Abstract
Immediately before parturition the cervix undergoes striking changes in structure (ripening) that facilitate dilatation and effacement. Cervical ripening shares many features in common with inflammation-associated tissue remodeling, making it a valuable process to explore with respect to the biochemical events in extracellular matrix restructuring. Cervical ripening can be pharmacologically induced with prostaglandin E2 (PGE2). Among the biochemical changes in the cervix at parturition is a marked increase in the hyaluronic acid (HA) content. HA and HA-binding proteins have been implicated in tissue hydration, release of collagenase, and leukocyte migration, but their roles in cervical ripening have not been explored. In the present study we examined the ability of PGE2 to induce expression of the HA-binding protein, tumor necrosis factor-stimulated gene (TSG)-6, in human cervical smooth muscle cells (hCSMCs) and compared the PGE2 response to that of tumor necrosis factor-α (TNF-α), an established inducer of TSG-6. TNF-α stimulated TSG-6 mRNA accumulation in a dose- and time-dependent manner, with the maximal response observed at 10 ng/ml after 6 hours of incubation. PGE2 stimulated TSG-6 mRNA expression, but the magnitude of response was substantially less than that produced by TNF-α, and it was maximal only after 24 hours of incubation. Quantitative real-time polymerase chain reaction was performed to assess the induction of TSG-6 mRNA and nascent transcripts at 24 hours of treatment. Induction of TSG-6 mRNA and nascent transcripts in response to 10 μmol/L of PGE2 was 5.7-fold and 6.3-fold greater than control values, respectively, whereas TNF-α (10 ng/ml) induced TSG-6 mRNA and nascent transcripts by 80-fold and 134-fold, respectively. TNF-α and PGE2 stimulated secretion of TSG-6 into the culture medium as detected by Western blotting. The effects of PGE2 on secretion of TSG-6 were delayed compared to TNF-α. A 1.3-kb fragment of the human TSG-6 proximal promoter drove luciferase expression in transfected hCSMCs. PGE2 increased TSG-6 promoter activity 1.75-fold. Paradoxically, TNF-α reduced TSG-6 promoter activity by 50%. We conclude that hCSMCs express the hyaladherin TSG-6; that TSG-6 expression in these cells is regulated by PGE2 as well as proinflammatory cytokines; responses of hCSMCs to TNF-α and PGE2 are distinct in terms of magnitude and the time course; and PGE2 and TNF-α exert different effects on the TSG-6 proximal promoter.
The ripening of the human cervix at term has been likened to an inflammatory process. The study of the biochemical events underlying this process could reveal important features of normal and pathological tissue remodeling. A substantial increase in the cervical content of hyaluronic acid (HA) at the onset of labor is thought to contribute to the inflammation-like remodeling of the cervix. The accumulation of HA promotes tissue hydration, 1-5 release of collagenase, 6,7 and leukocyte migration. 8,9 These actions of HA are probably mediated and/or modulated by hyaladherins, HA-binding proteins. Hyaladherins share a common motif, the link module, that confers binding affinity to HA. 10-12 Relatively little is known about the hyaladherins produced in the cervix during the ripening process. The inflammation-associated protein TSG-6 (tumor necrosis factor stimulated gene-6) is a hyaladherin expressed by fibroblasts, chondrocytes, monocytes, vascular smooth muscle cells, and cumulus-oocyte complexes in response to inflammatory mediators and growth factors. 12-15 The function of TSG-6 is not well understood, but it has been suggested to be an endogenous inhibitor of neutrophil migration, an anti-inflammatory molecule, and a modulator of extracellular matrix remodeling. 16,17 These activities are potentially important in the cervical ripening process that is characterized by infiltration of leukocytes and extensive reorganization of the cervical extracellular matrix.
In the present study we examined the expression of TSG-6 by human cervical smooth muscle cells (hCSMCs) in culture, focusing on the effects of molecules that are known to promote cervical ripening, proinflammatory cytokines, and prostaglandin E2 (PGE2).
Materials and Methods
Cell Culture
Proliferating human cervical smooth muscle cells (hCSMCs) (Clonetics, San Diego, CA) that we have previously demonstrated to retain the molecular phenotype of smooth muscle cells, 18 were grown in Smooth Muscle Cell Growth Medium-2 basal medium (Clonetics) supplemented with 5% fetal bovine serum. This medium contains human epidermal growth factor (0.5 ng/ml), human fibroblast growth factor (1.0 ng/ml), insulin (5 μg/ml), gentamicin (50 μg/ml), and amphotericin B (50 μg/ml). hCSMCs were cultured at 37°C under an atmosphere of 5% CO2 in air. Subcultures of hCSMCs from passages 3 to 6 were used in all of the experiments. Each experiment was reproduced on at least two occasions with similar results.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) and TSG-6 Probe Preparation
Five μg of total RNA extracted from hCSMCs was reverse-transcribed to produce cDNA using reverse transcriptase and oligo dT (Promega, Madison, WI) as a primer. The TSG-6 cDNA was amplified using 10% of the RT reaction in 100 μl containing 50 pmol of forward primer, 50 pmol of reverse primer, 5 U of Taq polymerase (Life Technologies, Inc., Grand Island, NY) with 0.2 mmol/L of dNTPs and 1.5 mmol/L of MgCl2. The sequences of the primers used to generate the TSG-6 probe were: forward primer 5′-ATTTGTGAGCAGCCCCTAAC-3′ and reverse primer 5′-AGTGAGATCAAAGGAGTTCC-3′. The expected size of the PCR product is 951 bp. PCR was performed in a 9600 GeneAmp PCR thermal cycler using the following conditions: 94°C (1 minute) for one cycle, 94°C (1 minute), 57°C (1 minute), 72°C (1.5 minutes) for 35 cycles, and a final incubation at 72°C for 7 minutes. The PCR product was subcloned into PCR 2.1 vector (Invitrogen, Carlsbad, CA) and sequenced to verify that it encoded the TSG-6 cDNA. To obtain the insert for Northern blotting, the plasmid containing the sequence-verified PCR product was digested with EcoRI and the insert was purified using a gel extraction kit (Qiagen, Valencia, CA) before labeling by the random primer method.
RNA Isolation and Northern Blot Analysis
Subconfluent cultures of hCSMCs were grown in medium supplemented with 1% fetal bovine serum for 24 hours before treatment with tumor necrosis factor (TNF)-α (0.01 to 20 ng/ml) (R&D System, Minneapolis, MN) or PGE2 (5 to 40 μmol/L) (Sigma Chemical Co., St. Louis, MO) for up to 72 hours. Total RNA was extracted from the cultures with Trizol reagent (Life Technologies, Inc.) using procedures recommended by the manufacturer. Equal amounts of RNA (30 to 50 μg/lane) were separated on 1% agarose-formaldehyde-denaturing gels and transferred to nylon membranes. Membranes were hybridized with 32P-labeled TSG-6 cDNA at 42°C for 16 to 18 hours, followed by two sequential washings for 15 minutes in 2× sodium chloride/sodium phosphate ethylenediaminetetraacetic acid (SSPE), 0.1% sodium dodecyl sulfate (SDS) at 37°C, and two washings in 0.1× SSPE, 0.1% SDS at 55°C. Blots were analyzed using a phosphoimager and then exposed to Kodak X-Omat AR film (Eastman-Kodak, Rochester, NY). The relative abundance of TSG-6 mRNA was normalized to 28S rRNA.
