Abstract
Vascular cell adhesion molecule-1 (VCAM-1; CD106), the receptor for VLA-4, is an important mediator of adhesive and co-stimulatory interactions that govern cutaneous immune responses. Initial studies designed to elucidate temporal aspects of endothelial adhesion molecule induction in murine acute graft-versus-host disease (aGVHD) revealed unexpected and novel VCAM-1 expression by cutaneous and mucosal epithelial cells. Immunohistochemical techniques confirmed VCAM-1 staining as early as 7 days after transplantation in a distinctive subpopulation of squamous epithelial cells that normally occupy focal domains within the epidermal basal cell layer, the follicular infundibulum, and the dorsal lingual epithelium. Specifically, VCAM-1 expression was intimately associated with rete ridge-like prominences in footpad epidermis and in dorsal lingual epithelium. VCAM-1, as evaluated by serial section-labeling techniques, was preferentially expressed at sites of early epithelial infiltration by CD4+ T cells. Western blot analysis confirmed expression of the 110-kd isoform of VCAM-1 in epithelium isolated from aGVHD animals, and immunoelectron microscopy demonstrated VCAM-1 reactivity restricted exclusively to epithelial cell plasma membranes. It is concluded that VCAM-1 is selectively expressed by discrete squamous epithelial subpopulations in murine aGVHD. As such, VCAM-1 may play a previously unrecognized role in mediating interactions between donor effector T lymphocytes and host epithelial cell targets.
Acute graft-versus-host disease (aGVHD) is a major complication of allogeneic bone marrow transplantation, accounting for significant morbidity and mortality in patients receiving bone marrow transplantation for treatment of hematopoietic malignancies and certain solid tumors. 1 Implicit to the development of aGVHD are 1) initial allostimulation of donor T cells by host histocompatibility antigens [often minor histocompatibility (mHC) antigens]; 2) homing of these effector T cells to microvascular beds in target tissues; and 3) migration and spatial association of effector T cells with specific cellular targets. 1 The precise molecular mechanisms that govern these events are incompletely understood, although it is likely that co-stimulatory and adhesion molecules play critical roles.
VCAM-1 (CD106) is an adhesion molecule that serves as the receptor for the leukocyte-associated integrin, VLA-4. 2 VCAM-1 is expressed by microvascular endothelial cells as a component of the adhesion cascade that orchestrates spatial localization and diapedesis of leukocytes during early stages of tissue inflammation. 2,3 Accordingly, expression of VCAM-1 has been demonstrated in activated human and murine endothelial cells in vitro 3-7 and in vivo in a variety of inflammatory disorders. 2,8-18 In addition, cells other than endothelium may express VCAM-1, including epithelial and fibrohistiocytic cells in the setting of tissue inflammation. 8-12,14,16,19-30 Induction of VCAM-1 involves cytokine pathways, with tumor necrosis factor-α playing a major role in determining VCAM-1 expression. 4,7,16,18,21,22,29-33 Other factors and agents known to induce VCAM-1 in a variety of experimental systems include interleukin (IL)-1, 4,7,21,22,29,30,34 IL-4, 4,21,25,29 interferon gamma, 22,30 IL-13, 4,5 lipopolysaccharide, 6,7,17,30 hyaluronan fragments, 23,24 β2-microglobulin, 27 substance P, 15 respiratory syncytial virus, 35 and rhinovirus. 36 In addition to its role in mediating endothelial-leukocyte interactions, VCAM-1 also may function as a co-stimulatory molecule in antigen-driven immune responses. 37 Indeed, VCAM-1 has been implicated as a mediator of both leukocyte adhesion and of allogeneic co-stimulation in the setting of experimental aGVHD. 38
In experimental aGVHD provoked by mHC antigenic differences, we and others have shown that initial leukocyte homing to skin is preceded by mast cell degranulation, 39 a potent source of preformed tumor necrosis factor capable of inducing adhesion cascades. 40,41 In the course of consequent studies examining the kinetics of adhesion molecule induction and expression during disease evolution, it was noted that in addition to endothelial VCAM-1 induction, this molecule also was expressed by a restricted population of epithelial cells destined for eventual apoptotic injury mediated by cytotoxic effector cells. 42 The expression of VCAM-1 by squamous epithelial cells in aGVHD heightens interest in this molecule as a potential key mediator of spatial restriction and selective targeting of specific epithelial subpopulations.
Materials and Methods
Animals
Two different strain combinations were used to produce aGVHD across mHC barriers. B10.D2/oSnJ (B10) and C57BL/6Byn (B6) male mice (Jackson Laboratory, Bar Harbor, ME) between the ages of 7 to 12 weeks were used as donors, and DBA/2Ncr (DBA; NCI Animal Production Program, Bethesda, MD) and C.B10-H2b/LiMcdJ (Balb.B; Jackson Laboratory) male mice between the ages of 9 to 16 weeks were used as recipients, respectively. The B10 → DBA and the B6 → Balb.B strain combinations have been previously shown to produce aGVHD as a result of allostimulation of CD4+ donor T cells by host mHC. 43 In addition, syngeneic transplants involving C57BL/6Byn (B6) → C57BL/6Byn (B6) were also performed as negative controls. Mice were housed in a sterile environment in microisolator cages (Lab Products, Maywood, NJ) and provided with acidified water (pH 2.5) and autoclaved food.
Transplantation Protocol
Antibodies
Anti-Thy-1.2 (J1j, ascites fluid, rat IgM) 44 and anti-CD8 (3.168, rat IgM) 45 monoclonal antibodies were used for donor transplant cell preparations. Anti-B-cell antibody (goat anti-mouse IgG) was purchased from ICN Immunobiologicals, Costa Mesa, CA. Guinea pig serum was used as a source of complement (C).
Preparation of Donor Cells
Donor cells were prepared according to previously described protocols. 43 Bone marrow cells were obtained from the femurs and tibiae of donor mice by flushing with buffered saline solution supplemented with 0.1% bovine serum albumin (Hyclone, Logan, UT). To prepare anti-Thy 1-treated (T cell-depleted) bone marrow (ATBM), cells were incubated with J1j monoclonal antibody (diluted 1:100) and C (diluted 1:25) for 45 minutes at 37°C, followed by four washes. T-cell-enriched donor cell populations were prepared by treating pooled spleen and lymph node cells with Gey’s balanced salt lysing solution, followed by a low ionic strength buffer to remove dead cells, and then panning on plastic Petri dishes precoated with goat anti-mouse IgG (40 μg/ml) for 1 hour at 4°C to remove B cells. This treatment generally resulted in a population of 90 to 95% Thy 1+ cells, as measured for phenotyping by flow cytometry.
