Abstract
The protein expression of the cyclin-dependent kinase inhibitor p27 is often deregulated in human tumors. In lymphomas the inactivation of p27 is achieved through either increased degradation 1 or sequestration via D cyclins, 2 and p27 protein levels have been shown to have a prognostic significance. 1,3 Recently, S-phase kinase-associated protein 2 (Skp2) has been proved to mediate p27 degradation in normal cells 4-7 and to have oncogenetic properties. 8,9 In this study, B-, T-, and myeloid hematopoietic cell lines and a well-characterized panel of human lymphomas (n = 244) were studied for the expression of Skp2. In human lymphomas, the expression of Skp2 strongly related to the grade of malignancy, being low in indolent tumors and very high in aggressive lymphomas. Moreover, the percentages of Skp2- and S-phase-positive cells, as measured by DNA content or BrdU labeling, strictly matched and closely parallel that of Ki-67 and cyclin A. An inverse correlation between Skp2 and p27 was found in the majority of lymphoma subtypes. Nonetheless, most mantle cell lymphomas and a subset of diffuse large cell lymphomas failed to show this correlation, suggesting that alternative pathway(s) for the regulation of p27 might exist. The detection of Skp2 protein either by flow cytometry or by immunohistochemistry represents a simple method to precisely assess the S phase of lymphomas. The potential diagnostic and prognostic value of Skp2 is discussed.
The S-phase kinase-associated protein 2 (Skp2) belongs to a family of proteins called F-box proteins (Fbps) characterized by an ∼40-amino acid long motif called F-box. Skp1, Cul1, and Roc1/Rbx1 associate with different Fbps to form E3 ubiquitin ligase complexes known as SCF. The Fbp subunits of the SCF complex ensure the specific recognition and ubiquitination of a large number of substrates that are in turn degraded by the proteasome. 10
Skp2 was first identified to interact with the cyclin A-Cdk2 complex in immortalized fibroblasts and transformed cells and required for G1 to S transition. 11 In vitro and in vivo studies have demonstrated that the SCF complexes containing Skp2, as Fbp (SCFSkp2), bind to p27, which is specifically ubiquitinated by the ubiquitin-conjugating enzyme Ubc3. 4-7 Skp2-deficient mice have smaller organs than littermate controls, their cells grow more slowly, and they display centrosome overduplication together with the accumulation of p27 and free cyclin E. 7 Importantly, in Skp2 transgenic mice, the forced expression of Skp2 in T cells cooperates with the N-ras oncogene leading to lymphomagenesis. 8 Finally, the tumorigenic role of Skp2 has been suggested by several investigators. 9,12
The deregulated expression of p27 plays a critical role in the pathogenesis of many human tumors. 13 The protein levels of p27 in normal cells are mainly regulated by an ubiquitin-mediated degradation. 14 Moreover, an enhanced protein degradation via the ubiquitin-proteasome pathway is also responsible for the low levels of p27 protein in aggressive human tumors 15-17 and in mantle cell lymphomas (MCLs). 1 We and others have demonstrated that the protein levels of p27 expression are different in specific subsets of human lymphomas, being high in low-grade and low in high-grade lymphomas. 1,3,18 More importantly, the partial or total loss of p27 function defines a group of patients that exhibit low overall survival and poor outcome. 1,3
In this study we have analyzed the expression of Skp2 in cell lines and human lymphomas and compared it to other proliferation indexes and to the expression of its substrate p27. Our findings demonstrate that Skp2 protein levels tightly correlate with the late G1 and S phase in neoplastic cell lines and tumors. We have found that there is a direct correlation among Skp2 and Ki-67 and cyclin A and an inverse relationship between Skp2 and p27 protein levels in the majority of human lymphomas. However, in a subset of lymphomas this correlation is lost, suggesting that alternative pathways of p27 inactivation might exist.
Materials and Methods
Pathological Samples
A panel of 244 well-characterized non-Hodgkin’s lymphomas (NHLs) was retrieved from the archives of the Division of Hematopathology of the New York University School of Medicine, from the Division of Morphology and Molecular Pathology of the Catholic University of Leuven in Belgium, and the Surgical Pathology Departments of the Universities of Torino and Verona in Italy. NHLs were classified according to the International Lymphoma Study Group, based on hematoxylin and eosin and immunoperoxidase stains, and clinical and molecular data, as previously described. 1 The NHLs characterized in this study included: 28 chronic lymphocytic leukemia/small lymphocytic lymphomas (CLL/SLLs), 51 follicular lymphomas (FL), 34 mucosa-associated lymphoid tissue B-cell lymphomas, 34 MCLs, and 76 diffuse large B-cell lymphomas (DLCLs), 15 lymphoblastic lymphomas (6 B-lymphoblastic lymphoma and 9 T-lymphoblastic lymphomas), 25 Burkitt’s lymphomas, and 5 Burkitt-like lymphomas. FL were classified based on the criteria of the revised World Health Organization classification 19 and of Mann and Berard. 20 A single case of plasma cell leukemia (>90% plasma cells) was also included for the flow cytometry staining. The following cell lines were also used: Namalwa, Jurkat, Karpas 299, DHL, JB, KM512, and K562.
Antibodies
The monoclonal antibodies (mAbs) applied for the immunohistochemistry in this study included: anti-p27 (KIP-1, 1:1000; Transduction Laboratories, Lexington, KY, USA), anti-p53 (DO-1, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-Ki-67 (MIB-1, 1:1000; Immunotech, Marseille, France), anti-cyclin E (1:50; Novocastra, CA, USA) and cyclin A (1:100, Santa Cruz Biotechnology). Mouse mAbs to Skp2 were produced in collaboration with Zymed Inc., South San Francisco, CA, USA. Immunohistochemical staining and Western blotting for Skp2 were performed using a cocktail of four different affinity-purified mAbs (clones 2C8D9, 2C2B12, 4A9B2, and 2G12E9) or a single mAb (clone 4A9B2), 8 as indicated.