Effects of Actinomycin D (Act D) and Cycloheximide (CHX) on TNF-α- and PGE2-Induced mRNA Expression
Subconfluent hCSMCs were cultured with 2 μg/ml of Act D or 50 μg/ml of CHX with 10 ng/ml of TNF-α or 10 μmol/L of PGE2 for 6 or 24 hours. Total RNA was subjected to Northern blotting as described above.
Western Blot Analysis
hCSMCs were cultured in serum-free medium for 24 hours, and then treated with TNF-α (10 ng/ml) for 24 hours or PGE2 (5 to 40 μmol/L) for up to 72 hours. The conditioned medium was subjected to 10% SDS-polyacrylamide gel electrophoresis and then Western blotting. The polyclonal antiserum used in Western blotting was raised as described in Carrette and colleagues 15 except that a 17-amino acid peptide (CTSTGNKNFLAGRFSHL) corresponding to the final 16-amino acid residues of human TSG-6 19 and a nonauthentic N-terminal Cys residue was used as the antigen. Supersignal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL) was used to detect antibody bound to antigen.
TSG-6 Promoter Analysis
The promoter region of the human TSG-6 gene was amplified by PCR using a forward primer spanning bp −1320 to −1301 with a KpnI linker (5′-CGAGGTACCTCACTAACCCTATCTGTGAA-3′) and a reverse primer spanning bp −20 to −1 with a NheI linker (3′-CTACACCTTTGGTCTACAAACGATCGAGC-5′). The 1.32-kb fragment spanning bp −1320 to −1 of the human TSG-6 gene was cloned into the pGL3 basic vector (Promega), which contains firefly luciferase as a reporter gene. 5′-deletion constructs were prepared by the subcloning of PCR products generated using various forward TSG-6-specific primers with attached linkers and the reverse primer described above. The forward primer used to generate the −756 TSG-6 promoter fragment spanned from bp −756 to −737 with a KpnI linker (5′-CGAGGTACCCCTTGATCGTCTTCTTCAAA-3′); the forward primer for the −160 TSG-6 promoter fragment spanned from bp −160 to −141 with a KpnI linker (5′-CGCGGTACCATTCTATCTCCTTAGTTTTG-3′); the forward primer for the −100 TSG-6 promoter fragment spanned from bp −100 to −81 with a KpnI linker (5′-CGCGGTACCTGAGATAATTGTGTAACTCT-3′). The sequences of all of these PCR-generated promoter fragments were confirmed to be identical to the corresponding TSG-6 promoter sequence previously reported by Lee and colleagues 20 with the following exceptions that insertions of A, G, C, T, A, C, and TG between nucleotides −1292 and −1291, −867 and −866, −681 and −680, −678 and −677, −631 and −630, −472 and −471, and −53 and −52, respectively, were consistently found. These same sequence variations were identified independently in Dr. Day’s laboratory and have been deposited in EMBL with accession codes of AJ413948 and AJ413949.
Transfection and Cell Stimulation
hCSMCs (1 × 105) were seeded in each well of 12-well plastic culture plates in medium supplemented with 1% fetal bovine serum. Cells were transfected using FuGENE 6 Transfection Reagent (Roche, Indianapolis, IN) with 0.5 μg/ml of each of the promoter constructs, 0.5 μg/ml of the CMV-β galactosidase plasmid, and 2 μl/ml of FuGENE. After 24 hours, hCSMCs were incubated in 0.1% ethanol (control), 10 ng/ml TNF-α, or 10 μmol/L PGE2 in medium supplemented with 1% fetal bovine serum for 24 hours to determine the effect on TSG-6 promoter activity. At the end of the culture period, cells were harvested and the cell lysates were assayed for luciferase and β-galactosidase activity as described below.
Luciferase and β-Galactosidase Assay
Luciferase activity was determined in a LUMAT LB 9507 luminometer (EG&G Berthold, Gaithersburg, MD) with Promega luciferin as substrate. β-galactosidase activity was determined by a standard colorimetric assay using 2-nitrophenyl β-d-galactopyranoside as substrate. The luciferase assay results were normalized to β-galactosidase activity to correct for variations in transfection efficiency. Each treatment group contained triplicate cultures and each experiment was repeated three to four times. Relative luciferase units (RLU) defined as luciferase light units/β-galactosidase activity are presented as means ± SE.
Reverse Transcription Reaction and Quantitative Real-Time PCR for TSG-6 mRNA
Proliferating hCSMCs were incubated with 0.1% ethanol vehicle or 10 μmol/L of PGE2 for 24 hours. Total RNA was extracted using Trizol reagent (Life Technologies, Inc.). To limit the possibility of detection of genomic DNA, total RNA was treated with RQ1-RNase-free DNase (Promega) for 30 minutes at 37°C before reverse transcription with Moloney murine leukemia virus-reverse transcriptase (Promega) as described by the manufacturer. Five μg of total RNA was reverse-transcribed to single-strand cDNA using 1 μl of oligo(dT)15 primer (Promega), 0.5 μl of RNase inhibitor from human placenta (Roche Molecular Biochemicals), and 1 μl of Moloney murine leukemia virus-reverse transcriptase (Promega) as described by the manufacturer.
Quantitative real-time PCR was performed to assess the induction of TSG-6 mRNA in hCSMCs in response to 10 μmol/L of PGE2. Primers for the analysis of the human TSG-6 gene elements were designed in the third exon: forward, 5′-TCATGTCTGTGCTGCTGGATG-3′; and reverse, 5′-GGGCCCTGGCTTCACAA-3′. The resulting cDNA was diluted 10-fold in sterile water and aliquots were subjected to quantitative real-time PCR. The real-time PCR reaction used 90 nmol/L of each primer and 12.5 μl of SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Agarose gel electrophoresis indicated the presence of a 67-bp single band for the TSG-6 PCR product. To account for differences in starting material, the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers and probe reagents from Applied Biosystems were used as described by the manufacturer. The target and GAPDH PCR reactions were performed in separate tubes in triplicate and the average threshold cycle for the triplicate was used in all subsequent calculations.
