Abstract
Endometriosis, the growth of ectopic endometrial tissue, is a chronic recurrent disease affecting 10% of the female population causing dyspareunia, pelvic pain, dysmenorrhea, and infertility. Suppression of ovarian activity is the cornerstone of medical therapy with limited benefit and severe adverse effects. Angiogenesis plays a major role in the development of endometriosis suggesting that anti-angiogenic therapy would offer a new therapeutic approach. We report successful treatment of endometriosis in estrogen-supplemented ovariectomized mice by transient overexpression (6 to 10 days of duration) of the gene for a natural angiogenesis inhibitor angiostatin, delivered to the peritoneum by a replication-deficient adenovirus vector (AdAngiostatin). Established endometriosis was eradicated in 14 of 14 AdAngiostatin-treated animals, whereas 11 of 13 control animals showed full disease development. Administered to normal cycling mice for the same transient period, AdAngiostatin caused impaired ovarian function with suppressed corpus luteum development, decreased production of estradiol and progesterone, decreased ovarian and uterine weight, and increased body weight. AdAngiostatin treatment lowered the levels of sex steroids but did not induce total castration. Gene therapy with angiogenic inhibitors is a highly effective treatment for endometriosis, even in a host with preserved estrogen levels. However, local or targeted delivery of the gene must be considered to avoid prolonged systemic effects and impaired ovarian function.
Endometriosis, the growth of functional endometrial tissue containing both glands and stroma outside the uterine cavity, is a chronic recurrent disease affecting a large number of women. Classic symptoms of endometriosis are dyspareunia, chronic pelvic pain, dysmenorrhea, and infertility. Affected women often have a poor quality of life and the disease is associated with significant health costs. 1 Approximately 10% of the general female population suffer from endometriosis 2 and almost 70% of young women with chronic pelvic pain have the disorder. 3 Among infertile women, the prevalence of endometriosis is 20 to 40% and presence of endometriosis decreases the success rate of in vitro fertilization. 4 Moreover, there is an association between untreated endometriosis and development of ovarian cancer. 5
Endometriosis is an estrogen-dependent disease and current therapeutic alternatives consist of various hormone treatments aimed at decreasing circulating estrogen to postmenopausal levels. This treatment has been shown to be of limited benefit and induces severe adverse effects, including hot flashes, weight gain, and mood disorders. 6 In fact, the treatment itself reduces the quality of life from a short-term perspective. 7 In the long term, lowered estrogen levels promote osteoporosis and increase the risk of heart disease and available therapies can therefore only be recommended for use for a maximum of 6 months. Consequently, there is a need to develop new therapeutic approaches to endometriosis.
Although the pathogenesis of endometriosis is controversial, it is generally accepted that endometriosis is the result of implantation of shed endometrial tissue on the peritoneal surface after retrograde menstruation. 8 Several factors are important in the progression of ectopic endometriotic implants such as an impaired immune defense, 9 stimulation of endometrial cell proliferation, 10 and the ability of the endometrium to adhere to and invade the peritoneal surface by production of adhesion molecules and matrix metalloproteinases. 11-13 A main feature of active endometriosis is pronounced vascularization within and around the tissue, which suggests that angiogenesis may be important in the maintenance of endometriosis. 14,15 This is supported by the findings that vascular endothelial growth factor, the most potent and specific angiogenic factor, is found in women with endometriosis at elevated levels in peritoneal fluid and macrophages, where it appears to be regulated by ovarian steroids. 16-19 Angiogenesis is also essential for the normal physiological function of the female reproductive system. 20,21 In other diseases, such as cancer and arthritis, angiogenesis seems to play a predominant role 22,23 and a number of inhibitors of angiogenesis have been demonstrated to modulate the progression of these diseases. 22,24,25 Angiostatin is one such endogenous angiogenesis inhibitor. Our recent studies have shown that delivery of angiostatin, by expression from a replication-deficient adenovirus vector (AdAngiostatin), results in high expression of biological active protein for up to 6 to 10 days in vivo. 26
Because angiogenesis plays a major role in the development of endometriosis it seems reasonable that anti-angiogenetic therapy would be a potential novel treatment for this disease. In this study we determined the effect of administration of an AdAngiostatin in a murine model of endometriosis and the effects of AdAngiostatin on normal ovarian function.
We show here that gene therapy with angiostatin is an effective treatment for endometriosis and that angiostatin gene therapy is effective even in the presence of physiological estrogen levels. We also show that prolonged expression of angiostatin in the normal cycling mouse causes decreased ovarian secretion of sex steroids with subsequent increased deposits of body fat.
Materials and Methods
Animals
C57BL/6 and FVB/n mice (6 to 8 weeks old) were purchased from Charles River (Troy, NY). They were housed in pathogen-free conditions with a light/dark cycle of 12/12 hours and fed with rodent chow and water ad libitum. Before any invasive procedure or euthanasia all mice were anesthetized with isofluorane (Abbot, Abbot Park, IL). The McMaster University animal ethics research board approved all animal work.
Induction of Endometriosis
Endometriosis was established in C57BL/6 female mice as previously described. 27 In this model, mice were ovariectomized (OVX) and 3-mm pellets containing 17 β-estradiol, 0.18 mg/60-day release (Innovative Research of America, Sarasota, FL) were implanted subcutaneously in the animal’s back 7 days before induction of endometriosis. The pellets provide continuous release of hormone at serum concentrations of 150 to 250 pmol/L (confirmed by serum analysis), which is in the range of physiological levels seen in mice during the estrous cycle. 28 This high stable physiological level of estrogen promotes the growth of endometrium and eliminates differences related to various stages of the estrous cycle. Half of the mice served as donor mice and half as recipient mice. The donor mice were killed by cervical dislocation and the uterus of each mouse was removed under sterile conditions. Both uterine horns were opened longitudinally and the endometrium was dissected off the uterine muscle with a scalpel. The endometrium was finely chopped and endometrial fragments were suspended in 0.4 ml of sterile normal saline and inoculated into the peritoneal cavity of a recipient mouse through a small midline incision.
Assessment of Lesions
Mice were killed 1 and 3 weeks after inoculation with endometrial fragments to assess the development of endometriosis. Others were examined from 2 to 3 to 18 days after virus injection (10 to 25 days after inoculation with endometrium) to assess the result of the treatment with AdAngiostatin. These time points were chosen when maximum expression of angiostatin protein was expected, days 2 to 3 and 1 week after the expected production of angiostatin had stopped. The peritoneum and visceral organs were examined using a dissecting microscope and lesions exhibiting the distinctive morphology of endometriosis were removed. Tissue was fixed in 10% buffered formalin and paraffin sections stained by hematoxylin and eosin (H&E). An experienced pathologist (CR) examined all slides in a blinded manner. Endometriosis was diagnosed if ectopic foci were present and if they contained both viable endometrial glands and stroma. Mice were weighed. Blood was collected by cardiac puncture. Where appropriate, uteri and ovaries were weighed and fixed in 10% formalin for examination of morphology.
