Abstract
Tumors often exhibit activation of specific tyrosine kinases, which may allow targeting of therapy through inhibition of tyrosine kinase signaling. This strategy has been used successfully in the development of STI571 (gleevec), an inhibitor of bcr-abl tyrosine kinase that has been used successfully in the treatment of chronic myelogenous leukemia. STI571 also shows activity against c-kit and platelet-derived growth factor receptor-β (PDGFRβ) tyrosine kinase signaling, thus potentially expanding the number of tumors that may respond to it. We describe a simple and rapid method to assess functional activity of tyrosine kinase signaling that is broadly applicable to tumor types. As proof of principle, we have applied it to cells that serve as models of the autosomal-dominant tumor syndrome tuberous sclerosis (TS). We found that TS model cells derived from tuberin heterozygous mice and from a human renal angiomyolipoma are highly sensitive to PDGFR antagonists and that these cells express PDGFRβ. Given that PDGFRβ signaling is inhibited by STI571, we found that SV7tert human angiomyolipoma cells are sensitive to STI571. Thus, we describe a novel but simple method of determining the functional tyrosine kinase profile of a neoplastic cell and our results suggest that STI571 might be useful in the treatment of neoplasms commonly seen in patients with TS.
Tuberous sclerosis (TS) is a common autosomal-dominant disorder that occurs because of the loss of one of two genes, hamartin (tsc1) and tuberin (tsc2). 1,2 TS, like other autosomal dominant cancer syndromes, including retinoblastoma, neurofibromatosis type 1, and multiple endocrine neoplasia, serves as an elegant confirmation of the Knudson and colleague’s 3 two-hit hypothesis, in which a second allele of the tumor suppressor is lost (loss of heterozygosity), resulting in tumorigenesis. However, this theory does not fully account for two findings. First, many of these syndromes show a distinct tissue tropism, despite the fact that expression of these genes is ubiquitous in most tissues. For example, tuberin and hamartin are widely expressed in the majority of human tissues, but tumors arise in specific organs, such as the kidney, brain, skin, and lung. 4-6 Second, loss of heterozygosity is not observed in all tumors from these patients. 7-10
Recently, high-level expression of the epidermal growth factor receptor has been observed in benign and malignant lesions of neurofibromatosis type 1. 11,12 Cells from these patients were found to be hypersensitive to epidermal growth factor receptor tyrosine-kinase antagonists. 11 Similarly, basal cell carcinomas arising in mice heterozygous for the tumor suppressor patched show activity of platelet-derived growth factor receptor α (PDGFRα). 13 We hypothesized that TS neoplasms may also show activation of a specific tyrosine kinase receptor, explaining in part the benign tissue-specific neoplasms observed in TS. We subjected TS-associated cell lines to a battery of small molecular weight tyrosine kinase inhibitors and found these cells to be highly sensitive to PDGFRβ tyrosine kinase inhibition. This approach may be generally applicable in determining potential contributions of tyrosine kinases to neoplastic processes through a rapid screen of tyrosine kinase inhibitors. We demonstrate that this simple method accurately predicts the presence of receptors and signaling partners in a given tumor type.
Materials and Methods
Derivation of Cell Lines
SV7tert [CRL 2461; American Type Culture Collection (ATCC), Rockville, MD] is a cell line derived from a human angiomyolipoma through the sequential introduction of SV40 large T antigen and telomerase into primary human angiomyolipoma cells. 14 Tsc2ang1 (ATCC CRL 2620) is a murine cell line derived from a cutaneous sarcoma arising in the extremity of a mouse heterozygous for tsc2. The sarcoma tissue was digested with collagenase and processed as described for SV7tert cells. 14 Mice heterozygous for tsc2 develop cutaneous sarcomas at a frequency of ∼10 to 15%. 15
Tyrosine Kinase Inhibitor Studies
The following tyrosine kinase inhibitors 16 were obtained from Calbiochem (San Diego, CA) and reconstituted as stock solutions in dimethyl sulfoxide immediately before use (AG9, AG17, AG18, AG30, AG82, AG99, AG112, AG370, AG490, AG879, AG957, AG1295, AG1296, AG1433, 2′thioadenosine, ST638, lavendustin C, oxindole 1, JAK3 inhibitors 1, 2, and 3, as well as JAK3 inhibitor-negative control. Ten thousand cells per well in a 24-well dish were plated on day 1 and were treated with inhibitors in doses ranging from 0 to 20 μg/ml. 17 Cells were counted 72 hours after treatment with inhibitors using a Coulter Counter (Coulter, Hialeah, FL).