For preparation of purified T-cell subset populations, as described elsewhere, 43 T-cell-enriched preparations were further treated with the appropriate monoclonal antibody directed against the undesirable subset plus C. To purify CD4+ T cells, lymphocytes were treated with anti-CD8 monoclonal antibody (3.168; diluted 1:50) plus C (1:25) for 45 minutes at 37°C, initially, and again for 30 minutes with a wash in between. Cells were phenotyped by flow cytometry and were routinely found to be negative for CD8.
Bone Marrow Transplantation and aGVHD Induction
The B10 → DBA (n = 3) and B6 → Balb.B (n = 2) strain combinations were used for studies of CD4+ T-cell-mediated aGVHD. Recipient mice were irradiated using a Gammacell 137Cs source (130 cGy/min) (Atomic Energy of Canada, Kanata, ON, Canada). Approximately 6 hours later, these recipients were injected intravenously with donor cells via the tail vein. The inoculum consisted of either ATBM cells (2 × 106) alone as a negative disease control or a mixture of ATBM (2 × 106) and donor-purified CD4+ T cells (at 2 to 5 × 106 cells, depending on the particular strain combination). Morbidity and mortality were monitored daily. Weights of the mice were monitored twice weekly.
Tissue Harvesting
Three animals per experimental group (ATBM-negative control group, CD4+ effector group) were sacrificed on days 4, 7, 14, and 21 after transplantation (sacrifice was limited to two animals in the CD4+ group on days 14 and 21 because of animal mortality). These time points have been previously shown to correlate with early and peak inductive phases of aGVHD pathology. 42,43 Tissue samples from the ear, tongue, and footpad were harvested for routine histology and frozen tissue analysis. Dorsal lingual squamous mucosa, unlike murine integument, is characterized by rete rete-like epithelial prominences that resemble the epidermal rete ridges 42,46,47 that are early targets in human aGVHD. 48 In one experiment, ear samples were fixed with 4% paraformaldehyde for 1 hour at 4°C, washed with 7% sucrose in phosphate-buffered saline (PBS), embedded in OCT compound (Miles Laboratories, Elkhart, IN), and then snap-frozen in liquid nitrogen for subsequent examination by immunofluorescence.
Immunohistochemistry
Cryosections (5 μm) were fixed in acetone and stained using a three-step avidin-biotin-horseradish peroxidase (ABC) method (Vector Laboratories, Burlingame, CA). To determine T cell/VCAM-1 co-localization, appreciation of spatial localization was enhanced by the use of serial sections, each separated by 5-μm intervals, evaluated by different primary antibodies. Briefly, tissue sections were incubated with primary monoclonal antibody or isotype-matched control antibody either overnight at 4°C or for 1 hour at room temperature. After washing in PBS, a species-specific biotinylated antibody was applied for 30 minutes at room temperature followed by ABC. Immunoreactivity was revealed using either 3,3′ diaminobenzidine (Sigma Chemical, St. Louis, MO), VIP (Vector), or NovaRED (Vector) chromagens. Primary and isotype-matched control antibodies were purchased from Pharmingen, San Diego, CA. Anti-CD4 and -CD8 rat monoclonal antibodies were used at concentrations of 1.25 μg/ml, hamster anti-intercellular adhesion molecule-1 (ICAM-1; CD54) was used at 2.5 μg/ml, rat anti-CD106 was used at 2.5 μg/ml, and rat anti-platelet-endothelial adhesion molecule-1 (PECAM-1; CD31) was used at 1.25 μg/ml. Polyclonal goat anti-mouse VCAM-1 antibody (R & D Systems, Inc., Minneapolis, MN) was used at 0.25 μg/ml for immunohistochemistry and at 0.8 μg/ml for Western blot analysis.
Immunofluorescence
Anti-CD106 monoclonal antibody was incubated on 4% paraformaldehyde-fixed and frozen ear tissue sections for 1 hour at room temperature. After thorough washing, biotinylated rabbit anti-rat antibody (1:200) was applied, followed by avidin-conjugated Texas Red or avidin-fluorescein isothiocyanate (Leinco, Ballwin, MO). Specimens were examined using a Nikon Microphot SA fluorescent microscope attached to an Optronics digital camera.
Immunoelectron Microscopy
Tongue and footpad cryosections were fixed with 4% paraformaldehyde in PBS for 10 minutes followed by three washes. Immunohistochemistry was performed as described above for CD106, and immunoreactivity was revealed using the chromagen, VIP. 49 After washing, the sections were refixed with 2.5% glutaraldehyde in PBS overnight at 4°C. The sections, with and without exposure to 1% osmium tetroxide for 15 minutes, were processed through graded alcohols and propylene oxide; infiltrated with epoxy resin; and embedded by an inverted Beem capsule method using Taab Epon 812 (Marivac, Ltd., Nova Scotia, Canada).
Western Blot Analysis
Cell-free lysates were prepared from epithelial sheets of either aGVHD or ATBM tongue tissue at day 10 after transplantation using the B10 → DBA strain combination. Lysates also were prepared from normal DBA tongue tissue and the SV-40 transformed murine endothelial cell line 3B-11 (American Type Culture Collection, Rockville, MD) as a source of VCAM-1-negative and -positive control lysate, respectively. Tongue tissue was cut into strips longitudinally and incubated overnight at 4°C in 2 mg/ml of dispase (Life Technologies, Inc., Grand Island, NY) in Dulbecco’s modified Eagle medium/nutrient mixture F-12 (Ham) 1:1 F12 medium with 100 IU/ml of penicillin and 100 μg/ml of streptomycin (Life Technologies). After overnight incubation, lingual epithelial sheets were readily separated from the underlying submucosa and floated onto lysate buffer [150 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 8.0 containing 1% Triton X-100 and protease inhibitor cocktail (Sigma)] for 1 hour at 4°C. The epithelial sheet remnants were removed and the supernatant cleared by centrifugation twice at 11,000 × g. VCAM-1-positive cell-free lysate was prepared by incubating 25 × 106 3B-11 cells in 1 ml of lysate buffer at 4°C for 1 hour. Lysates were cleared as described above. Protein concentration of lysate preparations was determined using the Micro BCA Protein Assay Kit per the manufacturer’s instructions (Pierce, Rockford, IL).