Adenoviral Vectors and Vector Administration
The vector-expressing murine angiostatin (AdAngiostatin) and the control vector Addl70-3 have been described previously. 29,30 One week after introduction of endometriosis the mice were treated intraperitoneally with AdAngiostatin or Addl70-3, 1 × 109 pfu in a total volume of 1 ml of phosphate-buffered saline (PBS). Normal cycling mice, C57BL/6 and FVB, were treated intraperitoneally with Addl70-3 or AdAngiostatin, 1 × 109 or 2 × 109 pfu in 1 ml of PBS.
Gene Expression in Peritoneal Lavage
AdAngiostatin or control virus Addl70-3, 1 × 109 in 1 ml of PBS, was administered intraperitoneally into C57BL/6 mice. Three days later 2 ml of PBS was injected intraperitoneally and after 1 hour the peritoneal fluid was collected via a small incision in the abdominal wall. A 15-μl aliquot was run on a 15% sodium dodecyl sulfate-polyacrylamide gel under nonreducing conditions, and transferred to Immobilon-P membranes (Millipore, Mississauga, ON). The membranes were probed with a 1/500 dilution using a chicken anti-rabbit-plasminogen IgY antibody, followed by a secondary rabbit anti-chicken alkaline phosphatase-conjugated antibody (Zymed, San Francisco, CA). A positive control, partially purified rabbit angiostatin derived from human urokinase-digested rabbit plasminogen was used as a positive control as previously described. 26 Prestained molecular weight markers were purchased from Novagen (Madison, WI).
Hormone Assays
Serum estradiol concentrations were assayed by Abbot Axsym Systems (Abbot) and serum progesterone concentrations by Immulite Progesterone enzyme immunoassay (Diagnostic Products Corp., Los Angeles, CA).
In Vitro Studies of Endometrial Cells
Endometrium was dissected off the uterine muscle in the same manner as for induction of endometriosis. The endometrial tissue was incubated at 37°C for 60 minutes in a collagenase/dispase solution (25 mg of collagenase and 250 mg of dispase in 500 ml of PBS; Boehringer Mannheim, Mannheim, Germany). After incubation the cells were centrifuged at 1200 rpm for 10 minutes at 4°C in a Beckman GPR centrifuge. The pellet was resuspended in Dulbecco’s modified essential medium (Life Technologies, Inc., Grand Island, NY) with 10% fetal bovine serum and penicillin/streptomycin and plated in a 24-well culture dish. After overnight incubation to allow the cells to adhere, the cells were washed with PBS and fresh medium was added. To determine the effect of gene transfer with angiostatin, cells were treated either with vehicle alone, AdAngiostatin or Addl70-3 at a multiplicity of infection of 10 pfu/cell. Under these conditions, in our experience transgene expression is detected within 4 to 6 hours and presence of gene product is maximal by 24 hours and extends throughout 4 to 5 days in vitro. After 3 days of incubation the cells were harvested by trypsinization and counted using trypan blue exclusion staining.
Immunohistochemistry
Formalin-fixed, paraffin-embedded endometriosis tissue were cut in 3-μm sections, deparaffinized, and pretreated with protease (Sigma Chemical Co., St. Louis, MO) at room temperature for 15 minutes. Slides were stained using a primary rabbit anti-human von Willebrand’s factor antibody (DAKO, Carpinteria, CA) at a dilution of 1:1000. This was followed by incubation with a biotinylated goat anti-rabbit antibody from a Histostain bulk kit (Zymed, San Francisco, CA). Thereafter sections were exposed to prediluted streptavidin conjugated to horseradish peroxidase and 3-amino-9-ethylcarbazole was added as a chromogen. Sections were counterstained with Mayer’s hematoxylin. Controls were run in parallel omitting the primary antibody as a negative control procedure. For quantification of vessel area and vessel number within the endometrial tissue, sections from day 7 after implantation (day 0 of vector administration) and at day 12 (5 days after AdAngiostatin administration) were stained for Factor VIII and examined under light microscopy (×100) with a Leica DMR microscope (Leica Microsystems, Wetzlar, Germany) and the area occupied by vessels measured using a software for quantitative morphometry, Leica QWin Image Processing System (Leica Imaging Systems, Cambridge, England). Data are expressed as mean ± SD and include the number of vessels encountered per mm2 of endometrial tissue, the average area of a vessel in the tissue and percentage of the tissue occupied by vessels.
Determination of Apoptosis
Sections sequential to those stained for H&E and for Factor VIII were stained for the presence of DNA fragmentation in apoptotic cells using an ApopTag detection kit (Intergen Co., Purchase, NY) under conditions recommended by the manufacturer.
Statistical Analysis
Data are expressed as mean ± SEM. Analysis of variance with Fisher’s post hoc test or Fisher’s exact test were used as appropriate to test for statistical significance between groups. When analyzing the physiologically not normally distributed estradiol concentrations the nonparametric Kruskal-Wallis and Mann Whitney U-test were used. A P < 0.05 was considered as statistically significant.
Results
Induction of Endometriosis
Of a total of 19 mice, 17 had developed well-established endometriosis by 7 days when the endometrial fragments obtained from both uterine horns of a donor mouse were implanted into the peritoneal cavity of a recipient mouse. There was no apparent progression of the endometriosis 3 weeks after inoculation compared with the development seen 1 week after inoculation. Typically, nodules, 4 to 5 mm in diameter, had formed on the peritoneal layer of the anterior abdominal wall. In a few mice endometriosis was also present on the surface of the intestine or in the pelvic peritoneum. On gross examination lesions varied from a single pink nodule, to multiple nodules, to dense tanned lesions, ie, the same gross variation as seen in human endometriosis. 14 Microscopic examination showed typical appearance of endometriosis with endometrial glandular epithelium surrounded by an intact basement membrane and adjacent endometrial stroma. Often hemosiderin-laden macrophages were associated with these tissue deposits (Figure 1A) ▶ . At this time, the endometrial tissue was highly vascularized with numerous well-formed, large vessels being detected throughout the tissue by Factor VIII staining (Figure 1, B and C) ▶ .
Figure 1.
