Abstract
Despite several advances in our basic understanding and in the clinical management of pancreatic cancer, virtually all patients who will be diagnosed with pancreatic cancer will die from this disease. The high mortality of pancreatic cancer is predominantly because of diagnosis at an advanced stage of disease and a lack of effective treatments. We used the Gene Logic Inc. BioExpress platform and Affymetrix GeneChip arrays to identify genes differentially expressed in pancreatic cancer. cDNA was prepared from samples of normal pancreas (n = 11), normal gastrointestinal mucosa (n = 22), resected pancreas cancer tissues (n = 14), and pancreas cancer cell lines (n = 8), and was hybridized to the complete Affymetrix Human Genome U95 GeneChip set (arrays U95 A, B, C, D, and E) for simultaneous analysis of 60,000 cDNA fragments, with 12,000 fragments covering full-length genes and 48,000 fragments covering expressed sequence tags (ESTs). Genes expressed at levels at least fivefold greater in the pancreatic cancers ascompared to normal tissues were identified. Serial analysis of gene expression (SAGE) libraries (http://www.ncbi.nlm.nih.gov/SAGE/) of two normal pancreatic ductal cell cultures (HX and H126) were used to exclude genes expressed in the normal ducts (more than five tags per library). Differential expression of selected candidate genes was validated by immunohistochemical analysis (n = 3), by in situ hybridization (n = 1), and by reverse transcriptase-polymerase chain reaction (n = 8). One hundred eighty fragments were identified as having fivefold or greater expression levels in pancreas cancer specimens as compared to normal tissue, of which 124 corresponded to known genes and 56 to ESTs. Of these 124 fragments, 10 genes were represented by two or more fragments, resulting in 107 known genes identified as differentially expressed in pancreatic cancer. An additional 10 genes were expressed in the SAGE libraries of normal pancreatic duct epithelium, and were excluded from further analysis. A literature search indicated that 28 of the remaining 97 genes have been reported in association with pancreatic cancer, validating this approach. The remaining 69 genes have not been implicated in pancreatic cancer before, and have immediate potential as novel therapeutic targets and tumor markers of pancreatic cancer.
Pancreatic cancer continues to have one of the highest mortality rates of any malignancy. Each year, 28,000 patients are diagnosed with pancreatic cancer, and nearly 28,000 will die of their disease. 1 The vast majority of patients are diagnosed at an advanced stage of disease because currently no tumor markers are known that allow reliable screening for pancreatic cancer at an earlier, potentially curative stage. This is a particular problem for those patients with a strong familial history of pancreatic cancer, who may have up to a 57-fold greater risk of developing pancreatic cancer in their lifetime. 2 New tumor markers of pancreatic cancer are urgently needed.
The utility of RNA-based global gene expression profiling biotechniques in identifying new markers of cancer is established. 3,4 For example, we have identified two new potential markers of pancreatic carcinoma, mesothelin and prostate stem cell antigen, using serial analysis of gene expression (SAGE). 5,6 Both markers are expressed specifically by the neoplastic epithelium of infiltrating carcinomas of the pancreas as compared to normal duct epithelium, and both offer new possibilities for the development of screening markers and therapeutic targets.
In an effort to identify additional potential markers of pancreatic carcinoma, we used the Gene Logic Inc. BioExpress platform and Affymetrix GeneChip arrays to identify genes differentially expressed in a large series of pancreatic cancers. Biocomputational tools were used to determine those genes most highly expressed within pancreatic cancer samples compared to normal pancreatic tissue. Genes found to be significantly expressed in SAGE libraries of normal pancreatic ductal cells were excluded, and the expression of selected genes was confirmed by immunohistochemical labeling, in situ hybridization and reverse transcriptase-polymerase chain reaction (RT-PCR). Here we report 97 genes differentially overexpressed in pancreatic cancer, 69 of which are novel.
Materials and Methods
Tissues
Samples (0.5 g) of normal pancreas (n = 11); normal duodenal, jejunal, or colonic mucosa (n = 22); or infiltrating pancreatic adenocarcinoma (n = 14) were collected from surgical specimens from patients at The Johns Hopkins Hospital. In each case, the specimens were harvested within 10 minutes of resection from the patient and snap-frozen in liquid nitrogen before storage at −80°C. The resected cancers were not microdissected because we were interested in not only identifying the genes expressed by neoplastic epithelial calls, but also the genes expressed as a result of the neoplastic cell-stroma interaction. Hematoxylin and eosin-stained sections of adjacent sections of the tissue were prepared before snap-freezing to confirm the diagnosis. The neoplastic cellularity of these tissue samples ranged from 5 to 55%. Normal gastrointestinal mucosa was included in the analyses to facilitate the identification of markers of pancreatic cancer that would be useful in screening secondary sources, such as in duodenal fluid samples.
Cell Lines
Human pancreatic cancer cell lines AsPc1, BxPc3, CAPAN1, CAPAN2, CFPAC1, COLO357, Hs766T, MiaPaCa2, Panc-1, and Su86.86, and human pancreatic normal duct epithelial line HPDE6, were obtained from the American Type Culture Collection, Rockville, MD. PL cell lines (PL1-6, PL8-14) were low-passage pancreatic carcinoma cell lines kindly provided by Dr. Elizabeth Jaffee from the Department of Oncology, The Johns Hopkins Hospital, Baltimore, MD. 7 Cell lines were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). CAPAN1 and CAPAN2 cell lines were cultured in RPMI 1640 medium (Life Technologies, Inc., Gaithersburg, MD) supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin), respectively. Use of different media minimized the variance in growth rates that would otherwise be exaggerated with a single medium. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in air.
mRNA Extractions and Affymetrix GeneChip Hybridization
Sample preparation and processing procedure was performed as described in the Affymetrix GeneChip Expression Analysis Manual (Santa Clara, CA). Briefly, each frozen tissue was crushed to powder by using the Spex Certiprep 6800 Freezer Mill (Metuchen, NJ). Total RNA was then extracted from the crushed normal and neoplastic tissues or cell pellets (BxPC3, COLO357, Hs766T, MiaPaCa2, Panc1, PL3, PL4, PL8) using TRIzol (Life Technologies, Inc., Rockville, MD) and cleaned using RNeasy columns according to the manufacturer’s protocol (Qiagen, Valencia, CA). Using 5 to 40 μg of total RNA, double-stranded cDNA was synthesized following SuperScript Choice system (Life Technologies, Inc., Rockville, MD). T7-(dT24) oligomer was used for priming the first-strand cDNA synthesis. The resultant cDNA was purified using Phase Lock Gel, phenol/chloroform extraction, and precipitated with ethanol. The cDNA pellet was collected and dissolved in appropriate volume. Using cDNA as template, cRNA was synthesized using a T7 MegaScript In Vitro Transcription (IVT) Kit (Ambion, Austin, TX). Biotinylate-11-CTP and 16-UTP ribonucleotides (Enzo Diagnostics Inc., Farmingdale, NY) were added to the reaction as labeling reagents. IVT reactions were performed at 37°C for 6 hours and, the labeled cRNA obtained was purified using RNeasy columns (Qiagen, Valencia, CA). The cRNA was fragmented in fragmentation buffer (40 mmol/L Tris-Acetate, pH 8.1, 100 mmol/L KOAc, 30 mmol/L MgOAc) for 35 minutes at 94°C. Fragmented cRNA prepared from each sample (10 to 11 μg/probe array) was hybridized to the human GeneChip set (HG_U95 A, B, C, D, and E) noncompetitively at 45°C for 24 hours in a hybridization oven with constant rotation (60 rpm). Fragmented cRNAs are hybridized to the GeneChip set by way of multiple 20 to 25 oligonucleotide probes specific for each gene, with each probe corresponding to a different region of the mRNA of interest. The probes specific for each mRNA are scattered across the surface of each GeneChip to control for technical issues that occur with each hybridization. The chips were washed and stained using Affymetrix fluidics stations. Staining was performed using streptavidin-phycoerythrin conjugate (SAPE; Molecular Probes, Eugene, OR), followed by the addition of biotinylated antibody to streptavidin (Vector Laboratories, Burlingame, CA), and finally with streptavidin-phycoerythrin conjugate. Probe arrays was scanned using fluorometric scanners (Hewlett Packard Gene Array Scanner; Hewlett Packard Corporation, Palo Alto, CA).
