Abstract
Extranodal marginal-zone B-cell lymphoma (MZBL) of mucosa-associated lymphoid tissue (MALT) arising in the thymus is rare, with the largest series in the literature including only three cases. In the present study, we investigated 15 cases of thymic MALT lymphoma to systematically characterize its clinical, histopathological, and molecular features. There was a marked female predilection (male:female = 1:4), with a mean age of 55 years at diagnosis. There was a strong association with autoimmune disease, especially Sjögren’s syndrome. Histologically, the thymic lymphoma showed the characteristic morphological features of extranodal MZBL of MALT type. Cysts were common. Prominent lymphoepithelial lesions were formed by centrocyte-like cells infiltrating and expanding the Hassall’s corpuscles and epithelium lining the cysts. Plasmacytic differentiation was apparent in all cases. Notably, 13 of 15 cases expressed immunoglobulin (Ig) A phenotype; IgA expression in thymic MALT lymphoma was in striking contrast with the IgM phenotype observed in most of the Sjögren’s syndrome-associated MZBLs and MALT lymphomas at other sites. Epstein-Barr virus was absent, and API2-MALT1 gene fusion, a recently reported MALT lymphoma-specific gene abnormality, was not detected in any case. Although one patient died of disease 85 months after the diagnosis, other patients were alive with overall 3-year and 5-year survival rates being 89% and 83%, respectively. Among the 22 patients reported previously and in the present series, at least 17 patients (77%) were Asians. These data indicate that thymic MALT lymphoma may represent a distinct subgroup of MALT lymphoma characterized by apparent predilection for Asians, a strong association with autoimmune disease, frequent presence of cysts, consistent plasma cell differentiation, tumor cells expressing IgA phenotype, and consistent lack of API2-MALT1 gene fusion.
Extranodal marginal-zone B-cell lymphoma (MZBL) of mucosa-associated lymphoid tissue (MALT), first described by Isaacson and Wright, 1 is currently recognized as a distinct clinicopathological entity in the category of MZBL according to the Revised European-American Lymphoma Classification 2 and the World Health Organization Classification for Tumors of Hematopoietic and Lymphoid Tissues. 3 This extranodal lymphoma is characterized by an indolent clinical course and characteristic histological appearances including centrocyte-like or monocytoid-like neoplastic cells, lymphoepithelial lesions, and follicular colonization. Multicentric involvement in the same mucosal site or several different mucosal organs is not uncommon. 4 Chronic inflammation or autoimmune disease, such as Helicobacter pylori gastritis, Hashimoto’s thyroiditis, and Sjögren’s syndrome (SS), apparently provides the soil for the emergence of the lymphoma; 5-7 this association suggests that proliferation of the lymphoma cells may depend on the presence of activated, antigen-driven T cells. 8,9
The commonest types of primary non-Hodgkin’s lymphoma of the thymus are T-cell lymphoblastic lymphoma 2,3 and primary mediastinal large B-cell lymphoma. 2,3,10,11 Primary low-grade B-cell MALT lymphoma is very rare in the thymus, with only 16 cases having been reported in the literature to date. 12-23 Because the largest series has included only three cases, 20 and follow-up information is limited, the clinicopathological and behavioral features of this lymphoma type have not been clearly delineated.
Recently, we and others showed that the API2 gene and the MALT1 gene are fused as a result of t(11;18)(q21;q21) in MALT lymphomas. 24-26 The fusion seems to be specific to MALT lymphoma, 27,28 and has been found in up to 50% of the cases. 29 Among MALT lymphomas of various anatomical sites, the lung shows the highest frequency of API2-MALT1 gene fusion. 30 However, thymic MALT lymphomas have not been examined.
In the present study, we investigated 15 cases of thymic MALT lymphoma by pooling case materials from several institutes, the largest series to date, to systematically characterize its clinical, histopathological, and molecular features.
Materials and Methods
Tissue and Clinical Data
From the files of Department of Pathology of Aichi Cancer Center Hospital (Japan), Nagoya City University (Japan), Queen Elizabeth Hospital (Hong Kong), and other collaborating institutes, 15 patients with thymic MALT lymphoma were identified. Nine of these cases have previously been reported. 12,13,15-17,21-23 All specimens were obtained at initial presentation and were reviewed by two independent pathologists (HI and SN). All tissue samples were fixed in 10% buffered formalin and embedded in paraffin. Tissue sections cut at 3 μm were prepared and stained with hematoxylin and eosin (H&E). The medical records of the patients were reviewed for the history, symptoms, laboratory data, and type of treatments. Follow-up was obtained from clinical records and attending physicians. For the cases included in this series that have been previously reported, follow-up information was updated. Staging of disease was assigned according to an adaptation of the Ann Arbor staging system for extranodal lymphomas. 31
Immunohistochemistry
The immunohistochemical study was performed using a streptavidin-biotin method as described elsewhere. 32 Deparaffinized tissue sections were treated with 3% hydrogen peroxide for 5 minutes, washed in phosphate-buffered saline, and then incubated with antibodies to the following antigens: cytokeratin (AE1/AE3; DAKO, Kyoto, Japan), CD20 (L26, DAKO), CD3 (polyclonal, DAKO), CD23 (1B12; Novocastra, Newcastle-on-Tyne, England), CD43 (MT1; Bio-Science, Emmerbrucke, Switzerland), CD5 (4C7, Novocastra), immunoglobulin (Ig) A (polyclonal, DAKO), IgD (polyclonal, DAKO), IgG (polyclonal, DAKO), IgM (polyclonal, DAKO), κ and λ light chains (polyclonal, DAKO), bcl2 protein (124, DAKO), cyclin D1 protein (5D4; MBL, Nagoya Japan), and ALK protein (polyclonal; Nichirei, Tokyo, Japan). Slides were incubated with biotinylated secondary antibody and then with peroxidase-streptavidin conjugate, followed by reaction with diaminobenzidine-hydrogen peroxide. The sections were counterstained with hematoxylin. Heat pretreatment was used for antigen retrieval except for antibodies against Ig.
