Abstract
The role of specific chemokine receptors during allergic asthmatic responses has been relatively undefined. A number of receptors are preferentially expressed on Th2 cells, including CCR4, CCR8, and CxCR4. In the present study, we have examined the role of CxCR4 in the development of cockroach allergen-induced inflammation and airway hyperreactivity in a mouse model of asthma. Using a specific inhibitor of CxCR4, AMD3100, our results indicate that blocking this receptor has a significant effect in down-regulating the inflammation and pathophysiology of the allergen-induced response. Treatment of allergic mice with AMD3100 significantly reduced airway hyperreactivity, peribronchial eosinophilia, and the overall inflammatory responses. In addition, there was a shift in the cytokine profile that was observed in the AMD3100-treated animals. Specifically, there was a significant reduction in interleukin-4 and interleukin-5 levels and a significant increase in interleukin-12 and interferon-γ levels within the lungs of treated allergic mice. Furthermore, there was a significant alteration in the local chemokine production of CCL22 (MDC) and CCL17 (TARC), two chemokines previously shown to be important in Th2-type allergen responses. Overall, specifically blocking CxCR4 using AMD3100 reduced a number of pathological parameters related to asthmatic-type inflammation.
Morbidity because of asthma continues to rise, especially among children. 1-3 A significant factor in these individuals is the chronic inflammation induced by Th2-type responses that accompanies and likely precedes the most severe episodes of asthmatic disease. 4-6 Thus, considerable efforts have been made to reduce the Th2-type responses that are detrimental to the asthmatic condition. Throughout the past several years the compliant use of inhaled steroids has proven to be important in the control of atopic asthma. 7-11 However, because of continued development of steroid resistance and side effects that have been attributed to long-term steroid use, other alternatives have been explored. In particular, an attractive set of targets is the chemokine family members and their receptors that are responsible for the recruitment of particular subsets of leukocytes to the inflamed lung. 12-14 Idealistically, the targeting of the correct chemokine or receptor may have a significant impact on the development of atopic asthmatic inflammation and aid in the diminished use of steroid compounds.
Initially, the targeting of chemokines was thought to be straightforward, however, the identification of multiple chemokines and receptors have, at the very least, complicated the issue. Originally, it was perceived that the targeting of eosinophil-specific factors and their receptors, predominantly eotaxin and CCR3, might have the greatest impact on development of severe disease. However, the realization that Th subsets, Th1 and Th2, may preferentially express specific chemokine receptors suggested that these cell types could be targeted and alter the recruitment of specific T cell subsets. 15-18 The Th1 cell subset has been shown to preferentially express CxCR3 and CCR5, whereas Th2 subsets express CCR3, CCR4, CCR8, and CxCR4. Although many of these observations have come from in vitro analysis of cytokine-skewed responses, recent observations in animal models and in human asthma studies have begun to confirm some of these issues. 19,20 Of these Th2 cell-expressed chemokine receptors, only CxCR4, and its ligand SDF-1, have been shown to be relevant during Th2-type allergic airway responses. 21 CxCR4, like many chemokine receptors, is a Gi-coupled receptor that is specific for SDF-1. 22,23 SDF has previously been shown to be chemotactic for a number of leukocyte populations, including neutrophils, monocytes, lymphocytes, and more recently, eosinophils. 24-29 Although G-protein-coupled receptors are a pharmaceutically attractive target, few compounds have been shown to be effective during in vivo studies. The studies outlined in this report investigated the use of a soluble CxCR4 inhibitor, AMD3100, in the allergic lung inflammatory responses. This compound has been shown to be effective and specific for blocking human immunodeficiency virus entry into CxCR4-positive cells 30,31 and has further proven to be safe in phase I clinical trials. 32 In a more recent study, AMD3100 was shown to specifically inhibit SDF-1-mediated responses and alter collagen type 1 model of arthritis in mice. 33 Thus, this antagonist may provide for an effective means to block allergen-induced airway inflammation and hyperreactivity.
Materials and Methods
Animals
Female CBA/J mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and were maintained under standard pathogen-free conditions
Sensitization and Induction of the Airway Response
To induce a Th2-type response, normal mice were sensitized and challenged with cockroach allergen as previously described. 34-36 Briefly, mice were immunized with 10 μg of cockroach allergen (Bayer Corp., Elkhart, IN) in incomplete Freund’s adjuvant on day 0. To localize the response to the lung the mice were given an intranasal administration of 10 μg of cockroach allergen in 10 μl of diluent on day 14. This initial intranasal challenge with antigen induced little cellular infiltrate. Mice were then challenged 6 days later by intratracheal administration of 10 μg of cockroach allergen in 50 μl of sterile.
Delivery of AMD3100
In our initial experiments a bolus injection of AMD3100 was administered intraperitoneally at various concentrations (0.1, 1, or 10 mg/kg, in 250 μl). AMD3100 was dissolved in saline and a saline control injection was administered in the control mice. AMD3100 was also used in chronic allergen models and levels needed to be maintained for ∼72 hours. To ensure sufficient levels of the antagonist throughout the 72-hour experimental period, we used osmotic Alzet (Alza Corporation, Palo Alto, CA) pumps to deliver AMD3100 at a constant rate of 250 μg/kg/hour. The Alzet (Alza) pumps loaded with AMD3100 or saline were implanted into the peritoneum of allergic mice 1 hour before initial intratracheal allergen challenge.
Morphometric Analysis of Airway and Peribronchial Eosinophil Accumulation
To assess migration of eosinophils into the airway, we subjected the mice to a 1-ml bronchoalveolar lavage with phosphate-buffered saline (PBS) containing 25 mmol/L of ethylenediaminetetraacetic acid at various time points after challenge. The cells were then dispersed using a cytospin (Shandon Scientific, Runcorn, UK) and differentially stained with Wright-Giemsa stain. The cell types (mononuclear phagocytes, lymphocytes, neutrophils, and eosinophils) were expressed as a percentage based on 200 total cells counted/sample. Lung tissue was preserved with 4% paraformaldehyde at various time points after challenge. The fixed lungs were embedded in paraffin and multiple 50-μm sections were differentially stained with Wright-Giemsa for the identification of eosinophils and viewed at ×1000. The individual eosinophils were counted from 100 high-powered fields per lung at each time point using multiple step sections of lung. The eosinophils counted were only in the peribronchial region, this assured the enumeration of only those eosinophils within or immediately adjacent to an airway. The inflammation observed in this model was completely associated with the airway with little or no alveolitis.