The scanned images were inspected and analyzed using established quality control measures, with the hybridization intensities reflecting in a linear manner the mRNA expression in the tissues or cells being assayed. Hybridization was controlled for each probe by the use of a mismatch control that has a single base mismatch. This mismatch control is analyzed using the GeneLogic informatics filter that compares the hybridization intensity of mismatched to perfect matched probes (to eliminate those that are nonspecific over a specified threshold) as well as different probes to the same gene.
Statistical Data Analysis
The GeneExpress Software System Fold Change Analysis tool was used to identify genes expressed at least fivefold greater in the pancreatic cancers compared to normal tissues. For each gene fragment, the ratio of the geometric means of the expression intensities in the normal control tissues and the pancreas cancer samples was calculated, and the fold change then calculated on a per fragment basis. Confidence limits were calculated using a two-sided Welch modified t-test on the difference of the means of the logs of the intensities.
SAGE
Short-term cultures of nonneoplastic pancreatic ductal epithelial cells (HX and H126) were prepared as described and validated as having the characteristics of ductal epithelium. 8 SAGE libraries were previously constructed as described by Ryu and colleagues, 9,10 and sequencing was performed by the CGAP SAGE consortium at the Lawrence Livermore National Laboratories and Washington University Human Genome Center (St. Louis, MO). SAGE library data from the short-term cultures of nonneoplastic pancreatic duct epithelial cells have been posted on the CGAP web site as part of the SAGEmap database (http://www.ncbi.nlm.nih.gov/SAGE).
In Situ Hybridization
Preparation of digoxigenin-labeled sense and antisense riboprobes and in situ hybridization were performed as previously described in detail. 11
RT-PCR
Total RNA was isolated from cultured cells by using TRIzol reagent (Life Technologies, Inc.). Cell lines used for RT-PCR were PL1-6, PL8-14, CAPAN1, CFPAC, AsPc1, BxPC3, Hs766T, MiaPaCa2, Panc1, and HPDE6. An aliquot of 1 μg of total RNA from each sample was reverse-transcribed to cDNA using the SuperScript II kit (Life Technologies, Inc.) according to the manufacturer’s instructions, with oligo(dT)12-18 primer. PCR primers were designed to amplify cDNA fragments with various sizes using standard PCR conditions. The PCR reaction products were resolved by electrophoresis in a 3% agarose gel and stained with ethidium bromide. Loading was controlled by the simultaneous PCR of glyceraldehyde-3-phosphate dehydrogenase cDNA.
Immunohistochemistry
Sections of infiltrating primary ductal adenocarcinoma of the pancreas were formalin-fixed and paraffin-embedded, and unstained 4-μm sections were then cut from the paraffin blocks. For detection of heat shock protein 47 (hsp47), sections were deparaffinized by routine techniques before placing in 200 ml of Target Retrieval Solution, pH 6.0 (Envision Plus Detection kit, DAKO, Carpinteria, CA) for 20 minutes at 100°C. After cooling for 20 minutes, slides were quenched with 3% H2O2 for 5 minutes, before incubating with a 1:800 dilution of monoclonal antibody (colligin m16.10A1) against heat shock protein 47 (Stressgen Biotechnologies, Victoria, BC, Canada) for 30 minutes using the DAKO Autostainer. Labeling was detected with the DAKO Envision system following the manufacturer’s protocol. For detection of topoisomerase IIα and fascin, slides were steamed for 20 minutes in sodium citrate buffer (diluted to 1× from 10× heat-induced epitope retrieval buffer; Ventana-Bio Tek Solutions, Tucson, AZ). After cooling for 5 minutes, slides were labeled with a 1:3200 dilution of mouse monoclonal antibody against topoisomerase II (clone TG100; Neomarkers, Freemont,CA) or a 1:500 dilution of mouse monoclonal antibody against fascin (DAKO) using the Bio Tek 1000 automated stainer (Ventana). Labeling was detected by adding biotinylated secondary antibodies, avidin-biotin complex, and 3,3′-diaminobenzidine. All sections were counterstained with hematoxylin, and staining was evaluated by three of the authors (CID, AM, and RHH) with agreement in all cases evaluated. Staining was considered positive if at least 10% of the cells showed immunolabeling.
Results
Data Filtering
RNA samples were hybridized to the complete Affymetrix Human Genome U95 GeneChip set (arrays U95 A, B, C, D, and E) for simultaneous analysis of 60,000 fragments, with 12,000 fragments covering full-length genes and 48,000 fragments covering ESTs. Affymetrix GeneChips were analyzed for all genes with a fivefold or greater increase in expression in the pancreatic adenocarcinoma tumor tissues or cell lines compared to all normal tissues, using a 95% confidence limit. We identified 180 fragments expressed at least fivefold greater in pancreatic cancer samples as compared to normal tissues, 12 of which were expressed greater than 10-fold. The level of significance for each gene fragment ranged from less than P = 0.00001 to P = 0.01 (modified Welch t-test).
Identification of Highly Expressed Genes in Pancreatic Cancer
Characterization of the 180 fragments identified revealed that 56 fragments corresponded to ESTs, and 124 fragments corresponded to known genes. Among these 124 fragments, 10 genes were represented by two or more fragments, resulting in 107 known genes identified as expressed at least fivefold or greater in pancreatic cancers as compared to normal (Table 1) ▶ .
Table 1.