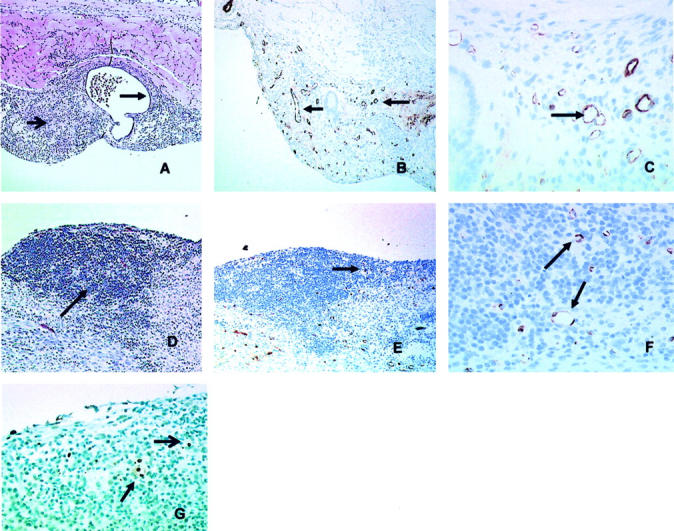
Morphology of experimental endometriosis. A: Formalin-fixed and paraffin-embedded endometriosis 7 days after inoculation of endometrium into the peritoneal cavity of mice demonstrates typical morphology of endometriosis with endometrial glandular epithelium surrounded by an intact basement membrane and adjacent endometrial stroma. Arrows indicate endometrial stroma (A and D) and endometrial glandular epithelium surrounding an endometrial cyst with secretion (A) (H&E staining; results shown are representative of n = 10). B: Immunohistochemical analysis with antibodies to Factor VIII demonstrates abundant microvessel staining in the 7-day (pretreatment) endometrial transplant. Arrows indicate intact vessel structures (C and F). C: Factor VIII staining at day 7 examined at higher power (×100). D: Five days after treatment with AdAngiostatin 1 × 109 pfu intraperitoneally there were no visible glands left and the stroma was less abundant (H&E staining). E: Microvessel staining with Factor VIII 5 days after AdAngiostatin treatment shows residual endometrial stroma with significantly reduced staining for microvessels (arrows). F: Factor VIII staining at day 5 of AdAngiostatin treatment at higher power (×100). G: Staining for apoptosis (Apoptag) at day 5 after AdAngiostatin treatment (×100) shows endothelial cells (closed arrowhead) and endometrial stromal cell (open arrowhead) undergoing apoptosis.
Eradication of Established Endometriosis by AdAngiostatin Treatment
One week after induction of endometriosis AdAngiostatin or the control vector Addl70-3 was injected intraperitoneally. Mice were sacraficed over a subsequent period of 2 to 18 days. Intraperitoneal administration of AdAngiostatin, 1 × 109 pfu, induced eradication of endometriosis in 14 of 14 mice whereas 11 of 13 animals showed full disease development after treatment with the control vector Addl70-3 (P < 0.0001, Table 1 ▶ ). Therapy progression after AdAngiostatin injection was followed at days 2, 5, and 14. Two days after the injection the cuboidal endometrial glands were somewhat flattened but the stroma was not visibly affected. After 5 days, one of three mice had only a few glands that still were visible within the residual tissue but reduced by ∼80%, in the other two animals only some stroma that was less abundant remained (Figure 1D) ▶ . After 14 days only scar tissue in the form of fibroblasts was present and no endometrial tissue remained.
Table 1.
Effect of Adenoviral Gene Delivery of Angiostatin on Endometriosis Induced in C57BL/6 Mice
| Treatment | Eradication of endometriosis | Response rate (%) |
|---|---|---|
| AdAngiostatin | 14/14 | 100 |
| Control (Addl70-3) | 2/13 | 15 |
Endometrium from syngenic mice were injected into the peritoneal cavity of OVX estrogen-supplemented recipient mice. One week after the injection endometriosis had developed and mice were treated intravenously with AdAngiostatin or control virus at 1 × 109 pfu. Sixteen to 18 days thereafter mice were killed and the presence of endometriosis was evaluated using a dissecting microscope. Where endometriosis was present the diagnosis was confirmed by histology. Fisher’s exact test was used for statistical analysis, P < 0.0001.
Reduction of Vasculature and Apoptosis of Endothelial Cells Induced by AdAngiostatin Treatment
Immunohistochemistry for Factor VIII demonstrated that at day 5 of AdAngiostatin treatment (12 days after implantation) not only had the bulk of the endometrial tissue decreased (Figure 1, D versus A) ▶ , but that the content of the vasculature had markedly decreased both in extent and in numbers of vessels (Figure 1, E versus B) ▶ . Examination at higher magnification (Figure 1, F versus C) ▶ showed that the number of vessels had decreased as had the average size of the vessels and the area occupied by the vasculature in the endometrial tissue. Quantification of these parameters by morphometry confirmed that the decreases seen in each measurement at this time (day 12 or 5 days after AdAngiostatin treatment) were significant, with the number of vessels decreasing from 133 ± 22 per mm2 at day 7 to 68 ± 17 per mm2 at day 12 (P < 0.015) and the average area per vessel as well as the total area occupied by vessels decreased approximately fourfold (Table 2) ▶ . Moreover, when the tissues were stained for the presence of cells exhibiting DNA damage indicative of apoptosis, at day 5 of AdAngiostatin treatment endothelial cells are seen to be undergoing apoptosis along with a few isolated endometrial stromal cells. The majority of stromal cells remaining at this time do not show evidence of apoptosis (Figure 1G) ▶ .
Table 2.
Quantification of Extent of Vasculature in Endometrial Tissue Implant in the Peritoneum before and after Adenoviral Gene Delivery of Angiostatin in C57BL/6 Mice
| Number of vessels per mm2 | Area/vessel μm2 | % Area vasculature | |
|---|---|---|---|
| Day 0 (seven days after implantation) | 133 ± 22 | 407 ± 154 | 5.5 ± 2.5 |
| Day 5 after vector | 68 ± 17 | 107 ± 49 | 0.7 ± 0.2 |
| P value (n = 3) | 0.015 | 0.032 | 0.027 |
In Vivo Expression of AdAngiostatin
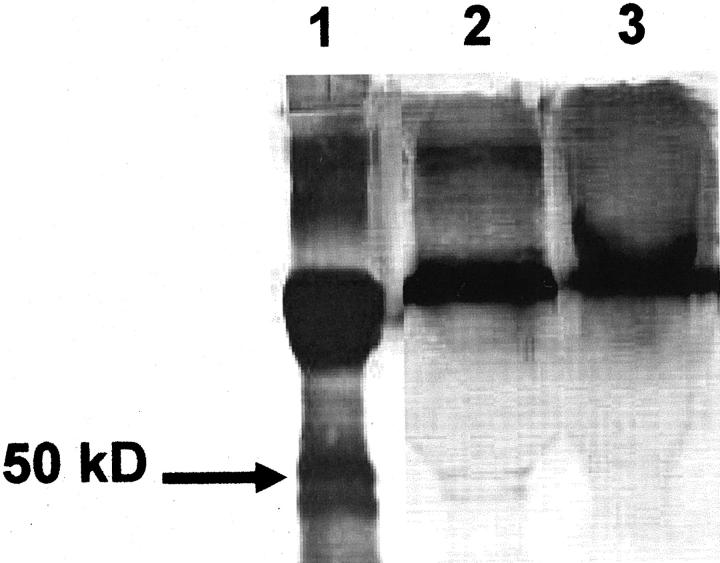
Examination by Western blot of peritoneal lavage taken 3 days after AdAngiostatin treatment demonstrated two distinct bands, between 40 and 50 kd, corresponding to murine angiostatin in the AdAngiostatin-treated mice, but not in the fluid recovered from control vector-treated mice (Figure 2) ▶ . These bands are consistent with our previous data showing bioactive angiostatin protein expression in murine lungs and in vitro cell culture of human umbilical endothelium and A549 cells. 26
Figure 2.