Quantitation of Inflammatory Mediators by Specific Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of cytokine and chemokine proteins in whole-lung homogenate and from cell-free supernatants were measured by specific ELISA. The interleukin (IL)-4, IL-5, eotaxin, and IL-12/23 (p40 subunit) antibodies were purchased and pretested by the company (R & D Systems, Rochester, MN), whereas interferon (IFN)-γ, CCL7, and CCL22 were set up using previously described methodology with antibodies made by our laboratory. 19,35,37 Briefly, lung tissue was homogenized on ice using a tissue-tearor (Biospec Products, Racine, WI) for 30 seconds in 1 ml of PBS containing 0.05% Triton X-100. The resulting supernatant was isolated after centrifugation (10,000 × g). Flat-bottomed 96-well microtiter plates (Nunc Immunoplate I 96-F; Nunc, Roskilde, Denmark) were coated with 50 μl/well of rabbit polyclonal antibodies, specific for the cytokine/chemokine in question, for 16 hours at 4°C and then washed with PBS and 0.05% Tween 20. Nonspecific binding sites were blocked with 2% bovine serum albumin in PBS and incubated for 90 minutes at 37°C. Plates were rinsed four times with wash buffer and cell-free supernatants were added (neat and 1/10) followed by incubation for 1 hour at 37°C. Plates were washed four times, a secondary, biotinylated cytokine-specific antibody was added for 30 minutes, followed by four washes. In a final step, streptavidin-peroxidase conjugate (Bio-Rad, Richmond, CA) was added, and the plates were incubated for 30 minutes at 37°C. Plates were washed again and chromogen substrate (Bio-Rad) was added and incubated at room temperature to the desired extinction. The reaction was terminated with 50 μl/well of 3 mol/L of H2SO4 solution and the plates were read at 490 nm in an ELISA reader. Standards were 0.5-log dilutions of recombinant protein from 1 pg/ml to 100 ng/ml. The ELISAs with purchased reagents were sensitive to 10 pg/ml; whereas the ELISAs developed using our own reagents were sensitive to 50 pg/ml.
Measurement of Airway Hyperreactivity
Airway hyperreactivity was measured using a Buxco mouse plethysmograph, which is specifically designed for the low tidal volumes (Buxco, Troy, NY) as previously described. 34-36,38 Briefly, the mouse to be tested was anesthetized with sodium pentobarbital and intubated via cannulation of the trachea with an 18-gauge metal tube. The mouse was subsequently ventilated with a Harvard pump ventilator (tidal volume = ∼0.2 ml, frequency = 120 breaths/minute, positive end-expiratory pressure 2.0 to 2.5 cm H2O) and the tail vein was cannulated with a 27-gauge needle for injection of the methacholine challenge. The plethysmograph was sealed and readings were monitored by computer. The trachea transducer was calibrated at a constant pressure of 20 cmH2O. Resistance is calculated by the Buxco software by dividing the change in pressure (Ptp) by the change in flow (F) (tp/F; units = cmH2O/ml/second) at two time points from the volume curve based on a percentage of the inspiratory volume. The mouse was attached to the box and ventilated for 5 minutes before acquiring readings. Once baseline levels were stabilized and initial readings were taken, a methacholine challenge was given via the cannulated tail vein. After determining a dose-response curve (10 to 500 μg/kg), an optimal dose was chosen, 100 μg/kg of methacholine. This dose was used throughout the rest of the experiments in this study and induced little change in resistance in normal, nonallergic mice. After the methacholine challenge, the response was monitored and the peak airway resistance was recorded as a measure of airway hyperreactivity.
Flow Cytometric Analysis of Lung T Lymphocytes
Flow cytometric analysis of lymphocyte subsets was performed in dispersed lung samples from normal and cockroach allergen-challenged mice. Lung homogenate leukocyte numbers were determined by enumerating the total cell number multiplied by the percentage of total lung leukocytes (as determined by differential cell staining) or individual subsets, such as CD4 or CD8 T lymphocytes as previously described. 39 The fluorescent staining procedure was performed on ice in Dulbecco’s phosphate-buffered saline (D-PBS) with 2% fetal bovine serum and 0.1% sodium azide. Total cells (1 × 106) were stained in 500 μl of buffer. Pelleted cells (5 minutes, 1400 rpm) were incubated for 30 minutes on ice with specific antibody, anti-CD4, anti-CD8, or a subclass control (Pharmingen, La Jolla, CA) directly conjugated with fluorescein isothiocyanate. After incubation an additional 2 ml of cold D-PBS was added and the cells pelleted by centrifugation (5 minutes at 1400 rpm at 4°C). The pelleted cells were washed twice with D-PBS and resuspended in 100 μl of 1% paraformaldehyde for 15 minutes. After incubation the cells were centrifuged with the addition of 2 ml of D-PBS and stored at 4°C in D-PBS containing 0.1% sodium azide until analyzed by flow cytometry. Cells were analyzed within 24 hours of staining procedure.
Statistics
Statistical significance was determined using analysis of variance with P values <0.05.
