Abstract
Myocardial hibernation refers to a state of prolonged impairment of left ventricular function in the presence of coronary artery disease, which may be reversed by revascularization. In this study we present evidence for a local inflammatory reaction in hibernating myocardial segments from patients undergoing coronary revascularization. We obtained transmural myocardial biopsies guided by transesophageal echocardiography from patients with ischemic ventricular dysfunction undergoing bypass surgery. Among the 28 biopsied segments included in the study, 23 showed evidence of systolic dysfunction. The majority of dysfunctional segments (85.7%) were viable (201Tl uptake ≥ 60%). The samples were stained with markers for mast cells, mature resident macrophages, and the monoclonal antibody Mac387 that labels newly recruited myeloid cells. Dysfunctional segments showed more extensive fibrosis and higher macrophage density than normal segments. Among the 23 dysfunctional segments, 12 recovered function as assessed with echocardiograms 3 months after revascularization. Segments with postoperative functional recovery had comparable macrophage and mast cell density with those showing persistent dysfunction. However, biopsied segments that subsequently recovered function contained significantly higher numbers of newly recruited Mac387-positive leukocytes (18.7 ± 3.1 cells/mm2, n = 12 versus 8.6 ± 0.9 cells/mm2, n = 11; P = 0.009). In addition, monocyte chemotactic protein-1, a potent mononuclear cell chemoattractant, was predominantly expressed in segments with recovery of function. Myocardial hibernation is associated with an inflammatory response leading to active leukocyte recruitment. Dysfunctional myocardial segments that show an active inflammatory reaction have a greater potential for recovery of function after revascularization. We postulate that revascularization may promote resolution of the ongoing inflammation, preventing further tissue injury and fibrosis.
Hibernating myocardium refers to a state of persistently impaired left ventricular function at rest, in the presence of coronary artery disease, that may be reversed by revascularization. 1-3 Chronically dysfunctional segments exhibit distinct morphological changes at both the cardiomyocyte and extracellular matrix levels. 4-6 Cardiomyocytes in hibernating areas demonstrate loss of contractile material, 7 glycogen accumulation, 4,8 and may undergo dedifferentiation, expressing contractile proteins specific to the fetal heart. 9,10 In addition, hibernating myocardial segments demonstrated increased expression of extracellular matrix proteins, 7 when compared with normal myocardial segments. Furthermore, dysfunctional myocardial segments with improved function after revascularization show significantly less tissue fibrosis 8,11,12 than myocardium with persistent dysfunction.
In recent years, substantial evidence has indicated an important role for inflammatory mechanisms in the pathophysiology of cardiovascular disease. 13-16 Triggering of the inflammatory process represents the response of vascular tissues to various types of injury. The cytokine cascade associated with myocardial infarction has been extensively studied and seems to be crucial for healing and scar formation, however the potential role of inflammation in mediating pathological changes associated with stable ischemic heart disease has not been adequately investigated. In this study we present evidence for a local inflammatory reaction in the myocardium from patients with myocardial dysfunction because of stable ischemic heart disease undergoing coronary revascularization. We hypothesized that myocardial hibernation may be associated with an active inflammatory process leading to leukocyte recruitment in the cardiac interstitium. We identified newly-recruited leukocytes in the human heart using immunohistochemical staining with the monoclonal antibody Mac387, which recognizes calgranulin, a protein rapidly down-regulated during monocyte to macrophage maturation. Our findings suggest that reversible ischemic myocardial dysfunction is a dynamic process associated with increased synthesis of mononuclear cell chemoattractants and continuous leukocyte recruitment.
Materials and Methods
Patient Population
We enrolled patients scheduled for coronary artery bypass surgery who had chronic ischemic resting left ventricular dysfunction in the distribution of ≥1 coronary artery (≥70% stenosis). A transthoracic two-dimensional echocardiogram, dobutamine stress echocardiography, and 201Tl single-photon emission tomography were performed 2 to 5 days before bypass surgery. During surgery, transmural myocardial biopsies were obtained from selected myocardial segments, guided by transesophageal echocardiogram. Patients underwent transthoracic two-dimensional echocardiography 3 months after surgery to evaluate changes in regional function. The Institutional Review Board of Baylor College of Medicine approved the study protocol, and all patients signed informed consent before enrollment.
Echocardiographic Studies
Imaging was performed in the standard parasternal and apical views with the patient in the left lateral position (Hewlett Packard Sonos 2500, 2.5- or 3.5-MHz transducer). Regional function was assessed according to the 16-segment model of the American Society of Echocardiography, and graded from 1 to 5 (1, normal; 2, mild hypokinesia; 3, severe hypokinesia; 4, akinesia; and 5, dyskinesia). Ejection fraction was quantified with the multiple diameter method. The echocardiographic studies were interpreted without knowledge of the histopathological data. Regional function recovery was defined by improvement of ≥1 grades in wall motion. To match myocardial segments with coronary distribution, the anterior wall, anterior septum, and apex were assigned to the left anterior descending coronary artery, the lateral wall to the circumflex, and the inferoposterior wall and inferior septum to the right coronary artery.
Dobutamine Echocardiography
Dobutamine infusion was started at 2.5 μg/kg/min and increased at 3-minute intervals to 5, 7.5, 10, 20, 30, and 40 μg/kg/min. Images at baseline, 5, and 7.5 μg/kg/min and peak dobutamine were digitized online in a quad-screen format to provide optimal assessment of viability. 17 The response of dysfunctional segments to dobutamine was classified as biphasic (improvement at a low dose with worsening at a high dose), worsening, no change, and sustained improvement (increased thickening without worsening later on). Any response during dobutamine echocardiography was considered indicative of viability.
Rest-Redistribution 201Tl
Rest and 4-hour redistribution 201Tl-single-photon emission tomography scans were performed after intravenous administration of 3 mCi of 201Tl before surgery. A large field-of-view rotating γ camera with a high-resolution parallel-hole collimator was used. Thirty-two frames were acquired over a 180° area (45° left posterior oblique to 45° left anterior oblique). The reconstructed images were oriented in the standard short axis, horizontal long axis, and vertical long axis for interpretation and quantification of 201Tl uptake by nuclear cardiologists unaware of all other data. Computerized polar maps of the three-dimensional myocardial radioactivity were generated. A 16-segment model comparable to that for echocardiography was used. Myocardial 201Tl activity was determined with a region of interest 40 × 40 pixels (matrix, 128 × 128). The activity in each segment was normalized to the segment with the highest uptake. A maximal uptake of ≥60% at rest or redistribution was considered indicative of viability, as previously demonstrated. 18
Transmural Left Ventricular Biopsies and Tissue Processing
Transmural myocardial biopsies were obtained with a 20-mm 14-gauge Tru-cut biopsy needle at the time of surgery, before cardioplegia. Biopsy of selected segments was directed by transesophageal echocardiography as previously described. 19,20 For patients who had segments with normal systolic function, biopsies were acquired from one normal and one dysfunctional segment. For all other patients two dysfunctional segments were biopsied. Segments with high likelihood for viability were targeted by avoiding very thin walls (<7 mm thickness) and echodense myocardium, usually indicative of a completed transmural infarction. 21
Experimental Ischemia/Reperfusion Protocols
To develop an immunohistological method of assessing leukocyte recruitment and inflammatory activity in the heart we used samples from infarcted canine hearts. An established protocol of canine circumflex coronary occlusion/reperfusion was used. 22,23 Healthy dogs were instrumented with a hydraulic occluder and underwent 1 hour of coronary occlusion, followed by reperfusion intervals ranging from 24 hours to 7 days. After the reperfusion periods, hearts were stopped by the rapid intravenous infusion of 30 meq of KCl and removed from the chest for sectioning from apex to base into four transverse rings ∼1 cm in thickness. The posterior papillary muscle and the posterior free wall were identified. Tissue samples were isolated from infarcted or normally perfused myocardium based on visual inspection. Myocardial segments were fixed in B*5 fixative 24 for histological analysis.