Expression of angiostatin in recovered peritoneal lavage of AdAngiostatin-treated mice. Western blot was used to detect murine angiostatin protein. Angiostatin was detected only in the AdAngiostatin-treated mice and could be seen as two bands at ∼40 to 50 kd, representing the two glycoforms of murine angiostatin (lane 2). No angiostatin bands could be detected in peritoneal lavage of Addl70-3 control-treated mice (lane 3). Positive control (lane 1) is rabbit angiostatin derived from human urokinase-digested rabbit plasminogen showing two angiostatin bands. A 50-kd marker position is shown (arrow).
Endometrial Cell Growth in Vitro Unaffected by AdAngiostatin Treatment
To exclude that AdAngiostatin had any direct effects on endometrial cell growth a single-cell suspension of the endometrium from the mice was prepared and plated at a density of 0.75 × 105 cells per well. The cells were either left uninfected or infected with control vector, Addl70-3 or the AdAngiostatin vector at an multiplicity of infection of 10 pfu/cell. Although we are unable to measure angiostatin production under these conditions, because of the lack of a suitable immunoassay, our experience with many other cytokine and growth factor vectors and similar cultures with Advector expressing LacZ, indicates that the gene product is maximally expressed in vitro by 24 hours and continues to be produced at that level throughout 3 to 4 days. Three days after infection there was no difference between the groups in the number of viable cells (controls, 0.9 ± 0.05; Addl70-3, 0.91 ± 0.01; AdAngiostatin, 0.92 ± 0.01 × 105 cells/well; n = 3).
AdAngiostatin Administration to Normal Cycling Mice Induced a 30% Increase in Body Weight
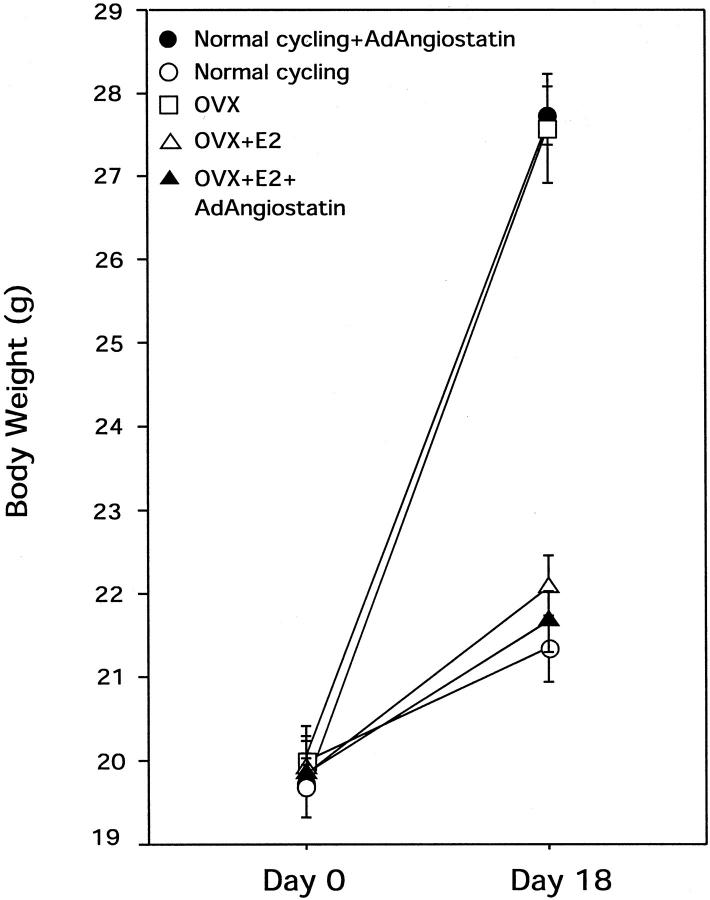
In our previous studies on the use of AdAngiostatin gene therapy for cancer, 26,30 we had noted a weight gain and abdominal fat deposition in female FVB mice (data not shown). A similar weight gain had been noted after OVX of FVB mice. To further investigate these findings, normal cycling mice were treated with AdAngiostatin. To rule out a strain-specific effect both FVB and C57BL/6 mice were treated. After an intraperitoneal injection of AdAngiostatin, 1 × 109 pfu or 2 × 109 pfu, to normal mice their body weight increased 30% compared with control mice throughout a period of 16 to 18 days (P < 0.0001, Figure 3 ▶ ). The weight gain was similar in both FVB and C57BL/6 mice. The increased body weight consisted mainly of fat tissue deposited in the abdominal cavity and to a lesser extent, subcutaneously. OVX animals showed a similar increase in body weight and deposition of body fat as the AdAngiostatin-treated mice. When physiological levels of estradiol were maintained in the OVX mice by a slow release pellet, the weight gain by AdAngiostatin failed to occur.
Figure 3.
Body weight in normal cycling mice and OVX mice after AdAngiostatin treatment. AdAngiostatin, 1 × 109 pfu/mouse was given intraperitoneally. After 16 to 18 days normal cycling untreated mice or normal cycling mice treated with AdAngiostatin, OVX, OVX + estradiol supplemented (E2), and OVX + E2 + AdAngiostatin mice were weighed. The OVX- and AdAngiostatin-treated normal cycling mice increased their body weight significantly compared to all other groups (P < 0.0001, n = 5 to 6).
AdAngiostatin Administration Suppressed Ovarian Function
Morphology
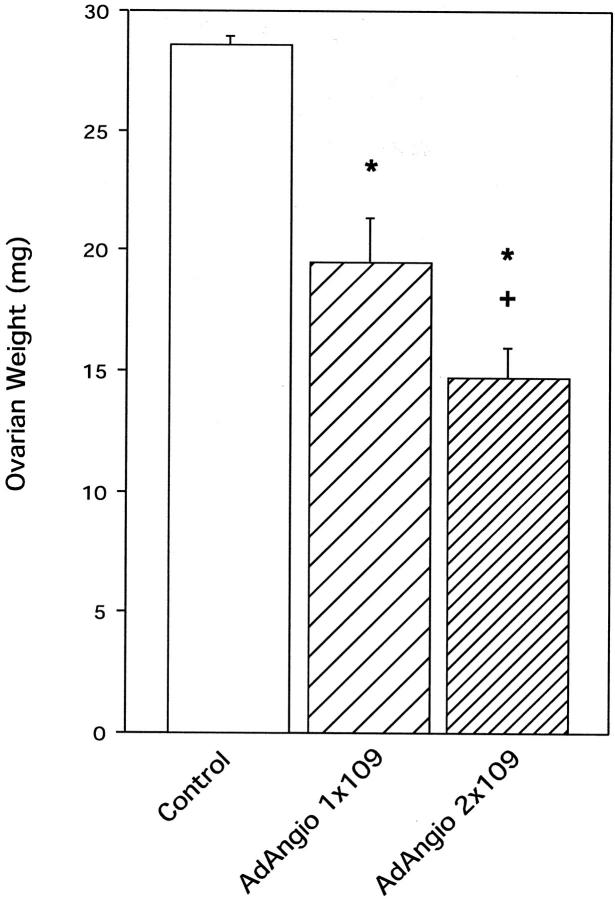
To further investigate the similarities in weight gain in AdAngiostatin-treated and OVX mice, ovarian function was measured. Three weeks after administration of AdAngiostatin to normal cycling FVB mice, both the size and weight of the ovaries had decreased up to 50% (P < 0.0001, Figure 4 ▶ ). When the dose of AdAngiostatin was increased from 1 × 109 pfu to 2 × 109 pfu resulting in greater angiostatin expression throughout the same 6- to 10-day transient period, ovarian weight further decreased significantly (P < 0.05, Figure 4 ▶ ). Histological evaluation and quantitative morphometry of ovaries in the AdAngiostatin-treated mice showed an overall decrease in secondary follicles, decreased corpus luteum development, and a loss of stroma (Figure 5 ▶ and Table 3 ▶ ). The decrease in ovarian weight was more pronounced in C57BL/6 mice where 1 × 109 pfu of AdAngiostatin caused a decrease from 28.2 ± 2.6 mg to 10 ± 0.6 mg compared to 28.6 ± 0.34 to 19.5 ± 1.8 in FVB mice.
