Abstract
Glucuronidation, mediated by UDP-glucuronosyltransferases (UGTs), affects the actions and disposition of diverse endo- and xenobiotics. In the case of catecholestrogens (CEs), glucuronidation is likely to block their oxidation to quinone estrogens that are the putative mediators of CEs’ actions as initiators of cancers. The goal of this study was to determine whether UGT2B7, the isoenzyme with a high affinity for 4-hydroxyestrone, is expressed in human breast parenchyma. Glucuronidation of 4-hydroxyestrone has relevance to breast carcinogenesis because quinone metabolites of 4-hydroxylated CEs can form potentially mutagenic depurinating DNA adducts, and because in breast tissue estrone is likely to be the predominant estrogen available for 4-hydroxylation. Using reverse transcriptase-polymerase chain reaction, immunocytochemistry, immunoblot analyses, and assays of glucuronidation of 4-hydroxyestrone, we show that UGT2B7 is expressed in human mammary epithelium, and that its expression is dramatically reduced in invasive breast cancers. In many in situ carcinomas, however, 4-hydroxyestrone immunostaining was not only preserved but even more intense than in normal mammary epithelium. The finding of reduced UGT2B7 protein and glucuronidation of 4-hydroxyestrone in invasive cancers suggests a tumor-suppressor function for the enzyme. Recent identification of all-trans retinoic acid as a substrate of UGT2B7 suggests that this function includes the generation of retinoyl-β-glucuronide, a potent mediator of actions of retinoids important for maintaining epithelia in a differentiated state. Current knowledge does not provide any ready explanation for the apparent increase in UGT2B7 expression in carcinomas in situ. However, this finding, together with reduced immunostaining at loci showing breach of the basement membrane (microinvasion), suggests involvement of UGT2B7-catalyzed reaction(s) in protection against invasion of surrounding tissue by cancer cells.
Glucuronidation of small lipophilic molecules, catalyzed by UDP-glucuronosyltransferase isoenzymes (UGTs), provides a mechanism for the regulation of the bioactivity and metabolic fate of a wide range of endo- and xenobiotics. 1 Glucuronidation, by increasing polarity, facilitates the partitioning of lipophilic molecules into the aqueous compartment and their ultimate excretion via the bile and kidney. In general, glucuronidation serves an inactivating or protective function by terminating or attenuating the biological activity of its substrates. There are, however, some well-characterized examples of glucuronidation resulting in the preservation or increase in the biological activity of its substrate, notably, that of morphine 2 and retinoic acid (RA). 3
This study was initiated because of our interest in identifying phase II enzymes expressed in human breast parenchyma that have the potential to block oxidation of catecholestrogens (CEs) and, thereby, prevent the generation of quinone estrogens (QEs) that are putative proximal carcinogens. 4,5 Oxidation of ring A of CEs generates two classes of QEs, 2,3,quinone-estrogens (2,3,QEs) and 3,4,quinone-estrogens (3,4,QEs), the metabolites of 2- and 4-hydroxylated CEs (2- and 4-OH-CEs), respectively. Although both classes of QEs are electrophiles that can form DNA adducts, 3,4,QEs are particularly suspect as mutagens, because they can form depurinating adducts. 5 This is in contrast to adducts formed by 2,3,QEs that are stable and, as such, may impede DNA replication. Oxidation of CEs can also initiate a process of redox cycling that generates potentially cytotoxic and genotoxic reactive oxygen species. 6 Therefore, the CE/QE pathway of estrogen metabolism has been proposed to provide a mechanism by which primary estrogens may contribute to the initiating stages of carcinogenesis. 5 A corollary of this postulate is that phase II-conjugating enzymes that can prevent oxidation of ring A of CEs serve a protective, tumor-suppressor function.
O-Methylation of CEs by the phase II enzyme catechol-O-methyltransferase is considered to play a major role in the inactivation of CEs. 7,8 A need for additional mechanism(s) for the inactivation of 4-OH-CEs, in particular in tissues in which both 2- and 4-OH-CEs are generated, is indicated by the observation that 2-OH-CEs inhibit the O-methylation of 4-OH-CEs. 9 We reasoned that in such tissues glucuronidation could provide the additional protection needed. One tissue in need for such protection is the human breast parenchyma. It is a tissue that has been shown to have the potential to generate both 2- and 4-OH-CEs and to express enzymes that can catalyze aromatic hydroxylation of primary estrogens at both C-2 and C-4. 10-12 In this study, we examined normal and neoplastic human breast parenchyma for the expression of UGT2B7, the UGT isoenzyme with a particularly high affinity for 4-OH-E1. 13,14 This property of the enzyme could be especially important for the human mammary epithelium because in human breast parenchyma 4-OH-E1 is likely to be the predominant 4-OH-CE generated. This supposition is based on evidence that in breast parenchyma the concentrations of estrone, free and conjugated, predominate over that of estradiol. 15,16 Hence, it is a tissue in which estrone can be assumed to be the principal primary estrogen available for aromatic hydroxylation. While this study was in progress, UGT2B7 was shown to also catalyze with high efficiency the glucuronidation of all-trans retinoic acid (atRA). 17 This property of the enzyme may also offer protection against carcinogenesis, because glucuronidation preserves rather than abrogates actions of RA known to be important for maintaining epithelia in a differentiated state. 3,18,19 The finding reported here that UGT2B7 is expressed in the normal mammary epithelium, but that its expression is decreased or abrogated in invasive cancers, supports the notion that UGT2B7 serves a protective, tumor-suppressor function in human breast parenchyma.
Materials and Methods
Breast Tissue
Breast tissue was obtained as described previously from tissue collected in the laboratory of one of the investigators (JW). Breast parenchyma for this tissue bank is obtained from surgical specimens. It is freed from excess fat cells by blunt dissection and by trimming with surgical scissors. Portions of the tissues intended for use in molecular biological and biochemical studies are snap-frozen at −80°C and stored at −80°C. Other portions of the tissues, intended for use in cytochemical studies, are fixed in 10% neutral-buffered formalin from 12 to 24 hours and then embedded in paraffin. Particular care is taken to minimize contamination with fat tissue those specimens that are snap-frozen for future use in molecular biological or biochemical studies. Collection and preparation of all tissues is performed by or under the supervision of one of the two participating investigators (JW or DAS). In this study, tissue was used from a total of 28 patients undergoing reduction mammoplasty for macromastia (normals), and neoplastic and nonneoplastic tissue from 37 patients undergoing mastectomy for breast cancer. Nonneoplastic tissue, shown histologically to be free of cancer, was obtained from the mastectomy specimens from sites distant from the primary cancers (normal from cancer). Age of patients with macromastia ranged from 16 to 46 years and that of patients with breast cancer from 28 to 81 years. All of the donors with macromastia were premenopausal. Five of these donors were using oral contraceptives and one of the postmenopausal breast cancer patients was receiving Premarin (Wyeth-Ayerst Laboratories, Philadelphia, PA) for hormone replacement. Authorization for the use of these tissues for research was obtained from the Institutional Review Board for Use of Human Tissues.
UGT2B7 Antibody Development and Specificity
A peptide, representing amino acids 519 to 529 of UGT2B7, was synthesized (Genemed, San Francisco, CA), coupled to keyhole limpet hemocyanin (KLH) and used to immunize chickens (Cocalico Biologicals, Inc., Reamstown, PA). Crude IgY was affinity purified, using the above peptide conjugated to Sulfo-Link columns (Pierce, Rockford, IL). The first fraction (2 ml) of the column effluent, ie, the fraction not adsorbed to the column, was used as a control for the specificity of the immunoreaction in the immunocytochemical studies. The specificity of the antibody was tested by immunoblot analysis of UGT2B7 and three other proteins from the UGT2B family expressed in HEK293 cells. Ten and 25 μg of total protein from each transfected cell line and untransfected HEK293 cells were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunoblot analysis was performed using affinity-purified UGT2B7 antibody (1:500 dilution) and rabbit anti-chicken horseradish-peroxidase secondary antibody (1:50,000), followed by chemiluminescent detection (Supersignal, Pierce). A commercially available polyclonal antibody developed in rabbits against UGT2B7 (Gentest, Woburn, MA) was used to confirm immunochemical findings obtained using the IgY chicken antibody.