Western Blot Analysis and in Vitro Protein Degradation Assay
For Western Blotting, cells were lysed (50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1% Triton X-100, 5 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L Na3VO4, and 1 mmol/L phenylmethyl sulfonyl fluoride and protease inhibitors) and 20 to 30 μg of proteins were electrophoresed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred onto nitrocellulose membranes. The membranes were first blocked and then incubated with the primary antibody (anti-Skp2, clone 4A9B2, 1:500; anti-p27, KIP-1, 1:2000; and anti-cyclin A, 1:1000) for 1 hour at room temperature. After three washes, filters were incubated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit antibodies (1:2000; Amersham, Arlington Heights, IL, USA) for 1 hour at room temperature. Detection of immunocomplexes was performed with an enhanced chemiluminescence system (ECL, Amersham). 1
In vitro degradation assays were performed on cryopreserved tissue samples. 1 Frozen tissues of MCLs (expressing low, intermediate, and high protein levels of p27), with >80% of tumor cells were sectioned and quickly disrupted by nitrogen decompression in 100 μl of lysing buffer (50 mmol/L Tris-HCl, pH 8.3, 5 mmol/L MgCl2, and 1 mmol/L dithiothreitol). Samples were then frozen and thawed three consecutive times. Lysates were spun down at 15,000 rpm and supernatants were collected and frozen at −80°C. Histidine-tagged p27 (100 μg) was incubated at 37°C for different intervals in 60 μl of degradation mix containing 30 μg of protein tissue homogenates, 50 mmol/L Tris-HCL (pH 8.0), 5 mmol/L MgCl2, 1 mmol/L dithiothreitol, 2 mmol/L ATP, 10 mmol/L creatine phosphokinase, and 10 mmol/L creatine phosphatase. The in vitro degradation of p27 was analyzed by immunoblotting with anti-p27 mAb.
Immunohistochemical Staining
For immunohistochemistry, slides were subjected to microwaving for 20 minutes in 10 mmol/L of citrate buffer (pH 8.0 for Skp2, cyclin E, and cyclin A; pH 6.0 for p27, p53, and Ki67). Immunostaining were performed on formalin-fixed, paraffin-embedded tissues using the avidin biotin peroxidase complex method and a semiautomated immunostainer (DAKO, Carpinteria, CA, USA or Ventana Systems, Tucson, AZ, USA) as described. 1 Three independent pathologists (RC, YF, and GI) evaluated the immunostaining. At least 10 high-power fields were randomly chosen and at least 100 cells/field were counted. Tumors were scored as percentage of positive cells for each antigen. The statistical significance of the means was calculated using a Student’s t-test. The statistical significance of Spk2 versus cyclin A expression was calculated with the chi-square test. P ≤ 0.05 was required for statistical significance.
Flow Cytometry and BrdU Labeling
For flow cytometric staining, untreated cell lines or lovastatin-treated (24 hours, 50 μmol/L; Sigma Chemical Co., St. Louis, MO, USA) cells were fixed and permeabilized according to the manufacturer’s instruction (Fix and Perm; Caltag, Burlingame, CA). Cells were subsequently stained using a cocktail of anti-Skp2 mAbs (1:100, Zymed). Anti-Skp2 mAbs were detected using phycoerythrin-conjugated horse anti-mouse antibody (1:200; Biosource, Camarillo, CA, USA). For DNA content determination, cells were fixed for 1 hour in 70% ethanol at 4°C. After washing, cells were treated with RNase (0.25 mg/ml) and stained with propidium iodide (50 μg/ml). The S-phase fraction was calculated using the Modfit program from Becton-Dickinson, Mountain View, CA.
For BrdU labeling, 1 × 10 6 cells or 1-mm-thick fresh tissues (three normal tonsils and seven randomly selected NHLs) were incubated in complete cell-culture media (RPMI-1640 with 10% fetal calf serum) in the presence of 10 μmol/L of BrdU for 30 minutes (suspension cells) or for 2 hours (tissue samples) at 37°C in 5% CO2. For BrdU determination using flow cytometry, cells were subsequently washed, fixed in 70% alcohol for 30 minutes, denatured with 2 N HCl, and then incubated with an anti-BrdU fluorescein isothiocyanate-labeled mAb (1:10, Becton-Dickinson) and propidium iodide (50 μg/ml). For BrdU determination using immunohistochemistry, tissue samples were rinsed in phosphate-buffered saline and fixed overnight in 10% buffered formalin. The incorporation of BrdU in proliferating cells was then detected by enzymatic digestion (0.05% proteinase K for 20 minutes at 37°C), denaturation (2 N HCl for 30 minutes at 37°C), and incubation with an anti-BrdU fluorescein isothiocyanate-conjugated antibody (30 minutes at room temperature, Becton-Dickinson). Bound anti-BrdU fluorescein isothiocyanate-conjugated antibodies were detected using an alkaline-conjugated anti-fluorescein isothiocyanate antibody (1:200; Roche, Indianapolis, IN) and NBT/BCIP as a substrate.
Results
Skp2 Expression in Human Lymphoid Tissues
The specificity of our anti-Skp2 antibodies was first evaluated in cell lines derived from human lymphomas by Western blotting. Skp2 mAbs detected only a single band migrating slightly faster than a tagged Skp2 recombinant protein (Figure 1A) ▶ . We subsequently evaluated by immunohistochemistry Skp2 protein expression in normal primary and secondary lymphoid tissues. In primary lymphoid organs, Skp2 staining was restricted to the nuclei of a subset of cortical thymocytes as well as of precursor myeloid and erythroid cells in the bone marrow (Figure 1, B and C) ▶ . In peripheral lymphoid organs, centroblasts or large centrocytes, within the dark zone of the germinal centers, were preferentially positive (Figure 1, D and E) ▶ . In contrast, small resting cells within the mantle of the B-cell follicle and interfollicular T lymphocytes were negative. Nonetheless, in the interfollicular areas some of the intermediate large cells were also Skp2-positive. In the oral mucosa as well as in the epidermis, Skp2-positive cells were confined, similarly to the Ki-67-positive cells, within the basal layer of the epithelium (data not shown).
