Abstract
We have developed an ultrasensitive bienzyme-substrate-recycle enzyme-linked immunosorbent assay for the measurement of Alzheimer’s disease (AD) abnormally hyperphosphorylated tau in cerebrospinal fluid (CSF). The assay, which recognizes attomolar amounts of tau, is ∼400 and ∼1300 times more sensitive than conventional enzyme-linked immunosorbent assay in determining the hyperphosphorylated tau and total tau, respectively. With this method, we measured both total tau and tau phosphorylated at Ser-396/Ser-404 in lumbar CSFs from AD and control patients. We found that the total tau was 215 ± 77 pg/ml in cognitively normal control (n = 56), 234 ± 92 pg/ml in non-AD neurological (n = 37), 304 ± 126 pg/ml in vascular dementia (n = 46), and 486 ± 168 pg/ml (n = 52) in AD patients, respectively. However, a remarkably elevated level in phosphorylated tau was only found in AD (187 ± 84 pg/ml), as compared with normal controls (54 ± 33 pg/ml), non-AD (63 ± 34 pg/ml), and vascular dementia (72 ± 33 pg/ml) groups. If we used the ratio of hyperphosphorylated tau to total tau of ≥0.33 as cutoff for AD diagnosis, we could confirm the diagnosis in 96% of the clinically diagnosed patients with a specificity of 95%, 86%, 100%, and 94% against nonneurological, non-AD neurological, vascular dementia, and all of the three control groups combined, respectively. It is suggested that the CSF level of tau phosphorylated at Ser-396/Ser-404 is a promising diagnostic marker of AD.
Alzheimer’s disease (AD) is the most common age-associated neurodegenerative disorder that affects an increasing number of the elderly around the world. Although significant progress in clinical and pathological diagnosis of the disease has been made recently, a definite diagnosis of the disease still relies on the demonstration of numerous neurofibrillary tangles and senile plaques in the brain, which is mostly done in autopsied tissue. Thus, for determining the efficacy of therapeutic drugs and for drug trials for AD, there is an urgent need for peripheral biochemical markers that represent specifically the brain lesions. Furthermore, a laboratory diagnostic marker can also add to the accuracy of the clinical diagnosis of the disease. Based on these needs, great efforts have been devoted in searching biochemical markers in cerebrospinal fluid (CSF) that can be diagnostic of AD. 1-6
Among all of the abnormalities described in the AD brain to date, those related to the hallmark neuropathological lesions, ie, formation of neurofibrillary tangles and deposition of amyloid β, are the best documented and the most promising diagnostic markers. In addition to a decreased level of Aβ1-42, 7 a pronounced increase in CSF tau has been found in most AD patients. 5,6,8-13 However, an increased level of total tau is also found in several neurological disorders other than AD.
It has been well studied and commonly accepted that abnormally phosphorylated tau is the major protein subunit of Alzheimer’s paired helical filaments (PHFs). 14,15 Among all of the phosphorylation sites found in PHF-tau, 16 C-terminal Ser-396 and Ser-404 represent a major Alzheimer’s epitope. Phosphorylation of tau at this epitope reduces its biological activity in promoting microtubule assembly, binding to microtubules, and the ability in stabilizing microtubules against nocodazole-induced depolymerization. 17-19 Dephosphorylation of AD abnormally hyperphosphorylated tau (AD P-tau) at these sites by protein phosphatases shifts its mobility to the position of normal tau in sodium dodecyl sulfate-polyacrylamide gel electrophoresis, restores its biological activity, and relaxes the structure of PHFs. 20,21 All these data strongly suggest that phosphorylation at Ser-396 and Ser-404 of tau might play a crucial role in AD pathology. However, the level of phosphorylated tau in CSF is relatively low compared with normal tau and has been difficult to quantitate. 22 To this end, we have modified and adapted the enzyme amplification method of Johannsson and colleagues, 23 and successfully developed a highly specific and ultrasensitive assay in the attomolar range for the quantitation of total tau and tau phosphorylated at Ser-396/Ser-404 in CSF, and have found a significant increase in the levels of tau, especially the phosphorylated protein in AD.
Materials and Methods
CSF Samples
Samples of lumbar CSF of living patients were obtained from The Netherlands Brain Bank and several teaching hospitals in China (THC) (Table 1) ▶ . The information on CSF samples obtained from The Netherlands Brain Bank is as follows: AD (n = 30), 13 male and 17 female with ages from 62 to 78 years (mean, 71 years), Mini-Mental State Examination (MMSE) score from 8 to 27 (mean, 21.4), and ApoE genotypes 4/4 (n = 4), 4/3 (n = 14), 3/3 (n = 11), and 3/2 (n = 1); vascular dementia (VaD, n = 18); non-AD (n = 13): spinal canal stenosis (n = 4), depression (n = 3), malignant lymphoma (n = 2), vertebro-basilar artery infarct (n = 1), diabetic neuropathy (n = 1), subclavian steal syndrome (n = 1) and polyneuritis and ataxia (n = 1). The samples collected in China were from the Tongji Medical University-affiliated Tongji Hospital: AD (n = 9), aged control (n = 14), Tongji Hospital-affiliated Qiaoko Military Hospital: aged control (n = 16), the second Hospital in Wuhan City: AD (n = 4), and the 187 Military Hospital in Haiko City: AD (n = 9), VaD (n = 28), age-matched non-AD neurological control (n = 24): meningeal hemangioma (n = 3), meningitis (n = 2), ataxia (n = 2), spinal canal stenosis (n = 1), diabetic polyneuropathy (n = 3), depression (n = 4), multiple sclerosis (n = 1), myodystrophy (n = 1), Parkinson syndrome (n = 2), acoustic neurinoma (n = 1), vertebro-basilar artery infarct (n = 2) and malignant lymphoma (n = 2). The diagnosis was made by different mental performance tests designed by different hospitals, computed tomography or magnetic resonance imaging, disease history, family history, clinical signs, and symptoms.
Table 1.
Source and Patient Diagnosis from Whom CSF Samples Were Examined
| Source | AD | VaD | Non-AD control | Aged control |
|---|---|---|---|---|
| NBB | 30 | 18 | 13 | 26 |
| THC | 22 | 28 | 24 | 30 |
| Total | 52 | 46 | 37 | 56 |
NBB, The Netherlands Brain Bank; THC, teaching hospitals in China.
