Abstract
Nonselenium glutathione peroxidase (NSGP) is a new member of the antioxidant family. Using antibodies to recombinant NSGP we have examined the distribution of this enzyme in normal, Parkinson’s disease (PD), and dementia with Lewy body disease (DLB) brains. We have also co-localized this enzyme with α-synuclein as a marker for Lewy bodies. In normal brains there was a very low level of NSGP staining in astrocytes. In PD and DLB there were increases in the number and staining intensity of NSGP-positive astrocytes in both gray and white matter. Cell counting of NSGP cells in PD and DLB frontal and cingulated cortices indicated there was 10 to 15 times more positive cells in gray matter and three times more positive cells in white matter than in control cortices. Some neurons were positive for both α-synuclein and NSGP in PD and DLB, and double staining indicated that NSGP neurons contained either diffuse cytoplasmic α-synuclein deposits or Lewy bodies. In concentric Lewy bodies, α-synuclein staining was peripheral whereas NSGP staining was confined to the central core. Immunoprecipitation indicated there was direct interaction between α-synuclein and NSGP. These results suggest oxidative stress conditions exist in PD and DLB and that certain cells have responded by up-regulating this novel antioxidant enzyme.
Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) are late-onset neurodegenerative diseases characterized by the presence of fibrillar cytoplasmic inclusions termed Lewy bodies (LBs) in the brain. In PD the brain regions primarily affected are the basal forebrains and brainstem especially the substantia nigra, whereas in DLB, abundant LBs are also found in widespread cerebral cortical regions. 1 LBs are intraneuronal inclusions composed of numerous modified proteins including the presynaptic protein α-synuclein. 2 In addition to proteins, lipids have been demonstrated in brainstem 3 and cortical LBs. 4
The formation of LBs in rare familial cases could be explained by mutations in α-synuclein, but the cause of the vast majority of cases is unknown. 5 Environmental and genetic factors may all play a role in the development of LBs in PD. Several hypotheses including a viral origin, dysfunction of mitochondrial complex 1 activity, and oxidative stress have been put forward to explain the development of PD. No one hypothesis fully explains the development of PD, but several observations are consistent with a role for oxidative stress in the development of PD. 6-8 A defect in mitochondrial complex 1 activity is reported in PD. 9,10 A high degree of nitration of tyrosine residues in α-synuclein has been reported in PD. 11 The nitrotyrosine could be because of the presence of the strong nitrating agent peroxynitrite, the product of superoxide and nitric oxide and strong evidence of oxidative stress conditions. There is a consistent decrease in the level of the antioxidant glutathione and increased lipid peroxidation in the substantia nigra in the brains of people dying with PD. 12,13 The reduced form of glutathione is an antioxidant used to remove hydrogen peroxide and protect against oxidation of proteins in a reaction catalyzed by glutathione peroxidase.
Glutathione peroxidase is a selenium-dependent enzyme important in the cellular antioxidant defense mechanism. Glutathione peroxidase has been extensively examined in human brain and in PD and there is wide variability in the reported levels but generally the levels and activity are not elevated. 14,15 The enzyme has been localized exclusively in glial cells. 16
Recently, we have identified a novel nonselenium glutathione peroxidase (NSGP) from rat lung. This enzyme has no amino acid sequence homology to any of the known selenium-dependent glutathione peroxidase enzymes. 17 This enzyme has antioxidant and phospholipase A2 activity that can reduce phospholipid hydroperoxides, 18,19 and thus could play a role in the antioxidant mechanism, especially toward oxidized lipids. The enzyme has not been previously examined in human brain. In this study, we raised and characterized an antibody that recognized human NSGP. We then examined the cellular locations of the enzyme in normal human brains, and in LB pathology in PD and DLB, using immunoblotting, immunocytochemistry, and immunoelectron microscopy.
Materials and Methods
Production of Recombinant NSGP
Screening of an adult rat lung cDNA library (Clonetech Laboratories, Palo Alto, CA) with rabbit polyclonal anti-NSGP 17 produced a 1435-bp cDNA. Sequencing of this clone demonstrated that it was identical to the published sequence of a rat lung acidic Ca2+-independent PLA2 19 that was later shown to possess both NSGP and phospholipase activity. 20 Using this cDNA as a template, polymerase chain reaction was performed using primers 5′-GGAATTCATGCCCGGA-GGGCTGCTTCTC-3′ and 5′-CCGCTCGAGCGGGTTCCCGCAGACTTAAGGCTG-3′, which included restriction sites for EcoR1 and XhoI (in bold) on the upstream and downstream primers, respectively. The amplicon generated by these primers spans the entire coding sequence of NGSP. After amplification, the products were treated with EcoR1 and XhoI to produce cohesive ends, purified, and cloned in frame into the glutathione S-transferase expression vector pGEX-6P (Amersham Pharmacia Biotech, Piscataway, NJ, USA); the expression construct was sequenced to ensure fidelity of polymerase chain reaction amplification. Transformation of bacteria, purification of the fusion protein, and cleavage of the glutathione S-transferase tail from the recombinant NGSP were all performed according to the manufacturer’s instructions. The purified protein was concentrated by lyophilization and used for antibody production.
Antibodies
Antibodies against NSGP
Antiserum was raised in New Zealand White rabbits using recombinant rat NSGP as antigen. The IgG fraction was obtained using Protein A affinity chromatography and concentrated using a Centriprep concentrator. The concentrated antibody was stored in phosphate-buffered saline (PBS) in small aliquots at −20°C. Chicken anti-NSGP antibodies were obtained from Antibody Technology Pty. Ltd., Stirling, South Australia.
Antibodies against α-Synuclein
α-Synuclein antibodies were raised in sheep against human α-synuclein peptide sequence 116 to 131. The antibodies were affinity-purified using the antigen and extensively characterized as described. 4,21-23
Antibodies against Glial Fibrillary Acidic Protein (GFAP)
GFAP antibodies were obtained from DAKO Pty. Ltd., Botany, NSW, Australia.