Demonstration of PDGFRβ Signal Transduction in SV7tert and tsc2ang1 Cells
Subconfluent cells in six-well plates were serum-starved overnight and stimulated for 8 minutes with 50 ng/ml of PDGF-BB (Peprotech EC, Ltd., London, UK). The cells were lysed and used for immunoprecipitation with anti-PDGFRβ antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA). Immunoprecipitates were immobilized on protein A-Sepharose beads that were washed and boiled in sodium dodecyl sulfate sample buffer. The eluted material was separated on 10% sodium dodecyl sulfate-polyacrylamide gels, transferred to filters, and immunoblotted using anti-phosphotyrosine antibodies (4G10; Transduction Laboratories, Lexington, KY) or anti-PDGFRβ antibodies. Immunoreactive proteins were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ). Western blot analysis of SV7tert and tsc2ang1 cells was also performed with a polyclonal phosphoPLC gamma 1 Ab (Biosource International, Camarillo, CA), following the instructions from the manufacturers.
Immunohistochemistry of Human Angiomyolipomas for Phosphorylated PDGFRβ
Sections of formalin-fixed, paraffin-embedded tissue (5 μm) were tested for the presence of immunohistochemically detectable antigen (phosphorylated PDGFRβ) with steam heat-induced antigen retrieval, polyclonal anti-phosphorylated PDGFRβ specific for phosphotyrosine 857 residue of PDGFRβ 18 (1/600), an avidin-biotinylated enzyme complex kit (LSAB; DAKO, Carpinteria, CA), and DAKO Autostainer. Sections were deparaffinized and rehydrated, then steamed in citrate buffer (pH 6) for 20 minutes and cooled for 5 minutes before immunostaining. All tissues were then exposed to 3% hydrogen peroxide for 5 minutes, primary antibody for 25 minutes, avidin-biotinylated enzyme complex for 25 minutes, diaminobenzidine as chromogen for 5 minutes, and hematoxylin as counterstain for 1 minute. These incubations were performed at room temperature. Between incubations, sections were washed with buffer. For the two negative controls, primary antibody was replaced by buffer.
Inhibition of Cell Proliferation by STI571
STI571 was generously provided by Dr. Elizabeth Buchdunger (Novartis, Basel, Switzerland) and was reconstituted in dimethyl sulfoxide as above. Cells were treated with STI571 as in the tyrosine kinase inhibitor studies above.
Results
To determine whether human angiomyolipoma and tumors arising in mice heterozygous for tsc2 showed hypersensitivity to tyrosine kinase inhibitors, we exposed relevant human and mouse cell lines to a battery of tyrosine kinase inhibitors. These are tyrosine inhibitors that inhibit specific kinases, including JAK2 kinase, JAK3 kinase, PDGFR, epidermal growth factor receptor, ERK1/2 inhibitor, p140 c-trk, p210 bcr-abl, p60 c-src, ErbB2, and vascular endothelial growth factor receptor 2 (VEGFR2/flk-1). Human SV7tert cells showed the highest sensitivity to AG17 (Figure 1A) ▶ , an inhibitor of PDGFR tyrosine kinase. Murine tsc2ang1 cells demonstrated hypersensitivity to AG17, and AG879, an inhibitor of p140 c-trk (Figure 1B) ▶ . AG957 showed potent inhibitory activity against human SV7tert cells, but has less effect on murine tsc2ang1 cells (Figure 1C) ▶ . Inhibitory compounds AG17 and AG879 are both substituted aromatic malononitriles, but they differ substantially from AG957 (Figure 2) ▶ . The left columns in Figure 2 ▶ represent concentrations of 5 μg/ml, the second from left columns represent 10 μg/ml, the second from right columns represent 15 μg/ml, and the right columns represent 20 μg/ml.