Major Reagents and Equipment
Recombinant human brain tau410 (tau 39 clone) and from an AD brain the AD P-tau were purified as described previously. 24,25 Resazurin, resorufin, NAD+-free nicotinamide adenine dinucleotide phosphate (NADP+), alkaline phosphatase (EC 3.1.3.1), NADH-dependent diaphorase (EC 1.8.1.4), and NAD+-dependent alcohol dehydrogenase (EC 1.1.1.1) were all purchased from Sigma (St. Louis, MO). The capture polyclonal antibody 92e was described previously. 26 Monoclonal antibodies Tau-1 (to tau unphosphorylated at Ser-198/199/202) and PHF-1 (to tau phosphorylated at Ser-396/404) were generous gifts from Drs. L. Binder (North Western University, Chicago, IL) and P. Davies (Albert Einstein College of Medicine, Bronx, NY), respectively. The alkaline phosphatase-conjugated goat anti-mouse IgG used as secondary antibody was purchased from Jackson (West Grove, PA). The major equipment used was Hitachi model 850 fluorescence spectrophotometer (Chiyoda-ku, Tokyo, Japan) and Shimadzu UV-120-02 spectrophotometer (Kyoto, Japan).
Procedure for Bienzyme-Substrate-Recycle Enzyme-Linked Immunosorbent Assay (ELISA)
Step 1: Coating with Capture Antibody
Coat the microtiter plate with tau antiserum 92e (1:2500), 100 μl/well for overnight at 4°C. Wash the plate five times with Tris-buffered saline (TBS), pH 8.5, containing 0.05% Tween 20 (TTBS).
Step 2: Blocking
Add blocking solution (TTBS plus 3% bovine serum albumin), 150 μl/well, and incubate at 37°C for 1 hour, wash as above.
Step 3: Addition of Antigen
Add 20 μl of CSF, diluted to 100 μl in TTBS, 3% bovine serum albumin, and 0.02% NaN3 to the plate, incubate overnight at 4°C, and wash as above.
Step 4: Addition of Reporter/Primary Antibody
Add 100 μl of Tau-1 (1:50,000) or PHF-1 (1:200) to the plate, incubate at 37°C for 1 hour, and wash as above.
Step 5: Addition of Secondary Antibody
Add 100 μl of alkaline phosphatase-conjugated goat anti-mouse IgG (1:10,000) to the plate, incubate at 37°C for 1 hour, and wash as above.
Step 6: Initiating Reaction
Add 100 μl of freshly prepared initiation buffer (225 mmol/L diethanolamine, pH 9.5, 0.04 mmol/L MgCl2, and 1 μmol/L NADP+) to the plate and incubate at 30°C for 45 minutes.
Step 7: Bienzyme Substrate Recycle
Transfer the initiation mixture to an Eppendorf tube and add 100 μl of bienzyme substrate recycling solution [0.1 mol/L phosphate buffer, pH 7.4, 6% alcohol, 0.5 U/ml alcohol dehydrogenase, 0.0125 U/ml diaphorase, and 8 μmol/L resazurin (the enzymes and the substrate prepared just before use)]. Incubate at 37°C for 30 minutes.
Step 8: Stopping the Reaction and Measuring the Fluorescence
The reaction is stopped by boiling the reaction mixture for 5 minutes. The fluorescence is then measured at excitation at 560 nm and emission at 590 nm. For conventional ELISA, 4-methylumbelliferyl phosphate (4-Mu-P) was used as the substrate of alkaline phosphatase-conjugated secondary antibody and fluorescence produced by 4-Mu was measured at excitation of 385 nmol/L and emission of 448 nmol/L.
For determination of total tau levels by antibody Tau-1, each CSF sample, before use in the above assay, was dephosphorylated by alkaline phosphatase (2000 U/ml) for 6 hours at 37°C in buffer, containing 50 mmol/L Tris-HCl, pH 8.0, 10 mmol/L MgCl2, 1% β-mercaptoethanol, and 0.5 mmol/L phenylmethyl sulfonyl fluoride. Recombinant human brain tau410, and ADP-tau purified from an AD brain were used as standards for total tau and phosphorylated (at Ser-396/404) tau, respectively.
Statistical Analysis
Assuming taus used for standard curves as 100% pure, and in the case of AD P-tau, 100% phosphorylation at Ser-396/404, the tau levels were calculated as means ± SD. The variation analysis and significance tests among the groups were made by clinical epidemiology statistic software STATE 5.0 (Harvard University, Boston, MA).
Results
Optimization of Bienzyme-Substrate Recycling
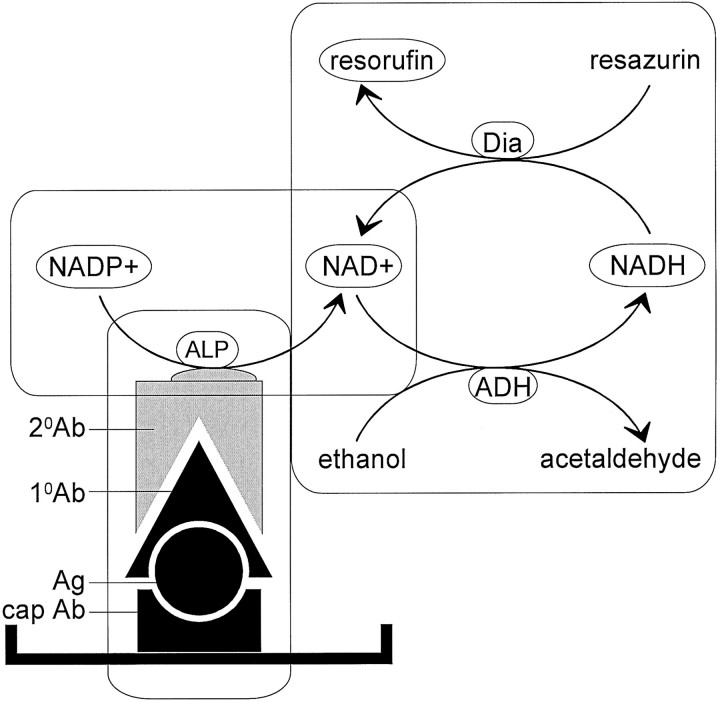
The ELISA for total tau and for tau phosphorylated at the PHF-1 site were made ultrasensitive by using bienzyme-substrate recycling. The principle of this assay is shown in Figure 1 ▶ . In this assay NADP+ used as a substrate is dephosphorylated by the alkaline phosphatase-conjugated secondary antibody into NAD+. The NAD+ then accepts hydrogen from alcohol and is reduced into NADH, and subsequently, NADH transfers its hydrogen to the substrate resazurin and forms the fluorescent product resorufin and thereby regenerates NAD+; this NAD+ then starts over again the cycle and this process continues in the presence of excess of resazurin. Bienzymes involved in this cycle are alcohol dehydrogenase and diaphorase. This last part of the assay is the key for the high sensitivity of the technique.