Figure 4.
Ovarian weight after AdAngiostatin treatment. Eighteen days after intraperitoneal injections of AdAngiostatin, 1 × 109 or 2 × 109 pfu, ovaries from FVB mice were removed and weighed. The weight decreased significantly in a dose-response pattern. *, P < 0.001 versus control; +, P < 0.05 versus AdAngiostatin 1 × 109 pfu (n = 5).
Figure 5.
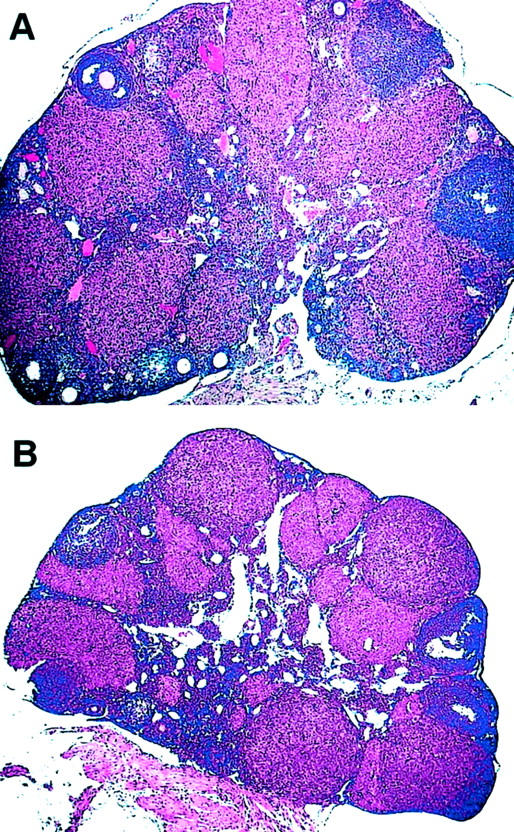
Morphology of ovaries after AdAngiostatin treatment. Eighteen days after intraperitoneal injections of AdAngiostatin, 1 × 109 or 2 × 109 pfu, ovaries from mice were removed and fixed in formalin and stained with H&E. A: Normal ovary with primary follicles and corpora lutea. B: The treated ovaries showed an overall decrease in corpus luteum development and a loss of stroma (results are representative of n = 5).
Table 3.
Ovarian Morphometry after Adenoviral Gene Delivery of Angiostatin in C57BL/6 Mice
| Control (Addl70-3) | AdAngiostatin | P value | |
|---|---|---|---|
| Primary follicles | 6.5 (5.5–7.5) | 3.5 (2.5–4) | <0.001 |
| Corpus luteum | 5 (3.5–5) | 2 (1.3–4) | <0.05 |
| Corpus luteum area (mm2) | 0.16 (0.13–0.2) | 0.07 (0.06–0.08) | <0.05 |
AdAngiostatin or control virus at 1 × 109 pfu were administered intravenously to normal cycling mice. Sixteen to 18 days thereafter mice were killed and the ovaries were fixed in formalin and H&E staining was performed. Numbers of primary follicles and corpora lutea were counted and the areas of the corpora lutea were measured. Results are medians and 25 to 75 percentiles.
Hormone Production
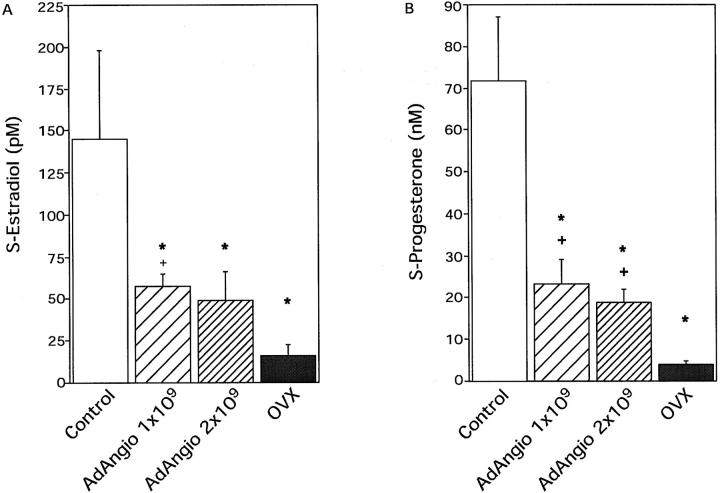
Serum levels of both estradiol and progesterone were decreased in AdAngiostatin-treated normal cycling mice. Control mice showed a physiological normal wide range of estradiol levels (46 to 401 pmol/L). Estradiol levels in AdAngiostatin-treated mice decreased by ∼60% from normal levels but did not fall to the levels seen in OVX animals (P < 0.05, Figure 6A ▶ ). The serum levels of progesterone decreased by ∼70% from normal levels but did not fall to the levels seen in OVX animals after AdAngiostatin treatment (P < 0.01, Figure 6B ▶ ).
Figure 6.
Serum hormone levels in normal cycling mice and OVX mice after AdAngiostatin treatment. A: Serum estradiol levels after AdAngiostatin treatment. Eighteen days after intraperitoneal injections of AdAngiostatin, 1 × 109 or 2 × 109 pfu, or after OVX, serum estradiol levels in mice were measured. In the control animals the serum levels showed a normal wide physiological range. *, P < 0.05 versus controls; +, P < 0.05 versus OVX (n = 4 to 6). B: Serum progesterone levels after AdAngiostatin treatment. Eighteen days after intraperitoneal injections of AdAngiostatin, 1 × 109 or 2 × 109 pfu, or after OVX, serum progesterone levels in mice were measured. In the control animals the serum levels showed a normal wide physiological range. *, P < 0.01 versus controls; +, P < 0.05 versus OVX (n = 4 to 6).
