Abstract
Inflammatory bowel disease of the colon is associated with a high osmolarity of colonic contents. We hypothesized that this hyperosmolarity may contribute to colonic inflammation by stimulating the proinflammatory activity of intestinal epithelial cells (IECs). The human IEC lines HT-29 and Caco-2 were used to study the effect of hyperosmolarity on the IEC inflammatory response. Exposure of IECs to hyperosmolarity triggered expression of the proinflammatory chemokine interleukin (IL)-8 both at the secreted protein and mRNA levels. In addition, hyperosmotic stimulation induced the release of another chemokine, GRO-α. These effects were because of activation of the transcription factor, nuclear factor (NF)-κB, because hyperosmolarity stimulated both NF-κB DNA binding and NF-κB-dependent transcriptional activity. Hyperosmolarity activated both p38 and p42/44 mitogen-activated protein kinases, which effect contributed to hyperosmolarity-stimulated IL-8 production, because p38 and p42/44 inhibition prevented the hyperosmolarity-induced increase in IL-8 production. In addition, the proinflammatory effects of hyperosmolarity were, in a large part, mediated by activation of Na+/H+ exchangers, because selective blockade of Na+/H+ exchangers prevented the hyperosmolarity-induced IEC inflammatory response. In summary, hyperosmolarity stimulates IEC IL-8 production, which effect may contribute to the maintenance of inflammation in inflammatory bowel disease.
Recent evidence indicates that in addition to its important role in the digestion and absorption of nutrients, the intestinal mucosa functions as an immunologically vital organ. In recent years, a large body of evidence has accumulated documenting that intestinal epithelial cells (IECs) play a central role in orchestrating gut immune responses to both exogenous and endogenous antigens. 1-6 An important feature of IEC immune processes is that in response to an antigenic challenge or signals received from neighboring immune cells, IECs produce a wide range of inflammatory cytokines. 7-12 In addition to the classical inflammatory signals represented by bacteria, bacterial products, or immune cell-derived cytokines, nonimmune factors have also been shown to elicit up-regulation of the IEC cytokine response. For example, there is now good evidence showing that hypoxia, hypoxia/reoxygenation, and acidosis can induce the formation of cytokines by IECs, 13-15 the process of which may have a pathophysiological role in inflammatory bowel disease or multiple organ failure. 9,16,17 In addition, mediators of the nervous system, such as acetylcholine 18 and neurotensin 19 as well as adenosine 20 have been reported to activate inflammatory pathways in IECs. On the other hand, relatively less attention has been focused on the role of another nonimmune stressful factor, hyperosmolarity. The idea that hyperosmolarity may regulate IEC production of inflammatory cytokines is based on evidence that hyperosmolarity has been shown to be associated with a variety of intestinal inflammatory conditions that are characterized by overproduction of cytokines of epithelial origin. These include Crohn’s disease and ulcerative colitis, 21,22 as well as the inflammatory bowel disease of the newborn, neonatal necrotizing enterocolitis. 23 Considering that in addition to renal epithelial cells, 24 IECs are exposed to the most extreme osmolar environments, it can be hypothesized that episodes of extreme hyperosmolarity may contribute to the exacerbation of intestinal inflammatory disease via up-regulation of the epithelial cytokine response.
In the present study, we examined the effect of hyperosmolarity on the intestinal epithelial inflammatory cytokine response. Because the chemokine interleukin (IL)-8 is one of the best investigated inflammatory cytokines released by IECs, we studied whether hyperosmolarity induced the expression of IL-8 by the human IEC lines HT-29 and Caco-2. Our results demonstrate that hyperosmotic stress activates the epithelial cell inflammatory cascade resulting in the production of IL-8. In addition, we provide evidence that the IL-8 response to hyperosmotic stress is mediated by activation of the transcription factor nuclear factor (NF)-κB as well as p38 and p42/44 MAP kinases. Finally, we show that an important proximal intracellular mediator of the IEC inflammatory response to hyperosmolarity is the Na+/H+ exchanger (NHE).
Materials and Methods
Cell Lines
The human colon cancer cell lines HT-29 and Caco-2 were obtained from American Type Culture Collection (Manassas, VA). HT-29 cells were grown in modified McCoy’s 5A Medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Caco-2 cells were grown in Dulbecco’s modified Eagle’s medium with high glucose containing 10% fetal bovine serum as well as 0.1 mmol/L of nonessential amino acids and 1 mmol/L of sodium pyruvate.