Figure 1.
Diagram showing the principle of the bienzyme-substrate-recycle ELISA. Alkaline phosphatase, which is linked to secondary antibody, dephosphorylates NADP+ to NAD+. Then, NAD+ enters a highly NAD+-specific redox cycle, in which NAD+ is reduced to NADH by alcohol dehydrogenase, and the NADH produced is oxidized back to NAD+ by diaphorase with the concomitant reduction of resazurin (a nonfluorescent substrate) to resorufin (a fluorescent product). The resorufin accumulates with each cycle of NAD+-NADH-NAD+ and the fluorescence of resorufin is measured at 560 nm excitation and 590 nm emission.
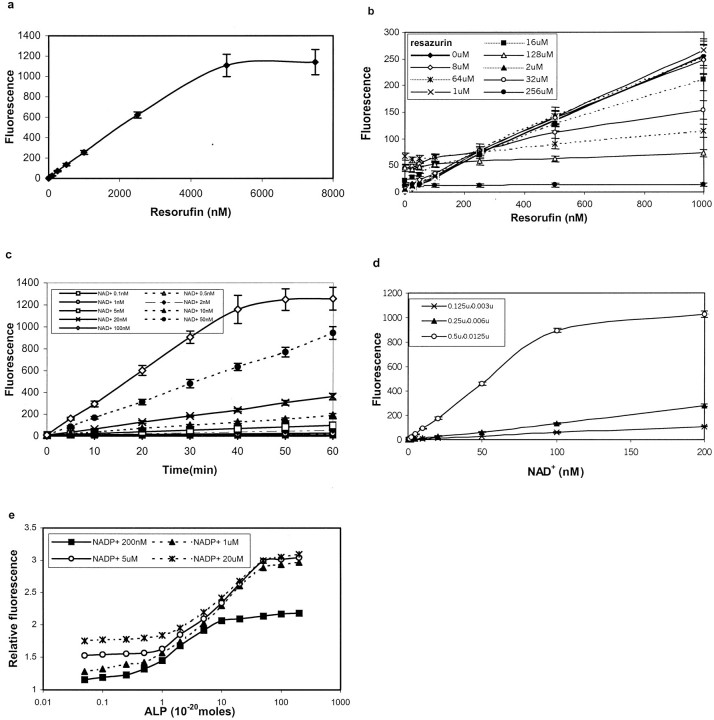
To find the optimum wavelength for quantitative analysis of the fluorescence produced through the recycling, we first made a scanning curve with the fluorescent product resorufin. The peak values were obtained at excitation of 560 nm and emission of 590 nm, respectively. At these wavelengths, the linear range of resorufin was from 10 nmol/L to 2.5 μmol/L (Figure 2a) ▶ . By mixing different concentration of substrate resazurin with resorufin and measuring the final fluorescence at above wavelength, we found that resazurin quenched the fluorescence produced by resorufin when the concentration was greater than 8 μmol/L (Figure 2b) ▶ . To determine the optimal concentration of the substrates involved in the recycling, we studied the fluorescent signal generated at different concentrations of NAD+ at resazurin concentrations of 2 μmol/L, 8 μmol/L, or 64 μmol/L. The most effective concentration match was found to be 8 μmol/L of resazurin and 100 nmol/L of NAD+ (Figure 2c) ▶ . At a resazurin concentration of 2 μmol/L, the fluorescent density did not increase even using a high concentration of NAD+, probably because of a rapid consumption of resazurin; and at resazurin concentrations greater than 8 μmol/L, a remarkable quenching and high background were observed (data not shown). Next we determined the optimal ratio of two enzymes involved in the recycle. When the ratio of alcohol dehydrogenase to diaphorase (Dia) was 0.5 U to 0.0125 U, the highest sensitivity and a widest linear range of NAD+ (0.1 nmol/L to 100 nmol/L) were reached (Figure 2d) ▶ . However, if the ratio of alcohol dehydrogenase to Dia was set at 0.25 U to 0.006 U or 0.125 U to 0.003 U, the linear range of NAD+ was shifted to 10 nmol/L to 500 nmol/L and 2 nmol/L to 500 nmol/L, respectively (not shown), but the sensitivity was markedly reduced (Figure 2d) ▶ .
Figure 2.
Optimization of the bienzyme-substrate-recycle ELISA. a: Linearity of resorufin fluorescence (standard curve) at 560 nm excitation and 590 nm emission. b: Quenching effect of different concentrations of resazurin at various concentrations of resorufin. A resazurin concentration of 8 μmol/L was found to be optimal. c: Concentration match for di-substrates, resazurin, and NAD+ at an alcohol dehydrogenase to diaphorase ratio of 0.5 U to 0.0125 U: the time kinetics of the generation of resorufin at 8 μmol/L of resazurin and different concentrations of NAD+; under these conditions 100 nmol/L of NAD+ resulted in largest linear range of the time of incubation. d: Units match for bienzyme: the generation of resorufin at different concentrations of NAD+ and at different ratios of alcohol dehydrogenase to diaphorase after incubation for 30 minutes; the ratio of 0.5 U to 0.0125 U was found to be optimal. e: Generation of resorufin at different concentrations of alkaline phosphatase (ALP) and of NADP+; at 1 μmol/L of NADP+ high sensitivity and wide linear range of ALP were achieved. In e the generation of resorufin fluorescence is shown as relative fluorescence, which is the logarithm value of the original fluorescence observed. It may be noted that background increased with an increase in NADP+ concentration. Not shown in this figure when the background was subtracted, at 1 μmol/L of NADP+ concentration, fluorescence of ∼20 and a relative fluorescence linear range of 0 to 3 were obtained.