Figure 1.

Skp2 expression in lymphoid cell lines and normal tissues. A: Western blot analysis on human lymphoid cell lines. Cells from the indicated cell lines were lysed and Western blotting was performed as described in Material and Methods. Lane 1, Namalwa cell line; lane 2, DHL cell line; lane 3, recombinant His-tagged Skp2 protein. B–E: Formalin-fixed, paraffin-embedded tissue sections were stained by immunohistochemistry with an anti-Skp2 mAb, as described in Materials and Methods. In the thymus (B) and in the bone marrow (C), Skp2 stained primarily the immature subcortical thymocytes and immature hematopoietic cells, respectively. In lymph nodes and tonsils, Skp2 labeled active proliferating cells within germinal centers and scattered immunoblasts in interfollicular areas (D and E).
Skp2 Is Expressed in S Phase in Neoplastic Lymphoid Cells
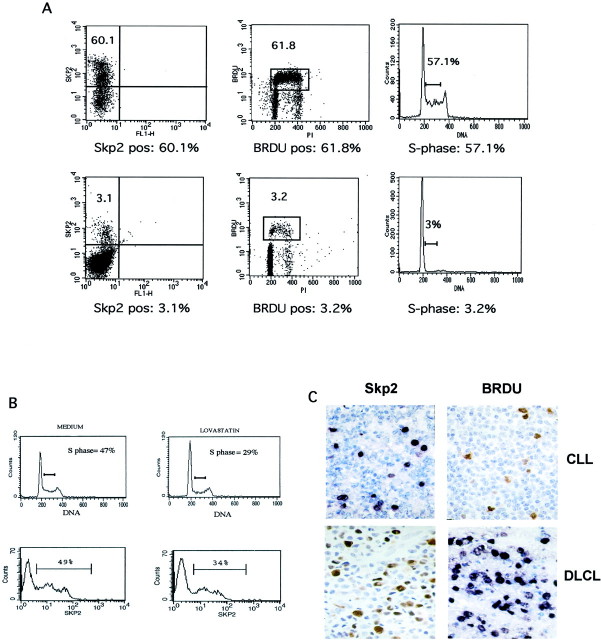
Since in normal cells the levels of Skp2 vary during the cell cycle, with a peak of expression at G1 to S through S phase, 11 we investigated the kinetics of Skp2 during cell-cycle progression in neoplastic lymphoid cells to seek for a possible aberrant pattern and/or deregulation. First, we compared Skp2 expression to the fraction of cells in S phase calculated by DNA content and BrdU incorporation in multiple and T-lymphoblastoid cell lines and in a case of plasma cell leukemia. In all of the cell lines the percentage of Skp2-positive cells always corresponded with the percentage of cells in the S phase (Table 1 ▶ , Figure 2A ▶ ). Moreover, flow cytometric analysis demonstrated two distinct subpopulations of Skp2-positive cells, with intermediate and high intensity of expression, suggesting that different levels of expression of Skp2 are achieved in different phases of the cell cycle (Figure 2B) ▶ . To further confirm the relation between Skp2 and S phase, we blocked the progression into the S phase of multiple lymphoid cell lines by inducing an early G1 cell-cycle arrest. In cells arrested by lovastatin in G1 phase, both the percentage of cells in S phase and those of Skp2-positive cells decreased in a comparable manner (Figure 2B) ▶ . These results were confirmed in all lymphoma-derived cell lines, demonstrating that Skp2 expression in neoplastic lymphoid cells as well in normal nonlymphoid cells, 11 peaks in late G1 to S phase and is maintained throughout the S phase. Next, we correlated the percentage and distribution of Skp2-positive cells with BrdU incorporation in fresh lymphoma samples. This approach showed a close correspondence between Skp2- and BrdU-positive cells (Figure 2C) ▶ , confirming that the majority of Skp2-positive cells are actively synthesizing the DNA in normal as well in neoplastic lymphoma cells.
Table 1.
Comparison between Skp2-Positive Cells and the Fraction of Cells in S Phase
| Cell line | Skp2* | DNA† | BrdU* |
|---|---|---|---|
| Karpas | 51 | 51 | 52 |
| Jurkat | 39 | 38 | ND |
| Namalwa | 49 | 42 | 46 |
| DHL | 55 | 47 | 51 |
| JB | 46 | 36 | 45 |
| KM512 | 60 | 50 | 56 |
| K562 | 60 | 62 | 57 |
| Plasma cell leukemia | 3 | 3 | 3 |
*, Percent of positive cells.
†, Percent of S phase.
Representative data of at least two experiments.
Figure 2.
Correlation among Skp2-, S phase-, and BrdU-positive cells. A: BrdU-labeled K562 cells (top) and a clinical case of plasma cell leukemia (bottom) were stained for anti-Skp2 and anti-BrdU mAbs. DNA content was also calculated, as described in Materials and Methods. Samples were analyzed by flow cytometry and the percentage of Skp2- and BrdU-positive cells was determined. The fraction of cells within the S phase was also itemized. B: The Namalwa cell line (Burkitt’s-derived cell line) was incubated with medium alone or 50 μmol/L of lovastatin for 24 hours. Cells were subsequently stained with anti-Skp2 mAbs of PI and analyzed by flow cytometry. The percentages of cells in S phase and positive for Skp2 were calculated as above. C: Tissue sections from cases of CLL or DLCL were incubated in medium containing BrdU and then processed as described in Materials and Methods. Sections showed a correlation between Skp2-expressing cells (left) and BrdU-positive cells (right). Original magnifications, ×200.
