature of 57°C. The wild-type allele resulted in a 1300-bp product and the null allele in a 600-bp product. 6
A sense primer for the p18 gene (5′-AGC CAT CAA ATT TAT TCA TGT TGC AGG) was used in combination with the wild-type antisense primer (5′-CCT CCA TCA GGC TAA TGA CC) and null antisense primer (5′-CCA GCC TCT GAG CCC AGA AAG CGA AGG). PCR amplification was performed at an annealing temperature of 60°C for 35 cycles and produced a 600-bp wild-type amplicon or a 400-bp null amplicon. 7
Anatomical and Histological Analysis
Animals were monitored daily and sacrificed at preplanned intervals or when they showed signs of morbidity. Body weight and specific organ weights were obtained at the time of sacrifice and genotyping was rechecked in some cases. Portions of specific tissues, including pituitaries and liver, were frozen in liquid nitrogen, stored at −70°C, and used for protein analysis and other studies.
For histological analysis tissues were fixed in 10% buffered formalin, dehydrated in increasing concentrations of ethanol, cleared in xylene, and embedded in paraffin blocks. Hematoxylin and eosin (H&E) stains were performed on all blocks and other sections were used for immunohistochemical analysis. Mitotic figures in the pituitary were counted in 50 fields at a final magnification of ×400.
Antibodies and Immunohistochemistry
Antibodies for prolactin (PRL) (used at 1/1000), growth hormone (GH) (used at 1/1000), thyroid stimulating hormone (TSH) (used at 1/500), and luteinizing hormone (LH) (used at 1/500) were obtained from the National Pituitary Distribution Agency, Bethesda, MD. Adrenocorticotropic hormone (ACTH) (used at 1/500) was obtained from DAKO (Santa Barbara, CA).
Antibodies to p27 (1/1000; Transduction Laboratories, Lexington, KY), SKP 2 (1/100; Santa Cruz Inc., Santa Cruz, CA), Jab 1 (1/500, Santa Cruz, Inc.), and Grb 2 (1/500; Santa Cruz, Inc.) were used for immunohistochemistry. Immunohistochemical staining was done as previously reported 23,24 with antigen retrieval used for p27, SKP 2, Jab 1, and Grb 2. Antigen retrieval was performed in an 800-W microwave oven using 0.1 mol/L of citrate buffer, pH 6.0, for 5 minutes followed by 20 minutes of cooling at room temperature. Immunohistochemical staining was done by incubation with the primary antibody overnight and the reaction product was developed with diaminobenzidine. 26,27 The avidin-biotin complex peroxidase kits were obtained from Vector Laboratories (Burlingame, CA). Semiquantitation of immunohistochemical staining was done by grading the nuclear immunoreactivity as 0, negative; +, rare or <5% cells positive; ++, focal or <25% cells positive; and +++, diffuse or >25% of cells positive. Four to five pituitaries were analyzed for each group.
Western Blotting
Western blots were done as previously reported. 27,28 Briefly, one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis was done and the electrophoresed proteins subsequently transferred to a polyvinylidene difluoride membrane (Bio-Rad, Richmond, CA) and subjected to immunoblot analysis for p27 (1/1000 dilution), SKP 2 (1/400), Jab 1 (1/500), Grb 2 (1/250), and β-actin (1/1000; Sigma Chemical Co., St. Louis, MO). The reaction product was detected with enhanced chemiluminescence (Amersham, Arlington Heights, IL). β-Actin was used to normalize for equal loading of the gels. Densitometric analysis of the bands was done with a Fluor-S multimager (Bio-Rad, Hercules, CA). Results were expressed as relative densitometry units, and all data were normalized with β-actin. A linear response was obtained using different amounts of proteins on the gel. Each experiment was repeated two to three times.
Statistical Analysis
Results were expressed as the mean ± SEM. Statistical analysis was done with the Wilcoxon rank sum test.
Results
Analysis of Null Mice
Genotype analyses by PCR were used to characterize the mice resulting from the various crosses (Figure 1) ▶ . Null mice that were deficient in the αSU gene developed anterior pituitary hyperplasia in a time-dependent manner as previously reported. 25,29 The p18- and p27-null mice developed enlarged intermediate lobes in a time-dependent manner. Animals sacrificed at 1 year of age from the αSU-null group had pituitaries weighing 73 ± 20 mg. The average weights of p18- and p27-null mice pituitaries were 6.1 ± 1.5 mg and 12.8 ± 5.4 mg, respectively.
Figure 1.
PCR analyses of α-glycoprotein hormone subunit wild-type αSU (top) and null (bottom) alleles. The αSU wild-type allele results in a 0.22-kb product and the null allele results in a 1.1-kb product. Lanes, from left to right: wild type, p18-null, p27-null, αSU-null, p18/αSU double-null, p18HT/αSU-null, p18-null/αSU HT, and negative control.