Quantitative Real-Time PCR of TSG-6 Nascent Transcripts
To limit the possibility of detection of genomic DNA, 5 μg of total RNA was treated with RQ1-RNase-free DNase (Promega) as described above. For the human TSG-6 and human GAPDH nascent transcript expression, primers for reverse transcription were designed in the third and fourth intron of each gene, respectively; TSG-6: 5′-CAACCTCTTAGCAGCATGGAACTGT-3′, GAPDH: 5′-TAGTTGCCTCCCCAAAGCAC-3′. Five μg of total RNA was reverse-transcribed to single-stranded cDNA as described above. The resulting amplicons derived from the specific TSG-6 and GAPDH intronic primers were diluted 10-fold in sterile water and aliquots were subjected to quantitative real-time PCR. The forward and reverse PCR primers for TSG-6 nascent RNA expression were the same as those described above for TSG-6 mRNA expression. For GAPDH nascent transcript expression, PCR primers were designed in the third exon (forward, 5′-GATTCCACCCATGGCAAATT-3′; reverse, 5′-AAGATGGTGATGGGATTTCCATT-3′). Optimization studies for detection of the nascent GAPDH transcript indicated that 90 nmol/L of each primer should be used in each 25-μl reaction. Agarose gel electrophoresis revealed the presence of a 83-bp single band for the nascent GAPDH transcript PCR product. PCR reactions for nascent TSG-6 and GAPDH transcripts were performed in separate tubes in triplicate and the average threshold cycle for the triplicates was used in all subsequent calculations.
Results
TNF-α and PGE2 Increase TSG-6 Gene Expression by hCSMCs
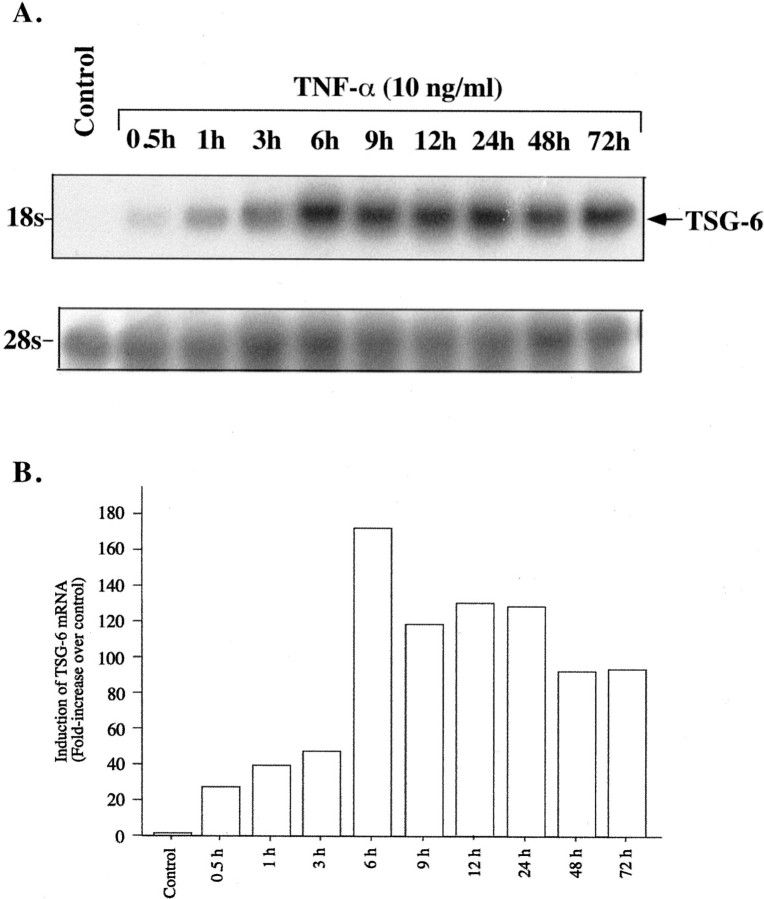
TNF-α is an established inducer of TSG-6 expression. We first defined the response of the hCSMCs to this proinflammatory cytokine. TNF-α had a dose-dependent stimulatory effect on expression of the 18S TSG-6 mRNA (Figure 1) ▶ . As little as 0.1 ng/ml of TNF-α raised TSG-6 message abundance, and a maximal response was observed with 10 ng/ml. Time-course studies conducted with 10 ng/ml of TNF-α (Figure 2) ▶ revealed a rapid increase in TSG-6 mRNA, detectable after 0.5 hours of treatment, the first time point we examined, and reaching a maximum at 6 hours, with mRNA levels declining modestly thereafter.
Figure 1.
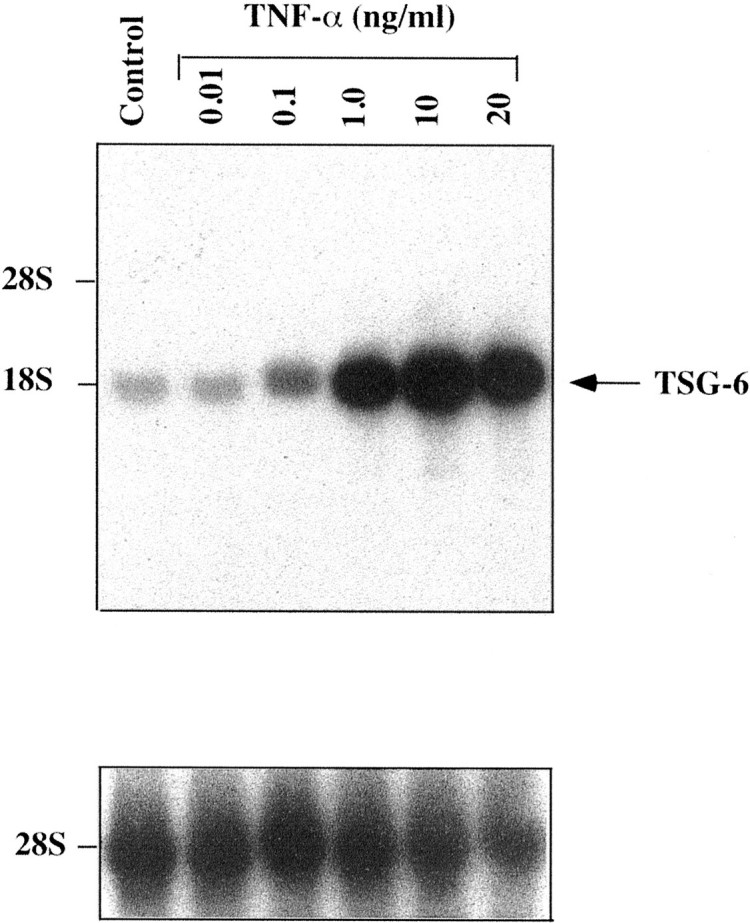
Effect of TNF-α on expression of TSG-6 mRNA. A Northern blot analysis of total RNA (40 μg/lane) extracted from hCSMCs cultured in the absence (control) or presence of different concentrations of TNF-α for 48 hours is shown. The blot was sequentially probed to detect 28S rRNA.
Figure 2.
Time course of TNF-α-induced expression of TSG-6 mRNA. A: Northern blot analysis of total RNA (40 μg/lane) extracted from hCSMCs cultured in the absence (control) or presence of TNF-α (10 ng/ml) for the indicated time periods is shown. The blot was also probed for 28S rRNA. B: Histogram showing induction of TSG-6 mRNA normalized to 28S rRNA relative to the control value that was arbitrarily set to 1.0. The results are representative of two separate studies.