Skp2 Expression in Human Lymphomas
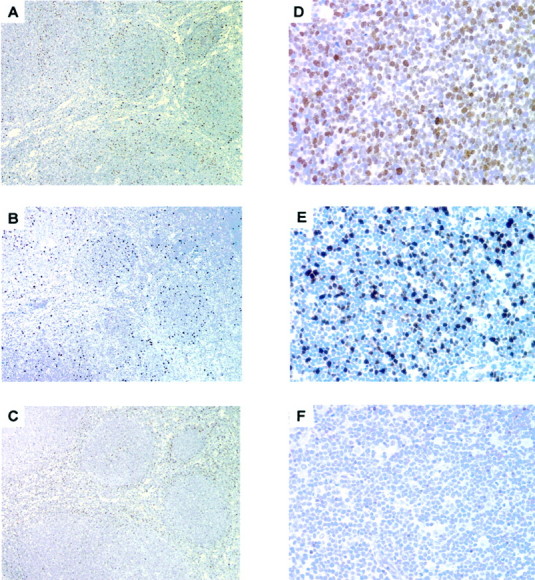
The expression of Skp2 was subsequently evaluated in B-cell NHLs representing all grades of malignancy. In CLLs and Skp2-positive cells were primarily localized within the proliferation centers primarily restricted to para-immunoblasts. (Figure 3B) ▶ . In mucosa-associated lymphoid tissue B-cell lymphomas only 5% of the neoplastic cells were found to be positive for Skp2. Skp2 expression in FL was restricted to a fraction of centroblasts and large centrocytes, but a variable number of interfollicular cells were also found positive. More importantly, the percentage of Skp2-positive cells significantly correlated with the histological grade of these lymphoma (mean percentage: FL grade I, 3.5%; grade II, 10.4%, and grade III, 20.1%). Notably the sole expression of Skp2 statistically correlated with the FL grading obtained using conventional cytological criteria 20 (grade I versus grade II, P = 0.0007; grade II versus grade III, P = 0.0006; grade I versus grade III, P < 0.0001) (Table 2) ▶ . A positive correlation was also found when the percentages of Ki-67-positive cells were correlated to the same groups of FL. In MCLs, Skp2-positive tumor cells were uniformly distributed (Figure 4, B and E) ▶ , with clusters of Skp2-positive cells corresponding to nonneoplastic cells within residual normal germinal centers. As expected, the blastoid and large cell variants of MCL 21 showed the highest percentages of Skp2-positive cells (Figure 4E) ▶ . Interestingly, among high-grade lymphomas, a very high percentage of Skp2-positive cells was found in Burkitt’s lymphomas (87 ± 8.8%) (Figure 5) ▶ . By contrast, in DLCL and Burkitt-like and lymphoblastic lymphomas the percentage of Skp2-positive cells ranged from 10 to 65%. These values were statistically different and we were able to separate these tumors from Burkitt’s lymphomas (P < 0.0001). Finally, when all types of lymphomas were compared, the percentage of Skp2-positive cells strictly correlated with the distinct groups of NHLs being low in low-grade lymphomas and higher in high-grade tumors (P < 0.001) (Table 2) ▶ .
Figure 3.

Skp2 labels proliferating cells in lymphomas. Sections obtained from human lymphomas fixed in formalin were stained with anti-Skp2, anti-Ki-67, and anti-p27 mAbs. Ki-67-positive (A) and Skp2-positive (B) cells reside in the proliferating centers of CLL/SLL, which are mainly p27-negative (C). In DLCL, Ki-67 labeled the majority of large cells (D) in contrast to Skp2 that stained only a fraction (E). The p27-positive cells (F) were almost exclusively limited to small lymphocytes.
Table 2.
Expression of Skp2, Ki-67, p27, p53, and Cyclin E in Different Types of Lymphomas
| Lymphoma subtype | No. of cases | Skp2 (%) | Ki-67 (%) | p27 (%) | Cyclin E (%) |
|---|---|---|---|---|---|
| Small lymphocytic lymphoma | 28 | 2.9 ± 2.8 | 5.5 ± 5.7 | 87.1 ± 11.2 | 7.7 ± 7.3 |
| Marginal zone lymphoma | 26 | 3.4 ± 2.5 | 4.7 ± 2.4 | 89.5 ± 5.2 | 3.6 ± 2.3 |
| Follicular center cell lymphoma | 35 | 12.2 ± 8.6 | 33.1 ± 19.9 | 45.3 ± 25.5 | 8.1 ± 5.7 |
| Grade I | 10 | 3.5 ± 2.4* | 15.8 ± 8.6† | 73.1 ± 12.2 | 3.7 ± 1.5 |
| Grade II | 11 | 10.4 ± 4.8* | 29.1 ± 12.4† | 47.0 ± 13.6 | 6.6 ± 3.2 |
| Grade III | 14 | 20.1 ± 6.8* | 48.6 ± 19.2† | 21.5 ± 18.1 | 13.0 ± 6.7 |
| Mantle cell lymphoma | 34 | 11.4 ± 8.0 | 25.9 ± 16.1 | 26.0 ± 30.4 | 9.9 ± 7.2 |
| Diffuse large-cell lymphoma | 76 | 20.9 ± 15.1 | 63.8 ± 24.7 | 23.3 ± 16.2 | 22.6 ± 12.3 |
| Lymphoblastic lymphoma | 15 | 36.3 ± 11.8 | 67.5 ± 14.8 | 20.0 ± 19.1 | 25.0 ± 9.4 |
| Burkitt-like lymphoma | 5 | 34.0 ± 8.2 | 82.0 ± 16.0 | 47.5 ± 38.4 | 23.7 ± 7.5 |
| Burkitt’s lymphoma | 25 | 87.0 ± 8.8 | 94.5 ± 5.5 | 16.8 ± 22.4 | 33.1 ± 13.8 |
*Statistical significance of Skp2 percentages and grade: grade I versus grade II, P = 0.0007; grade II versus grade III, P = 0.0006; grade I versus grade III, P < 0.0001.
†Statistical significance of Ki-67 percentages and grade: grade I versus grade II, P = 0.01; grade II versus grade III, P = 0.006; grade I versus grade III, P < 0.0001.