To verify the absence of endothelial contamination as a possible source of VCAM-1 reactivity in epithelial lysate preparations, dot blot analysis of aGVHD, ATBM, and normal DBA epithelial lysates was performed using the murine panendothelial cell antibody, clone MECA-32 (Pharmingen). All epithelial lysate preparations were negative using the MECA-32 antibody with the 3B-11 endothelial cell lysate serving as a positive control.
Lysate preparations diluted 1:1 in sample buffer or molecular weight markers (Sigma) were loaded onto a 7.5% Tris-glycine-sodium dodecyl sulfate gel (Ready Gel; Bio-Rad Laboratories, Hercules, CA) and run at 100 V constant voltage. Proteins were transferred to Immobilon-P polyvinylidene difluoride membrane at 280 mA constant current for 3 hrs and blocked with 5% milk, 10% horse serum, and 0.1% Tween 20 in PBS (blocking buffer) overnight at 4°C. Membranes then were incubated with goat anti-murine VCAM-1 polyclonal antibody (0.8 μg/ml) or normal goat IgG diluted in blocking buffer for 2 hours at room temperature, followed by washing in 0.1% Tween 20 in PBS (wash buffer). Horseradish peroxidase-conjugated horse anti-goat antibody at 1 μg/ml (Vector) was used as the secondary antibody for 1 hour at room temperature. Membranes were washed several times and left overnight in wash buffer at room temperature. Antibody reactivity was detected by chemiluminescence using an ECL Plus Western blotting detection kit (Amersham Pharmacia Biotech, Piscataway, NJ) with a 7.5-minute exposure to X-OMAT film (Eastman Kodak Co., Rochester, NY).
Results
Mice transplanted with purified CD4+ T cells in both strain combinations developed clinical evidence of aGVHD (weight loss, hunched posture, ruffled fur, and diarrhea) consistent with previous studies. 50-53 Histological examination of dorsal lingual epithelium, ear skin, and footpad from CD4+ T cell-transplanted mice demonstrated evidence of epithelial target cell injury and lymphocytic infiltration associated with aGVHD. 51,52,54 Control animals that received only ATBM cells did not develop clinical or histological evidence of disease at any time during the study.
By day 4 after transplantation, submucosal lingual up-regulation of endothelial VCAM-1 and increased vascular and diffuse epithelial basal and suprabasal cell layer expression of ICAM-1 were noted, as previously reported (data not shown). 55,56 By day 7, and with progressive intensity thereafter for the duration of the experiments, VCAM-1 expression was observed to selectively involve domains that correlated with rete rete-like prominences (RLPs) of the dorsal tongue (Figure 1, C and E) ▶ . VCAM-1+ RLPs accounted for <10% of total RLPs at day 7, and between 25 to 50% at both days 14 and 21.
Figure 1.
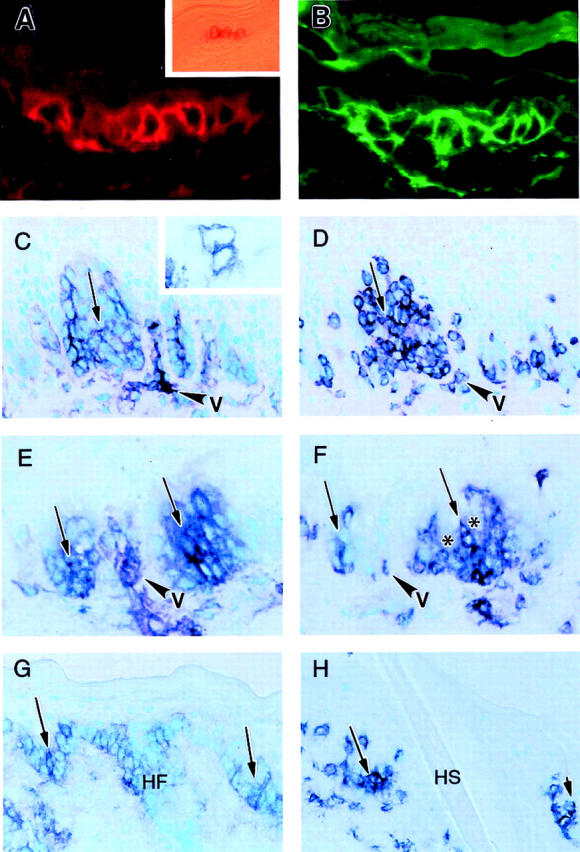
Immunohistochemical patterns of VCAM-1 expression by epidermal and lingual squamous cells in murine aGVHD. A and B: Ear skin 21 days after transplant, characteristically devoid of rete ridges, demonstrates intermittent single cell (A, Texas red) and contiguous zonal (B, fluorescein isothiocyanate) reactivity for VCAM-1 in two separate regions of murine epidermis. Inset in A represents a 1-μm section showing a similar contiguous patch of VCAM-1 reactivity in lateral foot skin also devoid of rete ridges. The cells in this field were further sectioned for immunoelectron microscopy (see Figure 3C ▶ ). C and D: VCAM-1 (C) and CD4 (D) expression in lingual squamous epithelium in adjacent sections 14 days after transplant. VCAM-1 staining is preferentially restricted to RLPs (C, arrow) that also are infiltrated by CD4 T cells (D, arrow; v = capillary loops that characteristically separate the epithelial ridges). Inset in C shows isolated individual VCAM-1-positive lingual squamous cells observed as early as day 7 after transplantation. E and F: VCAM-1 (E) and CD4 (F) expression in lingual squamous epithelium in adjacent sections 21 days after transplant. In addition to localization of VCAM-1 to RLPs (E, arrows) and spatially-associated CD4+ T cell infiltration of these prominences identified in adjacent sections (F, arrows), central unstained foci consistent with cellular targeting (satellitosis) are also seen (F, asterisks). G and H: VCAM-1 (G) and CD4 (H) expression in ventrolateral footpad epithelium 14 days after transplant. Note site-related admixture of rete ridges (arrows) and hair follicle (HF) infundibular epithelium, both showing preferential expression of VCAM-1. The adjacent section shows CD4+ T cell infiltration of these VCAM-1-positive sites; the follicular epithelium is replaced by hair shaft (HS) at this level. Chromagen = VIP in C–H. Original magnifications: ×400 (A, B, and inset in C); ×125 (inset in A); ×250 (C–H). Note that arrows in C/D, E/F, and G/H pairs identify adjacent regions separated by ∼5 μm in adjacent serial sections.