Results
AMD3100 Treatment of Mice Reduces Allergen-Induced Airway Hyperreactivity (AHR)
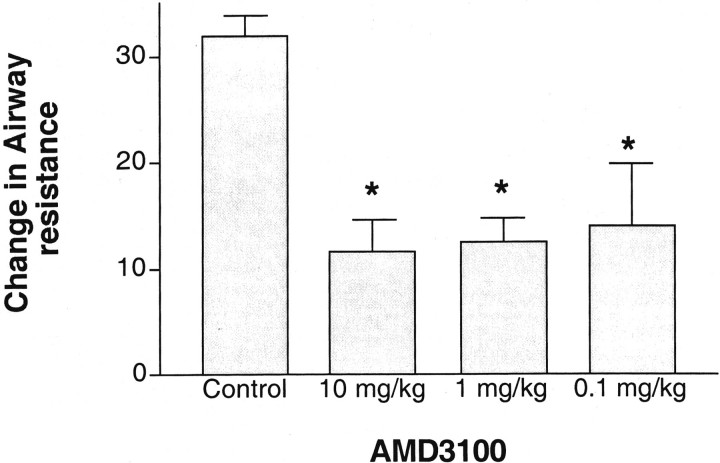
Previous data has shown that blocking CxCL12 (SDF-1) can significantly alter airway responses. Thus, the inhibition of CXCR4 may have a significant effect on the induction of AHR because of its presence on Th2-type cells as well as on other cell populations involved in allergic responses. The ability of AMD3100 to block development of allergen-induced AHR was examined using various doses given intraperitoneally immediately before (within 15 minutes) a single allergen rechallenge. The data in Figure 1 ▶ indicates that AMD3100 has activity for inhibition of airway hyperreactivity over a broad range of doses. Although the effect of inhibiting airway hyperreactivity was diminished slightly at the lowest dose (0.1 mg/kg) it still demonstrated a significant decrease in airway hyperreactivity at 24 hours after allergen challenge. In separate studies a lower dose of AMD3100 (0.01 mg/kg) had no significant effect on the response (data not shown).
Figure 1.
AMD3100 treatment significantly reduces development of AHR. Animals sensitized and challenged with cockroach allergen were given various doses of AMD3100 and assessed for development of airway hyperreactivity 24 hours after challenge. The data represents means ± SE from five to six mice per group.
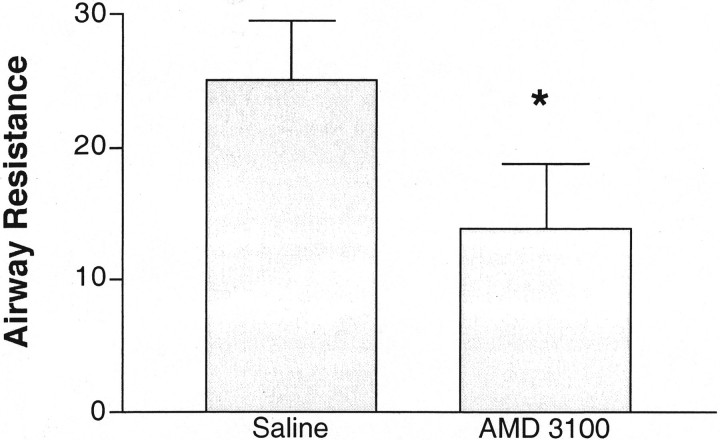
In additional studies we have used multiple intratracheal exposures with cockroach allergen to set up a model that induces a fully Th2-induced eosinophil-dependent airway hyperreactivity response. In these studies, we have assessed the effectiveness of AMD3100 delivered by an osmotic Alzet pump deposited into the peritoneum of the allergic mice for constant delivery. This allergen model was set up with an initial intratracheal challenge given after 21 days of the sensitization process. Forty-eight hours after the initial intratracheal allergen challenge a second intratracheal allergen rechallenge was given. This was at a time when there was a significant amount of peribronchial inflammation, including mononuclear cells and eosinophils, as previously described. 35 Peak airway hyperreactivity and cytokine responses occur in the lungs of sensitized mice 24 hours after the second challenge. AMD3100 was given to the mice via a 3-day Alzet pump implanted into the peritoneum 2 hours before the initial intratracheal allergen challenge. The data in Figure 2 ▶ indicates that treatment of the mice with AMD3100 (250 μg/kg/hour), via the Alzet osmotic pump, significantly reduces the severity of the airway hyperreactivity response. Thus, these results correspond to the above data using a single allergen challenge and may allow a more chronic exposure protocol to be assessed.
Figure 2.
Inhibition of CxCR4 with AMD3100 during chronic allergic responses significantly attenuates airway hyperreactivity in allergic mice. Animals were implanted intraperitoneally with a 3-day osmotic pump that delivered 250 μg/kg/hour of AMD3100 or the saline vehicle. The cockroach allergen-sensitized mice were given two antigenic airway challenges 48 hours apart and assessed for airway hyperreactivity 24 hours after the final allergen challenge. The data represent means ± SE from six mice in each group. Control nonallergic mice treated for 3 days with Alzet pumps containing either AMD3100 or saline followed by an allergen challenge had no alteration of AHR compared to untreated control mice (airway resistance range was between 2.5 and 4.0 cmH2O/ml/second).
Analysis of Leukocyte Infiltration in Allergic Mice Treated with AMD3100
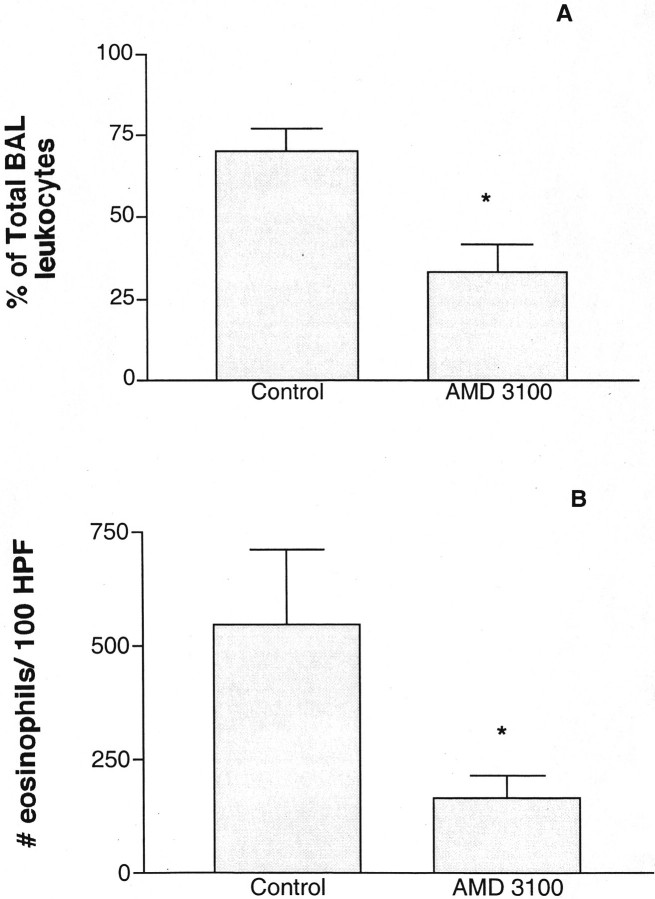
Because AMD3100 is specifically directed toward CxCR4, a chemokine receptor involved in leukocyte recruitment, we next examined whether there was an alteration in total leukocyte infiltrates and, if so, which subsets were affected? We observed a significant alteration in the accumulation of peribronchial and airway eosinophils during the chronic allergen model in the AMD3100 treatment group (Figure 3) ▶ . These changes can be readily observed by histological examination of the lungs from the treated animals (Figure 4) ▶ . In addition, the histology indicates an overwhelming inhibition in the overall inflammatory response. The alteration in peribronchial eosinophil accumulation and the histologically apparent alteration in the overall accumulation of cells suggested an across-the-board effect on recruitment.