Immunohistochemistry, Histology, and Morphometric Analysis
Samples from human and canine myocardium were fixed in B*5 fixative, to improve antigen preservation, 24 and embedded in paraffin. Sections were cut at 3 μm and stained with picrosirius red to identify areas of collagen deposition. 25 Serial sections were stained immunohistochemically with the following antibodies: monoclonal anti-tryptase antibody, monoclonal anti-chymase antibody (both from Chemicon, Temecula, CA), monoclonal antibody PM-2K (Biogenesis, Brentwood, NH) that labels mature resident macrophages, monoclonal antibody Mac387 (DAKO, Carpinteria, CA), which identifies newly recruited myeloid cells, 26 monoclonal antibody to CD31 (DAKO), 27 sheep polyclonal antibody to MMP-9 (The Binding Site, Birmingham, UK), and goat polyclonal antibody to MCP-1 (R&D, Minneapolis, MN). The Mac387 antibody recognizes two calcium-binding myeloid-associated proteins, termed calgranulins, that are not expressed by differentiated monocyte-derived macrophages. 28 Staining was performed using a peroxidase-based technique with the Vectastain mouse kit (Vector Laboratories, Burlingame, CA) and developed with diaminobenzidine and nickel (Vector Laboratories). Slides were counterstained with eosin and examined in a Zeiss microscope. Dual immunohistochemical staining was performed combining a peroxidase-based technique for the PM-2K antibody developed with diaminobenzidine and nickel (black), and an alkaline phosphatase-based method for the Mac387 antibody developed with the alkaline phosphatase substrate kit I (Vector Laboratories) (red). Each section was scanned at ×200 magnification using a Leaf Lumina digital camera and Adobe Photoshop software (Adobe Systems, San Jose, CA).
Quantitative Analysis
Quantitative analysis was performed using Zeiss Image software. The entire stained biopsied sample (mean area, 3.3 + 0.4 mm2, n = 28) was scanned using Adobe Photoshop software (Adobe Systems), and a Leaf Microlumina digital camera. Collagen staining was expressed as the percentage of the picrosirius-red stained area to the total area of the segment. Macrophage and mast cell density were expressed as cells/mm2. The density of extravascular Mac387-positive cells was used as an index of inflammatory activity. MCP-1 expression was semiquantitated as follows: 0, absent staining; 1, focal staining; 2, diffuse staining in less than half of the section; 3, staining present in more than half of the specimen.
Statistics
Data are presented as mean ± SEM. Unpaired t-test was used to compare the pathological variables between dysfunctional and nondysfunctional segments and segments with and without recovery of function after revascularization. In addition, collagen percent staining was correlated with wall motion score and with macrophage density using the Spearman’s correlation coefficient. Significance was set at P < 0.05.
Results
Patient Population
Fifteen patients (13 men) with ischemic heart disease were enrolled in the study. The mean age was 62 years (range, 50 to 73 years). The mean left ventricular ejection fraction was 29.4 ± 2%. Ten patients (67%) had hypertension, 9 (60%) were diabetic, 10 (67%) had angina pectoris, and 8 (56%) had congestive heart failure. Twenty-eight myocardial segments were used for the study. Two additional segments were excluded because of inadequate sampling. Five segments showed normal wall motion, 4 were mildly hypokinetic, 13 severely hypokinetic, and 6 were akinetic. Thirteen patients underwent rest-redistribution thallium single-photon emission tomography before bypass surgery. Mean myocardial 201Tl uptake was 70.2 ± 2.5%. The majority of biopsied segments were viable [18 of 21 (85.7%) dysfunctional segments had a thallium uptake ≥60% and all except one had an uptake of ≥50%]. Thirteen patients underwent dobutamine echocardiography before revascularization, demonstrating that the majority of segments (17 of 20 dysfunctional segments or 85%) were viable. All patients underwent complete revascularization without complications. Of the 23 dysfunctional segments, 12 segments recovered function after revascularization. The mean left ventricular ejection fraction significantly increased after revascularization (29.4 ± 2% to 38.6 ± 3.3%; P < 0.001, n = 15).
Identification of Mast Cells and Macrophages in the Myocardium—Relation of Macrophage and Mast Cell Density with Collagen Staining and Wall Motion
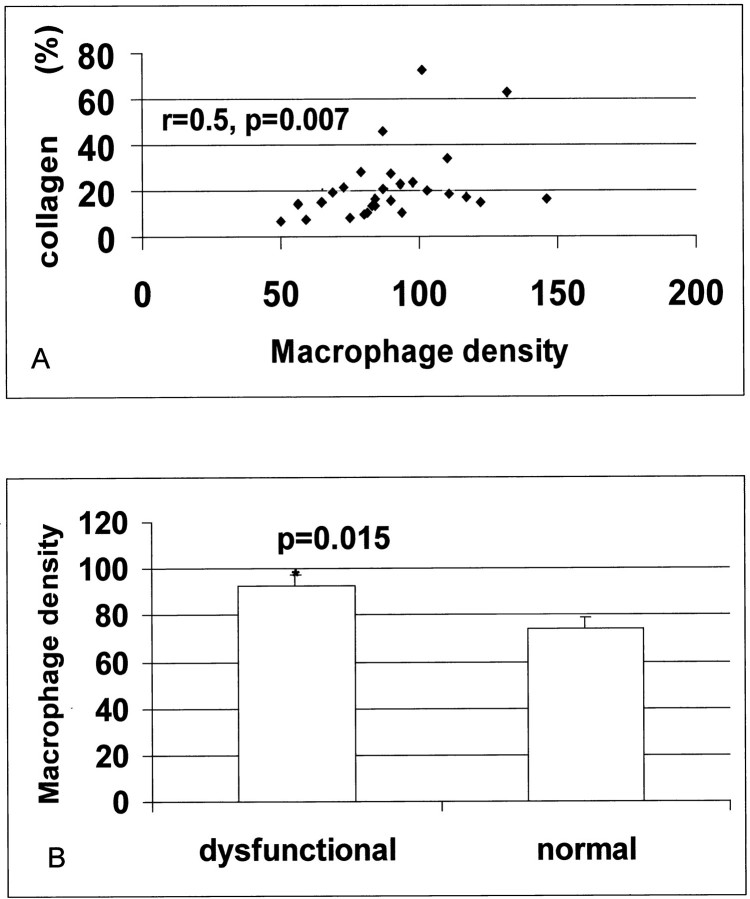
Collagen content ranged between 6.32% and 72.4%, and correlated directly with wall motion score (r = 0.55, P = 0.026, n = 28). Immunohistochemical staining with the antibody PM-2K (Figure 1) ▶ , that specifically labels mature tissue macrophages, demonstrated a significant number of macrophages, predominantly located in areas of fibrosis. Mast cells were identified as granular interstitial cells with intense expression of the mast cell-specific proteases tryptase (Figure 1A ▶ and Figure 2A ▶ ) and chymase (Figure 2B) ▶ . Macrophage density directly correlated with percent collagen staining (r = 0.50, P = 0.007, n = 28) (Figure 3A) ▶ . In addition, macrophage density was higher in segments with contractile dysfunction when compared with normal segments (92.7 ± 4.8 cells/mm2, n = 23, versus 73.8 ± 4.8 cells/mm2, n = 5; P = 0.015) (Figure 3B) ▶ . Mast cell density did not correlate with fibrosis,however mast cell numbers were significantly higher in akinetic segments (12.2 ± 2.7 cells/mm2, n = 6) compared to nonakinetic segments (6.57 ± 0.69 cells/mm2, n = 22, P = 0.038).