CD4+ T cells preferentially infiltrated the RLPs, as previously described 42,57,58 (Figure 1, D and F) ▶ . Anti-CD8 antibody staining was uniformly negative. Apparent VCAM-1 keratinocyte reactivity was also documented in focal basal cell layer domains of involved ear skin (Figure 1, A and B) ▶ and lateral foot pad (Figure 1G) ▶ . In these regions, focal staining of follicular infundibular keratinocytes was also noted. Although CD4+ T cell infiltration preferentially involved VCAM-1-positive epithelial domains (Figure 1, C to H ▶ , and Figure 2, C and D ▶ ), occasional VCAM-1-negative RLPs and epidermal basal layer domains were also infiltrated by CD4+ T cells (Figure 2, A and B) ▶ , consistent with expression of VCAM-1 exclusively by epithelial cells and not by infiltrating T cells. Recipients of syngeneic transplants and ATBM-treated recipients of allogeneic transplants failed to show VCAM-1 expression or CD4+ T cell infiltration of RLPs at any of the sites or time points examined.
Figure 2.
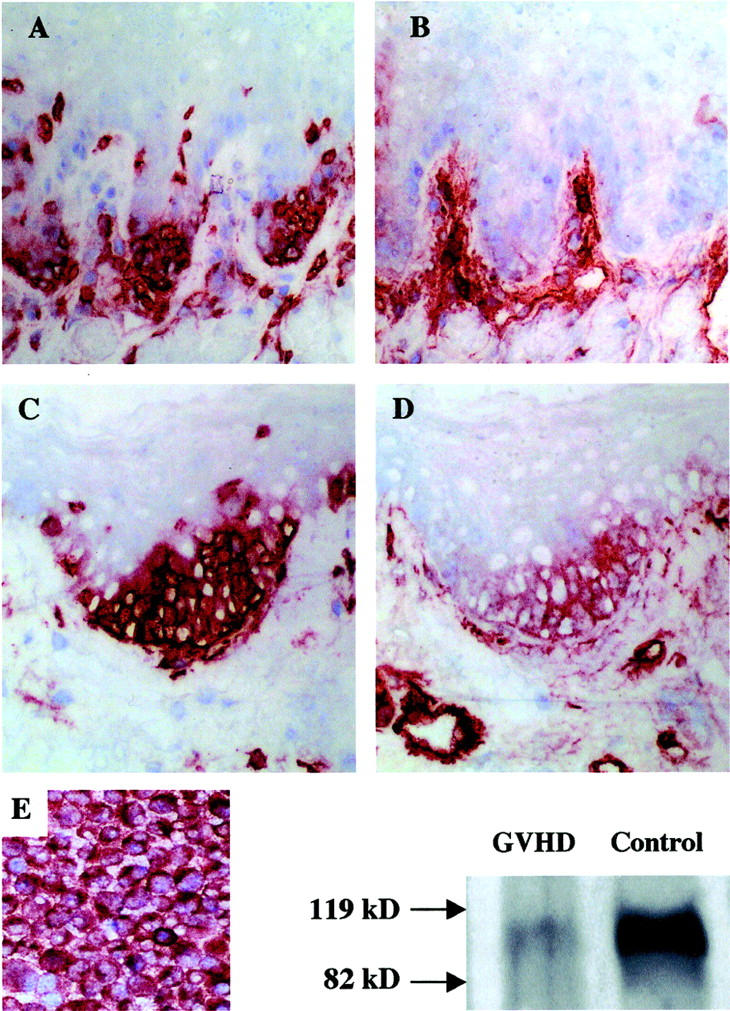
Correlative immunohistochemistry and Western blot analysis of VCAM-1 in murine GVHD lingual mucosa and the SV-40-transformed 3B-11 murine endothelial cell line. T cells consistently infiltrated RLPs (A and C), which were either VCAM-1-negative (B) or -positive (D) in corresponding areas of adjacent sections. Note VCAM-1 reactivity in submucosal vessels (B and D). This demonstrates that migrating T cells are not reactive for VCAM-1 and that VCAM-1 epithelial expression is coincident with T cell infiltration of lingual retia, but not vice versa. Lysates obtained from isolated lingual epithelial sheets from day 10 aGVHD animals (50 μg total protein) and transformed endothelial cells (2.5 μg total protein) that constitutively express VCAM-1, as confirmed by immunohistochemistry in cytospin preparations (E), demonstrated similar 110-kd bands by Western blot analysis consistent with VCAM-1 protein. Chromagen = NovaRED. Original magnifications, ×400.
The SV-40 transformed murine endothelial cell line 3B-11, which uniformly expresses VCAM-1 constitutively, 59 was used as a positive control for both immunohistochemistry and Western blot analysis (Figure 2E) ▶ using a commercially available polyclonal antibody to murine VCAM-1. Cell-free lysate prepared from 3B-11 cells demonstrated an immunoreactive band with a molecular weight of ∼110 kd (Figure 2E) ▶ corresponding to that reported for murine VCAM-1. 17,33,34 A similar, less intense, immunoreactive band also was detected in cell-free lysates prepared from separated lingual epithelial sheets of aGVHD mice at day 10 after transplantation. Lysate preparations from mice receiving ATBM-treated transplants and normal control mice were negative for VCAM-1 by Western blot analysis (results not shown).
Immunoultrastructural techniques were used to localize subcellular VCAM-1 expression in aGVHD. The submucosal lingual vasculature demonstrated prominent lumenal and focal ablumenal VCAM-1 membrane reactivity (Figure 3A) ▶ . Ultrastructural localization of VCAM-1 on lingual squamous epithelium was restricted to cells exclusively within the RLPs, which showed circumferential expression of VCAM-1 (Figure 3B) ▶ . Similarly, cell membranes of keratinocytes predominantly within the basal layer of footpad epidermis showed circumferential cell membrane reactivity with patchy accentuation along the basal lamina interface (Figure 3 ▶ ; C to E). Melanocytes, Langerhans cells, epidermotropic lymphocytes, and dendritic cells were consistently negative for VCAM-1.
Figure 3.