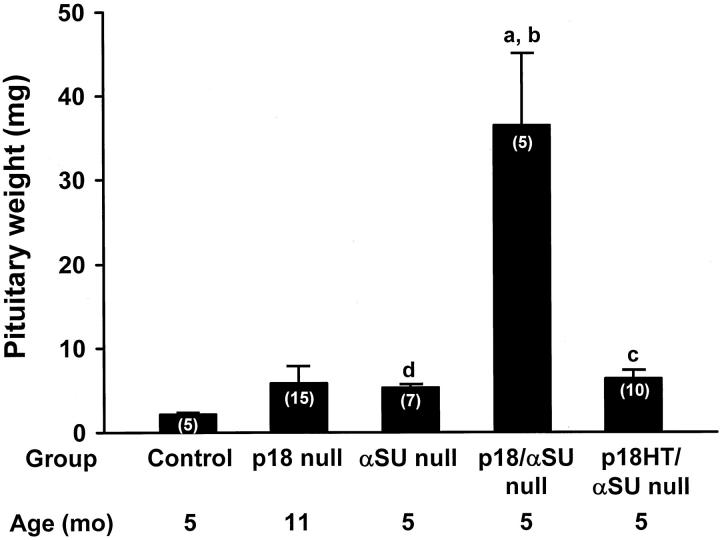
The p18/αSU double-null mice had a more rapid growth of their anterior pituitaries compared to either the p18-null or αSU-null animals (Figure 2) ▶ . When the mice were sacrificed by 5 months of age, the pituitaries were sevenfold larger than the αSU-null mice sacrificed at the same time (P < 0.05) and fivefold larger than the p18-null mice sacrificed at 11 months of age on average (Figure 2) ▶ . Mice that were p18 heterozygous and αSU-null sacrificed at 5 months of age had pituitaries that were slightly larger than those of the αSU-null mice (Figure 2) ▶ .
Figure 2.
Analysis of pituitaries from mice with targeted disruption of the p18 and αSU genes. Mice were sacrificed at 5 months for most groups except the p18-null mice that were sacrificed at 11 months of age. The pituitaries were weighed and divided into aliquots for histological and immunohistochemical studies. Numbers in parentheses indicate the numbers of animals in each group. a, P < ] 0.05 compared to controls; b, P < 0.05 compared to αSU-null; c, P < 0.01 compared to controls; d, P < 0.01 compared to controls.
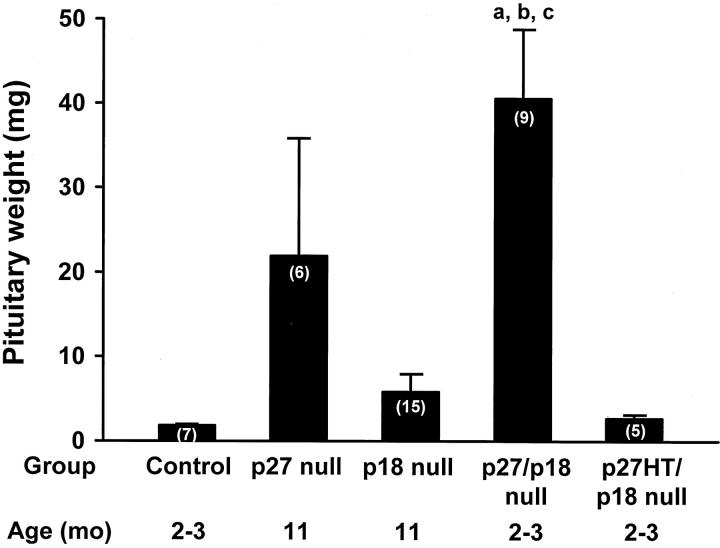
The p27/p18 double-null mice developed large pituitaries by 2 to 3 months of age and animals became moribund at this age because of the enlarged pituitaries, unlike their control littermates. Examination of the pituitaries showed that at 2 to 3 months of age, they were sevenfold larger than the p18-null mice at 11 months of age and nearly twofold larger than the p27-null mice at 11 months of age (Figure 3) ▶ . The pituitaries of p27 heterozygous/p18-null mice were slightly larger than the control mice at the same age (Figure 3) ▶ .
Figure 3.
Analysis of pituitaries from mice with targeted disruption of the p18 and p27 genes. Most mice were sacrificed at 11 months. The p27/p18 double-null mice that were sacrificed at 2 to 3 months because of increasing morbidity because of rapidly enlarging pituitaries. The p27-null and p18-null mice were sacrificed at 11 months of age. The pituitaries were weighed and divided into aliquots for histological and immunohistochemical studies. Numbers in parentheses indicate the numbers of animals in each group. a, P < 0.01 compared to controls; b, P < 0.01 compared to p18-null mice; c, P < 0.01 compared to p27HT/p18-null mice.