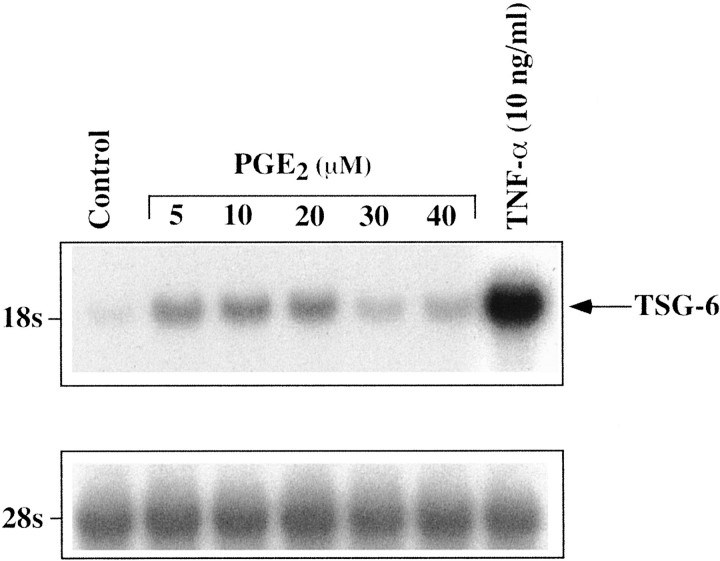
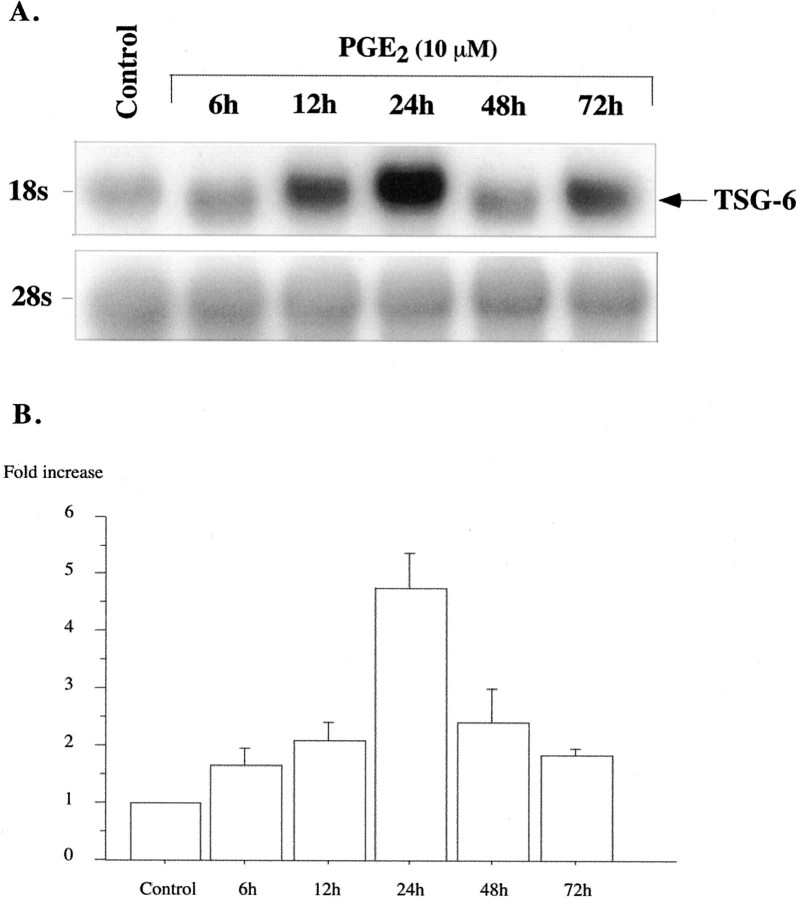
PGE2 also caused a dose-dependent increase in TSG-6 mRNA expression at 5 to 20 μmol/L; a lesser response was observed with higher PGE2 concentrations of 30 and 40 μmol/L (Figure 3) ▶ that may reflect desensitization or cell demise. In contrast to TNF-α, which rapidly induced TSG-6 mRNA, the response of hCSMCs to PGE2 was more sluggish. A modest response was seen at 6 hours and a peak in TSG-6 mRNA was observed at 24 hours of treatment (Figure 4) ▶ . Notably, TSG-6 mRNA induction by PGE2 after 24 hours of treatment was substantially lower than that observed with 10 ng/ml of TNF-α based on the signal strength of simultaneously hybridized blots and blots containing RNA from TNF-α- and PGE2-treated cells.
Figure 3.
Effect of PGE2 on expression of TSG-6 mRNA. A Northern blot analysis of total RNA (40 μg/lane) extracted from hCSMCs cultured in the absence (control) or presence of different concentrations of PGE2 or TNF-α (10 ng/ml) for 24 hours is shown. The blot was sequentially probed with 28S rRNA.
Figure 4.
Time course of PGE2-induced TSG-6 mRNA expression. A: Northern blot analysis of total RNA (40 μg/lane) extracted from hCSMCs cultured in the absence (control) or presence of PGE2 (10 μmol/L) for up to 72 hours is shown. The blots were sequentially probed for 28S rRNA. B: Histogram showing induction of TSG-6 mRNA normalized to 28S rRNA relative to the control value that was arbitrarily set to 1.0. Values are means ± SE from five separate experiments.
Quantitative Real-Time PCR for TSG-6 mRNA and Nascent Transcripts
We performed quantitative real-time PCR to assess the induction of TSG-6 mRNA and nascent transcripts, the latter being an index of TSG-6 gene transcription, in hCSMCs treated with 10 μmol/L of PGE2 for 24 hours (Table 1) ▶ . The increase in TSG-6 mRNA and nascent transcripts in response to 10 μmol/L of PGE2 was 5.7-fold and 6.3-fold compared to the 0.1% ethanol control group, respectively. In simultaneously conducted experiments TNF-α treatment increased TSG-6 mRNA and nascent transcript levels by ∼80-fold and 134-fold, respectively. These observations are consistent with the significant differences in TSG-6 induction in response to the prostanoid and the cytokine demonstrated by Northern blotting. We also examined the effects of 8-Br-cAMP because PGE2 is thought to act on hCSMCs via the EP4 receptor, which is coupled to adenylate cyclase. 21 The response to 8-Br-Br-cAMP was basically similar to that produced by PGE2.
Table 1.
Effect of PGE2 on TSG-6 mRNA and Nascent Transcripts
| Fold increase of TSG-6 mRNA and nascent transcripts ratio | |||
|---|---|---|---|
| PGE2/ control | TNF-α/ control | 8-Br-cAMP/ control | |
| mRNA | 5.7 ± 1.2 | 79.5 ± 16.5 | 4.3 ± 0.6 |
| Nascent transcripts | 6.3 ± 2.6 | 133.7 ± 50.4 | 2.9 ± 0.5 |
Quantitative real-time PCR was used to measure the fold increase in TSG-6 mRNA and nascent transcripts in hCSMCs treated with PGE2 (10 μM), TNF-α (10 ng/ml), or 8-Br-cAMP (1 mmol/L) or the ethanol vehicle. The values presented are from three independent experiments, each conducted in triplicate.