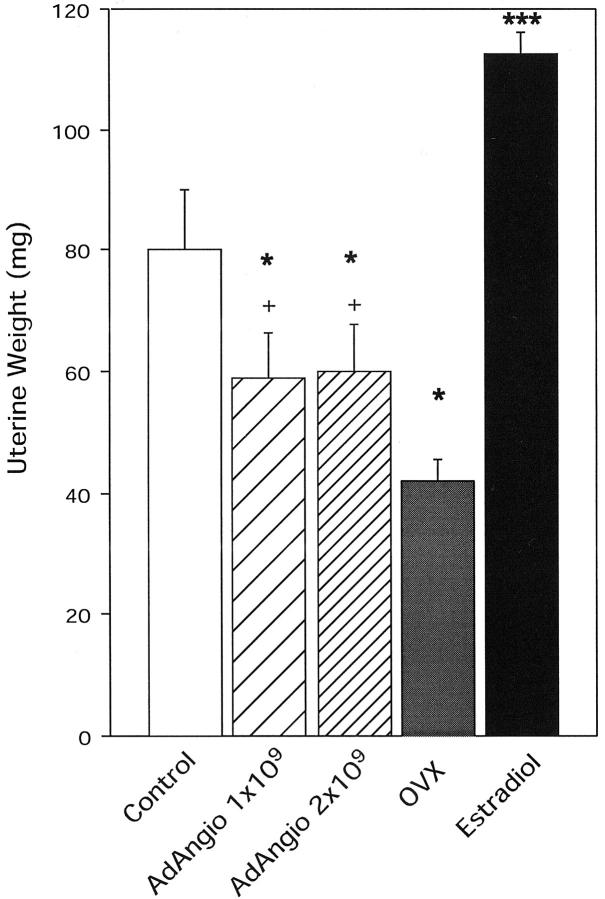
Uterine Weight
Uterine weight is another accurate assessment of systemic estrogen exposure. 31 In normal cycling mice uterine weight decreased significantly after AdAngiostatin treatment (P < 0.0001, Figure 7 ▶ ). The decrease, however, did not seem to be dose-dependent. The endometrium in the treated mice showed some loss of thickness and the glands exhibited decreased proliferative and secretory activity (data not shown). As seen with ovarian weight, uterine weight decrease was more pronounced in C57BL/6 mice, 86 ± 11 mg to 42 ± 3 mg compared to 80 ± 10 mg to 59 ± 7 mg in FVB mice after administration of 1 × 109 pfu of AdAngiostatin.
Figure 7.
Uterine weight after AdAngiostatin treatment. Eighteen days after intraperitoneal injection of AdAngiostatin, 1 × 109 or 2 × 109 pfu, or OVX or estradiol supplement, uteri from FVB mice were removed and weighed. *, P < 0.05 versus controls; +, P < 0.05 versus OVX; ***, P < 0.001 versus all other groups (n = 5 to 10).
Discussion
Our data demonstrate, we believe for the first time, that angiogenesis inhibition through gene transfer of angiostatin is a powerful treatment for endometriosis. Transient overexpression of angiostatin, delivered to the peritoneum by a replication-deficient adenovirus vector (AdAngiostatin), successfully eradicated established endometriosis. Angiostatin is an endogenous peptide fragment of plasminogen and has been shown to have potent anti-angiogenic properties. 22 Within the peritoneal cavity, AdAngiostatin infects a wide series of cells, including the mesothelium and the endometrial stromal cells, producing bioactive angiostatin throughout the period of the experiment (6 to 10 days). In our study the AdAngiostatin vector had no apparent direct effect on endometrial cell growth in vitro contrary to our earlier studies showing a direct effect on endothelial cells. 26 Moreover, when the tissue was examined for evidence of apoptosis after AdAngiostatin treatment, vascular endothelial cells were the main cells seen to be undergoing apoptosis with only a few endometrial cells showing such evidence. If the AdAngiostatin were to have a direct effect on endometrial cells causing regression, we would expect greater evidence of apoptosis in the endometrial cell tissue at this time. Thus, the lack of apparent effect in vitro on endometrial cells and evidence of induction of endothelial cell apoptosis, strongly suggests that the mechanism involved in regression of endometrial tissue growth induced by angiostatin treatment was the effect on angiogenesis inhibition as previously described in other systems.
Equally important, the quantification of vessel content (Table 2) ▶ indicates that angiostatin treatment caused a decrease in the number of vessels, the average size of individual vessels and the density of vessels in the endometrial tissue. In particular, the fourfold decrease in vessel size and area occupied by vessels relative to endometrial stroma shows that the vasculature is affected and reduced before the endometrial tissue is regressed by the treatment. We would expect that other inhibitors of angiogenesis would have a similar effect on established endometrial tissue causing regression and resorption, secondary to reduction of vasculature. Although it may be argued that introduction of a virus with a gene product may cause inflammation resulting in inhibition of angiogenesis, our previous experience with a large number of cytokines and growth factors introduced with adenovirus vectors in various animal models have not shown effects on angiogenesis. Only those vectors expressing factors known to modulate endothelial cell behavior, such as the chemokines IP-10 and Mig, along with angiostatin, alter angiogenesis in vivo. 26,32 Data from our group as well as others have shown that angiostatin interferes with vascular growth by inhibiting endothelial cell proliferation and induces apoptosis in endothelial cells. 22,26,30 This effect on angiogenesis is likely to occur in all tissues and could affect all new vessel growth including physiological normal angiogenesis in the reproductive system. Our results also confirm and extend the data from other studies that, among other factors, angiogenesis plays a major role in the establishment and progression of endometriosis. 14-19
Endometriosis is an estrogen-dependent condition that becomes quiescent after menopause and may recur with estrogen replacement. Current available therapies are aimed at chemically inducing menopause, which is associated with severe adverse effects. Therefore, a drug that is effective while maintaining normal circulating estrogen levels would be an important advancement in the treatment of patients with endometriosis. The murine endometriosis model used in our experiments enables studies in a host with normal estrogen levels, because the OVX mice were substituted with estrogen to a physiological level. Our data further show that angiogenesis inhibition can be effective even with maintained physiological estrogen levels. This suggests that in a clinical setting, anti-angiogenic gene therapy may be effective with a preserved endogenous ovarian estrogen production or in combination with estrogen supplementation.
AdAngiostatin given to non-OVX normal cycling mice had an effect on endogenous estrogen production. This was demonstrated both by decreased serum hormone levels and by decreased uterine weight, which is a good assessment of systemic estrogen exposure. 31 Both serum estradiol levels and uterine weight were significantly lowered by sustained systemic angiostatin expression (Figures 6 and 7) ▶ ▶ but did not reach the decreased levels seen in OVX animals. Taken together these data indicate that estrogen production was clearly reduced but not totally abolished by this prolonged systemic AdAngiostatin treatment. These impacts on ovarian function would also apply to gene therapy for cancer using similar systemic angiostatic treatments.