Histology and Immunohistochemistry
Histological analysis revealed diffuse and nodular or adenomatous hyperplasia of the pituitary (Figure 4 ▶ ; A to F). For p18- and p27-null mice, this was mainly intermediate lobe ACTH cell hyperplasia (Table 1 ▶ and Figure 4, A and B ▶ ), although one PRL-positive hyperplastic gland was present in 1 of 10 p18-null mice examined by immunohistochemistry (Figure 4E) ▶ . The αSU-null mice had TSH cell hyperplasia in the anterior lobe of the pituitary. The p18/αSU double-null mice had mainly anterior lobe TSH cell hyperplasia when sacrificed at 5 months of age with less developed intermediate lobe ACTH cell hyperplasia at this age (Figure 4, C and D) ▶ .
Figure 4.
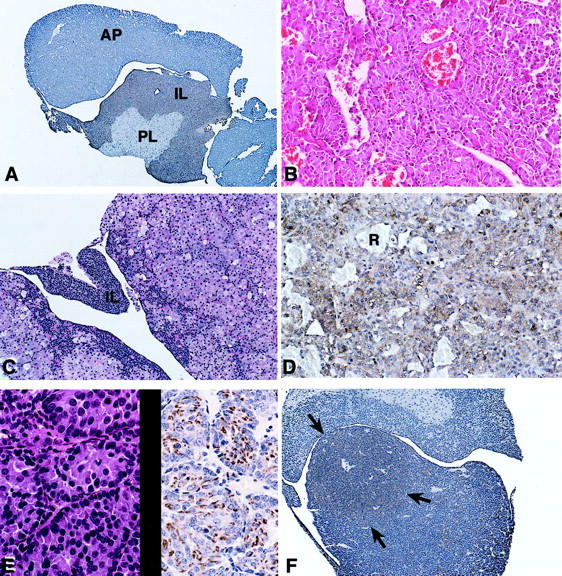
Histological and immunohistochemical analysis of hyperplastic pituitaries. A: p18-null mouse showing slight ACTH cell hyperplasia of the intermediate lobe (IL). B: Marked ACTH intermediate lobe hyperplasia in a p27/p18 double-null mouse. C: p18/αSU double-null mouse showing marked hyperplasia of anterior lobe TSH cells at 5 months of age. The intermediate lobe (IL) is not very hyperplastic at this age. D: αSU-null mouse pituitary with marked TSH cell hyperplasia. Red blood cells (R) are present in sinusoidal spaces. E: PRL cell hyperplasia on H&E (left) and after staining with a PRL antibody (right) in a p18-null mouse. F: Hyperplastic LH nodule in the anterior pituitary of a p27 heterozygous/p18-null mouse. The arrows indicate the LH-positive nodule after immunostaining with anti-LH. Original magnifications: ×75 (A); ×200 (B); ×150 (C); ×300 (D, E); ×100 (F).
Table 1.
Immunohistochemical Analysis of Hyperplastic Pituitaries
| Group | n | Intermediate lobe | Anterior Lobe | ||||
|---|---|---|---|---|---|---|---|
| ACTH | PRL | GH | TSH | LH | ACTH | ||
| p27-null | 12 | 12 | — | — | — | — | — |
| p18-null | 10 | 9 | 1 | — | — | — | — |
| αSU-null | 5 | — | — | — | 5 | 0 | 0 |
| p27/p18 | — | — | — | — | — | ||
| double-null | 5 | 5 | |||||
| p18/αSU | — | — | — | — | |||
| double-null | 9 | 4 | 9 | ||||
n = number of pituitaries immunostained in each group.
Ten wild-type pituitaries were analyzed as controls.
Hyperplastic LH nodules were identified in 2 of 6 p27 heterozygous/p18 null mouse pituitaries.
The p27/p18 double-null mice had markedly enlarged pituitaries with intermediate lobe ACTH cell hyperplasia (Table 1 ▶ , Figure 4B ▶ ) at the time of sacrificing at 2 to 3 months of age. Nodular LH cell hyperplasia was present in two of six pituitaries from p27 heterozygous/p18-null mice (Table 1 ▶ , Figure 4F ▶ ) along with intermediate lobe ACTH cell hyperplasia.
Analysis of mitotic activity showed between one to five mitoses per 50 high-power fields in the p27-null, p18-null, αSU-null, as well as the double-null mice whereas none to one mitoses per 50 high-power fields were present in the anterior pituitary of control mice.