In Situ Hybridization for Epstein-Barr Virus-Encoded Small RNA (EBER)
Epstein-Barr virus was detected on paraffin sections byin situ hybridization using fluorescein isothiocyanate-labeled EBER oligonucleotide probes (DAKO) and the DAKO Hybridization Detection Kit (DAKO).
Clonality Analyses by Southern Blot and Polymerase Chain Reaction (PCR)
Clonality analysis of Ig gene rearrangements was performed by Southern blot analysis or PCR as previously described. 13,21,33 Southern blot was used when frozen materials were available, and the blots were hybridized with probes to the joining region of the Ig heavy-chain (IgH) gene. 13 For PCR, DNA extracted from paraffin sections was amplified with primers (FR2A, FR3A, LJH, and VLJH) to detect rearrangement occurring between frame regions and the joining region of IgH. 21,33 Amplification of β-globin DNA fragment (268 bp) was used to indicate satisfactory preservation of DNA in the sample. DNA obtained from fresh placental tissue and that from fixed normal lymph nodes served as negative controls.
API2-MALT1 Fusion Transcript
API2-MALT1 fusion transcript was detected using paraffin sections according to the method we recently reported. 34 All eight variant fusion transcripts having been reported can be detected in this assay. Briefly, total RNA was extracted from the paraffin sections by proteinase K digestion. RNA was subjected to first-round multiplex reverse transcriptase-PCR, then to second-round nested multiplex PCRs (three parallel; second PCR-A, second PCR-B, and second PCR-C). The final PCR products were stained with ethidium bromide and run on 8% polyacrylamide gels. The band size ranges from 80 to 179 bp. RNA samples known to possess API2-MALT1 gene fusion were used as positive controls. As an internal control for RNA quality, the ubiquitously expressed β-actin mRNA fragment (190 bp) was amplified.
Results
Clinical Findings
The clinical features, treatment, and outcome are summarized in Table 1 ▶ . The study participants included 11 Japanese and 4 Chinese patients. Three patients were male and 12 were female (male:female = 1:4). The ages ranged from 36 to 75 years (mean and median, 55 years). Ten patients were asymptomatic, and the mediastinal masses were found on screening. Five patients presented with chest-related symptoms such as chest pain, shortness of breath, and hemoptysis. Systemic symptoms such as weight loss and fever, ie, B symptoms, were not present. Nine patients had an underlying autoimmune disease, most commonly SS (eight patients). The mean duration between the diagnosis of the autoimmune disease and lymphoma was 8.9 years. Although the other six patients were not clinically diagnosed as having autoimmune disease, two (patients 9 and 12) had serological findings suggestive of autoimmune disease. Patient 5 had concurrent MALT lymphomas in the salivary gland and lung, and patient 11 in the stomach. In both patients, the extrathymic lymphomas were discovered simultaneously with thymic lymphomas.
Table 1.
Clinical Features of Patients with Thymic MALT Lymphoma
| Patient | Nationality | Age (year)/sex | Symptoms | Concurrent tumors | Autoimmunity and duration (year) | Stage | Hyperglobulinemia (increased Ig type) | Treatment | Survival (months) | Response | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Japanese | 47/F | No | No | SS (4) | IA | M (IgA) | CTx, RTx | 8 | PR | AWD | 23 |
| 2 | Japanese | 61/M | No | No | SS (5) | IA | M (IgA) | S, RTx | NA | CR | Lost | 17 |
| 3 | Japanese | 75/F | No | No | SS (5) | IIA | M (IgA) | S, RTx | 24 | CR | NED | 17 |
| 4 | Japanese | 47/F | No | No | SS (2) | IA | P (IgG, A) | S, RTx | 48 | CR | NED | New case |
| 5 | Japanese | 46/F | No | Salivary, lung | SS (13) | IIA | No | S, CTx | 63 | CR | NED | New case |
| 6 | Japanese | 59/F | Chest pain | No | SS (25) | IIA | P (IgG, A, M) | S, RTx | 101 | CR | NED | 13 |
| 7 | Japanese | 67/M | No | No | No | IA | No | CTx, RTx | 85 | PR | DOD | New case |
| 8 | Japanese | 55/F | Back pain | No | RA (2) | IA | NA | S | 73 | CR | NED | 16 |
| 9 | Japanese | 63/F | No | No | Yes* | IA | P (IgG, A) | S | 60 | CR | NED | 22 |
| 10 | Japanese | 64/F | Back pain | No | No | IIA | No | S | 36 | CR | NED | 15 |
| 11 | Japanese | 36/F | No | Stomach | SS (11) | IIIA | P (IgG, A, M) | S, CTx | 8 | CR | DOC | 21 |
| 12 | Chinese | 55/F | No | No | Yes* | IIA | M (IgA) | S, CTx | 252 | CR | NED | 12 |
| 13 | Chinese | 42/F | No | No | No | IA | NA | S | NA | CR | Lost | New case |
| 14 | Chinese | 57/M | Short breath | No | No | IVA | No | CTx | 36 | CR | NED | New case |
| 15 | Chinese | 45/F | Hemoptysis | No | SS (13) | IIA | M (IgA) | CTx, RTx | 16 | CR | NED | New case |
NA, information not available; Yes*, probable autoimmune disease; CTx, chemotherapy; RTx, radiotherapy; S, surgery; AWD, alive with disease; NED, no evidence of disease; DOD, died of disease; DOC, died of other causes.
Physical examination did not reveal lymphadenopathy or splenomegaly. An anterior mediastinal mass was demonstrated radiographically. Laboratory tests showed that nine patients had hypergammaglobulinemia: polyclonal in four patients and monoclonal (M-protein) in five; the serum IgA titer was elevated in all of these patients. No patients had elevated lactate dehydrogenase level. Seven patients had stage I disease, six had stage II disease, and one each had stage III and stage IV disease.