Highly Expressed Genes Identified in Pancreatic Cancer Cell Lines and Tissues
| Affymetrix fragment name | Known gene name | Fold change | P value | eNorthern pattern* | SAGE normal tags† | Reported in pancreas | Ref. | Cellular location§ |
|---|---|---|---|---|---|---|---|---|
| 39829_at | ADP-ribosylation factor-like 7 | 7.17 | <0.00001 | A | 0,0 | no | C | |
| 37403_at | Annexin A1 | 5.66 | <0.00001 | A | 1,0 | no | C | |
| 89917_at | Apolipoprotein C-I | 8.09 | 0.00008 | B | 1,1 | yes | 11 | EM,M |
| 88518_at | Aspartate beta-hydroxylase | 5.59 | <0.00001 | A | 1,2 | no | C | |
| 1898_at | Ataxia-telangiectasia group D-associated protein | 5.21 | <0.00001 | A | 0,0 | no | C | |
| 91017_at | Baculoviral IAP repeat-containing 3 | 7.5 | <0.00001 | A | 0,0 | no | ||
| 74989_at | Biglycan | 12.27 | 0.00011 | B | 0,0 | yes | 36 | EM |
| 36976_at | Cadherin 11, type 2, OB-cadherin (osteoblast) | 5.93 | <0.00001 | B | 0,0 | no | M | |
| 38391_at | Capping protein (actin filament), gelsolin-like | 5.08 | <0.00001 | B | 4,1 | no | C | |
| 74707_at | Capping protein (actin filament), gelsolin-like | 11.66 | <0.00001 | B | 4,1 | no | C | |
| 53708_at | Cation-chloride cotransporter-interacting protein | 5.42 | <0.00001 | A | 2,0 | no | M | |
| 339_at | Caveolin 2 | 5.95 | <0.00001 | A | 0,0 | no | C | |
| 2036_s_at | CD44 antigen | 5.3 | <0.00001 | A | 0,2 | yes | 37 | M |
| 89856_at | CD83 antigen | 6.51 | <0.00001 | B | 0,0 | no | M | |
| 38112_g_at | Chondroitin sulfate proteoglycan 2 (versican) | 5.04 | 0.00009 | B | 0,0 | no | EM | |
| 46260_at | Claudin 1 | 5.61 | <0.00001 | A | 0,0 | no | M | |
| 35474_s_at | Collagen, type I, alpha 1 | 7.07 | 0.00002 | B | 1,1 | yes | 9;10 | EM |
| 32305_at | Collagen, type I, alpha 2 | 8.84 | 0.00001 | B | 4,2 | yes | 9;10 | EM |
| 32306_g_at | Collagen, type I, alpha 2 | 5.3 | 0.00128 | B | 4,2 | yes | 9;10 | EM |
| 63596_f_at | Collagen, type I, alpha 2 | 5.69 | 0.00286 | B | 4,2 | yes | 9;10 | EM |
| 60071_s_at | Collagen, type I, alpha 2 | 5.18 | 0.0048 | B | 4,2 | yes | 9;10 | EM |
| 37892_at | Collagen, type XI, alpha 1 | 6.88 | 0.00001 | B | 0,0 | yes | 9;10 | EM |
| 73132_r_at | Cyclin-dependent kinase inhibitor 2A (p16) | 5.87 | <0.00001 | A | 0,0 | yes | 38 | C |
| 40490_at | DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 21 | 8.31 | <0.00001 | A | 8,9‡ | no | ||
| 73128_at | Dihydropyrimidinase-like 3 | 8.33 | 0.00001 | B | 1,0 | no | ||
| 39959_at | Diubiquitin | 5.49 | 0.00001 | B | 0,0 | no | C | |
| 48740_s_at | DKFZP564G013 protein | 6.61 | <0.00001 | A | 0,0 | no | ||
| 37981_at | Drebrin 1 | 5.24 | <0.00001 | A | 0,1 | no | C | |
| 1379_at | EphA2 | 5.07 | <0.00001 | A | 7,6‡ | no | M | |
| 56226_at | Eukaryotic translation initiation factor 2C, 2 | 5.84 | <0.00001 | A | 1,1 | no | C | |
| 34678_at | fer-1 (C. elegans)-like 3 (myoferlin) | 9.03 | <0.00001 | A | 0,0 | no | M | |
| 39945_at | Fibroblast activation protein, alpha | 5.57 | 0.00001 | B | 0,0 | no | M | |
| 311_s_at | Fibronectin 1 | 6.53 | 0.00005 | B | 2,1 | no | M,EM | |
| 65830_at | FXYD domain-containing ion transport regulator 5 | 8.69 | <0.00001 | A | 5,7‡ | no | ||
| 91306_s_at | Gap junction protein, beta 2, 26kD (connexin 26) | 7.32 | <0.00001 | A | 0,0 | yes | 39 | M |
| 91309_r_at | Gap junction protein, beta 2, 26kD (connexin 26) | 5.37 | <0.00001 | A | 0,0 | yes | 39 | M |
| 89908_f_at | GDP dissociation inhibitor 1 | 7.58 | <0.00001 | A | 0,1 | yes | 40 | C |
| 40365_at | Guanine nucleotide binding protein (G protein), alpha 15 | 5.18 | <0.00001 | A | 0,0 | no | ||
| 43366_at | Hypothetical protein FLJ10261 | 5.61 | <0.00001 | A | 0,1 | no | ||
| 43963_at | Hypothetical protein FLJ10261 | 6.35 | <0.00001 | A | 0,1 | no | ||
| 58235_at | Hypothetical protein FLJ10540 | 5.45 | <0.00001 | A | 0,0 | no | ||
| 44062_at | Hypothetical protein FLJ10849 | 5.59 | <0.00001 | A | 2,1 | no | ||
| 85285_at | Hypothetical protein FLJ11183 | 5.6 | <0.00001 | A | 0,0 | no | ||
| 54030_at | Hypothetical protein FLJ20373 | 7.08 | <0.00001 | A | 1,1 | no | ||
| 47427_at | Hypothetical protein FLJ20539 | 5.8 | <0.00001 | A | 0,0 | no | ||
| 74810_s_at | Hypothetical protein FLJ22569 | 13.05 | <0.00001 | A | 2,0 | no | ||
| 75276_at | Hypoxia-inducible factor 1, alpha subunit | 5.66 | <0.00001 | A | 1,0 | yes | 41 | C |
| 37558_at | IGF-II mRNA-binding protein 3 | 5.91 | <0.00001 | A | 1,1 | yes | 42 | |
| 88957_at | Integrin, beta-like 1 (with EGF-like repeat domains) | 7.49 | <0.00001 | A | 0,0 | no | M | |
| 89882_at | Interferon induced transmembrane protein 1 (9-27) | 6.51 | <0.00001 | A | 0,0 | yes | 43 | M |
| 35372_r_at | Interleukin-8 | 6.53 | <0.00001 | A | 0,0 | yes | 35 | C |
| 1369_s_at | Interleukin-8 | 5.14 | 0.00004 | A | 0,0 | yes | 35 | C |
| 41294_at | Keratin 7 | 10.77 | <0.00001 | A | 2,4 | yes | 18 | C |
| 33893_r_at | KIAA0470 gene product | 5.45 | <0.00001 | A | 0,0 | no | ||
| 35832_at | KIAA1077 protein | 6.99 | 0.00001 | B | 0,0 | no | ||
| 57215_at | KIAA1078 protein | 5.3 | <0.00001 | A | 1,1 | no | ||
| 36070_at | KIAA1199 protein | 5.61 | <0.00001 | A | 1,2 | no | ||
| 50402_at | KIAA1265 protein | 5.05 | <0.00001 | A | 0,0 | no | ||
| 60289_at | KIAA1323 protein | 5.18 | <0.00001 | A | 0,0 | no | ||
| 77022_at | KIAA1363 protein | 5 | <0.00001 | A | 0,0 | no | ||
| 41438_at | KIAA1451 protein | 5.83 | <0.00001 | A | 0,1 | no | ||
| 75014_i_at | KIAA1533 protein | 6.22 | 0.00002 | A | 93,123‡ | no | ||
| 78484_at | KIAA1577 protein | 5.36 | <0.00001 | A | 0,0 | no | ||
| 74535_at | Lamin B2 | 11.53 | <0.00001 | A | 0,0 | no | N | |
| 35280_at | Laminin, gamma 2 | 5.28 | <0.00001 | A | 0,0 | yes | 44 | EM |
| 91124_i_at | Leukemia-associated phosphoprotein p18 (stathmin) | 6.69 | <0.00001 | A | 5,3 | no | ||
| 32821_at | Lipocalin 2 (oncogene 24p3) | 8.86 | <0.00001 | A | 5,2 | yes | 45 | S |
| 73002_at | Matrix metalloproteinase 14 (membrane-inserted) | 7.27 | 0.00003 | B | 0,0 | yes | 11 | M |
| 668_s_at | Matrix metalloproteinase 7 (matrilysin, uterine) | 8.79 | 0.00001 | B | 0,0 | yes | 9 | S |
| 75026_s_at | Methylene tetrahydrofolate dehydrogenase | 5.97 | <0.00001 | A | 14,14‡ | no | C | |
| 35694_at | Mitogen-activated protein kinase kinase kinase kinase 4 | 5.6 | <0.00001 | A | 0,0 | no | C | |
| 38272_at | MKP-1 like protein tyrosine phosphatase | 5.63 | <0.00001 | B | 0,0 | no | ||