Expression of p27 Regulatory Proteins
When pituitary tissues for p27-null, p18-null, and αSU-null mice were analyzed by Western blots for p27, SKP 2, the highest levels of Grb 2 and Jab 1, the αSU-null mice had the lower levels of SKP 2 and Grb 2 protein that were 3.5-fold higher than the control wild-type liver. The p27-null mice pituitary tissue had Grb 2 levels that were 2.3-fold higher than the control mice (Figure 5, A and B) ▶ . The levels of Jab 1 were not significantly different in any of the pituitary tissues from p27-, p18-, or αSU-null mice by Western blot analysis (Figure 5) ▶ .
Figure 5.
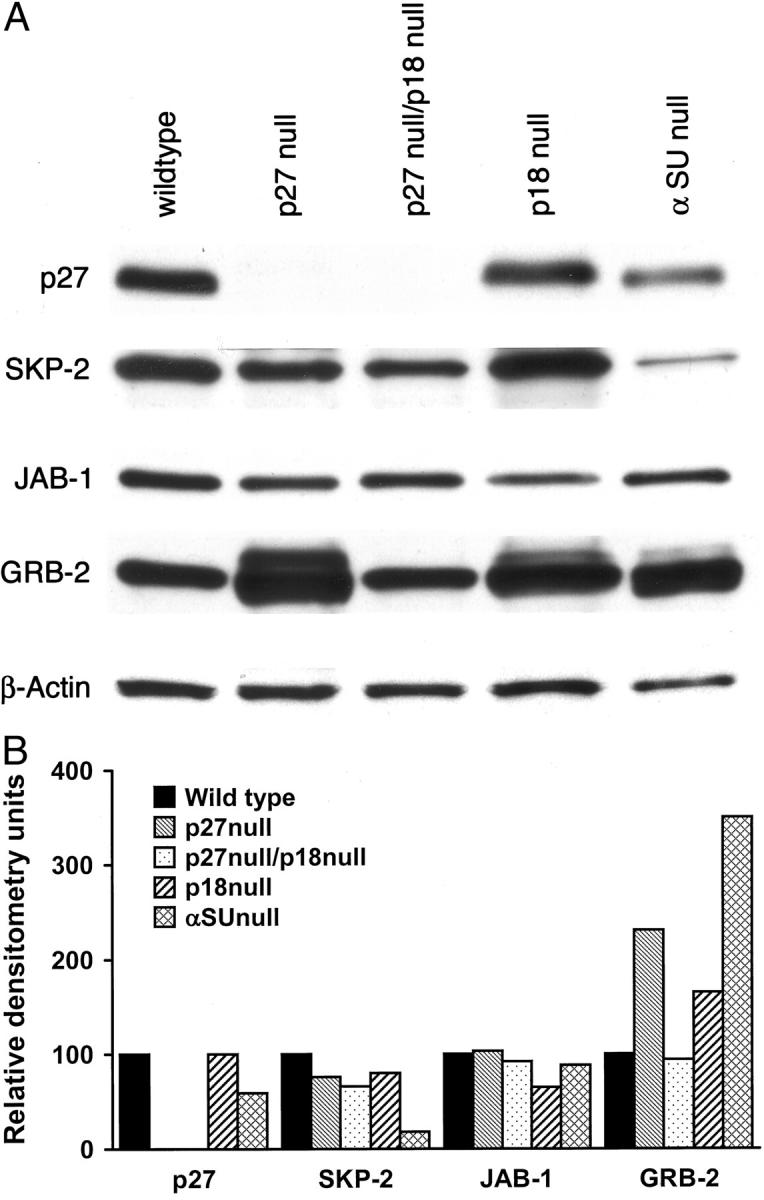
A: Western blot of p27, SKP-2, GR-2, and JAB-1 analysis of pituitary tissue from null mice. Fifty μg of protein was used in each lane. Each experiment was repeated twice. B: Densitometric analysis of the Western blot showed decreased levels of SKP-2 and elevated levels GRB-2 in αSU-null liver compared to the wild-type mice. JAB-1 was relatively unchanged.
Analysis of immunohistochemical staining showed mainly nuclear, but some cytoplasmic, staining (Figure 6) ▶ . Only nuclear staining was evaluated. Staining of the pituitaries showed the highest levels of Grb 2 in the αSU- and p18/αSU double-null mice (Table 2) ▶ . p27 immunoreactivity was not present in the pituitaries of the p27-null and p27/p18 double-null mice. Jab 1 levels were similar to wild type in the pituitary by Western blotting and by immunohistochemistry.
Figure 6.
