Abstract
Chronic rejection is the leading cause of late renal transplant failure. Various structural lesions are observed in grafts undergoing chronic rejection including glomerular basement membrane (GBM) duplications. The well-established Fisher (F344) to Lewis (LEW) rat renal transplant model for chronic rejection was used to assess the presence and role of the humoral immune response against graft antigens during chronic rejection. LEW recipients of F344 allografts develop transplant glomerulopathy and produce IgG1 antibodies directed against F344 GBM preparations that are detectable 3 weeks after transplantation. Glomerular IgG1 deposition was observed that in vitro co-localized with a rabbit anti-rat GBM antiserum in rejecting F344 grafts; elution experiments of isolated glomeruli yielded IgG1 antibodies reactive in vitro with F344 GBM, but not LEW GBM. Prevention of acute rejection by transient treatment of the recipients with cyclosporin A completely abrogated the production of anti-GBM antibodies. Using proteomic techniques we identified the antigens recognized by the LEW posttransplant sera as being the heparan sulfate proteoglycan perlecan and the α1 chain of collagen type VI in association with the α5 chain of collagen type IV. In conclusion, LEW recipients of F344 kidney grafts produce IgG1 antibodies against donor type perlecan and α1(VI)/α5(IV) collagen and develop transplant glomerulopathy. These data implicate an important role for the humoral immune response in the development of glomerulopathy during chronic rejection.
Chronic rejection (CR) is the most prevalent cause of renal transplant failure after the first few posttransplant (Tx) months. Clinically it is characterized by a gradual decline in glomerular filtration rate, usually in conjunction with proteinuria and arterial hypertension. 1 The glomeruli may show a myriad of lesions, including chronic transplant glomerulopathy, which is characterized by duplication of the glomerular basement membrane (GBM) with interposition of electron-lucent material. 2,3 Transplant glomerulopathy is observed in up to 20% of kidney grafts with CR. 4 It has been postulated that CR results from immune reactions of the recipient against yet poorly defined antigens exposed in the graft. 5 Nonimmune factors, such as hypertension or ischemia/reperfusion injury, may lead to unmasking or alteration of graft antigen(s). 1 In syngeneic transplants with comparable degrees of nonimmune injury, CR does not develop within the same time span compared with allogeneic grafts, underlining the importance of immunological mechanisms. 6-8 We hypothesize that immune reactions such as antibody formation after previous damage play a role in the perpetuation of CR in renal allografts. In a mouse model of chronic cardiac graft rejection, antibodies are crucial for disease development. 7 Immunoglobulin heavy chain (IGH) knockout mice that receive a cardiac allograft do not develop CR in contrast to immunoglobulin heavy chain wild-type mice. 7 Moreover, transfer of posttransplantation (Tx) IgG antibodies or antigen-reactive immune serum into transplanted SCID mice results in transplant atherosclerosis. 6,8
A well-established model to study CR in renal allografts is the F344 to LEW rat model. All LEW recipients of F344 grafts develop acute rejection at approximately day 30 resulting in 50% graft loss. The surviving animals show histopathological and functional characteristics of CR from day 50. The reverse combination, ie, LEW kidneys transplanted into F344 rats all exhibit long-term surviving kidney grafts in the absence of histological abnormalities, despite early acute rejection episodes. In this model, antibody responses specific for lymph node-derived lymphocytes have been described. 9 These antibodies disappeared at 8 weeks after Tx and were described to activate neutrophils and resulted in T cell activation. In addition, a humoral immune response against undefined tissue antigens has been reported previously in this model. 10 However, the nature, kinetics, and the specificity of these kidney-specific antibodies has remained elusive.
In chronic cardiac allograft rejection with graft vasculopathy, alloantibodies are mainly directed against the endothelium. In a previous report of chronic renal allograft rejection with transplant glomerulopathy in the rat using the same model, hardly any antibodies against donor endothelial cells were detected. 11
Because the GBM is frequently duplicated in CR, we hypothesize that it may be a target of the humoral immune response in CR. In the present study, we investigated the kinetics and specificity of the anti-GBM antibody response after Tx.
Previous experiments have shown the development of anti-tubular basement membrane (TBM) antibodies after allogeneic kidney transplantation but such anti-TBM antibodies did not result in tissue damage or tubulointerstitial inflammation. 12 In the present study we focus on anti-GBM antibodies generated after Tx, because these antibodies could play a role in the pathogenesis of glomerular lesions.
We observed that anti-GBM antibodies in the LEW recipients of F344 grafts are exclusively of the IgG1 isotype, donor-type GBM specific, and are reactive with F344 GBM preparations after elution from transplanted rat kidneys. Furthermore, proteomics revealed that the antigens recognized by post Tx sera are the heparan sulfate proteoglycan perlecan and the α1 chain of collagen type VI in association with the α5 chain of collagen type IV.
Materials and Methods
Animals
Male inbred Fisher (F344, RT1lv1) and Lewis (LEW, RT1l) rats weighing 250 g were purchased from Harlan, Horst, The Netherlands. Animals had free access to water and standard rat chow. Animal care and experimentation were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Kidney Transplantation
Kidney transplantations were performed under halothane anesthesia as previously described. 10,11 The left kidney of the recipient was removed and a donor kidney was transplanted in the orthotopic position; a patch of the donor aorta and of the inferior vena cava were anastomosed to the recipient aorta and inferior vena cava, respectively. The donor ureter was anastomosed end-to-end to the ureter of the recipient. The remaining native right kidney was removed 7 days after transplantation. Postoperatively, animals received 1 mg/kg body weight of temgesic subcutaneously (buprenorphine-hydrochloride; Schering-Plough B.V., Amstelveen, The Netherlands) for pain relief.
Blood samples were collected weekly by tail vein puncture and sera were stored at −80°C. All rats were housed in metabolic cages once a week to collect urine for assessment of microalbuminuria that was measured on a Hitachi-911 nephelometer (Hitachi, Tokyo, Japan).
LEW rats that had received a F344 kidney graft were sacrificed on days 7 (n = 3), 14 (n = 3), 30 (n = 6), 60 (n = 6), and 90 (n = 6) after transplantation and sera and kidneys were collected. Similarly, F344 rats received a LEW kidney and were sacrificed on days 60 (n = 6) and 100 (n = 2), respectively.
To investigate the effect of acute rejection on antibody formation and development of transplant glomerulopathy three LEW recipients of F344 grafts received low-dose cyclosporine A (CsA) subcutaneously (Sandimmune; Novartis Pharma, Basel, Switzerland, 1.5 mg/kg body weight) 5 days a week for 4 weeks and remained afterward without further treatment until sacrifice on day 100.
Histology
Tissue samples were fixed in methyl Carnoy’s solution, 11 embedded in paraffin, sectioned, and stained with periodic acid-Schiff, hematoxylin and eosin, or trichrome. All kidney sections were scored blindly by a renal pathologist using a semiquantitative scale (0 to 3); mesangiolysis was scored as described previously; 13 and glomerulitis, glomerulosclerosis, and transplant glomerulopathy were scored as described in the Banff working classification. 13,14 Histological changes were compared using the Kruskal-Wallis one-way analysis of variance on ranks using Duna’s comparison between multiple groups. P values <0.05 were considered significant.
Electron Microscopy
Tissue samples were diced into 0.5-mm 3 cubes, fixed in 2% glutaraldehyde, and postfixed by immersion in 2% osmium tetroxide solution. After fixation, tissues were washed in 0.1 mol/L (pH 7.4) sodium cacodylate buffer, dehydrated in graded acetone, and embedded in epoxy resin (epon 812), according to the usual procedure, with polymerization being performed at 60°C. One-μm-thick sections were cut by glass knives on a Reichert-Jung Ultracut-E ultramicrotome and stained with 0.5% toluidine blue solution. Ninety- to 100-nm-thin sections were cut on a Reichert-Jung Ultracut-E ultramicrotome with a Diatome diamond knife, stained with uranylacetate (ultrostain 1 solution; Leica Co., Canada) and lead solution (ultrostain 2, Leica Co.). The sections were viewed under a Hitachi 600 electron microscope at 50 kW.