We performed several experiments to determine whether PGE2 (10 μmol/L) acts synergistically with TNF-α at either low concentrations (0.1 ng/ml) or concentrations that maximally stimulate TSG-6 expression (10 ng/ml). Cells were treated with each agent alone or in combination for 24 hours. We found no evidence for PGE2 augmentation of the TSG-6 mRNA response to TNF-α at either low or high TNF-α concentrations (data not shown).
TNF-α and PGE2 Stimulate TSG-6 Secretion, But with Different Temporal Patterns
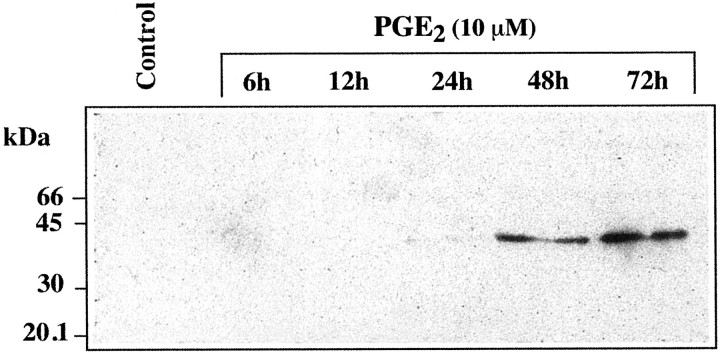
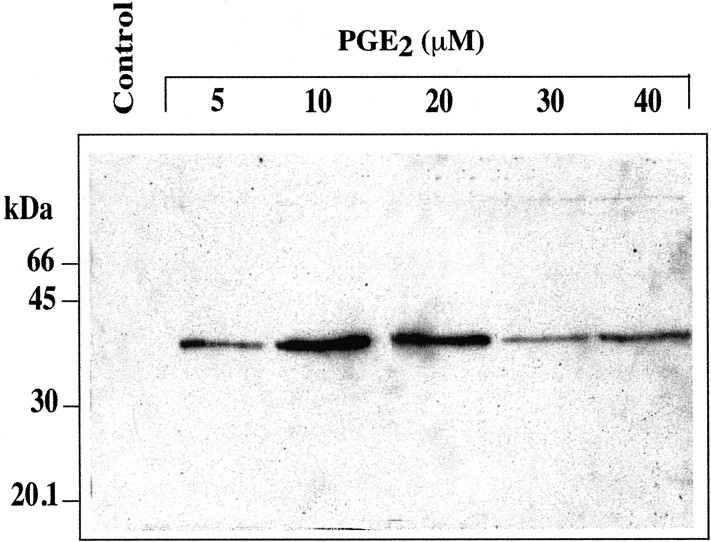
Exposure of hCSMCs to TNF-α at 10 ng/ml for 24 hours resulted in the release of the 39-kd TSG-6 protein into the culture medium as detected by Western blotting (Figure 5) ▶ . In contrast, there was no detectable release of TSG-6 by hCSMCs exposed to 10 μmol/L of PGE2 for 24 hours. However, when hCSMCs were cultured with 10 μmol/L of PGE2 for up to 72 hours, detectable TSG-6 was present in the conditioned medium by 48 hours of incubation (Figure 6) ▶ . The effects of PGE2 on secretion of TSG-6 after 72 hours of culture were dose-dependent with maximal secretion observed with 10 and 20 μmol/L of PGE2, and as with TSG-6 mRNA expression, lower production of TSG-6 was observed with concentrations of 30 and 40 μmol/L (Figure 7) ▶ .
Figure 5.
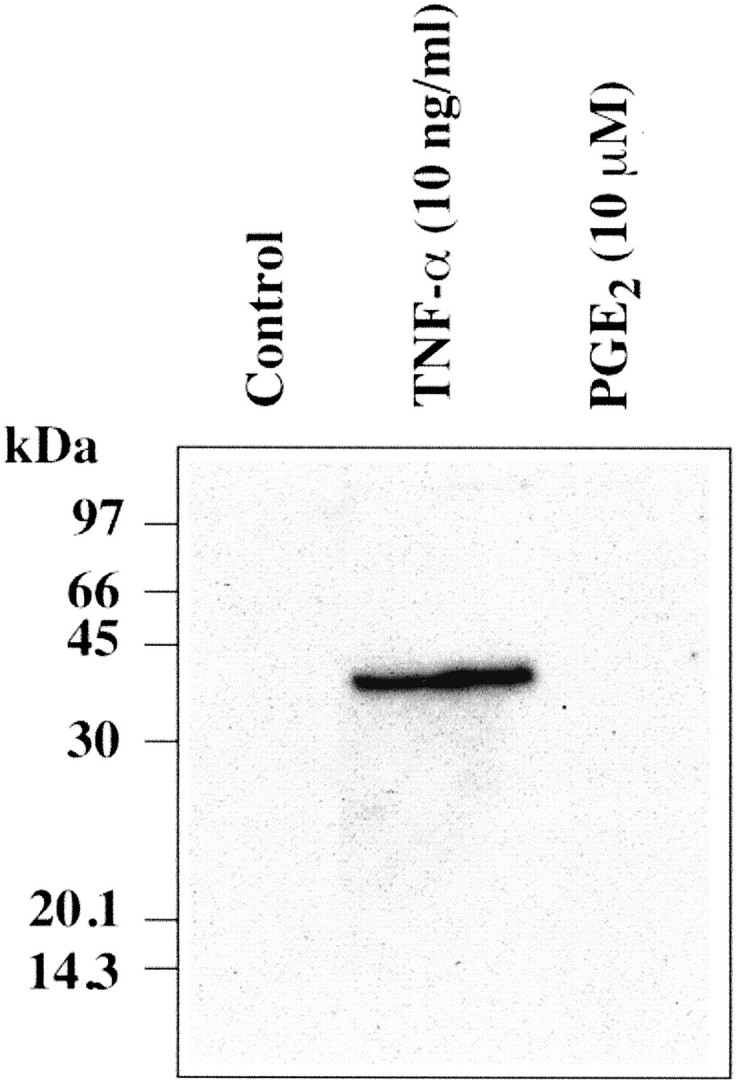
TSG-6 production by hCSMCs treated with TNF-α or PGE2. hCSMCs were incubated with TNF-α at 10 ng/ml or PGE2 at 10 μmol/L for 24 hours in serum-free medium and aliquots of conditioned media (80 μl/lane) were subjected to SDS-polyacrylamide gel electrophoresis and then Western blotting.
Figure 6.
Time course of TSG-6 production by hCSMCs treated with PGE2. hCSMCs were incubated without or with PGE2 (10 μmol/L) for up to 72 hours in serum-free medium and aliquots of conditioned media (80 μl/lane) were subjected to SDS-polyacrylamide gel electrophoresis and then Western blotting.