Eleven tumors were surgically resected with or without additional treatment including chemotherapy and radiotherapy. The other four tumors were treated mainly by chemotherapy and radiotherapy. Most patients showed favorable clinical response: complete remission achieved in 13 patients (87%) and partial response in 2 (13%; patients 1 and 7). Recurrence occurred in two patients (patient 5 in the salivary gland at 20 months and patient 11 in the mediastinum at 8 months), both of whom presented initially with multicentric disease. During the follow-up (8 to 252 months; average, 62 months), there was one tumor-related death: patient 7, who showed a partial response to chemoradiotherapy, died of the tumor 85 month after the diagnosis. The overall 3-year and 5-year survival rates were 89% and 83%, respectively.
Gross Appearance
The pathological findings are summarized in Table 2 ▶ . The size of the mediastinal tumor ranged from 3 to 17 cm (average, 9.2 cm) in the largest diameter. The weight ranged from 30 to 160 g (average, 89 g). Macroscopically, many of the tumors were solid with multiple cystic spaces. Figure 1 ▶ shows the representative gross appearance.
Table 2.
Macroscopical, Histological, and Molecular Features of Thymic MALT Lymphomas
| Patient | Specimen | Size (cm) | Weight (g) | Regional node metastasis | Gross appearance | Ig class/light chain | EBER | Clonality | API2-MALT1 (beta-actin) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Biopsy | 7 | NA | No | Solid and cystic | IgA/L | No | M, PCR | No (Yes) |
| 2 | Resection | 9.5 | NA | No | Partially cystic | IgA/K | No | M, Southern | No (Yes) |
| 3 | Resection | 13.1 | NA | Yes | Homogeneous | IgA/K | No | M, Southern | No (Yes) |
| 4 | Resection | 6 | 30 | No | Partially cystic | IgA/K | No | M, PCR | No (Yes) |
| 5 | Resection | 7 | NA | Yes | Cystic | IgG/K | No | Smear, PCR | No (Yes) |
| 6 | Resection | 13 | 150 | Yes | Partially cystic | IgA/K | No | M, Southern | No (Yes) |
| 7 | Biopsy | 8.5 | NA | No | Solid | IgG/L | No | M, PCR | No (Yes) |
| 8 | Resection | 7.5 | 50 | No | Homogeneous | IgA/K | No | M, PCR | No (Yes) |
| 9 | Resection | 17 | 120 | No | Solid and cystic | IgA/K | No | M, PCR | No (Yes) |
| 10 | Resection | 5 | 44 | Yes | Solid | IgA/L | No | M, PCR | No (Yes) |
| 11 | Resection | 3 | 68 | Yes | Solid | IgA/L | No | Smear, PCR | No (Yes) |
| 12 | Resection | 10 | 160 | No | Solid and cystic | IgA/K | No | M, PCR | No (Yes) |
| 13 | Resection | 11 | 90 | No | Solid and cystic | IgA/L | No | M, PCR | No (Yes) |
| 14 | Biopsy | 17 | NA | No | NA | IgA/L | No | M, PCR | No (Yes) |
| 15 | Biopsy | 3 | NA | Yes | NA | IgA/K | No | M, PCR | No (Yes) |
NA, information not available; K, kappa; L, lambda; M, monoclonal; Southern, Southern blot.
Figure 1.
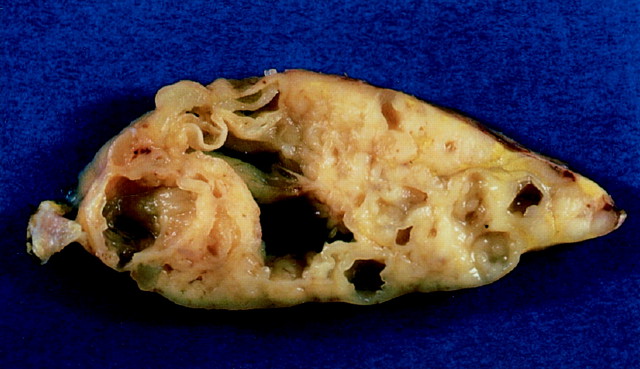
The cut surface of the thymic tumor shows multiple cystic spaces with intervening pale tan tissue.
Histological Findings
The thymic architecture was totally effaced by a dense infiltration of small- to medium-sized lymphoid cells (Figure 2) ▶ . There were interspersed reactive lymphoid follicles with preserved mantle zones. The lymphoid infiltrate showed a variegated appearance at low magnification because of presence of different populations of cells, which included centrocyte-like (CCL) cells, small lymphocytes, plasma cells, and rare large transformed cells. The most prominent population was CCL cells forming broad, pale-staining bands and sheets (Figure 3A) ▶ . These cells had indented or folded nuclei and a moderate amount of pale to clear cytoplasm, sometimes reminiscent of monocytoid B cells. Lymphoepithelial lesions were a prominent feature, being formed by expanded Hassall’s corpuscles (Figure 3B) ▶ or cystic epithelium infiltrated extensively by the CCL cells with pale cytoplasm (Figure 3C) ▶ . Variable-sized epithelium-lined cysts containing eosinophilic proteinaceous fluid, occasionally with cholesterol crystals, were recognized in all of the cases including those in which cystic change was not apparent macroscopically. In all cases, plasma cell differentiation was recognized. Plasma cells were often found to aggregate around the vessels, which frequently showed mild to moderate sclerosis (Figure 3D) ▶ . The plasma cells showed minimal nuclear atypia, and nuclear and cytoplasmic Ig inclusion bodies were rarely recognized. Focal calcification was occasionally observed, and follicular colonization was not evident on H&E-stained sections.
Figure 2.