Tissue
The human brain tissue used in this study is listed in Table 1 ▶ . Brain tissue was obtained from the National Health and Medical Research Council South Australian Brain Bank. These brains were removed at autopsy, generally within 24 hours of death. Brains were bisected and one half was snap-frozen at −70°C while the other half was either perfusion-fixed or immersion-fixed with 4% formaldehyde and 2% picric acid as previously described. 4 Tissue blocks were embedded in paraffin and 5-μm sections obtained for each brain. Each case was examined from a clinicopathological perspective to determine the pathology. In this article brain tissue from seven PD cases, five DLB cases, and five normal cases were examined. Brain regions examined include the frontal cortex, cingulate, and the brain stem.
Table 1.
Human Brain Tissues Used in Study
| Case | Age | Gender | Region |
|---|---|---|---|
| PD (123/93) | 65 | Male | BS |
| PD (52/99) | 84 | Male | BS |
| PD (7/95) | 68 | Female | BS |
| PD (P21) | 80 | Female | BS, C, MFC |
| PD (P22) | 85 | Female | C, MFC |
| PD (P25) | 83 | Female | MFC |
| PD (P31) | 81 | Female | C, MFC |
| DLB (P23) | 67 | Male | C, MFC |
| DLB (P26) | 91 | Female | C, MFC |
| DLB (P30) | 81 | Female | C, MFC |
| DLB (P36) | 80 | Male | C, MFC |
| DLB (P40) | 86 | Male | C, MFC |
| Normal (N10) | 79 | Female | C, FC |
| Normal (N18) | 69 | Male | C, FC |
| Normal (N19) | 61 | Female | C, FC |
| Normal (N20) | 84 | Female | C, FC |
| Normal (N24) | 71 | Female | C, FC |
Abbreviations: PD, Parkinson’s disease; DLB, dementia with Lewy bodies; BS, brain stem; C, cingulate; MFC, mid-frontal cortex; FC, frontal cortex.
Homogenates
Frozen tissue from the cortex, cingulate, and substantia nigra from each case was carefully dissected to obtain white and gray matter and homogenized in PBS using a motorized Wheaton Teflon pestle tissue grinder (clearance, 0.15 to 0.23 mm). The homogenate was centrifuged at 8000 × g for 30 minutes to remove particulate matter and the supernatant was assayed for total protein and frozen at −70°C.
LB Isolation
Cortical LBs were isolated using magnetic bead immunoprecipitation from fresh frozen cortices from DLB cases as described. 4,22 For immunoelectron microscopy, isolated LBs were pelleted in a microcentrifuge at 13,000 × g for 5 minutes, fixed for 10 minutes with 0.25% glutaraldehyde-2% formaldehyde in PBS, pH 7.4, postfixed in 0.5% osmium tetroxide for 30 minutes, and processed as described in the Electronmicroscopy section.
One-Dimensional Electrophoresis and Western Blotting
Proteins from brain homogenates were separated using a Bio-Rad Minigel System (Bio-Rad, Richmond, CA) and 4 to 20% precast polyacrylamide gel electrophoresis gels (Gradipore Ltd., Frenchs Forrest, NSW, Australia). The separated proteins were transferred onto nitrocellulose using a Semidry Transfer Unit (model TE70 SemiPhor; Hoeffer Scientific Instruments, Pharmacia) using a transfer buffer of 0.25 mol/L Tris, 0.192 mol/L glycine, and 20% methanol for 90 minutes. The nitrocellulose membrane containing the transferred protein was blocked with milk proteins and incubated overnight with a primary antibody to NSGP. The antigen:antibody complex was visualized using a Vector ABC kit (Vector Laboratories Inc., Burlingame, CA).
Immunohistochemistry
Co-Localization of NSGP and α-Synuclein
Sections from each brain were incubated with either sheep anti-α-synuclein as a marker for LBs, rabbit anti-NSGP, or both antibodies for 18 hours. For 3,3′ diaminobenzidine tetrahydrochloride dihydrate (DAB) staining, the single antibodies were visualized with either donkey anti-sheep-conjugated horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA) or in the case of NSGP donkey anti-rabbit conjugated to biotin (Jackson ImmunoResearch) and the antibody complex was visualized using a Vector ABC kit. Sections were examined and photographed with an Olympus BX50 microscope linked to a Kodak EOS-DCS digital camera. For fluorescence staining the α-synuclein was visualized with donkey anti-sheep-conjugated fluorescein isothiocyanate (Jackson ImmunoResearch) and NSGP with donkey anti-rabbit-conjugated Cy5 (Jackson ImmunoResearch). Sections were examined using a Bio-Rad confocal laser scanning microscope and software package (Bio-Rad MRC 1024, Bio-Rad).
Immunoabsorption of Antibody
To confirm that the staining observed was specific, two aliquots of NSGP antibody as used in the previous staining were prepared, with one aliquot being immunoabsorbed for 24 hours with excess NSGP antigen. Both aliquots were then centrifuged and the supernatants used to stain identical tissue and Western blots.
Co-Localization of NSGP and GFAP
Sections from each brain were incubated for 18 hours with antibodies to NSGP and GFAP to determine whether NSGP was specifically up-regulated in astrocytes. For fluorescence staining, NSGP was detected using donkey anti-rabbit-conjugated Cy5 (Jackson ImmunoResearch) and GFAP with donkey anti-mouse-conjugated Cy3 (Jackson ImmunoResearch). Sections were examined using a Bio-Rad confocal laser-scanning microscope and software package.
Electronmicroscopy
Fresh brain pieces and pellets from magnetic bead immunoisolation were immersion-fixed with 2.0% paraformaldehyde, 0.25% glutaraldehyde (w/v) in 0.1 mol/L phosphate buffer at pH 7.4 for 3 hours and postfixed in 0.5% osmium tetroxide (w/v) for 1 hour at 4°C. Blocks were dehydrated through graded acetone at 4°C and embedded in L.R. White resin (London Resin, Basingstoke, UK). Ultrathin sections were incubated for 3 hours with rabbit anti-NSGP and visualized with donkey anti-rabbit-conjugated 12-nm gold (Jackson ImmunoResearch). NSGP preimmune serum was used as a negative control. The sections were stained with lead citrate and uranyl acetate and examined in a JEM 1200 EX electron microscope (Jeol, Tokyo, Japan).