Initiation Reaction
This reaction provides substrate NAD+ through the hydrolysis of NADP+ that is catalyzed by the secondary antibody-conjugated alkaline phosphatase. It is a critical step to link ELISA with the bienzyme-substrate-recycling reaction. To optimize the conditions for this reaction, we first checked the factors, which affect the activity of alkaline phosphatase. It was shown that the activity of alkaline phosphatase was decreased with an increased concentration of Zn++. Increased activity was observed by adding Mg++ to the reaction system. The peak activity was reached when the concentration of Mg++ and diethylamine was set at 0.04 mmol/L and 225 mmol/L in the absence of Zn++ (data not shown). In addition, the optimum reaction time and temperature for alkaline-phosphatase activity were determined as 45 minutes at 30°C. When the temperature was increased to 43°C, the background was high and an inactivation of the enzyme started to occur at 30 minutes (data not shown). To achieve low background and reproducible results, it was found extremely important to keep alkaline-phosphatase substrate NADP+ in an NAD+-free condition. Therefore, we carefully determined the condition that would lead to a spontaneous hydrolysis of NADP+. It was found that a significant hydrolysis of NADP+ occurred when stored at room temperature (25 ∼ 30°C) as solution in water or diethylamine buffer. Whereas, only a 10 to 15% hydrolysis of NADP+ was observed when its solution was stored at 4°C for 48 hours. The optimal storage condition was found to be −20°C because even 10 times freeze and thaw cycles did not induce any detectable hydrolysis of the compound (data not shown). The highest sensitivity and the widest linear range for alkaline-phosphatase activity were observed at the NADP+ concentration of 1 μmol/L. At 1 μmol/L of NADP+ concentration, when the background was subtracted, a fluorescence of ∼20 and a relative fluorescence (logarithm value of fluorescence) linear range of 0 to 3 were obtained (data not shown). If the concentration was lower than 1 μmol/L, a high sensitivity but a narrow linear range were seen. On the other hand, a higher concentration of NADP+ (>1 μmol/L) induced a lower sensitivity and a higher background (Figure 2e) ▶ .
Optimization of the Immunoreaction in the Bienzyme-Substrate-Recycle ELISA
We optimized the ELISA component of the above assay using 4-methylumbelliferyl phosphate (4-Mu-P) as substrate of alkaline phosphatase and quantitated the fluorescent product 4-Mu. The assay was linear when the concentration of standard 4-Mu was between 0.15 μmol/L to 160 μmol/L (data not shown). The linear range was also observed by using 0.8 mmol/L 4-Mu-P and dilution of alkaline phosphatase-conjugated secondary antibody at 1:1.5 × 10 7 to 1:2.5 × 10 5 (data not shown). Additionally, no cross-reaction was found between capture antibody 92e (1:2500 and 1:10,000) and the secondary antibody (1:10,000 and 1:30,000), or Tau-1 (1:10,000 and 1:50,000) or PHF-1 (1:100 and 1:200). Similarly no cross-reaction of Tau-1 to phosphorylated tau purified from AD brain or of PHF-1 to recombinant tau was found (not shown).
Comparison of the Sensitivity of the Bienzyme-Substrate-Recycle ELISA with the Conventional ELISA
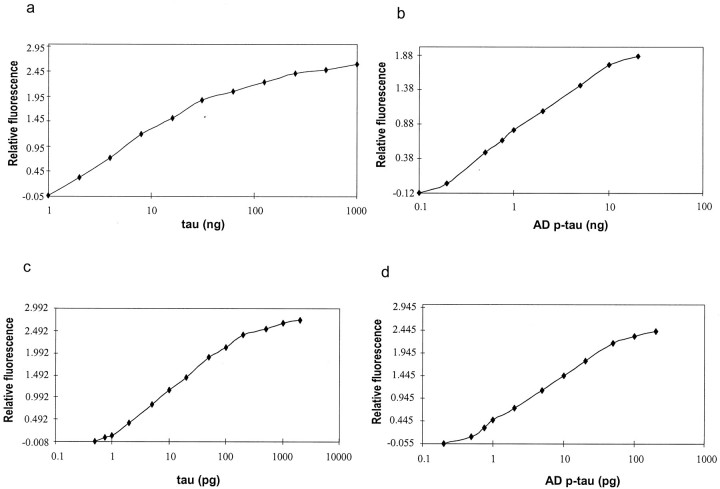
After optimizing all above conditions, and by linking the above three portions of the procedure together we established the bienzyme-substrate-recycle ELISA. We then compared this assay with the conventional ELISA using the standard recombinant human tau and AD P-tau. We found that the sensitivity of the conventional ELISA was 1 ng tau with a linear range of 1 ng to 32 ng (20 fmol to 640 fmol for average molecular weight of 50,000) (Figure 3a) ▶ and 0.2 ng AD P-tau with a linear range of 0.2 ng to 10 ng (4 fmol to 200 fmol) (Figure 3b) ▶ . In contrast, by the bienzyme-substrate-recycle ELISA, the detection range for normal tau was 0.75 pg to 200 pg (15 amol to 4 fmol) (Figure 3c) ▶ , which stands for ∼1300 times increase in sensitivity and more than five times enlargement of the detection range compared with conventional ELISA. For AD P-tau, the detectable range was 0.5 pg to 50 pg (10 amol to 1 fmol) (Figure 3d) ▶ , which equals an ∼400 times increase in sensitivity and two times enlargement of the detection range.
Figure 3.
Standard curves of recombinant tau and AD P-tau analyzed by the bienzyme-substrate-recycle ELISA and by conventional ELISA. The sensitivity and linear range for recombinant tau (a) and AD P-tau (b) were 1 ng to 32 ng and 0.2 ng to 10 ng, respectively, by conventional ELISA, and 0.75 pg to 200 pg for recombinant tau (c) and 0.5 pg to 50 pg for AD P-tau (d) by bienzyme-substrate-recycle ELISA.