Figure 1.
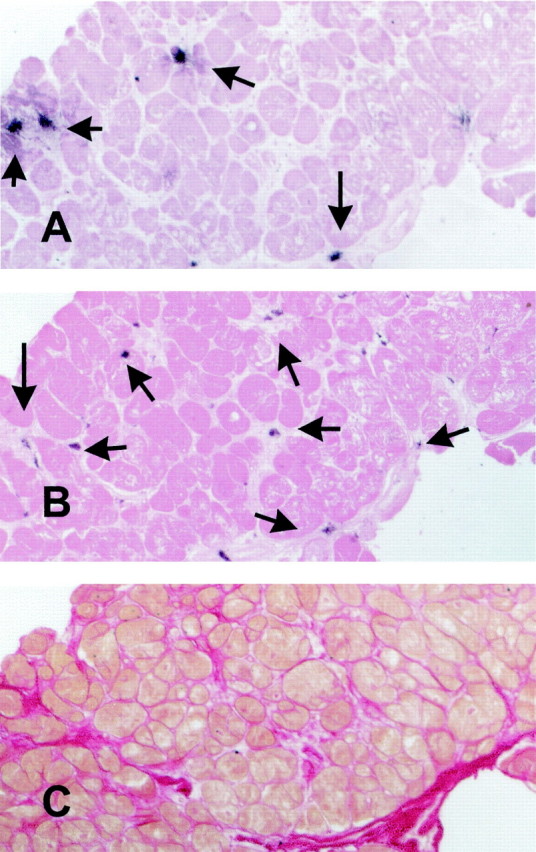
Labeling of mast cells and macrophages in the human myocardium. Serial myocardial sections were stained for tryptase (A) to identify mast cells (arrows) and for the monoclonal antibody PM-2K (B) to label mature resident macrophages (arrows). Both slides were counterstained with eosin. Adjacent sections were stained with picrosirius red for collagen (C). Original magnifications, ×200.
Figure 2.
Mast cells in the human heart are identified as granular interstitial cells with intense immunoreactivity for tryptase (A and C) and chymase (B).
Figure 3.
Macrophage density correlates with collagen deposition and is higher in dysfunctional myocardial segments. A significant correlation between macrophage density and collagen percent staining was noted (r = 0.5, P = 0.007) (A). Macrophage numbers were higher in dysfunctional myocardial segments when compared with segments showing normal regional function (*, P = 0.015) (B).
The Monoclonal Antibody Mac387 as an Index of Inflammatory Activity in the Heart
Immunohistochemical staining with the monoclonal antibody Mac387 was used as a marker of active inflammation as previously described. Mac387 detects an epitope of the calcium-binding protein MRP14, 29 labeling newly recruited myeloid cells and not mature macrophages. To demonstrate the ability of Mac387 immunohistochemistry to identify newly recruited inflammatory leukocytes in the injured myocardium, we used sections from the experimental canine myocardial infarcts. After 1 hour of coronary occlusion and 24 hours of reperfusion a large number of Mac387-positive cells was identified in the infarcted area, reflecting extensive infiltration of the injured territory with myeloid cells (neutrophils and monocytes) (Figure 4A) ▶ . In contrast, after 7 days of reperfusion, only a few Mac387-positive cells were found in the healing area, which was filled with mature macrophages, identified with the monoclonal antibody PM-2K (Figure 4B) ▶ . Mature macrophages did not stain for Mac387, indicating that this method can be used as a marker for new recruitment of leukocytes in the heart.
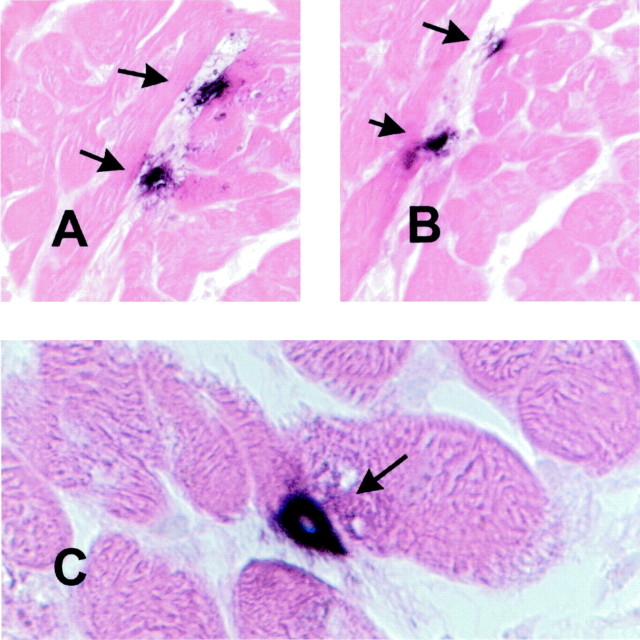
Figure 4.