Counting of Positive Glial Cells
NSGP-positive glial cells were counted at ×40 magnification with a graticule eyepiece (250 μm2 or 0.0625 mm2) using an Olympus HO-2 microscope from five fields from seven control, six DLB, and four PD cases. In each case, the regions were selected from the white and gray matter of the frontal cortex and cingulate. The positive cells from the five randomly selected regions were averaged and divided by the area of the graticule (0.0625 mm2) and expressed as cells/mm2. The mean and SE of the positive cells from each case are shown in Figure 4 ▶ . Significance at the 0.05 level of probability was determined between grouped data using Student’s t-tests. The brain stem of another three PD cases were examined histologically but were not included here as the cingulate and cortex were not available.
Figure 4.
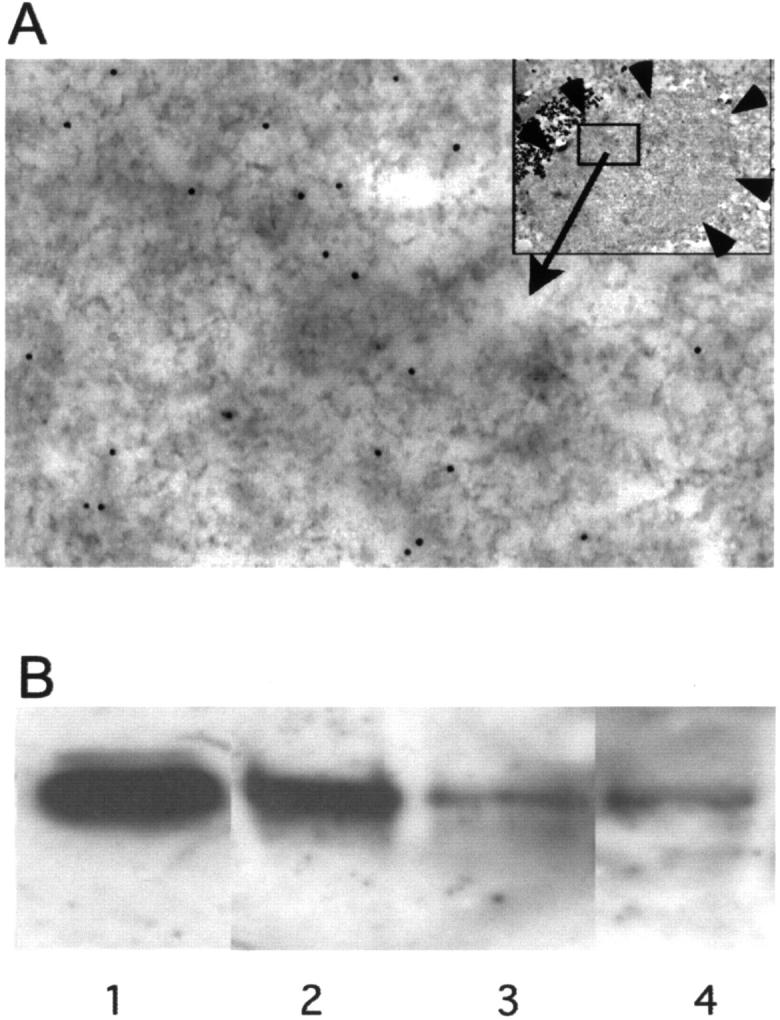
A: Electronmicrograph of a LB purified using magnetic beads and immunostained with a NSGP primary antibody and visualized with a secondary antibody conjugated to 12-nm gold. Top right inset shows the intact LB bound to magnetic beads (MB), small arrows indicate the boundary of LB. The area indicated was taken at higher power and shows the distribution of the gold particles. B: Immunoprecipitation of NSGP by anti-α-synuclein antibodies from a cytosol fraction of normal control human brain. Immunoblotting was conducted by using chicken IgY raised against recombinant NSGP, followed by biotinylated donkey anti-chicken IgY, and streptavidin-biotin-HRP complex. The membrane was developed using X-ray film and a enhanced chemiluminescence kit (Amersham Pharmacia Biotec, Sydney, Australia). Lane 1, Recombinant NSGP (∼15 ng). Lane 2, Precipitates using antibody to α-synuclein C-terminal 116 to 131 amino acids. Lane 3, Precipitates using antibody to α-synuclein N-terminal 11 to 26 amino acids. Lane 4, Precipitates from acetone-extracted cytosol using antibody to α-synuclein N-terminal 11 to 26 amino acids.
Immunoprecipitation
Fresh-frozen cortical tissue from two cases without evidence of neurological disease were homogenized in 10 vol of PBS (pH 7.4) containing a cocktail of protease inhibitors, using a motorized Wheaton Teflon pestle tissue grinder. The homogenate was centrifuged at 8000 × g for 30 minutes to obtain a cytosol fraction. In some experiments, the cytosol fraction was extracted to remove residue lipids. Affinity-purified sheep antiserum was raised against α-synuclein C-terminal (116 to 131 amino acids) and affinity-purified rabbit antiserum against N-terminal (11 to 26 amino acids) was covalently coated to tosylactivated magnetic beads (Dynabeads M-500) according to the manufacturer’s instructions. Coated beads were mixed with the human brain cytosol fraction (∼2 × 107beads/ml) and incubated overnight at 4°C. The beads were washed four times with PBS, and bound material was eluted with sodium dodecyl sulfate sample buffer. The sample was run on a one-dimensional PAGE gel and Western blotted as described above. The blot was probed with a chicken anti-nonselenium glutathione peroxidase antibody (Antibody Technology Pty. Ltd.).