| 37032_at | Nicotinamide N-methyltransferase | 5.04 | 0.00056 | A | 0,2 | no | C | |
| 78518_at | Nuclear receptor subfamily 2, group F, member 1 | 7.48 | <0.00001 | A | 0,0 | no | C | |
| 75321_f_at | Nucleosome assembly protein 1-like 1 | 5.4 | <0.00001 | A | 0,3 | no | C | |
| 73229_at | Nucleosome assembly protein 1-like 1 | 6.29 | <0.00001 | A | 0,3 | no | C | |
| 91187_s_at | Nucleosome assembly protein 1-like 1 | 8.57 | <0.00001 | A | 0,3 | no | C | |
| 91189_r_at | Nucleosome assembly protein 1-like 1 | 8.48 | <0.00001 | A | 0,3 | no | C | |
| 91546_r_at | Nucleosome assembly protein 1-like 1 | 7.54 | <0.00001 | A | 0,3 | no | C | |
| 1451_s_at | Osteoblast specific factor 2 (fasciclin I-like) | 5.3 | 0.00025 | B | 0,0 | no | ||
| 78711_at | Paraneoplastic antigen MA1 | 5.49 | <0.00001 | A | 0,1 | no | N | |
| 81926_at | Peptidylarginine deiminase type I | 5.87 | <0.00001 | A | 0,0 | no | ||
| 37310_at | Plasminogen activator, urokinase | 5.71 | <0.00001 | A | 1,2 | yes | 46 | S |
| 189_s_at | Plasminogen activator, urokinase receptor | 6.18 | <0.00001 | A | 0,1 | yes | 46 | M |
| 74696_r_at | PDGF receptor, beta polypeptide | 6.14 | 0.00005 | B | 0,0 | yes | 47 | M |
| 91311_at | Pleckstrin homology-like domain, family A, member 1 | 14.66 | <0.00001 | A | 5,2 | no | ||
| 90442_at | Plectin 1, intermediate filament binding protein, 500kD | 6.69 | 0.00015 | A | 0,1 | no | C | |
| 49666_s_at | PRO1073 protein | 6.27 | <0.00001 | A | 1,5 | no | ||
| 63958_at | Prostate stem cell antigen | 5.34 | 0.00001 | B | 0,0 | yes | 6 | M |
| 40078_at | Protease, serine, 23 | 5.62 | <0.00001 | A | 0,0 | yes | 48 | |
| 40079_at | Protease, serine, 23 | 7.07 | <0.00001 | A | 0,0 | yes | 48 | |
| 80688_at | Protein kinase C-like 1 | 5.23 | <0.00001 | A | 0,1 | yes | 49 | C |
| 80463_at | Putative protein | 5.05 | <0.00001 | A | 0,2 | no | ||
| 46683_at | RAB6 interacting, kinesin-like (rabkinesin6) | 5.09 | <0.00001 | A | 0,0 | no | C | |
| 49125_at | Ras GTPase activating protein-like | 7.01 | <0.00001 | A | 4,4 | no | ||
| 49125_at | Ras GTPase activating protein-like | 7.01 | <0.00001 | A | 4,4 | no | ||
| 33730_at | Retinoic acid induced 3 | 5.53 | <0.00001 | A | 3,1 | no | M | |
| 57027_at | Retinoic acid induced 3 | 9.25 | <0.00001 | A | 3,1 | no | M | |
| 52123_at | Rho guanine nucleotide exchange factor (GEF) 1 | 5.35 | <0.00001 | A | 0,0 | no | C | |
| 89969_at | Ribosomal protein S15a | 5.55 | <0.00001 | A | 0,0 | no | C | |
| 74736_f_at | RNA binding motif, single stranded interacting protein 1 | 9.41 | <0.00001 | A | 0,0 | no | N | |
| 39421_at | Runt-related transcription factor 1 (aml1 oncogene) | 5.92 | <0.00001 | A | 0,4 | no | N | |
| 34319_at | S100 calcium-binding protein P | 8.73 | <0.00001 | A | 0,0 | no | N | |
| 74815_at | Secreted phosphoprotein 1 (osteopontin) | 7.98 | 0.01276 | B | 0,0 | no | S | |
| 38125_at | Serine (or cysteine) proteinase inhibitor, clade E, member 1 | 6.68 | <0.00001 | A | 10,10‡ | yes | 27 | |
| 39166_s_at | Heat shock protein 47 | 6.41 | <0.00001 | B | 1,4 | no | ||
| 41544_at | Serum-inducible kinase | 6.23 | <0.00001 | A | 0,0 | no | ||
| 39070_at | Singed (Drosophila)-like (sea urchin fascin homolog like) | 13.31 | <0.00001 | A | 1,1 | no | C | |
| 33143_s_at | Solute carrier family 16, member 3 | 8.23 | <0.00001 | A | 0,0 | no | M | |
| 87860_s_at | Solute carrier family 21, member 12 | 8.92 | <0.00001 | A | 0,0 | no | ||
| 32186_at | Solute carrier family 7, member 5 | 6.27 | <0.00001 | A | 0,0 | no | M | |
| 658_at | Thrombospondin 2 | 6.94 | 0.00001 | B | 0,0 | no | EM | |
| 659_g_at | Thrombospondin 2 | 9.92 | <0.00001 | B | 0,0 | no | EM | |
| 43353_at | Thrombospondin 2 | 5.37 | 0.00006 | B | 0,0 | no | EM | |
| 43353_at | Tissue inhibitor of metalloproteinase 1 | 8.14 | <0.00001 | A | 22,24‡ | yes | 28 | S |
| 74096_at | Topoisomerase (DNA) II alpha (170kD) | 5.28 | 0.00001 | A | 1,0/2,0 | no | N | |
| 69053_at | Transcription factor BMAL2 | 7.03 | <0.00001 | A | 0,0 | no | N | |
| 231_at | Transglutaminase 2 | 5.22 | <0.00001 | A | 5,11‡ | yes | 50 | C |
| 41531_at | Transmembrane 4 superfamily member 1 | 6.19 | <0.00001 | A | 7,5‡ | no | M | |
| 892_at | Transmembrane 4 superfamily member 1 | 6.22 | <0.00001 | A | 7,5‡ | no | M | |
| 46644_at | Transmembrane, prostate androgen induced RNA | 5.33 | <0.00001 | A | 1,2 | no | ||
| 91095_s_at | Transmembrane, prostate androgen induced RNA | 9.54 | <0.00001 | A | 1,2 | no | ||
| 31888_s_at | Tumor suppressing subtransferable candidate 3 | 5.99 | <0.00001 | A | 9,11‡ | no | C | |
| 291_s_at | Tumor-associated calcium signal transducer 2 | 9.29 | <0.00001 | A | 0,0 | yes | 10 | M |
| 82782_at | Zinc finger protein 267 | 5.53 | <0.00001 | A | 0,0 | no | C |
*A, elevated expression in cell lines and tumor tissues; B, elevated expression in tumor tissues only.
†Values listed are the total number of tags present in the two SAGE libraries of normal pancreatic duct epithelium (HX and H126) for each known gene identified. Genes with >5 tags present in at least one of the two libraries are indicated by an ‡, and were excluded from further analyses.
§Putative location. C, cytoplasmic; M, cell membrane; EM, extracellular matrix; N, nuclear; S, secreted.
The GeneExpress platform allows for an e-Northern analysis of Affymetrix fragments to estimate the levels of expression of any fragment among the normal and cancer samples analyzed. An e-Northern was then generated for each of the 124 Affymetrix fragments to determine levels of expression of each fragment within the normal tissues, pancreas cancer cell lines, and pancreas cancer tumor tissues studied. Two prominent patterns of expression were identified (Figure 1) ▶ . The first pattern (the A or cancer-specific pattern) demonstrated elevated expression of the fragment in both pancreas cancer cell lines and in resected pancreatic cancer tissues compared to normal tissues. Ninety-five fragments showed this pattern. The second pattern (the B or invasion-specific pattern) showed elevated expression of the fragment in the resected pancreatic cancer tissues only, but not in the cancer cell lines or normal tissues. This B pattern was observed for 29 fragments. Genes that were represented by more than one fragment showed the same e-Northern pattern for each fragment analyzed.
Figure 1.