Immunocytochemistry
Formalin-fixed, paraffin-embedded human breast tissue from reduction mammoplasty (n = 20) or mastectomy (neoplastic, n = 17; nonneoplastic, n = 15) was sectioned at 4 μm and transferred onto ProbOn+ slides (Fisher Biotech, Atlanta, GA). Sections from more than one tissue block were examined from six of the mastectomy specimens (two blocks from three donors, three blocks from one donor, and four blocks from two donors). A low-temperature antigen retrieval procedure 20 was applied to deparaffinized and rehydrated tissue sections using 0.1 mol/L citrate buffer, pH 6.0, for 1 hour at 80°C and immunocytochemistry was performed as described previously. 21 The sections were incubated with affinity-purified UGT2B7 antibody overnight at 4°C. The signal from the biotinylated goat anti-chicken secondary antibody was amplified and visualized using Vectastain ABC with alkaline phosphatase as the reporter, and Vector Red Alkaline Phosphatase Substrate Kit I reagent (Vector Laboratories, Burlingame, CA) as the chromogen. Endogenous alkaline phosphatase was inhibited using 0.2 N HCl. Sections incubated with only the secondary antibody served as routine controls. Additional controls were performed using the first fraction of the effluent from the affinity columns in place of the affinity-purified primary antibody and at the same protein concentration. This fraction represents the portion of the IgY that was not adsorbed by the peptide used as antigen that was conjugated to the column used for affinity purification. It is equivalent to IgY preabsorbed with the peptide. Representative sections from several mammoplasty specimens, and from histologically normal and neoplastic tissue from mastectomy specimens, were included in each analysis. The sections were viewed using a Nikon Eclipse E600 microscope and recorded using either a Spot digital camera (Diagnostic Instruments, Inc.) and Image Pro Plus version 3.0 software or a Nikon Digital Camera DXM 1200 and Nikon, ACT1 version 2 software. The sections were reviewed systematically by three of the authors (JW, SAG, and DAS) and recorded by digital photomicrography by two of the authors (JW and SG). Sections from the cancer patients were reviewed with the collaborating pathologist (EF).
Immunoblot Analysis
Flash-frozen tissues from reduction mammoplasty specimens (n = 6) and mastectomy specimens (neoplastic, n = 8; nonneoplastic, n = 6) were homogenized with a Polytron (Brinkman) in buffer containing 50 mmol/L Tris, pH 7.4, 5 mmol/L MgCl2, 25 mmol/L KCl, ethylenediaminetetraacetic acid, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPs), and protease inhibitors. Total protein (50 μg) from each was separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunoblot analysis was performed using affinity-purified UGT2B7 IgY antibody (1:750) as the primary antibody, biotinylated anti-chicken antibody as the secondary antibody, followed by horseradish peroxidase Vectastain ABC detection system (Vector Laboratories). When using the commercially available rabbit IgG antibody, the site(s) of hybridization was identified using goat anti-rabbit horseradish-peroxidase secondary antibody (1:500) (Gentest), followed by chemiluminescent detection (Supersignal, Pierce).
UDP-Glucuronosyltransferase Activity
Enzyme activity was measured in dispersed cryosections. Twenty 20-μm-thick cryosections of human breast tissue from reduction mammoplasty (n = 9) or mastectomy (cancer, n = 8; nonneoplastic, n = 10) were suspended in phosphate-buffered saline (pH 7.4) containing 0.5 mmol/L dithiothreitol. The sections were disrupted first by vortexing, followed by brief homogenization using Tissue Tearer homogenizer. Protein concentrations were determined using the BioRad Protein Assay (BioRad Laboratories, Hercules, CA). Radioassays of UDP-glucuronosyltransferase activity were performed as described by Matern and colleagues, 22 using 100 μmol/L of 4-OH-E1 and 500 μmol/L of UDP-glucuronic acid. Control incubations did not contain steroid substrate. Reaction mixtures were incubated at 37°C for 30 minutes. Under these conditions, the limit of 4-OH-E1 glucuronide formation was ∼0.2 pmol/mg protein/minute.
Estimation of Cell Density in Tissues Used in Enzyme Assays: Measurement of Percentage of Tissue Section Occupied by Cell Nuclei Using Computerized Image Analysis
Nonneoplastic breast parenchyma of humans is comprised mainly of collagenous matrix. Mammary epithelial cells, although the predominant cell type in normal breast parenchyma and in which UGT2B7 was localized, account for only a small and variable portion of the tissue. Neoplastic tissue, on the other hand, is characterized by a much higher cell density, principally because of the presence of neoplastic epithelial cells. Using total protein as the denominator when calculating enzyme activity fails to take into account these differences in cell density. To obtain an estimate of enzyme activity of epithelial cells, we measured the area occupied by nuclei in a representative tissue section from each tissue specimen used in the enzyme assays. These values were then used to estimate cell density, as described below.
Representative 20-μm-thick cryostat sections, obtained from each of the frozen blocks of tissue used in the UGT-glucuronidation enzyme assay, were stained with Mayer’s hematoxylin (a nuclear stain). These sections were then viewed on a Nikon Eclipse E600 microscope using ×1 or ×2 power objectives. The images were captured using a Spot digital camera (Diagnostic Instruments, Inc.) and the measurements were obtained using Image Pro Plus version 3.0 software. Spatial calibration was performed using a micrometer slide captured at ×1 and ×2 magnification. Briefly, the hematoxylin-stained nuclei were selected by their blue color and the area they occupied was determined. The total area of each section was outlined by tracing along the outside border of the section using a freehand measuring tool. The percentage of the sections occupied by nuclei was obtained using the following formula:
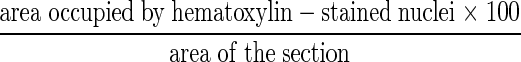 |
These values were then applied as a correction factor to the values obtained in the enzyme assays. This approach provides a practical and relatively simple means to deal with the problem of variations in epithelial cell density that characterizes normal human breast tissue, and the large differences in cell density between normal and neoplastic breast tissue. The method clearly does not take into account the larger size of nuclei in cancer cells. Nor does it take into account cellular heterogeneity in normal and neoplastic tissue, eg, the presence of stromal cells in normal tissue and stromal and inflammatory cells in cancer tissue. To deal with these variables will require more complex and discriminating approaches. However, in both normal and neoplastic tissue, epithelial cells clearly constitute the majority.
Statistical Analysis
Statistical significance of differences in glucuronidation of 2-OH-E1 by the three types of tissues used in the enzyme assays (normal, normal from cancer, and cancer) were determined by one-way analysis of variance followed by Neuman-Keuls t-test, as well as simple polynomal linear regression analysis. Statistical significance of differences between area occupied by cell nuclei in normal and neoplastic breast tissue was determined by Student’s t-test.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was obtained from flash-frozen normal and neoplastic human breast tissue using Trizol Reagent (Life Technologies, Inc., Rockville, MD) according to the manufacturer’s instructions. Briefly, tissue was kept on dry-ice until addition of Trizol reagent (1 ml/100 mg tissue) and then immediately homogenized (Polytron, Brinkman). The tissue homogenates were centrifuged at 12,000 × g for 10 minutes at 4°C and any fat that collected at the surface was removed. The Trizol layer was transferred to a new tube, mixed with chloroform, and the phases separated by centrifugation. The RNA in the aqueous phase was precipitated using isopropyl alcohol and resuspended in diethyl pyrocarbonate-treated water. RNA was treated with DNase using DNA-free (Ambion, Austin, TX).