Levels of Total and Abnormally Hyperphosphorylated Tau (P-Tau) in the CSF of AD and Control Patients as Determined by the Bienzyme-Substrate-Recycle ELISA
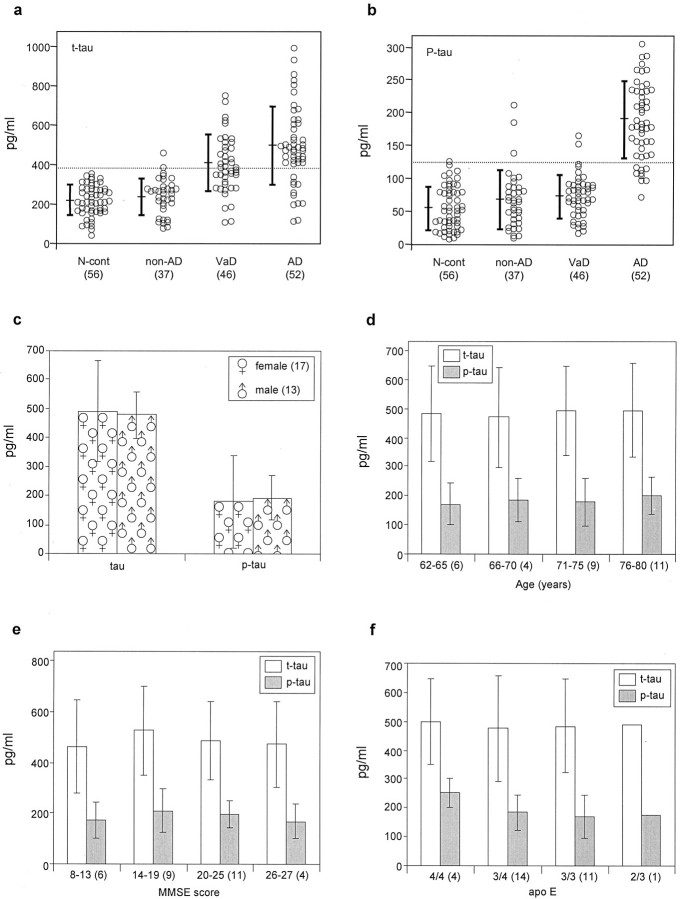
We measured the levels of total normal tau and of P-tau in lumbar CSF of patients collected from The Netherlands and from the Peoples Republic of China by using the assay established in the present study. It was found that both total tau and P-tau were significantly increased in AD as compared to cognitively normal, non-AD, and VaD controls (Figure 4, a and b) ▶ . However, a statistically significant higher level of total tau was also seen in VaD than normal controls. In contrast to total tau, a markedly higher level of P-tau was only seen in AD but not in VaD (Figure 4b) ▶ . The absolute values for phosphorylated tau in the present study might be overestimated because AD P-tau used as a standard might not be 100% phosphorylated at Ser-396/Ser-404. However, the relative values among different groups should not be affected. No correlation was detected in AD patients from The Netherlands Brain Bank between the sex (Figure 4c) ▶ , age (Figure 4d) ▶ , MMSE score (Figure 4e) ▶ , and ApoE genotype (Figure 4f) ▶ with the level of tau or P-tau in CSF. Because ApoE genotyping data were available only on AD patients from The Netherlands Brain Bank only these cases were used for all correlation analyses. Consistent with previous studies 5,11,12 no significant changes in the elevation of CSF tau levels were observed between mild (MMSE scores, 26 to 27) and severely impaired (MMSE scores, 8 to 13) AD patients.
Figure 4.
CSF levels of total tau and P-tau as determined by bienzyme-substrate-recycle ELISA. The level of total tau in AD was significantly higher than normal control, non-AD, and VaD, and it was significantly higher in VaD than normal control (a). A significant increase in P-tau was only seen in AD but not in VaD CSF (b). Correlation analysis (not shown) of tau or P-tau of AD patients from The Netherlands Brain Bank with sex (c), age (d), MMSE score (e), and ApoE genotype (f) revealed no correlation with any one of these parameters. Because patients from teaching hospitals in China were assessed by different mental performance tests at each hospital, and these patients were not ApoE genotyped, they were not included in the correlation analysis.
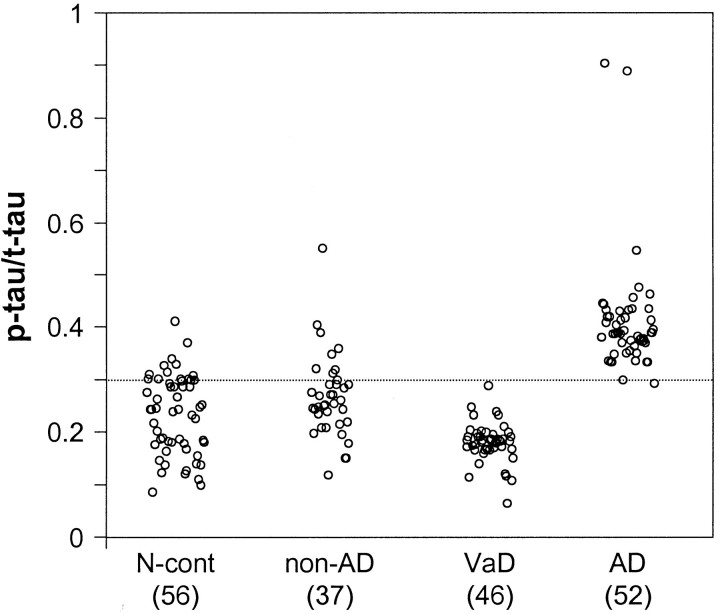
To analyze the sensitivity and specificity of the diagnostic value of the CSF total tau and P-tau levels, we used the following formulas for the calculations: % sensitivity = [true-positive/(true-positive + false-negative)] × 100; % specificity = [true-negative/(true-negative + false-positive)] × 100. At total tau ≥ 370 pg/ml (mean + 1.72 SD of normal control value) as cutoff for AD, 79% sensitivity and specificity of 100%, 95%, and 50% toward normal, non-AD neurological, and VaD samples, respectively, were achieved (Table 2) ▶ . If we used P-tau as a diagnostic marker of AD, the sensitivity and especially the specificity toward VaD group were markedly increased. A P-tau to total tau ratio of ≥0.33 as cutoff for AD diagnosis resulted in sensitivity of 96% and specificity of 95%, 86%, 100%, and 94% toward normal aged control, non-AD neurological, VaD, and all these three groups combined, respectively (Figure 5 ▶ , Table 2 ▶ ).
Table 2.