Figure 1.
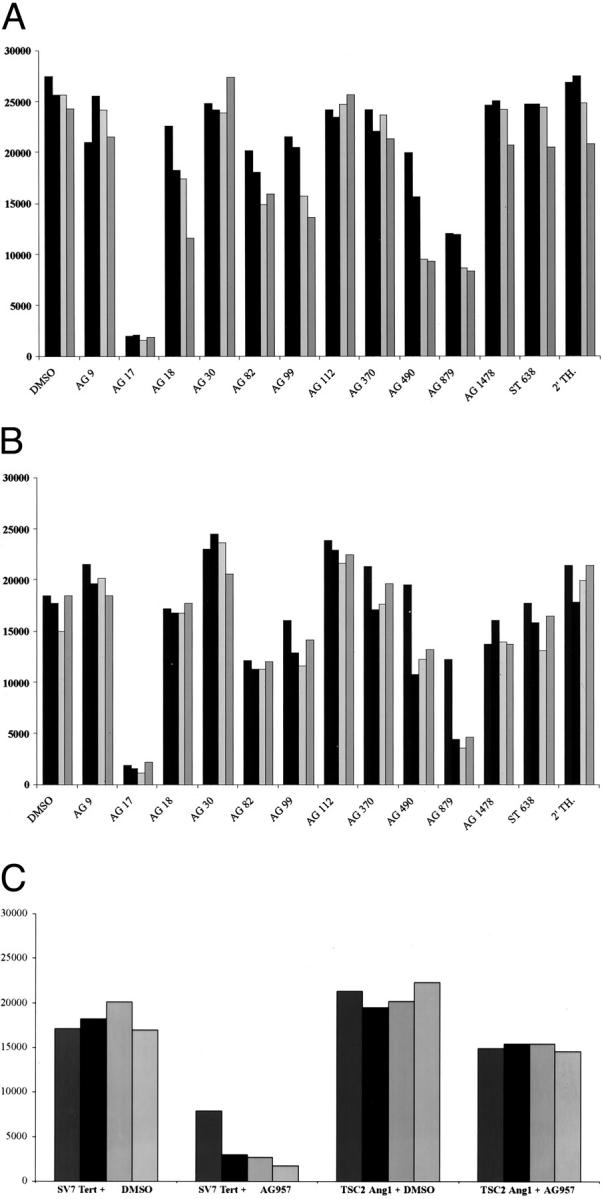
Inhibition of proliferation of SV7 tert and tsc2ang1 cells by tyrosine kinase inhibitors. A: Inhibition of SV7 tert cells using a battery of inhibitors. B: Inhibition of tsc2ang1 cells. C: Inhibition of both cell lines by AG957. The x axis displays the name of the tyrosine kinase inhibitor and the y axis represents the number of cells after 72 hours. Left columns represent concentrations of 5 μg/ml (or equal concentration of dimethyl sulfoxide), second from left columns represent concentrations of 10 μg/ml, second from right columns represent concentrations of 15 μg/ml, and right columns represent concentrations of 20 μg/ml.
Figure 2.