Total RNA (2 μg) from normal or neoplastic breast was subjected to reverse transcription at 42°C for 50 minutes using Superscript II reverse transcriptase (Life Technologies, Inc.) and oligo dT primer. Human liver mRNA was used as a positive control. PCR was performed using one-sixth of the reverse transcription product, 1.25 U Platinum pfx DNA polymerase (Life Technologies, Inc.) and primers corresponding to nucleotides 275 to 383 of human UGT2B7 (accession no. J05428, upstream primer 5′-AGAATTTCATCATGCAACAG-3′ and the reverse complimentary downstream primer 5′-GTTATGTCACCAAATATTG-3′). Primers for β-actin, which span an intron, were also included as an internal control for quantity and quality of RNA and for DNA contamination. The cycling conditions used were: 94°C for 3 minutes; 35 cycles of 30 seconds at 94°C for denaturing; 45 seconds at 51°C for annealing; 1 minute at 72°C for elongation followed by 10 minutes at 72°C for final extension, and a 4°C hold. The PCR products were electrophoresed on a 2% agarose gel. PCR products were isolated from the gel using QIAquick gel extraction kit (Qiagen, Valencia, CA) and sequenced.
Results
Antibody Specificity
By immunoblot analysis, the affinity-purified antibody was shown to cross-react with UGT2B7-expressed protein but not with expressed protein for any of the three other members of the UGT2B family tested, or with protein from untransfected HEK293 cells (Figure 1) ▶ . There was also no cross-reactivity with expressed protein of four members of the UGT1A family of isoenzymes, 1A1, 1A4, 1A6, and 1A8 (not shown). There was no immunostaining of breast tissue processed for immunocytochemistry using the first fraction of the effluent from the affinity columns in place of the affinity-purified primary antibody and at the same protein concentration (see Materials and Methods).
Figure 1.
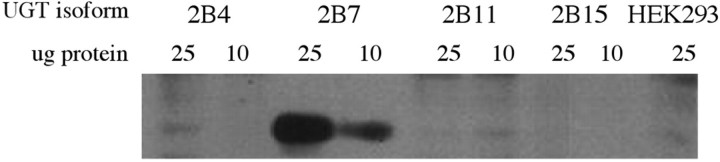
Specificity of UGT2B7 antibody. Immunoblot of UGT2B7 antibody tested against four members of the UGT2B family of isoenzymes, UGT2B4, UGT2B7, UGT2B11, and UGT2B15, expressed in HEK293 cells. Ten and 25 μg of the four expressed proteins, and protein from untransfected HEK293 cells, were electrophoresed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, as described in Materials and Methods. The antibody can be seen to recognize UGT2B7 protein, but not the other three UGT proteins or protein from untransfected HEK293 cells.
Immunocytochemical Localization of UGT2B7 in Normal Breast Parenchyma and in Invasive and in Situ Breast Cancers
In histologically normal tissues, there was consistent immunostaining in the epithelium lining the mammary ductal system (Figure 2) ▶ . This was seen in both tissue obtained from patients with macromastia and in tissue obtained from breast cancer patients at sites distant from the cancers. Within the mammary epithelium, immunostaining was maximal, and in some cases clearly restricted, to luminal epithelial cells (Figure 2) ▶ . The intensity of immunostaining of the mammary epithelium in tissue obtained from different individuals varied somewhat (Figure 2) ▶ . In some sections there were also noticeable differences in the intensity of immunostaining of epithelial cells within the same terminal ductal lobular units (Figure 2) ▶ . These findings suggest interindividual differences in the level of expression of UGT2B7 in the mammary epithelium, as well as differences in its level of expression among epithelial cells in any one tissue. The differences seemed to be unrelated to the degree of differentiation of the ductal system (type II or type III lobule), or the age or reproductive history of the tissue donor. Vacuolation of mammary epithelial cells was seen in sections from some of the patients. This phenomenon is reported to be a characteristic of the luteal phase of the menstrual cycle. 23 The intensity of immunostaining in such vacuolated epithelial cells was comparable to that in nonvacuolated epithelial cells in tissue sections from other patients. There were no obvious, consistent differences between either the pattern or the intensity of immunostaining in histologically normal tissue obtained from donors with breast cancer and those with macromastia.
Figure 2.
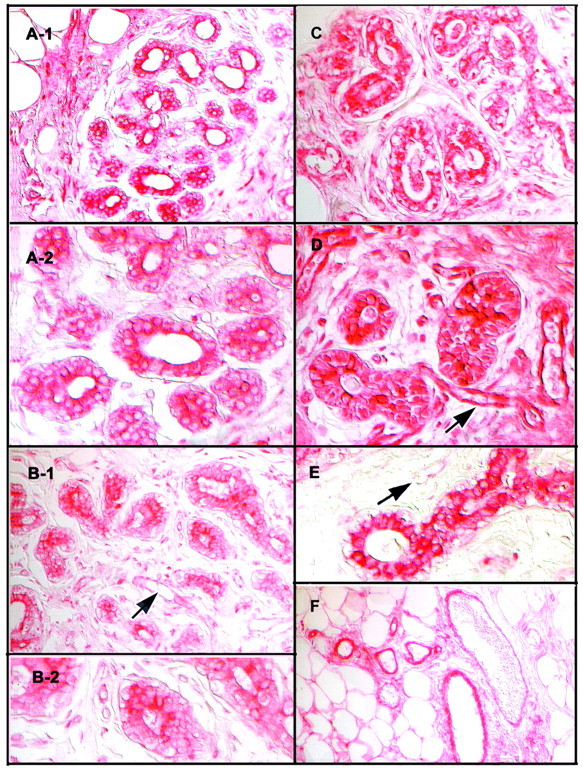
Localization of UGT2B7 by immunocytochemistry in nonneoplastic human breast parenchyma. Immunocytochemistry was performed on tissue obtained from patients undergoing reduction mammoplasty for simple macromastia as described in Materials and Methods. A-1 and A-2: Terminal ductal elements showing immunostaining of moderate intensity of mammary epithelium [shown at low (A-1) and high (A-2) magnification]. Note localization of immunostaining in luminal epithelial cells and some variation in intensity of immunostaining in these cells. B-1 and B-2: Terminal ductal elements showing overall immunostaining of relatively low intensity in a representative section from a second patient [shown at low (B-1) and high (B-2) magnification]. Arrow points to blood vessel showing immunostaining of what appear to be endothelial cells that is also of relatively low intensity. C: Immunostaining of moderate intensity in a tissue section from a third donor showing vacuolated appearance of the mammary epithelium seen in the luteal phase of the menstrual cycle. 23 D and E: Sections from two additional patients showing more intense immunostaining in the mammary epithelium at low (D) and high (E) magnification, respectively. Intensity of immunostaining of blood vessels in D (arrow), is comparable to that in the mammary epithelium. In contrast, the intensity of immunostaining of blood vessels in E (arrow) is much less than in epithelial cells in ductal elements adjacent to it. In intensely immunoreactive sections, such as shown in D as well as in sections with vacuolated basal epithelial cells, such as shown in C, there appeared to be some immunostaining associated with cell nuclei. This was likely to be because of cytoplasm of overlying cells, because there was no immunostaining associated with cell nuclei when immunocytochemistry was performed on thinner (0.2 μm) sections (not shown). F: A group of blood vessels among fat cells showing immunostaining. Sections were not counterstained and images were captured using Nomarski optics, as described in Materials and Methods.
In more intensely immunopositive sections, some immunostaining seemed to be associated with nuclei (Figure 2) ▶ . This association was no longer seen when immunocytochemistry was performed on thinner (2 μm) sections (not shown). Hence, the appearance of nuclear immunostaining in 4-μm sections was likely to be because of cells overlapping. This suggestion needs to be confirmed using confocal microscopy.
