Abstract
α-Methylacyl-CoA racemase (AMACR) has previously been shown to be a highly sensitive marker for colorectal and clinically localized prostate cancer (PCa). However, AMACR expression was down-regulated at the transcript and protein level in hormone-refractory metastatic PCa, suggesting a hormone-dependent expression of AMACR. To further explore the hypothesis that AMACR is hormone regulated and plays a role in PCa progression AMACR protein expression was characterized in a broad range of PCa samples treated with variable amounts and lengths of exogenous anti-androgens. Analysis included standard slides and high-density tissue microarrays. AMACR protein expression was significantly increased in localized hormone-naive PCa as compared to benign (P < 0.001). Mean AMACR expression was lower in tissue samples from patients who had received neoadjuvant hormone treatment but still higher compared to hormone-refractory metastases. The hormone-sensitive tumor cell line, LNCaP, demonstrated stronger AMACR expression by Western blot analysis than the poorly differentiated cell lines DU-145 and PC-3. AMACR protein expression in cells after exposure to anti-androgen treatment was unchanged, whereas prostate-specific antigen, known to be androgen-regulated, demonstrated decreased protein expression. Surprisingly, this data suggests that AMACR expression is not regulated by androgens. Examination of colorectal cancer, which is not hormone regulated, demonstrated high levels of AMACR expression in well to moderately differentiated tumors and weak expression in anaplastic colorectal cancers. Taken together, these data suggest that AMACR expression is not hormone-dependent but may in fact be a marker of tumor differentiation.
Prostate cancer (PCa) is the most common non-skin cancer diagnosed in men in the United States. 1 One explanation for the rapid increase in the incidence of PCa diagnosis has been the advent of prostate-specific antigen (PSA) screening. PSA screening has led to earlier detection of PCa. 2 However, the impact of PSA screening on cancer-specific mortality is still unknown pending the results of prospective randomized screening studies. 3-5 A major limitation of the serum PSA test is lack of PCa sensitivity and specificity especially in the intermediate range of PSA detection (4 to 10 ng/ml). Our group has concentrated on developing and validating novel PCa biomarkers using a combined expression and tissue microarray (TMA) approach. 6 This approach by our group and others has led to the identification of hepsin, a serine protease up-regulated in PCa. 6-10 Furthermore, our group was able to use high-density TMAs to determine associations of hepsin protein and another protein, pim-1 kinase, with clinical outcome. 6
Using a similar approach, α-methylacyl-CoA racemase (AMACR), an enzyme that plays an important role in bile acid biosynthesis and β-oxidation of branched-chain fatty acids, 11,12 was also recently identified. AMACR was determined to be up-regulated in PCa after examination of several independent gene expression data sets, including our own. 6,8,10,13 These findings were supported by different groups on the protein level even when using different types of antibodies for immunoblot analysis and high-density TMAs. 13-15 Interestingly, hormone-refractory metastatic PCa demonstrated lower AMACR expression than hormone-naive-localized PCa. This observation suggested that AMACR protein expression is regulated by androgens. It is extremely important to identify PCa biomarkers, which portend an aggressive clinical course, given that hormone-refractory tumors are virtually all lethal. However, currently no clinical marker is available to identify a subgroup of localized tumors that may eventually develop into lethal PCa. To examine the intriguing possibility that the PCa biomarker, AMACR, might play a role in hormone dysregulation of localized PCa, we undertook the current study.
Materials and Methods
Sample Collection, cDNA Array, and TMA Construction and Evaluation
Clinical samples were taken from the Radical Prostatectomy Series and from the Rapid Autopsy Program at the University of Michigan. 16 Both are part of the University of Michigan Prostate Cancer Specialized Program of Research Excellence (SPORE). Primary PCa of metastatic cases as well as lymph node metastases were contributed in collaboration from the University of Ulm, Ulm, Germany. Detailed clinical expression analyses as well as TMA data were acquired and are maintained on a secure relational database 17 according to the Institutional Review Board protocol of both institutions.