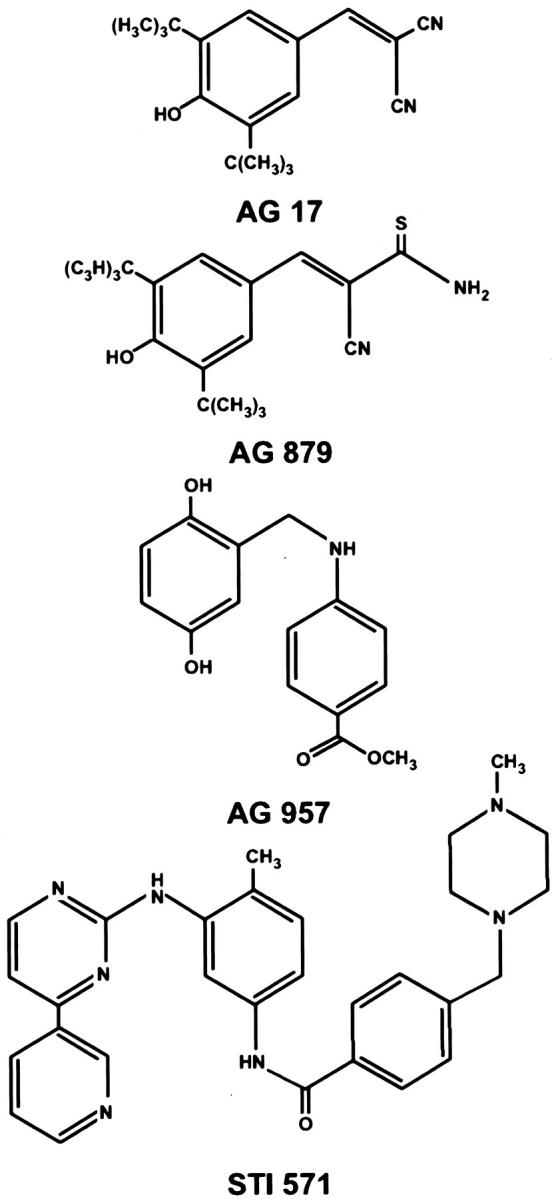
Chemical structures of compounds showing inhibitory activity against SV7tert and tsc2ang1 cells.
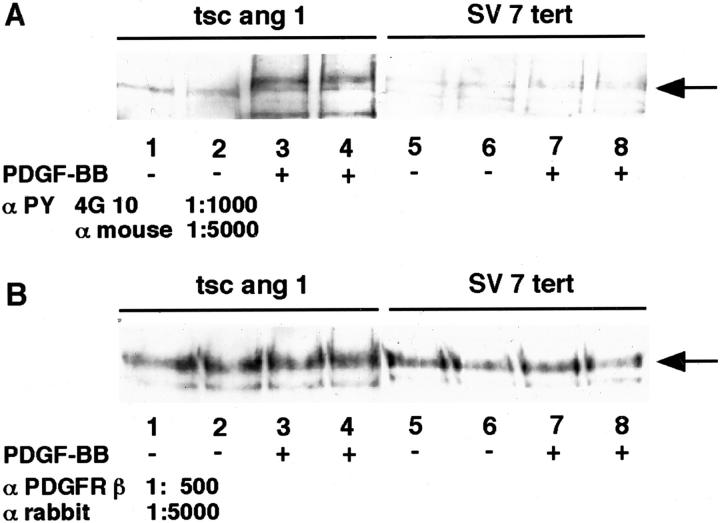
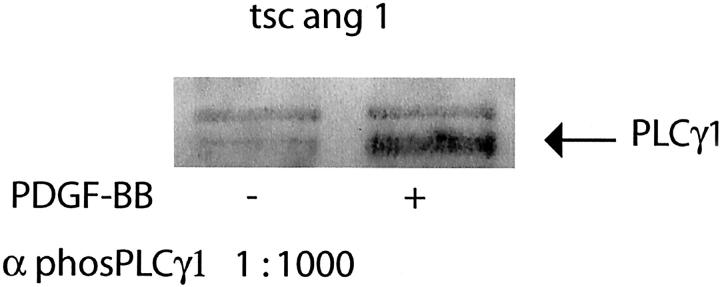
We chose to focus on PDGFR signal transduction because AG17, a PDGFR kinase inhibitor, strongly inhibited both murine and human cell lines. In addition, the newly approved drug STI571 inhibits PDGFRβ tyrosine kinase in addition to bcr-abl and c-kit tyrosine kinases. 19,20 The expression of functional PDGFRβ on both human SV7tert cells as well as on the murine tsc2ang1 cells was demonstrated by immunoblotting. Cells were treated with PDGF-BB or vehicle, and PDGFRβ was immunoprecipitated, followed by immunoblotting with anti-phosphotyrosine antibodies. As shown in Figure 3 ▶ , the expression levels of PDGFRβ was slightly higher in tsc2ang1 cells compared with the SV7tert cells. In both cases, PDGF-BB treatment resulted in increased levels of phosphotyrosine-containing PDGFRβ (Figure 3) ▶ . Furthermore, PDGF-BB treatment of tsc2ang1 cells resulted in activation of phospholipase C gamma (PLCγ), as an increase in phosphorylated PLCγ was noted after stimulation with PDGF-BB (Figure 4) ▶
Figure 3.