Direct Immunofluorescence
Kidneys removed at different time points were snap-frozen in precooled isobutanol and stored at −150°C. Cryostat sections of 3 μm were acetone-fixed for 10 minutes at room temperature and stored at −20°C. To detect specific rat immunoglobulin subclasses, monoclonal antibodies specific for rat IgA, IgG1, IgG2a, IgG2b, IgG2c, and IgM (Prof. H. Bazin, Leuven University, Leuven, Belgium) were used. These monoclonals were either directly fluorescein isothiocyanate-conjugated or used in combination with a tyramide-fluorescein isothiocyanate amplification 15 and kidney sections were subsequently embedded in DABCO-glycerol (1,4-diazabicyclo(2,2,2) octane; Sigma Chemical Co,, St. Louis, MO). Double labeling was performed using a rabbit anti-rat GBM antiserum (prepared in our own laboratory 10 ) and bound rabbit antibodies were detected using tetramethyl-rhodamine isothiocyanate-conjugated goat anti-rabbit IgG antibodies (Nordic, Tilburg, The Netherlands).
GBM Isolation
Collagenase-digested GBM preparations of F344 or LEW origin were isolated as described previously. 10 Briefly, glomeruli were isolated by dissecting the renal cortex, followed by homogenization and pressing through a series of sieves with decreasing pore size (150 and 106 nm; glomeruli were harvested on a 75-nm sieve). After sonication, membrane fragments were digested with collagenase (Collagenase Type Ia, Sigma) overnight at 37°C in 100 mmol/L of Tris/HCl (pH 7.4) and10 mmol/L of CaCl2. The proteins in the supernatant after centrifugation were used for enzyme-linked immunosorbent assay (ELISA) and Western blot analysis.
GBM ELISA
Ninety-six well ELISA plates (Greiner, Alphen aan de Rÿn, The Netherlands) were coated overnight with collagenase-digested F344 or LEW GBM preparations (0.3 μg total protein/well) in carbonate buffer (pH 9.6) at room temperature. After blocking with phosphate-buffered saline (PBS)/1% bovine serum albumin (w/v), plates were incubated with serial dilutions of post Tx sera or normal rat sera in PBS/0.05% (v/v) Tween-20/1% bovine serum albumin (w/v) for 1 hour at 37°C. Because more than 95% of rat immunoglobulins have κ light chains, 16 antibody binding was detected using a digoxigenin (DIG)-conjugated (Boehringer-Mannheim, Mannheim, Germany) mouse monoclonal antibody specific for rat κ light chains (His8; Prof. Dr. P. Nieuwenhuis, University of Groningen, Groningen, The Netherlands) for 1 hour at 37°C. After washing, the wells were incubated with horseradish peroxidase-conjugated sheep F(ab′) fragments anti-DIG (Boehringer Mannheim) for another hour at 37°C. Finally, wells were stained with the peroxidase substrate ABTS (2,2′-amino-bis−3-ethylbenzthiazoline-6-sulfonic acid; Sigma) in the presence of H2O2 for 1 hour before the optical density was measured at 415 nm using a Titertek multiscan plate reader. To detect specific rat immunoglobulin subclasses, various monoclonal antibodies were used. As a positive control, purified rat Ig was coated to the plates and stained with these monoclonal antibodies. To test for GBM-binding to ELISA plates, coated wells were incubated with a rabbit anti-rat GBM antiserum.
Elution of Kidney Bound Antibodies
To investigate the specificity of in vivo kidney-bound antibodies, F344 (n = 4) or LEW (n = 3) kidney allografts removed on day 60 were subjected to acid elution. Normal F344 (n = 2) and LEW (n = 2) kidneys were used as controls. Kidneys were perfused with 100 ml of PBS in vivo, removed, and homogenized by pressing pieces of cortex through an 80-mesh sieve generating a sample containing predominantly glomeruli. After sonication, homogenates were incubated for 1 hour at room temperature in citrate buffer (pH 2.5) followed by neutralization with 1 mol/L of NaOH. Samples were tested after overnight dialysis against PBS in the GBM ELISA.
Western Blot Analysis
Collagenase-digested, or nondigested LEW and F344 GBM preparations (6.5 μg total protein) were subjected to 4 to 15% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Criterion Precastgel, Tris-HCL; Bio-Rad Laboratories, Richmond, CA) under reducing or nonreducing conditions, followed by semidry blotting to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA). Blots were blocked for 2 hours in PBS/1% bovine serum albumin (w/v) at room temperature and washed in PBS/0.05% Tween-20. Blots were subsequently incubated with a 1 in 5 dilution of pooled post Tx sera (day 60) in PBS/Tween-20/0.5% bovine serum albumin overnight at 4°C. After washing, blots were incubated with a DIG-conjugated mouse monoclonal antibody against rat IgG1 for 1 hour at room temperature. Subsequently, blots were incubated with horseradish peroxidase-conjugated sheep F(ab′) fragments anti-DIG for another hour at room temperature and after extensive washing bands were visualizedwith DAB (diaminobenzidine hydrochloride, Sigma)/nickel/imidazole. 17
Proteomics
Collagenase-digested F344 GBM preparations were subjected to two-dimensional SDS-PAGE (8%) in duplicate; one of the gels was stained for total protein content using SYPRO Ruby (Bio-Rad Laboratories) whereas the other gel was blotted semidry to polyvinylidene difluoride membranes (as described in “Western Blot Analysis”). Isoelectric focusing was performed on an IPGphor (Amersham Pharmacia Biotech AB, Uppsala, Sweden) using 7-cm Immobiline isoelectric focusing strips of pH range 3 to 10. Before performing the second dimension, strips were treated with dithioerythritol 2,3-dihydroxybutane-1, 4-dithiol (DTE) and iodoacetamide. Spots recognized by the LEW post Tx antibodies were excised from the duplicate gel and digested following the protocol of Shevchenko and colleagues. 18 Identification was performed by on-line nano LC-electrospray mass spectrometry on a Q-TOF (Micromass, Manchester, UK) using a 300-μm ID × 5 mm C18-Pepmap trapping column (LC-Packings, Amsterdam, The Netherlands) for clean-up. Tryptic peptides were step-eluted into the mass spectrometer and subjected to MS/MS and their sequences were determined by interactive use of PeptideSearch 19 and manual interpretation.
Results
Direct Immunofluorescence of F344 Grafts Showed IgG1 Antibodies on the GBM
F344 kidney grafts were stained for the presence of rat immunoglobulins using the different mouse monoclonal antibodies. Kidney grafts removed after 7 days showed no immunoglobulin staining. A prominent rat IgG1 staining was observed in the glomeruli and on the TBM of F344 grafts removed on days 14 and afterwards and was impressively more intense at later time points (Figure 1A) ▶ . As the diffuse glomerular staining did not look like the typical linear pattern of an anti-GBM antibody deposition, the sections were incubated with a rabbit anti-rat GBM antiserum followed by a tetramethyl-rhodamine isothiocyanate-conjugated goat anti-rabbit antiserum (Figure 1B) ▶ . The glomerular staining pattern was very similar to that of the rat IgG1 staining (Figure 1A) ▶ .
Figure 1.