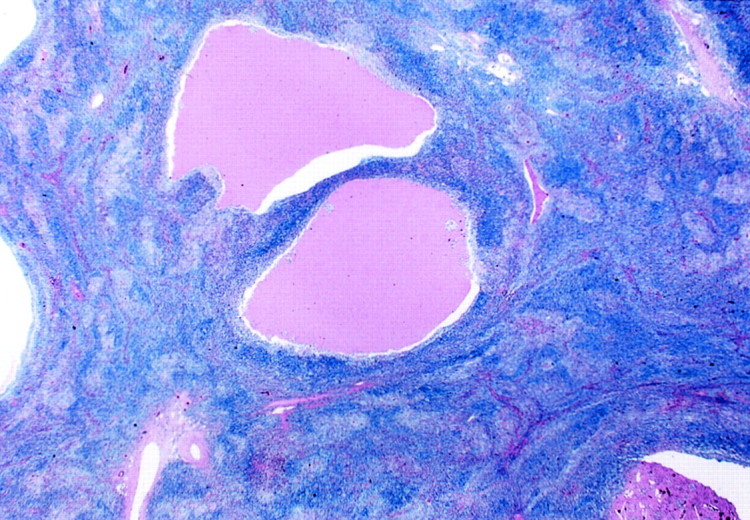
The low-power view of thymic MALT lymphoma shows totally effaced thymic architecture, interspersed reactive lymphoid follicles with preserved mantle zones, and variable-sized cysts containing eosinophilic fluid, occasionally with cholesterol crystals (H&E; original magnification, ×2.5).
Figure 3.
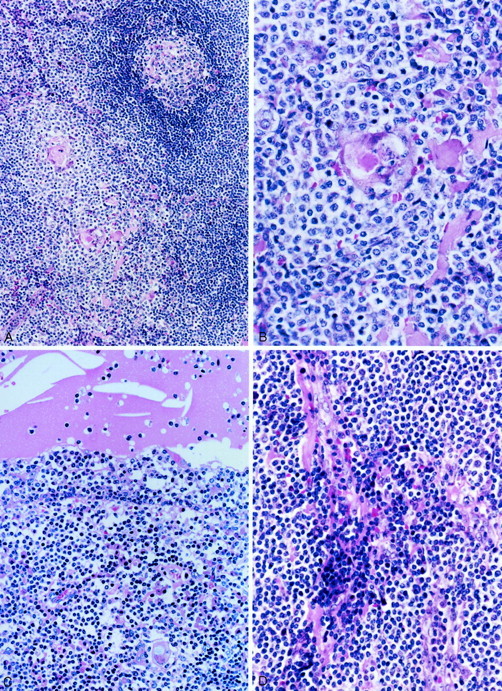
Histological appearance of thymic MALT lymphoma. A: A lymphoid follicle with intact mantle zone is surrounded by a diffuse small lymphoid infiltrate. B: Lymphoepithelial lesion formed by a Hassall’s corpuscle infiltrated by CCL cells. C: The epithelium lining the cyst is invaded by CCL cells forming the lymphoepithelial lesion. The cyst contains eosinophilic material and cholesterol crystals. D: Mature plasma cells are found around the vessels that show mild sclerosis. H&E; original magnifications: ×40 (A), ×100 (B), ×66 (C), ×80 (D).
Immunohistochemistry
Immunostaining for cytokeratin highlighted the lymphoepithelial lesions that were formed predominantly by the expanded Hassall’s corpuscles and epithelium lining the cysts. The immunohistochemical features of lymphoma cells are detailed in Table 2 ▶ . The tumor cells were immunoreactive for CD20 (Figure 4A) ▶ and bcl2, but not CD3, CD5, CD23, CD43, cyclin D1, or ALK protein. The plasma cells showed monotypic Ig light chain (κ type in nine cases and λ type in six; Figure 4, B and C ▶ ). Notably, 13 of 15 cases showed IgA heavy-chain type (Figure 4D) ▶ , and the remaining 2 cases (patients 5 and 7) IgG type. Patient 5 had concurrent salivary and pulmonary MALT lymphomas in addition to the thymic tumor, and patient 7 was the only case that showed tumor-related death. Extrathymic lymphomas found in patients 5 and 11 showed identical Ig heavy-chain type with that of thymic lymphoma.
Figure 4.
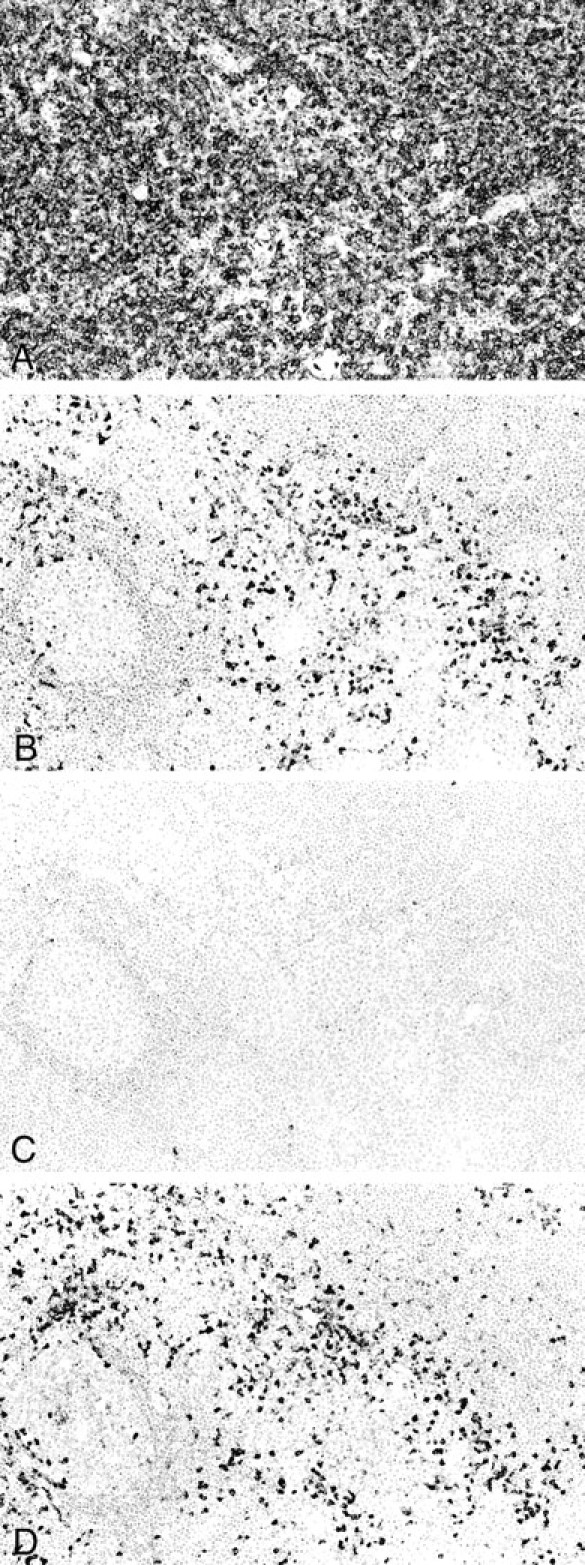
Immunohistochemical studies. A: The lymphoma cells are positive for the B-cell marker CD20. B: Plasmacytic cells show κ light chain restriction. C: Only a few plasmacytic cells express λ light chain. D: Most plasmacytic cells express IgA heavy chain. Serial sections, hematoxylin; original magnifications, ×33 (A–D).