Results
NSGP Is Present in Normal and Diseased Brain Tissue
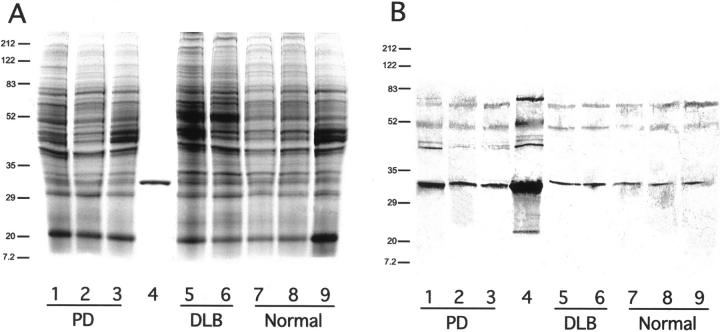
On one-dimensional reducing PAGE gels the recombinant NSGP protein ran as a clearly defined band below the prestained carbonic anhydrase standard at 33.4 kd at ∼31 kd on all tissues examined (Figure 1, A and B) ▶ . The stained gels were loaded with 10 μg of total protein per well and all bands showed staining of similar intensities. The Western blot gels were all loaded with 2.5 μg of total protein. Although breakdown products or reaggregations were not observed on the stained gels they were detected on the Western blots (Figure 1B) ▶ . The recombinant protein showed a series of reaggregations between 45 and 100 kd and a breakdown fragment at ∼15 kd. Interestingly, the human brain samples exhibited a similar range of higher molecular weight reaggregations but the lower molecular weight fragment was not seen. Western blotting indicated that there might be elevated levels in PD. After the antibody was preabsorbed with excess antigen no labeling was observed with Western blotting or immunohistochemistry, indicating that the antibody is specific.
Figure 1.
A: PAGE analysis of soluble brain proteins from white and gray matter of normal, PD, and DLB brains. Lane 1, PD, FC, and GM; lane 2, PD, FC, and WM; lane 3, PD and SN; lane 4, NSGP recombinant protein; lane 5, DLB, FC, and GM; lane 6, DLB, FC, and WM; lane 7, N, FC, and GM; lane 8, N, FC, and WM; and lane 9, N and SN. Gel was stained with Coomassie blue. Molecular weights in kd are indicated at the left. Abbreviations: FC, frontal cortex; GM, gray matter; WM, white matter; SN, substantia nigra; DLB, dementia with LBs, N, normal. B: Western blot of the same samples as indicated in A. Primary antibody was protein A-purified rabbit anti-NSGP IgG, secondary antibody was a biotin-labeled donkey anti-rabbit (711-065-152, Jackson ImmunoResearch) and visualized with a Vector ABC kit using DAB.
NSGP Is Primarily Localized in Astrocytes in Human Brains
Light immunohistochemistry of normal cortical tissue using the NSGP antibody indicated that this protein is present in the cytoplasm of a population of round cells with a circular or ovoid nucleus with small projections that resembled astrocytes (Figure 2; A, B, and E) ▶ . Not all glial cells were positive and in some tissues identical adjacent cells showed different staining. No staining was observed in any neurons in normal cortex (Figure 2A) ▶ . In addition, no staining was seen with the preimmune serum.
Figure 2.
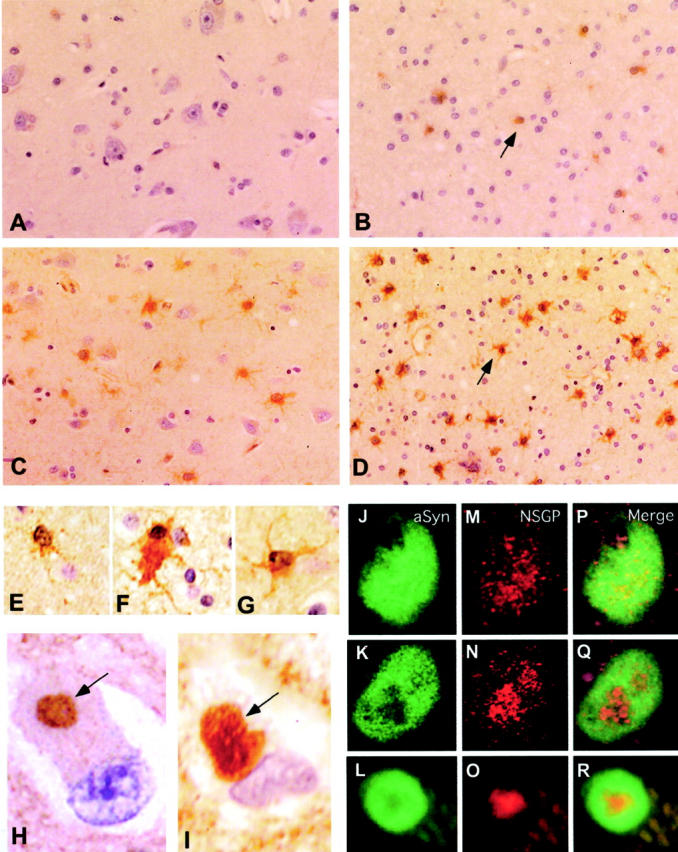
A–D: Low-power images (original magnifications, ×400) of the NSGP immunocytochemistry of gray and white gray matter from normal (A and B) and DLB (C and D) brain tissue. NSGP was visualized using a biotinylated donkey anti-rabbit secondary antibody and a Vector ABC staining kit with DAB. Sections were lightly counterstained with hematoxylin. NSGP-positive glial cells were markedly increased in both gray and white matter of DLB compared to normal. The arrows in C and D indicate NSGP-positive glial cells. E–G: Comparison of astrocyte labeling (oil immersion; original magnifications, ×1000) with NSGP antibodies in normal white cortical matter (E), and white (F) and gray matter (G) of DLB cortical tissue. H and I: LB labeling with rabbit anti-NSGP antibodies (H), and labeling with sheep anti-α-synuclein antibodies (I) within the gray matter of DLB tissue (oil immersion; original magnifications, ×1000). Immunoreactivity was visualized with biotinylated secondary antibodies and a Vector ABC kit using DAB. Arrows indicate the positively stained LBs within the cytoplasm of neurons. J–R: J to L show α-synuclein labeling with fluorescein isothiocyanate in homogeneous LBs (J) and two forms of concentric LBs (K and L); M to O show NSGP labeling with Cy5 in the same three LBs; and P to R depict the merged image showing the co-localization.