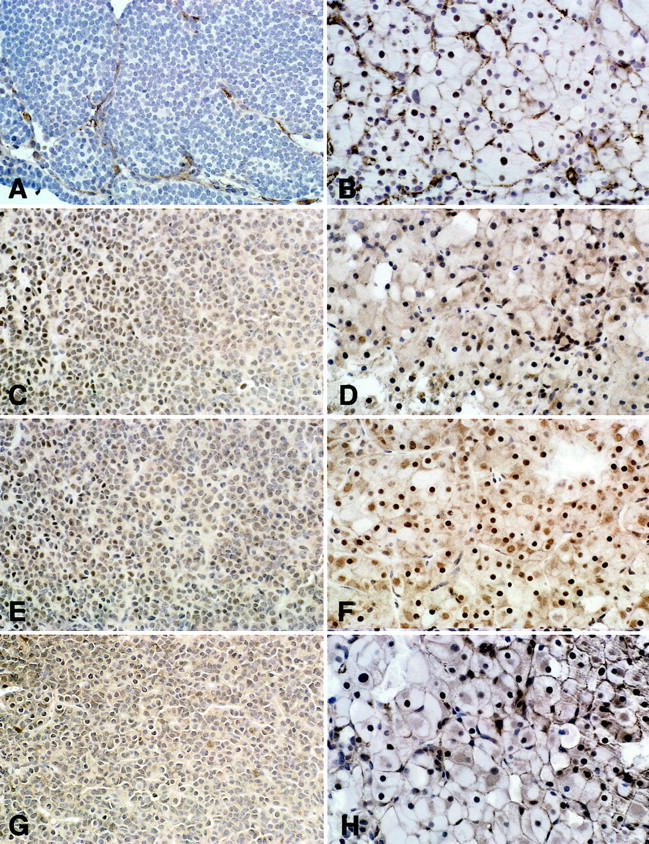
Immunohistochemical analysis of stains for various proteins in pituitary tissues from p27/p18 double-null (A, C, E, G) and p18/αSU double-null mice (B, D, F, H). p27 was absent in the p27/p18 double-null pituitary (A), whereas the p18/αSU pituitary shows diffuse (+++) nuclear staining (B). C and D: SKP-2 was diffusely (+++) expressed in the nucleus of the hyperplastic pituitaries. E and F: GRB-2 was expressed in the nucleus with focal (++) expression in the nucleus of p27/p18 double-null and diffuse (+++) expression in the nucleus of p18/αSU double-null mice. G and H: JAB-1 showed nuclear and cytoplasmic staining in pituitaries. The p18/αSU double-null mice and the p27/p18 double-null mice showed diffuse (+++) expression. Original magnifications, ×300.
Table 2.
Immunohistochemical Analysis of Pituitary Tissues in Wild-Type and Null Mice
| Group | p27 | SKP 2 | Jab 1 | Grb 2 |
|---|---|---|---|---|
| Wild type | ++ | + | +++ | ++ |
| p27-null | 0 | ++ | +++ | ++ |
| p18-null | +++ | +++ | +++ | ++ |
| p27/p18 | ||||
| double-null | 0 | +++ | +++ | ++ |
| αSU-null | +++ | +++ | +++ | +++ |
| p18/αSU | ||||
| double-null | +++ | +++ | +++ | +++ |
Analysis of staining: 0, negative; +, rare or <5% cells positive; ++, focal or <25% cells positive; and +++, diffuse or >25% of cells positive.
Between four to five pituitaries per group were analyzed.
Discussion
Pituitary hyperplasia and tumor development is most commonly seen in the anterior pituitary of rodents and humans. These lesions often develop spontaneously, 30,31 although they can also be induced by chemicals such as hormonal treatment. 32 Mice with targeted disruption of the Rb, p27, and p18 genes often develop pituitary lesions in the intermediate lobe. 1-8,33 We designed experiments to target the anterior pituitary gland in mice with targeted disruption of the p18 and αSU genes. This new model more closely simulates pituitary lesions developing spontaneously in rodents and humans and serves as a model to analyze the pathogenesis of anterior pituitary neoplasia.
The TSH cell hyperplasia in the αSU-null mice and the p18/αSU double-null mice was a secondary hyperplasia resulting from the knockout of the α-subunit gene that led to thyroid atrophy and pituitary TSH cell hyperplasia. 23,25
Although ACTH intermediate lobe hyperplasia was most common in our studies, one hyperplastic PRL cell lesion was found in a p18-null male mouse. In the p27 heterozygous/p18-null mice two animals developed nodular hyperplasia of LH-producing cells. These findings indicate that hyperplastic lesions with these models involve various pituitary cell lineage, including ACTH, TSH, PRL, and LH. The presence of increased numbers of mitoses in the pituitary tissues of null mice compared to the controls supports the observation that there was pituitary cell hyperplasia and not just hypertrophy in these tissues.