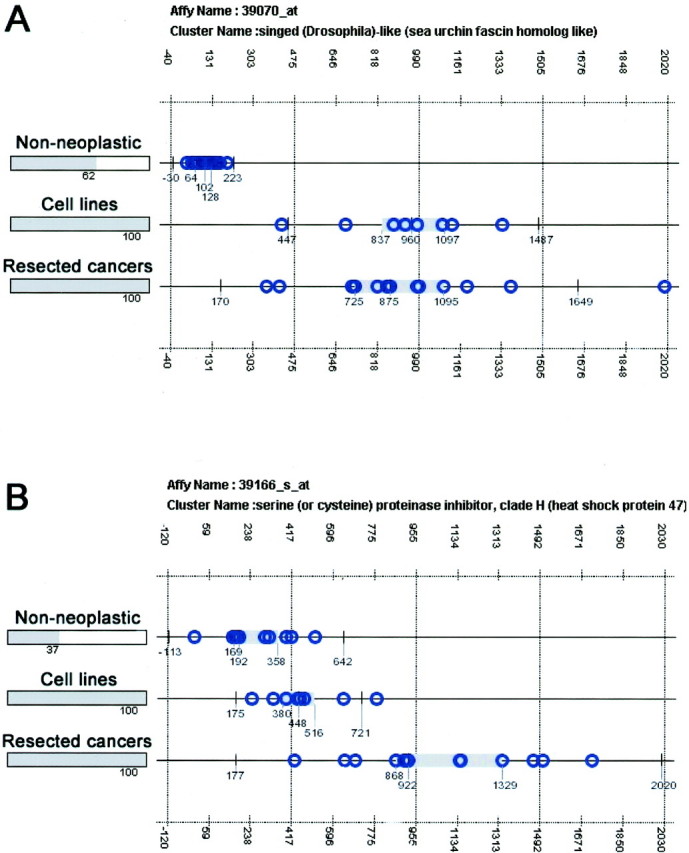
e-Northern analysis of highly expressed Affymetrix gene fragments identified by the GeneExpress platform. A: Affymetrix fragment for sea urchin fascin homolog, highly expressed in both pancreas cancer cell lines and tumor tissues compared to normal (A pattern). B: Affymetrix fragment for heat shock protein 47, specifically overexpressed in pancreas cancer tumor tissues but not pancreas cancer cell lines or normal tissues (B pattern).
The normal pancreas contains a predominance of acinar cells and islets relative to normal duct epithelium. The normal pancreatic duct epithelium is therefore underrepresented in gene expression analyses of bulk normal pancreas. Therefore, the candidate genes identified by Affymetrix GeneChip were further refined to exclude genes highly expressed in cultures of normal pancreatic ductal epithelial cells. For each gene identified as differentially expressed by Affymetrix GeneChip, the corresponding SAGE tag was identified, and the total number of SAGE tags present in the SAGEmap database (http://www.ncbi.nlm.nih.gov/SAGE/) of normal pancreas duct epithelium libraries HX and H126 was determined. Any gene having more than five tags in at least one of these two SAGE libraries was then excluded from further analysis. Using this approach, 10 genes were identified as having high levels of expression in normal pancreatic duct epithelium (DEAD/H box polypeptide 21, EphA2, FXYD domain-containing ion transport regulator 5, KIAA1577 protein, methylene tetrahydrofolate dehydrogenase, serine/cysteine proteinase inhibitor, clade E1, TIMP1, transglutaminase 2, transmembrane 4 superfamily member 1, and tumor-suppressing subtransferable candidate 3). These genes were excluded, leaving 97 remaining differentially expressed genes (Table 1) ▶ . Thus, based on the initial results of e-Northern analysis and SAGE filtering, 97 candidate genes were identified as differentially overexpressed in pancreatic cancer.
Literature Search of Genes Highly Expressed in Pancreatic Cancer
For each of the 97 genes identified, a search was performed using the online NCBI database PubMed using the known gene name together with the terms “pancreas” or “pancreas cancer.” Of the 97 genes analyzed, 28 genes were previously reported to be associated with pancreatic cancer, whereas 69 genes were not (Table 1) ▶ . Of these 69 genes not identified in this PubMed search as having been reported in pancreatic cancer, 21 have been reported before in association with tumor types other than pancreatic cancer, whereas 48 genes have not been reported in association with any neoplasm.
These 97 candidate tumor markers of pancreatic cancer represented a variety of cellular functions. Genes identified included those involved in cell membrane junctions (claudin 1, connexin 26), 12,13 signal transduction (tumor-associated calcium signal transducer 2, ras GTPase-activating protein-like), 14,15 calcium homeostasis (S100 calcium-binding protein P), 16 cytoskeletal assembly (fascin, keratin 7, rabkinesin6 and pleckstrin), 17-20 cell surface adhesion and recognition (integrin β-like 1), 21 DNA transcription (topoisomerase IIα, transcription factor BMAL2, and AML1), 22-24 DNA repair (ATDC), 25 or extracellular matrix remodeling and function (collagens 1α1, 1α2, and X1α1, heat shock protein 47, MMP14, and MMP7). 11,26,27 The cellular localization of the corresponding gene products was also determined using the online database OMIM available through the NCBI web site (http://www.ncbi.nlm.nih.gov/entrez/query). Genes were found to encode membrane-bound proteins (prostate stem cell antigen, OB-cadherin), cytoplasmic proteins (fascin, ATDC), nuclear proteins (topoisomerase IIα, paraneoplastic antigen MA1), as well as extracellular proteins, such as those involved in extracellular matrix homeostasis (hsp47, thrombospondin 2) or secreted protein products (osteopontin).
Verification of Selected Candidate Tumor Markers
Candidate genes were selected for verification of expression in samples of pancreatic cancer tissues or cell lines (Figures 2 and 3) ▶ ▶ . Four genes were selected for analysis by immunohistochemical or in situ hybridization techniques: fascin, topoisomerase IIα, hsp47, and pleckstrin.
Figure 2.
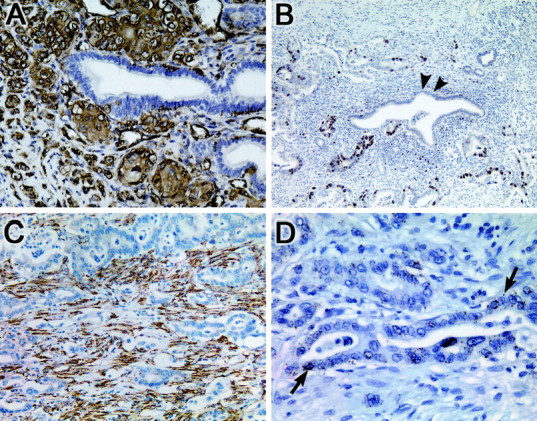
Validation of gene expression by immunohistochemical and in situ hybridization in primary pancreatic cancers. A: Fascin. Strong cytoplasmic immunolabeling is noted within the infiltrating neoplastic epithelium, in contrast to the normal pancreatic duct epithelium that is negative. B: Topoisomerase IIα. Strong nuclear immunolabeling is noted within the neoplastic epithelium, in contrast to the normal pancreatic duct epithelium (black arrows) and desmoplastic stroma that are negative. C: Heat shock protein 47. Strong immunolabeling is noted of the desmoplastic stroma of the tumor, in contrast to the neoplastic epithelium that is negative. D: Pleckstrin. mRNA expression is detected within the neoplastic epithelium by in situ hybridization (black arrows), in contrast to the surrounding desmoplastic stroma that is negative. The nonneoplastic epithelium also did not label.
Figure 3.
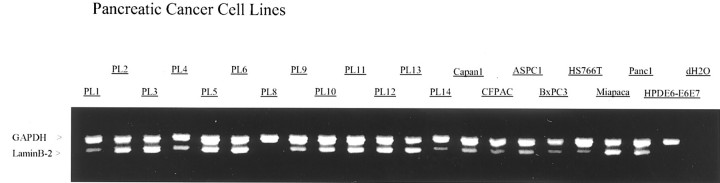
Validation of gene expression by RT-PCR in 20 pancreatic cancer cell lines, an immortal human pancreatic ductal epithelial cell line (HPDE6), and a water control. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serves as an RNA control. Lamin B2 is overexpressed in 19 of the 20 pancreatic cancer cell lines.