Increased Glial Staining in Brain Regions Affected in PD and DLB
In DLB and PD, there was a much greater staining in the cortex than in normal tissue from the same regions. There was staining in the cytoplasm of most astrocytes. Some astrocytes resembled those found in normal tissues whereas many took on a large star-shaped appearance with intense staining (Figure 2, C and D) ▶ . The positive astrocytes appeared larger with more processes, indicative of hyperplastic changes (Figure 2, F and G) ▶ . The NSGP staining appeared to be regional. In the white matter of most regions examined, some regions had intense astrocyte staining whereas other areas on the same section showed far less staining. The gray matter closer to the white matter boundary showed more intense staining than the gray areas more distant. The staining seen with the NSGP antibody was completely blocked when the antibody was immunoabsorbed with the antigen confirming the staining was indeed specific.
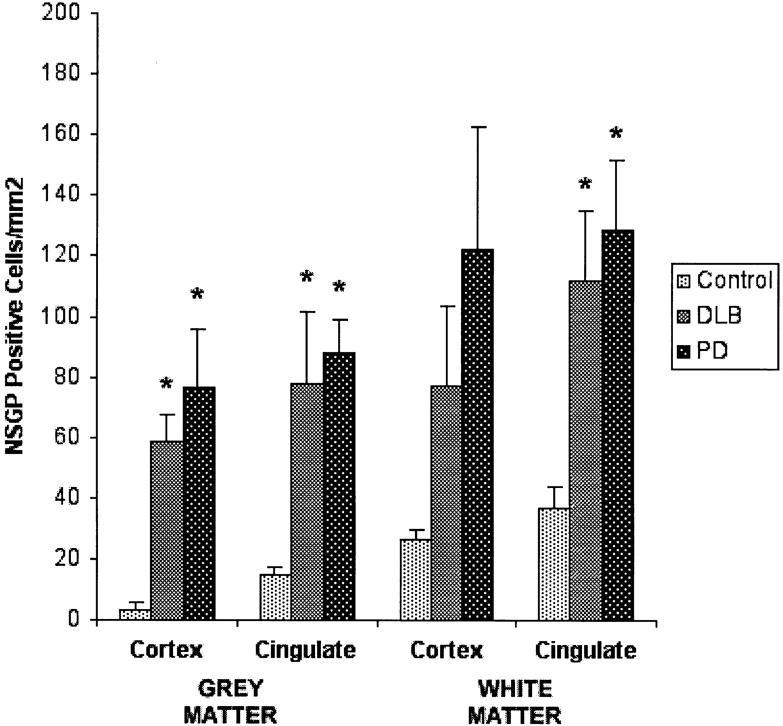
Counting of NSGP-positive glial cells in white and gray matter of frontal and cingulated cortical regions from different cases confirmed the histological observations. There was ∼15 times more positive cells in the gray matter and four times more in the white matter in the frontal cortex in both DLB and PD (Figure 3) ▶ . In the cingulate gray and white matter there was a threefold to fivefold increase in the number of positive cells in DLB and PD. The increases in DLB and PD gray matter astrocyte numbers were highly significant. Similarly, the increases in positive astrocytes in the cingulate white matter were highly significant, but the cortical data were more varied and not significant at the 0.05 level of probability. The low level of astrocyte staining was very consistent throughout all control cases as indicated by the tight standard errors. Similarly, the increase number of NSGP-positive cells in the gray mater was consistent throughout all cases. The increase in positive astrocytes in cortical white mater in both DLB and PD was much more varied. Some cases showed very high levels of astrocyte activation whereas other cases were similar to the control cases.
Figure 3.
Number of NSGP-positive glial cells in the white and gray matter of control (n = 7), DLB (n = 6), and PD (n = 4) brains. Mean ± SE. Significance was determined using unpaired Student’s t-tests and the asterisks indicate significance at the 0.05 level of probability.
Although the increase in the number of positive cells in the white matter was not as great as in the gray matter, the level of astrocyte activation was far greater. The white matter astrocytes were much larger with approximately five times the level of NSGP staining (see Figure 2; E, F, and G ▶ ). This increase in staining taken with the increase in the number of astrocytes indicates a similar total increase as in the gray matter. The gray mater astrocytes had increased in number but were not activated to the same level as the white matter astrocytes.
Confocal microscopy supported the findings at the light microscopy level. Very little staining was observed in normal brain tissue or disease tissue with the preimmune serum. A population of cells with a circular nucleus with few projections (astrocytes) were positive for NSGP but not for α-synuclein in both normal and disease brain tissue. In addition, a population of star-shaped cells with an abundant cytoplasm and many extensive projections (activated astrocytes) stained very strongly in the white matter of disease brain tissue. These were also observed in the gray matter adjacent to the white matter in disease tissue.
Co-localization of GFAP and NSGP indicated that both antigens were present in astrocytes and that the staining was much more intense in disease brains. NSGP stained more cells than GFAP, and many cells that had the appearance of astrocytes were labeled with NSGP but not with GFAP.
NSGP in Neurons with LBs in PD and DLB
In PD and DLB, the neurons containing LB-like inclusions, as compared with those determined by α-synuclein staining, were also positive for NSGP (Figure 2, H and I) ▶ . When the general outline of the LB could be seen, the NSGP staining was clearly more intense toward the central core of the LB (Figure 2H) ▶ . Double staining confirmed the co-localization of the LB marker α-synuclein and NSGP in LBs (Figure 2; P, Q, and R) ▶ . Confocal microscopy analysis indicated that NSGP was detected in ∼50% of α-synuclein-positive LBs in both PD and DLB. Under the confocal microscope, LBs showed different staining patterns for α-synuclein and NSGP. In general, the staining area for α-synuclein was greater than for NSGP. Some LBs had an even distribution of α-synuclein staining (homogenous LBs, Figure 2J ▶ ), whereas others had more peripheral labeling with an unstained central region or core (concentric LBs; Figure 2, K and L ▶ ). The NSGP staining appeared granular, compared to α-synuclein staining, and staining was more intense in the central region although there was a light staining throughout the entire LB in homogeneous LBs (Figure 2M) ▶ . In concentric LBs, NSGP was very intense in the central core where α-synuclein was lacking (Figure 2, N and O) ▶ . When the two images are merged the area of co-localization was clearly evident (Figure 2; P, Q, and R) ▶ .
Although there was an increase in the level of staining of astrocytes in the cingulate and cortex in PD, we found very few LBs in these regions. In the brain stem in PD, many large LBs and Lewy neurites were observed in the substantia nigra. Approximately 50% of the LBs were positive for NSGP. Only the occasional large Lewy neurite had some labeling, most Lewy neurites were not positive for NSGP.