Tissue procurement for expression analysis on RNA level was described in detail elsewhere. 6 For the development of TMA, samples were embedded in paraffin. The study pathologist (MAR) reviewed slides of all cases and circled areas of interest. These slides were used as a template for construction of the six TMAs used in this study. All TMAs were assembled using the manual tissue arrayer (Beecher Instruments, Silver Spring, MD). At least three tissue cores were sampled from each donor block. Histological diagnosis of the tissue cores was verified by standard hematoxylin and eosin (H&E) staining of the initial TMA slide. Standard biotin-avidin complex immunohistochemistry was performed using a polyclonal anti-AMACR antibody (kind gift of Ronald J. A. Wanders, University of Amsterdam, Amsterdam, The Netherlands). Digital images were acquired using the BLISS Imaging System (Bacus Laboratory, Lombard, IL). Staining intensity was scored as negative (score = 1), weak (score = 2), moderate (score = 3), and strong (score = 4). For exploration of the treatment effect by the means of hormonal withdrawal before radical prostatectomy, standard slides were used for regular H&E staining and consecutive sections for detection of AMACR expression. To test AMACR expression in poorly differentiated colon cancers, cases were used from a cohort of well-described colon tumors. 18 In addition to well-differentiated colon cancers, a recently described subset of poorly differentiated colon carcinomas with a distinctive histopathological appearance, termed “large-cell minimally differentiated carcinomas,” was used. As previously described, these poorly differentiated colon carcinomas had a high frequency of the microsatellite instability phenotype. 18
Cell Culture and Immunoblot Analysis
Prostate cell lines (RWPE-1, LNCaP, PC-3, and DU-145) were obtained from the American Type Culture Collection (Rockville, MD). Cells were maintained in RPMI 1640 with 8% decomplemented fetal bovine serum, 0.1% glutamine, and 0.1% penicillin and streptomycin (BioWhittaker, Walkersville, MD). Cells were grown to 75% confluence and then treated for 24 and 48 hours with the anti-androgen bicalutamide (Casodex, Zeneca Pharmaceuticals, Plankstadt, Germany) at a final concentration of 20 μmol/L or with methyltrienolone (synthetic androgen) (R1881; NEN, Life Science Products, Boston, MA) at a final concentration of 1 nmol/L. Cells were harvested and lysed in Nonidet P-40 lysis buffer containing 50 mmol/L Tris-HCl, pH 7.4, 1% Nonidet P-40 (Sigma, St. Louis, MO) and complete proteinase inhibitor cocktail (Roche, Indianapolis, IN). Fifteen μg of protein extracts were mixed with sodium dodecyl sulfate sample buffer and electrophoresed onto a 10% sodium dodecyl sulfate-polyacrylamide gel under reducing conditions. After transferring, the membranes (Amersham Pharmacia Biotech, Piscataway, NJ) were incubated for 1 hour in blocking buffer (Tris-buffered saline with 0.1% Tween and 5% nonfat dry milk). The AMACR antibody (kind gift of Dr. Wanders) was applied at 1:10,000 diluted blocking buffer overnight at 4°C. After three washes with TBS-T buffer, the membrane was incubated with horseradish peroxidase-linked donkey anti-rabbit IgG antibody (Amersham Pharmacia Biotech) at 1:5000 for 1 hour at room temperature. The signals were visualized with the ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ). For β-tubulin blots, membranes were stripped with Western Re-Probe buffer (Geno-tech, St. Louis, MO) and blocked in Tris-buffered saline with 0.1% Tween with 5% nonfat dry milk, and incubated with rabbit anti-β-tubulin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:500 for 2 hours. For PSA expression the membranes were reprobed in the described manner with PSA antibody (rabbit polyclonal; DAKO Corporation, Carpinteria, CA) at 1:1000 dilution and further processed.
Statistical Analysis
Primary analysis of the cDNA expression data were done with the Genepix software. Cluster analysis with the program Cluster and generation of figures with TreeView was performed as described earlier. 6 AMACR protein expression was statistically evaluated using the mean score result for each prostate tissue type (ie, benign prostate, naive localized or advanced PCa, hormone-treated and hormone-refractory PCa). To test for significant differences in AMACR protein expression between all tissue types, we performed a one-way analysis of variance test. To determine differences between all pairs a post hoc analysis using the Scheffé method was applied as described earlier. 14 For comparison of naive primaries to their corresponding lymph node metastases with respect to AMACR protein expression, a nonparametric analysis (Mann-Whitney test) was performed. To compare AMACR expression intensity to the scored hormonal effect of the pretreated localized PCa cases the Mantel-Haenszel chi-square test was applied. AMACR expression scores are presented in a graphical format using error bars with 95% confidence intervals (CIs). P values <0.05 were considered statistically significant.