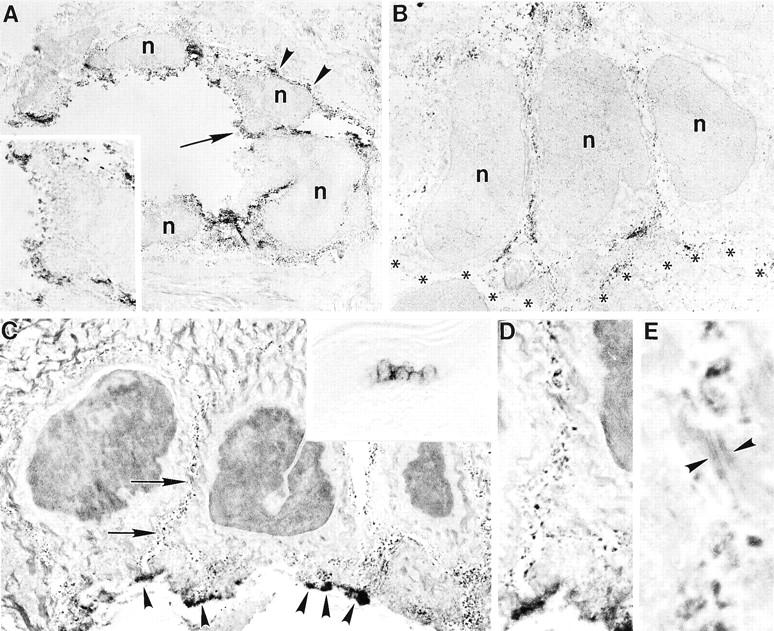
Immunoultrastructural localization of VCAM-1 reactivity in murine acute GVHD. A: Submucosal lingual blood vessel with prominent lumenal (arrow and inset) and focal ablumenal (arrowheads) membrane reactivity (n = nucleus). B: Circumferential basal cell membrane expression of VCAM-1 involving lingual squamous mucosa (basal lamina region indicated by asterisks). C: Similar circumferential staining is present in basal cells of footpad epidermis (arrows), with patchy accentuation along the inferior membrane that interfaces with the basal lamina (arrowheads). Inset represents light microscopic view of VCAM-1-positive cell cluster that was sampled. D and E: Higher magnifications of cell membrane VCAM-1 expression by keratinocytes. Note that desmosomes (E, arrowheads) fail to demonstrate staining. Original magnifications: ×3800 (A); ×7500 (inset in A); ×5700 (B); ×5000 (C); ×400 (inset in C); ×11,000 (D); ×33,750 (E).
Discussion
In this study, we have shown that: 1) VCAM-1 is expressed by epidermal and follicular keratinocytes, and by lingual squamous epithelial cells, in addition to microvascular endothelial cells, in experimental aGVHD; 2) VCAM-1 expression is restricted to epithelial cell membranes at an ultrastructural level; and 3) VCAM-1 is restricted to epithelial subpopulations within RLPs that are preferentially infiltrated by effector T cells.
VCAM-1 expression has been observed previously in the setting of human and experimental GVHD, although not on squamous epithelium. Specifically, VCAM-1 has been demonstrated in human aGVHD on dermal, 55,60 intestinal, 61,62 and salivary gland 14 endothelium; dermal perivascular dendritic cells and macrophages; 56,63 and salivary gland ductular epithelium. 14 VCAM-1 immunoreactivity and mRNA has been documented, however, by neoplastic cells in the setting of undifferentiated nasopharyngeal carcinoma. 64 In addition to squamous epithelial cells, other potential sources of VCAM-1 expression in aGVHD could include dendritic Langerhans cells, intraepidermal dermal dendrocytes, 65 and dissociated endothelial VCAM-1 bound to its ligand on the surfaces of epidermotropic T cells. The immunohistochemical finding of T cell infiltration focally independent of VCAM-1 expression in serially-sectioned rete-like domains of lingual epithelium argues against the possibility of shed VCAM-1 on T cell surfaces, and immunoelectron microscopy of the involved epidermis and lingual epithelium confirmed VCAM-1 expression exclusively on the plasma membranes of epithelial cells.
Evidence for a pathogenic role for VCAM-1 in aGVHD comes from murine models, in which antibodies to both VCAM-1 and its receptor, VLA-4, have been shown to inhibit disease. 38,66 This effect could result from blockade of adhesive pathways that regulate homing of effector T cells to activated microvascular beds in target tissues. However, inhibition of VCAM-1/VLA-4-mediated co-stimulation also seems to be involved, because VCAM-1 blockade effectively abrogates mixed lymphocyte cultures across mHC barriers as well as aGVHD in recipients of antigenically relevant bone marrow transplants. 38 Moreover, allostimulated donor T cells capable of eliciting murine aGVHD show increased VLA-4 expression during allogeneic expansion, and in vivo inhibition of this molecule via IL-12 treatment is associated with amelioration of disease. 67
In squamous epithelia, the functional significance of VCAM-1 expression by discrete subpopulations could involve effector T cell adhesion to spatially-restricted epithelial domains, or co-stimulatory pathways involving recognition of relevant mHC determinants expressed by specific target cells. In this regard, it is of note that the restricted areas of VCAM-1 expression observed in this study (eg, RLPs of lingual epithelium) have been previously observed to correlate precisely with early effector T cell infiltration and eventual apoptotic injury. 42 The spatial association between VCAM-1 expression by epithelial cells and T cell infiltration was further confirmed in this study. Because VCAM-1 epithelial expression was invariably coincident with T cell infiltration of lingual retia, but not vice versa, it is inferred that VCAM-1 induction follows, not precedes, spatially-directed intraepithelial T cell homing. Cytokines and factors potentially responsible for epithelial VCAM-1 induction at sites of effector T cell infiltration include tumor necrosis factor, interferon gamma, IL-1, IL-4, IL-13, and lipopolysaccharide. Preliminary in vitro experiments have failed to demonstrate VCAM-1 induction on murine epithelium in lingual organ cultures using recombinant tumor necrosis factor, interferon gamma, IL-4, or IL-13 29 (GF Murphy, unpublished observations). Additional studies are underway to examine further the potential roles of these and other candidate molecules in epithelial VCAM-1 induction.
It has not escaped our notice that VCAM-1 expression in lingual epithelium is restricted to RLPs that have been previously shown to harbor epithelial stem cells. 46,47 Moreover, VCAM-1 induction was observed to involve follicular infundibular keratinocytes and focal domains within the basal cell layer of the epidermis, both possessing potential for overlap with stem cell compartments previously elucidated in these regions. 68,69 Sale and co-workers 48,58 have provided compelling evidence for correlation of target cell injury and squamous epithelial stem cell compartments in aGVHD. Although we have established correlation between sites of epithelial VCAM-1 expression, T cell infiltration, and epithelial apoptosis in lingual aGVHD in this and other studies, 42 we have not as yet determined whether VCAM-1 expression precisely defines label-retaining cells with stem cell characteristics or whether it directly correlates with individual cells undergoing apoptosis. Nonetheless, it is conceptually intriguing to speculate that cytotoxic targeting in aGVHD may be focused initially at cells critical to epithelial self-renewal. Further studies are therefore indicated to elucidate the molecular basis for epithelial basal cell layer heterogeneity and its relationship to cytotoxic targeting in aGVHD.