Drugs and Reagents
Amiloride HCl, 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), 5-(N-methyl-N-isobutyl)amiloride (MIA), urea, mannitol, sucrose, and MTT, as well as the nonselective NHE inhibitors cimetidine, harmaline, and clonidine were purchased from Sigma (St. Louis, MO). Human IL-1β was obtained from R&D Systems (Minneapolis, MN). The p38 inhibitor SB203580 and p42/44 inhibitor PD98059 were purchased from Calbiochem (San Diego, CA). Stock solutions of SB203580, PD98059, amiloride, EIPA, MIA, cimetidine, harmaline, and clonidine were prepared using dimethyl sulfoxide (DMSO, Sigma). The final DMSO concentration in the medium for all concentrations of all agents was 0.5%. Vehicle controls also contained 0.5% DMSO.
IL-8 and GRO-α Measurement
To study the effect of hyperosmolarity on IL-8 and GRO-α production by IECs, HT-29 or Caco-2 cells in 96-well plates were treated with isosmolar medium or varying hyperosmolar medium prepared by dissolving increasing amounts of mannitol, NaCl, or sucrose in isosmolar growth medium described above (McCoy’s for HT-29 and Dulbecco’s modified Eagle’s medium for Caco cells). The cells were incubated with hyperosmolar or isosmolar medium for 18 hours. To test the effect of NHE or MAP kinase inhibitors, these inhibitors or their vehicle (0.5% DMSO in medium) were added to the cells immediately after treatment with hyperosmolar medium, followed by an incubation period of 18 hours. However, because harmaline was toxic to the cells when the incubation lasted for 18 hours, the effect of this agent on hyperosmolarity-induced IL-8 production was tested 4 hours after stimulation. In experiments investigating the effect of pH changes on IL-8 production, 25 mmol/L of HEPES was added to normal McCoy’s medium to increase the buffering capacity of the medium. pH was then adjusted using NaOH. Human IL-8 and GRO-α levels were determined from the cell supernatants using commercially available enzyme-linked immunosorbent assay kits (BD Pharmingen, San Diego, CA, and R&D Systems, Minneapolis, MN, respectively) and according to the manufacturers’ instructions.
RNA Isolation and Reverse Transcriptase-Polymerase Chain Reaction (PCR)
Total RNA was isolated from HT-29 cells using TRIzol reagent (Invitrogen). Reverse transcription of the RNA was performed using MuLV reverse transcriptase from Perkin Elmer (Foster City, CA). Five μg of RNA was transcribed in a 20-μl reaction containing 2 μl of 10× PCR buffer, 2 μl of 10 mmol/L dNTP mix, 2 μl of 25 mmol/L MgCl2, 2 μl of 100 mmol/L dithiothreitol, 1 μl of 50 μmol/L oligo d(T)16, and 0.3 μl of MuLV reverse transcriptase (50 U/μl). The reaction mix was incubated at 42°C for 15 minutes for reverse transcription. Thereafter, the reverse transcriptase was inactivated at 99°C for 5 minutes. Reverse transcriptase-generated DNA was amplified using the Expand High Fidelity PCR System (Boehringer Mannheim, Indianapolis, IN). The PCR reaction mix (25 μl) contained 2 μl of cDNA, water, 2.5 μl of 10× PCR buffer, 1.5 μl of 25 mmol/L MgCl2, 1 μl of 10 mmol/L dNTP mix, 0.5 μl of 10 μmol/L oligonucleotide primer (each), and 0.2 μl of DNA polymerase. cDNA was amplified using the following primers and conditions: IL-8 25 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ (sense) and 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ (antisense) and GAPDH-5′-CGGAGTCAACGGATTTGGTCGTAT-3′ (sense) and 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ (antisense), an initial denaturation at 94°C × 5 minutes, 27 and 23 cycles of 94°C × 30 seconds for IL-8 and GAPDH, respectively, 58°C × 45 seconds, 72°C × 45 seconds; a final dwell at 72°C × 7 minutes. The expected PCR products were IL-8 at 289 bp and GAPDH at 306 bp. PCR products were resolved on a 1.5% agarose gel and stained with ethidium bromide.