Results
Hierarchical clustering of 76 prostate tissues including benign prostate, benign prostatic hyperplasia, localized PCa, and metastatic PCa and filtering for only those genes with a 1.5-fold expression difference or greater, clustered the samples into histologically distinct groups as previously described. 6 As demonstrated by a TreeView presentation of this data (Figure 1) ▶ , AMACR was one of several genes that demonstrated overexpression at the cDNA level of PCa samples with respect to benign pooled prostate tissue. Interestingly, although some of the hormone-refractory cases demonstrated overexpression, as depicted by the red shading, the highest level of overexpression by cDNA analysis was in the clinically localized PCa cases.
Figure 1.
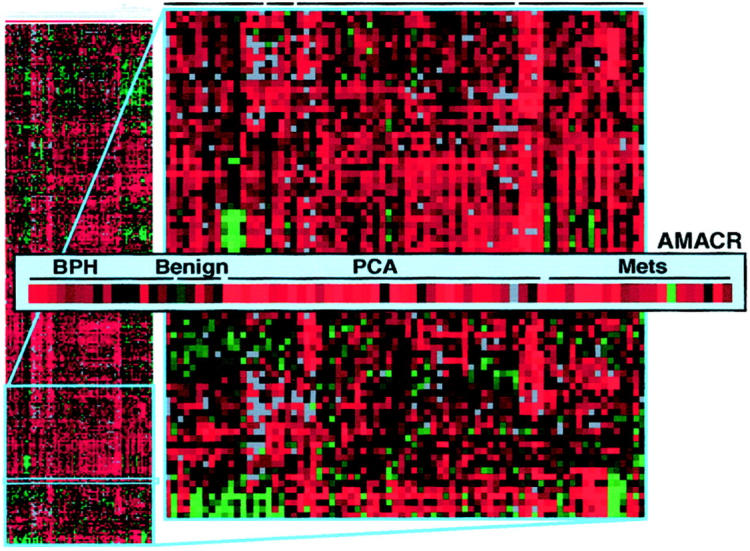
Seventy-six prostate tissue samples including benign prostate, benign prostatic hyperplasia, localized PCa, and metastatic PCa, were analyzed using cDNA expression array analysis. Hierarchical clustering was performed, filtering for only those genes with at least a 1.5-fold expression difference compared to a pool of normal prostate. AMACR was one of the genes that was found to be significantly overexpressed in PCa. Data given are a measure of relative gene expression of AMACR in each sample. Red and green represent up- and down-regulation, respectively. Lighter shading correlates with increased expression.
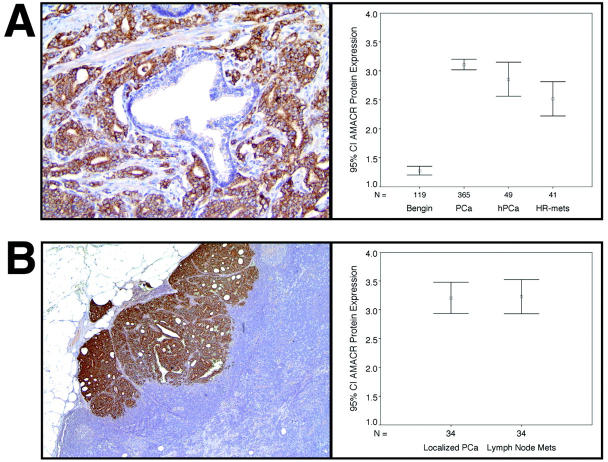
To further investigate the role of AMACR protein expression in samples with variable differentiation and exposure to anti-androgen treatment, several TMAs with a wide range of PCa were constructed: a total of 119 benign prostate samples, 365 primary hormone-naive PCa samples, 37 naive PCa lymph node metastases, and 41 hormone-refractory metastatic PCa samples were evaluated. An additional 49 hormone-treated primary PCas (including 22 on standard slides) were examined for histological changes associated with anti-androgen treatment and AMACR protein expression. In our hands the percentage of stained cancer cells per sample was 95% in the cases studied. This was independent from the scored intensity of AMACR staining. The mean AMACR protein expression levels for each tissue category is presented in Figure 2A ▶ . Benign prostate, naive primary PCa, hormone-treated primary cancer, and hormone-refractory metastatic tissue had a mean staining intensity of 1.28 [standard error (SE), 0.038; 95% CI, 1.20 to 1.35], 3.11 (SE, 0.046; CI, 3.02 to 3.20), 2.86 (SE, 0.15; CI, 2.56 to 3.15), and 2.52 (SE, 0.15; CI, 2.22 to 2.28), respectively. One-way analysis of variance analysis revealed a P value of <0.0001. To specifically examine the difference between different tissue types, a post hoc pair-wise comparison was performed. Clinically localized PCa demonstrated a significantly stronger AMACR protein expression as compared to benign prostate tissue (post hoc analysis using Scheffé method; mean difference = 1.83; P < 0.0001; CI, 1.53 to 2.13). As expected from previous