The development of hyperplastic pituitaries in mice expressing one allele of a CDKI gene such as p27 or p18 in our studies is consistent with the concept of haplo-insufficiency for tumor suppressor genes observed by Fero and colleagues. 34 In their studies of mice heterozygous for p27, animals treated with radiation or carcinogens subsequently developed enlarged pituitaries more rapidly than the untreated control littermates. These observations indicate that the absence of one copy of a tumor suppressor gene may be sufficient to lead to hyperplasia/neoplasia in specific target tissues. 34
Although enlarged pituitaries in rodents are often referred to as tumors or adenomas, 4-8 the distinction between pituitaries with diffuse and adenomatous hyperplasia from true neoplasms is often difficult. Although the use of X chromosomal-linked genes such as the androgen receptor and other genes have been used successfully to support clonality of pituitary tumors in humans, 35,36 these techniques have not been used extensively in rodents. We are currently developing methods to determine clonality in hyperplastic and neoplastic rodent pituitaries. However, for the purpose of the current studies, the morphological features of the enlarged pituitaries in mice with αSU deletions and in the p27- and p18-null mice were considered to represent hyperplastic glands. These observations are supported by our observations that pituitaries weighing 100 mg in αSU-null mice were reduced to 10 to 15 mg in 2 weeks of thyroxine treatment (unpublished observations). 25,29
We analyzed the expression of various proteins that influence p27 ubiquitin-mediated degradation in the pituitary of p27-, p18-, and αSU-null mice. In the pituitary of p27-null mice, Grb 2 levels were 2.3 times greater than control. Interestingly, the αSU-null mice had markedly elevated levels of Grb 2, but decreased levels of SKP 2 by Western blotting.
Immunohistochemical analysis done with the pituitary tissues also showed high levels of Grb 2 in the pituitary of αSU-null and p18/αSU double-null mice. Although several studies have indicated an inverse relationship between p27 and SKP 2 expression in cultured cells and tissues, 17,18,20 we did not detect a marked increase in SKP 2 in p27-null mice in the pituitary. The Jab 1/CSN5 protein is a regulator of p27 transport from the nucleus to the cytoplasm and Jab 1 has an important role in G1 progression and cell proliferation. 16 More recent studies have shown that overexpression of Grb 2 accelerates Jab 1-mediated degradation of p27, 21 indicating that Grb 2 participates in a negative regulation of p27 that may link the signal transduction pathway with the cell-cycle regulatory pathway. 22 The markedly elevated levels of Grb 2 in the pituitary of αSU-null mice in the presence of relatively normal levels of Jab 1 suggests an important role of Grb 1 in p27 degradation.
In the pituitary of αSU-null and p18/αSU double-null mice Grb 2 levels were higher than in the control wild-type mice, suggesting that Grb 2 expression with possible increased degradation of p27. The immunoreactivity for SKP 2 was relatively high in the pituitaries of most groups compared to wild-type mice except for the αSU-null mice by Western blotting. A growing body of evidence has implicated SKP 2 as an oncogenic factor in human cancers. 17 Other studies have shown that SKP 2 was required for the ubiquitin-mediated degradation of p27. 18 Our analysis in the pituitary suggests that SKP 2 may have a role in pituitary p27 regulation, but it probably has other functions in these cells, because it was highly expressed in p27-null mice. Previous studies from our laboratory showed lower levels of p27 in anterior pituitary ACTH cells compared to other cell types. 37,38 Because of these differences, which were also observed by other investigators subsequently, 39 it is possible that the ubiquitin-mediated degradation of p27 by proteins such as Grb 2, Jab 1, and SKP 2 may vary in different types of anterior pituitary cell types and this may influence pituitary hyperplasia and tumor. In situ studies with double localization of proteins or laser capture microdissection combined with immunophenotyping 26 will be needed to address this question.
Other recent studies with a mouse knock-in model have observed a second proteolytic pathway for controlling p27, which is activated by mitogens and degrades p27 exclusively during the G1 phase of the cell cycle indicating the complexity of p27 regulation. 40
In summary, we have described a model of p18- and αSU double-null mice that develop accelerated anterior pituitary TSH cell hyperplasia and shows alterations in some proteins related to p27 and other CDKI ubiquitin-mediated degradation. These null mice can be used as a model to study the molecular pathogenesis of anterior pituitary hyperplasia and tumor development.
Acknowledgments
We thank Drs. D. S. Franklyn and Xiang from the University of North Carolina, Chapel Hill, NC, for the p18-null mice; Drs. M. L. Fero and J. L. Roberts from the Fred Hutchinson Cancer Center, Seattle, WA, for the p27-null mice; Dr. Sally Camper from the University of Michigan, Ann Arbor, MI, for the αSU-null mice; and the National Pituitary Agency for antibodies to pituitary hormones.
Footnotes
Address reprint requests to R. V. Lloyd, M.D., Ph.D., Dept. of Laboratory Medicine and Pathology, Mayo Clinic, 200 First St., SW, Rochester, MN 55905. E-mail: lloyd.ricardo@mayo.edu.