Blood vessels also showed immunoreactivity. The immunostaining seemed to be associated with endothelial cells, as well as with the smooth muscle layer surrounding larger blood vessels (Figure 2, D and F) ▶ . Additional studies will be needed to identify the exact cell type(s) and tissue component(s) within the smooth muscle layer showing UGT2B7 immunoreactivity. The intensity of immunoreactivity associated with blood vessels, such as that associated with the mammary epithelium, varied among patients. There were clear instances of dissociation in intensity of immunostaining in the two tissue compartments (Figure 2, D and E) ▶ . This finding suggests regulation of UGT2B7 expression in blood vessels independent from that in the mammary epithelium.
The intensity of immunostaining of cancer cells within invasive lesions was consistently much less than that of normal mammary epithelial cells (Figure 3) ▶ . Immunostaining in these cells ranged from faint to virtually absent. In contrast, immunostaining of cancer cells within in situ lesions was not only maintained but often of an intensity rarely seen in histologically normal tissues (Figure 4) ▶ . In situ cancers were seen in sections from tissue obtained from the primary neoplastic site from 11 of the 17 cancer patients. In all but one of these, cancer cells with intense immunostaining predominated. There was obvious heterogeneity. Some cancer cells with reduced immunoreactivity could be seen within, as well as at the edge of some of the intensely immunoreactive in situ lesions. Interestingly, in two cases the site of reduced immunoreactivity at the edge of an in situ lesion corresponded to the site of apparent microinvasion, ie, a breach in the basement membrane (Figure 4, F and G) ▶ . In situ lesions comprised predominantly or solely of cancer cells with reduced or minimal immunostaining were seen in 4 of the 11 cases. A solitary in situ lesion with intense immunostaining was also seen in a section from tissue obtained from a site distant from the cancer.
Figure 3.
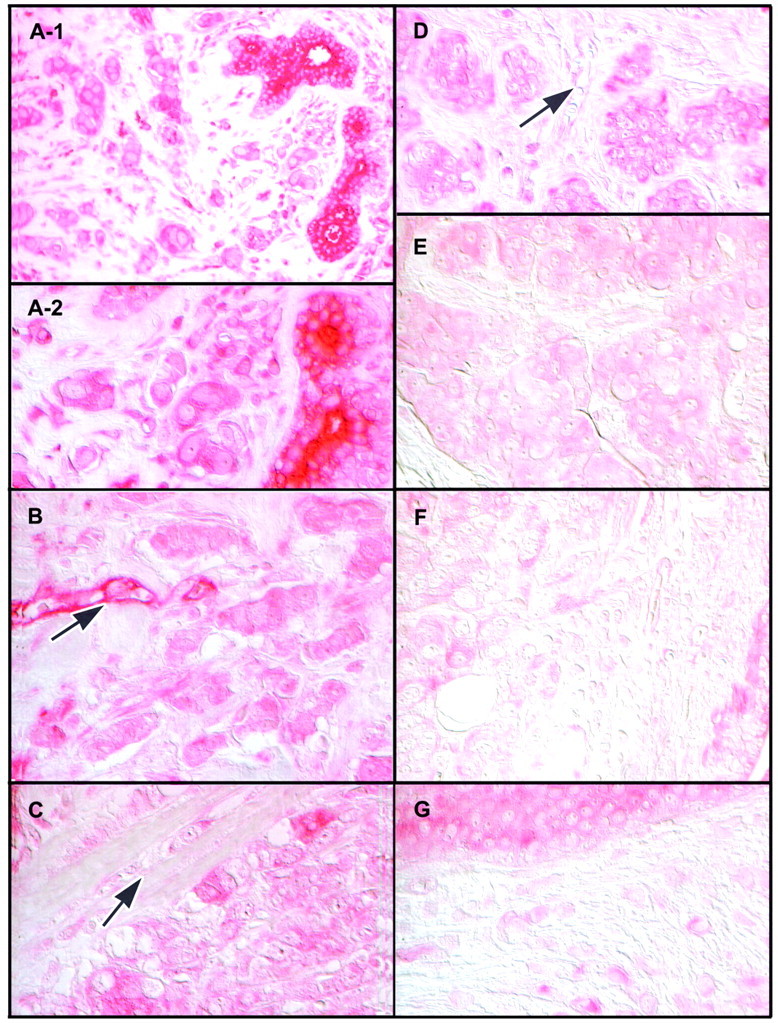
Localization of UGT2B7 by immunocytochemistry in invasive breast cancer. A-1 and A-2: Two histologically normal, strongly immunopositive mammary ducts in the midst of cancer cells with minimal immunostaining [shown at low (A-1), and high (A-2) magnification]. B–F: Tissue sections from additional patients illustrating the striking decrease or loss of immunostaining in invasive cancers. Arrow in B points to a blood vessel that is strongly immunopositive, whereas arrows in C and D point to blood vessels that are only weakly immunopositive. G: Cancer cells with minimal or no immunostaining adjacent to what appears to be an in situ lesion that shows relatively weak immunostaining. Sections were not counterstained and images were captured using Nomarski optics, as described in Materials and Methods.
Figure 4.
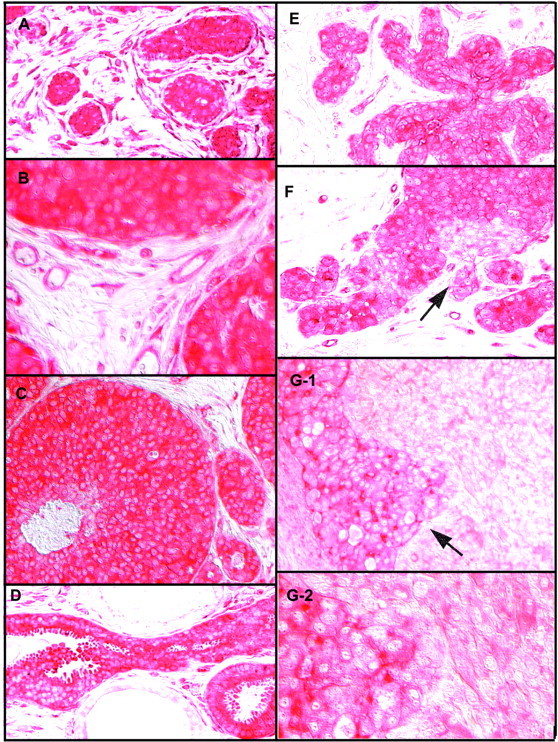
Localization of UGT2B7 by immunocytochemistry in intraductal and in situ breast cancers. A: A group of small in situ lesions showing intense immunostaining, surrounded by a ring of strongly immunopositive blood vessels. B: A section from tissue from another patient, viewed at high magnification, showing edge of three small in situ lesions and blood vessels associated with them. C: Commedo carcinoma with intense immunostaining. D: Strongly immunopositive intraductal carcinoma with apocrine features. E: A lobular in situ carcinoma. F: An in situ carcinoma comprised of groups of weakly immunopositive cancer cells in the midst of strongly immunopositive cancer cells. Arrow points to what appears to be a site of microinvasion. G-1 and G-2: An in situ lesion, comprised of cancer cells in which immunostaining ranges from strong to minimal, in the midst of minimally immunopositive invasive cancer [shown at low (G-1) and high (G-2) magnification]. Arrow points to a site suggestive of microinvasion where the in situ and invasive components of the cancer appear to be merging. Sections were not counterstained, and images were captured using Nomarski optics, as described in Materials and Methods.
In most cases, blood vessels associated with invasive cancers showed little or no immunoreactivity (Figure 3) ▶ . In contrast, the ring of blood vessels surrounding some in situ cancers was characterized by intense immunostaining, comparable to that in cancer cells in the adjacent in situ lesion (Figure 4, A-1 and A-2) ▶ ▶ ▶ . Occasionally, strongly immunopositive blood vessels were seen next to essentially immunonegative invasive cancers (Figure 3B) ▶ . This finding provides further evidence of a potential for regulation of UGT2B7 expression in blood vessels independent from that in neighboring epithelial cells, whether normal or neoplastic.