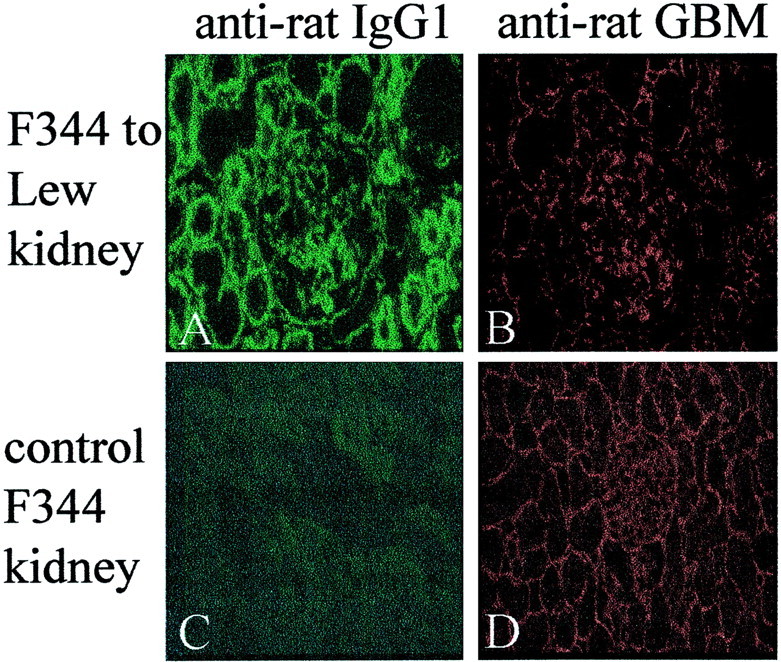
Immunofluorescent staining for in vivo immunoglobulin deposition of antibodies after Tx. A: F344 kidney graft removed on day 60 after Tx from a LEW recipient and stained for rat IgG1. B: Same kidney section (A) incubated with a rabbit anti-rat GBM antiserum and stained for rabbit IgG. C: Normal F344 kidney section stained for rat IgG1. The staining is similar to LEW kidney grafts removed from F344 recipients. D: Normal F344 kidney section incubated with rabbit anti-rat GBM antiserum and stained for rabbit IgG. Original magnifications, ×250.
Rat IgG2a was only observed on the TBM, not in glomeruli, of rejecting F344 kidneys, whereas trace amounts of rat IgM were only observed in glomeruli of rejecting F344 kidney grafts. Stainings for rat IgA, IgG2b, and IgG2c were completely negative in all kidney compartments. The LEW grafts removed from F344 recipients and normal F344 kidneys did not show any staining using the same panel of monoclonal antibodies (Figure 1C) ▶ . Staining of these kidneys using the polyclonal rabbit anti-rat GBM antiserum resulted in a linear, more intense GBM-like staining pattern (Figure 1D) ▶ .
LEW Recipients of F344 Grafts Develop Microalbuminuria
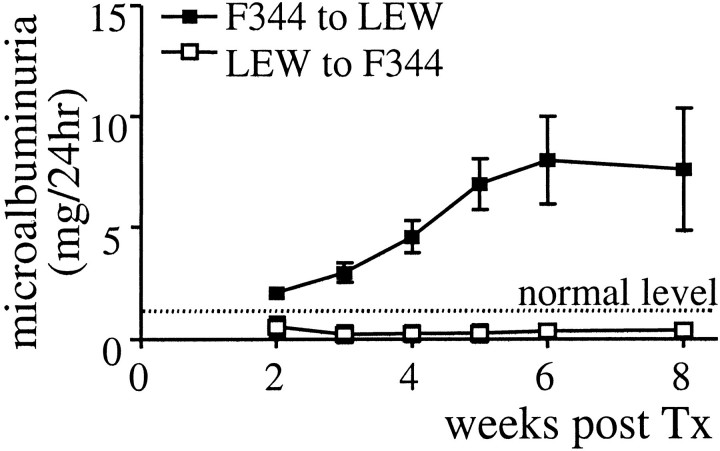
LEW recipients of F344 grafts excreted on average 8 mg/24 hours of albumin in their urine from 3 to 4 weeks after Tx (Figure 2) ▶ that persisted during the observed time period, whereas nonrejecting F344 recipients of LEW grafts excreted less than 1 mg/24 hours, comparable to normal LEW or F344 rats. In urine of LEW recipients of F344 grafts we were able to detect rat IgG from 5 weeks after Tx (data not shown).
Figure 2.
Microalbuminuria measured in weekly collected urine samples of LEW recipients of F344 grafts (open squares) and F344 recipients of LEW kidney grafts (closed squares). Protein excretion is measured using a nephelometer and expressed as the mean (±SEM) of six (F344 to LEW) or three (LEW to F344) rats.
Histological Analysis of F344 Kidney Grafts Removed from LEW Recipients Showed Lesions Characteristic of CR and Transplant Glomerulopathy
F344 kidneys transplanted into non-immunosuppressed LEW recipients showed changes characteristic of acute rejection that evolved throughout time to CR. Seven- and 14-day grafts contained prominent mononuclear cell infiltrates in the interstitium and within dilated peritubular capillaries. In 30-day grafts significant interstitial inflammation, mild tubulitis, and vasculitis were observed. Sixty- and 90-day grafts revealed a progressive increase in interstitial fibrosis, tubular atrophy, and glomerulosclerosis, as in previous experiments 14 (data not shown). A significant glomerulitis was present at 7, 14, and 60 days in F344 allografts removed from LEW recipients but decreased with time to levels comparable to LEW kidneys removed from F344 recipients (Figure 3A ▶ , P < 0.05). Grafts removed on days 30 and 60 exhibited pronounced mesangiolysis (Figure 3A ▶ , P < 0.05). Glomerulosclerosis increased with time after transplantation and involved on average 25 to 50% of glomeruli on day 90 (Figure 3A ▶ , P < 0.05). LEW kidneys removed from F344 recipients showed only mild glomerulitis, tubulitis, and interstitial inflammation but did not develop transplant glomerulopathy.
Figure 3.
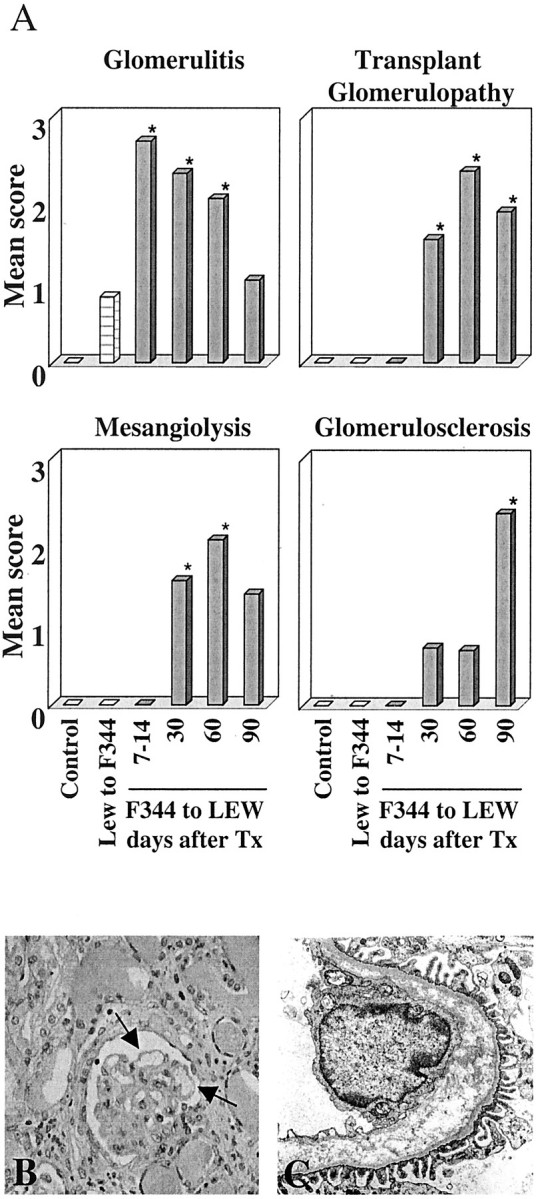
Histological scores in F344 kidney grafts removed from LEW recipients at various time points after Tx. A: Quantification of glomerular changes in F344 (black bars) and LEW (hatched bars) kidney grafts, compared to normal kidneys (open bars). All glomerular alterations were significantly different between rejecting F344 kidneys and normal F344 or LEW rat kidneys (*, P < 0.05). B: Periodic acid-Schiff staining of a F344 renal allograft removed from a LEW recipient 30 days after Tx (original magnification, ×200). Arrows indicate local GBM duplication. C: Electron micrograph of a F344 renal allograft removed from a LEW recipient 30 days after Tx (original magnification, ×8000).