In conclusion, VCAM-1 is expressed by spatially-defined, discrete epithelial subpopulations that potentially represent target cells in aGVHD. This observation may facilitate understanding of the pathogenic underpinnings of lesion formation and suggests that significant target cell heterogeneity exists at a molecular level within epithelial tissues. Evolving recognition of the relationship of squamous epithelial heterogeneity to cellular targeting should foster development of novel strategies to specifically insulate these subpopulations from cytotoxic injury in aGVHD.
Footnotes
Address reprint requests to George F. Murphy, M.D., Director, The Jefferson Center for Dermatopathology, Suite 545 Jefferson Alumni Hall, 1020 Locust St., Philadelphia, PA 19107-6799. E-mail: george.murphy@mail.tju.edu.
Supported by National Institutes of Health grants CA 40358 and CA 77401.
References
- 1.Gilliam AC, Murphy GF: Cellular pathology of cutaneous graft-versus-host disease. Ferrara JLM Deeg HI Burakoff SJ eds. Graft-vs-Host Disease: Immunology, Pathophysiology, and Treatment. 1996:pp 291-313 Marcel Dekker, New York
- 2.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowsky S, Hemler M, Lobb RR: VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA/fibronectin binding site. Cell 1990, 60:577-584 [DOI] [PubMed] [Google Scholar]
- 3.Honda S, Campbell JJ, Andrew DP, Engelhardt B, Butcher BA, Warnock RA, Ye RD, Butcher EC: Ligand-induced adhesion to activated endothelium and to vascular cell adhesion molecule-1 in lymphocytes transfected with the N-formyl peptide receptor. J Immunol 1994, 152:4026-4035 [PubMed] [Google Scholar]
- 4.Jahnsen FL, Brandtzaeg P, Haye R, Haraldsen G: Expression of functional VCAM-1 by cultured nasal polyp-derived microvascular endothelium. Am J Pathol 1997, 150:2113-2123 [PMC free article] [PubMed] [Google Scholar]
- 5.Weigel G, Bertalanffy P, Dubsky P, Griesmacher A, Wolner E: Mycophenolic acid influences T helper (Th2) cytokine induced expression of intercellular cell adhesion molecule-1 (ICAM-1) on human endothelial cells. Clin Chem Lab Med 1999, 37:253-257 [DOI] [PubMed] [Google Scholar]
- 6.Hahne M, Lenter M, Jager U, Vestweber D: A novel soluble form of mouse VCAM-1 is generated from a glycolipid-anchored splicing variant. Eur J Immunol 1994, 24:421-428 [DOI] [PubMed] [Google Scholar]
- 7.Sikorski EE, Hallman R, Berg EL, Butcher EC: The Peyer’s patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-alpha and IL-1. J Immunol 1993, 151:5239-5250 [PubMed] [Google Scholar]
- 8.Ogawa T, Yorioka N, Ito T, Taniguchi Y, Kumagai J, Awaya Y, Yamakido M: Ultrastructural localization of vascular cell adhesion molecule-1 in proliferative and crescentic glomerulonephritis. Virchows Arch 1996, 429:283-291 [DOI] [PubMed] [Google Scholar]
- 9.Veale D, Rogers S, Fitzgerald O: Immunolocalization of adhesion molecules in psoriatic arthritis, psoriatic and normal skin. Br J Dermatol 1995, 132:32-38 [DOI] [PubMed] [Google Scholar]
- 10.de Boer OJ, Wakelkamp IM, Pals ST, Claessen N, Bos JD, Das PK: Increased expression of adhesion receptors in both lesional and non-lesional psoriatic skin. Arch Dermatol Res 1994, 286:304-311 [DOI] [PubMed] [Google Scholar]
- 11.Groves RW, Ross EL, Barker JNWN, MacDonald DM: Vascular cell adhesion molecule-1: expression in normal and diseased skin and regulation in vivo by interferon gamma. J Am Acad Dermatol 1993, 29:67-72 [DOI] [PubMed] [Google Scholar]
- 12.Walton LJ, Thornhill MH, Farthing PM: VCAM-1 and ICAM-1 are expressed by Langerhans cells, macrophages and endothelial cells in oral lichen planus. J Oral Pathol Med 1994, 23:262-268 [DOI] [PubMed] [Google Scholar]
- 13.Regezi JA, Dekker NP, MacPhail LA, Lozada-Nur F, McCalmont TH: Vascular adhesion molecules in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontics 1996, 81:682-690 [DOI] [PubMed] [Google Scholar]
- 14.Hiroki A, Nakamura S, Shinohara M, Gondo H, Ohyama Y, Hayashi S, Harada M, Niho Y, Oka M: A comparison of glandular involvement between chronic graft-versus-host disease and Sjogren’s syndrome. Int J Oral Maxillofac Surg 1996, 25:298-307 [DOI] [PubMed] [Google Scholar]
- 15.Quinlan KL, Song I, Naik SM, Letran EL, Olerud JE, Bunnett NW, Armstrong CA, Caughmann SW, Ansel JC: VCAM-1 expression on human dermal microvascular endothelial cells is directly and specifically up-regulated by substance P. J Immunol 1999, 162:1656-1661 [PubMed] [Google Scholar]
- 16.Groves RW, Allen MH, Ross EL, Barker JN, MacDonald DM: Tumour necrosis factor alpha is pro-inflammatory in normal human skin and modulates cutaneous adhesion molecule expression. Br J Dermatol 1995, 132:345-352 [DOI] [PubMed] [Google Scholar]
- 17.Mc Gilvray MD, Rotstein OD: Antioxidant modulation of skin inflammation: preventing inflammatory progression by inhibiting neutrophil influx. Can J Surg 1999, 42:109-115 [PMC free article] [PubMed] [Google Scholar]
- 18.McHale JF, Olivier AH, Marshall D, Haskard DO: Vascular endothelial cell expression of ICAM-1 and VCAM-1 at the onset of eliciting contact hypersensitivity in mice: evidence for a dominant role of TNF-α. J Immunol 1999, 162:1648-1655 [PubMed] [Google Scholar]
- 19.Uccini S, Ruco LP, Monardo F, La Parola IL, Cerimele D, Baroni CD: Molecular mechanisms involved in intraepithelial lymphocyte migration: a comparative study in skin and tonsil. J Pathol 1993, 169:413-419 [DOI] [PubMed] [Google Scholar]
- 20.Hemmerlein B, Scherbening J, Kugler A, Radzun HJ: Expression of VCAM-1, ICAM-1, E-and P-selectin and tumour-associated macrophages in renal cell carcinoma. Histopathology 2000, 37:78-83 [DOI] [PubMed] [Google Scholar]