Figure 4.
Skp2 and p27 stains are not inversely correlated in MCLs. Serial sections from MCLs were stained with anti-Skp2, anti Ki-67, and anti-p27 mAbs. The fraction of cells stained for Skp2 (B) was low and similar to the percentage of Ki-67-positive cells (A) and not complementary to the fraction of cells stained with p27 (C), thus underlining an absence of inverse correlation. In the blastoid variant of MCL, both stains for Ki-67 (D) and Skp2 (E) were higher than in the classical MCLs and p27 (F) was negative.
Figure 5.
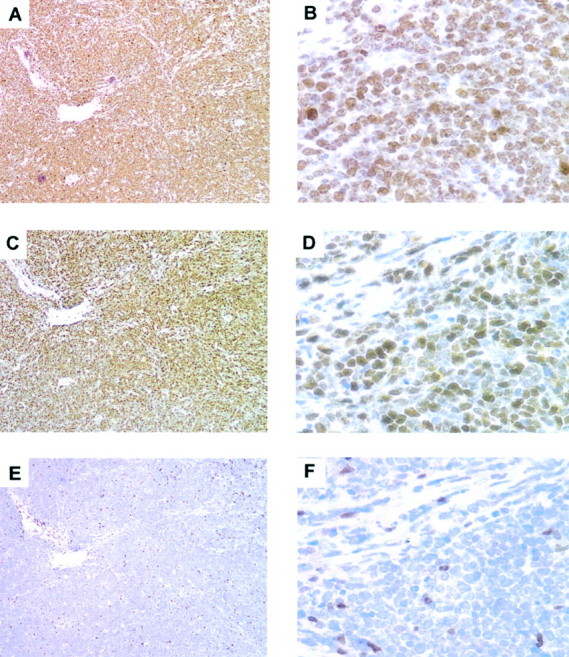
Burkitt’s lymphomas display high expression of Skp2. Serial sections from Burkitt’s lymphomas were stained for Ki-67 (A and B), Skp2 (C and D), and p27 (E and F). High-power magnification (B, D, and F) demonstrates that almost 100% of the cells are Ki-67-positive, and the vast majority are positive for Skp2. p27 was not expressed in the neoplastic cells, rare and few normal cells were p27-positive.
Correlation of Skp2 Expression with Other Cell-Cycle Markers
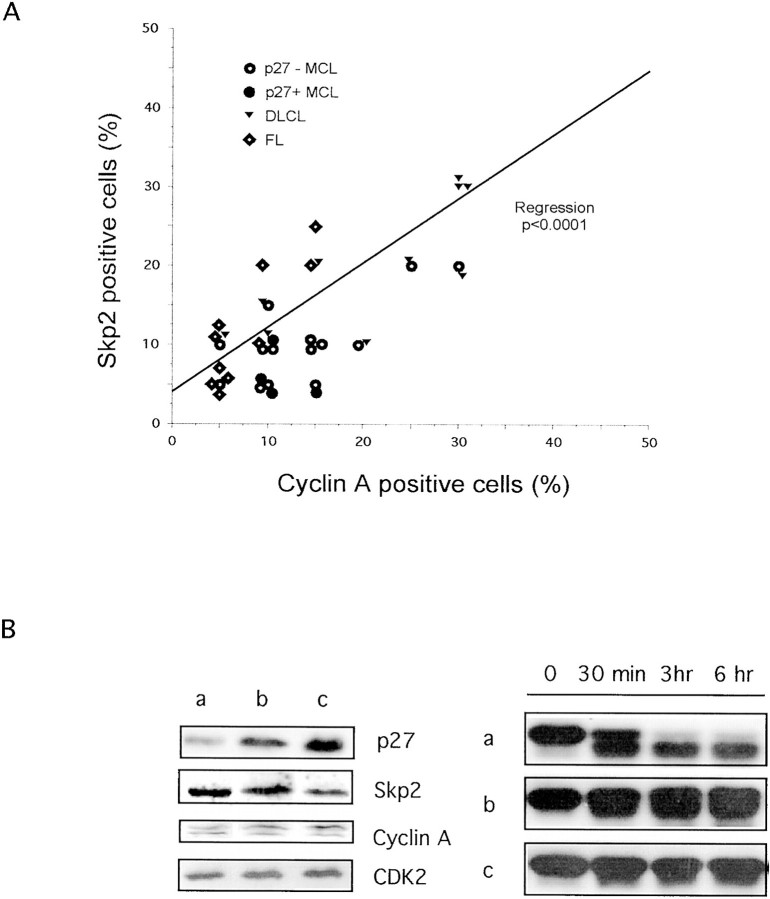
We next compared the expression of Skp2 and Ki-67, the most commonly used proliferation marker, with those of p27, cyclin E, and cyclin A. Skp2 expression showed a statistical correlation with Ki-67 and cyclin E both in low-grade lymphomas, such as CLLs (r = 0.47, P = 0.01), and in high-grade lymphomas such as DLCL (r = 0.63 and r = 0.48, respectively; P < 0.0001). Nevertheless, the percentage of Skp2-positive cells always corresponded to a subset of Ki-67-positive cells. As previously described, in high-grade lymphomas (lymphoblastic lymphoma, DLCL, Burkitt’s and Burkitt-like lymphomas), Ki-67 was expressed by the very large majority of tumor cells (mean percentage ≥64%), whereas the percentage of Skp2-positive cells varied among these categories (Table 2) ▶ . Interestingly, in lymphoblastic lymphomas, DLCL, and Burkitt-like lymphomas anti-Skp2 mAb identified only a fraction (mean percentage ≤36% of the total tumors cells) of the Ki-67-positive cells. Conversely, the percentages of Skp2- and Ki-67-positive cells were very similar in Burkitt’s lymphomas (87% versus 92.6%, respectively). As shown above, the expression of Skp2 in lymphoid cell lines highly correlates with the percentage of cells within the S phase and BrdU-positive cells. To corroborate this finding, we further investigated the relationship between Skp2 expression and the expression of cyclin A. A total of 38 cases, including FL (n = 10), MCL (n = 18), and DLCL (n = 10) lymphomas, were studied. We decided to study the expression of cyclin A because its expression is largely limited to S to G2 phases 22,23 and it correlates with the percentages of S to G2 and BrdU in normal and transformed cells. 24 To identify whether the p27, and cyclin A and Skp2 expression were correlated, we selected p27-negative (n = 14) and p27-positive (n = 4) MCL lymphomas. Using this approach, we demonstrated that Skp2 and cyclin A expression directly correlated in all tumors, including p27-negative lymphomas (Figure 6A) ▶ . Overall, these findings further support the hypothesis that in human lymphomas the percentage of Skp2-positive cells is closely correlated to the fraction of proliferating cells.