The Diagnostic Potential of CSF Total Tau and Phosphorylated Tau Levels for AD
| Marker (pg/ml) | Sensitivity | Specificity, % | |||
|---|---|---|---|---|---|
| N. control | Non-AD | VaD | Combined | ||
| t-tau ≥370 | 79 | 100 | 95 | 50 | 82 |
| P-tau ≥120 | 83 | 98 | 92 | 93 | 95 |
| P-tau ≥100 | 94 | 89 | 84 | 87 | 87 |
| P-tau to t-tau ≥0.33 | 96 | 95 | 86 | 100 | 94 |
Figure 5.
CSF levels of P-tau normalized against levels of total tau (P-tau to t-tau ratio). The rest of the details are same as in Figure 4 ▶ . The P-tau to t-tau ratio in AD CSF was significantly higher than each of the three control groups and as well as the control groups combined as one group (P < 0.01). The diagnostic value of P-tau to t-tau ratio at ≥0.33 as cutoff for AD had a sensitivity of 96% and specificity of 95%, 86%, 100%, and 94% in comparison with N. cont., non-AD, VaD, and all controls combined, respectively (see Table 2 ▶ ).
Discussion
Currently a definite diagnosis of AD can be made only histopathologically which is mostly done on autopsied brains and rarely on brain biopsies from living patients. The clinical diagnosis of AD is very cumbersome, expensive, and has a varying degree of accuracy that is highest around 90% in AD research centers. The current clinical diagnosis does not allow discrimination between the efficacy of test drugs that inhibit neurodegeneration and drugs that result in only symptomatic improvement, ie, symptomatic drugs. Thus, recognizing this urgent and strong need a number of laboratories have been investigating the potential of a number of molecular markers in the CSF as a diagnostic test for the disease. Among many of the potential molecules, microtubule-associated protein tau is one of the most promising and best documented. 6 Although the level of total tau in CSF has been found to be increased in AD, there is a considerable overlap with control cases. Because practically all of the increase in tau level in AD brain is in the abnormally hyperphosphorylated form, 27,28 measurement of this tau is likely to improve the diagnostic accuracy. In the present study we have found that the CSF levels of total tau are elevated not only in AD but also in VaD in comparison with normal (nonneurological) control and non-AD neurological control cases. Similar levels of total tau observed in both normal control and non-AD groups suggested that the rate of neurodegeneration in the latter was similar to that which occurred in the nonneurological aged controls. However, the CSF levels of tau phosphorylated at Ser-396/Ser-404 (PHF-1 site), especially relative to the total tau levels are elevated only in AD and neither in VaD, normal control, or non-AD neurological cases. Levels of the phosphorylated tau normalized against total tau levels ie, P-tau to t-tau ratio at 96% sensitivity of AD yielded a specificity of 95%, 86%, 100%, and 94% against normal control, non-AD, VaD, and all these three groups combined, respectively. The small number of normal control and non-AD cases found to have P-tau to t-tau ratio in the AD range might represent preconverters of AD. The increase in both P-tau and total tau levels was seen early in the disease, ie, AD patients with MMSE scores of 26 to 27 and as well scores of 8 to 13 had similar increases in both forms of tau. These findings are consistent with previous reports in which elevated CSF levels of total tau were observed at the early stages of AD. 5,11,12
The level of the hyperphosphorylated tau is too low to be detected by conventional methods. To solve this problem, in the present study we have adapted the enzyme amplification immunoassay of Johansson and colleagues 23 that links conventional ELISA with bienzyme substrate recycling and have established a highly specific and ultrasensitive method that can measure AD abnormal tau in CSF. By using this method, we have compared the level of total tau and tau phosphorylated at Ser-396/Ser-404 (PHF-1 site) in AD with VaD, non-AD, and normal-aged control CSFs collected from two different countries. Our findings suggest that the assay developed is sensitive and specific enough for the detection of micro amounts of tau in CSF and that the increased level in CSF of tau phosphorylated at the PHF-1 site is specific to AD and might be used as a diagnostic aid for AD. It may be noted that none of the control groups of patients had any lesions of abnormally hyperphosphorylated tau in their brains and thus consequently low CSF levels of abnormally hyperphosphorylated tau. Cases with non-AD tauopathies such as frontotemporal dementia, corticobasal degeneration, and Pick disease will most likely have elevated CSF levels of abnormally hyperphosphorylated tau.
Tau in AD brain is known to be abnormally hyperphosphorylated at more than 21 sites. 16,29 The PHF-1 (Ser-396/404) site investigated in the present study is one of the major and most well-studied sites. Other major abnormally phosphorylated sites include Thr-181, Ser-199, and Ser-231/Thr-235. Similar to our findings the levels of tau phosphorylated at Ser-231/Thr-235, 22,30 Ser-199, 31 and at Thr-181 32 have been found to be specifically elevated in AD CSF. Thus, there is increasing evidence for the promising diagnostic potential of CSF abnormally hyperphosphorylated tau levels in AD.
Conventional ELISA has been widely used to assay micro amounts of antigens and antibodies since it was established in 1966. 33 Many efforts have been made to improve the sensitivity of the assay. The main strategies used for this particular purpose have been to amplify the signal produced by antibody-conjugated enzymes, such as to increase the number of copies of the reporter enzyme itself or using better substrates. The sensitivity of the conventional ELISA was increased 2 to 100 times by using the biotin-avidin system because avidin has high affinity to biotin (one avidin binds four biotins), and multiple biotins can be conjugated to the secondary antibodies or enzymes. 34 However, nonspecific binding caused by positive charge of the avidin molecule is the major disadvantage of this system. Therefore, neutral streptavidin was introduced to reduce the background. 35 In respect to the improvement in sensitivity made with respect to substrates, various approaches, such as the use of fluorescent-, chemiluminescent-, or radioisotope-labeled substrates, have been tested. Fluorescent substrate enlarged the sensitivity of the method by 5 to 100 times, 35 and radioimmunoassay made the technique a thousand times more sensitive than conventional ELISA. 36 Although radioimmunoassay is reasonably sensitive enough for the determination of micro amounts of proteins, it is limited in use, especially in the clinical application in developing countries, because of the special requirements in reagents, instruments, and proper facilities where radiation can be used. The method developed in the present study increased the sensitivity to 400 or 1300 times toward P-tau or normal recombinant tau, respectively, and it does not need special reagents or equipment. Therefore, it has great potential in clinical application and in drug trials.