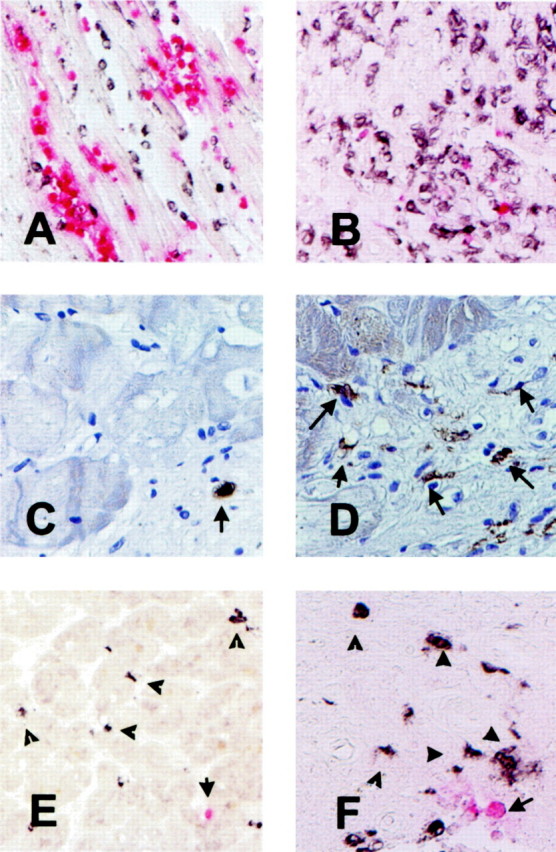
Use of the monoclonal antibody Mac387 as a marker of acute inflammation in the heart. A: Canine myocardial infarction after 1 hour of circumflex coronary artery occlusion and 24 hours of reperfusion. Peroxidase-based immunohistochemistry for the macrophage-specific antibody PM-2K (black) was followed by alkaline phosphatase-based staining for Mac387 a marker of newly recruited myeloid cells (red). Note the abundance of Mac387-positive cells in the infarcted area. B: Canine infarct after 1 hour of coronary occlusion and 7 days of reperfusion. The healing infarct is now filled with mature macrophages (black); whereas only a few newly recruited leukocytes are noted (red). C: Immunohistochemical staining of a biopsied human myocardial sample with the Mac387 antibody followed by hematoxylin counterstaining identified newly recruited leukocytes. D: Immunostaining of a biopsied human myocardial sample with the macrophage-specific antibody PM-2K labeled mature interstitial macrophages. E and F: Dual immunohistochemical staining combining peroxidase-based staining for PM-2K (black) and alkaline phosphatase-based staining for Mac387 (red) demonstrating that macrophages (arrowheads) significantly outnumbered newly recruited leukocytes (arrow). Original magnifications: ×200 (A, B, E); ×400 (C, D, F).
Immunohistochemical staining of biopsied human myocardial samples with Mac387 labeled infiltrating leukocytes (Figure 4C) ▶ . Hematoxylin and eosin staining demonstrated that the vast majority of these cells had mononuclear cell morphology. Dual immunohistochemical staining combining peroxidase-based labeling with PM-2K and alkaline phosphatase-based staining with Mac387 demonstrated that mature resident macrophages significantly outnumbered newly recruited leukocytes in all biopsied samples (Figure 4, E and F) ▶ . Figure 4, C to F ▶ , shows representative sections from dysfunctional human myocardial samples; quantitative analysis of the findings will be presented in the next section.
Relation of Functional Recovery with Macrophage and Mast Cell Density—Active Recruitment of Mononuclear Cells in Segments with Functional Recovery
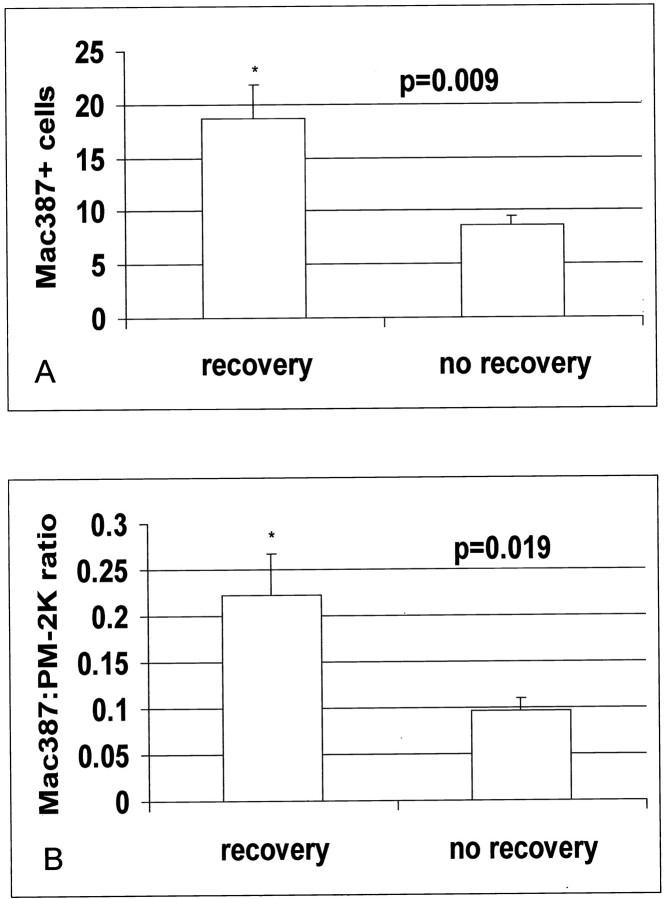
Twelve dysfunctional segments demonstrated recovery of function after revascularization and 11 segments did not recover. Thallium uptake was not significantly different between segments with recovery and those without recovery of function (recovery, 73.1 ± 2.7%; no recovery, 66.8 ± 4.6%; P = 0.25) Compared with segments showing persistent dysfunction, those with recovery of function after revascularization had similar macrophage density (88.7 ± 4.76 cells/mm2, n = 12, versus 96.8 ± 8.86 cells/mm2, n = 11; P = NS) and mast cell density (9.2 ± 1.6 cells/mm2, n = 12, versus 6.2 ± 1.03 cell/mm2, n = 11, versus P = NS) (Figure 5) ▶ . However, segments with recovery had higher numbers of newly recruited Mac387-positive cells (18.7 ± 3.1 cells/mm2, n = 12, versus 8.6 ± 0.9 cells/mm2, n = 11; P = 0.009) and a higher ratio of newly recruited Mac387-expressing cells to mature resident macrophages (recovery, 0.223 ± 0.045 cells/mm2, n = 12, versus no recovery, 0.0965 ± 0.014 cells/mm2, n = 11; P = 0.019) (Figure 6 ▶ and Figure 7 ▶ ). Serial section staining with an antibody to matrix metalloproteinase (MMP)-9 demonstrated that a significant number of newly recruited Mac-387-positive cells showed intense MMP-9 immunoreactivity (Figure 8) ▶ . Expression of proteolytic enzymes may suggest the migratory potential of these cells.
Figure 5.