No immunostaining was seen in tissue sections incubated with secondary antibody only, or with effluent from columns used for affinity purification of the UGT2B7 antibody (Figure 5) ▶ . The same pattern of immunostaining was obtained with the commercial available polyclonal UGT2B7 antibody (Gentest) as with affinity-purified IgY antibody. There was no background staining in sections incubated with rabbit IgG used as a control for incubations performed with the commercial UGT2B7 antibody.
Figure 5.
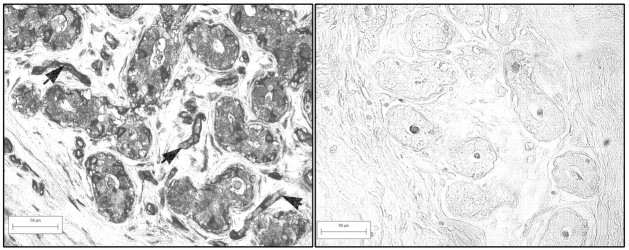
Controls for immunocytochemistry. Immunocytochemistry performed on the section shown on the left with the affinity-purified UGT2B7 antibody, showing strong immunostaining, and on the section shown on the right with eluate from column used to purify the UGT2B7 at the same protein concentration as the UGT2B7 antibody. In section on the right, there is strong immunostaining of epithelial cells and blood vessels (arrows point to such blood vessels). In control section, immunostaining is restricted to the acellular material in the lumen of the duct.
Immunoblot Analysis
A representative immunoblot of protein obtained from histologically normal and neoplastic tissue specimens is shown in Figure 6 ▶ . A single intense band of appropriate size for UGT2B7 was identified by immunoblot analysis of protein prepared from all histologically normal tissue samples, whether obtained from reduction mammoplasty (n = 8) or mastectomy specimens (n = 9). The variations in width of the immunopositive band and chemiluminescence intensity among samples could be because of differences in proportion of tissue occupied by mammary epithelial cells, as well as level of expression of UGT2B7 in these cells. Only a very faint immunoreactive band was seen in immunoblots of protein prepared from six of the nine breast cancer specimens. A wider, more intense, immunopositive band was seen in the other three cancer cases. The higher level of UGT2B7 protein in these three cancers could be because of the presence of in situ lesions with a high level of UGT2B7 expression, a prevalent phenomenon in our breast cancer patient population. Indeed, immunocytochemistry performed on cancerous tissue from two of these three patients identified some intensely immunoreactive in situ neoplastic foci in the midst of invasive cancer with reduced or no immunostaining. Findings obtained with the IgY UGT2B7 antibody developed in chickens paralleled those obtained with the UGT2B7 antibody developed in rabbits.
Figure 6.

Immunoblot analysis of protein from normal and neoplastic breast tissue for UGT2B7. Protein was prepared from tissue obtained at reduction mammoplasty (normal), and from tissue obtained from mastectomy specimens from sites distant from the primary cancer (normal from cancer,) and from the primary cancer (cancer), and electrophoresed as described in Materials and Methods. The one cancer sample showing an intense immunopositive band was from a patient with evidence of widespread, strongly immunopositive, in situ lesions.
Identification of UGT2B7 mRNA by RT-PCR
Expression of UGT2B7 in human breast parenchyma was confirmed by RT-PCR. Amplicons of the appropriate size were obtained from total RNA isolated from both normal and neoplastic breast tissue (Figure 7) ▶ . The sequence of the amplicon was shown to correspond to the expected sequence for UGT2B7. Transcripts of UGT2B7 were present in all nonneoplastic samples, but absent or weak in four of six neoplastic samples. The presence of transcripts in the two cancers could be because of the presence of in situ lesions with a high level of UGT2B7 expression of nonneoplastic mammary epithelium or UGT2B7 expressed in cells associated with blood vessels.
Figure 7.
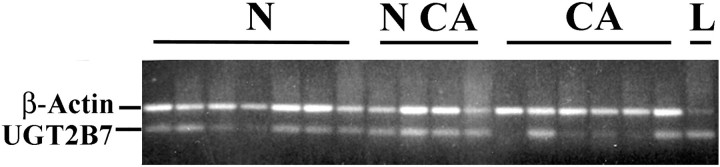
RT-PCR of total RNA from normal and neoplastic breast tissue for UGT2B7 transcripts. RNA was prepared from tissue obtained at reduction mammoplasty (N, normal), and from tissue obtained from mastectomy specimens from sites distant from the primary cancer (NCA, normal from cancer), and from the primary cancer (CA, cancer). RNA from liver (L) was included as a positive control. Primers for β-actin, spanning an intron, were included as an internal control for quantity and quality of RNA, as well as to test for presence of genomic DNA contamination. UGT2B7 transcripts were identified in all normal tissue samples, whether from mammoplasty or mastectomy specimens, but not in four of six of the neoplastic samples.
UDP-Glucuronosyltransferase Activity
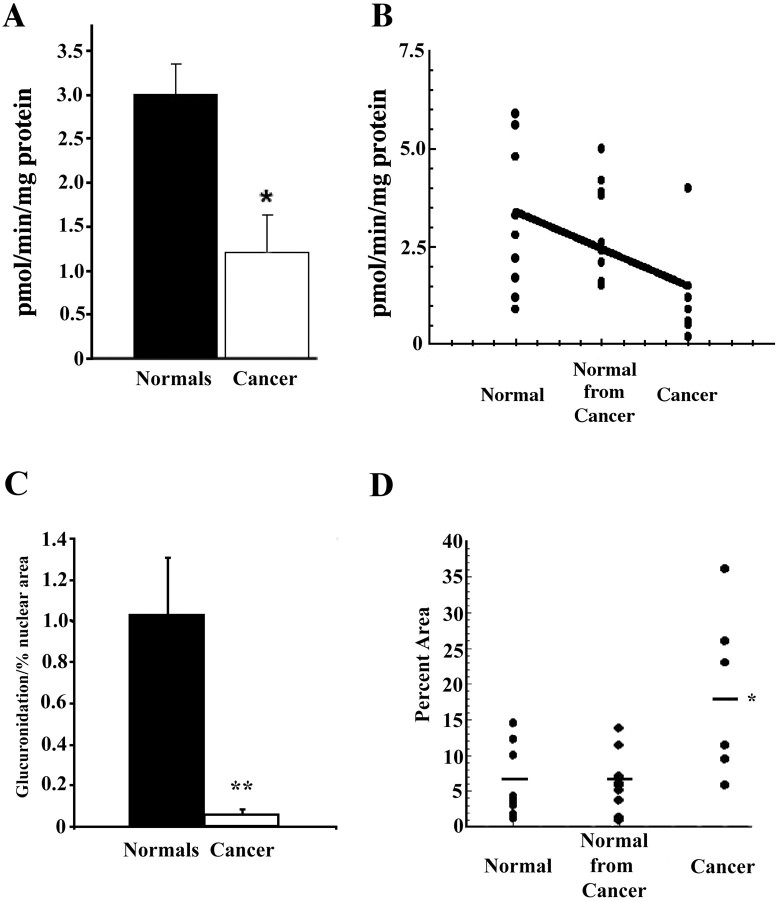
Data on glucuronidation of 4-OH-E1 by tissue from mammoplasty specimens (normal), and histologically normal (normal from cancers), and cancerous tissue from mastectomy specimens, are presented in Figure 8 ▶ . Enzyme activity of normal tissue ranged from 0.9 to 5.9 pmol/mg protein/minute (mean ± SEM = 3.12 ± 0.63), and that of normal tissue from cancers from 1.5 to 5.0 pmol/mg protein/minute (mean ± SEM = 2.87 ± 0.4). Enzyme activity in these two groups did not differ from each other statistically. Therefore, for statistical purposes, values from these two groups could be combined. UDP-glucuronosyltransferase activity of neoplastic tissue ranged from 0.2 to 4.0 pmol/mg protein/minute (mean ± SEM = 1.23 ± 0.42). These values are significantly different from those obtained using normal tissue from mammoplasty and mastectomy specimens combined (P = <0.01). The single tissue sample with high activity in the cancer group (4 pmol/mg protein/minute) was found to be from a patient with extensive in situ lesions with intense UGT2B7 immunostaining (not shown). No UGT-glucuronosyltransferase activity was detected when 4-OH-E2 was substituted for 4-OH-E1 as the substrate in the assay.