work, a significant decrease in AMACR protein expression was observed in the metastatic hormone-refractory PCa samples with respect to clinically localized PCa (0.59; P = 0.002; CI, 0.15 to 1.03). Hormone-treated primaries had a mean AMACR expression of 2.86, which was between the expression levels of naive primaries (3.11) and hormone-refractory cases (2.52) (post hoc analysis using Scheffé method; P = 0.51; CI, −0.66 to 0.16; and P = 0.56; CI, −0.23 to 0.91). Interestingly there was no significant difference in AMACR expression in the 37 naive primary prostate samples and lymph node metastases derived from the same patient (Mann-Whitney test, P = 0.8). In other words, matched primaries and lymph node metastases showed a similar AMACR expression pattern (Figure 2B) ▶ . Examples of in situ AMACR protein expression in a naive primary PCa and a naive lymph node metastasis are presented in Figure 2 ▶ .
Figure 2.
A: AMACR protein expression in localized hormone-naive PCa. The specificity of AMACR is demonstrated by the near complete absence of AMACR expression in benign prostate glands (center). Error bars (right) representing the 95% CI of the mean expression level of AMACR for the four tissue types: benign prostate (benign), localized hormone-naive PCa (PCa), hormone-treated localized PCa (hPCa), and hormone-refractory distant PCa metastases (HR mets). B: Strong AMACR expression in a naive lymph node metastasis. Error bars (right) representing the 95% CI of the mean expression of the primary naive PCa and corresponding lymph node metastases. Original magnifications, ×400.
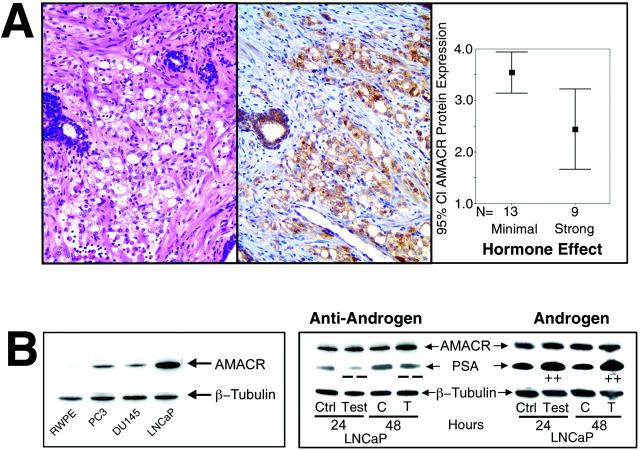
Because there seems to be a wider variability in AMACR protein expression in the hormone-treated PCa samples as compared to naive cases (Figure 2) ▶ , we further investigated this observation to correlate the hormone treatment effect with AMACR protein expression. We examined a subset of 22 PCa cases in which the patients received variable amounts and types of anti-androgen treatment before surgery. These cases were evaluated blindly with respect to treatment protocol for histological evidence of hormone treatment (H&E slide) and AMACR protein expression. The hormonal effect visible on the H&E slides was classified from 1 to 4 with 1 representing “no effect” and 4 showing a “very strong effect.” Thirteen cases demonstrated either no or moderate hormonal effect and nine cases had a very strong hormonal effect. Statistical analysis revealed a significant difference between these two groups with respect to AMACR expression intensity (Figure 3 ▶ , Mantel-Haenszel chi-square, P = 0.009). Figure 3 ▶ presents an example of a PCa case treated before surgery with anti-androgens, which has a strong hormonal effect appreciated on H&E and decreased AMACR protein expression (Figure 3A) ▶ . In these cases, no association was detected between the type of hormone treatment (ie, monotherapy or complete hormonal withdrawal for hormone deprivation) or duration of treatment and AMACR expression.