Figure 3.
Allergic animals treated with AMD3100 have a significant reduction in eosinophil accumulation in and around the airway. Chronically treated allergic animals from Figure 2 ▶ were assessed for the level of eosinophil recruitment into the airway and lungs by examining the levels in bronchoalveolar lavage fluid (A) and enumerating the peribronchial eosinophils morphometrically (B). The total numbers of bronchoalveolar lavage leukocytes were 2.3 ± 1 × 10 5 for control and 1.9 ± 2.0 × 10 5 for AMD3100. The data represent the means ± SE for six mice in each group. Nonallergic control mice treated in a similar manner followed by a cockroach allergen challenge had minimal peribronchial eosinophil accumulation (<10 eosinophils/100 high-powered fields).
Figure 4.
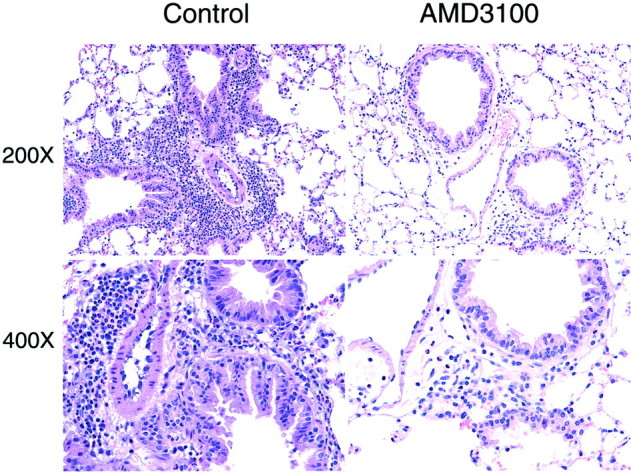
Inhibition of CxCR4 with AMD3100 during chronic allergic responses significantly attenuates the overall airway inflammatory responses. The photo represents the significant reduction in the leukocyte recruitment responses in the lungs of the allergic mice.
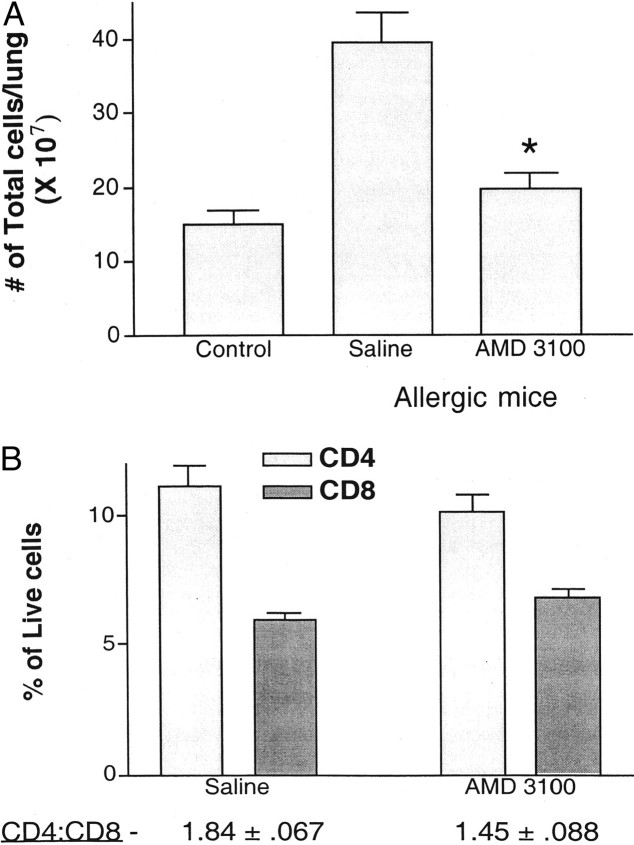
To further study the nature of the altered inflammation, multiple allergen-challenged mice were implanted with Alzet pumps intraperitoneally with either saline or AMD3100 (250 μg/kg/hour). Lungs of chronic allergen-challenged mice were dispersed with collagenase and the total leukocytes were assessed along with individual subsets. Figure 5A ▶ demonstrates that AMD3100 significantly decreased the total number of leukocytes that were migrating into the lungs of chronically challenged allergic mice. We also examined whether AMD3100 altered particular T cell populations. The collagenase-dispersed lung samples from above were used for flow cytometric analysis and lymphocyte subsets were examined. The data in Figure 5B ▶ indicates that treatment of allergic mice with AMD3100 altered CD4+ cell content based on percentage of total cells that migrated into the lungs. In addition, the ratio of CD4:CD8 T cells was significantly reduced (P < 0.05). Together with the overall decrease in inflammation, these data strongly suggest that blocking CxCR4 with AMD3100 affects the T cell numbers and ratios of subsets and likely all subsequent responses, including eosinophil accumulation and airway hyperreactivity.