Supported in part by National Institutes of Health grant CA90249 and a grant from the Jarislowsky Foundation.
References
- 1.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weingberg RA: Effects of Rb mutation in the mouse. Nature 1992, 359:295-300 [DOI] [PubMed] [Google Scholar]
- 2.Hu N, Gutsman A, Herbert DC, Bradley A, Lee WH, Lee EY: Heterozygous Rb-1 delta 20/+ mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene 1994, 9:1021-1027 [PubMed] [Google Scholar]
- 3.Harvey M, Vogel H, Lee EY, Bradley A, Donehower LA: Mice deficient in both p53 and Rb develop tumors primarily of endocrine origin. Cancer Res 1955, 55:1146-1151 [PubMed] [Google Scholar]
- 4.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama KI: Mice lacking p27kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia and pituitary tumors. Cell 1996, 85:707-720 [DOI] [PubMed] [Google Scholar]
- 5.Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A: Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27 (kip1). Cell 1996, 85:721-732 [DOI] [PubMed] [Google Scholar]
- 6.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM: A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis and female sterility in p27kip1-deficient mice. Cell 1996, 85:733-744 [DOI] [PubMed] [Google Scholar]
- 7.Franklin DS, Godfrey VL, Lee H, Kovalev GI, Schoonhoven R, Chen-Kiang S, Su L, Xiong Y: CKD inhibitors p18INK4C and p27kip1 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev 1998, 12:2899-2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin DS, Godfrey VL, O’Brien DA, Deng C, Xiong Y: Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol Cell Biol 2000, 20:6147-6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahl JH, Kendall SK, Brinkmeier ML, Greco TL, Watkins-Chow DE, Campos-Barros A, Lloyd RV, Camper SA: Thyroid hormone is essential for pituitary somatotropes and lactotropes. Endocrinology 1999, 140:1884-1892 [DOI] [PubMed] [Google Scholar]
- 10.Teixeira LT, Kiyokawa H, Peng XD, Christov KT, Frohman LA, Kineman RD: p27kip1-deficient mice exhibit accelerated growth hormone-releasing hormone (GHRH)-induced somatotrope proliferation and adenoma formation. Oncogene 2000, 19:1875-1884 [DOI] [PubMed] [Google Scholar]
- 11.Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW: p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol 1999, 154:313-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desdouets C, Bréchot C: p27: a pleiotropic regulator of cellular phenotype and a target for cell cycle dysregulation in cancer. Pathol Biol 2000, 48:203-210 [PubMed] [Google Scholar]
- 13.Krek W: Proteolysis and the G1-S transition: the SCF connection. Curr Opin Genet Dev 1998, 8:36-42 [DOI] [PubMed] [Google Scholar]
- 14.Ciechanover A: The ubiquitin-proteasome proteolytic pathway. Cell 1994, 79:13-21 [DOI] [PubMed] [Google Scholar]
- 15.Montagnoli A, Fiore F, Eyton E, Carrano AC, Draetta GF, Hershiko A, Pagono M: Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev 1999, 13:1181-1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomoda K, Kubota Y, Kato J: Degradation of the cyclin-dependent kinase inhibitors. p27kip1 is instigated by Jab 1. Nature 1999, 398:160-165 [DOI] [PubMed] [Google Scholar]
- 17.Gstaiger M, Jordan R, Lim M, Catzavelos C, Mestan J, Slingerland J, Krek W: Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA 2001, 98:5043-5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrano AC, Eytan E, Hershko A, Pagano M: SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol 1999, 1:193-199 [DOI] [PubMed] [Google Scholar]
- 19.Chiaur DS, Murthy S, Cenciarelli C, Parks W, Loda M, Inghirami G, Demetrick D, Pagano M: Five human genes encoding F-box proteins: chromosomal mapping and analysis in human tumors. Cytogenet Cell Genet 2000, 88:255-258 [DOI] [PubMed] [Google Scholar]
- 20.Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, Kitigawa M, Nakayama K, Hatakeyama S: Targeted disruption of SKP2 results in accumulation of cyclin E and p27kip1, polyploidy and centrosome overduplication. EMBO J 2000, 19:2069-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiyama Y, Tomoda K, Tanaka T, Arata Y, Yoneda-Kato N, Kato J: Direct binding of the signal-transducing adaptor Grb2 facilitates down-regulation of the cyclin-dependent kinase inhibitor p27kip1. J Biol Chem 2001, 276:12084-12090 [DOI] [PubMed] [Google Scholar]