Figure 6.
Correlation between cyclin A and Skp2 and p27 in vitro degradation. A: Expression of Skp2 and cyclin A in NHLs. A representative panel of low- and high-grade lymphomas were stained using anti-Skp2 and anti-cyclin A antibodies as described in Material and Methods. Values are reported as percentage of positive tumor cells. Single regression analysis demonstrates a positive correlation between Skp2 and cyclin A expression (P < 0.0001). B: The protein expression profiles of MCLs were determined using Western blot with the indicated antibodies (left). Purified recombinant p27 was incubated in vitro with the tissue extracts of MCLs with low (a), intermediate (b), and high (c) protein levels of p27 for the indicated intervals (right). Levels of recombinant p27 were evaluated by Western blotting using anti-p27 mAb. Tumor cells represented more than 80% of the total cells.
We and others have recently demonstrated that p27 protein expression not only inversely correlates with the grade of lymphomas, but it also has a prognostic significance. 1,3,18 Because in normal cells p27 protein levels are tightly regulated during the cell cycle via Skp2, we investigated whether the protein levels of p27 also inversely correlated with Skp2 in human lymphomas. As anticipated, in a very large majority of lymphomas there was an inverse correlation between p27 and Skp2 (Table 2) ▶ (r = 0.81, P = 0.01). Overall, these findings parallel and expand our previous results obtained from the analysis of Ki-67 and p27 expression in human NHLs. 1 However in MCLs and in a subset of DLCLs and lymphoblastic lymphomas this relationship was not found. In fact, the percentage of p27-negative cells did not always correlate with that of Skp2-positive cells, being MCL mostly negative for p27 expression and showing a relatively low percentage of Skp2-positive cells (Figure 4) ▶ . Moreover, a fraction of cells of DLCLs was negative for both p27 and Skp2 (Figure 3, E and F) ▶ . Because the protein levels of p27 in MCL correlate with the degree of p27 protein degradation in vitro, 1 we compared the expression of p27 and Skp2 and p27 degradation in vitro in a small number of MCLs with high, intermediate, and low levels of p27. As shown in Figure 6 ▶ , the protein levels of Skp2 were quite similar despite the different expression of p27 and they correlated quite closely to those of cyclin A and to the percentage of positive cells stained with anti-cyclin A and anti-Ki-67 antibodies (data not shown). These findings further support the hypothesis that Skp2 is not overexpressed in tumors lacking p27 and that p27 protein levels in these lymphomas may be regulated via alternative mechanisms.
Discussion
In recent years the cell-cycle-related proteins have gained increasing importance in the understanding of the pathogenesis and prognosis of lymphomas. In lymphomas, alterations of the cell cycle are related to the overexpression of molecules driving the progression through the cell cycle (such as cyclin D1 in MCL), and/or to the decreased expression of proteins that inhibit cell-cycle progression (ie, p16INK4 and p27KIP1). Among these inhibitors, increasing importance has been attributed to p27. 1,3,18,25 In quiescent cells the levels of p27 are high, whereas in response to mitogenic stimuli, p27 is phosphorylated in threonine-187 and subsequently degraded via the ubiquitin-proteasome pathway. 13 The F-box protein of the E3 ligase responsible for p27 degradation has been recently demonstrated to be Skp2, 4-7 a protein required for the G1 to S transition in both transformed cells and diploid fibroblasts. 11
In human lymphomas p27 has been shown to inversely correlate with the proliferation index and to have prognostic significance. 1,3 Since p27 degradation in normal cells is dependent on the expression of Skp2 and because the loss of p27 expression in some lymphomas is due to an increased rate of p27 degradation, 1 we tested whether the expression of Skp2 could correlate with the levels of p27 protein in human lymphomas. We analyzed a large series of B-cell lymphomas derived from different B cells frozen at different stages of differentiation and representing unique histological and clinical grades. As hypothesized, we could observe an inverse correlation between p27 and Skp2 in most human lymphomas. Overall, these findings are in agreement with the notion that the protein levels of p27 are—at least in normal cells—primarily regulated by the SCFSkp2 ligase. On the other hand MCLs, in which p27 levels are often undetectable and do not correlate with the cell-cycle progression, 1 showed only a low percentage of Skp2-positive cells. Moreover 30 to 35% of the cells of high-grade lymphomas displayed no staining for both Skp2 and p27. These findings argue in favor of a down-regulation of p27 that is not solely dependent on Skp2. In favor of this hypothesis are data that indicate that other factors might influence p27 protein levels, such as decreased transcription or translation, abnormal localization or sequestration, 13 and cleavage via nonapoptotic caspases. 26 Finally the recent demonstration that p27 can also be degraded via a Skp2-independent pathway sheds new light on the high and complex regulation of this cell-cycle inhibitor. 27
By comparing the expression of Skp2 with that of other cell-cycle proteins, such as Ki-67, cyclin E, and cyclin A, we have also shown that Skp2 directly correlates with the proliferative index in all types of lymphomas. Interestingly, the fraction of cells stained with anti-Skp2 antibody was always lower than the percentage of cells stained using anti-Ki-67 mAb. These findings are in accordance with the different patterns of expression of these proteins during the cell cycle. In cycling cells, in fact, the Ki-67 protein is expressed throughout the cell cycle, ie, from the G1 phase, through the entire S phase, up to the G2 to M phase, 28 whereas Skp2 is expressed only during the late G1 and S phase. 11,29 Thus, Skp2 expression defines a narrower window in the cell-cycle progression compared with that identified by anti-Ki-67 mAbs. This difference may offer a clear advantage in the analysis of neoplastic processes. It is not simple, in fact, to separate lymphoma subtypes or to predict biological evolutions within specific groups considering only the percentage of Ki-67-positive cells. This is particularly true in tumors with high proliferating indexes such as Burkitt’s lymphoma, Burkitt-like lymphomas, and many DLCLs, whose percentages of Ki-67-positive cells are uniformly too high. By contrast, Skp2 determination could provide a real advantage in clinical settings permitting discrimination between Burkitt’s lymphomas (87%), and other high-grade neoplasms (Burkitt-like lymphomas and DLCLs, 34% and 21%, respectively). Nonetheless, the mechanisms responsible for these differences are unclear. Toward this end, we could speculate that the high expression of Skp2 in Burkitt’s lymphomas might result from its deregulated expression or might simply reflect a very fast cell-cycle progression.