Figure 5.
Blocking CxCR4 significantly reduced the accumulation of total leukocytes in the lungs of allergic mice (A) and significantly altered the level of CD4 + cell recruitment (B). Lungs from chronically allergen-challenged mice treated with AMD3100 (250 μg/kg/hour) or saline were dispersed by collagenase treatment and the single-cell suspensions assessed for leukocyte numbers and subset analysis by flow cytometry. Data represents means ± SE from four mice in each group.
Alteration of Cytokine and Chemokine Levels after Blocking CxCR4 Interactions
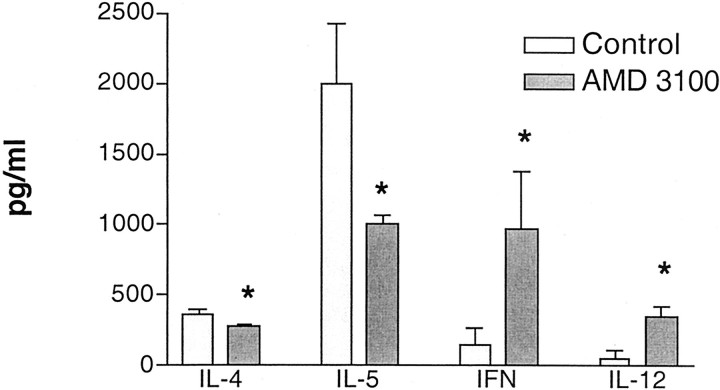
In these latter studies we also assessed the local cytokine responses in the lungs of the challenged animals. The ability to reduce the appropriate cytokines (Th2 type), while increasing others (Th1 type) may be the most effective strategy to alter the long-term effects of asthmatic disease. Analysis of pulmonary levels of Th1-associated (IL-12 p40 subunit and IFN-γ) and Th2-associated (IL-4 and IL-5) cytokines indicates that AMD3100 clearly affected the cytokine profile of the lungs after multiple allergen challenges (Figure 6) ▶ . Both IL-4 and IL-5 were significantly decreased, whereas IL-12 (p40 subunit) and IFN-γ were significantly increased after multiple allergen challenges in the AMD3100 treatment group. These data indicate that the phenotype of the response was drastically altered from a predominant Th2 allergic response to a more clinically attractive Th1-type response. These data help explain why there were fewer eosinophils and lower airway hyperreactivity responses.
Figure 6.
Alteration of cytokine profiles in allergic animals treated with AMD3100. The Th1- and Th2-associated cytokines, IL-12 (p40), IFN-γ (Th1 type), and IL-4 and IL-5 (Th2 type) were assayed in whole-lung homogenates of chronically challenged allergic mice by specific ELISA. The data represents means ± SE from five mice in each group. *, P < 0.05.
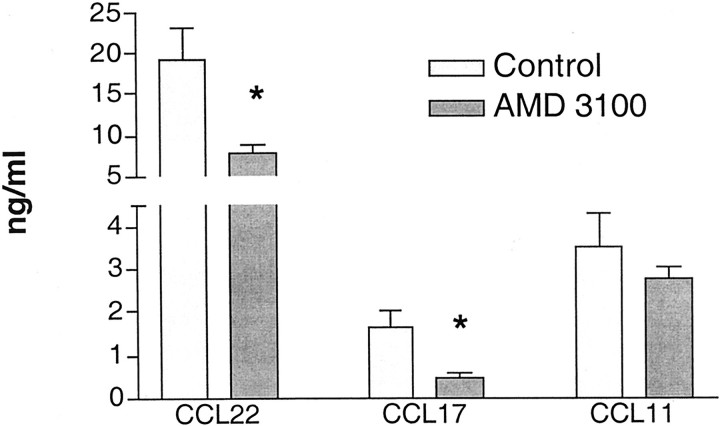
In addition to examining Th-1 and Th2-associated cytokines, we were also interested in assessing chemokines that may be involved in the allergen-induced inflammatory responses. We were especially interested in some of those chemokines that had previously been shown to be associated with the chronic immune responses. The data in Figure 7 ▶ illustrates that when we examined CCL11 (CCR3 ligand), CCL17, and CCL22 (CCR4 ligands), both CCL17 and CCL22 were significantly reduced in the AMD3100-treated group. These data indicated that along with the Th2-type cytokines, chemokines were also significantly reduced, correlating with the overall reduction of inflammation and airway hyperreactivity.
Figure 7.
Reduction of chemokine production in the lungs of allergic mice treated with AMD3100. CCL11, CCL17, and CCL22 levels were assessed in the lung homogenates from mice treated with either AMD3100 (250 μg/kg/hour) or saline using specific ELISAs. The data represents means ± SE from five mice in each group. *, P < 0.05.
Discussion
The rationale behind using chemokine receptor antagonists in inflammatory diseases is to selectively alter the recruitment of particular subsets of cells that initiate and maintain the inflammatory responses. In these studies, AMD3100 (a specific CxCR4 antagonist) was examined in a cockroach allergen-induced animal model of asthma. Several lines of evidence support the idea that CxCR4 may play a role in this Th2-type immune response. Possibly the most interesting evidence to date indicates that IL-4 up-regulates, whereas IFN-γ down-regulates CxCR4 expression on the surface of T lymphocytes. 18,40-42 In further support are studies indicating that Th1-type lymphocytes have very little or no expression of CxCR4, whereas Th2-type lymphocytes express CxCR4 on their surface. 20,41 In agreement with our data is a recent study that indicates that SDF-1 and CxCR4 are directly involved in the asthmatic response in an ovalbumin model of asthma. 21 Thus, the role of CxCR4 during an allergic/asthmatic pulmonary response may reflect the altered immune environment leading to increased lung damage and airway reactivity. The present experiments add further information to the possible mechanism of how CxCR4 is involved. When AMD3100 was administered to the allergic mice Th1-type cytokines were increased in the lung after allergen rechallenge. These data correlate well with the inflammation analysis, including decreased peribronchial eosinophil accumulation and the reduction in total leukocyte accumulation in allergen-challenged animals, as assessed by lung dispersion.