SV7 tert and tsc2ang1 cells express functional PDGFRβ. Subconfluent cultures of Tsc2ang1 or SV7tert cells were treated with PDGF-BB or vehicle for 8 minutes and aliquots of total cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to filters. One filter was incubated with anti-phosphotyrosine antibody 4G10 (αPY) and the secondary antibody against mouse immunoglobulin (α mouse) (A). A parallel filter was incubated with anti-PDGFRβ antiserum (α PDGFRβ), followed by the secondary antibody against rabbit immunoglobulin (α rabbit), at indicated dilutions. Immunoreactivity was detected by enhanced chemiluminescence (B).
Figure 4.
PDGF stimulation results in phosphorylation of PLCγ. Tsc2ang1 cells were treated with PDGF-BB as in Figure 3 ▶ , and lysates examined with antibodies specific for phosphorylated PLCγ1. The lane marked (−) represents control cells and the lane marked (+) represents cells treated with PDGF-BB.
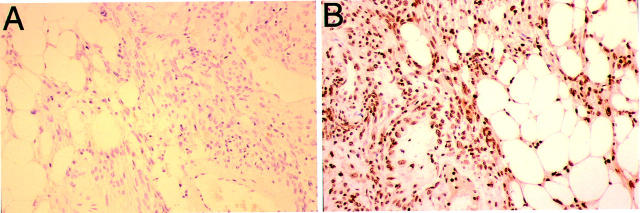
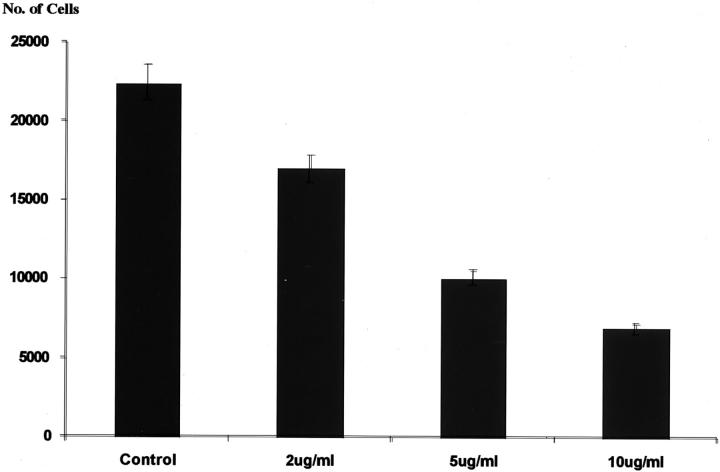
To determine whether our findings of PDGFRβ activation was truly associated with human angiomyolipomas in vivo, we stained authentic human angiomyolipoma tissue with an antibody specific for phosphorylated PDGFRβ. We observed strong staining for activated PDGFRβ in the tumor sections, indicating that PDGF-mediated signal transduction events occur in human angiomyolipomas in vivo (Figure 5) ▶ . We then tested the ability of STI571 to inhibit the growth of SV7tert and tsc2ang1 in vitro. We found that human SV7tert angiomyolipoma cells demonstrated increased sensitivity to STI571 compared to murine tsc2ang1, and this sensitivity occurred in a dose-dependent manner (Figure 6) ▶ .