Figure 3.
A: PCa demonstrating strong hormonal effect (right) because of anti-androgen treatment (H&E). Weak AMACR expression (middle) seen in the corresponding immunostained section. Comparison of the mean AMACR expression between PCa demonstrating minimal and strong hormonal effects by H&E examination (right, 95% CI). B: Left: Western blot analysis representing the baseline AMACR expression in different prostate cell lines. Internal loading control with β-tubulin. Right: Western blot analysis of LNCaP cells for AMACR and PSA expression after treatment with an androgen or an anti-androgen for 24 hours and 48 hours. Results of the nontreated control samples (C) and the hormone-treated (T) cells are shown. Original magnification, ×200 (A).
For further exploration of the hormonal effect on AMACR expression, primary cell culture experiments and Western blot analysis were performed. As demonstrated in Figure 3B ▶ , LNCaP cells, derived from a metastatic lesion but considered hormone responsive, showed a higher baseline AMACR expression as compared to PC-3 and DU-145 cells, which are both hormone-independent cell lines derived from metastatic lesions. A benign cell line, RWPE-1, 19 showed near absent AMACR expression, which is consistent with our in situ protein expression data. To simulate an anti-androgen treatment, we used the hormone-responsive cell line, LNCaP, and treated the cells with bicalutamide in a final concentration of 20 μmol/L for a time period of 24 and 48 hours. Interestingly, AMACR expression in cell lysates of LNCaP cells did not change at either time point when exposed to anti-androgen therapy. Under the same conditions, PSA, a gene known to be regulated by the androgen receptor, showed decreased protein expression. In addition, when LNCaP cells were exposed to a synthetic androgen R1881, no increase in AMACR expression was observed (Figure 3B) ▶ . Therefore, these cell culture experiments provide evidence that AMACR expression may not be directly regulated by the androgen pathway.
However, another explanation for these observations was that AMACR overexpression occurred in PCa, but as these tumors became poorly differentiated, as in the hormone-refractory PCa, AMACR expression was down-regulated either directly or indirectly because of the process of dedifferentiation. To elucidate this potential correlation we examined colon cancer samples for AMACR expression. As we have recently identified, 20 AMACR protein expression is also observed in some other tumor types, with the highest overall expression in colorectal cancers. Colorectal cancers are not known to be regulated by androgens and therefore represent a good control to test this hypothesis. Twenty well-differentiated and nine anaplastic colon cancer samples were chosen. As previously described, the poorly differentiated tumors have distinct molecular alterations distinguishing them from the common well to moderately differentiated colorectal tumors. 18 Figure 4 ▶ demonstrates strong AMACR protein expression in a moderately differentiated colon cancer. This tumor still forms well-defined glandular structures. The surrounding benign colonic tissue does not express AMACR. The anaplastic colon cancers demonstrated weak AMACR protein expression (Figure 4) ▶ . Moderate to strong AMACR expression was seen in 18 of 20 differentiated cases but in only 2 of 9 anaplastic colonic cancers (chi-square, P < 0.001). Seven of nine of the anaplastic colon cancers had weak to moderate expression. For comparative purposes examples of metastatic hormone-refractory PCa from the TMA experiments are shown in Figure 4 ▶ . Both of these cases demonstrate weak AMACR protein expression.
Figure 4.