The efficacy of AMD3100 was initially tested by measuring the change in airway resistance in mice after a single allergen challenge. AMD3100 exhibited a broad dose-dependent reduction in airway resistance with significant activity as low as 0.1 mg/kg. Our subsequent studies were focused on the effect of AMD3100 during multiple allergen challenges, which may be more representative of the responses in chronic asthmatics that constantly are exposed to allergen stimulation. Because we were concerned with the availability of AMD3100 during chronic allergen exposure, we also examined the efficacy of giving AMD3100 to animals via osmotic pumps, thus allowing a constant release instead of a bolus administration. These studies demonstrated significant decreases in the airway responses and a striking alteration in the development of the peribronchial lung inflammation. Of greatest concern in asthmatics are the long-term consequences of allergen-induced inflammation and damage to the airway. 20,43 Possibly reflecting the responses in chronic asthmatics, our exposure of animals to multiple allergen challenges has previously indicated an extreme dependency on lymphocyte and eosinophil accumulation and activation. 34,35,39 The constant inflammation associated with this response may be the most devastating long-term problem to these patients. Managing this latter aspect may be the key to altering the progression of the asthmatic disease. Using AMD3100, the observed effects were broad-based and demonstrated several favorable aspects of the response. Firstly, and probably most importantly, inhibiting CxCR4 with AMD3100 significantly reduced the airway hyperreactivity response by nearly 50%. Subsequent experiments indicated an across the board reduction in inflammation, including a significant reduction in peribronchial and airway eosinophilia and an alteration in lymphocyte and mononuclear phagocyte numbers. In fact, the analysis of total leukocytes indicated that there was an ∼80% reduction in recruited leukocytes over background numbers in AMD3100-treated animals compared to control saline-treated animals. This effect may be because of blocking CxCR4 interactions on multiple cell populations, including lymphocytes and eosinophils, both of which have been described to express CxCR4. 29,44 In addition, recent studies have identified CxCR4 on basophils, and its ligand, SDF-1, was able to induce activation and degranulation of those cells. 45 However, the data from the present studies may build the strongest case for alteration of T cell accumulation and activation, based on the alteration of the cytokine profiles. Previous studies have clearly indicated that administration of IL-12 and/or IFN-γ in the lungs attenuates airway hyperreactivity induced by allergens. 46-48 The results from these studies not only demonstrated that IL-12 (p40 subunit) and IFN-γ levels were increased, but the administration of the CxCR4 antagonist, AMD3100, reduced IL-4 and IL-5 levels. This shift toward Th1-type cytokines may be the most favorable response because previous studies have demonstrated that Th2 cell transfer increases airway hyperreactivity, whereas transfer of Th1-type cells does not increase or alter airway hyperreactivity even though a significant response to allergen is observed. 5,49 Thus, the Th1-type cytokines, although possibly damaging, do not promote an asthmatic-type response on their own. Altogether, the alteration of cytokine profiles constitute a significant aspect of this compound that throughout time could reduce the airway damage and inflammation in patients that potentially leads to end-stage disease.
The role of chemokines in asthma is central to the chronic recruitment of leukocytes that migrate into and around the airway during asthmatic responses. A number of chemokines have been identified as possible targets using animal models of asthma and hyperreactivity, including CCL2 (MCP-1), CCL7 (MCP-3), CCL11 (eotaxin), CCL17 (TARC), CCL22 (MDC), and CxCL12 (SDF-1). 14,34,35,39,50-52 The reduction of CCL17 and CCL22 after blocking CxCR4 may represent an additional aspect of the attenuated response. The reduction of these chemokine mediators may be a direct result of the alteration of the T cell-derived cytokines, Th1 versus Th2. A number of chemokine receptors have also been shown to be important in the development of the airway responses, including CCR1, CCR2, and CCR8. 53-55 Relevant data for blocking other receptors, such as CCR3 and CCR4, are still lacking. However, it is likely that multiple receptors contribute to the recruitment of the various cell populations that migrate into the airways after allergen challenge. It seems that CxCR4 may be a pivotal receptor used during the allergen-induced responses. The anti-inflammatory response observed with AMD3100 treatment has been observed in a previous publication using a type I collagen-induced arthritis model, 33 but no alteration in specific subsets was seen. The present study seems to demonstrate an altered immune environment that may be because of an overall decrease in inflammation as observed in the arthritis model. In addition, CxCR4 has a significant role in leukocyte maturation in the bone marrow. 56 Thus, further studies will need to more thoroughly address whether AMD3100 has an effect on leukocyte differentiation in the bone marrow.
The specific CxCR4 inhibitor used in these studies, AMD3100, has been shown to specifically inhibit human immunodeficiency virus entry into cells via blocking CxCR4 interactions. 30,31 The pharmacokinetics and safety have already been assessed in humans and AMD3100 was found to be well tolerated. 32 There are a number of observations from the present studies that may be advantageous in other diseases as well, including the skewing of the immune response from Th2- toward a Th1-type response. Altogether, these data indicate that AMD3100 may be a desirable compound to pursue in clinical trials for efficacy in asthmatic patient populations.
Footnotes
Address reprint requests to Nicholas W. Lukacs, Department of Pathology, University of Michigan Medical School, 1301 Catherine St., Ann Arbor, MI 48109-0602. E-mail: nlukacs@umich.edu.
Supported in part by National Institutes of Health grants AI36302 and HL31963.