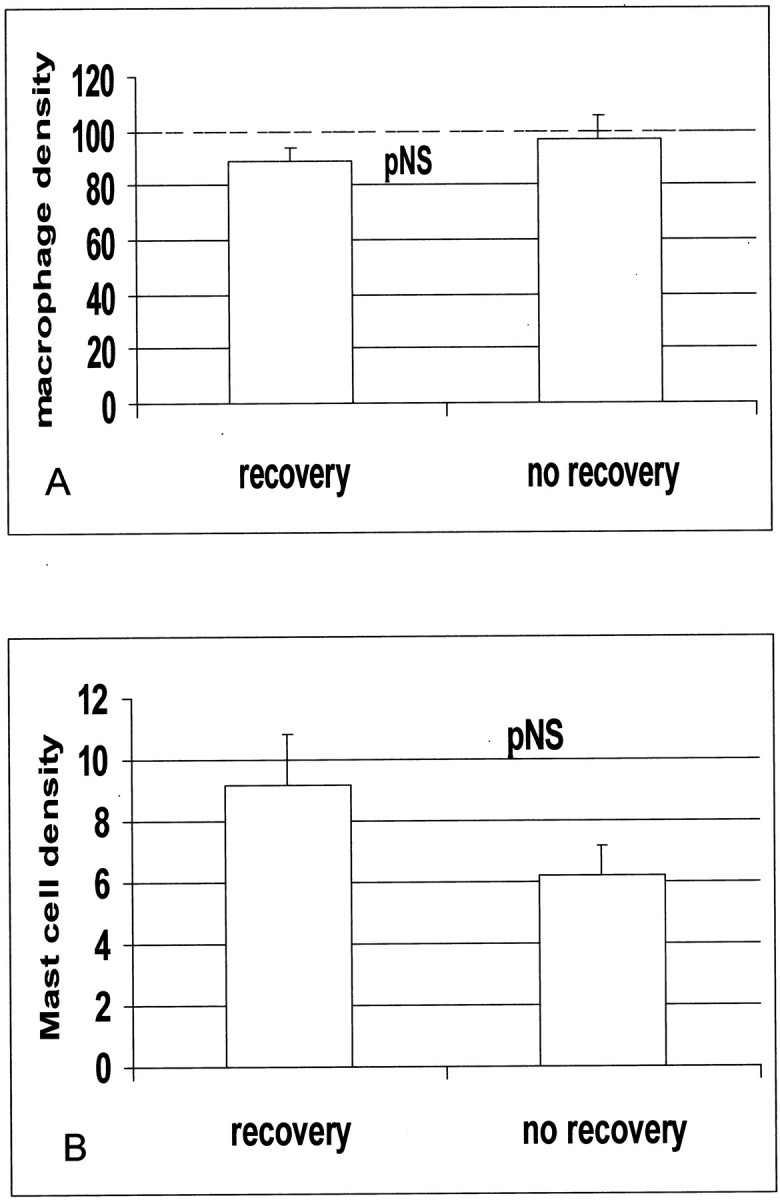
Macrophage (A) and mast cell (B) density is similar in dysfunctional segments with and without recovery of function after revascularization.
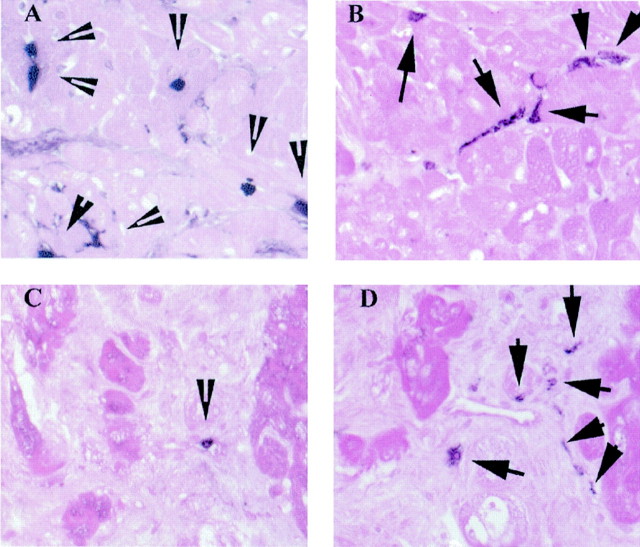
Figure 6.
Immunohistochemical labeling of newly recruited Mac387-positive myeloid cells (A and C) and mature PM-2K-positive resident macrophages (B and D) in the myocardium. Representative sections from a dysfunctional segment that showed recovery of function after revascularization are shown (A and B). Note the significant number of newly recruited Mac-387-positive cells (A, arrowheads), compared to the number of mature resident macrophages (B, arrows). In contrast, segments with persistent dysfunction (C and D) had rare newly recruited cells (C, arrowhead) and a high number of resident macrophages (D, arrows). The slides were counterstained with eosin. Original magnifications, ×400.
Figure 7.
A: Segments with recovery of function show higher numbers of Mac387-positive cells when compared with those without recovery (P = 0.009). B: In addition, the ratio of newly recruited cells to mature macrophages was significantly higher in segments with recovery (P = 0.019).
Figure 8.
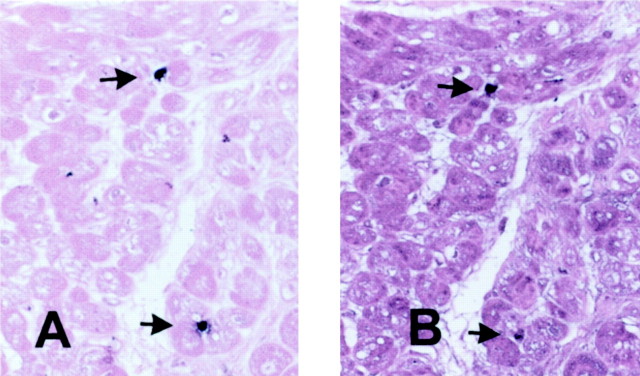
Newly recruited leukocytes express MMP-9, exhibiting a protein-degrading migratory phenotype. Immunohistochemical staining of a biopsied myocardial sample with Mac387 detects two newly recruited leukocytes (arrows) (A). Serial section staining demonstrates MMP-9 expression by these cells (arrows) (B). Original magnifications, ×400.
MCP-1 Expression in Hibernating Myocardial Segments
Using immunohistochemical staining we examined MCP-1 protein localization in the biopsied myocardial samples. Segments with recovery of function had more intense MCP-1 expression (1.08 ± 0.25 versus 0.27 ± 0.19; P = 0.0179), when compared with segments without recovery. MCP-1 immunoreactivity was predominantly localized in cardiomyocytes and microvascular endothelial cells (identified with CD31 staining) (Figure 9) ▶ from hibernating segments.
Figure 9.