Apart from some labeling in LBs, the substantia nigra did not exhibit the same level of up-regulation of NSGP as other brain regions. The large neurons in the oculomotor nucleus however, showed more staining than any other neurons in any region examined. These neurons were not positive for α-synuclein, and contained an even distribution of NSGP.
NSGP Is Localized in Purified LBs and Interacts with α-Synuclein
To examine whether NSGP is incorporated into LBs, immunoelectron microscopy was conducted on magnetic bead-immunopurified LBs. Under the electron microscope, the distribution of immunogold labeling for NSGP was patchy within given LBs (Figure 4A) ▶ , consistent with the distribution observed at the confocal level. It was difficult to differentiate between concentric and homogenous LBs at this level, but the center of some LBs did appear to contain vesicles that were more heavily labeled with gold particles than the surrounding LB material and were possibly of the concentric type. These results indicate NSGP is incorporated into LB filamentous structures.
Localization of NSGP in LBs suggests the protein may interact with α-synuclein, abnormal α-synuclein filamentous aggregates, or any other LB components. To examine if NSGP interacts with normal α-synuclein, the cytosol fractions from normal control brains without neurodegenerative pathology were immunoprecipitated using affinity-purified α-synuclein antibodies. A 31-kd NSGP-immunopositive band was co-precipitated with α-synuclein (Figure 4B ▶ , lanes 1 to 4). No high molecular species were detected. Both N- or C-terminal α-synuclein antibodies were able to pull down NSGP (Figure 4B ▶ , lanes 2 and 3) but the C-terminal antibodies appeared more effective than N-terminal antibodies, suggesting the N-terminal portion of α-synuclein is more important in mediating the interaction. Both NSGP and α-synuclein interact with lipids, 18,24,25 and α-synuclein N-terminal portion contains the lipid binding sites, 24,25 suggesting lipids could be involved in the interaction between α-synuclein and NSGP. To further examine if there is direct interaction between α-synuclein and NSGP, the cytosol fractions were extracted with acetone to remove any residue lipids, and immunoprecipitated with α-synuclein C-terminal antibodies. A smaller amount of NSGP was still co-precipitated with α-synuclein (Figure 4B ▶ , lane 4), indicating some direct binding between NSGP and α-synuclein.
Discussion
We have shown for the first time that NSGP is present in human brain tissue and that it is up-regulated in astrocytes and in neurons containing LBs, possibly as a result of oxidative stress. NSGP was first identified in human skin where it was implicated in wound repair, 26 and in rat lung where it lines the entire conducting airways. 17 In the human brain it is present as a 31-kd protein in both gray and white matter in all tissues examined. This was slightly higher than the native protein (26 kd) previously observed in rat tissue. 17 It did run however, identical to the rat recombinant protein used as a marker. This was probably because of different gel conditions, in this study 4 to 20% precast gradient gels were used as opposed to standard 12% gels in the previous study and unstained standards were used. NSGP is an antioxidant enzyme catalyzing the conversion of hydrogen peroxide to H2O using reduced glutathione and has no sequence homology with the selenium-dependent forms of glutathione peroxidase (SGP) that are reported to be exclusively located in glial cells. 16 A significant feature of NSGP is that it is able to reduce phospholipid hydroperoxides unlike SGP. 18 This activity might be highly significant and explain the up-regulation and localization of NSGP in neurons. In addition, this enzyme is reported to be bifunctional with some phospholipase activity and both active sites have been mapped. 20 Although it is not known what initiates oxidative stress in PD or DLB the resulting generation of reactive oxygen species leads to damage to DNA, proteins, and lipids. 27
In the lung this enzyme is a secreted protein and the possibility exists that this is also a secreted protein in the brain. Although the antibody was protein G-purified there was a much higher background than with α-synuclein or the preimmune serum suggesting this protein might also be secreted by some brain cells. Although PAGE gels and Western blots are only a crude measure of specific protein levels, there did appear to be increased levels in the PD samples shown in Figure 1 ▶ but not in DLB. Further studies using an enzyme-linked immunosorbent assay are required to accurately determine tissues levels.
The oxidative stress that has lead to the formation of LBs would appear to be more widespread than just affecting neurons. In normal brain tissue the astrocytes are small round cells with little cytoplasm with only a few processes evident. NSGP staining is much more intense in disease brain tissue than in normal tissue with widespread activation of astrocytes from the brain stem to the cortex. In PD, the pigmented cells of the substantia nigra were not heavily labeled with NSGP. We suspect that this was because the susceptible neurons had been previously destroyed. The large neurons of the occulomotor region of the brainstem were more intensely labeled with NSGP even though they were not positive for α-synuclein. The ability to up-regulate the antioxidant enzymes such as NSGP may determine whether certain neurons are sensitive or more resilient to oxidative stress conditions.
Despite the fact that the substantia nigra is one of the major regions devastated in PD, this region was not heavily stained for NSGP. Intense astrocyte staining in the white matter was observed in some but not all regions of the brain stem. Although very few LBs were seen in other brain regions in PD, the cingulate and cortex regions examined showed extensive, regional up-regulation of NSGP in astrocytes. In PD and DLB many of the astrocytes were large star-shaped cells with many processes with intense staining for NSGP and appeared to be activated. This would also support the model that oxidative stress conditions exist in these disease brains. The intense staining of astrocytes was seen mainly in the white matter although there was a much greater increase in the number of positive cells in the gray matter. The staining in the gray matter was highest at the boundary with the white matter, which decreased further from the white matter boundary. This would infer that the oxidative stress conditions are not confined to the gray matter where LBs are found and that both white and gray matter are involved. The up-regulation of this enzyme is regional. Some regions were affected much more than others in close proximity. It is difficult to explain these findings but they could possibly be related to regional blood flow. Our preliminary studies suggests there is also an increase in NSGP in glia but not in neurons in Alzheimer’s disease, indicating that up-regulation of NSGP in glia is not unique to PD and DLB but the neuronal response seems to be unique.