Figure 5.
Immunohistochemistry of human angiomyolipoma tissue for anti-phospho-PDGFRβ. A: Negative control for staining (performed in the absence of primary antibody). B: Markedly positive staining of human angiomyolipoma with phosphospecific PDGFRβ.
Figure 6.
Inhibition of SV7tert cells by STI571. The x axis displays the concentration of STI571 and the y axis represents the number of cells after 72 hours.
Discussion
Autosomal dominant tumor predisposition syndromes include TS, neurofibromatosis, Cowden’s disease, and multiple endocrine neoplasias. Recently, activation of tyrosine kinase receptors have been described in autosomal-dominant tumor predisposition syndromes, including NF1 and basal cell nevus syndrome. 11 This finding led us to hypothesize that a similar mechanism of tyrosine kinase activation may be operative in TS. To find relevant tyrosine kinase pathways, we used a simple strategy of exposing relevant cell lines to tyrosine kinase inhibitors. In a study of both human and murine cell lines, we found increased sensitivity to inhibitors of PDGF receptor tyrosine kinases. We further demonstrate that PDGFRβ is functionally active in our cell lines, and that functional PDGFRβ is present in authentic human angiomyolipoma tissue. This strategy is broadly applicable to other neoplastic disorders and may help elucidate activation of tyrosine kinase pathways in tumors in which the tumor suppressor or oncogene is yet unknown.
TS is a common autosomal-dominant disorder that leads to neoplasms of the kidney, brain, skin, and lung in children and adults. 21 Cardiac rhabdomyomas occur around birth and usually resolve spontaneously. Renal tumors most commonly include angiomyolipomas, which are a distinct neoplasm of perivascular epithelioid cells, and exhibit phenotypic markers of smooth muscle, fat, and melanocytes. 4 Multifocal renal lesions are the major cause of morbidity and mortality in adults with TS, resulting in hemorrhage, renal failure, and occasional malignant transformation. A malignant version of angiomyolipoma occurs sporadically in pancreas, omentum, and female genitourinary tissues, and these lesions may demonstrate loss of tuberin. 22,23 In addition, similar lesions occur in the lungs of young women and are known as lymphangiomyomatosis. Lymphangiomyomatosis is a hormone-sensitive neoplasm, and can sometimes be ameliorated with hormonal therapy. 24,25 The finding that these neoplasms can be treated with hormone-mediated signal transduction therapy led to the hypothesis that renal lesions may also be amenable to signal transduction therapy. However, the primary modality for treatment of TS is surgical, and effective medical therapies are lacking for TS.
Recently, STI571 gained approval as the first specific tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia. 26 In addition to the inhibition of bcr-abl, STI571 inhibits activated c-kit and PDGF-mediated signal transduction. This has led to the successful therapy of gastrointestinal stromal tumors, which exhibits an activated kit receptor. 27,28 PDGF receptors are expressed in a wide variety of neoplasms, including sarcomas and gliomas, 29-31 leading to the hope that these lesions will respond to the orally available STI571.
Our study has accomplished three aims. First, we have demonstrated the feasibility of tyrosine kinase inhibitor profiling in that we have discovered tyrosine kinase pathways activated in cells without previous knowledge of their tyrosine kinase phenotype. Second, we have demonstrated that TS may predispose to activation of PDGFR. Finally, our results suggest a rationale for clinical trials of STI571 in the treatment and prevention of tuberous sclerosis-associated neoplasms.
Footnotes
Address reprint requests to Jack L Arbiser, M.D., Department of Dermatology, Emory University School of Medicine, WMB 5309, 1639 Pierce Dr., Atlanta, GA 30322. E-mail: jarbise@emory.edu.