Extensive duplication of the GBM, characteristic of transplant glomerulopathy was seen in 30-, 60-, and 90-day grafts (Figure 3, B and C) ▶ . Duplication of the GBM, with interposition of electron-lucent material (Figure 3C) ▶ was evident from ultrastructural examination of F344 grafts removed from LEW recipients 30 days after Tx; the morphology of podocytes and endothelium appeared normal.
IgG1 Anti-GBM Antibodies in Sera of LEW Recipients of F344 Kidney Grafts
Because rat IgG1 antibodies were bound to the glomeruli of rejecting kidneys and the GBM appeared severely damaged by ultrastructural examination we investigated if an IgG1 antibody response against the GBM was present in the LEW recipients of F344 grafts. To test for anti-GBM reactivity in serum of transplanted rats an anti-GBM ELISA was developed. In this ELISA, we coated collagenase-digested F344 or LEW GBM to the plates, incubated the wells with post Tx sera and subsequently stained for immunoglobulin binding. Serum samples collected weekly after Tx were tested. LEW recipient rats produced variable amounts of antibodies that were strongly reactive with F344 GBM from 3 weeks after Tx up to 100 days (Figure 4A) ▶ . The antibodies reactive with F344 GBM were of the IgG1 isotype. These IgG1 antibodies were specific for F344 GBM, and were not reactive with LEW GBM (Figure 4B) ▶ . When the ELISA was performed using κ light chain-specific antibodies similar results were obtained (data not shown). The reverse combination, F344 recipients of LEW grafts, did not produce detectable IgG1 (Figure 4, A and B) ▶ or other κ light chain containing antibodies that were reactive with F344 or LEW GBM (not shown).
Figure 4.
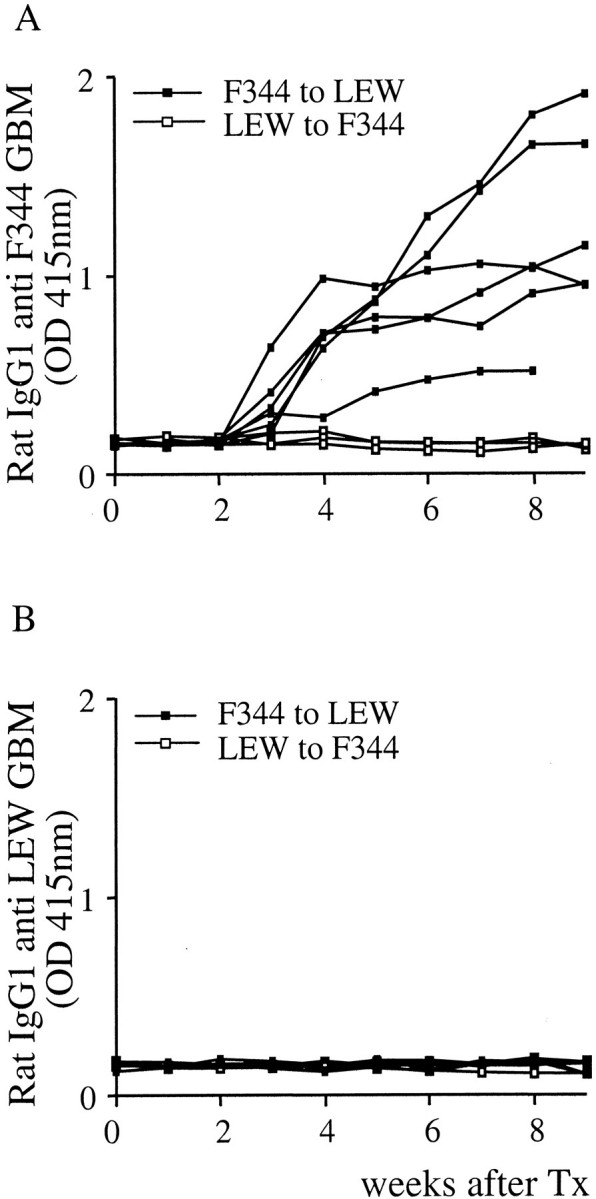
Antibodies against GBM in sera after Tx as detected by ELISA. Coated collagenase-digested GBM preparations were incubated with 1/25 dilutions of sera of LEW recipients of a F344 graft (closed squares, n = 6) or F344 recipients of LEW grafts (open squares, n = 3) and stained with a mouse monoclonal antibody specific for rat IgG1.
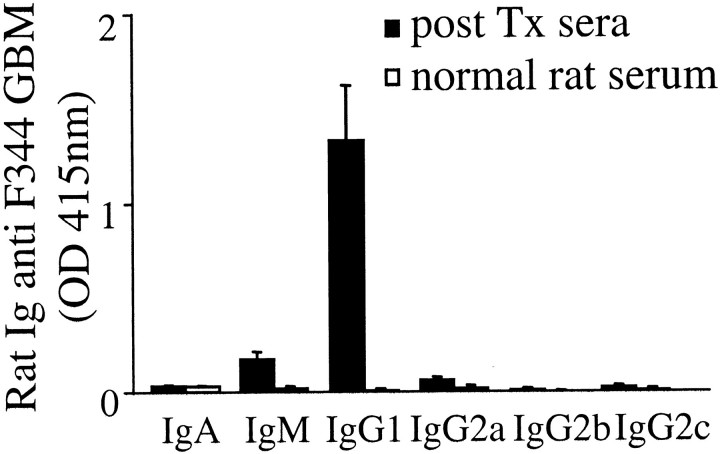
To investigate whether other immunoglobulin isotypes were also reactive with GBM preparations, binding of various rat isotypes in the GBM ELISA was tested. The ELISA showed that antibodies reactive with F344 GBM in pooled post Tx LEW sera collected at day 60 were predominantly of the IgG1 isotype (Figure 5) ▶ . Only a marginal, but nonsignificant, binding of IgM antibodies to F344 GBM was observed. Normal LEW serum did not contain antibodies reactive with either F344 or LEW GBM.
Figure 5.
Identification of isotype of rat anti-GBM antibodies in serum after Tx. Collagenase-digested F344 GBM preparations were coated in an ELISA plate, incubated with sera after Tx (1/25 dilution, closed bars) or normal LEW sera (open bars) and stained for immunoglobulin (sub)classes (mean of three sera ±SEM).
We also tested our collagenase-digested GBM preparations for MHC class I content using the OX-18 monoclonal antibody (gift of Dr. PJK Kuppen, Leiden University Medical Center, Leiden, The Netherlands), for mesangial cell contamination using the monoclonal IgG2a anti-Thy1.1 antibody (ER4G 15 ) and for podocyte contamination using a rabbit anti-rat podocyte antibody (gift of Dr. T. Palmen, University of Helsinki, Helsinki, Finland). No MHC class I, Thy1.1, or podocyte reactivity was detected in the GBM ELISA.
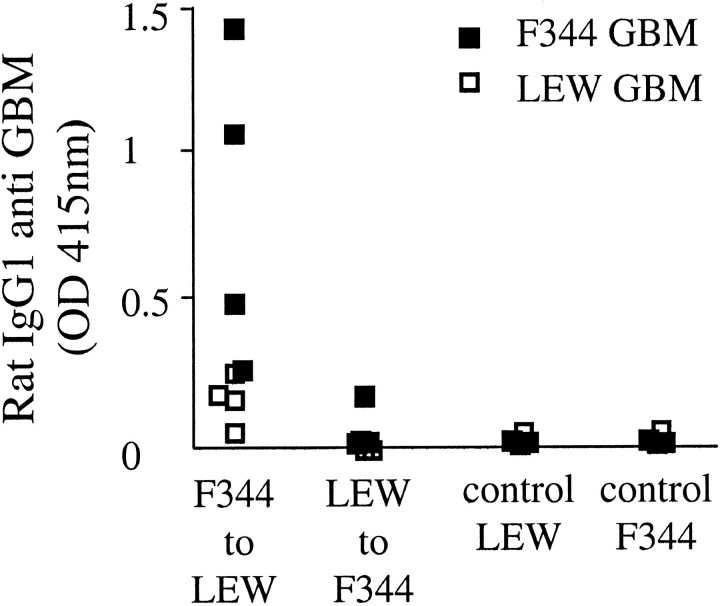
Elution of Kidney-Bound Antibodies Yielded IgG1 Antibodies Reactive with F344 GBM Preparations
To further confirm that IgG1 antibodies deposited in glomeruli of chronically rejecting F344 kidneys were indeed reactive to the GBM, isolated glomeruli were subjected to acid elution. Only eluates prepared from PBS-perfused Tx F344 kidneys contained IgG1 antibodies that bound in the ELISA coated with F344 GBM only (Figure 6) ▶ . In contrast, eluates obtained from LEW kidneys removed from F344 recipients and eluates harvested from normal F344 and LEW kidneys did not contain any detectable anti-GBM antibodies. Finally, antibodies eluted from rejecting F344 grafts reacted only with F344 GBM preparations and not with LEW GBM (Figure 6) ▶ . Eluates were negative for IgM and IgG2a binding.