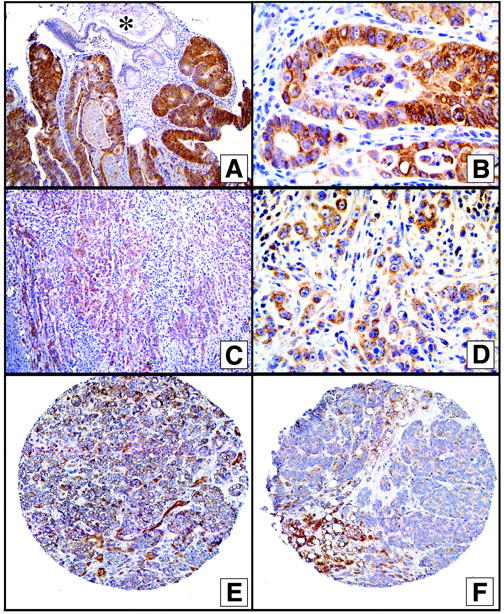
Top: AMACR expression in well-differentiated colon cancer. Cancer specificity demonstrated by the lack of staining in benign colonic glands (*). Middle: Weak AMACR expression in an anaplastic colon cancer case. Bottom: Weak AMACR expression in representative hormone-refractory PCa metastases. Original magnifications: ×400 (A, C); ×1000 (B); ×100 (D); ×200 (E and F).
Discussion
High-throughput technologies such as cDNA microarrays and TMA have opened a gateway for research. The combination of a tool for discovery with one for verification allows surveying target genes in a very efficient manner. By exploring gene expression data it could be shown that AMACR is consistently overexpressed in PCa. 14 These findings are supported by AMACR expression data described recently in at least two more independent studies using cDNA microarrays. 8,10 Even combined analysis across the three studies revealed statistically significant differential expression of AMACR between benign prostate and PCa. 14 This study focuses on the AMACR expression in different groups of PCa including the aspect of neoadjuvant hormonal withdrawal in localized disease. The most interesting finding in this context was that AMACR expression is decreased in hormone-refractory metastatic tissue samples compared to localized PCa. From the clinical point of view, this observation is very important because localized PCa can be controlled well by different means such as surgery or radiotherapy or even watchful waiting. 21,22 On the other hand, there is no consensus as how to treat metastatic disease successfully to positively impact life expectancy. 23 Therefore, identifying PCa biomarkers that are up-regulated in localized PCa but decrease again in far advanced hormone-refractory PCa could help to understand the mechanism involved with the ultimate goal of identifying new treatments.
A surprising finding is that AMACR expression seemed to be hormone-independent in the setting of our cell culture experiments. PSA, a gene known to be regulated by androgens, demonstrated hormone-related alterations in expression under the same conditions. These findings may provide evidence that AMACR is not regulated by the androgen pathway. Still, because we see a decreased AMACR expression in hormone-refractory tissue, it might be postulated that AMACR could play a role as a biomarker for hormone resistance. Considering the fact that hormone treatment in the mean of hormonal withdrawal did not affect AMACR expression in the cell culture, may lead to the conclusion that some othermechanism than the androgen pathway is responsible for AMACR down-regulation in the integrity of cancer tissue. These findings are considered to be very important and require further investigation.
An alternative hypothesis would suggest that AMACR is overexpressed in the development of cancer, perhaps playing an important role in providing energy for the neoplastic cells. However as the tumors become dedifferentiated, they no longer require these sources of energy. Poorly differentiated tumors may take over other pathways to accomplish this same activity of branched fatty acid oxidation. Recent work failed to identify an association with the proliferative rate of the tumor cells and AMACR expression 14 or with the Gleason score and AMACR expression. 13,14 Examination of other tumors demonstrated that colon cancer has the highest AMACR expression (unpublished observations). As colorectal cancers are not known to be hormonally regulated, the fact that dedifferentiation and decreased AMACR expression went hand in hand, further supports the hypothesis that dedifferentiation leads to decreased AMACR expression in the hormone-refractory metastatic PCa. Hormone treatment is also a front line therapy in metastatic PCa but is known to loose efficacy, selecting out hormone-insensitive clones. Anticipating the selection of potentially more dedifferentiated cells because of hormone treatment, an observed strong treatment effect may be consistent with decreased AMACR expression because of the selection of more dedifferentiated cells.