Supported by the Tuberous Sclerosis Alliance and the National Institutes of Health (grant RO1 AR47901) (to J. L. A.) and Emory Skin Disease Research Core Center Grant P30AR42687.
References
- 1.: The European Chromosome 16 Tuberous Sclerosis Consortium: Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993, 75:1305-1315 [DOI] [PubMed] [Google Scholar]
- 2.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den OA, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Kwiatkowski DJ: Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 1997, 277:805-808 [DOI] [PubMed] [Google Scholar]
- 3.Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG: Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci USA 1994, 91:11413-11416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eble JN: Angiomyolipoma of kidney. Semin Diagn Pathol 1998, 15:21-40 [PubMed] [Google Scholar]
- 5.Wienecke R, Maize JC, Jr, Reed JA, de Gunzburg J, Yeung RS, DeClue JE: Expression of the TSC2 product tuberin and its target Rap1 in normal human tissues. Am J Pathol 1997, 150:43-50 [PMC free article] [PubMed] [Google Scholar]
- 6.Plank TL, Logginidou H, Klein-Szanto A, Henske EP: The expression of hamartin, the product of the TSC1 gene, in normal human tissues and in. Mod Pathol 1999, 12:539-545 [PubMed] [Google Scholar]
- 7.Perry A, Roth KA, Banerjee R, Fuller CE, Gutmann DH: Nf1 deletions in s-100 protein-positive and -negative cells of sporadic and neurofibromatosis 1 (nf1)-associated plexiform neurofibromas and malignant peripheral nerve sheath tumors. Am J Pathol 2001, 159:57-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajenaru ML, Donahoe J, Corral T, Reilly KM, Brophy S, Pellicer A, Gutmann DH: Neurofibromatosis 1 (NF1) heterozygosity results in a cell-autonomous growth advantage for astrocytes. Glia 2001, 33:314-323 [DOI] [PubMed] [Google Scholar]
- 9.Henske EP, Scheithauer BW, Short MP, Wollmann R, Nahmias J, Hornigold N, van Slegtenhorst M, Welsh CT, Kwiatkowski DJ: Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am J Hum Genet 1996, 59:400-406 [PMC free article] [PubMed] [Google Scholar]
- 10.Henske EP, Neumann HP, Scheithauer BW, Herbst EW, Short MP, Kwiatkowski DJ: Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosom Cancer 1995, 13:295-298 [DOI] [PubMed] [Google Scholar]
- 11.DeClue JE, Heffelfinger S, Benvenuto G, Ling B, Li S, Rui W, Vass WC, Viskochil D, Ratner N: Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J Clin Invest 2000, 105:1233-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zelkowitz M: Neurofibromatosis fibroblasts: abnormal growth and binding to epidermal growth factor. Adv Neurol 1981, 29:173-189 [PubMed] [Google Scholar]
- 13.Xie J, Aszterbaum M, Zhang X, Bonifas JM, Zachary C, Epstein E, McCormick F: A role of PDGFRalpha in basal cell carcinoma proliferation. Proc Natl Acad Sci USA 2001, 98:9255-9259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbiser JL, Yeung R, Weiss SW, Arbiser ZK, Amin MB, Cohen C, Frank D, Mahajan S, Herron GS, Yang J, Onda H, Zhang HB, Bai X, Uhlmann E, Loehr A, Northrup H, Au P, Davis I, Fisher DE, Gutmann DH: The generation and characterization of a cell line derived from a sporadic renal angiomyolipoma: use of telomerase to obtain stable populations of cells from benign neoplasms. Am J Pathol 2001, 159:483-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ: Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest 1999, 104:687-695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazit A, Yaish P, Gilon C, Levitzki A: Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem 1989, 32:2344-2352 [DOI] [PubMed] [Google Scholar]