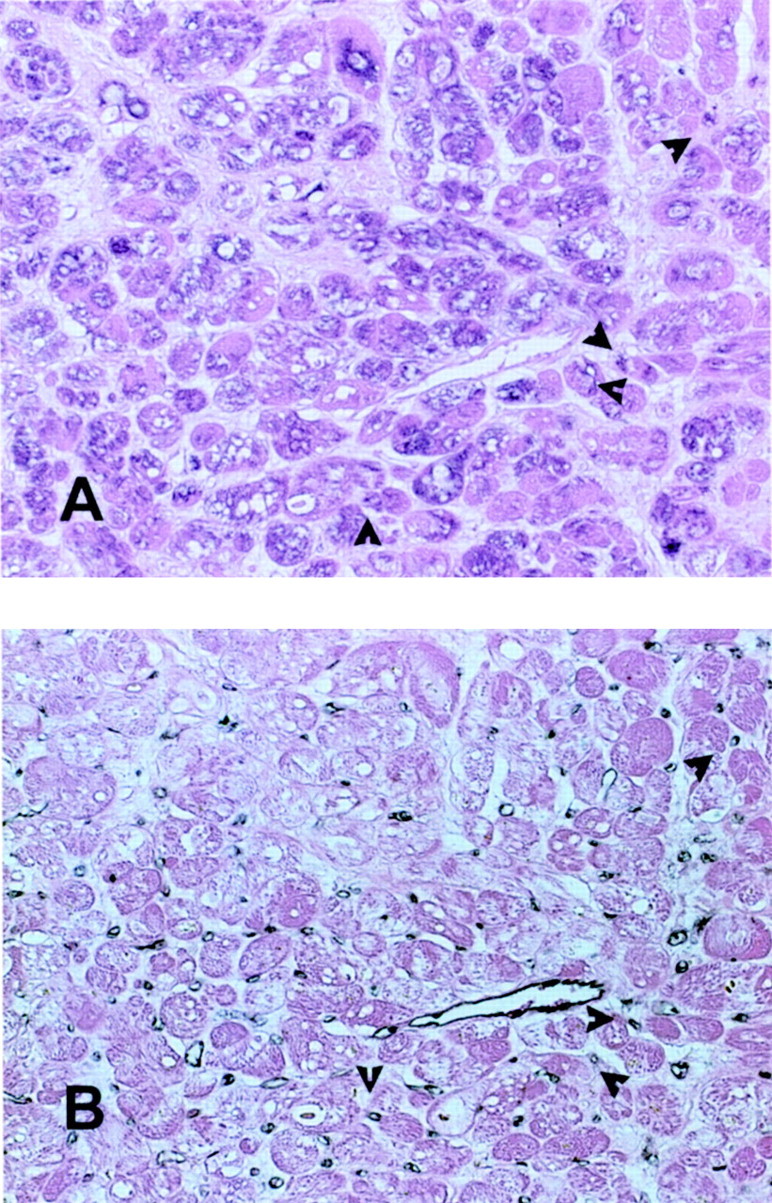
Immunohistochemical staining for MCP-1 in a myocardial segment with recovery of function after revascularization (A). Diffuse staining is noted in many cardiomyocytes and occasional microvascular endothelial cells (arrows) identified by serial section staining with a monoclonal antibody to CD31 (B). Original magnifications, ×400.
Discussion
Hibernating myocardium has been defined as a persistent impairment of contractile function resulting from reduced coronary flow, that can be partially or completely resolved once coronary perfusion is restored. 30,31 The pathophysiology of hibernation seems to be quite complex: it most likely involves repetitive postischemic dysfunction causing phenotypic changes in myocardial cells leading to myocyte degeneration and reparative fibrosis. Our study protocol excluded dysfunctional myocardial segments with an end-diastolic thickness <7 mm, ensuring that we examine segments with viable myocardium and a substantial chance of recovery after revascularization. The viability of the biopsied myocardial segments was supported by 201Tl single-photon emission tomography, showing a 201Tl uptake ≥60% in 85.7% (18 of 21 cases) of the dysfunctional segments, and by dobutamine echocardiography demonstrating a sustained or biphasic response in 85% (17 of 20 cases) of the dysfunctional segments. Our study suggests that hibernating myocardium is associated with a dynamic inflammatory process characterized by myocardial expression of the monocyte chemoattractant MCP-1 and continuous leukocyte infiltration. Monocyte-derived macrophages serve as sources of fibrogenic factors and may mediate fibrosis and contractile dysfunction.
Macrophages and Mast Cells in Myocardial Fibrosis
Macrophages and mast cells are thought to play an important role in fibrotic processes through the production of fibrogenic growth factors and proteases. Macrophages accumulate in healing myocardial infarcts 23 and their secretory products may regulate extracellular matrix remodeling. In addition, the number of mast cells is increased after experimental myocardial infarction 32 and in cardiomyopathic hearts. 33 Activated mast cells may be involved in tissue repair through the release of a wide variety of performed and newly synthesized mediators, such as the potent fibrogenic protease tryptase and a number of growth factors including basic fibroblast growth factors, vascular endothelial growth factor, and transforming growth factor-β. 34 Both macrophages and mast cells are capable of producing matrix metalloproteinases, 35,36 critical factors in extracellular matrix metabolism. Accumulation of extracellular matrix proteins adversely affects myocardial viscoelasticity, leading to diastolic and systolic dysfunction. 37,38 Our findings documented a good correlation between collagen content and wall motion score. Macrophage density was higher in dysfunctional myocardial segments and correlated directly with collagen expression (Figure 2) ▶ . Macrophages appeared to accumulate predominantly in areas of collagen deposition and may have a crucial role in regulating extracellular matrix metabolism in the cardiac interstitium through the production of growth factors and metalloproteinases. In addition, mast cell numbers were higher in akinetic segments, but did not correlate with collagen expression.
Recovering Myocardial Segments Demonstrate MCP-1 Expression and Active Leukocyte Infiltration
We did not find a significant difference in macrophage and mast cell density between segments with recovery of function after revascularization and segments showing persistent dysfunction. We then examined new recruitment of Mac387-positive leukocytes in the biopsied samples as an index of an active inflammatory process. This technique was validated in a model of experimental canine myocardial infarction (Figure 4) ▶ , showing a large number of Mac387-positive cells during the early inflammatory phase, that decreased with maturation of the healing infarct. In the biopsied human myocardial samples, newly recruited Mac387-positive leukocytes with morphological characteristics of mononuclear cells were more frequently found in recovering segments, suggesting an active dynamic process of monocyte infiltration in these areas (Figures 6 and 7) ▶ ▶ . Mac387-positive cells often demonstrated expression of MMP-9, exhibiting a matrix-degrading phenotype with a potential importance in leukocyte migration. 39 Segments with recovery also showed high expression of MCP-1 (Figure 9) ▶ , an additional indicator of an active inflammatory response. MCP-1 is a potent monocyte chemoattractant with a significant role in numerous fibrotic processes 40,41 and may be responsible for continuous recruitment of mononuclear cells in the myocardium. MCP-1 synthesis is up-regulated in patients with dilated cardiomyopathy, 42,43 experimental models of heart failure, 44 and after experimental myocardial infarction. 45,46 Targeted expression of the MCP-1 gene in murine cardiac muscle induced marked macrophage infiltration, leading to depressed contractile function, hypertrophy, dilation, and myocardial fibrosis. 47 In addition to its potent mononuclear cell chemotactic activity, MCP-1 may directly mediate fibrosis through stimulation of fibroblast collagen and transforming growth factor-β expression. 48
Inflammation as a Dynamic Reversible Process Mediating Myocardial Hibernation
Fibrosis is the endpoint of an inflammatory process characterized by leukocyte recruitment and activation, fibroblast proliferation, and increased extracellular matrix production. We suggest that myocardial hibernation is characterized by a dynamic, continuous inflammatory process associated with MCP-1 expression in the myocardium and active leukocyte recruitment. Recent experiments from our laboratory demonstrated that a single brief (15 minutes) episode of coronary occlusion induces MCP-1 synthesis in the canine myocardium for at least 5 hours after reperfusion. 49 It has been recently suggested that the phenotype of hibernating myocardium may arise from repetitive episodic ischemia with or without an underlying reduction in baseline blood flow. We propose that in the hibernating myocardium repetitive nonlethal ischemic insults may lead to prolonged MCP-1 synthesis and continuous recruitment of mononuclear cells. This dynamic inflammatory process may slowly induce tissue injury, extracellular matrix remodeling, and fibrosis through the production of growth factors and metalloproteinases by monocyte-derived macrophages.