- 22.Birge RB, Knudsen BS, Besser D, Hanafusa H: SH2- and SH3-containing adaptor proteins: redundant or independent mediators of intracellular signal transduction. Genes Cells 1996, 276:595-613 [DOI] [PubMed] [Google Scholar]
- 23.Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA: Targeted disruption of the pituitary glycoprotein hormone α-subunit produces hypogonadal and hypothyroid mice. Genes Dev 1995, 9:2007-2019 [DOI] [PubMed] [Google Scholar]
- 24.Gubbay J. Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R: A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 1990, 346:245–250 [DOI] [PubMed]
- 25.Kulig E, Camper SA, Kuecker S, Jin L, Lloyd RV: Remodeling of hyperplastic pituitaries in hypothyroid α-subunit knockout mice after thyroxine and 17 β-estradiol treatment: role of apoptosis. Endocr Pathol 1998, 9:261-274 [DOI] [PubMed] [Google Scholar]
- 26.Jin L, Tsumanuma I, Ruebel KH, Bayliss JM, Lloyd RV: Analysis of homogeneous populations of anterior pituitary folliculostellate cells by laser capture microdissection and reverse transcription-polymerase chain reaction. Endocrinology 2001, 142:1703-1709 [DOI] [PubMed] [Google Scholar]
- 27.Jin L, Kulig E, Qian X, Scheithauer BW, Eberhardt NL, Lloyd RV: A human pituitary cell adenoma line proliferates and maintains some differentiated functions following expression of SV40 large T antigen. Endocr Pathol 1998, 9:169-184 [Google Scholar]
- 28.Qian X, Jin L, Grande JP, Lloyd RV: Transforming growth factor-β and p27 expression in pituitary cells. Endocrinology 1996, 137:3051-3060 [DOI] [PubMed] [Google Scholar]
- 29.Stahl JH, Kendall SK, Brinkmeier ML, Greco TL, Watkins-Chow DE, Campos-Barros A, Lloyd RV, Camper SA: Thyroid hormone is essential for pituitary somatotropes and lactotropes. Endocrinology 1999, 140:1884-1892 [DOI] [PubMed] [Google Scholar]
- 30.Lloyd RV: Tumors of the Pituitary. Stinson SF Schuller HM Reznik G eds. Atlas of Tumor Pathology of the Fischer Rat. 1990, :pp 265-289 CRC Press, Boca Raton [Google Scholar]
- 31.Horvath E, Kovacs K: Fine structural cytology of the adenohypophysis in rat and man. J Electr Micro Tech 1998, 8:401-432 [DOI] [PubMed] [Google Scholar]
- 32.Lloyd RV: Estrogen-induced hyperplasia and neoplasia in the rat anterior pituitary gland. An immunohistochemical study. Am J Pathol 1983, 113:198-206 [PMC free article] [PubMed] [Google Scholar]
- 33.Park MS, Rosai J, Nguyen HT, Capodieci P, Cordon-Cardo G, Koff A: p27 and Rb are on overlapping pathways suppressing tumorigenesis in mice. Proc Natl Acad Sci USA 1999, 96:6382-6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ: The murine gene p27kip1 is haplo-insufficient for tumor suppression. Nature 1998, 396:177-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander JM, Biller BMK, Bikkal H, Zervas NT, Arnold A, Klibanski A: Clinically non-functioning pituitary tumors are monoclonal in origin. J Clin Invest 1990, 86:336-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herman V, Fagin J, Gonsky R, Kovaks K, Melmed S: Clonal origin of pituitary adenomas. J Clin Endocrinol Metab 1990, 71:1427-1433 [DOI] [PubMed] [Google Scholar]
- 37.Jin L, Qian X, Kulig E, Sanno N, Scheithauer BW, Kovacs K, Young Jr WF, Lloyd RV: Transforming growth factor-beta, transforming growth factor-beta receptor II and p27kip1 expression in nontumorous and neoplastic human pituitaries. Am J Pathol 1997, 151:509–519 [PMC free article] [PubMed]
- 38.Erickson LA: p27kip1 and other cell cycle protein expression in normal and neoplastic endocrine tissues. Endocr Pathol 2000, 11:109-122 [DOI] [PubMed] [Google Scholar]
- 39.Lidhar K, Korbonits M, Jordan S, Khalimova Z, Kaltsas G, Lu X, Clayton RN, Jenkins PJ, Monson JP, Besser GM, Lowe DG, Grossman AB: Low expression of the cell cycle inhibitor p27kip1 in normal corticotroph cells, corticotroph tumors, and malignant pituitary tumors. J Clin Endocr Metab 1999, 84:3823-3830 [DOI] [PubMed] [Google Scholar]
- 40.Malek NP, Sundberg H, McGrew S, Nakayama K, Kriakidis TR, Roberts JM: A mouse knock-in model exposes sequential proteolytic pathways that regulate p27 Kip1 in G1 and S phase. Nature 2001, 413:323-327 [DOI] [PubMed] [Google Scholar]