The analysis of Skp2 levels may also provide a powerful tool for a more precise grading of follicular cell lymphomas. So far, the grading system of follicular cell lymphomas remains still imprecise and often irreproducible, being dependent on the subjective count of centroblasts or large centrocytes. 19,30 Because Skp2 staining gives the precise measurement of cells committed to proliferating its quantification, particularly by using automatic microscopic scanning, it might offer a more objective grading. A reproducible classification of FL will allow studying the efficacy of conventional and new therapeutic approaches, such as anti-idiotype vaccine strategies in the treatment of lymphomas.
Finally, flow cytometry has also shown that the percentage of Skp2-positive cells matches with the number of cells in S phase. Because Skp2 has a precise concordance with S phase, it could be the best candidate to replace Ki-67, proliferating cell nuclear antigen, and other commonly used markers for the determination of the proliferation index in human tumors. In particular, it could be extremely convenient in the determination of the fraction of proliferating cells in tumors in which BrdU incorporation still has an important prognostic value, such as multiple myeloma. BrdU incorporation analysis, in fact, is technically demanding, time consuming, and limited only to specialized laboratories. The reproducibility and technical simplicity of the flow cytometry and immunohistochemical stains using anti-Skp2 antibodies gives a real edge for the analysis of many human tumors in routine clinical settings.
In conclusion, we have shown that Skp2 is a useful and precise marker to quantify cells committed to or in S phase. Thus, the analysis of Skp2 expression may represent a useful tool with both diagnostic and possibly prognostic applications in lymphomas. Notably, Kudo and colleagues 31 have recently demonstrated that Skp2 expression can be used as a prognostic marker in oral squamous cell carcinomas. We also found that the expression of Skp2 seems not to be deregulated in the large majority of human lymphomas. Nonetheless, more studies are requested to confirm our observations, particularly in lymphomas with very high Skp2 expression, ie, Burkitt’s lymphomas. In fact, because Skp2 overexpression may occur in other neoplasms and high levels of Skp2 seem to play an important role in tumorigenesis 8,9,12,31 a more extensive analysis of Skp2 may be necessary. Finally, the apparent discrepancy between low levels of Skp2 and p27 seen in MCLs suggests that an enhanced degradation of p27 may occur via alternative F-box proteins or additional E3 ligase(s). In fact, recent studies have pointed out that the protein regulation of p27 and other SCF substrates may be achieved by different pathways involving multiple players. 27,32,33 The discovery of the molecular mechanisms regulating the protein expression of p27 in lymphomas might bring new light in the understanding of the pathogenesis of human tumors. Moreover, the recognition of these defects will allow the design of new and more specific approaches for the treatment of human malignancies.
Acknowledgments
We thank E. Zhu and D. Corino for their excellent technical assistance.
Footnotes
Address reprint requests to Dr. Giorgio Inghirami, New York University, Department of Pathology and Kaplan Comprehensive Cancer Center, 550 First Ave., New York, NY 10016. E-mail: inghig01@med.nyu.edu.
Supported by National Institutes of Health grants ROI-CA90773 (to G.I.), ROI-CA76584, and ROI-GM57587 (to M.P.).
R. C. and Y. F. both contributed equally to this work.
R. C. is on leave from the University of Torino, Department of Pathology, Via Santena, 10126, Torino, Italy.
Present address of J. S.: Department of Pediatrics, Boston Children’s Hospital, 300 Longwood Avenue, Boston, MA 02115.