Fascin and topoisomerase II both showed an A pattern of expression on e-Northern, corresponding to elevated expression in both the resected neoplastic tissues and cancer cell lines. Immunohistochemical labeling of fascin showed intensely positive cytoplasmic labeling of the neoplastic epithelium in eight of eight samples of paraffin-embedded pancreatic duct adenocarcinomas studied (100%). In all cases, normal duct epithelium and desmoplastic stroma did not express fascin protein (Figure 2A) ▶ .
Topoisomerase IIα showed strong positive nuclear immunolabeling within eight of eight pancreatic duct adenocarcinomas studied (100%). Normal duct epithelium and the surrounding desmoplastic stroma did not express detectable levels of topoisomerase IIα (Figure 2B) ▶ .
In contrast to fascin and topoisomerase IIα, hsp47 showed a B pattern of expression on e-Northern, indicating elevated expression of hsp47 in the resected neoplastic tissues only, but not in the cancer cell lines or normal tissues. (Figure 2C) ▶ . In concordance with this pattern, immunolabeling for hsp47 showed strong labeling of the desmoplastic stroma within the invasive cancer in eight of eight pancreatic duct adenocarcinomas studied (100%). In one of the eight cases, the neoplastic epithelium also labeled. No expression of hsp47 was detected within normal pancreatic duct epithelium or within the intralobular stroma of normal pancreas tissue within the same paraffin-embedded tissue sections.
Pleckstrin was also identified as differentially expressed in pancreatic cancer and displayed an A pattern of expression by e-Northern. No commercially available antibody for pleckstrin was available. Therefore, a digoxigenin-labeled probe was generated to match the coding region of the pleckstrin gene for use in in situ hybridization. In situ hybridization using the anti-sense probe showed expression within the neoplastic epithelium in all eight cases (100%), seen as variably sized granules throughout the cytoplasm of the neoplastic epithelium, in contrast to normal duct epithelium or the surrounding desmoplastic stroma, which did not express this gene (Figure 3D) ▶ .
Eight additional genes were selected for validation by an RT-PCR study of 20 pancreas cancer cell lines and the immortal human pancreatic ductal epithelial cell line (HPDE6) (Figure 3) ▶ . Genes selected for validation using RT-PCR were claudin 1, S100 calcium-binding protein P (S100P), interferon-induced transmembrane protein 1 (IFITM1), lamin B2, DKFZP564G013 protein, KIAA0470 gene product, KIAA1265 protein, and KIAA1363 protein. Expression of these eight genes were detected in 19 of the 20 cell lines analyzed, in support of their initial identification as differentially expressed genes by Affymetrix GeneChip.
Discussion
The 5-year-survival rate of patients with ductal adenocarcinoma of the pancreas is 4%, one of the lowest of any neoplasm. 1 Unfortunately, most patients are diagnosed at an advanced stage of disease that is incurable with existing therapy. The identification of genes differentially expressed in pancreatic cancer is critical to the development of novel therapeutics and new markers to detect this disease at an earlier, potentially curable stage. We used the Gene Logic Inc. BioExpress platform and Affymetrix GeneChip arrays to identify 97 genes differentially expressed in pancreatic carcinoma. The differential expression of 12 selected genes was confirmed by in situ hybridization, immunohistochemical labeling, or RT-PCR. These 97 genes may form the basis for the development of screening methods, diagnostic markers, and therapeutic targets for this highly lethal cancer.
The finding of 97 genes significantly overexpressed in infiltrating pancreatic duct carcinomas has immediate diagnostic potential. Overexpression of these novel tumor markers of pancreatic cancer can be used to differentiate infiltrating pancreatic duct adenocarcinoma from chronic pancreatitis, particularly in small tissue samples or cytological material. Our initial studies to validate these markers support this possibility. Immunohistochemical and in situ labeling for these differentially expressed genes, including fascin, topoisomerase IIα, and pleckstrin, specifically label the neoplastic epithelium of infiltrating pancreatic duct adenocarcinomas, but not by normal duct epithelium included in the same tissue sections.
These 97 differentially expressed markers of pancreatic cancer also have potential for the development of new screening tests for pancreatic cancer. For example, the development of tagged antibodies to one or more of these genes may be useful in the diagnostic radiological imaging of small primary pancreatic cancers or metastases before they become clinically apparent. Several of these genes were found to be membranous or secreted proteins, suggesting they may be shed into the blood or pancreatic secretions. If so, these proteins may also serve as diagnostic markers in such specimens, not only for identification of primary pancreatic cancers at an earlier stage, but also for the identification of recurrent disease at an earlier phase when it may be more responsive to adjuvant therapies. In addition, whereas use of any one marker individually may have a limited sensitivity or specificity in detecting pancreatic cancer, the development of a panel of markers may significantly increase the specificity of detecting clinically inapparent pancreatic cancers without decreasing the sensitivity. 28
The identification of these differentially expressed genes in pancreatic cancer also has important therapeutic applications for pancreatic cancer. For example, Jaffee and colleagues 29 have recently shown that cell-mediated immunotherapy can be both safe and effective in patients with pancreatic cancer, and each of the differentially expressed genes represents a potential target for the development of a cell-mediated vaccine. Similarly, as a number of the genes identified were found to encode for cell surface proteins (ie, OB-cadherin, CD83, claudin 1, prostate stem cell antigen, and retinoic acid-induced 3), these proteins hold promise for the development of antibody-based immunotherapy against pancreatic carcinoma. 7,30 In addition, signal transduction pathways in which these differentially expressed genes may function are potential targets for molecular therapeutics.
Overexpression of several of the genes found in pancreatic duct adenocarcinomas, such as ataxia-telangiectasia group D-associated protein (ATDC), topoisomerase IIα (TOP2A), and transglutaminase II (TGM2), may offer new insights into the biology of pancreatic cancer. ATDC protein has been shown to be induced by ionizing radiation and to suppress the radiosensitivity of ataxia telangiectasia (A-T) fibroblast cell lines. 31 The overexpression of ATDC in pancreatic cancers may therefore contribute to the radioresistance often observed for this tumor type. 32 Chemotherapeutic resistance in pancreatic cancers may also, in part, be contributed to by genes such as TOP2A or TGM2. 22 TOP2A is a target for several chemotherapeutic agents, including doxorubicin, that have been used for treatment of advanced pancreatic cancer. 33 The high levels of TOP2A expression in some pancreatic cancers might indicate amplification of this gene, an occurrence that contributes to the ineffectiveness of this chemotherapeutic agent in other tumor types. 22,33 Similarly, the overexpression of TGM2 has also been associated with drug resistance. 34
Other highly expressed genes in pancreatic cancer, such as interleukin-8 (IL-8) or the AML1 oncogene, may contribute to the aggressiveness of this tumor by alternative mechanisms. IL-8 overexpression in pancreatic cancers is thought to result from low oxygen tension and hypoxia of the tumor microenvironment. Consequently, IL-8 overexpression is thought to contribute to the aggressiveness of pancreatic cancer by inducing angiogenesis and promoting tumor metastasis. 35 The AML1 oncogene is a transcription factor that is commonly overexpressed by translocation in acute myeloid leukemias. 24 The overexpression of AML1 in pancreatic cancer suggests that this gene may also play a role in the pathogenesis of this tumor type. Thus, our finding of differentially expressed genes related to the aggressiveness of pancreatic cancers may be used to develop more effective therapeutic protocols for this tumor type.