- 21.Atsuta J, Sterbinsky SA, Plitt J, Schwiebert LM, Bochner BS, Schleimer RP: Phenotyping and cytokine regulation of the BEAS-2B human bronchial epithelial cell: demonstration of inducible expression of the adhesion molecules VCAM-1 and ICAM-1. Am J Respir Cell Mol Biol 1997, 17:571-582 [DOI] [PubMed] [Google Scholar]
- 22.Sanmugalingham D, De Vries E, Gauntlett R, Symon FA, Bradding P, Wardlaw AJ: Interleukin-5 enhances eosinophil adhesion to bronchial epithelial cells. Clin Exp Allergy 2000, 30:255-263 [DOI] [PubMed] [Google Scholar]
- 23.Schawalder A, Oertli B, Beck-Schimmer B, Wuthrich RP: Regulation of hyaluronan-stimulated VCAM-1 expression in murine renal tubular epithelial cells. Nephrol Dial Transplant 1999, 14:2130-2136 [DOI] [PubMed] [Google Scholar]
- 24.Oertli B, Beck-Schimmer B, Fan X, Wuthrich RP: Mechanisms of hyaluronan-induced up-regulation of ICAM-1 and VCAM-1 expression by murine kidney tubular epithelial cells: hyaluronan triggers cell adhesion molecule expression through a mechanism involving activation of nuclear factor-κB and activating protein-1. J Immunol 1998, 161:3431-3437 [PubMed] [Google Scholar]
- 25.Bobryshev YV, Lord RS: Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immuno-inflammatory reactions. Cardiovasc Res 1998, 37:799-810 [DOI] [PubMed] [Google Scholar]
- 26.Bobryshev YV, Lord RS, Reiner SP, Munro VF: VCAM-1 expression and network of VCAM-1 positive vascular dendritic cells in advanced atherosclerotic lesions of carotid arteries and aorta. Acta Histochem 1996, 98:185-194 [DOI] [PubMed] [Google Scholar]
- 27.Jaradat MI, Schnizlein-Bick CT, Singh GK, Moe SM: β2-microglobulin increases expression of vascular cell adhesion molecule on human synovial fibroblasts. Kidney Int 2001, 59:1951-1959 [DOI] [PubMed] [Google Scholar]
- 28.Bombara MP, Webb DL, Conrad P, Marlor CW, Sarr T, Ranges GE, Aune TM, Greve JM, Blue M: Cell contact between T cells and synovial fibroblasts causes induction of adhesion molecules and cytokines. J Leukoc Biol 1993, 54:399-406 [DOI] [PubMed] [Google Scholar]
- 29.Croft D, McIntyre P, Wibulswas A, Kramer I: Sustained elevated levels of VCAM-1 in cultured fibroblast-like synoviocytes can be achieved by TNF-α in combination with either IL-4 or IL-13 through increased mRNA stability. Am J Pathol 1999, 154:1149-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steffen BJ, Breier G, Butcher EC, Schulz M, Engelhardt B: ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol 1996, 148:1819-1838 [PMC free article] [PubMed] [Google Scholar]
- 31.Rosseau S, Selhorst J, Wiechmann K, Leissner K, Maus U, Mayer K, Grimminger F, Seeger W, Lohmeyer J: Monocyte migration through the alveolar epithelial barrier: adhesion molecule mechanisms and impact of chemokines. J Immunol 2000, 164:427-435 [DOI] [PubMed] [Google Scholar]
- 32.Tu Z, Kelley VR, Collins T, Lee FS: IκB kinase is critical for TNF-α-induced VCAM1 gene expression in renal tubular epithelial cells. J Immunol 2001, 166:6839-6846 [DOI] [PubMed] [Google Scholar]
- 33.Kinashi T, St. Pierre Y, Springer TA: Expression of glycophosphatidylinositol-anchored and -non-anchored isoforms of vascular cell adhesion molecule 1 in murine stromal and endothelial cells. J Leukoc Biol 1995, 57:168-173 [DOI] [PubMed] [Google Scholar]
- 34.Terry RW, Kwee L, Levine JF, Labow MA: Cytokine induction of an alternatively spliced murine vascular cell adhesion molecule (VCAM) mRNA encoding a glycosylphosphatidylinositol-anchored VCAM protein. Proc Natl Acad Sci USA 1993, 90:5919-5923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang SZ, Hallsworth PG, Dowling KD, Alpers JH, Bowden JJ, Forsyth KD: Adhesion molecule expression on epithelial cells infected with respiratory syncytial virus. Eur Respir J 2000, 15:358-366 [DOI] [PubMed] [Google Scholar]
- 36.Papi A, Johnston SL: Respiratory epithelial cell expression of vascular cell adhesion molecule-1 and its up-regulation by rhinovirus infection via NF-κB and GATA transcription factors. J Biol Chem 1999, 274:30041-30051 [DOI] [PubMed] [Google Scholar]
- 37.Damle NK, Klussman K, Linsley PS, Aruffo A: Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J Immunol 1992, 148:1985-1992 [PubMed] [Google Scholar]
- 38.Schlegel PG, Vaysburd M, Chen Y, Butcher EC, Chao NJ: Inhibition of T cell costimulation mediated by VCAM-1 prevents murine graft-versus-host disease across minor histocompatibility barriers. J Immunol 1995, 155:3856-3865 [PubMed] [Google Scholar]
- 39.Sueki H, Whitaker D, Buchsbaum M, Murphy GF: Novel interactions between dermal dendrocytes and mast cells in human skin. Implications for hemostasis and matrix repair. Lab Invest 1993, 69:160-172 [PubMed] [Google Scholar]
- 40.Klein LM, Lavker RM, Matis WL, Murphy GF: Degranulation of human mast cells induces an endothelial antigen central to leukocyte adhesion. Proc Natl Acad Sci USA 1989, 86:8972-8976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF: Human mast cells contain and release tumor necrosis factor α, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci USA 1991, 88:4220-4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilliam AC, Whitaker-Menezes D, Korngold R, Murphy GF: Apoptosis is the predominant form of epithelial target cell injury in acute experimental graft-versus-host disease. J Invest Dermatol 1996, 107:377-383 [DOI] [PubMed] [Google Scholar]
- 43.Korngold R, Sprent J: Variable capacity of L3T4+ T cells to cause graft-vs-host disease across minor histocompatibility barriers in mice. J Exp Med 1987, 165:1552-1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruce J, Symington FW, McKearn TJ, Sprent J: A monoclonal antibody discriminating between subsets of T and B cells. J Immunol 1981, 730:118-133 [PubMed] [Google Scholar]