Figure 7.
TSG-6 production by hCSMCs treated with different concentrations of PGE2. hCSMCs were incubated in the absence or presence of different concentrations of PGE2 for 72 hours in serum-free medium and aliquots of conditioned media (80 μl/lane) were subjected to SDS-polyacrylamide gel electrophoresis and then Western blotting.
TSG-6 Expression in Response to TNF-α and PGE2 Requires On-Going RNA But Not Protein Synthesis
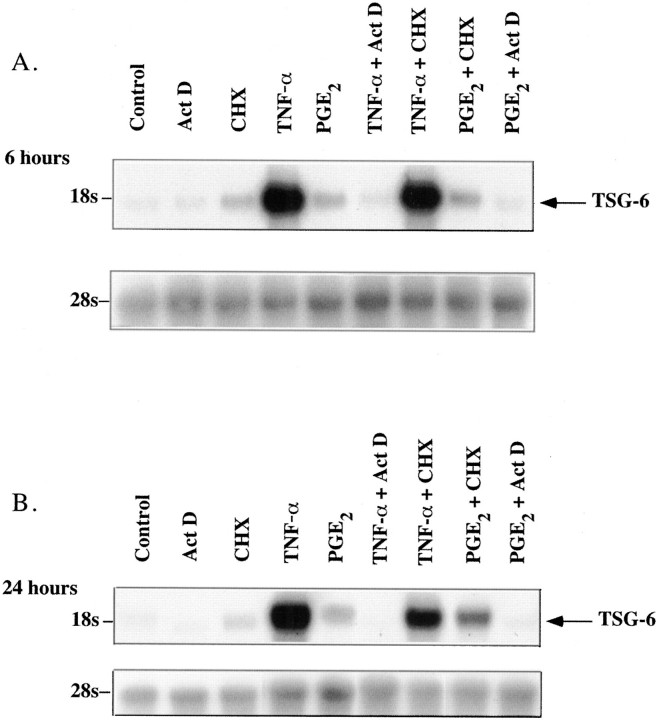
The TNF-α- and PGE2-stimulated rise in TSG-6 mRNA was not dependent on on-going protein synthesis as it occurred in the presence of the inhibitor, CHX that blocks de novo protein synthesis by >95% at the concentration used in this study (50 μg/ml) (Figure 8) ▶ . In fact, CHX alone caused a modest increase in TSG-6 mRNA (2.4-fold greater than control, normalized to 28S rRNA) and augmented the effects of PGE2 (4.3-fold at 6 hours of treatment and 14.67-fold at 24 hours), but not those of TNF-α. However, TNF-α and PGE2-stimulated TSG-6 mRNA expression required on-going RNA synthesis as Act D completely blocked the rise in TSG-6 mRNA in both TNF-α- and PGE2-treated hCSMCs without affecting basal levels at 6 hours of treatment and reducing basal mRNA abundance normalized to 28S rRNA by ∼60% after 24 hours of treatment.
Figure 8.
Effect of CHX and Act D on TNF-α and PGE2 induction of TSG-6 mRNA in hCSMCs. A: Northern blot analysis of total RNA (50 μg/lane) extracted from hCSMCs treated with 10 ng/ml of TNF-α, 10 μmol/L of PGE2, 50 μg/ml of CHX, 2 μg/ml of Act D, or the combination of these for 6 hours is shown. The blot was sequentially probed for 28S rRNA. B: hCSMCs were exposed to the same treatments for a period of 24 hours and Northern blot analysis was conducted as described above.
PGE2 Stimulates TSG-6 Promoter Activity
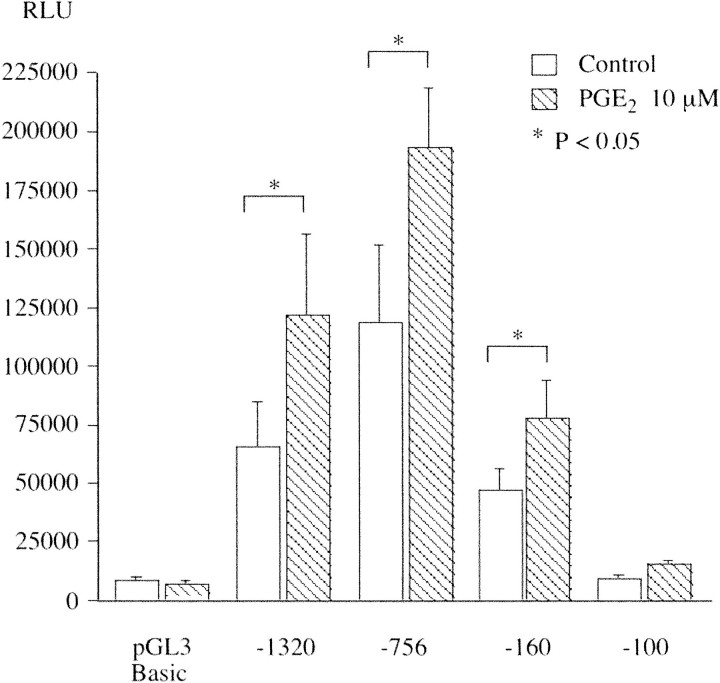
To examine the regulation of TSG-6 gene transcription, we cloned the TSG-6 promoter from hCSMC genomic DNA. A 1.3-kb fragment of the human TSG-6 proximal promoter drove luciferase expression in transfected hCSMCs. Treatment of the cells with 10 μmol/L of PGE2 after 24 hours of incubation increased TSG-6 promoter activity 1.75-fold (Table 2) ▶ . Paradoxically, TNF-α (10 ng/ml) reduced TSG-6 promoter activity by 64%, indicating that PGE2 and TNF-α regulate TSG-6 transcription by different mechanisms. We also examined four 5′-deletion constructs (Figure 9) ▶ . Ten μmol/L of PGE2 increased the activity of the −756 and −160 constructs, but not the −100 construct that had only modest promoter activity compared to the empty pGL3 vector. Notably removal of sequences between positions −1320 bp and −756 bp tended to enhance promoter function. A similar result has been reported by Lee and colleagues 20 who studied TSG-6 promoter activity in human FS-4 foreskin fibroblasts.
Table 2.
Transcriptional Activation of TSG-6 Promoter in Response to TNF-α and PGE2
| Treatment | RLU × 10−3 (mean ± SE) |
|---|---|
| Control | 35.71 ± 2.1 |
| TNF-α (10 ng/ml) | 22.71 ± 2.9 |
| PGE2 (10 μM) | 64.42 ± 3.6 |
Effect of TNF-α and PGE2 on 1.32-kb TSG-6 promoter activity in hCSMCs. Transfection and cell stimulation were performed as described in Materials and Methods. The relative luciferase units (RLU) presented are from three independent experiments, each conducted with triplicate cultures. Values with different superscripts are significantly different (P < 0.05) by the Tukey-Kramer test.