According to the principle of the assay shown in Figure 1 ▶ , the following three elements must be carefully addressed to detect micro amounts of NAD+ produced by the recycling. 1) The concentration of alcohol and resazurin must be in excess to keep the reaction velocity reaching to its maximum. 2) The concentration of NAD+ and NADH must be lower than the Km value to keep the enzymatic reaction at its first order kinetics. 3) The amount of alcohol dehydrogenase and diaphorase must be titered to the concentration of NAD+ and NADH to match the basic Michaelis-Menten’s equation of enzyme kinetics. To fulfill the first requirement, we optimized the concentration of resazurin and found that 8 μmol/L satisfied both the excess substrate requirement and had minimal quenching effect on the fluorescent product. To match the requirement of the Michaelis-Menten kinetics for the formation of NADH and NAD+, we titrated the ratio of alcohol dehydrogenase and diaphorase. The widest detection linear range (0.1 ∼ 100 nmol) was obtained when the ratio of the two enzymes was 0.5 U to 0.0125 U. These concentrations were 20 to 1000 times lower than that of NAD+ and NADH. The reaction catalyzed by alkaline phosphatase was also carefully studied. To optimize alkaline-phosphatase activity itself, we also studied the optimal concentration of NADP+ for an efficient production of NAD+. We found that both NAD+ and the background were increased with an increase in NADP+ and alkaline phosphatase. When the concentration of NADP+ was 1 μmol/L and alkaline phosphatase 0.25 ∼ 20 × 10−20 mol, a relatively high sensitivity with a wide linear range and a low background were achieved.
It has been generally accepted that abnormal phosphorylation of tau is the first and most critical step for the formation of PHF and neurofibrillary tangles in AD brain. 25,37 It is not known exactly how long it takes from abnormal phosphorylation of tau to the hallmark lesion of tangle formation in AD brain. However, various clinical and histopathological studies have suggested that AD is a chronic and progressive neurodegenerative disorder, and thus, the pathological processes might occur long before the appearance of clinical signs and symptoms. Therefore, early diagnosis of the disease is important both for establishing prevention and for evaluating the efficacy of therapeutic drugs. In this regard, the information provided in the present study is highly significant.
Acknowledgments
We thank the Biomedical Photography Unit of NYS Institute for Basic Research for the preparation of figures; Janet Biegelson and Sonia Warren for help with typing the manuscript; The Netherlands Brain Bank at The Netherlands Institute for Brain Research, Amsterdam, The Netherlands (coordinator, Dr. Rivka Ravid) and from different teaching hospitals in China for providing the CSF samples from AD and control cases; and the Brain Tissue Resource Center (Public Health Service grant MH/NS 31862) McLean Hospital, Belmont, MA, and the New York State Institute for Basic Research Tissue Bank (Dr. P. Kozlowski) for the human autopsied brain samples.
Footnotes
Address reprint requests to Dr. Jianzhi Wang, Pathophysiology Department, Tongji Medical College, Huazhong University of Science and Technology, 13 Hong Kang Rd., Wuhan, 430030 P. R. China. E-mail: wangjz@tjmu.edu.cn.
Supported in part by the Natural Science Foundation of China (grants 39925012, G1999054007, and 39870767), the Science and Technology Committee of China, the National Educational Committee of China, the National Institutes of Health (grants AG08076 and TW00703), and the New York State Office of Mental Retardation and Developmental Disabilities.
References
- 1.Growdon JH: Biomarkers of Alzheimer disease. Arch Neurol 1999, 56:281-283 [DOI] [PubMed] [Google Scholar]
- 2.Garlind A, Brauner A, Hojeberg B, Basun H, Schultzberg M: Soluble interleukin-1 receptor type II levels are elevated in cerebral fluid in Alzheimer disease. Brain Res 1999, 826:112-116 [DOI] [PubMed] [Google Scholar]
- 3.Galasko D: Cerebrospinal fluid opens a window on Alzheimer disease. Arch Neurol 1999, 56:655-656 [DOI] [PubMed] [Google Scholar]
- 4.Hock C, Heese K, Muller-Spahn F, Huber P, Riesen W, Nitsch RM, Otten U: Increased CSF level of never growth factor in patients with Alzheimer disease. Neurology 2000, 54:2009-2011 [DOI] [PubMed] [Google Scholar]
- 5.Hampel H, Buerger K, Kohnken R, Teipel SJ, Zinkowski R, Rappoport SI, Davies P: Tracking of Alzheimer disease progression with CSF tau protein phosphorylated at threonine 231. Ann Neurol 2001, 49:545-546 [PubMed] [Google Scholar]
- 6.Andreasen N, Minthon L, Davidsson P, Vanderstichele H, Vanmechelen E, Winblad B, Blennow K: Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol 2001, 58:373-379 [DOI] [PubMed] [Google Scholar]
- 7.Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin B, Vanderstichele H, Vanmechelen E, Blennow K: Cerebrospinal fluid beta-amyloid (1-42) in Alzheimer disease: differences between early and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol 1999, 56:673-680 [DOI] [PubMed] [Google Scholar]
- 8.Arai H, Terajima M, Terajima M, Miura M, Higuchi S, Muramatsu T, Machida N, Seki H, Takase S, Clark CM, Lee VMY, Trojanowski JQ: Tau in cerebrospinal fluid: a potential diagnostic marker in Alzheimer’s disease. Ann Neurol 1995, 389:649-652 [DOI] [PubMed] [Google Scholar]
- 9.Jensen M, Basun H, Lannfelt L: Increased cerebrospinal fluid tau in patients with Alzheimer disease. Neurosci Lett 1995, 186:189-191 [DOI] [PubMed] [Google Scholar]
- 10.Mori H, Hosoda K, Matsubara E, Nakamoto T, Furiya Y, Endoh R, Usami M, Shoji M, Maruyama S, Hirai S: Tau in cerebrospinal fluid: establishment of the sandwich ELISA with antibody specific to the repeat sequence in tau. Neurosci Lett 1995, 186:181-183 [DOI] [PubMed] [Google Scholar]