Figure 6.
Eluates prepared from F344 kidney grafts bind to F344 GBM only. Eluates were prepared from F334 or LEW kidney grafts removed after day 60. Eluates were tested in the GBM-ELISA using collagenase-digested F344 (closed squares) or LEW GBM preparations (open squares) and stained for IgG1 binding.
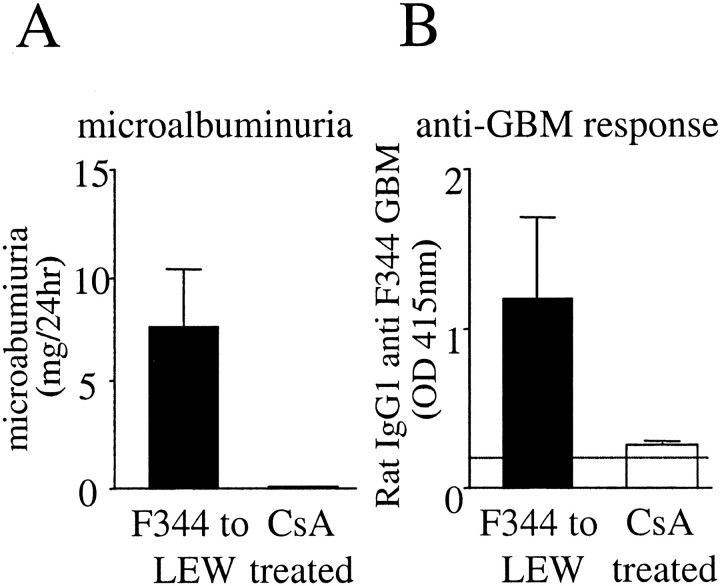
Treatment with CsA Abolished Antibody Production and Disease Development
LEW recipients of F344 grafts treated with low-dose CsA to prevent acute rejection did not produce microalbuminuria (<1 mg/24 hours; Figure 7A ▶ ) and the histology of these kidneys showed only mild interstitial inflammation. CsA treatment abolished production of kidney-bound (data not shown) or circulating (Figure 7B) ▶ anti-GBM antibodies, whereas the total amount of circulating antibodies is comparable to normal rats (data not shown).
Figure 7.
Comparison of nontreated and CsA-treated LEW recipients of F344 grafts for microalbuminuria and anti-GBM antibody production. A: Microalbuminuria (mean ± SEM) at day 60 after Tx in urine of nontreated and of LEW recipients of F344 grafts (closed bar, n = 6) or of LEW rats treated with CsA (open bar, n = 3). B: Measurement of anti-GBM antibody levels in sera of nontreated LEW recipients of F344 grafts (closed bar, n = 6) and in sera of CsA-treated LEW recipients (open bar, n = 3) using the GBM-ELISA with F344 GBM as coating (mean ± SD).
Western Blot Analysis Resulted in Specific Antigen Recognition after Incubation with LEW Sera after Tx
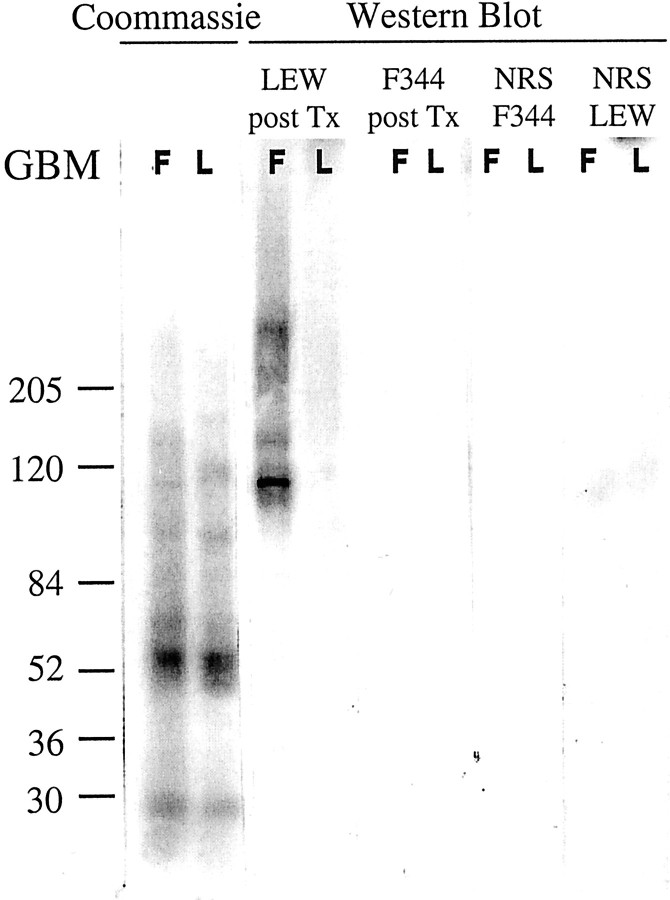
To further analyze the F344 GBM antigens recognized by LEW IgG1 antibodies, Western blot analysis was performed using collagenase-digested GBM preparations. When the collagenase-digested GBM preparations were electrophoresed under nonreducing conditions, F344 and LEW GBM showed similar protein bands in the Coomassie-stained blot (Figure 8) ▶ . After blotting and incubation with LEW anti-F344 sera after Tx (day 60) several bands were observed in F344, but not in LEW GBM (Figure 8) ▶ . These bands were not detected using control F344 anti-LEW sera after Tx or using normal LEW or F344 rat sera. The proteins recognized in collagenase-digested F344 GBM preparations by posttransplant LEW sera are ∼120, 165, and ≫200 kd.
Figure 8.
Western blot analysis of collagenase-digested F344 and LEW GBM (on 4 to 15% gradient SDS-PAGE under nonreducing conditions) incubated with pooled LEW sera after Tx and stained for rat IgG1 antibodies. Total protein content on the blots was visualized using Coomassie blot staining.
Proteomics Identified the Heparan Sulfate Proteoglycan Perlecan and the α1 Chain of Collagen Type VI Together with the α5 Chain of Collagen Type IV
Analysis of collagenase-digested F344 GBM preparations on two-dimensional SDS-PAGE resulted in a strongly reactive spot of ∼120 kd (Figure 9A ▶ , spot 1) containing the peptides: VDSYGGFLR, GMVFGIPDGVLELVPQR, and LSFDQPSDFK that were unique for perlecan. A weak spot of ∼40 kd (Figure 9A ▶ , spot 2)contained the peptides: VPNYQALLR, VAVVQYSGQGQQQPGR, and GVLYQTVSR unique for the α1 chain of collagen type VI and peptide GQSIQPFISR unique for the α5 chain of collagen type IV. Thus, one spot contained the heparan sulfate proteoglycan (HSPG) perlecan and the other contained peptides corresponding to both collagen α1 type VI and α5type IV.
Figure 9.