It is possible that this dynamic process has several stages and that we are sampling in a continuum. The early stage of high inflammatory activity may be associated with chemokine induction and leukocyte recruitment. Reversibility through revascularization may depend on timely down-regulation of the inflammatory process, decreased MCP-1 expression and diminished monocyte infiltration. This could lead gradually to lower numbers of resident macrophages and diminished synthesis of fibrogenic substances. In contrast, segments with persistent dysfunction may have reached a point of no return, where long-standing hypoxia-mediated inflammatory reaction has led to extensive tissue injury, lower levels of MCP-1 expression, and decreased active infiltration with inflammatory cells. In this stage there is no reversible inflammatory component and revascularization may have little to offer.
Conclusions
This study demonstrates for the first time that myocardial hibernation is an active dynamic process associated with increased synthesis of mononuclear cell chemoattractants and continuous leukocyte recruitment. Monocyte-derived macrophages may accumulate in the dysfunctional myocardium, inducing tissue damage and fibrosis. Early revascularization may be effective, at least in part, by suppressing the ischemia-mediated inflammatory process, preventing irreversible injury.
Acknowledgments
We thank Alida Evans and Stephanie Butcher for their outstanding technical assistance and Concepcion Mata and Sharon Malinowski for their expert secretarial assistance in preparing the manuscript.
Footnotes
Address reprint requests to Nikolaos G. Frangogiannis, M.D., Section of Cardiovascular Sciences, Department of Medicine, Baylor College of Medicine, One Baylor Plaza M/S F-602, Houston TX 77030. E-mail: ngf@bcm.tmc.edu.
Supported by the National Institutes of Health (grant HL-42550 to M. L. E. and N. G. F.), the DeBakey Heart Center, the John S. Dunn, Sr., Trust Fund (to W. A. Z.), and the Methodist Hospital Foundation (to N. G. F.).
References
- 1.Rahimtoola SH: Concept and evaluation of hibernating myocardium. Annu Rev Med 1999, 50:75-86 [DOI] [PubMed] [Google Scholar]
- 2.Rahimtoola SH: A perspective on the three large multicenter randomized clinical trials of coronary bypass surgery for chronic stable angina. Circulation 1985, 72:V123-V135 [PubMed] [Google Scholar]
- 3.Heusch G: Hibernating myocardium. Physiol Rev 1998, 78:1055-1085 [DOI] [PubMed] [Google Scholar]
- 4.Vanoverschelde JL, Pasquet A, Gerber B, Melin JA: Pathophysiology of myocardial hibernation. Implications for the use of dobutamine echocardiography to identify myocardial viability. Heart 1999, 82(Suppl 3):III1-III7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsasser A, Schaper J: Hibernating myocardium: adaptation or degeneration? Basic Res Cardiol 1995, 90:47-48 [DOI] [PubMed] [Google Scholar]
- 6.Lim H, Fallavollita JA, Hard R, Kerr CW, Canty Jr JM: Profound apoptosis-mediated regional myocyte loss and compensatory hypertrophy in pigs with hibernating myocardium. Circulation 1999, 100:2380–2386 [DOI] [PubMed]
- 7.Elsasser A, Schlepper M, Klovekorn WP, Cai WJ, Zimmermann R, Muller KD, Strasser R, Kostin S, Gagel C, Munkel B, Schaper W, Schaper J: Hibernating myocardium: an incomplete adaptation to ischemia. Circulation 1997, 96:2920-2931 [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Vaduganathan P, Ali N, Blaustein A, Verani MS, Winters Jr WL, Zoghbi WA: Identification of hibernating myocardium: comparative accuracy of myocardial contrast echocardiography, rest-redistribution thallium-201 tomography and dobutamine echocardiography. J Am Coll Cardiol 1997, 29:985–993 [DOI] [PubMed]
- 9.Ausma J, Thone F, Dispersyn GD, Flameng W, Vanoverschelde JL, Ramaekers FC, Borgers M: Dedifferentiated cardiomyocytes from chronic hibernating myocardium are ischemia-tolerant. Mol Cell Biochem 1998, 186:159-168 [PubMed] [Google Scholar]
- 10.Ausma J, Cleutjens J, Thone F, Flameng W, Ramaekers F, Borgers M: Chronic hibernating myocardium: interstitial changes. Mol Cell Biochem 1995, 147:35-42 [DOI] [PubMed] [Google Scholar]
- 11.Depre C, Vanoverschelde JL, Melin JA, Borgers M, Bol A, Ausma J, Dion R, Wijns W: Structural and metabolic correlates of the reversibility of chronic left ventricular ischemic dysfunction in humans. Am J Physiol 1995, 268:H1265-H1275 [DOI] [PubMed] [Google Scholar]
- 12.Hennessy T, Diamond P, Holligan B, O’Keane C, Hurley J, Codd M, McCarthy C, McCann H, Sugrue D: Correlation of myocardial histologic changes in hibernating myocardium with dobutamine stress echocardiographic findings. Am Heart J 1998, 135:952-959 [DOI] [PubMed] [Google Scholar]
- 13.Frangogiannis NG, Youker KA, Rossen RD, Gwechenberger M, Lindsey MH, Mendoza LH, Michael LH, Ballantyne CM, Smith CW, Entman ML: Cytokines and the microcirculation in ischemia and reperfusion. J Mol Cell Cardiol 1998, 30:2567-2576 [DOI] [PubMed] [Google Scholar]
- 14.Hansen PR: Inflammatory alterations in the myocardial microcirculation. J Mol Cell Cardiol 1998, 30:2555-2559 [DOI] [PubMed] [Google Scholar]
- 15.Herskowitz A, Choi S, Ansari AA, Wesselingh S: Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol 1995, 146:419-428 [PMC free article] [PubMed] [Google Scholar]
- 16.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML: Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 1998, 98:699-710 [DOI] [PubMed] [Google Scholar]
- 17.Afridi I, Kleiman NS, Raizner AE, Zoghbi WA: Dobutamine echocardiography in myocardial hibernation. Optimal dose and accuracy in predicting recovery of ventricular function after coronary angioplasty. Circulation 1995, 91:663-670 [DOI] [PubMed] [Google Scholar]
- 18.Qureshi U, Nagueh SF, Afridi I, Vaduganathan P, Blaustein A, Verani MS, Winters Jr WL, Zoghbi WA: Dobutamine echocardiography and quantitative rest-redistribution 201T1 tomography in myocardial hibernation. Relation of contractile reserve to 201T1 uptake and comparative prediction of recovery of function. Circulation 1997, 95:626–635 [DOI] [PubMed]
- 19.Shan K, Bick RJ, Poindexter BJ, Nagueh SF, Shimoni S, Verani MS, Keng F, Reardon MJ, Letsou GV, Howell JF, Zoghbi WA: Altered adrenergic receptor density in myocardial hibernation in humans: a possible mechanism of depressed myocardial function. Circulation 2000, 102:2599-2606 [DOI] [PubMed] [Google Scholar]