EBER, Clonality Analysis, and API2-MALT1 Fusion Transcript
In situ hybridization for EBER was totally negative in 14 cases; EBER signals were found in less than 2% of lymphoma cells in 1 case (case 13). In all three cases studied by Southern blot, clonal IgH rearrangement was demonstrated. In the other 12 cases, PCR assay for IgH rearrangement showed distinct rearranged bands indicating monoclonality in 10 cases and a smear pattern in 2 cases. DNA quality was confirmed in all cases by successful amplification of the β-globin gene. Multiplex reverse transcriptase-PCR failed to detect API2-MALT1 fusion transcript in any cases (Figure 5) ▶ . The negative results were confirmed by repeat assays. Positive controls yielded definite bands, and β-actin mRNA fragment was successfully amplified in all cases indicating good preservation of RNA in the tested samples.
Figure 5.
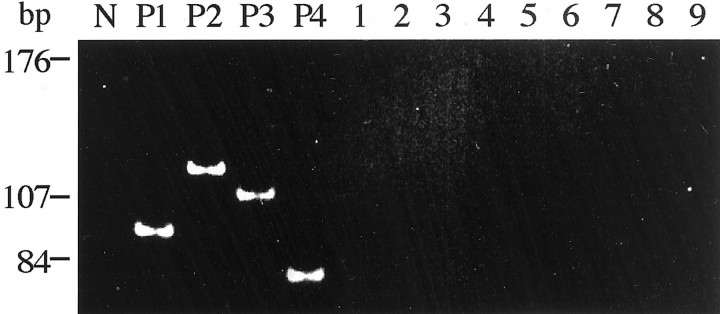
Multiplex reverse transcriptase-PCR assay for API2-MALT1 fusion transcript (second PCR-B). N, no template RNA; P1 to P4, four positive controls with breakpoints A1446 to M541 (94 bp), 1446 to M814 (119 bp), A1446 to M1123 (107 bp), and A1446 to M1150 (80 bp), respectively (see Materials and Methods and Inagaki et al 34 ). Lanes 1–9, thymic MALT lymphomas showing no detectable PCR products.
Discussion
Including the current series, 22 cases of thymic MALT lymphoma have been described in the literature. 12-23 It is noteworthy that at least 17 (77%) of these patients are Asians. The apparent rarity of this lymphoma type in Caucasians suggests that there may be racial and/or environmental factors influencing its development.
Pathologically, thymic MALT lymphoma commonly manifests as a multicystic mediastinal mass. Even in cases with no cysts detected macroscopically, cystic spaces are recognized in all cases microscopically. Although cysts formed by dilated ducts can be observed in some cases of salivary gland MALT lymphomas, epithelium-lined cysts appear to be a constant and distinctive feature of MALT lymphoma of the thymus. 35 Cyst formation may be related to the tendency for cystic transformation of medullary duct-epithelium-derived structures (including Hassall’s corpuscles) when tumor grows in the thymic gland. 36 All cases otherwise show the typical morphological and immunophenotypic features of MALT lymphoma. The tumor is composed predominantly of CCL cells, admixed with variable components of plasma cells, small lymphocytes, and a few large lymphoid cells. Within and around epithelial structures, the lymphoid cells usually resemble monocytoid B cells. Lymphoepithelial lesions are always prominent. Plasma cell differentiation is recognized in all cases, a feature that is not so frequently observed in MALT lymphomas of other sites (∼40%). 4 The plasmacytic differentiation in this tumor can aid in the diagnosis because it renders demonstration of light chain restriction using immunohistochemistry relatively easy. On the other hand, detection of monoclonality by IgH rearrangement using PCR 33 is not always successful, as well shown in this study, attributable to poor annealing of primers to the Ig gene because of partial rearrangement or somatic hypermutations.
There is a remarkable association of thymic MALT lymphoma with autoimmune disease. In this series of 15 cases, 8 cases (53%) had SS, and 1 had rheumatoid arthritis. Interestingly, two additional patients showed blood test abnormalities suggestive of autoimmunity, although they were asymptomatic. If these latent cases are also counted, 11 of 15 patients (73%) developed thymic MALT lymphomas in a setting of autoimmunity. The mean interval between the diagnosis of autoimmune disease and thymic MALT lymphoma is 8.9 years. It is conceivable that chronic inflammation in the thymic gland caused by autoimmunity may play an important role in pathogenesis of thymic MALT lymphoma, although the pathological alterations in the thymic glands of patients with SS have not been well documented. It has been reported that the majority of lymphomas occurring in SS patients are nodal or extranodal low-grade B-cell lymphomas. Royer and colleagues 37 reported that 12 of 16 lymphomas that occurred in association with SS were MZBLs including 9 cases of MALT lymphoma. In their series, one thymic MALT lymphoma case arose concurrently with salivary and gastric MALT lymphomas. Shin and colleagues 38 described the clinicopathological features of 13 SS patients with monocytoid B-cell lymphoma (MZBL) of either nodal or extranodal origin. Combining the clinical profile of the SS patients with MZBLs from these two reports, the mean age was 58 years, there was marked female predilection (male:female = 1:7.3), and the SS diagnosis was established 8.2 years before the diagnosis of MZBL. These profiles are similar to those observed in the patients with thymic MALT lymphoma reported in this series, suggesting that thymic MALT lymphoma may be a rare form of MZBL arising in association with SS. Nonetheless, despite these similarities, there is a striking difference between thymic MALT lymphoma and other SS-associated MZBL: 13 of 15 cases (87%) of thymic MALT lymphoma show an IgA phenotype, whereas SS-associated nonthymic MZBLs have been reported to express exclusively an IgM phenotype. 37-40 IgA phenotype is also commonly observed in thymic MALT lymphomas reported from the West; two of the four such cases have expressed IgA phenotype. 14,20 This IgA expression also discriminates thymic MALT lymphoma from nonthymic MALT lymphomas, which typically express IgM, and less often IgG or IgA. 2,3 The reason of IgA restriction in thymic MALT lymphoma is not clear, and IgA restriction in lymphoma is limited to α-heavy-chain disease, namely, immunoproliferative small intestinal disease, 41,42 and a rare type of nodal large B-cell lymphoma characterized by frequent sinusoidal involvement, immunoblastic/plasmablastic morphology, negative staining for CD20 and CD79a, expression of full-length ALK protein, and lack of t(2;5) translocation. 3,43 The former is endemic in Middle-Eastern and Mediterranean countries and considered as a variant of MALT lymphoma by some researchers. It is certainly worthwhile to pursue whether thymic MALT lymphoma is incited by an infective process such as immunoproliferative small intestinal disease. However, thymic MALT lymphoma is immunopathologically different from α-chain disease in that Ig light chains are expressed.