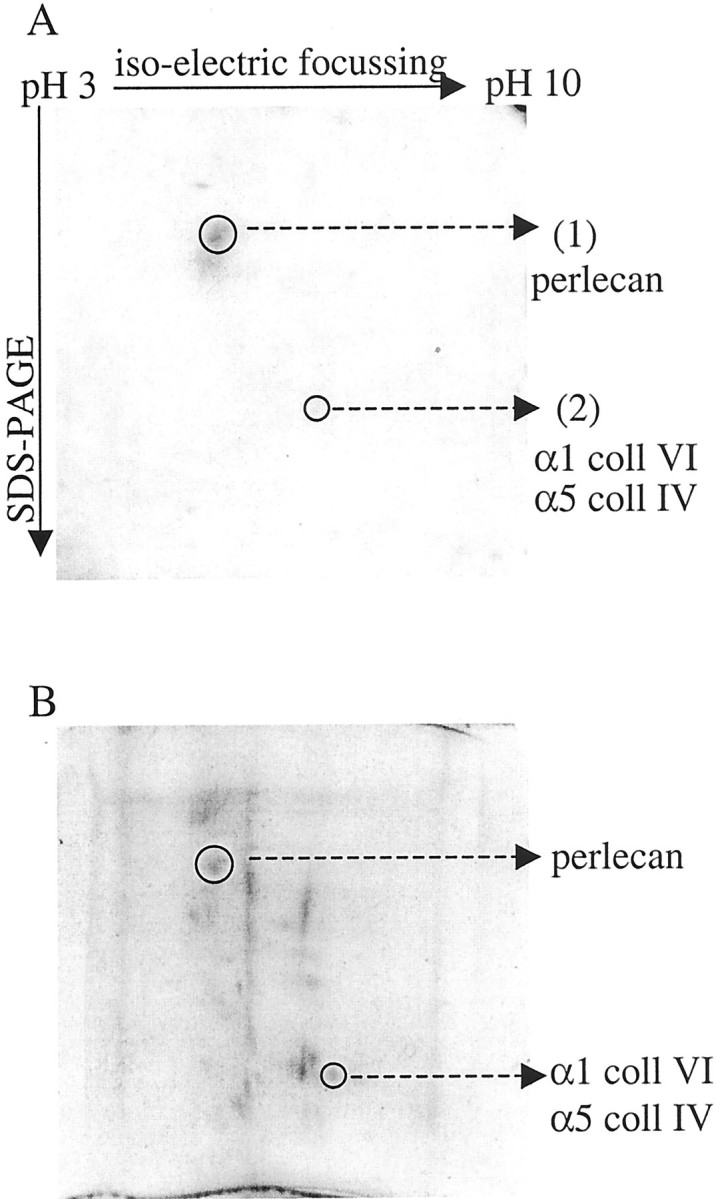
Proteomics. A: Western blot of two-dimensional gel electrophoresis of collagenase-digested F344 GBM, incubated with LEW sera after Tx and stained for rat IgG1, resulting in a strong immunoreactive spot containing peptides that were resolved by MS/MS. B: SYPRO Ruby stain of two-dimensional gel containing collagenase-digested F344 GBM showing the total amount of protein present on the gel.
Discussion
Upon transplantation of a F344 kidney graft into a LEW recipient microalbuminuria develops (>8 mg/24 hours) starting at 3 weeks after Tx. Histopathological changes characteristic of CR are present in these grafts including vascular fibrointimal thickening, interstitial fibrosis, tubular atrophy, transplant glomerulopathy with characteristic GBM duplication, mesangiolysis, and glomerulosclerosis. Using this model, we demonstrated that IgG1 antibodies directed against the HSPG perlecan and the α1 chain of collagen type VI in association with the α5 chain of collagen type IV are deposited along the GBM of F344 kidney allografts. Transient treatment with CsA prevented both acute and CR as well as IgG1 anti-GBM antibody production by LEW recipients.
In the F344 to LEW renal allograft model proteinuria is present, one of the characteristics of CR. This urinary protein leakage is considered to be of glomerular origin, because we detected large molecular weight proteins (ie, IgG) in urine (data not shown), suggesting that the glomerular filtration barrier is damaged. Electron microscopic analysis of F344 kidney grafts revealed an intact podocyte architecture, but a duplicated GBM. This suggests indeed that the GBM is one of the main structural targets of the immune response during CR.
Transplant glomerulopathy develops in ∼5 to 10% of human renal transplants, in ∼20% of patients with CR and is associated with proteinuria and poor graft survival. 3,4 Its pathogenesis is unknown, but it is thought to be related to glomerular endothelial cell injury, as described for transplant glomerulitis and antibody-mediated rejection. 20-22 However, by electron microscopy the endothelium does not always appear to be damaged, as is the case in our rat renal allograft model.
Perlecan is essential for maintaining the integrity of basement membranes (BM). 23 Injection of rats with monoclonal antibodies against HSPG resulted in proteinuria and BM thickening, 24,25 suggesting that antibodies against the HSPG perlecan may contribute to the proteinuria observed in our model for CR. BM alterations, duplications, and abnormal matrix depositions including collagen type VI and HSPG have been associated with various (chronic) human diseases involving BM changes and proteinuria. 26 Increased expression of perlecan has been described in various kidney diseases including diabetic nephropathy, 27,28 membranous nephropathy, 29,30 minimal change nephrotic syndrome, 31 and diffuse mesangial sclerosis. 32 In addition, accumulation of collagen VI has also been described in various diseases including diabetic nephropathy, 33 diffuse mesangial sclerosis, 32 and membranous glomerulonephritis. 34
Perlecan and α1(VI)/α5(IV) collagen are both located on the endothelial side of the GBM, limited to focal accumulations, 31 and to the mesangial matrix. 31,35-37 In addition, type VI collagen is produced by endothelial cells. 38 Together this suggests that initial antibody-mediated GBM damage can occur independent of endothelial cell injury, but may in the long term affect endothelial cell architecture and/or function as observed in patient specimens. 39
In the F344 kidney grafts, IgG1 staining along the GBM appeared nonlinear in contrast to classical anti-GBM antibody disease in which anti-GBM antibody deposits are linear. Our observed nonlinear staining pattern may be explained by limited focal accumulations of perlecan and α1(VI)/α5(IV) collagen. Staining kidney sections with an antibody directed against the heparan sulfate side chain of HSPG has previously also resulted in a nonlinear pattern, again supporting focal accumulation sites of perlecan. 40 Furthermore, staining for collagen type IV would normally also result in a linear GBM staining, in contrast to the F344 kidney allografts exhibiting a nonlinear staining. However, in the proteomics experiment we found collagen type IV in association with collagen type VI, suggesting that the LEW antibodies are directed against the associated proteins rather than the individual proteins, thereby possibly explaining the observed localization.
A strong interaction of the α1 chain of collagen VI with the carboxyl terminal globular domain of type IV collagen has been described 36 and it has been suggested that this collagen interaction anchors endothelial BMs to the extracellular matrix. The identification of both α1(VI) and α5(IV) collagen in one spot of the two-dimensional gel suggests the presence of an interaction between those two collagen molecules in renal BMs. The size of the bands on SDS-PAGE for both collagen and perlecan is smaller than expected on basis of molecular weights described in literature being 150 to 160 and 467 kd, respectively. However, we have used GBM preparations obtained after various isolation steps, including sonification, which might contribute to partial degradation of the large extracellular matrix proteins. It has been reported that perlecan is fragmented during isolation into smaller proteolytic fragments with different sizes, including fragments of 95 to 130, 150, and 250 kd. 41 The peptide sequences obtained by proteomics are all derived from domains II and III of the perlecan protein, suggesting that only a small part of the perlecan molecule was represented in the identified protein spot.