NF-κB EMSA and Supershift Assay
HT-29 cells were stimulated with mannitol added to isosmotic medium or isosmotic medium for varying lengths of time and nuclear protein extracts were prepared as described previously. 26 To determine the effect of NHE blockade, cells were treated with amiloride (300 μmol/L, Sigma) or its vehicle (0.5% DMSO) concomitantly with mannitol. All nuclear extraction procedures were performed on ice with ice-cold reagents. Cells were washed twice with phosphate-buffered saline (PBS) and harvested by scraping into 1.5 ml of PBS and pelleted at 1500 × g for 10 minutes. The pellet was resuspended in three-packed cell volume of cytosolic lysis buffer [20% v/v glycerol, 10 mmol/L HEPES, pH 8.0, 10 mmol/L KCl, 0.5 mmol/L ethylenediaminetetraacetic acid (EDTA), pH 8.0, 1.5 mmol/L MgCl2, 0.6% v/v Nonidet P-40, 0.5 mmol/L dithiothreitol, and 0.2 mmol/L phenylmethyl sulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin A] and incubated for 15 minutes on ice with occasional vortexing. After centrifugation at 4500 × g for 10 minutes, supernatants (cytosolic extracts) were saved for Western blotting studies, while the pellet was further processed to obtain nuclear extracts. Two cell pellet volume of nuclear extraction buffer (20% v/v glycerol, 20 mmol/L HEPES, pH 8.0, 420 mmol/L NaCl, 0.5 mmol/L EDTA, pH 8.0, 1.5 mmol/L MgCl2, 50 mmol/L glycerol-phosphate, 0.5 mmol/L dithiothreitol, 0.2 mmol/L phenylmethyl sulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin A) was added to the nuclear pellet and incubated on ice for 30 minutes with occasional vortexing. Nuclear proteins were isolated by centrifugation at 14,000 × g for 15 minutes. Protein concentrations were determined using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Nuclear extracts were aliquoted and stored at −80°C until used for EMSA. The NF-κB consensus oligonucleotide probe used for the EMSA was purchased from Promega (Madison, WI). Oligonucleotide probes were labeled with [γ-32P]-ATP using T4 polynucleotide kinase (Invitrogen) and purified in MicroSpin G-50 columns (Amersham Pharmacia, Piscataway, NJ). For the EMSA analysis, 12 μg of nuclear proteins were preincubated with EMSA-binding buffer (8% glycerol v/v, 10 mmol/L Tris-HCl, pH 8.0, 1 mmol/L MgCl2, 0.5 mmol/L EDTA, pH 8.0, and 0.5 mmol/L dithiothreitol) as well as 15 ng/μl poly(dI)-poly(dC), 0.4 ng/μl of single-strand DNA, and 0.2 mg/ml bovine serum albumin at room temperature for 10 minutes before addition of the radiolabeled oligonucleotide for an additional 25 minutes. The specificities of the binding reactions were tested by incubating samples with a 50-fold molar excess of the unlabeled oligonucleotide probe. Protein-nucleic acid complexes were resolved using a nondenaturing polyacrylamide gel consisting of 4% acrylamide (29:1 ratio of acrylamide:bisacrylamide) and run in 0.5× TBE buffer (45 mmol/L Tris-base, 45 mmol/L boric acid, 1 mmol/L EDTA) for ∼2.5 hours at constant current (25 mA). Gels were transferred to Whatman 3M paper, dried under vacuum at 80°C for 40 minutes, and exposed to photographic film at −80°C with an intensifying screen. For supershift studies, before addition of the radiolabeled probe, samples were incubated for 30 minutes with 2 μg of isotype control (rabbit polyclonal IgG Mad 1 antibody, sc-222X), p65 (sc-109X), or p50 (sc-114X) antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Western Blot Analysis
HT-29 cells in six-well plates were treated with mannitol (100 mmol/L) containing medium for varying lengths of time. Ten μg of cytosolic protein extracts were separated on 8 to 16% Tris-glycine gel (Invitrogen) and transferred to a nitrocellulose membrane. The membrane was probed with anti-phospho-p38 or anti-phospho-p42/44 MAP kinase antibody (Promega) and subsequently incubated with a secondary horseradish peroxidase-conjugated donkey anti-rabbit antibody (Boehringer). Bands were detected using the ECL Western Blotting Detection Reagent (Amersham Life Science, Arlington Heights, IL).
Transient Transfection and Luciferase Activity
For transient transfections, 2 to 4 × 105 HT-29 cells were seeded per well of a 24-well tissue culture dish 1 day before transient transfection. Cells were transfected with serum-free RPMI 1640 medium containing 25 μl/ml of Lipofectamine 2000 reagent (Invitrogen) and 10 μg/ml of plasmid DNA including a NF-κB luciferase promoter construct or the empty vector (Clontech, San Diego, CA) according to the manufacturer’s instructions. This NF-κB luciferase vector contains four tandem copies of the NF-κB consensus sequence fused to a TATA-like promoter region from the Herpes simplex virus thymidine kinase promoter. After 5 hours of incubation, medium was replaced with McCoy’s 5A medium containing 10% fetal bovine serum. Cells were allowed to recover at 37°C for 20 hours, and subsequently were stimulated with mannitol (100 mmol/L) containing medium or isosmotic medium for 16 hours. Luciferase activity was measured by the Luciferase Reporter Assay System (Promega) and normalized relative to μg of protein.
Measurement of Mitochondrial Respiration
Mitochondrial respiration, an indicator of cell viability, was assessed by the mitochondria-dependent reduction of MTT to formazan. Cells in 96-well plates were incubated with MTT (0.5 mg/ml) for 60 minutes at 37°C. Culture medium was removed by aspiration, and the cells were solubilized in DMSO (100 μl). The extent of reduction of MTT to formazan within cells was quantitated by measurement of optical density at 550 nm using a Spectramax 250 microplate reader (Molecular Devices, Sunnyvale, CA). 27,28
Statistical Evaluation
Values in the figures and in the text are expressed as mean ± SEM of n observations. Statistical analysis of the data was performed by one-way analysis of variance followed by Dunnett’s test, as appropriate.