- 45.Sarmiento M, Glasebrook AL, Fitch FW: IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt-2 antigen block T-cell mediated cytolysis in the absence of complement. J Immunol 1980, 125:2665-2672 [PubMed] [Google Scholar]
- 46.Lavker RM, Sun T-T: Heterogeneity in epidermal basal keratinocytes: morphological and functional correlations. Science 1982, 215:1239-1241 [DOI] [PubMed] [Google Scholar]
- 47.Lavker RM, Sun T-T: Epidermal stem cells. J Invest Dermatol 1983, 81:121S-127S [DOI] [PubMed] [Google Scholar]
- 48.Sale GE, Schulman HM, Gallucci BB, Thomas ED: Young rete ridge keratinocytes are preferred targets in cutaneous graft-vs-host disease. Am J Pathol 1985, 118:278-287 [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou M, Grofova I: The use of peroxidase substrate vector VIP in electron microscopic single and double antigen localization. J Neurosci Methods 1995, 62:149-158 [DOI] [PubMed] [Google Scholar]
- 50.Korngold R, Sprent J: Lethal graft-vs-host disease following bone marrow transplantation across minor histocompatibility barriers in mice: prevention by removing mature T cells from marrow. J Exp Med 1978, 148:1687-1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrara JLM, Marion A, McIntyre JF, Murphy GF: Amelioration of acute graft-vs-host disease due to minor histocompatibility antigens by in vivo administration of anti-interleukin 2 receptor antibody. J Immunol 1986, 137:1874-1877 [PubMed] [Google Scholar]
- 52.Murphy GF, Whitaker D, Sprent J, Korngold R: Characterization of target injury of murine acute graft-vs-host disease directed to multiple minor histocompatibility antigens elicited by either CD4+ or CD8+ effector cells. Am J Pathol 1991, 138:983-990 [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy GF, Sueki H, Teuscher C, Whitaker D, Korngold R: Role of mast cells in early epithelial target cell injury in experimental acute graft-versus-host disease. J Invest Dermatol 1994, 102:451-461 [DOI] [PubMed] [Google Scholar]
- 54.Ferrara J, Guillen FJ, Sleckman B, Burakoff SJ, Murphy GF: Cutaneous acute graft-vs-host disease to minor histocompatibility antigens in a murine model: histologic analysis and correlation to clinical disease. J Invest Dermatol 1986, 86:371-375 [DOI] [PubMed] [Google Scholar]
- 55.Shen N, Ffrench P, Guyotat D, Ffrench M, Fiere D, Bryon PA, Dechavanne M: Expression of adhesion molecules in endothelial cells during allogeneic bone marrow transplantation. Eur J Haematol 1994, 52:296-301 [DOI] [PubMed] [Google Scholar]
- 56.Norton J, Sloane P: A prospective study of cellular and immunologic changes in skin of allogeneic bone marrow recipients. Relationship to clinical and histologic features of acute graft-versus-host disease. Am J Clin Pathol 1994, 101:597-602 [DOI] [PubMed] [Google Scholar]
- 57.Cotsarelis G, Cheng S-Z, Dong G, Sun T-T, Lavker RM: Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 1989, 57:201-209 [DOI] [PubMed] [Google Scholar]
- 58.Sale GE, Raff RF, Storb R: Stem cell regions in filiform papillae of tongue as targets of graft-vs-host disease. Transplantation 1994, 58:1273-1287 [PubMed] [Google Scholar]
- 59.O’Connell KA, Rudmann AA: Cloned spindle and epithelioid cells from murine Kaposi’s sarcoma-like tumors are of endothelial origin. J Invest Dermatol 1993, 100:742-745 [DOI] [PubMed] [Google Scholar]
- 60.Arico M, Noto G, Pravata G, Bongiorno MR, Mirto S, Malizia G: Transfusion-associated graft-versus-host disease—report of two further cases with an immunohistochemical analysis. Clin Exp Dermatol 1994, 19:36-42 [DOI] [PubMed] [Google Scholar]
- 61.Roy J, Platt JL, Weisdorf DJ: The immunopathology of upper gastrointestinal acute graft-versus-host disease. Lymphoid cells and endothelial adhesion molecules. Transplantation 1993, 55:572-578 [DOI] [PubMed] [Google Scholar]
- 62.Norton J, Sloane JP, Al-Saffar N, Haskard DO: Expression of adhesion molecules in human intestinal graft-versus-host disease. Clin Exp Immunol 1992, 87:231-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norton J, Sloane JP, Al-Saffar N, Haskard DO: Vessel associated adhesion molecules in normal skin and acute graft-versus-host disease. J Clin Pathol 1991, 44:586-591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruco LP, Stoppacciaro A, Uccini S, Breviario F, Dejana E, Gallo A, DeVincentiis M, Pileri S, Nicholls JM, Baroni CD: Expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in undifferentiated nasopharyngeal carcinoma (lymphoepithelioma) and in malignant epithelial tumors. Hum Pathol 1994, 25:924-928 [DOI] [PubMed] [Google Scholar]
- 65.Yoo YH, Park BS, Whitaker-Menezes D, Korngold R, Murphy GF: Dermal dendrocytes participate in the cellular pathology of experimental acute graft-versus-host disease. J Cutan Pathol 1998, 25:426-434 [DOI] [PubMed] [Google Scholar]
- 66.Itoh S, Matsuzaki Y, Kimura T, Ikegami T, Shoda J, Doy M, Fujiwara M, Tanaka N: Suppression of hepatic lesions in a murine graft-versus-host reaction by antibodies against adhesion molecules. J Hepatol 2000, 32:587-595 [DOI] [PubMed] [Google Scholar]
- 67.Dey BR, Yang YG, Szot GL, Pearson DA, Sykes M: Interleukin-12 inhibits graft-versus-host disease through an Fas-mediated mechanism associated with alterations in donor T-cell activation and expansion. Blood 1998, 91:3315-3322 [PubMed] [Google Scholar]
- 68.Cotsarelis G, Sun TT, Lavker RM: Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61:1329-1337 [DOI] [PubMed] [Google Scholar]
- 69.Watt FM: Epidermal stem cells: markers, patterning and the control of stem cell fate. Philos Trans R Soc Lond Biol 1998, B353:831-837 [DOI] [PMC free article] [PubMed] [Google Scholar]