- 17.LaMontagne KR, Jr, Moses MA, Wiederschain D, Mahajan S, Holden J, Ghazizadeh H, Frank DA, Arbiser JL: Inhibition of MAP kinase kinase causes morphological reversion and dissociation between soft agar growth and in vivo tumorigenesis in angiosarcoma cells. Am J Pathol 2000, 157:1937-1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernard A, Kazlauskas A: Phosphospecific antibodies reveal temporal regulation of platelet-derived growth factor beta receptor signaling. Exp Cell Res 1999, 253:704-712 [DOI] [PubMed] [Google Scholar]
- 19.Sjoblom T, Shimizu A, O’Brien KP, Pietras K, Dal Cin P, Buchdunger E, Dumanski JP, Ostman A, Heldin CH: Growth inhibition of dermatofibrosarcoma protuberans tumors by the platelet-derived growth factor receptor antagonist STI571 through induction of apoptosis. Cancer Res 2001, 61:5778-5783 [PubMed] [Google Scholar]
- 20.Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB: Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 2000, 295:139-145 [PubMed] [Google Scholar]
- 21.Shepherd CW, Gomez MR, Lie JT, Crowson CS: Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 1991, 66:792-796 [DOI] [PubMed] [Google Scholar]
- 22.Martignoni G, Pea M, Rigaud G, Manfrin E, Colato C, Zamboni G, Scarpa A, Tardanico R, Roncalli M, Bonetti F: Renal angiomyolipoma with epithelioid sarcomatous transformation and metastases: demonstration of the same genetic defects in the primary and metastatic lesions. Am J Surg Pathol 2000, 24:889-894 [DOI] [PubMed] [Google Scholar]
- 23.Zavala-Pompa A, Folpe AL, Jimenez RE, Lim SD, Cohen C, Eble JN, Amin MB: Immunohistochemical study of microphthalmia transcription factor and tyrosinase in angiomyolipoma of the kidney, renal cell carcinoma, and renal and retroperitoneal sarcomas: comparative evaluation with traditional diagnostic markers. Am J Surg Pathol 2001, 25:65-70 [DOI] [PubMed] [Google Scholar]
- 24.Ferrans VJ, Yu ZX, Nelson WK, Valencia JC, Tatsuguchi A, Avila NA, Riemenschn W, Matsui K, Travis WD, Moss J: Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J Nippon Med Sch 2000, 67:311-329 [DOI] [PubMed] [Google Scholar]
- 25.Desurmont S, Bauters C, Copin MC, Dewailly D, Tonnel AB, Wallaert B: Treatment of pulmonary lymphangioleiomyomatosis using a GnRH agonist. Rev Mal Respir 1996, 13:300-304 [PubMed] [Google Scholar]
- 26.Sawyers CL, Druker B: Tyrosine kinase inhibitors in chronic myeloid leukemia. Cancer J Sci Am 1999, 5:63-69 [PubMed] [Google Scholar]
- 27.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, Demetri GD: Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001, 344:1052-1056 [DOI] [PubMed] [Google Scholar]
- 28.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad TG, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y: Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279:577-580 [DOI] [PubMed] [Google Scholar]
- 29.Shimizu A, O’Brien KP, Sjoblom T, Pietras K, Buchdunger E, Collins VP, Heldin CH, Dumanski JP, Ostman A: The dermatofibrosarcoma protuberans-associated collagen type Ialpha1/platelet-derived growth factor (PDGF) B-chain fusion gene generates a transforming protein that is processed to functional PDGF-BB. Cancer Res 1999, 59:3719-3723 [PubMed] [Google Scholar]
- 30.Nister M, Heldin CH, Wasteson A, Westermark B: A platelet-derived growth factor analog produced by a human clonal glioma cell line. Ann NY Acad Sci 1982, 397:25-33 [DOI] [PubMed] [Google Scholar]
- 31.Shamah SM, Stiles CD, Guha A: Dominant-negative mutants of platelet-derived growth factor revert the transformed phenotype of human astrocytoma cells. Mol Cell Biol 1993, 13:7203-7212 [DOI] [PMC free article] [PubMed] [Google Scholar]