- 11.Kurz A, Riemensschneider M, Buck K, Willoch F, Bartenstein P, Muller U, Guder W: Tau protein in cerebrospinal fluid is significantly increased at the earliest clinical stage of Alzheimer disease. Alzheimer Dis Assoc Disord 1998, 12:372-377 [DOI] [PubMed] [Google Scholar]
- 12.Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, De Deyn PP, Bancher C, Cras P, Wiltfang J, Mehta PD, Iqbal K, Pottel H, Vanmechelen E, Vanderstichele H: Improved discrimination of AD patients using β-amyloid(1-42) and tau levels in CSF. Neurology 1999, 52:1555-1562 [DOI] [PubMed] [Google Scholar]
- 13.Kahle PJ, Jakowec M, Teipel SJ, Hampel H, Petzinger GM, Di Monte DA, Silverberg GD, Moller HJ, Yesavage JA, Tinklenberg JR, Shooter EM, Murphy Jr GM: Combined assessment of tau and neuronal thread protein in Alzheimer’s disease CSF. Neurology 2000, 54:1498–1504 [DOI] [PubMed]
- 14.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM: Microtubule-associated protein tau: a component of Alzheimer paired helical filaments. J Biol Chem 1986, 261:6084-6089 [PubMed] [Google Scholar]
- 15.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI: Abnormal phosphorylation of the microtubule associated protein τ (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 1986, 83:4913-4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal K, Grundke-Iqbal I: Alzheimer abnormally phosphorylated tau is more hyperphosphorylated than the fetal tau and causes the disruption of microtubules. Neurobiol Aging 1995, 16:375-379 [Google Scholar]
- 17.Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM: Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 1993, 10:1089-1099 [DOI] [PubMed] [Google Scholar]
- 18.Song JS, Yang SD: Tau protein kinase I/GSK-3beta/kinase FA in heparin phosphorylates tau on Ser199, Thr231, Ser235, Ser262, Ser396 and Ser400 sites phosphorylated in Alzheimer disease brain. J Protein Chem 1995, 14:95-105 [DOI] [PubMed] [Google Scholar]
- 19.Wang JZ, Wu QL, Smith A, Grundke-Iqbal I, Iqbal K: Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS Lett 1998, 436:28-34 [DOI] [PubMed] [Google Scholar]
- 20.Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal K: Dephosphorylation of Alzheimer paired helical filaments with protein phosphatase 2A and 2B. J Biol Chem 1995, 270:4854-4860 [DOI] [PubMed] [Google Scholar]
- 21.Wang JZ, Grundke-Iqbal I, Iqbal K: Restoration of biological activity of Alzheimer abnormally phosphorylated tau by dephosphorylation with protein phosphatase-2A, -2B and -1. Mol Brain Res 1996, 38:200-208 [DOI] [PubMed] [Google Scholar]
- 22.Ishiguro K, Ohdeto H, Arai H, Yamaguchi H, Urakami K, Park JM, Sato K, Kohno H, Imahori K: Phosphorylated tau in human cerebrospinal fluid is a diagnostic marker for Alzheimer’s disease. Neurosci Lett 1999, 270:91-94 [DOI] [PubMed] [Google Scholar]
- 23.Johannsson A, Ellis DH, Bates DL, Plumb AM, Stanley CJ: Enzyme amplification for immunoassays. Detection limit of one hundredth of an attomole. J Immunol Methods 1986, 87:7-11 [DOI] [PubMed] [Google Scholar]
- 24.Singh TJ, Haque N, Grundke-Iqbal I, Iqbal K: Rapid Alzheimer-like phosphorylation of tau by the synergistic actions of non-proline-dependent protein kinases and GSK-3. FEBS Lett 1995, 358:267-272 [DOI] [PubMed] [Google Scholar]
- 25.Köpke E, Tung YC, Shaikh S, Alonso A del C, Iqbal K, Grundke-Iqbal I: Microtubule associated protein tau: abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem 1993, 268:24374–24384 [PubMed]
- 26.Grundke-Iqbal I, Vorbrodt AW, Iqbal K, Tung YC, Wang GP, Wisniewski HM: Microtubule-associated polypeptides tau are altered in Alzheimer paired helical filaments. Mol Brain Res 1988, 4:43-52 [DOI] [PubMed] [Google Scholar]
- 27.Khatoon S, Grundke-Iqbal I, Iqbal K: Brain levels of microtubule associated protein tau are elevated in Alzheimer’s disease brain: a radioimmunoslot-blot assay for nanograms of the protein. J Neurochem 1992, 59:750-753 [DOI] [PubMed] [Google Scholar]
- 28.Khatoon S, Grundke-Iqbal I, Iqbal K: Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Lett 1994, 351:80-84 [DOI] [PubMed] [Google Scholar]
- 29.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y: Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem 1995, 270:823-829 [DOI] [PubMed] [Google Scholar]
- 30.Kohnken R, Buerger K, Zinkowski R, Miller C, Kerkman D, DeBernardis J, Shen J, Moller HJ, Davies P, Hampel H: Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer’s disease patients. Neurosci Lett 2000, 287:187-190 [DOI] [PubMed] [Google Scholar]
- 31.Itoh N, Arai H, Urakami K, Ishiguro K, Ohno H, Hampel H, Buerger K, Wiltfang J, Otto M, Kretzschmar H, Moeller H-J, Imagawa M, Kohno H, Nakashima K, Kuzuhara S, Sasaki H, Imahori K: Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 1999 for the antemortem diagnosis of Alzheimer’s disease. Ann Neurol 2001, 50:150-156 [DOI] [PubMed] [Google Scholar]
- 32.Sjogren M, Davidsson P, Tullberg M, Minthon L, Wallin A, Wikkelso C, Granerus AK, Vanderstichele H, Vanmechelen E, Blennow K: Both total and phosphorylated tau are increased in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2001, 70:624-630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avrameas S, Guilbert B: A method for quantitative determination of cellular immunoglobulins by enzyme-labeled antibodies. Eur J Immunol 1971, 1:394-399 [DOI] [PubMed] [Google Scholar]
- 34.Wilchek M, Bayer EA: The avidin-biotin complex. Immunol Today 1984, 5:39-43 [DOI] [PubMed] [Google Scholar]
- 35.Wilchek M, Bayer EA: Avidin-biotin technology. Methods in Enzymology, 1990, vol. 184. Academic Press, San Diego [DOI] [PubMed]
- 36.Harris CC, Yolken RH, Krokan H, Hsu IC: Ultrasensitive enzymatic radioimmunoassay: application to detection of cholera toxin and rotavirus. Proc Natl Acad Sci USA 1979, 76:5336-5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K: Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA 2001, 98:6923-6928 [DOI] [PMC free article] [PubMed] [Google Scholar]