The AMACR gene product is an enzyme, that plays an important role in bile acid biosynthesis and β-oxidation of branched-chain fatty acids. 12,24 The link of AMACR expression and neoplasia, however, has only recently been made. AMACR overexpression appears to occur in tumors that have been linked to high-fat diet such as PCa and colorectal cancer. 25 The relationship between fatty acid consumption and cancer is a controversial subject in the development of PCa and colorectal cancer. 26,27 An essential role for AMACR in the oxidation of bile acid intermediates has been demonstrated. AMACR encodes an enzyme that catalyzes the racemization of α-methyl-branched carboxylic coenzyme A thioesters and is localized in peroxisomes and mitochondria. 28 As AMACR is involved in the metabolism of lipids it may be speculated that this could lead to alterations in the oxidant balance of a cell. These changes might be associated with DNA damage, malignant transformation, and other parameters of cell disturbance. It is still not clear if AMACR is directly involved in carcinogenesis via a degradation pathway of branched chain fatty acids or if it represents an epiphenomenon, because it is overexpressed in some human tumors. These hypotheses need to be further elucidated.
Acknowledgments
We thank Dr. Ronald J. A. Wanders (University of Amsterdam) for the anti-AMACR antibody; Robin Kunkel with assistance in preparing figures; Dr. Henry D. Appelman (Pathology, University of Michigan) for pathology support; Prof. Dr. Peter Möller, Chairman Department of Pathology, Ulm, Germany, for providing tissue samples; and Dr. Kenneth J. Pienta, Associate Professor, University of Michigan, for his support through the Rapid Autopsy Program and the SPORE affiliation.
Footnotes
Address reprint requests to Mark A. Rubin, M.D., Department of Pathology, University of Michigan Medical School, 1301 Catherine Rd., Ann Arbor, MI 48109-0602. E-mail: marubin@umich.edu.
Supported by the Specialized Program in Research Excellence in Prostate Cancer (P50 CA69568), the National Cancer Institute, and the University of Michigan Bioinformatics Program (pilot grant 379206).
References
- 1.Dennis LK, Resnick MI: Analysis of recent trends in prostate cancer incidence and mortality. Prostate 2000, 42:247-252 [DOI] [PubMed] [Google Scholar]
- 2.Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, deKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL, et al: Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol 1994, 151:1283-1290 [DOI] [PubMed] [Google Scholar]
- 3.Etzioni R, Legler JM, Feuer EJ, Merrill RM, Cronin KA, Hankey BF: Cancer surveillance series: interpreting trends in prostate cancer—part III: quantifying the link between population prostate-specific antigen testing and recent declines in prostate cancer mortality. J Natl Cancer Inst 1999, 91:1033-1039 [DOI] [PubMed] [Google Scholar]
- 4.Maattanen L, Auvinen A, Stenman UH, Rannikko S, Tammela T, Aro J, Juusela H, Hakama M: European randomized study of prostate cancer screening: first-year results of the Finnish trial. Br J Cancer 1999, 79:1210-1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroder FH, van der Maas P, Beemsterboer P, Kruger AB, Hoedemaeker R, Rietbergen J, Kranse R: Evaluation of the digital rectal examination as a screening test for prostate cancer. Rotterdam section of the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst 1998, 90:1817-1823 [DOI] [PubMed] [Google Scholar]
- 6.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM: Delineation of prognostic biomarkers in prostate cancer. Nature 2001, 412:822-826 [DOI] [PubMed] [Google Scholar]
- 7.Magee JA, Araki T, Patil S, Ehrig T, True L, Humphrey PA, Catalona WJ, Watson MA, Milbrandt J: Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res 2001, 61:5692-5696 [PubMed] [Google Scholar]
- 8.Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, Frierson HF, Jr, Hampton GM: Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res 2001, 61:5974-5978 [PubMed] [Google Scholar]