Invasive pancreatic cancers represent an aggregate of diverse cell types, such as invasive neoplastic epithelial cells, fibroblasts, inflammatory cells, smooth muscle cells, endothelial cells, and cells of residual nonneoplastic pancreatic parenchyma. 9 Thus, comparative studies of gene expression in pancreatic cancer tissues and cell cultures provide valuable information of gene expression by the different cellular compartments of the neoplasm. By e-Northern analysis, we found that 29 genes were overexpressed in pancreas cancer tumor tissues only, as compared to cancer cell lines or normal tissues. Studies suggest that most of these genes are likely to be expressed by the nonneoplastic host stromal response to the neoplasm. 11 Genes with this pattern of expression (B pattern on e-Northern), which included hsp47; apolipoprotein C-1; collagens type 1α1, 1α2, and XIα1; osteopontin, and thrombospondin 2, highlight the prominent host stromal response characteristic of infiltrating pancreatic duct adenocarcinomas. In some instances, however, the gene expression identified in association with pancreas cancer tumor tissues does not always indicate stromal gene expression, but instead may reflect the gene expression of epithelial cells only when such cells are within a tumor in vivo (as opposed to the environment of cell culture). 11 Prostate stem cell antigen exemplifies this observation. It had a B pattern of expression by e-Northern analysis (Table 1) ▶ , and a striking epithelial-specific pattern of expression by immunohistochemical labeling in a majority of resected infiltrating pancreatic cancers. 6
In summary, we have identified 97 differentially expressed genes in infiltrating pancreatic cancer, all with immediate potential utility for the development of screening tools, radiological imaging techniques, or therapies for pancreatic cancer. Approximately one-third of these 97 known genes have previously been reported in association with pancreatic cancer, and an additional 12 genes were confirmed by immunohistochemical labeling, in situ hybridization, or RT-PCR, thus validating our approach in identifying these new markers. These genes not only provide insights into the complex cellular biology of pancreatic duct adenocarcinoma, but also represent novel clinical targets for this tumor type.
Footnotes
Address reprint requests to Ralph H. Hruban, M.D., The Johns Hopkins Hospital-Surgical Pathology, The Harry and Jeanette Weinberg Building, 401 North Broadway, Room 2242, Baltimore, MD 21231-2410. E-mail: rhruban@jhmi.edu.
Supported by the National Institutes of Health Specialized Programs of Research Excellence in Gastrointestinal Cancer (grant CA62924), the Michael Rolfe Fund for Pancreatic Cancer Research, and a grant from GeneLogic Inc.
Presented at the 70th meeting of the United States and Canadian Academy of Pathology, February 23 to March 1, 2002.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M: Cancer statistics, 2001. CA Cancer J Clin 2001, 51:16-36 [DOI] [PubMed] [Google Scholar]
- 2.Tersmette AC, Petersen GM, Offerhaus GJA, Falatko FC, Goggins M, Rosenblum E, Wilentz RE, Yeo CJ, Cameron JL, Kern SE, Hruban RH: Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res 2001, 7:738-744 [PubMed] [Google Scholar]
- 3.Nacht M, Ferguson AT, Zhang W, Petroziello JM, Cook BP, Gao YH, Maguire S, Riley D, Coppola G, Landes GM, Madden SL, Sukumar S: Combining serial analysis of gene expression and array technologies to identify genes differentially expressed in breast cancer. Cancer Res 1999, 59:5464-5470 [PubMed] [Google Scholar]
- 4.Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, Cho KR, Riggins GJ, Morin PJ: Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res 2000, 60:6281-6287 [PubMed] [Google Scholar]
- 5.Argani P, Iacobuzio-Donahue C, Ryu B, Goggins M, Rosty C, Wilentz RE, Murugesan S, Kaushal M, Leach SD, Jaffee E, Yeo CJ, Cameron JL, Kern SE, Hruban RH: Mesothelin is expressed in the vast majority of adenocarcinomas of the pancreas: identification of a new cancer marker by serial analysis of gene expression (SAGE). Clinical Cancer Res 2001, 7:3862-3868 [PubMed] [Google Scholar]
- 6.Argani P, Rosty C, Reiter RE, Wilentz RE, Murugesan SD, Leach SD, Ryu B, Skinner HG, Goggins M, Jaffee EM, Yeo CJ, Cameron JL, Kern SE, Hruban RH: Discovery of new markers of cancer through serial analysis of gene expression (SAGE): prostate stem cell antigen (PSCA) is overexpressed in pancreatic adenocarcinoma. Cancer Res 2001, 61:4320-4324 [PubMed] [Google Scholar]
- 7.Jaffee EM, Schutte M, Gossett J, Morsberger LA, Adler AJ, Thomas M, Greten TF, Hruban RH, Yeo CJ, Griffin CA: Development and characterization of a cytokine-secreting pancreatic adenocarcinoma vaccine from primary tumors for use in clinical trials. Cancer J Sci Am 1998, 4:194-203 [PubMed] [Google Scholar]
- 8.Kolar C, Caffrey T, Hollingsworth M, Scheetz M, Sutherlin M, Weide L, Lawson T: Duct epithelial cells cultured from human pancreas processed for transplantation retain differentiated ductal characteristics. Pancreas 1997, 15:265-271 [DOI] [PubMed] [Google Scholar]
- 9.Ryu B, Jones J, Hollingsworth MA, Hruban RH, Kern SE: Invasion-specific genes in malignancy: serial analysis of gene expression comparisons of primary and passaged cancers. Cancer Res 2001, 61:1833-1838 [PubMed] [Google Scholar]
- 10.Ryu B, Jones J, Hollingsworth MA, Hruban RH, Kern SE: Relationships and differentially expressed genes among pancreatic cancers examined by large-scale serial analysis of gene expression. Cancer Res 2002, 62:819-826 [PubMed] [Google Scholar]
- 11.Iacobuzio-Donahue CA, Ryu B, Hruban RH, Kern SE: Exploring the host desmoplastic response to pancreatic carcinoma: gene expression of stromal and neoplastic cells at the site of primary invasion. Am J Pathol 2002, 160:91-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita K, Furuse M, Fujimoto K, Tsukita S: Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 1999, 96:511-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrio M, Romagosa A, Mercade E, Mazo A, Nadal M, Gomez-Foix AM, Fillat C: Enhanced pancreatic tumor regression by a combination of adenovirus and retrovirus-mediated delivery of the herpes simplex virus thymidine kinase gene. Gene Ther 1999, 6:547-553 [DOI] [PubMed] [Google Scholar]
- 14.Ripani E, Sacchetti A, Corda D, Alberti S: Human Trop-2 is a tumor-associated calcium signal transducer. Int J Cancer 1998, 76:671-676 [DOI] [PubMed] [Google Scholar]
- 15.Namima M, Takeuchi K, Watanabe Y, Yamano M, Saito M, Sasa H, Okamoto K: Localization of GTPase-activating protein-(GAP) like immunoreactivity in mouse cerebral regions. Mol Chem Neuropathol 1998, 35:157-172 [DOI] [PubMed] [Google Scholar]
- 16.Donato R: S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 2001, 33:637-668 [DOI] [PubMed] [Google Scholar]
- 17.Tseng Y, Fedorov E, McCaffery JM, Almo SC, Wirtz D: Micromechanics and ultrastructure of actin filament networks crosslinked by human fascin: a comparison with alpha-actinin. J Mol Biol 2001, 310:351-366 [DOI] [PubMed] [Google Scholar]
- 18.Goldstein NS, Bassi D: Cytokeratins 7, 17, and 20 reactivity in pancreatic and ampulla of vater adenocarcinomas. Percentage of positivity and distribution is affected by the cut-point threshold. Am J Clin Pathol 2001, 115:695-702 [DOI] [PubMed] [Google Scholar]