Normal cycling non-OVX mice treated with AdAngiostatin showed a substantial increase in body weight and this increase in body weight and fat deposition was similar to that seen in castrated (OVX) animals without treatment. OVX in rodents induces obesity and this model has been used to mimic postmenopausal obesity in women. 33 The increased bodyweight in OVX animals can be reversed by estrogen replacement and has been explained by the close relationship between estrogen and leptin expression. 33 Our data suggest that a reduction in circulating estrogen levels, not total castration, is sufficient to induce this weight gain.
The effect of AdAngiostatin on ovarian function in normal cycling mice was repeated in two different strains. The differences in reduction of ovarian and uterine weights between the strains could be the result of genetic heterogeneity of both angiogenesis and/or responsiveness to anti-angiogenic treatment in mice, as previously shown. 34
After ovulation, the estrogen-producing primary follicles are transformed to corpora lutea, which start progesterone secretion, both of which are events highly dependent on angiogenesis. 21,35 We show in this study that systemic angiostatin gene overexpression for a period of 6 to 10 days results in decreased number of primary follicles, decreased number and size of corpora lutea, decreased overall weight of the ovaries, decreased production of estradiol and progesterone, decreased uterine weight, and decreased endometrial thickness and proliferative activity. Taken together these data strongly suggest that AdAngiostatin also affects normal physiological angiogenesis in the ovary and uterus, although further studies are needed to elucidate the precise underlying mechanism(s).
When preserved fertility is not a concern, inhibition of ovulation may have beneficial effects. The numbers of lifetime ovulations and endometriosis are separate risk factors for ovarian cancer, which is a commonly fatal disease. 36 Decreased numbers of ovulations by pregnancy, oral contraceptive use, or prolonged breast-feeding reduce the risk of developing ovarian cancer. 37 It could therefore be speculated that by using anti-angiogenic therapies for endometriosis the risk of ovarian cancer might be reduced by eradication of endometriosis as well as by reducing the numbers of ovulations.
Endometriosis does not occur in non-primates and research has previously been limited to transplantation of human endometrium into nude mice. However, by using normal immunocompetent mice, endometriosis that has both macroscopic and histological features that resembles human endometriosis can be induced. Because various cells in the immune system secrete many regulatory factors of angiogenesis, an immunocompetent host is essential in models for investigation of pathogenesis and therapy of endometriosis. 16,38 The position of the lesions in the anterior abdominal wall in this rodent model reflects the influence of gravity, similar to human endometriosis, which mainly occurs in the pelvic peritoneum. 27
By administrating angiostatin through Adenovector gene therapy a transient prolonged overexpression of a correctly glycosylated protein is achieved. This protein should have a longer half-life compared to the recombinant protein, which may be fragile and lose biological activity. 39 Moreover, it has been shown that the angiostatin protein has a very short half-life in plasma 40 which would require repeated administration to achieve similar prolonged raised systemic levels. Gene transfer with replication-deficient adenovirus vectors induces production of bioactive angiostatin in vivo up to 10 days. 26,30 This transient expression was enough to eradicate endometriosis in this current model. In patients, endometriosis is diagnosed by laparoscopy or laparotomy, thereby endometriotic lesions become available for direct injection of anti-angiogenic compounds. In those circumstances in which endometriosis appears on vital organs and surgery is not an option, this treatment could be especially beneficial, particular as it can be delivered and maintained locally and may avoid possible systemic effects of raised angiostatin levels. Because laparoscopy, for obvious reasons, is not done repeatedly, a therapy expressed throughout a period of time, as seen using the AdAngiostatin vector, would be preferred.
Our data, and that from earlier studies, 26,30 indicate that angiostatin expressed from an adenovirus vector administered to the peritoneal cavity has no direct effect on endometrial cell viability, inhibits endothelial cell proliferation and induces endothelial cell apoptosis, and blocks angiogenesis. Through these effects on vascular structures angiogenesis is inhibited in established murine endometriosis, inducing regression and resorption of the endometrial tissue.
We conclude that whereas inhibition of angiogenesis represents a functional drug target in endometriosis, care must be taken to minimize the deleterious side effects on normal reproductive function associated with this therapy. Controlled local or targeted delivery of angiostatic factors could prove very effective in therapy of endometriosis with therapeutic effects even in a host with preserved estrogen levels.
Acknowledgments
We thank Duncan Chong, Xueya Feng, and Mary Jo Smith for expert technical assistance.
Footnotes
Address reprint requests to Jack Gauldie, Ph.D., F.R.S.C., Department of Pathology and Molecular Medicine, McMaster University, 1200 Main St. West, Hamilton, Ontario, Canada L8N 3Z5. E-mail: gauldie@mcmaster.ca.
Supported by Canadian Institutes for Health Research (CIHR), Hamilton Health Science, St. Joseph’s Healthcare, and the Swedish Institute.