- 9.Stamey TA, Warrington JA, Caldwell MC, Chen Z, Fan Z, Mahadevappa M, McNeal JE, Nolley R, Zhang Z: Molecular genetic profiling of Gleason grade 4/5 prostate cancers compared to benign prostatic hyperplasia. J Urol 2001, 166:2171-2177 [PubMed] [Google Scholar]
- 10.Luo J, Duggan DJ, Chen Y, Sauvageot J, Ewing CM, Bittner ML, Trent JM, Isaacs WB: Human prostate cancer and benign prostatic hyperplasia: molecular dissection by gene expression profiling. Cancer Res 2001, 61:4683-4688 [PubMed] [Google Scholar]
- 11.Ferdinandusse S, Denis S, Ijlst J, Dacremont G, Waterham HR, Wanders RJ: Subcellular localization and physiological role of alpha-methylacyl-CoA racemase. J Lipid Res 2000, 41:1890-1896 [PubMed] [Google Scholar]
- 12.Kotti TJ, Savolainen K, Helander HM, Yagi A, Novikov DK, Kalkkinen N, Conzelmann E, Hiltunen JK, Schmitz W: In mouse alpha-methylacyl-CoA racemase, the same gene product is simultaneously located in mitochondria and peroxisomes. J Biol Chem 2000, 275:20887-20895 [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z, Woda BA, Rock KL, Xu Y, Savas L, Khan A, Pihan G, Cai F, Babcook JS, Rathanaswami P, Reed SG, Xu J, Fanger GR: P504S: a new molecular marker for the detection of prostate carcinoma. Am J Surg Pathol 2001, 25:1397-1404 [DOI] [PubMed] [Google Scholar]
- 14.Rubin MA, Zhou M, Dhanasekaran SM, Varambally S, Barrette TR, Sanda MG, Pienta KJ, Ghosh D, Chinnaiyan AM: Alpha-methylacyl coenzyme A racemase as a tissue biomarker for prostate cancer. JAMA 2002, 287:1662-1670 [DOI] [PubMed] [Google Scholar]
- 15.Luo J, Zha S, Gage WR, Dunn TA, Hicks JL, Bennett CJ, Ewing CM, Platz EA, Ferdinandusse S, Wanders RJ, Trent JM, Isaacs WB, De Marzo AM: Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res 2002, 62:2220-2226 [PubMed] [Google Scholar]
- 16.Rubin MA, Putzi M, Mucci N, Smith DC, Wojno K, Korenchuk S, Pienta KJ: Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin Cancer Res 2000, 6:1038-1045 [PubMed] [Google Scholar]
- 17.Manley S, Mucci NR, De Marzo AM, Rubin MA: Relational database structure to manage high-density tissue microarray data and images for pathology studies focusing on clinical outcome: the prostate specialized program of research excellence model. Am J Pathol 2001, 159:837-843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinoi T, Tani M, Lucas PC, Caca K, Dunn RL, Macri E, Loda M, Appelman HD, Cho KR, Fearon ER: Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am J Pathol 2001, 159:2239-2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS: Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis 1997, 18:1215-1223 [DOI] [PubMed] [Google Scholar]
- 20.Zhou M, Chinnaiyan A, Kleer C, Lucas P, Rubin MA: Alpha-methyl-CoA racemase: a novel tumor marker over expressed in several human cancers and their precursor lesions. Am J Surg Pathol 2002, 26:926-931 [DOI] [PubMed] [Google Scholar]
- 21.Krisch EB, Koprowski CD: Deciding on radiation therapy for prostate cancer: the physician’s perspective. Semin Urol Oncol 2000, 18:214-225 [PubMed] [Google Scholar]
- 22.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC: Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin N Am 2001, 28:555-565 [DOI] [PubMed] [Google Scholar]
- 23.Altwein JE: Therapeutic options in locally defined or advanced prostate cancer. Eur Urol 1999, 35:9-16 [PubMed] [Google Scholar]
- 24.Ferdinandusse S, Overmars H, Denis S, Waterham HR, Wanders RJ, Vreken P: Plasma analysis of di- and trihydroxycholestanoic acid diastereoisomers in peroxisomal alpha-methylacyl-CoA racemase deficiency. J Lipid Res 2001, 42:137-141 [PubMed] [Google Scholar]
- 25.Bartsch H, Nair J, Owen RW: Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis 1999, 20:2209-2218 [DOI] [PubMed] [Google Scholar]
- 26.Moyad MA: Fat reduction to prevent prostate cancer: waiting for more evidence? Curr Opin Urol 2001, 11:457-461 [DOI] [PubMed] [Google Scholar]
- 27.Willett WC: Diet and cancer. Oncologist 2000, 5:393-404 [DOI] [PubMed] [Google Scholar]
- 28.Schmitz W, Albers C, Fingerhut R, Conzelmann E: Purification and characterization of an alpha-methylacyl-CoA racemase from human liver. Eur J Biochem 1995, 231:815-822 [DOI] [PubMed] [Google Scholar]