Figure 8.
UDP-glucuronosyltransferase activity of tissue obtained from mammoplasty specimens (normal), and of tissue obtained from mastectomy specimens from sites distant from the cancer (normal from cancer) and from the cancers. Assays were performed on disrupted cryostat sections, as described in Materials and Methods. Statistical significance of differences in glucuronidation of 4-OH-E1 by tissue from the three groups was determined by one-way analysis of variance, followed by Neuman-Keuls t-test. Because values for glucuronosyltransferase activity of normal tissue from mammoplasty and tissues distant from the cancers did not differ significantly from each other, they were pooled. Top (A and B): Enzyme activity normalized for mg protein. Bottom (C): Enzyme activity normalized for mg tissue protein corrected for differences in percent area occupied by cell nuclei. A: Pooled values for tissue from mammoplasty specimens and from tissue distant from the cancer from mastectomy specimens, compared with values for the cancer tissue. Differences were significant at the P < 0.01 level by Student’s t-test. B: Individual values plotted for the three groups. Line indicates finding from simple polynomal linear regression analysis (slope significant at the P < 0.01 level). The single high value in the cancer group was from a patient with extensive, strongly immunopositive in situ lesions. C: UDP-glucuronosyltransferase activity normalized for mg protein corrected for differences in percent area occupied by cell nuclei. The percent area occupied by hematoxylin-stained cell nuclei was estimated in representative sections from the tissue used for the enzyme assays, using computerized image analysis, as described in Materials and Methods. **, Significantly different from histologically normal specimens from mammoplasty and mastectomy specimens combined (P < 0.01 by Student’s t-test). D: Individual values for percentage of area occupied by cell nuclei in representative sections. In histologically normal tissues, these values ranged from 0.93 to 13.8 (mean ± SEM = 6.28 ± 1.1) and in sections from breast cancers from 5.96 to 36.1 (mean ± SEM = 18.7 ± 4.7) and were significantly different from each other (P < 0.01).
As indicated in Materials and Methods, using total protein as the denominator in calculating enzyme activity fails to take into account the differences in epithelial cell density found among normal tissues specimens. More importantly, it fails to take into account the fact that epithelial cell density in neoplastic tissue is consistently much higher than that in normal tissue. To address this issue we measured the percent area occupied by cell nuclei in representative cryosections from tissues used in the enzyme assays (see Materials and Methods). The percent area occupied by cell nuclei ranged from 0.93 to 13.80 (mean ± SEM = 6.28 ± 1.1) in sections from normal tissues and from 5.96 to 36.10 (mean and SEM = 18.7 ± 4.7) in sections from breast cancers. As shown in Figure 8D ▶ , values for glucuronidation of 4-OH-E1 adjusted for these differences in percent nuclear area magnified the difference in enzyme activity between normal and neoplastic tissue.
Discussion
The findings presented suggest a role for UGT2B7 in the regulation of levels in the mammary epithelium of its two major known endogenous substrates, 4-OH-E1 and atRA. 13,14,17 The marked reduction in expression of UGT2B7 in invasive breast cancers suggests an anti-carcinogenic function for the enzyme. The proposition that this function encompasses the glucuronidation of 4-OH-E1 and RA can be readily supported by available evidence.
Glucuronidation of CEs, by blocking oxidation of ring A of CEs, can provide an important line of defense against the generation of procarcinogenic electrophiles from these prevalent estrogen metabolites. 4,5 Our findings support the notion that in normal human mammary epithelium, UGT2B7 serves such a protective function with respect to 4-OH-E1, and that this line of defense is primarily abrogated in invasive cancers. Increased availability of ring A of 4-OH-E1 for oxidation in invasive cancers, resulting from decrease glucuronidation, could contribute to the extensive oxidative DNA damage identified by Malins and co-workers 24 in breast cancers. How the pro-oxidant state implied by these observations contributes to cancer progression remains to be defined.
To assess fully the functional consequence of reduced glucuronidation of 4-OH-E1 in breast cancers will require determining whether the glucuronide of 4-OH-E1 is biologically active. A precedent for conjugates of CEs having potentially important tumor-suppressor functions is provided by evidence of anti-angiogenic, anti-proliferative actions of O-methylated metabolites of 2-OH-CEs. 25-29
The finding of no detectable glucuronidation of 4-OH-E2 suggest that, in contrast to 4-OH-E1, protection against oxidation of 4-OH-E2 by glucuronidation is not available in human breast parenchyma. This could pose a problem when there is a shift in balance between estrone and estradiol present in this tissue. Normally, 17β-hydroxysteroid dehydrogenase isoenzyme(s) expressed in breast tissue favors the conversion of estradiol to estrone. In breast cancer and under conditions of oxidative stress, however, the balance may shift to favor estradiol formation. 30-32
A second putative tumor suppressor function for UGT2B7, that of glucuronidation of atRA, is suggested by the recent identification of atRA as a second endogenous substrate of this isoenzyme. 17 Glucuronidation of atRA, like that of 4-OH-E1, can be viewed as a tumor-suppressor function: retinoyl-β-glucuronide, the product of the reaction, has been shown in diverse studies to be a potent mediator of actions of RA, and glucuronidation may protect RA from oxidative metabolism. 3,18,19 UGT2B7 is the first and, thus far, the only member of the UGT family of isoenzymes known to have the potential to catalyze the glucuronidation of atRA with high catalytic efficiency. Therefore, our finding implies reduced formation of retinoyl-β-glucuronide in invasive cancers. To determine whether this is the case will require comparing glucuronosyltransferase activity of normal and neoplastic breast tissue using atRA as the substrate. Reduced formation of a potent derivative of RA in breast cancer would fit with emerging evidence suggesting disruption of in situ RA biosynthesis and reduced availability of RA in cancers of epithelial origin. 33-36
Current knowledge of neither CE nor RA homeostasis, however, offers any ready explanation for the intense UGT2B7 immunoreactivity seen in many in situ neoplastic foci. It could reflect a defensive response in the preinvasive stage of carcinogenesis, in particular, a defense against invasion of surrounding tissue by cancer cells. This interpretation is supported by the finding of cancer cells with minimal UGT2B7 immunoreactivity at sites of microinvasion. It is also consistent with the known role of RA in inhibiting expression of matrix metalloproteinases, enzymes critical for the invasion of tissues by cancer cells. 37-40 Interestingly, expression of RXR, a second gene product relevant to retinoid action, has also been reported to be elevated within in situ breast cancers to levels greater than those found in either normal breast tissue or breast cancer. 41
In situ neoplastic lesions are considered to represent early stages in the multistep process of carcinogenesis. 42,43 A positive correlation has been suggested to exist between incidence of in situ breast cancers and overall breast cancer incidence in a particular population. 44 The prognosis among patients with histologically similar in situ lesions varies. Hence, the need for molecular markers that identify lesions that are more likely to become invasive. Such markers may serve not only to assist in choosing among treatment options, but also to gain insight into the process of carcinogenesis and to identify new potential targets for therapeutic intervention. The findings presented here suggest that expression of UGT2B7 may represent one such marker.