- 19.Lai F, Fernald AA, Zhao N, Le Beau MM: cDNA cloning, expression pattern, genomic structure and chromosomal location of RAB6KIFL, a human kinesin-like gene. Gene 2000, 248:117-125 [DOI] [PubMed] [Google Scholar]
- 20.Sato TK, Overduin M, Emr SD: Location, location, location: membrane targeting directed by PX domains. Science 2001, 294:1881-1885 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz MA: Integrin signaling revisited. Trends Cell Biol 2001, 11:466-470 [DOI] [PubMed] [Google Scholar]
- 22.Tanner M, Jarvinen P, Isola J: Amplification of HER-2/neu and topoisomerase IIalpha in primary and metastatic breast cancer. Cancer Res 2001, 61:5345-5348 [PubMed] [Google Scholar]
- 23.Ikeda M, Yu W, Hirai M, Ebisawa T, Honma S, Yoshimura K, Honma KI, Nomura M: cDNA cloning of a novel bHLH-PAS transcription factor superfamily gene, BMAL2: its mRNA expression, subcellular distribution, and chromosomal localization. Biochem Biophys Res Commun 2000, 275:493-502 [DOI] [PubMed] [Google Scholar]
- 24.Downing JR: AML1/CBFbeta transcription complex: its role in normal hematopoiesis and leukemia. Leukemia 2001, 15:664-665 [DOI] [PubMed] [Google Scholar]
- 25.Hosoi Y, Kapp LN: Expression of a candidate ataxia-telangiectasia group D gene in cultured fibroblast cell lines and human tissues. Int J Radiat Biol 1994, 66:S71-S76 [PubMed] [Google Scholar]
- 26.Gress TM, Muller-Pillasch F, Lerch MM, Friess H, Buchler M, Beger HG, Adler G: Balance of expression of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in chronic pancreatitis. Gastroenterology 1994, 32:221-225 [PubMed] [Google Scholar]
- 27.Dafforn TR, Della M, Miller AD: The molecular interactions of heat shock protein 47 (Hsp47) and their implications for collagen biosynthesis. J Biol Chem 2001, 276:49310-49319 [DOI] [PubMed] [Google Scholar]
- 28.Zhou W, Sokoll LJ, Bruzek DJ, Zhang L, Velculescu VE, Goldin SB, Hruban RH, Kern SE, Hamilton SR, Chan DW, Vogelstein B, Kinzler KW: Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol Biomarkers Prev 1998, 7:109-112 [PubMed] [Google Scholar]
- 29.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, Lillemoe KD, O’Reilly S, Abrams RA, Pardoll DM, Cameron JL, Yeo CJ: Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001, 19:145-156 [DOI] [PubMed] [Google Scholar]
- 30.McDevitt MR, Ma D, Lai LT, Simon J, Borchardt P, Frank RK, Wu K, Pellegrini V, Curcio MJ, Miederer M, Bander NH, Scheinberg DA: Tumor therapy with targeted atomic nanogenerators. Science 2001, 294:1537-1540 [DOI] [PubMed] [Google Scholar]
- 31.Laderoute KR, Knapp AM, Green CJ, Sutherland RM, Kapp LN: Expression of the ATDC (ataxia telangiectasia group D-complementing) gene in A431 human squamous carcinoma cells. Int J Cancer 1996, 66:772-778 [DOI] [PubMed] [Google Scholar]
- 32.Abrams RA, Grochow LB, Chakravarthy A, Sohn TA, Zahurak ML, Haulk TL, Ord S, Hruban RH, Lillemoe KD, Pitt HA, Cameron JL, Yeo CJ: Intensified adjuvant therapy for pancreatic and periampullary adenocarcinoma: survival results and observations regarding patterns of failure, radiotherapy dose and CA19–9 levels. Int J Radiat Oncol Biol Phys 1999, 44:1039-1046 [DOI] [PubMed] [Google Scholar]
- 33.Jarvinen TA, Tanner M, Rantanen V, Barlund M, Borg A, Grenman S, Isola J: Amplification and deletion of topoisomerase IIalpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am J Pathol 2000, 156:839-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han JA, Park SC: Reduction of transglutaminase 2 expression is associated with an induction of drug sensitivity in the PC-14 human lung cancer cell line. J Cancer Res Clin Oncol 1999, 125:89-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K: Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res 1999, 5:3711-3721 [PubMed] [Google Scholar]
- 36.Weber CK, Sommer G, Michl P, Fensterer H, Weimer M, Gansauge F, Leder G, Adler G, Gress TM: Biglycan is overexpressed in pancreatic cancer and induces G1-arrest in pancreatic cancer cell lines. Gastroenterology 2001, 121:657-667 [DOI] [PubMed] [Google Scholar]
- 37.Rall CJN, Rustgi AK: CD44 isoform expression in primary and metastatic pancreatic adenocarcinoma. Cancer Res 1995, 55:1831-1835 [PubMed] [Google Scholar]
- 38.Wilentz RE, Geradts J, Maynard R, Offerhaus GJA, Kang M, Goggins M, Yeo CJ, Kern SE, Hruban RH: Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res 1998, 58:4740-4744 [PubMed] [Google Scholar]
- 39.Carrio M, Mazo A, Lopez-Iglesias C, Estivill X, Fillat C: Retrovirus-mediated transfer of the herpes simplex virus thymidine kinase and connexin26 genes in pancreatic cells results in variable efficiency on the bystander killing: implications for gene therapy. Int J Cancer 2001, 94:81-88 [DOI] [PubMed] [Google Scholar]
- 40.Caillol N, Pasqualini E, Lloubes R, Lombardo D: Impairment of bile salt-dependent lipase secretion in human pancreatic tumoral SOJ-6 cells. J Cell Biochem 2000, 79:628-647 [DOI] [PubMed] [Google Scholar]
- 41.Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, Niizeki H, Kawamura K, Hosokawa M, Asaka M: Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res 2001, 61:6548-6554 [PubMed] [Google Scholar]
- 42.Mueller-Pillasch F, Lacher U, Wallrapp C, Micha A, Zimmerhackl F, Hameister H, Varga G, Friess H, Buchler M, Beger HG, Vila MR, Adler G, Gress TM: Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene 1997, 14:2729-2733 [DOI] [PubMed] [Google Scholar]
- 43.Wen Y, Yan DH, Wang B, Spohn B, Ding Y, Shao R, Zou Y, Xie K, Hung MC: p202, an interferon-inducible protein, mediates multiple antitumor activities in human pancreatic cancer xenograft models. Cancer Res 2001, 61:7142-7147 [PubMed] [Google Scholar]
- 44.Fukushima N, Sakamoto M, Hirohashi S: Expression of laminin-5-gamma-2 chain in intraductal papillary-mucinous and invasive ductal tumors of the pancreas. Mod Pathol 2001, 14:404-409 [DOI] [PubMed] [Google Scholar]
- 45.Friedl A, Stoesz SP, Buckley P, Gould MN: Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J 1999, 31:433-441 [DOI] [PubMed] [Google Scholar]
- 46.Ellenrieder V, Hendler SF, Ruhland C, Boeck W, Adler G, Gress TM: TGF-beta-induced invasiveness of pancreatic cancer cells is mediated by matrix metalloproteinase-2 and the urokinase plasminogen activator system. Int J Cancer 2001, 93:204-211 [DOI] [PubMed] [Google Scholar]
- 47.Ebert M, Yokoyama M, Friess H, Kobrin MS, Buchler MW, Korc M: Induction of platelet-derived growth factor A and B chains and over-expression of their receptors in human pancreatic cancer. Int J Cancer 1995, 62:529-535 [DOI] [PubMed] [Google Scholar]
- 48.Ohta T, Tajima H, Fushida S, Kitagawa H, Kayahara M, Nagakawa T, Miwa K, Yamamoto M, Numata M, Nakanuma Y, Kitamura Y, Terada T: Cationic trypsinogen produced by human pancreatic ductal cancer has the characteristics of spontaneous activation and gelatinolytic activity in the presence of proton. Int J Mol Med 1998, 1:689-692 [DOI] [PubMed] [Google Scholar]
- 49.Trauzold A, Wermann H, Arlt A, Schutze S, Schafer H, Oestern S, Roder C, Ungefroren H, Lampe E, Heinrich M, Walczak H, Kalthoff H: CD95 and TRAIL receptor-mediated activation of protein kinase C and NF-kappaB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene 2001, 20:4258-4269 [DOI] [PubMed] [Google Scholar]
- 50.Elsasser HP, MacDonald R, Dienst M, Kern HF: Characterization of a transglutaminase expressed in human pancreatic adenocarcinoma cells. Eur J Cell Biol 1993, 61:321-328 [PubMed] [Google Scholar]