Primary mediastinal large B-cell lymphoma is a distinctive high-grade lymphoma of the thymus. 2,3,10,11 Is it the high-grade counterpart of thymic MALT lymphoma? Although there has been one case of thymic MALT lymphoma (IgM phenotype and lacking association with autoimmunity) reported to show transformation to a large cell lymphoma (case 3), 20 several findings suggest that they are not related: 1) mediastinal large B-cell lymphoma does not show such a striking female predilection; 2) the patients are much younger (most frequent in the third decades of life); 3) there is no association with autoimmune disease; 4) none of the cases in this series shows a large cell component at presentation or on follow-up; and 5) mediastinal large B-cell lymphoma usually lacks expression of Ig (including IgA).
There is no evidence that Epstein-Barr virus plays a role in the pathogenesis of thymic MALT lymphoma, as all 15 cases in this series were negative for EBER by in situ hybridization. This finding is also consistent with the lack of Epstein-Barr virus association in previous studies on MALT lymphomas of other sites, including patients from Western and Eastern countries. 32,44 Using a recently developed multiplex reverse transcriptase-PCR assay that can be applied to routinely-processed paraffin sections, 34 we did not find API2-MALT1 gene fusion in any of the cases of thymic MALT lymphoma, suggesting that other oncogenic events may be responsible for the development of thymic MALT lymphoma. As we and others recently reported, API2-MALT1 gene fusion may confer inflammation-independent growth to gastric MALT lymphomas and hence resistance to H. pylori eradication therapy. 45-47 The alternative explanation for lack of API2-MALT1 gene fusion in thymic MALT lymphoma is that it is an early lymphoma in the evolution chain. 48 However, this does not seem likely because most of the thymic MALT lymphomas already form bulky masses at diagnosis.
Surgical excision and/or chemoradiotherapy is usually effective, and long-term survival is common. Among the 15 cases, there was 1 tumor-related death 85 months after the diagnosis (patient 7) with overall 3-year and 5-year survival rates being 89% and 85%, respectively. When we consider six cases with long follow-up (≥5 years, patients 5 to 9 and 12), five patients were well and alive after surgery. However, patient 7 underwent no surgical intervention, partially responded to chemoradiotherapy, and finally died of the disease. This patient had an IgG-type lymphoma unlike most other lymphomas, however, there was no other clinical or pathological characteristic that would be helpful to predict the aggressive evolution of the lymphoma. Although more cases have to be studied, we would recommend surgical treatment at least when complete remission is not achieved by nonsurgical treatment.
In summary, thymic MALT lymphoma seems to be clinicopathologically distinctive from MALT lymphomas in other sites: prevalence in Asians, strong association with autoimmune disease, marked female predominance, frequent presence of epithelium-lined cysts, almost invariable presence of a neoplastic plasma cell component, expression of IgA phenotype, and absence of API2-MALT1 gene fusion.
Acknowledgments
We thank Seizo Nagaya, Hisashi Takino, and Chika Ando for their excellent technical assistance.
Footnotes
Address reprint requests to Hiroshi Inagaki, M.D., Department of Pathology, Nagoya City University Medical School, 1 Kawasumi, Mizuho-ku, Nagoya, 467-8601, Japan. E-mail: hinagaki@med.nagoya-cu.ac.jp.
Supported in part by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan (grant no. 13670185).