The differences in level of expression of UGT2B7 in tissue obtained from different individuals, although less striking than between normal and neoplastic tissue, are worth noting. Expression of UGT2B7, like that of other phase II enzymes, is likely to be inducible by endobiotics as well as xenobiotics and in a tissue-specific manner. 1,45,46 Hence, the apparent interindividual differences in level of expression of UGT2B7 could reflect differences in hormonal milieu among patients, as well as in their exposure to xenobiotics and drugs. To begin to interpret the significance of the interindividual differences for cancer susceptibility will require much more knowledge of the regulation of UGT2B7 expression than what is currently available. In addition, it will require large, population based molecular epidemiological studies in which expression of UGT2B7 is quantified and exposure of individuals to potential inducers is known.
An additional finding is the presence of UGT2B7 immunoreactivity in blood vessels. This finding parallels the observation of UGT2B7 expression in blood vessels in the human prostate. 47 Immunostaining was seen consistently in blood vessels in nonneoplastic tissue, but often lost in blood vessels associated with invasive cancers. In contrast, in the ring of blood vessels often seen surrounding in situ cancers, 48 UGT2B7 immunostaining was often very intense. Together, these observations suggest regulation of CE and/or retinoid homeostasis in blood vessels by glucuronidation.
Glucuronidation has a profound effect on the actions and disposition of a wide range of lipophilic endo- and xenobiotics. The physiological substrates of members of the UGT family of isoenzymes encompass estrogens, androgens, as well as RAs, hormonal agents with important regulatory functions that are also implicated in carcinogenesis. 2,49 There have been remarkably few studies aimed at documenting the expression of UGTs in extra-hepatic tissues that are targets of hormonal carcinogenesis, their tissue- and cell type-specific regulation, and the effect of the process of carcinogenesis on their expression. Such studies may provide new insights into defensive mechanism that might be marshaled to impede cancer progression. The availability of sequence information on a growing number of members of the UGT superfamily, as well as of other phase II enzymes, together with new microtechniques, make such studies now feasible.
Acknowledgments
We thank Dr. G. Clawson, Gittlen Cancer Institute, The Pennsylvania State University College of Medicine, for his help and support for this study.
Footnotes
Address reprint requests to Judith Weisz, M.B., B. Chir, Pennsylvania State University, Department of Obstetrics and Gynecology, the MS Hershey Medical Center, H103, 500 University Dr., Hershey, PA 17033. E-mail: jxw7@email.psu.edu.
Supported by National Institutes of Health grants CA65532 (to J. W.), GM26221 (to T. R. T.), and CA40145 (to G. C.) (that provided the system used for capturing immunocytochemical images and performing image analysis).
References
- 1.Tukey RH, Strassburg CP: Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 2000, 40:581-616 [DOI] [PubMed] [Google Scholar]
- 2.Coffman BL, King CD, Rios GR, Tephly TR: The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268). Drug Metab Dispos Biol Fate Chem 1998, 26:73-77 [PubMed] [Google Scholar]
- 3.Formelli F, Barua AB, Olson JA: Bioactivities of N-(4-hydroxyphenyl) retinamide and retinoyl beta-glucuronide. FASEB J 1996, 10:1014-1024 [DOI] [PubMed] [Google Scholar]
- 4.Yager JD, Liehr JG: Molecular mechanisms of estrogen carcinogenesis. Annu Rev Toxicol Pharmacol 1996, 36:203-232 [DOI] [PubMed] [Google Scholar]
- 5.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D: Estrogens as endogenous genotoxic agents—DNA adducts and mutations. J Natl Cancer Inst Monogr 2000, 27:75-93 [DOI] [PubMed] [Google Scholar]
- 6.Liehr JG, Roy D: Free radical generation by redox cycling of estrogens. Free Radic Biol Med 1990, 8:415-423 [DOI] [PubMed] [Google Scholar]
- 7.Weisz J, Clawson GA, Creveling CR: Biogenesis and inactivation of catecholestrogens. Adv Pharmacol 1998, 42:828-833 [DOI] [PubMed] [Google Scholar]
- 8.Raftogianis R, Creveling C, Weinshilboum R, Weisz J: Estrogen metabolism by conjugation. J Natl Cancer Monographs 2000, 27:113-124 [DOI] [PubMed] [Google Scholar]
- 9.Roy D, Weisz J, Liehr JG: The O-methylation of 4-hydroxyestradiol is inhibited by 2-hydroxyestradiol: implications for estrogen-induced carcinogenesis. Carcinogenesis 1990, 11:459-462 [DOI] [PubMed] [Google Scholar]
- 10.Levin M, Weisz J, Bui QD, Santen RJ: Peroxidatic catecholestrogen production by human breast cancer tissue in vitro. J Steroid Biochem 1987, 28:513-520 [DOI] [PubMed] [Google Scholar]
- 11.Hellmold H, Rylander T, Magnusson M, Reihner E, Warner M, Gustafsson JA: Characterization of cytochrome P450 enzymes in human breast tissue from reduction mammaplasties. J Clin Endocrinol Metab 1998, 83:886-895 [DOI] [PubMed] [Google Scholar]
- 12.Muskhelishvili L, Thompson PA, Kusewitt DF, Wang C, Kadlubar FF: In situ hybridization and immunohistochemical analysis of cytochrome P450 1B1 expression in human normal tissues. J Histochem Cytochem 2001, 49:229-236 [DOI] [PubMed] [Google Scholar]
- 13.Turgeon D, Carrier J, Levesque E, Hum DW, Belanger A: Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology 2001, 142:778-787 [DOI] [PubMed] [Google Scholar]
- 14.Ritter JK, Chen F, Sheen YY, Lubet RA, Owens IS: Two human liver cDNAs encoded UDP-glucuronosyltransferase with 2 log differences in activity towards parallel substrates including hyodeoxycholic acid and certain estrogen derivatives. Biochemistry 1992, 31:3409-3414 [DOI] [PubMed] [Google Scholar]
- 15.Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, Maloche C: Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J Clin Endocrinol Metab 1996, 81:1460-1464 [DOI] [PubMed] [Google Scholar]
- 16.Pasqualini JR, Cortes-Prieto J, Chetrite G, Talbi M, Ruiz A: Concentrations of estrone, estradiol and their sulfates, and evaluation of sulfatase and aromatase activities in patients with breast fibroadenoma. Int J Cancer 1997, 70:639-643 [DOI] [PubMed] [Google Scholar]
- 17.Samokyszyn VM, Gall WE, Zawada G, Freyaldenhoven MA, Chen G, Mackenzie PI, Tephly TR, Radominska-Pandya A: 4-hydroxyretinoic acid, a novel substrate for human liver microsomal UDP-glucuronosyltransferase(s) and recombinant UGT2B7. J Biol Chem 2000, 275:6908-6914 [DOI] [PubMed] [Google Scholar]
- 18.Sietsema WK, DeLuca HF: A new vaginal smear assay for vitamin A in rats. J Nutr 1982, 112:1481-1489 [DOI] [PubMed] [Google Scholar]
- 19.Miller DA, DeLuca HF: Biosynthesis of retinoyl-beta-glucuronide, a biologically active metabolite of all-trans-retinoic acid. Arch Biochem Biophys 1986, 244:179-186 [DOI] [PubMed] [Google Scholar]