The pathological hallmark of PD and DLB is the presence of LBs, which are an ordered collection of damaged lipids and proteins, many of which are aggregated, ubiquinated, and nitrated. 4,11,28 α-Synuclein is a major modified LB protein and we have used this as a marker for LBs in the current study. We have previously shown that LBs are not uniform and that α-synuclein is located more peripherally than ubiquitin and that the concentric types have a lipid core. 4 In concentric LBs, NSGP has a similar distribution to the lipid and this might be because of its role in reducing lipid peroxides. Light, confocal, and electron microscopy all confirmed the presence of NSGP in LBs. NSGP was present in the cell bodies of some neurons that were positive for diffuse cytoplasmic α-synuclein but did not have LBs, suggesting that NSGP is present before LBs form. Approximately 50% of the LBs were positive for NSGP, and LBs that were negative tended to be larger. We suggest that the neuron responds to the oxidative stress conditions by unregulating NSGP as a protective mechanism. As these conditions persist cellular damage occurs leading to the formation of LBs. When the cell dies NSGP is no longer expressed and only the protease resistant LB remains. The NSGP staining in neurons and LBs is not as intense as the staining for α-synuclein and was not evident at the conventional fluorescence microscopic level, but was clearly present at the confocal level and with DAB staining. The confocal microscopy was performed using Cy5-conjugated donkey anti-rabbit IgG and fluorescein isothiocyanate-conjugated donkey anti-sheep IgG, to eliminate cross reactivity and bleach through. Each secondary antibody was immunoabsorbed against several other species, and there is little overlap in the emission spectrums of these two fluorophores.
Although the LB pathology in DLB and PD are essentially the same, why these have developed LBs in different regions is still a major question. Although speculative, it would appear from this study that NSGP is up-regulated in specific cells early in the disease process as part of the neurons defense against oxidative stress because it is only present when LBs are present in viable neurons as indicated by an intact nucleus. The trigger for the up-regulation of this enzyme might be the change in cellular redox potential, which is primarily determined by the level of reduced glutathione. Low levels of reduced glutathione in affected regions has been a consistent finding in the PD brain. 29,30 Changes in cellular redox potential are reported to activate cell-signaling pathways including transcription factors. 31,32
Our results indicate the NSGP is a component of LBs, and the interaction between NSGP and α-synuclein may at least partially account for the accumulation of this protein in LBs. LBs also contain many other proteins as well as lipids and these could be the potential binding partner for NSGP. It is interesting to note that NSGP staining is more widespread in homogenous LBs, whereas it is concentrated in the core in concentric LBs. These patterns suggest that after recruitment into LBs, NSGP may undergo redistribution to certain subdomains that have higher binding affinity to NSGP. The LB core contains concentrated lipids that could be the substrates for NSGP. However, it remains to see if such accumulation and redistribution of NSGP in LBs has any physiological or pathological consequence.
There is considerable evidence that oxidative stress and α-synuclein are involved in neurodegeneration in PD. There are however, many unresolved questions relating to the roles of oxidative stress and α-synuclein in the formation of inclusions in PD and other neurodegenerative diseases. One question is whether oxidative stress causes damage to RNA, DNA, proteins, and lipids and results in protein and lipid aggregation and the formation of inclusion bodies and α-synuclein is just one of many proteins involved, or, whether α-synuclein overexpression results in the oxidative stress that then damages DNA, RNA, proteins, and lipids. Hsu and colleagues 33 using a murine hypothalamic cell line have reported that α-synuclein overexpression results in α-synuclein-positive inclusions, mitochondrial modification, and the generation of free radicals, which suggests α-synuclein plays an active role in producing oxidative stress. Using the same murine hypothalamic cell line transfected with α-synuclein, they found the cells were more resistant to oxidative stress, which suggests α-synuclein has a protective role. 34 Overexpression of α-synuclein may well deplete cellular energy reserves and result in oxidative stress and may be a similar process to the genetic forms of PD. Whether α-synuclein has an active role in neurodegeneration or is just an innocent bystander remains to be determined. Nunomura and co-workers 35 have reported that 8-hydroxyguanisone, an oxidized nucleoside from RNA, is elevated well before the formation of plaques and tangles in Alzheimer’s disease and that oxidative stress is an early event in this disease. We would also suggest that the elevated levels of NSGP in neurons is a early event in PD as its up-regulation is more widespread than LBs.
In conclusion, we have demonstrated for the first time that NSGP is present in the human brain and that it is present in neurons that contain LBs. In addition, we have demonstrated widespread gliosis in many brain regions without LB pathology. Because this only occurred in PD or DLB and not in control brains we suggest that the most likely explanation for the up-regulation of NSGP is in response to increased oxidative stress conditions in these diseases.
Acknowledgments
We thank Rebecca McNamara for skilled assistance; and the National Health and Medical Research Council of Australia, the Flinders Medical Centre Foundation, and the Flinders Medical Research Institute Imaging Facility for their support.
Footnotes
Address reprint requests to John H. T. Power, Ph.D., Department of Human Physiology, School of Medicine, Flinders University of South Australia, Sturt Rd., Bedford Park, Australia 5042. E-mail: john.power@flinders.edu.au.
Supported by the National Health and Medical Research Council of Australia (project number 102170) and the Flinders Medical Centre Foundation.