References
- 1.Chiarle R, Budel LM, Skolnik J, Firzzera G, Chilosi M, Corato A, Pizzolo G, Magidson J, Montagnoli A, Pagano M, Maes B, De Wolf-Peeters C, Inghirami G: Increased proteasome degradation of cyclin-dependent kinase inhibitor p27 is associated with a decreased overall survival in mantle cell lymphoma. Blood 2000, 95:619-626 [PubMed] [Google Scholar]
- 2.Sanchez-Beato M, Camacho FI, Martinez-Montero JC, Saez AI, Villuendas R, Sanchez-Verde L, Garcia JF, Piris MA: Anomalous high p27/KIP1 expression in a subset of aggressive B-cell lymphomas is associated with cyclin D3 overexpression. p27/KIP1-cyclin D3 colocalization in tumor cells. Blood 1999, 94:765–772 [PubMed]
- 3.Erlanson M, Portin C, Linderholm B, Lindh J, Roos G, Landberg G: Expression of cyclin E and the cyclin-dependent kinase inhibitor p27 in malignant lymphomas—prognostic implications. Blood 1998, 92:770-777 [PubMed] [Google Scholar]
- 4.Carrano AC, Eytan E, Hershko A, Pagano M: SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol 1999, 1:193-199 [DOI] [PubMed] [Google Scholar]
- 5.Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H: p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol 1999, 9:661-664 [DOI] [PubMed] [Google Scholar]
- 6.Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W: p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol 1999, 1:207-214 [DOI] [PubMed] [Google Scholar]
- 7.Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, Kitagawa M, Hatakeyama S: Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J 2000, 19:2069-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latres E, Chiarle R, Schulman BA, Pavletich NP, Pellicer A, Inghirami G, Pagano M: Role of the F-box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci USA 2001, 98:2515-2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gstaiger M, Jordan R, Lim M, Catzavelos C, Mestan J, Slingerland J, Krek W: Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA 2001, 98:5043-5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kipreos ET, Pagano M: The F-box protein family. Genome Biol 2000, 1:3002.1-3002.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Kobayashi R, Galaktionov K, Beach D: p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell 1995, 82:915-925 [DOI] [PubMed] [Google Scholar]
- 12.Hershko D, Bornstein G, Ben-Izhak O, Carrano A, Pagano M, Krausz MM, Hershko A: Inverse relation between levels of p27(Kip1) and of its ubiquitin ligase subunit Skp2 in colorectal carcinomas. Cancer 2001, 91:1745-1751 [DOI] [PubMed] [Google Scholar]
- 13.Slingerland J, Pagano M: Regulation of the Cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol 2000, 183:10-17 [DOI] [PubMed] [Google Scholar]
- 14.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M: Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 1995, 269:682-685 [DOI] [PubMed] [Google Scholar]
- 15.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M: Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med 1997, 3:231-234 [DOI] [PubMed] [Google Scholar]
- 16.Esposito V, Baldi A, De Luca A, Groger AM, Loda M, Giordano GG, Caputi M, Baldi F, Pagano M, Giordano A: Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res 1997, 57:3381-3385 [PubMed] [Google Scholar]
- 17.Piva R, Cancelli I, Cavalla P, Bortolotto S, Dominguez J, Draetta GF, Schiffer D: Proteasome-dependent degradation of p27/kip1 in gliomas. J Neuropathol Exp Neurol 1999, 58:691-696 [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Beato M, Saez AI, Martinez-Montero JC, Sol Mateo M, Sanchez-Verde L, Villuendas R, Troncone G, Piris MA: Cyclin-dependent kinase inhibitor p27KIP1 in lymphoid tissue: p27KIP1 expression is inversely proportional to the proliferative index. Am J Pathol 1997, 151:151-160 [PMC free article] [PubMed] [Google Scholar]
- 19.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD: The World Health Organization classification of hematological malignancies report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Mod Pathol 2000, 13:193-207 [DOI] [PubMed] [Google Scholar]
- 20.Mann RB, Berard CW: Criteria for the cytologic subclassification of follicular lymphomas: a proposed alternative method. Hematol Oncol 1983, 1:187-192 [DOI] [PubMed] [Google Scholar]
- 21.Zoldan MC, Inghirami G, Masuda Y, Vandekerckhove F, Raphael B, Amorosi E, Hymes K, Frizzera G: Large-cell variants of mantle cell lymphoma: cytologic characteristics and p53 anomalies may predict poor outcome. Br J Haematol 1996, 93:475-486 [DOI] [PubMed] [Google Scholar]
- 22.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G: Cyclin A is required at two points in the human cell cycle. EMBO J 1992, 11:961-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henglein B, Chenivesse X, Wang J, Eick D, Brechot C: Structure and cell cycle-regulated transcription of the human cyclin A gene. Proc Natl Acad Sci USA 1994, 91:5490-5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlandsson F, Linnman C, Ekholm S, Bengtsson E, Zetterberg A: A detailed analysis of cyclin A accumulation at the G(1)/S border in normal and transformed cells. Exp Cell Res 2000, 259:86-95 [DOI] [PubMed] [Google Scholar]
- 25.Quintanilla-Martinez L, Thieblemont C, Fend F, Kumar S, Pinyol M, Campo E, Jaffe ES, Raffeld M: Mantle cell lymphomas lack expression of p27Kip1, a cyclin-dependent kinase inhibitor. Am J Pathol 1998, 153:175-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frost V, Al-Mehairi S, Sinclair AJ: Exploitation of a non-apoptotic caspase to regulate the abundance of the cdkI p27(KIP1) in transformed lymphoid cells. Oncogene 2001, 20:2737-2748 [DOI] [PubMed] [Google Scholar]
- 27.Hara T, Kamura T, Nakayama K, Oshikawa K, Hatakeyama S, Nakayama KI: Degradation of p27Kip1 at the G0–G1 transition mediated by a Skp2-independent ubiquitination pathway. J Biol Chem 2001, 276:48937-48943 [DOI] [PubMed] [Google Scholar]
- 28.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H: Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984, 133:1710-1715 [PubMed] [Google Scholar]
- 29.Carrano AC, Pagano M: Role of the f-box protein skp2 in adhesion-dependent cell cycle progression. J Cell Biol 2001, 153:1381-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin AR, Weisenburger DD, Chan WC, Ruby EI, Anderson JR, Vose JM, Bierman PJ, Bast MA, Daley DT, Armitage JO: Prognostic value of cellular proliferation and histologic grade in follicular lymphoma. Blood 1995, 85:3671-3678 [PubMed] [Google Scholar]
- 31.Kudo Y, Kitajima S, Sato S, Miyauchi M, Ogawa I, Takata T: High expression of S-phase kinase-interacting protein 2, human F-box protein, correlates with poor prognosis in oral squamous cell carcinomas. Cancer Res 2001, 61:7044-7047 [PubMed] [Google Scholar]
- 32.Malek NP, Sundberg H, McGrew S, Nakayama K, Kyriakidis TR, Roberts JM: A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature 2001, 413:323-327 [DOI] [PubMed] [Google Scholar]
- 33.Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, Harper JW, Elledge SJ: Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 2001, 294:173-177 [DOI] [PubMed] [Google Scholar]