Figure 9.
Analysis of TSG-6 promoter function. The indicated promoter constructs were transfected into hCSMCs and incubated with 0.1% ethanol or 10 μmol/L of PGE2 as described in Materials and Methods. The relative light units (RLU) presented are means ± SE from four independent experiments, each conducted with triplicate cultures.
Discussion
Cervical ripening during parturition involves biochemical and cellular changes that are similar to those occurring in tissue inflammation. 22 The ripening process is associated with a marked increase in the level of HA. 23-25 Proinflammatory cytokines and PGE2 induce glycosaminoglycan production by cervical fibroblasts. 26-28 HA promotes tissue hydration, 1-5 release of collagenase, 6,7 and leukocyte migration, all components of the cervical ripening process. 8,9 Collectively, these observations suggest that HA plays an important role in the process of cervical ripening.
Although the functions of HA in the cervix during parturition are becoming clear, there has been little attention given to the HA-binding proteins that are thought to mediate and/or modulate the actions of HA. TSG-6 is one of the HA-binding proteins containing a single link module composed of two α-helices and two triple-stranded β-sheets arranged around a large hydrophobic core. 11 TSG-6 interacts with HA via its link module and amino acid residues involved in ligand binding have recently been determined. 17,29 TSG-6 has been implicated in the regulation of leukocyte migration and its pattern of expression suggests that it may be involved in extracellular matrix remodeling. 16,30 Elevated TSG-6 protein levels have been found in the synovial fluid of arthritic patients, 31 and recombinant TSG-6 has potent anti-inflammatory activity in vivo. 16 Based on these results, it was suggested that TSG-6 constitutes part of a cytokine-initiated feedback loop that operates to down-regulate the inflammatory response. 16 TSG-6 has been shown to form a stable, probably covalent, 120-kd complex with the serine protease inhibitor, inter-α inhibitor (IαI). 32 It has been reported that this complex has significantly increased anti-plasmin activity over IαI alone. 16 Thus TSG-6 may be important in regulating leukocyte migration and matrix remodeling because plasmin enhances the activation of latent metalloproteinases involved in extracellular matrix breakdown. 13,16,33
Our observations demonstrate that hCSMCs express TSG-6, and that TSG-6 expression in these cells is regulated, as expected, by proinflammatory cytokines. However, we have also established for the first time that PGE2 controls the TSG-6 gene. Notably, the responses of hCSMCs to TNF-α and PGE2 are distinct in terms of both magnitude and time course. We found that TNF-α and PGE2 treatment of hCSMCs increased nascent TSG-6 transcripts demonstrating that the rise in TSG-6 mRNA is at least in part the result of increased TSG-6 transcription. This observation is consonant with the fact that Act D blocked the induction of TSG-6 mRNA. Yet, the effect of PGE2 and TNF-α on TSG-6 proximal promoter activity suggest that the regulatory elements responsible for increased transcription are different. This is not unanticipated because PGE2 acts on cervical fibroblasts through the EP4 receptor that couples to adenylate cyclase, 21 whereas proinflammatory cytokines activate receptors that do not immediately engage the cAMP signaling cascade. The ability of cAMP-mediated signaling pathways to influence TSG-6 expression was verified by demonstrating that 8-Br-cAMP (1 mmol/L for 24 hours) increased TSG-6 mRNA and nascent transcript accumulation. The modest increase in TSG-6 mRNA abundance in CHX-treated cells and the augmentation of the response to PGE2 may suggest an effect of CHX on TSG-6 mRNA stability or possibly inhibition of synthesis of a transcriptional silencer. The fact that CHX did not block the effects of PGE2 on TSG-6 gene expression argues against the involvement of a prostanoid-induced protein autocrine factor in the TSG-6 response.
Lee and colleagues 20 reported that sequences between −165 to +78 in the TSG-6 promoter confer regulation by proinflammatory cytokines on this gene. This region contains a potential binding site for AP-1 as well as other transcription factors. However, the transcriptional response of the TSG-6 promoter construct reported by Lee and colleagues 20 in FS-4 cells to TNF-α treatment was modest at best compared to the response to interleukin-1. The magnitude of stimulated promoter activity in the study of Lee and colleagues 20 also did not correspond to the change in TSG-6 mRNA or the results of nuclear run-on assays. Our findings suggest that elements in the TSG-6 gene outside of those included in our promoter constructs and those of Lee and colleagues 20 must contribute to the transcriptional response to TNF-α as well as the maximal response to PGE2.
The production of TSG-6 in the cervix driven by proinflammatory cytokines and PGE2, molecules that promote cervical ripening, implicates TSG-6 in the cervical ripening process. Based on existing information, we speculate that TSG-6 modulates the inflammation-like biochemical and cellular changes in the cervix by retaining leukocytes in the cervix while at the same time exerting anti-inflammatory actions and restricting proteolytic activity so that alterations in the extracellular matrix are spatially and temporally regulated.
TSG-6 can be detected in human cervical fluid of women who present with symptoms of preterm labor (T Fujimoto and JF Strauss, unpublished observations). This finding is consistent with the notion that the cervix produces TSG-6 in situ. It also raises the possibility that detection of TSG-6 could serve as marker for impending cervical changes leading to preterm birth.
Acknowledgments
We thank Professor Mike Bayliss and Ms. Jo Flannelly (Royal Veterinary College, London, UK) for their help in preparing the anti-TSG-6 antiserum, and Ms. Judith Wood for assistance in preparation of this manuscript.
Footnotes
Address reprint requests to Jerome F. Strauss, III, M.D., Ph.D., 1354 BRB II, 421 Curie Blvd., Philadelphia, PA 19104. E-mail: jfs3@mail.med.upenn.edu.
Supported by the National Institutes of Health (grant HD34612) and the Bill and Melinda Gates Foundation.