Antibody deposition along the TBM, 42 the GBM, 43 and in glomeruli 44,45 have been observed in human renal allografts, but so far no follow-up information is available on clinical outcome. In our model, IgG1 and IgG2a antibody binding to TBM antigens was observed in combination with glomerular IgG1 deposition. We cannot exclude that sharing of antigens between TBM and GBM 30 causing IgG1 deposits in both compartments. Perlecan and collagen VI are known to be expressed in most renal BMs, including the TBM. 30,35,46
The induction of antibodies against perlecan or α1(VI)/α5(IV) collagen might be the result of the formation of new epitopes exposed in the GBM after injury. Alternatively, antibodies might be induced as a result of (genetic) differences in perlecan or α1(VI)/α5(IV) collagen between donor and recipient. Alternative splicing of human collagen type VI has been reported 47 and restriction fragment polymorphisms are described for perlecan. 48,49 Finally, heparan sulfate side chains of perlecan might be altered by transplantation-related processes, such as ischemia/reperfusion injury resulting in generation of reactive oxygen species. 27 The question remains unanswered as to what the difference is between the molecules of the donor and recipient that ultimately result in antibody production. Once antibodies are formed they may play a role in maintaining the GBM abnormalities and thereby result in urinary protein loss. Binding of antibodies results in activation of the complement system and may subsequently induce the release of inflammatory mediators and recruitment of inflammatory cells leading to graft rejection. In the F344 to LEW renal allograft model we were able to detect C3 and C5b-9 in the glomeruli and on the TBM (data not shown) of rejecting F344 grafts removed from LEW recipients, indicating that the complement activation cascade is involved.
LEW recipients of F344 grafts were transiently treated with low-dose CsA to investigate the requirement of acute rejection episodes for antibody production and disease development. The total amount of antibodies in serum was comparable to normal rat serum. Because the CsA-treated rats did not develop disease, whereas all other LEW recipients of F344 grafts did, we hypothesize that acute rejection is the initial event and is a prerequisite for CR. Alternatively or in addition, CsA treatment might have induced tolerance for the GBM antigens. LEW post Tx sera bound to the GBM and TBM of normal F344 kidney sections in vitro (data not shown). In addition, preliminary data show that LEW post Tx antibodies bind to kidneys of unilaterally nephrectomized F344 rats on intra-arterial injection, suggesting that the epitopes of perlecan and collagen are not induced as a consequence of CR-mediated changes. 11 Therefore, we hypothesize that the local intragraft immune activation status of the recipient, which is associated with acute rejection, is required for production of IgG1 antibodies directed against the GBM. This antibody response could then conceivably contribute to the pathogenesis of CR. Thus, LEW recipients of F344 kidney allografts produce antibodies against the novel epitope whereas F344 recipients that encounter LEW antigens do not produce antibodies. It is not known what differences exist between F344 and LEW recipients, but there might be a difference in cytokine production during the initial phase of rejection that results in antibody production only in LEW recipients. This cytokine production pattern might be altered after CsA treatment abolishing formation of damaging antibodies recognizing graft antigens.
Th2 cytokines are known to skew the humoral response into production of IgG1 antibodies. 5 This would be consistent with the hypothesis that activated CD4+ Th2 cells late after transplantation play the most critical role in the initiation and/or maintenance of chronic allograft rejection. 5 Type 2 cytokines stimulate antibody production, are associated with the regulation of some of the effector mechanisms of CR, and may influence the disease process directly through the regulation of matrix metabolism involved in tissue restructuring. 50
In conclusion, we found circulating and kidney graft-bound IgG1 antibodies against the GBM in LEW recipients of F344 grafts undergoing CR, recognizing perlecan and α1(VI)/α5(IV) collagen of the GBM. The F344 grafts showed histological signs of transplant glomerulopathy, including the characteristic BM duplications. Long-term surviving LEW grafts in F344 recipients or F344 grafts in LEW recipients treated with low-dose CsA showed no signs of CR and no production of anti-GBM antibodies was detected.
We conclude that IgG1 antibodies recognizing perlecan and α1(VI)/α5(IV) collagen play a crucial role in the pathogenesis of transplant glomerulopathy observed during CR in rats.
Acknowledgments
We thank Allan Thompson and Arnoud H. de Ru for technical assistance and Prof. Dr. M. R. Daha and Dr. A. Roos for critical reading of the manuscript.
Footnotes
Address reprint requests to S. A. Joosten, M.Sc., Department of Nephrology, C3P, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands. E-mail: s.a.joosten@lumc.nl.
Supported by a grant from the Dutch Kidney Foundation (grant no. C98.1783).
References
- 1.Paul LC: Chronic renal transplant loss. Kidney Int 1995, 47:1491-1499 [DOI] [PubMed] [Google Scholar]
- 2.Yilmaz A, Yilmaz S, Kallio E, Rapola J, Häyry P: Evolution of glomerular basement membrane changes in chronic rejection. Transplantation 1995, 60:1314-1322 [PubMed] [Google Scholar]
- 3.Shu KH, Lu YS, Chang CH, Yang CR, Chan LP, Cheng CH, Sheu SS, Hsu JH, Lian JD: Transplant glomerulopathy—a clinicopathological study. Transplant Proc 1996, 28:1527-1528 [PubMed] [Google Scholar]
- 4.Kupin W, Nakhleh R, Lee M, Venkat KK, Goggins M, Mozes M, Escobar F, Abouljoud M: Separate risk factors for the development of transplant glomerulopathy vs chronic tubulointerstitial rejection. Transplant Proc 1997, 29:245-246 [DOI] [PubMed] [Google Scholar]
- 5.Shirwan H: Chronic allograft rejection. Do the Th2 cells preferentially induced by indirect alloantigen recognition play a dominant role? Transplantation 1999, 68:715-726 [DOI] [PubMed] [Google Scholar]
- 6.Waaga AM, Gasser M, Laskowski I, Tilney NL: Mechanisms of chronic rejection. Curr Opin Immunol 2000, 12:517-521 [DOI] [PubMed] [Google Scholar]
- 7.Hancock WW, Buelow R, Sayegh MH, Turka LA: Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med 1998, 4:1392-1396 [DOI] [PubMed] [Google Scholar]
- 8.Cramer DV, Shirwan H: The importance of humoral immune responses in chronic rejection. Transplant Rev 1998, 12:166-176 [Google Scholar]
- 9.Hancock WW, Whitley WD, Baldwin III WM, Tilney NL: Cells, cytokines, adhesion molecules, and humoral responses in a rat model of chronic renal allograft rejection. Transplant Proc 1992, 24:2315–2316 [PubMed]
- 10.De Heer E, Davidoff A, Van der Wal A, Van Geest M, Paul LC: Chronic renal allograft rejection in the rat. Transplantation-induced antibodies against basement membrane antigens. Lab Invest 1994, 70:494-502 [PubMed] [Google Scholar]
- 11.Paul LC, Muralidharan J, Muzaffar SA, Manting EH, Valentin JF, De Heer E, Kashgarian M: Antibodies against mesangial cells and their secretory products in chronic renal allograft rejection in the rat. Am J Pathol 1998, 152:1209-1223 [PMC free article] [PubMed] [Google Scholar]
- 12.Hart DN, Fabre JW: Kidney-specific alloantigen system in the rat. Characterization and role in transplantation. J Exp Med 1980, 151:651-666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Häyry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 1999, 55:713-723 [DOI] [PubMed] [Google Scholar]