- 20.Nagueh SF, Mikati I, Weilbaecher D, Reardon MJ, Al Zaghrini GJ, Cacela D, He ZX, Letsou G, Noon G, Howell JF, Espada R, Verani MS, Zoghbi WA: Relation of the contractile reserve of hibernating myocardium to myocardial structure in humans. Circulation 1999, 100:490-496 [DOI] [PubMed] [Google Scholar]
- 21.Cwajg JM, Cwajg E, Nagueh SF, He ZX, Qureshi U, Olmos LI, Quinones MA, Verani MS, Winters WL, Zoghbi WA: End-diastolic wall thickness as a predictor of recovery of function in myocardial hibernation: relation to rest-redistribution T1–201 tomography and dobutamine stress echocardiography. J Am Coll Cardiol 2000, 35:1152-1161 [DOI] [PubMed] [Google Scholar]
- 22.Frangogiannis NG, Mendoza LH, Lewallen M, Michael LH, Smith CW, Entman ML: Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J 2001, 15:1428-1430 [DOI] [PubMed] [Google Scholar]
- 23.Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML: IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol 2000, 165:2798-2808 [DOI] [PubMed] [Google Scholar]
- 24.Beckstead JH: A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem 1994, 42:1127-1134 [DOI] [PubMed] [Google Scholar]
- 25.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ: Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol 1995, 147:325-338 [PMC free article] [PubMed] [Google Scholar]
- 26.Rugtveit J, Scott H, Halstensen TS, Norstein J, Brandtzaeg P: Expression of the L1 antigen (calprotectin) by tissue macrophages reflects recent recruitment from peripheral blood rather than upregulation of local synthesis: implications for rejection diagnosis in formalin-fixed kidney specimens. J Pathol 1996, 180:194-199 [DOI] [PubMed] [Google Scholar]
- 27.Frangogiannis NG, Michael LH, Entman ML: Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res 2000, 48:89-100 [DOI] [PubMed] [Google Scholar]
- 28.Chilosi M, Mombello A, Montagna L, Benedetti A, Lestani M, Semenzato G, Menestrina F: Multimarker immunohistochemical staining of calgranulins, chloroacetate esterase, and S100 for simultaneous demonstration of inflammatory cells on paraffin sections. J Histochem Cytochem 1990, 38:1669-1675 [DOI] [PubMed] [Google Scholar]
- 29.Goebeler M, Roth J, Teigelkamp S, Sorg C: The monoclonal antibody MAC387 detects an epitope on the calcium-binding protein MRP14. J Leukoc Biol 1994, 55:259-261 [DOI] [PubMed] [Google Scholar]
- 30.Redwood SR, Ferrari R, Marber MS: Myocardial hibernation and stunning: from physiological principles to clinical practice. Heart 1998, 80:218-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorge H, Schulz R, Heusch G: Pathophysiology of hibernation, stunning, and ischemic preconditioning. Thorac Cardiovasc Surg 1998, 46(Suppl 2):255-262 [DOI] [PubMed] [Google Scholar]
- 32.Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML: Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation 1998, 98:687-698 [DOI] [PubMed] [Google Scholar]
- 33.Patella V, Marino I, Arbustini E, Lamparter-Schummert B, Verga L, Adt M, Marone G: Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation 1998, 97:971-978 [DOI] [PubMed] [Google Scholar]
- 34.Metcalfe DD, Baram D, Mekori YA: Mast cells. Physiol Rev 1997, 77:1033-1079 [DOI] [PubMed] [Google Scholar]
- 35.Galis ZS, Sukhova GK, Kranzhofer R, Clark S, Libby P: Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc Natl Acad Sci USA 1995, 92:402-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang KC, Wolters PJ, Steinhoff M, Bidgol A, Blount JL, Caughey GH: Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J Immunol 1999, 162:5528-5535 [PubMed] [Google Scholar]
- 37.Weber KT, Brilla CG, Janicki JS: Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res 1993, 27:341-348 [DOI] [PubMed] [Google Scholar]
- 38.Weber KT: Targeting pathological remodeling: concepts of cardioprotection and reparation. Circulation 2000, 102:1342-1345 [DOI] [PubMed] [Google Scholar]
- 39.Stuve O, Chabot S, Jung SS, Williams G, Yong VW: Chemokine-enhanced migration of human peripheral blood mononuclear cells is antagonized by interferon beta-1b through an effect on matrix metalloproteinase-9. J Neuroimmunol 1997, 80:38-46 [DOI] [PubMed] [Google Scholar]
- 40.Antoniades HN, Neville-Golden J, Galanopoulos T, Kradin RL, Valente AJ, Graves DT: Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 1992, 89:5371-5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith RE, Strieter RM, Zhang K, Phan SH, Standiford TJ, Lukacs NW, Kunkel SL: A role for C-C chemokines in fibrotic lung disease. J Leukoc Biol 1995, 57:782-787 [DOI] [PubMed] [Google Scholar]
- 42.Lehmann MH, Kuhnert H, Muller S, Sigusch HH: Monocyte chemoattractant protein 1 (MCP-1) gene expression in dilated cardiomyopathy. Cytokine 1998, 10:739-746 [DOI] [PubMed] [Google Scholar]
- 43.Aukrust P, Ueland T, Muller F, Andreassen AK, Nordoy I, Aas H, Kjekshus J, Simonsen S, Froland SS, Gullestad L: Elevated circulating levels of C-C chemokines in patients with congestive heart failure. Circulation 1998, 97:1136-1143 [DOI] [PubMed] [Google Scholar]
- 44.Behr TM, Wang X, Aiyar N, Coatney RW, Li X, Koster P, Angermann CE, Ohlstein E, Feuerstein GZ, Winaver J: Monocyte chemoattractant protein-1 is upregulated in rats with volume-overload congestive heart failure. Circulation 2000, 102:1315-1322 [DOI] [PubMed] [Google Scholar]
- 45.Kumar AG, Ballantyne CM, Michael LH, Kukielka GL, Youker KA, Lindsey ML, Hawkins HK, Birdsall HH, MacKay CR, LaRosa GJ, Rossen RD, Smith CW, Entman ML: Induction of monocyte chemoattractant protein-1 in the small veins of the ischemic and reperfused canine myocardium. Circulation 1997, 95:693-700 [DOI] [PubMed] [Google Scholar]
- 46.Kakio T, Matsumori A, Ono K, Ito H, Matsushima K, Sasayama S: Roles and relationship of macrophages and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the ischemic and reperfused rat heart. Lab Invest 2000, 80:1127-1136 [DOI] [PubMed] [Google Scholar]
- 47.Kolattukudy PE, Quach T, Bergese S, Breckenridge S, Hensley J, Altschuld R, Gordillo G, Klenotic S, Orosz C, Parker-Thornburg J: Myocarditis induced by targeted expression of the MCP-1 gene in murine cardiac muscle. Am J Pathol 1998, 152:101-111 [PMC free article] [PubMed] [Google Scholar]
- 48.Gharaee-Kermani M, Denholm EM, Phan SH: Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem 1996, 271:17779-17784 [DOI] [PubMed] [Google Scholar]
- 49.Lakshminarayanan V, Lewallen M, Frangogiannis NG, Evans AJ, Wedin KE, Michael LH, Entman ML: Reactive oxygen intermediates induce monocyte chemotactic protein-1 in vascular endothelium after brief ischemia. Am J Pathol 2001, 159:1301-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]