References
- 1.Isaacson PG, Wright DH: Malignant lymphoma of mucosa-associated lymphoid tissue: a distinctive type of B-cell lymphoma. Cancer 1983, 52:1410-1416 [DOI] [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Müller-Hermelink HK, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: a proposal from the international Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 3.Jaffe ES, Harris NL, Stein H, Vardiman JW (Eds): Tumours of haematopoietic and lymphoid tissues. World Health Organization Classification of Tumors, Pathology, and Genetics. Lyon, IARC Press, 2001
- 4.Isaacson PG, Spencer J: Malignant lymphoma of mucosa-associated lymphoid tissue. Histopathology 1987, 11:445-462 [DOI] [PubMed] [Google Scholar]
- 5.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG: Helicobacter pylori-associated gastritis, and primary B-cell gastric lymphoma. Lancet 1991, 338:1175-1176 [DOI] [PubMed] [Google Scholar]
- 6.Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD: Helicobacter pylori infection, and gastric lymphomas. N Engl J Med 1994, 330:1267-1271 [DOI] [PubMed] [Google Scholar]
- 7.Isaacson PG: Mucosa-associated lymphoid tissue lymphoma. Semin Hematol 1999, 36:139-147 [PubMed] [Google Scholar]
- 8.Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG: Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993, 342:575-577 [DOI] [PubMed] [Google Scholar]
- 9.Greiner A, Knorr C, Qin Y, Sebald W, Schimpl A, Banchereau J, Müller-Hermelink HK: Low-grade B cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signaling, and Th2-type cytokines for in vitro growth and differentiation. Am J Pathol 1997, 150:1583-1593 [PMC free article] [PubMed] [Google Scholar]
- 10.Suster S: Primary large-cell lymphomas of the mediastinum. Semin Diagn Pathol 1999, 16:51-64 [PubMed] [Google Scholar]
- 11.Cazals-Hatem D, Lepage E, Brice P, Ferrant A, d’Agay MF, Baumelou E, Briere J, Blanc M, Gaulard P, Biron P, Schlaifer D, Diebold J, Audouin J: Primary mediastinal large B-cell lymphoma. A clinicopathological study of 141 cases compared with 916 nonmediastinal large B-cell lymphomas, a GELA study. Am J Surg Pathol 1996, 20:877-888 [DOI] [PubMed] [Google Scholar]
- 12.Isaacson PG, Chan JKC, Tang C, Addis B: Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue arising in the thymus: a thymic lymphoma mimicking myoepithelial sialadenitis. Am J Surg Pathol 1990, 14:342-351 [DOI] [PubMed] [Google Scholar]
- 13.Takagi N, Nakamura S, Yamamoto K, Kunishima K, Takagi I, Suyama M, Shinoda M, Sugiura T, Oyama A, Suzuki H, Koshikawa T, Kontani K, Ueda R, Takahashi T, Ariyoshi Y, Suchi T: Malignant lymphoma of mucosa-associated lymphoid tissue arising in the thymus of a patient with Sjogren’s syndrome. A morphologic, phenotypic, and genotypic study. Cancer 1991, 69:1347-1355 [DOI] [PubMed] [Google Scholar]
- 14.DiLoreto C, Mariuzzi L, DeGrassi A, Beltrami CA: B cell lymphoma of the thymus and salivary gland. J Clin Pathol 1996, 49:595-597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimosato Y, Mukai K: Tumor of the mediastinum. Atlas of Tumor Pathology, third series, fascicle 21. 1997, Armed Forces Institute of Pathology, Washington DC
- 16.Yokose T, Kodama T, Matsuno Y, Shimosato Y, Nishimura M, Mukai K: Low-grade B cell lymphoma of mucosa-associated lymphoid tissue in the thymus of a patient with rheumatoid arthritis. Pathol Int 1998, 48:74-81 [DOI] [PubMed] [Google Scholar]
- 17.Yamasaki S, Matsushita H, Tanimura S, Nakatani T, Hara S, Endo Y, Hara M: B-cell lymphoma of mucosa-associated lymphoid tissue of the thymus: a report of two cases with a background of Sjogren’s syndrome and monoclonal gammopathy. Hum Pathol 1998, 29:1021-1024 [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Nam ES, Yi JG, Shin HS, Kim IS: Mucosa-associated lymphoid tissue (MALT) lymphoma of the thymus. Case report and literature review. Int J Surg Pathol 1998, 6:229-234 [Google Scholar]
- 19.McCluggage WG, McManus K, Qureshi R, McAleer S, Wotherspoon AC: Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) of thymus. Hum Pathol 2000, 31:255-259 [DOI] [PubMed] [Google Scholar]
- 20.Lorsbach RB, Pinkus GS, Shahsafaei A, Forfman DM: Primary marginal zone lymphoma of the thymus. Am J Clin Pathol 2000, 113:784-791 [DOI] [PubMed] [Google Scholar]
- 21.Nagasaka T, Lai R, Harada T, Chen YY, Chen WG, Arber DA, Weiss LM: Coexisting thymic and gastric lymphomas of mucosa-associated lymphoid tissues in a patient with Sjogren’s syndrome. Arch Pathol Lab Med 2000, 124:770-773 [DOI] [PubMed] [Google Scholar]
- 22.Moriyama E, Yokose T, Kodama T, Matsumoto Y, Hojo F, Takahashi K, Nagai K, Nishiwaki Y, Ochiai A: Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue in the thymus of a patient with pulmonary amyloid nodules. Jpn J Clin Oncol 2000, 30:349-353 [DOI] [PubMed] [Google Scholar]
- 23.Kamimura K, Nakamura N, Ishibashi T, Maruyama Y, Abe M: Somatic hypermutation of immunoglobulin heavy chain variable region genes in thymic marginal zone B cell lymphoma of MALT type of a patient with Sjogren’s syndrome. Histopathology 2002, 40:294-296 [DOI] [PubMed] [Google Scholar]
- 24.Dierlamm J, Baens M, Wlodarska I, Stefanova-Ouzounova M, Hernandez JM, Hossfeld DK, De Wolf-Peeters C, Hagemeijer A, Van den Berghe H, Marynen P: The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood 1999, 93:3601-3609 [PubMed] [Google Scholar]
- 25.Akagi T, Tamura A, Motegi M, Suzuki R, Hosokawa Y, Nakamura S, Morishima Y, Seto M: A novel gene, MALT1 at 18q21, is involved in t(11;18)(q21;q21) found in low-grade B-cell lymphoma of mucosa-associated lymphoid tissue. Oncogene 1999, 18:5785-5794 [DOI] [PubMed] [Google Scholar]