References
- 1.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH: Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology 1996, 47:1113-1124 [DOI] [PubMed] [Google Scholar]
- 2.Goedert M: Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci 2001, 2:492-501 [DOI] [PubMed] [Google Scholar]
- 3.Den Hartog Jager WA: Sphingomyelin in Lewy inclusions bodies in Parkinson’s disease. Arch Neurol 1969, 21:615-619 [DOI] [PubMed] [Google Scholar]
- 4.Gai WP, Yuan HX, Li XQ, Power JHT, Blumbergs PC, Jensen PH: In situ and in vitro study of colocalization and segregation of α-synuclein, ubiquitin and lipids in cortical Lewy bodies. Exp Neurol 2000, 166:324-333 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Dawson V, Dawson T: Oxidative stress and genetics in the pathogenesis of Parkinson’s disease. Neurobiol Dis 2000, 7:240-250 [DOI] [PubMed] [Google Scholar]
- 6.Foley P, Riederer P: Influence of neurotoxins and oxidative stress on the onset and progression of Parkinson’s disease. J Neurol 2000, 247:82-94 [DOI] [PubMed] [Google Scholar]
- 7.Fahn S, Cohen G: The oxidative stress hypothesis in Parkinson’s disease: evidence supporting it. Ann Neurol 1992, 32:804-812 [DOI] [PubMed] [Google Scholar]
- 8.Jenner P, Dexter DT, Sian J, Schapira AHV, Marsden CD: Oxidative stress as cause of nigral cell death in Parkinson’s disease and incidental Lewy body disease. Ann Neurol 1992, 32:S82-S87 [DOI] [PubMed] [Google Scholar]
- 9.Davey GP, Peuchen S, Clark JB: Energy thresholds in brain mitochondria. J Biol Chem 1998, 273:12753-12757 [DOI] [PubMed] [Google Scholar]
- 10.Poirer J, Thiffcault C: Are free radicals involved in the pathogenesis of idiopathic Parkinson’s disease. Eur Neurol 1993, 33:38-43 [DOI] [PubMed] [Google Scholar]
- 11.Duda JE, Giasson BI, Chen Q, Gur TL, Hurtig HI, Stern MB, Gollomp SM, Ischiropoulos H, Lee V M-Y, Trojanowski JQ: Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am J Pathol 2000, 157:1439-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch E: Does oxidative stress participate in nerve cell death in Parkinson’s disease. Eur Neurol 1993, 33:52-59 [DOI] [PubMed] [Google Scholar]
- 13.Schulz J, Lindenau J, Seyfried J, Dichgans J: Glutathione, oxidative stress and neurodegeneration. Eur J Biochem 2000, 267:4904-4911 [DOI] [PubMed] [Google Scholar]
- 14.Sian J, Dexter DT, Lees AJ, Daniel S, Jenner P, Marsden CD: Glutathione-related enzymes in brain in Parkinson’s disease. Ann Neurol 1994, 36:356-361 [DOI] [PubMed] [Google Scholar]
- 15.Johannsen P, Velander G, Mai J, Thorling EB, Dupont E: Glutathione peroxidase in early and advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry 1991, 54:679-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F: Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 1993, 52:1-6 [DOI] [PubMed] [Google Scholar]
- 17.Power JHT, Nicholas TE: Immunohistochemical localization and characterization of a rat Clara cell 26kD protein (CC26) with similarities to glutathione peroxidase and phospholipase A2. Exp Lung Res 1999, 25:379-391 [DOI] [PubMed] [Google Scholar]
- 18.Fisher AB, Dodia C, Manevich Y, Chen J-W, Feinstein SI: Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J Biol Chem 1999, 274:21326-21334 [DOI] [PubMed] [Google Scholar]
- 19.Kim TS, Dodia C, Chen X, Hennigan BB, Jain M, Feinstein SI, Fisher AB: Cloning of a rat lung acidic Ca (2+)-independent PLA2 and its organ distribution. Am J Physiol 1998, 274:L750-L761 [DOI] [PubMed] [Google Scholar]
- 20.Chen J-W, Dodia C, Feinstein SI, Jain MK, Fisher AB: 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem 2000, 275:28421-28427 [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Sandmann-Keil D, Gai WP, Braak E: Extensive axonal Lewy neurites in Parkinson’s disease: a novel pathological feature revealed by α-synuclein immunocytochemistry. Neurosci Let 1999, 265:67-69 [DOI] [PubMed] [Google Scholar]
- 22.Gai WP, Power JHT, Blumbergs PC, Culvenor JG, Jensen PH: α-Synuclein immunoisolation of glial inclusions from multiple system atrophy brain tissue reveals multiprotein components. J Neurochem 1999, 73:2093-2100 [PubMed] [Google Scholar]
- 23.Jenson PH, Islam K, Kenney JM, Nielson MS, Power JHT, Gai WP: Microtubule-associated protein 1B is a component of cortical Lewy bodies and bind α-synuclein filaments. J Biol Chem 2000, 275:21500-21507 [DOI] [PubMed] [Google Scholar]
- 24.Davidson WS, Jonas A, Clayton DF, George JM: Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 1998, 16:9443-9449 [DOI] [PubMed] [Google Scholar]
- 25.Jensen PH, Nielson MS, Jakes R, Dotti CG, Goedert M: Binding of α-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. J Biol Chem 1998, 273:26292-26294 [DOI] [PubMed] [Google Scholar]
- 26.Munz B, Frank S, Hubner G, Olsen E, Werner S: A novel type of glutathione peroxidase: expression and regulation during wound repair. Biochem J 1997, 326:579-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ: Parkinson disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol 1999, 154:1423-1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwatsubo T, Yamaguchi H, Fujimuro M, Yokosaawa H, Ihara Y, Trojanowski JQ, Lee M: Purification and characterization of Lewy bodies from brains of patients with diffuse Lewy body disease. Am J Pathol 1996, 148:1517-1529 [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch E: Does oxidative stress participate in nerve cell death in Parkinson’s disease. Eur Neurol 1993, 33:52-59 [DOI] [PubMed] [Google Scholar]
- 30.Schulz J, Lindenau J, Seyfried J, Dichgans J: Glutathione, oxidative stress and neurodegeneration. Eur J Biochem 2000, 267:4904-4911 [DOI] [PubMed] [Google Scholar]
- 31.Ruddock LW, Klappa P: Oxidative stress: protein folding with a novel redox switch. Curr Biol 1999, 9:400-402 [DOI] [PubMed] [Google Scholar]
- 32.Kamata H, Hirata H: Redox regulation of cell signalling. Cell Signal 1999, 11:1-14 [DOI] [PubMed] [Google Scholar]
- 33.Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E: α-Synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol 2000, 157:401-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto M, Hsu LJ, Rockenstien E, Takenouchi T, Mallory M, Masliah E: α-Synuclein protects against oxidative stress via inactivation of the c-Jun N-terminal kinase stress-signaling pathway in neuronal cells. J Biol Chem 2002, 277:11465-11472 [DOI] [PubMed] [Google Scholar]
- 35.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA: Oxidative damage is the earliest event in Alzheimers disease. J Neuropathol Exp Neurol 2001, 60:759-767 [DOI] [PubMed] [Google Scholar]