References
- 1.Schuh S, Johnson D, Stephens D, Callahan S, Canny G: Hospitalization patterns in severe acute asthma in children. Pediatr Pulmonol 1997, 23:184-192 [DOI] [PubMed] [Google Scholar]
- 2.Bates DV: Observations on asthma. Environ Health Perspect 1995, 103(Suppl 6):S243-S247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manian P: Genetics of asthma: a review. Chest 1997, 112:1397-1408 [DOI] [PubMed] [Google Scholar]
- 4.Boyce JA: The pathobiology of eosinophilic inflammation. Allergy Asthma Proc 1997, 18:293-300 [DOI] [PubMed] [Google Scholar]
- 5.Umetsu DT, DeKruyff RH: Th1 and Th2 CD4+ cells in the pathogenesis of allergic diseases. Proc Soc Exp Biol Med 1997, 215:11-20 [DOI] [PubMed] [Google Scholar]
- 6.Polito AJ, Proud D: Epithelia cells as regulators of airway inflammation. J Allergy Clin Immunol 1998, 102:714-718 [DOI] [PubMed] [Google Scholar]
- 7.Burke CM, Sreenan S, Pathmakanthan S, Patterson J, Schmekel B, Poulter LW: Relative effects of inhaled corticosteroids on immunopathology and physiology in asthma: a controlled study. Thorax 1996, 51:993-999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djukanovic R, Homeyard S, Gratziou C, Madden J, Walls A, Montefort S, Peroni D, Polosa R, Holgate S, Howarth P: The effect of treatment with oral corticosteroids on asthma symptoms and airway inflammation. Am J Respir Crit Care Med 1997, 155:826-832 [DOI] [PubMed] [Google Scholar]
- 9.Gauvreau GM, Doctor J, Watson RM, Jordana M, O’Byrne PM: Effects of inhaled budesonide on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med 1996, 154:1267-1271 [DOI] [PubMed] [Google Scholar]
- 10.Majori M, Piccoli ML, Bertacco S, Cuomo A, Cantini L, Pesci A: Inhaled beclomethasone dipropionate downregulates CD4 and CD8 T-lymphocyte activation in peripheral blood of patients with asthma. J Allergy Clin Immunol 1997, 100:379-382 [DOI] [PubMed] [Google Scholar]
- 11.Denburg JA, Woolley MJ, Ellis R, Dahlback M, O’Byrne PM: Allergen-induced changes in bone marrow progenitors and airway responsiveness in dogs. Int Arch Allergy Immunol 1995, 107:239-241 [DOI] [PubMed] [Google Scholar]
- 12.Baggiolini M, Dewald B, Moser B: Human chemokines: an update. Annu Rev Immunol 1997, 15:675-705 [DOI] [PubMed] [Google Scholar]
- 13.Moser B, Loetscher M, Piali L, Loetscher P: Lymphocyte responses to chemokines. Int Rev Immunol 1998, 16:323-344 [DOI] [PubMed] [Google Scholar]
- 14.Lukacs NW, Oliveira SH, Hogaboam CM: Chemokines and asthma: redundancy of function or a coordinated effort? J Clin Invest 1999, 104:995-999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A: Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med 1998, 187:875-883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D’Ambrosio D, O’Garra A, Robinson D, Rocchi M, Santoni A, Zlotnik A, Napolitano M: The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol 1998, 161:547-551 [PubMed] [Google Scholar]
- 17.Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, Qin S, Forster R, Lipp M, Lanzavecchia A: Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol 1999, 29:2037-2045 [DOI] [PubMed] [Google Scholar]
- 18.Annunziato F, Cosmi L, Galli G, Beltrame C, Romagnani P, Manetti R, Romagnani S, Maggi E: Assessment of chemokine receptor expression by human Th1 and Th2 cells in vitro and in vivo. J Leukoc Biol 1999, 65:691-699 [DOI] [PubMed] [Google Scholar]
- 19.Burdick MD, Kunkel SL, Lincoln PM, Wilke CA, Strieter RM: Specific ELISAs for the detection of human macrophage inflammatory protein-1 alpha and beta. Immunol Invest 1993, 22:441-449 [DOI] [PubMed] [Google Scholar]
- 20.Williams CM, Galli SJ: Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med 2000, 192:455-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalo JA, Lloyd CM, Peled A, Delaney T, Coyle AJ, Gutierrez-Ramos JC: Critical involvement of the chemotactic axis CXCR4/stromal cell-derived factor-1 alpha in the inflammatory component of allergic airway disease. J Immunol 2000, 165:499-508 [DOI] [PubMed] [Google Scholar]
- 22.Well TN, Proudfoot AE, Power CA: Chemokine receptors and their role in leukocyte activation. Immunol Lett 1999, 65:35-40 [DOI] [PubMed] [Google Scholar]
- 23.Zlotnik A, Morales J, Hedrick JA: Recent advances in chemokines and chemokine receptors. Crit Rev Immunol 1999, 19:1-47 [PubMed] [Google Scholar]
- 24.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC: The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med 1997, 185:111-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA: A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor (SDF-1). J Exp Med 1996, 184:1101-1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O’Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman RM: Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med 1996, 184:2433-2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maghazachi AA: Role of the heterotrimeric G proteins in stromal-derived factor-1alpha-induced natural killer cell chemotaxis and calcium mobilization. Biochem Biophys Res Commun 1997, 236:270-274 [DOI] [PubMed] [Google Scholar]
- 28.Tanabe S, Heesen M, Yoshizawa I, Berman MA, Luo Y, Bleul CC, Springer TA, Okuda K, Gerard N, Dorf ME: Functional expression of the CXC-chemokine receptor-4/fusin on mouse microglial cells and astrocytes. J Immunol 1997, 159:905-911 [PubMed] [Google Scholar]
- 29.Nagase H, Miyamasu M, Yamaguchi M, Fujisawa T, Ohta K, Yamamoto K, Morita Y, Hirai K: Expression of CXCR4 in eosinophils: functional analyses and cytokine-mediated regulation. J Immunol 2000, 164:5935-5943 [DOI] [PubMed] [Google Scholar]