- 26.Morgan JA, Yin Y, Borowsky AD, Kuo F, Nourmand N, Koontz JI, Reynolds C, Soreng L, Griffin CA, Graeme-Cook F, Harris NL, Weisenburger D, Pinkus GS, Fletcher JA, Sklar J: Breakpoint of the t(11;18)(q21;q21) in mucosa-associated lymphoid tissue (MALT) lymphoma lie within or near the previously undescribed gene MALT1 in chromosome 18. Cancer Res 1999, 59:6205-6213 [PubMed] [Google Scholar]
- 27.Rosenwald A, Ott G, Stilgenbauer S, Kalla J, Bredt M, Katzenberger T, Greiner A, Ott MM, Gawin B, Dojner H, Konrad H, Müller-Hermelink HK: Exclusive detection of the t(11;18)(q21;q21) in extranodal marginal zone B cell lymphomas (MZBL) of MALT type in contrast to other MZBL and extranodal large B cell lymphomas. Am J Pathol 1999, 155:1817-1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remstein ED, James CD, Kurtin PJ: Incidence and subtype specificity of API2-MALT1 fusion translocations in extranodal, nodal, and splenic marginal zone lymphomas. Am J Pathol 2000, 156:1183-1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baens M, Maes B, Steyls A, Geboes K, Marynen P, De Wolf-Peeters C: The product of the t(11;18), an API2-MLT fusion, marks nearly half of gastric MALT type lymphomas without large cell proliferation. Am J Pathol 2000, 156:1433-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motegi M, Yonezumi M, Suzuki H, Suzuki R, Hosokawa Y, Hosaka S, Kodera Y, Morishima Y, Nakamura S, Seto M: API2-MALT1 chimeric transcripts involved in mucosa-associated lymphoid tissue type lymphoma predict heterogeneous products. Am J Pathol 2000, 156:807-812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musshoff K: Stadieneinteilung der nicht-Hodgkin lymphome. Strahlentherapie 1977, 153:218-221 [PubMed] [Google Scholar]
- 32.Inagaki H, Banno S, Wakita A, Ueda R, Eimoto T: Prognostic significance of CD44v6 in diffuse large B-cell lymphoma. Mod Pathol 1999, 12:546-552 [PubMed] [Google Scholar]
- 33.Inagaki H, Nonaka M, Nagaya S, Tateyama H, Sasaki M, Eimoto T: Monoclonality in gastric lymphoma detected in formalin-fixed, paraffin-embedded endoscopic biopsy specimens using immunohistochemistry, in situ hybridization, and polymerase chain reaction. Diagn Mol Pathol 1995, 4:32-38 [DOI] [PubMed] [Google Scholar]
- 34.Inagaki H, Okabe M, Seto M, Nakamura S, Ueda R, Eimoto T: API2-MALT1 fusion transcripts involved in mucosa-associated lymphoid tissue lymphoma. Multiplex RT-PCR detection using formalin-fixed paraffin embedded specimens. Am J Pathol 2001, 158:699-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaacson PG, Norton AJ: Extranodal lymphomas. 1994:pp 70-81 Churchill-Livingstone, Edinburgh
- 36.Chan JKC: Tumors of the lymphoreticular system, including spleen and thymus. ed 2 Fletcher CDM eds. Diagnostic Histopathology of Tumors, 2000, :pp 1306-1307 Churchill-Livingstone, London [Google Scholar]
- 37.Royer B, Cazals-Hatem D, Sibilia J, Agbalika F, Cayuela JM, Soussi T, Maloisel F, Clauvel JP, Brouet JC, Mariette X: Lymphomas in patients with Sjögren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood 1997, 90:766-775 [PubMed] [Google Scholar]
- 38.Shin SS, Sheibani K, Fishleder A, Ben-Ezra J, Bailey A, Koo CH, Burke JS, Tubbs R, Rappaport H: Monocytoid B-cell lymphoma in patients with Sjogren’s syndrome: a clinicopathological study of 13 patients. Hum Pathol 1991, 22:422-430 [DOI] [PubMed] [Google Scholar]
- 39.Zulman J, Jaffe R, Talal N: Evidence that malignant lymphoma of Sjogren’s syndrome is a monoclonal B-cell neoplasm. N Engl J Med 1978, 299:1215-1220 [DOI] [PubMed] [Google Scholar]
- 40.McCurley TL, Collins RD, Ball E, Collins RD: Nodal and extranodal lymphoproliferative disorders in Sjogren’s syndrome: a clinical and immunopathological study. Hum Pathol 1990, 21:482-492 [DOI] [PubMed] [Google Scholar]
- 41.Isaacson PG, Dogan A, Price SK, Spencer J: Immunoproliferative small-intestinal disease: an immunohistochemical study. Am J Surg Pathol 1989, 13:1023-1033 [DOI] [PubMed] [Google Scholar]
- 42.Fine KD, Stone MJ: Alpha-heavy chain disease, Mediterranean lymphoma, and immunoproliferative small intestinal disease. Am J Gastroenterol 1999, 94:1139-1152 [DOI] [PubMed] [Google Scholar]
- 43.Delsol G, Lamant L, Mariame B, Pulford K, Dastugue N, Brousset P, Rigal-Huguet F, Saati TA, Cerretti DP, Morris SW, Mason D: A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking 2;5 translocation. Blood 1997, 89:1483-1490 [PubMed] [Google Scholar]
- 44.Xu WS, Chan AC, Lee JM, Liang RH, Ho FC, Srivastava G: Epstein-Barr virus infection and its gene expression in gastric lymphoma of mucosa-associated lymphoid tissue. J Med Virol 1998, 56:342-350 [DOI] [PubMed] [Google Scholar]
- 45.Nakamura T, Nakamura S, Yonezumi M, Suzuki T, Matsuura A, Yatabe Y, Yokoi T, Ohshi K, Seto M: Helicobacter pylori and the t(11;18)(q21;q21) translocation in gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Jpn J Cancer Res 2000, 91:301-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, Ruskon-Fourmestraux, Lavergne-Slove A, Ye H, Molina T, Bouhnik Y, Hamoudi RA, Diss TC, Dogan A, Megraud F, Rambaud JC, Du MQ, Isaacson PG: Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001, 357:39-40 [DOI] [PubMed] [Google Scholar]
- 47.Sugiyama T, Asaka M, Nakamura S, Yonezumi S, Seto M: API2-MALT1 chimeric transcript is a predictive marker for the responsiveness of H. pylori eradication treatment in a low-grade gastric MALT lymphoma. Gastroenterology 2001, 120:1884-1885 [DOI] [PubMed] [Google Scholar]
- 48.Alpen B, Neubauer A, Dierlamm J, Marynen P, Thiede C, Bayerdörffer E, Stolte M: Translocation t(11;18) absent in early gastric marginal zone B-cell lymphoma of MALT type responding to eradication of Helicobacter pylori infection. Blood 2000, 95:4014-4015 [PubMed] [Google Scholar]