- 20.Elias J: Low temperature antigen restoration of steroid hormone receptor proteins in routine paraffin sections. J Histotechnol 1997, 20:155-158 [Google Scholar]
- 21.Weisz J, Fritz-Wolz G, Gestl S, Clawson G, Creveling CR, Liehr JG, Dabbs D: Nuclear localization of catechol-O-methyltransferase in neoplastic and nonneoplastic mammary epithelial cells. Am J Pathol 2000, 156:1841-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matern H, Heinemann H, Matern S: Radioassay of UDP-glucuronosyltransferase activities toward endogenous substrates using labeled UDP-glucuronic acid and an organic solvent extraction procedure. Anal Biochem 1994, 219:182-188 [DOI] [PubMed] [Google Scholar]
- 23.Vogel PM, Georgiade NA, Fetter BF, Vogel PS, McCarty KS: The correlation of histological changes in the human breast with menstrual cycle. Am J Pathol 1981, 104:23-34 [PMC free article] [PubMed] [Google Scholar]
- 24.Malins DC, Polissar NL, Schaefer S, Su Y, Vinson M: A unified theory of carcinogenesis based on order-disorder transitions in DNA structure as studied in the human ovary and breast. Proc Natl Acad Sci USA 1998, 95:7637-7642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arbiser JL, Panigrathy D, Klauber N, Rupnick M, Flynn E, Udagawa T, D’Amato RJ: The antiangiogenic agents TNP-470 and 2-methoxyestradiol inhibit the growth of angiosarcoma in mice. J Am Acad Dermatol 1999, 40:925-929 [DOI] [PubMed] [Google Scholar]
- 26.Pico C, Puigserver P, Oliver P, Palou A: 2-Methoxyestradiol, an endogenous metabolite of 17beta-estradiol, inhibits adipocyte proliferation. Mol Cell Biochem 1998, 189:1-7 [DOI] [PubMed] [Google Scholar]
- 27.Reiser F, Way D, Bernas M, Witte M, Witte C: Inhibition of normal and experimental angiotumor endothelial cell proliferation and cell cycle progression by 2-methoxyestradiol. Proc Soc Exp Biol Med 1998, 219:211-216 [DOI] [PubMed] [Google Scholar]
- 28.Schumacher G, Kataoka M, Roth JA, Mukhopadhyay T: Potent antitumor activity of 2-methoxyestradiol in human pancreatic cancer cell lines. Clin Cancer Res 1999, 5:493-499 [PubMed] [Google Scholar]
- 29.Tsukamoto A, Kaneko Y, Yoshida T, Han K, Ichinose M, Kimura S: 2-Methoxyestradiol, an endogenous metabolite of estrogen, enhances apoptosis and beta-galactosidase expression in vascular endothelial cells. Biochem Biophys Res Commun 1998, 248:9-12 [DOI] [PubMed] [Google Scholar]
- 30.Peltoketo H, Vihko P, Vihko R: Regulation of estrogen action: role of 17 beta-hydroxysteroid dehydrogenases. Vitam Horm 1999, 55:353-398 [PubMed] [Google Scholar]
- 31.Purohit A, Singh A, Reed MJ: Regulation of steroid sulphatase and oestradiol 17 beta-hydroxysteroid dehydrogenase in breast cancer. Biochem Soc Trans 1999, 27:323-327 [DOI] [PubMed] [Google Scholar]
- 32.Abplanalp W, Rymaszewski M, Adamski J, Subbiah MT: Evidence for interference in estradiol-17beta inactivation to estrone by oxidized low-density lipoprotein and selected lipid peroxidation products. J Lab Clin Med 1999, 134:253-259 [DOI] [PubMed] [Google Scholar]
- 33.Xu XC, Zile MH, Lippman SM, Lee JS, Lee JJ, Hong WK, Lotan R: Anti-retinoic acid (RA) antibody binding to human premalignant oral lesions, which occurs less frequently than binding to normal tissue, increases after 13-cis-RA treatment in vivo and is related to RA receptor beta expression. Cancer Res 1995, 55:5507-5511 [PubMed] [Google Scholar]
- 34.Chen AC, Guo X, Derguini F, Gudas LJ: Human breast cancer cells and normal mammary epithelial cells: retinol metabolism and growth inhibition by the retinol metabolite 4-oxoretinol. Cancer Res 1997, 57:4642-4651 [PubMed] [Google Scholar]
- 35.Pasquali D, Thaller C, Eichele G: Abnormal level of retinoic acid in prostate cancer tissues. J Clin Endocrinol Metab 1996, 81:2186-2191 [DOI] [PubMed] [Google Scholar]
- 36.Cain JMZ, Shearer D, Bennett AR, Olt G, Weisz J: Expression of the retinol dehydrogenase hRoDH4, an enzyme implicated in retinoic acid biosynthesis (hRoDH-4), in normal and neoplastic endometria. Am J Obstet Gynecol (in press) [DOI] [PubMed]
- 37.Pan L, Eckhoff C, Brinckerhoff CE: Suppression of collagenase gene expression by all-trans and 9-cis retinoic acid is ligand dependent and requires both RARs and RXRs. J Cell Biochem 1995, 57:575-589 [DOI] [PubMed] [Google Scholar]
- 38.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z: The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999, 98:137-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoenermark MP, Mitchell TI, Rutter JL, Reczek PR, Brinckerhoff CE: Retinoid-mediated suppression of tumor invasion and matrix metalloproteinase synthesis. Ann NY Acad Sci 1999, 878:466-486 [DOI] [PubMed] [Google Scholar]
- 40.Fisher GJ, Talwar HS, Lin J, Voorhees JJ: Molecular mechanisms of photoaging in human skin in vivo and their prevention by all-trans retinoic acid. Photochem Photobiol 1999, 69:154-157 [DOI] [PubMed] [Google Scholar]
- 41.Lawrence JA, Merino MJ, Simpson JF, Manrow RE, Page DL, Steeg PS: A high-risk lesion for invasive breast cancer, ductal carcinoma in situ, exhibits frequent overexpression of retinoid X receptor. Cancer Epidemiol Biomarkers Prev 1998, 7:29-35 [PubMed] [Google Scholar]
- 42.Page DL, Simpson JF: Pathology of preinvasive and excellent-prognosis breast cancer. Curr Opin Oncol 2000, 12:526-531 [DOI] [PubMed] [Google Scholar]
- 43.Allred DC, Mohsin SK, Fuqua SA: Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer 2001, 8:47-61 [DOI] [PubMed] [Google Scholar]
- 44.Stoll BA: Biological mechanisms in breast cancer invasiveness: relevance to preventive interventions. Eur J Cancer Prev 2000, 9:73-79 [DOI] [PubMed] [Google Scholar]
- 45.Munzel PA, Schmohl S, Heel H, Kalberer K, Bock-Hennig BS, Bock KW: Induction of human UDP glucuronosyltransferases (UGT1A6, UGT1A9, and UGT2B7) by t-butylhydroquinone and 2,3,7,8-tetrachlorodibenzo-p-dioxin in Caco-2 cells. Drug Metab Dispos Biol Fate Chem 1999, 27:569-573 [PubMed] [Google Scholar]
- 46.Strassburg CP, Kneip S, Topp J, Obermayer-Straub P, Barut A, Tukey RH, Manns MP: Polymorphic gene regulation and interindividual variation of UDP-glucuronosyltransferase activity in human small intestine. J Biol Chem 2000, 275:36164-36171 [DOI] [PubMed] [Google Scholar]
- 47.Barbier O, Lapointe H, El Alfy M, Hum DW, Belanger A: Cellular localization of uridine diphosphoglucuronosyltransferase 2B enzymes in the human prostate by in situ hybridization and immunohistochemistry. J Clin Endocrinol Metab 2000, 85:4819-4826 [DOI] [PubMed] [Google Scholar]
- 48.Engels K, Fox SB, Whitehouse RM, Gatter KC, Harris AL: Distinct angiogenic patterns are associated with high-grade in situ ductal carcinomas of the breast. J Pathol 1997, 181:207-212 [DOI] [PubMed] [Google Scholar]
- 49.Mackenzie PI, Mojarrabi B, Meech R, Hansen A: Steroid UDP glucuronosyltransferases: characterization and regulation. J Endocrinol 1996, 150(Suppl):S79-S86 [PubMed] [Google Scholar]