References
- 1.Laurent TC, Fraser JR: Hyaluronan. FASEB J 1992, 6:2397-2404 [PubMed] [Google Scholar]
- 2.Comper WD, Laurent TC: Physiological function of connective tissue polysaccharides. Physiol Rev 1978, 58:255-315 [DOI] [PubMed] [Google Scholar]
- 3.Toole BP: Hyaluronan is not just a goo! J Clin Invest 2000, 106:335-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camenisch TD, McDonald JA: Hyaluronan: is bigger better? Am J Respir Cell Mol Biol 2000, 23:431-433 [DOI] [PubMed] [Google Scholar]
- 5.Lee JY, Spicer AP: Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol 2000, 12:581-586 [DOI] [PubMed] [Google Scholar]
- 6.Knudson W: Tumor-associated hyaluronan. Providing an extracellular matrix that facilitates invasion. Am J Pathol 1996, 148:1721-1726 [PMC free article] [PubMed] [Google Scholar]
- 7.Hiro D, Ito A, Matsuta K, Mori Y: Hyaluronic acid is an endogenous inducer of interleukin-1 production by human monocytes and rabbit macrophages. Biochem Biophys Res Commun 1986, 140:715-722 [DOI] [PubMed] [Google Scholar]
- 8.Savani RC, Bagli DJ, Harrison RE, Turley EA: The role of hyaluronan-receptor interactions in wound repair. Garg HG Longaker MT eds. Scarless Wound Healing. 2000, :pp 115-142 Marcel Dekker, New York [Google Scholar]
- 9.Hakansson L, Hallgren R, Venge P, Artursson G, Vedung S: Hyaluronic acid stimulates neutrophil function in vitro and in vivo. A review of experimental results and a presentation of a preliminary clinical trial. Scand J Infect Dis 1980, 24(Suppl):S54-S57 [PubMed] [Google Scholar]
- 10.Day AJ, Aplin RT, Willis AC: Overexpression, purification, and refolding of link module from human TSG-6 in Escherichia coli: effect of temperature, media, and mutagenesis on lysine misincorporation at arginine AGA codons. Protein Expr Purif 1996, 8:1-16 [DOI] [PubMed] [Google Scholar]
- 11.Kohda D, Morton CJ, Parkar AA, Hatanaka H, Inagaki FM, Campbell ID, Day AJ: Solution structure of the link module: a hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell 1996, 86:767-775 [DOI] [PubMed] [Google Scholar]
- 12.Day AJ: The structure and regulation of hyaluronan-binding proteins. Biochem Soc Trans 1999, 27:115-121 [DOI] [PubMed] [Google Scholar]
- 13.Wisniewski HG, Vilcek J: TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev 1997, 8:143-156 [DOI] [PubMed] [Google Scholar]
- 14.Fulop C, Kamanth RV, Li Y, Otto JM, Salustri A, Olsen BR, Glant TT, Hascall VC: Coding sequence, exon-intron structure and chromosomal localization of murine TNF-stimulated gene 6 that is specifically expressed by expanding cumulus cell-oocyte complexes. Gene 1997, 202:95-102 [DOI] [PubMed] [Google Scholar]
- 15.Carrette O, Nemade RV, Day AJ, Brickner A, Larsen WJ: TSG-6 is concentrated in the extracellular matrix of mouse cumulus oocyte complexes through hyaluron and inter-alpha-inhibitor binding. Biol Reprod 2001, 65:301-308 [DOI] [PubMed] [Google Scholar]
- 16.Wisniewski HG, Hua JC, Poppers DM, Naime D, Vilcek J, Cronstein BN: TNF/IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-alpha-inhibitor and exerts a strong anti-inflammatory effect in vivo. J Immunol 1996, 156:1609-1615 [PubMed] [Google Scholar]
- 17.Parkar AA, Kahmann JD, Howat SL, Bayliss MT, Day AJ: TSG-6 interacts with hyaluronan and aggrecan in a pH-dependent manner via a common functional element: implications for its regulation in inflamed cartilage. FEBS Lett 1998, 428:171-176 [DOI] [PubMed] [Google Scholar]
- 18.Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, Strauss III JF: Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol 1999, 154:1755–1762 [DOI] [PMC free article] [PubMed]
- 19.Lee TH, Wisniewski HG, Vilcek J: A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J Cell Biol 1992, 116:545-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee TH, Klampfer L, Shows TB, Vilcek J: Transcriptional regulation of TSG6, a tumor necrosis factor- and interleukin-1-inducible primary response gene coding for a secreted hyaluronan-binding protein. J Biol Chem 1993, 268:6154-6160 [PubMed] [Google Scholar]
- 21.Narumiya S, Sugimoto Y, Ushikubi F: Prostanoid receptors: structures, properties, and functions. Physiol Rev 1999, 79:1193-1226 [DOI] [PubMed] [Google Scholar]
- 22.Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM: Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol 1980, 138:273-281 [DOI] [PubMed] [Google Scholar]
- 23.Osmers R, Rath W, Pflanz MA, Kuhn W, Stuhlsatz HW, Szeverenyi M: Glycosaminoglycans in cervical connective tissue during pregnancy and parturition. Obstet Gynecol 1993, 81:88-92 [PubMed] [Google Scholar]
- 24.Golichowski AM, King SR, Mascaro K: Pregnancy-related changes in rat cervical glycosaminoglycans. Biochem J 1980, 192:1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu T, Endo M, Yosizawa Z: Glycoconjugates (glycosaminoglycans and glycoproteins) and glycogen in the human cervix uteri. Tohoku J Exp Med 1980, 131:289-299 [DOI] [PubMed] [Google Scholar]
- 26.Ogawa M, Hirano H, Tsubaki H, Kodama H, Tanaka T: The role of cytokines in cervical ripening: correlations between the concentrations of cytokines and hyaluronic acid in cervical mucus and the induction of hyaluronic acid production by inflammatory cytokines by human cervical fibroblasts. Am J Obstet Gynecol 1998, 179:105-110 [DOI] [PubMed] [Google Scholar]
- 27.Carbonne B, Jannet D, Dallot E, Pannier E, Ferre F, Cabrol D: Synthesis of glycosaminoglycans by human cervical fibroblasts in culture: effects of prostaglandin E2 and cyclic AMP. Eur J Obstet Gynecol Reprod Biol 1996, 70:101-105 [DOI] [PubMed] [Google Scholar]
- 28.Schmitz T, Dallot E, Leroy MJ, Breuiller-Fouche M, Ferre F, Cabrol D: EP4 receptors mediate prostaglandin E2-stimulated glycosaminoglycan synthesis in human cervical fibroblasts in culture. Mol Hum Reprod 2001, 7:397-402 [DOI] [PubMed] [Google Scholar]
- 29.Mahoney DJ, Blundell CD, Day AJ: Mapping the hyaluronan-binding site on the link module from human tumor necrosis factor-stimulated gene-6 by site-directed mutagenesis. J Biol Chem 2001, 276:22764-22771 [DOI] [PubMed] [Google Scholar]
- 30.Bayliss MT, Howat SL, Dudhia J, Murphy JM, Barry FP, Edwards JC, Day AJ: Up-regulation and differential expression of the hyaluronan-binding protein TSG-6 in cartilage and synovium in rheumatoid arthritis and osteoarthritis. Osteoarthritis Cartilage 2001, 9:42-48 [DOI] [PubMed] [Google Scholar]
- 31.Wisniewski HG, Maier R, Lotz M, Lee S, Klampfer L, Lee TH, Vilcek J: TSG-6: a TNF-α, IL-1β, and LPS-inducible secreted glycoprotein associated with arthritis. J Immunol 1993, 151:6593-6601 [PubMed] [Google Scholar]
- 32.Wisniewski HG, Burgess WH, Oppenheim JD, Vilcek J: TSG-6, an arthritis-associated hyaluronan binding protein, forms a stable complex with the serum protein inter-alpha-inhibitor. Biochemistry 1994, 33:7423-7429 [DOI] [PubMed] [Google Scholar]
- 33.Matrisian LM: The matrix-degrading metalloproteinases. Bioessays 1992, 14:455-463 [DOI] [PubMed] [Google Scholar]