- 14.Benediktsson H, Chea R, Davidoff A, Paul LC: Antihypertensive drug treatment in chronic renal allograft rejection in the rat. Effect on structure and function. Transplantation 1996, 62:1634-1642 [DOI] [PubMed] [Google Scholar]
- 15.Van Dixhoorn MG, Sato T, Muizert Y, Van Gijlswijk-Janssen DJ, De Heer E, Daha MR: Combined administration of IgA and IgG anti-Thy-1 antibodies enhances renal inflammation in rats. Kidney Int 1999, 55:2299-2309 [DOI] [PubMed] [Google Scholar]
- 16.Zhou D, Leslie GA, Guo K, Gutman GA: Expression of immunoglobulin lambda chains in the laboratory rat. J Immunogenet 1986, 13:299-307 [DOI] [PubMed] [Google Scholar]
- 17.Harlow E, Lane D: Immunoblotting-DAB staining. Harlow E Lane D eds. Antibodies, A Laboratory Manual. 1997, :p 508 Cold Spring Harbor Laboratory, Cold Spring Harbor [Google Scholar]
- 18.Shevchenko A, Wilm M, Vorm O, Mann M: Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 1996, 68:850-858 [DOI] [PubMed] [Google Scholar]
- 19.Mann M, Wilm M: Error-tolerant identification of peptides in sequence databases by peptide sequence tags. Anal Chem 1994, 66:4390-4399 [DOI] [PubMed] [Google Scholar]
- 20.Suri DL, Tomlanovich SJ, Olson JL, Meyer TW: Transplant glomerulopathy as a cause of late graft loss. Am J Kidney Dis 2000, 35:674-680 [DOI] [PubMed] [Google Scholar]
- 21.Briner J: Transplant glomerulopathy. Appl Pathol 1987, 5:82-87 [PubMed] [Google Scholar]
- 22.Maryniak RK, First MR, Weiss MA: Transplant glomerulopathy: evolution of morphologically distinct changes. Kidney Int 1985, 27:799-806 [DOI] [PubMed] [Google Scholar]
- 23.Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fassler R: Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol 1999, 147:1109-1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miettinen A, Stow JL, Mentone S, Farquhar MG: Antibodies to basement membrane heparan sulfate proteoglycans bind to the laminae rarae of the glomerular basement membrane (GBM) and induce subepithelial GBM thickening. J Exp Med 1986, 163:1064-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van den Born J, Van den Heuvel LP, Bakker MA, Veerkamp JH, Assmann KJ, Berden JH: A monoclonal antibody against GBM heparan sulfate induces an acute selective proteinuria in rats. Kidney Int 1992, 41:115-123 [DOI] [PubMed] [Google Scholar]
- 26.Vleming LJ, Baelde JJ, Westendorp RG, Daha MR, Van Es LA, Bruijn JA: Progression of chronic renal disease in humans is associated with the deposition of basement membrane components and decorin in the interstitial extracellular matrix. Clin Nephrol 1995, 44:211-219 [PubMed] [Google Scholar]
- 27.Raats CJ, Van den Born J, Berden JH: Glomerular heparan sulfate alterations: mechanisms and relevance for proteinuria. Kidney Int 2000, 57:385-400 [DOI] [PubMed] [Google Scholar]
- 28.Iozzo RV, Cohen IR, Grassel S, Murdoch AD: The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J 1994, 302:625-639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oomura A, Nakamura T, Arakawa M, Ooshima A, Isemura M: Alterations in the extracellular matrix components in human glomerular diseases. Virchows Arch A Pathol Anat Histopathol 1989, 415:151-159 [DOI] [PubMed] [Google Scholar]
- 30.Miner JH: Renal basement membrane components. Kidney Int 1999, 56:2016-2024 [DOI] [PubMed] [Google Scholar]
- 31.Groffen AJ, Veerkamp JH, Monnens LA, Van den Heuvel LP: Recent insights into the structure and functions of heparan sulfate proteoglycans in the human glomerular basement membrane. Nephrol Dial Transplant 1999, 14:2119-2129 [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Zhang SY, Sich M, Beziau A, Van den Heuvel LP, Gubler MC: Glomerular extracellular matrix and growth factors in diffuse mesangial sclerosis. Pediatr Nephrol 2001, 16:429-438 [DOI] [PubMed] [Google Scholar]
- 33.Nerlich AG, Schleicher ED, Wiest I, Specks U, Timpl R: Immunohistochemical localization of collagen VI in diabetic glomeruli. Kidney Int 1994, 45:1648-1656 [DOI] [PubMed] [Google Scholar]
- 34.Cai Y, Beziau A, Sich M, Kleppel MM, Gubler MC: Collagen distribution in human membranous glomerulonephritis. Pediatr Nephrol 1996, 10:14-21 [DOI] [PubMed] [Google Scholar]
- 35.Groffen AJ, Hop FW, Tryggvason K, Dijkman H, Assmann KJ, Veerkamp JH, Monnens LA, Van den Heuvel LP: Evidence for the existence of multiple heparan sulfate proteoglycans in the human glomerular basement membrane and mesangial matrix. Eur J Biochem 1997, 247:175-182 [DOI] [PubMed] [Google Scholar]
- 36.Kuo HJ, Maslen CL, Keene DR, Glanville RW: Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem 1997, 272:26522-26529 [DOI] [PubMed] [Google Scholar]
- 37.Zhu D, Kim Y, Steffes MW, Groppoli TJ, Butkowski RJ, Mauer SM: Application of electron microscopic immunocytochemistry to the human kidney: distribution of type IV and type VI collagen in normal human kidney. J Histochem Cytochem 1994, 42:577-584 [DOI] [PubMed] [Google Scholar]
- 38.Tan EM, Glassberg E, Olsen DR, Noveral JP, Unger GA, Peltonen J, Chu ML, Levine E, Sollberg S: Extracellular matrix gene expression by human endothelial and smooth muscle cells. Matrix 1991, 11:380-387 [DOI] [PubMed] [Google Scholar]
- 39.Ball B, Mousson C, Ratignier C, Guignier F, Glotz D, Rifle G: Antibodies to vascular endothelial cells in chronic rejection of renal allografts. Transplant Proc 2000, 32:353-354 [DOI] [PubMed] [Google Scholar]
- 40.Van den Born J, Van den Heuvel LP, Bakker MA, Veerkamp JH, Assmann KJ, Weening JJ, Berden JH: Distribution of GBM heparan sulfate proteoglycan core protein and side chains in human glomerular diseases. Kidney Int 1993, 43:454-463 [DOI] [PubMed] [Google Scholar]
- 41.Iozzo RV, San Antonio JD: Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest 2001, 108:349-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orfila C, Durand D, Vega-Vidalle C, Suc JM: Immunofluorescent deposits on the tubular basement membrane in human renal transplant. Nephron 1991, 57:149-155 [DOI] [PubMed] [Google Scholar]
- 43.Habib R, Antignac C, Hinglais N, Gagnadoux MF, Broyer M: Glomerular lesions in the transplanted kidney in children. Am J Kidney Dis 1987, 10:198-207 [DOI] [PubMed] [Google Scholar]
- 44.Colvin RB: The renal allograft biopsy. Kidney Int 1996, 50:1069-1082 [DOI] [PubMed] [Google Scholar]
- 45.Hancock WH, Whitley WD, Tullius SG, Heemann UW, Wasowska B, Baldwin III WM, Tilney NL: Cytokines, adhesion molecules, and the pathogenesis of chronic rejection of rat renal allografts. Transplantation 1993, 56:643–650 [DOI] [PubMed]
- 46.Murdoch AD, Liu B, Schwarting R, Tuan RS, Iozzo RV: Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissues as detected by a novel monoclonal antibody against domain III and by in situ hybridization. J Histochem Cytochem 1994, 42:239-249 [DOI] [PubMed] [Google Scholar]
- 47.Zanussi S, Doliana R, Segat D, Bonaldo P, Colombatti A: The human type VI collagen gene. mRNA and protein variants of the alpha 3 chain generated by alternative splicing of an additional 5-end exon. J Biol Chem 1992, 267:24082-24089 [PubMed] [Google Scholar]
- 48.Dodge GR, Kovalszky I, Chu ML, Hassell JR, McBride OW, Yi HF, Iozzo RV: Heparan sulfate proteoglycan of human colon: partial molecular cloning, cellular expression, and mapping of the gene (HSPG2) to the short arm of human chromosome 1. Genomics 1991, 10:673-680 [DOI] [PubMed] [Google Scholar]
- 49.Dodge GR, Kovalszky I, Hassell JR, Iozzo RV: Transforming growth factor beta alters the expression of heparan sulfate proteoglycan in human colon carcinoma cells. J Biol Chem 1990, 265:18023-18029 [PubMed] [Google Scholar]
- 50.Postlethwaite AE, Holness MA, Katai H, Raghow R: Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest 1992, 90:1479-1485 [DOI] [PMC free article] [PubMed] [Google Scholar]