C. D. was a post-doctoral fellow from the Division of Obstetrics and Gynecology, Linköping University, Sweden.
References
- 1.Garry R, Clayton R, Hawe J: The effect of endometriosis and its radical laparoscopic excision on quality of life indicators. Br J Obstet Gynaecol 2000, 107:44-54 [DOI] [PubMed] [Google Scholar]
- 2.Strathy JH, Molgaard CA, Coulam CB, Melton LJ: Endometriosis and infertility: a laparoscopic study of endometriosis among fertile and infertile women. Fertil Steril 1982, 38:667-672 [DOI] [PubMed] [Google Scholar]
- 3.Laufer MR, Goitein L, Bush M, Cramer DW, Emans SJ: Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J Pediatr Adolesc Gynecol 1997, 10:199-202 [DOI] [PubMed] [Google Scholar]
- 4.Yanushpolsky EH, Best CL, Jackson KV, Clarke RN, Barbieri RL, Hornstein MD: Effects of endometriomas on oocyte quality, embryo quality, and pregnancy rates in in vitro fertilization cycles: a prospective, case-controlled study. J Assist Reprod Genet 1998, 15:193-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A: Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol 1997, 176:572-579 [DOI] [PubMed] [Google Scholar]
- 6.Hughes E, Fedorkow D, Collins J, Vandekerckhove P: Ovulation suppression for endometriosis (Cochrane review). The Cochrane Library, Issue 2 2002. Update Software, Oxford
- 7.Bergqvist A: Current drug therapy recommendations for the treatment of endometriosis. Drugs 1999, 58:39-50 [DOI] [PubMed] [Google Scholar]
- 8.Sampson JA: Peritoneal endometriosis due to menstrual dissemination of endometrial tissues into the peritoneal cavity. Am J Obstet Gynecol 1927, 14:422-469 [Google Scholar]
- 9.Lebovic DI, Mueller MD, Taylor RN: Immunobiology of endometriosis. Fertil Steril 2001, 75:1-10 [DOI] [PubMed] [Google Scholar]
- 10.Surrey ES, Halme J: Effect of peritoneal fluid from endometriosis patients on endometrial stromal cell proliferation in vitro. Obstet Gynecol 1990, 76:792-797 [DOI] [PubMed] [Google Scholar]
- 11.Prifti S, Sillem M, Arslic T, Monga B, Rehberger S, Runnebaum B: In vitro expression of soluble and cell surface-associated CD44 on endometrial cells from women with and without endometriosis. Eur J Clin Invest 1998, 28:1055-1060 [DOI] [PubMed] [Google Scholar]
- 12.Chung H, Wen Y, Chun S, Nezhat C, Woo B, Lake Polan M: Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: a rationale for endometriotic invasiveness. Fertil Steril 2001, 75:152-159 [DOI] [PubMed] [Google Scholar]
- 13.Sillem M, Prifti S, Neher M, Runnebaum B: Extracellular matrix remodelling in the endometrium and its possible relevance to the pathogenesis of endometriosis. Hum Reprod Update 1998, 4:730-735 [DOI] [PubMed] [Google Scholar]
- 14.Nisolle M, Casanas-Roux F, Anaf V, Mine JM, Donnez J: Morphometric study of the stromal vascularization in peritoneal endometriosis. Fertil Steril 1993, 59:681-684 [PubMed] [Google Scholar]
- 15.Fujimoto J, Sakaguchi H, Hirose R, Wen H, Tamaya T: Angiogenesis in endometriosis and angiogenic factors. Gynecol Obstet Invest 1999, 48(Suppl 1):14-20 [DOI] [PubMed] [Google Scholar]
- 16.McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Muller KH, Sharkey AM, Smith SK: Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest 1996, 98:482-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN: Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab 1996, 81:3112-3118 [DOI] [PubMed] [Google Scholar]
- 18.Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M: Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod 1998, 13:1686-1690 [DOI] [PubMed] [Google Scholar]
- 19.McLaren J: Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update 2000, 6:45-55 [DOI] [PubMed] [Google Scholar]
- 20.Hyder SM, Stancel GM: Regulation of angiogenic growth factors in the female reproductive tract by estrogens and progestins. Mol Endocrinol 1999, 13:806-811 [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN, Peers D, Chisholm V, Hillan KJ, Schwall RH: Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med 1998, 4:336-340 [DOI] [PubMed] [Google Scholar]
- 22.O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J: Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994, 79:315-328 [DOI] [PubMed] [Google Scholar]
- 23.Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, Pope RM, Ferrara N: Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol 1994, 152:4149-4156 [PubMed] [Google Scholar]
- 24.Giavazzi R, Taraboletti G: Angiogenesis and angiogenesis inhibitors in cancer. Forum (Genova) 1999, 9:261-272 [PubMed] [Google Scholar]
- 25.de Bandt M, Grossin M, Weber AJ, Chopin M, Elbim C, Pla M, Gougerot-Pocidalo MA, Gaudry M: Suppression of arthritis and protection from bone destruction by treatment with TNP-470/AGM-1470 in a transgenic mouse model of rheumatoid arthritis. Arthritis Rheum 2000, 43:2056-2063 [DOI] [PubMed] [Google Scholar]
- 26.Gyorffy S, Palmer K, Gauldie J: Adenoviral vector expressing murine angiostatin inhibits a model of breast cancer metastatic growth in the lungs of mice. Am J Pathol 2001, 159:1137-1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somigliana E, Vigano P, Rossi G, Carinelli S, Vignali M, Panina-Bordignon P: Endometrial ability to implant in ectopic sites can be prevented by interleukin-12 in a murine model of endometriosis. Hum Reprod 1999, 14:2944-2950 [DOI] [PubMed] [Google Scholar]
- 28.Bronson FH, Desjardins C: Circulating concentrations of FSH, LH, estradiol, and progesterone associated with acute, male-induced puberty in female mice. Endocrinology 1974, 94:1658-1668 [DOI] [PubMed] [Google Scholar]
- 29.Bett AJ, Haddara W, Prevec L, Graham FL: An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA 1994, 91:8802-8806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GyorffyS, Palmer K, Podor TJ, Hitt M, Gauldie J: Combined treatment of a murine breast cancer model with type 5 adenovirus vectors expressing murine angiostatin and IL-12: a role for combined anti-angiogenesis and immunotherapy. J Immunol 2001, 166:6212-6217 [DOI] [PubMed] [Google Scholar]
- 31.Mobbs CV, Cheyney D, Sinha YN, Finch CE: Age-correlated and ovary-dependent changes in relationships between plasma estradiol and luteinizing hormone, prolactin, and growth hormone in female C57BL/6J mice. Endocrinology 1985, 116:813-820 [DOI] [PubMed] [Google Scholar]
- 32.Palmer K, Hitt M, Emtage P, Gyorffy S, Gauldie J: Combined CXC chemokine and interleukin-12 gene transfer enhances antitumor immunity. Gene Therapy 2001, 8:282-290 [DOI] [PubMed] [Google Scholar]
- 33.Chu SC, Chou YC, Liu JY, Chen CH, Shyu JC, Chou FP: Fluctuation of serum leptin level in rats after ovariectomy and the influence of estrogen supplement. Life Sci 1999, 64:2299-2306 [DOI] [PubMed] [Google Scholar]
- 34.Rohan RM, Fernandez A, Udagawa T, Yuan J, D’Amato RJ: Genetic heterogeneity of angiogenesis in mice. EMBO J 2000, 14:871-876 [DOI] [PubMed] [Google Scholar]
- 35.Klauber N, Rohan RM, Flynn E, D’Amato RJ: Critical components of the female reproductive pathway are suppressed by the angiogenesis inhibitor AGM-1470. Nat Med 1997, 3:443-446 [DOI] [PubMed] [Google Scholar]
- 36.Ness RB, Cottreau C: Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst 1999, 91:1459-1467 [DOI] [PubMed] [Google Scholar]
- 37.Gwinn ML, Lee NC, Rhodes PH, Layde PM, Rubin GL: Pregnancy, breast feeding, and oral contraceptives and the risk of epithelial ovarian cancer. J Clin Epidemiol 1990, 43:559-568 [DOI] [PubMed] [Google Scholar]
- 38.Coughlin CM, Salhany KE, Wysocka M, Aruga E, Kurzawa H, Chang AE, Hunter CA, Fox JC, Trinchieri G, Lee WM: Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest 1998, 101:1441-1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sim BK, O’Reilly MS, Liang H, Fortier AH, He W, Madsen JW, Lapcevich R, Nacy CA: A recombinant human angiostatin protein inhibits experimental primary and metastatic cancer. Cancer Res 1997, 57:1329-1334 [PubMed] [Google Scholar]
- 40.Hatton MW, Day S, Southward SM, Dereske M, Ross B, Seidlitz E, Singh G, Richardson M: Metabolism of rabbit angiostatin glycoforms I and II in rabbits: angiostatin-I leaves the intravascular space faster and appears to have greater anti-angiogenic activity than angiostatin-II. J Lab Clin Med 2001, 138:83-93 [DOI] [PubMed] [Google Scholar]