- 30.Schols D, Struyf S, Van Damme J, Este JA, Henson G, De Clercq E: Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med 1997, 186:1383-1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schols D, Este JA, Henson G, De Clercq E: Bicyclams, a class of potent anti-HIV agents, are targeted at the HIV coreceptor fusin/CXCR-4. Antiviral Res 1997, 35:147-156 [DOI] [PubMed] [Google Scholar]
- 32.Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, Bridger G, Henson GW: Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother 2000, 44:1667-1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthys P, Hatse S, Vermeire K, Wuyts A, Bridger G, Henson GW, De Clercq E, Billiau A, Schols D: Amd3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor cxcr4, inhibits autoimmune joint inflammation in ifn-gamma receptor-deficient mice. J Immunol 2001, 167:4686-4692 [DOI] [PubMed] [Google Scholar]
- 34.Campbell EM, Charo IF, Kunkel SL, Strieter RM, Boring L, Gosling J, Lukacs NW: Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/− mice: the role of mast cells. J Immunol 1999, 163:2160-2167 [PubMed] [Google Scholar]
- 35.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW: Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol 1998, 161:7047-7053 [PubMed] [Google Scholar]
- 36.Campbell E, Kunkel SL, Strieter RM, Lukacs NW: Differential roles of IL-18 in allergic airway disease: induction of eotaxin by resident cell populations exacerbates eosinophil accumulation. J Immunol 2000, 164:1096-1102 [DOI] [PubMed] [Google Scholar]
- 37.Evanoff HL, Burdick MD, Moore SA, Kunkel SL, Strieter RM: A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest 1992, 21:39-45 [DOI] [PubMed] [Google Scholar]
- 38.Campbell E, Hogaboam C, Lincoln P, Lukacs NW: Stem cell factor-induced airway hyperreactivity in allergic and normal mice. Am J Pathol 1999, 154:1259-1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukacs NW, Strieter RM, Warmington K, Lincoln P, Chensue SW, Kunkel SL: Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C-C family chemokines in allergic airway inflammation. J Immunol 1997, 158:4398-4404 [PubMed] [Google Scholar]
- 40.Wang J, Harada A, Matsushita S, Matsumi S, Zhang Y, Shioda T, Nagai Y, Matsushima K: IL-4 and a glucocorticoid up-regulate CXCR4 expression on human CD4+ T lymphocytes and enhance HIV-1 replication. J Leukoc Biol 1998, 64:642-649 [DOI] [PubMed] [Google Scholar]
- 41.Jourdan P, Vendrell JP, Huguet MF, Segondy M, Bousquet J, Pene J, Yssel H: Cytokines and cell surface molecules independently induce CXCR4 expression on CD4+ CCR7+ human memory T cells. J Immunol 2000, 165:716-724 [DOI] [PubMed] [Google Scholar]
- 42.Jourdan P, Abbal C, Noraz N, Hori T, Uchiyama T, Vendrell JP, Bousquet J, Taylor N, Pene J, Yssel H, Nora N: IL-4 induces functional cell-surface expression of CXCR4 on human T cells. J Immunol 1998, 160:4153-4157 [PubMed] [Google Scholar]
- 43.Corrigan CJ, Kay AB: T cells and eosinophils in the pathogenesis of asthma. Immunol Today 1992, 13:501-507 [DOI] [PubMed] [Google Scholar]
- 44.Kim CH, Broxmeyer HE: Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol 1999, 65:6-15 [DOI] [PubMed] [Google Scholar]
- 45.Jinquan T, Jacobi HH, Jing C, Reimert CM, Quan S, Dissing S, Poulsen LK, Skov PS: Chemokine stromal cell-derived factor 1alpha activates basophils by means of CXCR4. J Allergy Clin Immunol 2000, 106:313-320 [DOI] [PubMed] [Google Scholar]
- 46.Lee YL, Fu CL, Ye YL, Chiang BL: Administration of interleukin-12 prevents mite Der p 1 allergen-IgE antibody production and airway eosinophil infiltration in an animal model of airway inflammation. Scand J Immunol 1999, 49:229-236 [DOI] [PubMed] [Google Scholar]
- 47.Hogan SP, Foster PS, Tan X, Ramsay AJ: Mucosal IL-12 gene delivery inhibits allergic airways disease and restores local antiviral immunity. Eur J Immunol 2000, 28:413-423 [DOI] [PubMed] [Google Scholar]
- 48.Li XM, Chopra RK, Chou TY, Schofield BH, Wills-Karp M, Huang SK: Mucosal IFN-gamma gene transfer inhibits pulmonary allergic responses in mice. J Immunol 1996, 157:3216-3219 [PubMed] [Google Scholar]
- 49.Randolph DA, Carruthers CJ, Szabo SJ, Murphy KM, Chaplin DD: Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J Immunol 2000, 162:2375-2383 [PubMed] [Google Scholar]
- 50.Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TNC, Proudfoot A, Martinez AC, Dorf M, Bjerke T, Coyle AJ, Gutierrez-Ramos JC: The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med 1998, 188:157-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, Powers CA, Proudfoot AE, Coyle AJ, Gearing D, Gutierrez-Ramos JC: Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol 1999, 163:403-411 [PubMed] [Google Scholar]
- 52.Elsner J, Petering H, Hochstetter R, Kimmig D, Wells TN, Kapp A, Proudfoot AE: The CC chemokine antagonist Met-RANTES inhibits eosinophil effector functions through the chemokine receptors CCR1 and CCR3. Eur J Immunol 1997, 27:2892-2898 [DOI] [PubMed] [Google Scholar]
- 53.Blease K, Mehrad B, Standiford TJ, Lukacs NW, Gosling J, Boring L, Charo IF, Kunkel SL, Hogaboam CM: Enhanced pulmonary allergic responses to Aspergillus in CCR2−/− mice. J Immunol 2000, 165:2603-2611 [DOI] [PubMed] [Google Scholar]
- 54.Campbell EM, Charo IF, Kunkel SL, Strieter RM, Boring L, Gosling J, Lukacs NW: Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/− mice: the role of mast cells. J Immunol 1999, 163:2160-2167 [PubMed] [Google Scholar]
- 55.Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, Kung T, Wiekowski MT, Hedrick JA, Cook DN, Zingoni A, Narula SK, Zlotnik A, Barrat FJ, O’Garra A, Napolitano M, Lira SA: Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med 2001, 193:573-584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagasawa T: A chemokine, SDF-1/PBSF, and its receptor, CXC chemokine receptor 4, as mediators of hematopoiesis. Int J Hematol 2000, 72:408-411 [PubMed] [Google Scholar]