Results
Hyperosmolarity Induces IL-8 Protein Secretion by HT-29 and Caco-2 Cells
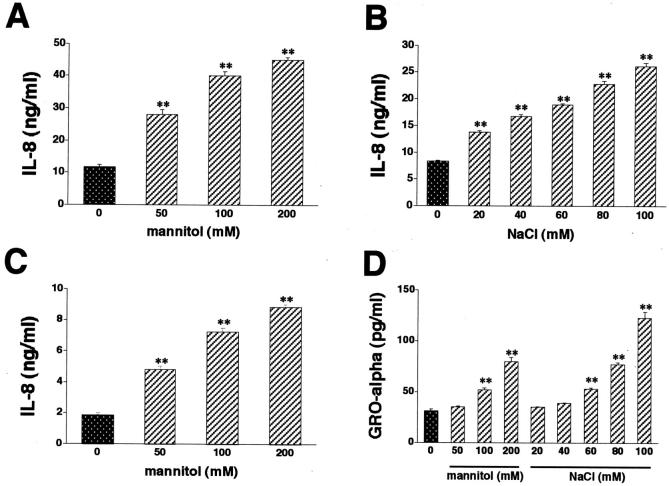
Exposure of HT-29 cells to hyperosmotic medium containing mannitol stimulated IL-8 production when compared to isosmolar medium (Figure 1A) ▶ . Mannitol stimulated IL-8 production in a concentration-dependent manner. Increasing medium osmolarity with NaCl (Figure 1B) ▶ or sucrose (data not shown) reproduced the stimulatory effect of mannitol on IL-8 production, also in a concentration-dependent manner. Mannitol was also tested in Caco-2 cells, where similar to results in HT-29 cells, it increased the release of IL-8 (Figure 1C) ▶ .
Figure 1.
Hyperosmolarity stimulates IL-8 production by both HT-29 (A and B) and Caco-2 (C) cells. Cells were incubated with hyperosmotic medium prepared by the addition of mannitol (A and C) or NaCl (B) to isosmolar growth medium. NaCl concentrations depicted on the x axis represent NaCl amounts added to isosmolar growth medium that already contains ∼140 mmol/L of NaCl (B). In control wells, cells were incubated with isosmolar growth medium. D: Hyperosmotic medium containing either mannitol or NaCl also stimulates GRO-α production by HT-29 cells. Supernatants for IL-8 and GRO-α measurement were taken 18 hours after the hyperosmolar challenge. Data are mean ± SEM of n = 6 to 12 wells from two different experiments. *, P < 0.05; **, P < 0.01.
In contrast to the membrane impermeable agents mannitol, sucrose, and NaCl, the membrane permeable agent urea (100 mmol/L) failed to augment the production of IL-8 (5.17 ± 0.39 ng/ml in control HT-29 cells versus 4.11 ng/ml ± 0.31 ng/ml in 100 mmol/L urea-treated HT-29 cells, n = 6, P > 0.05).
To determine, whether the hyperosmotic inflammatory response of IECs resulted in the production of other inflammatory mediators, we next tested the effect of osmolytes on the production of another chemokine, GRO-α. Similar to results with IL-8, hyperosmotic medium containing either mannitol or NaCl induced the release of GRO-α into the medium from HT-29 cells (Figure 1D) ▶ . These results support the idea that hyperosmolarity stimulates a full-blown inflammatory response in IECs.
Hyperosmolarity-Induced IL-8 Production by HT-29 Cells Is Dependent on NHE Activation and Is Not Mediated by an Increase in pH
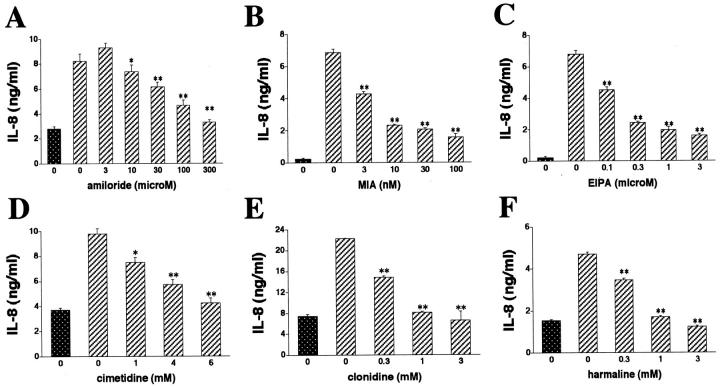
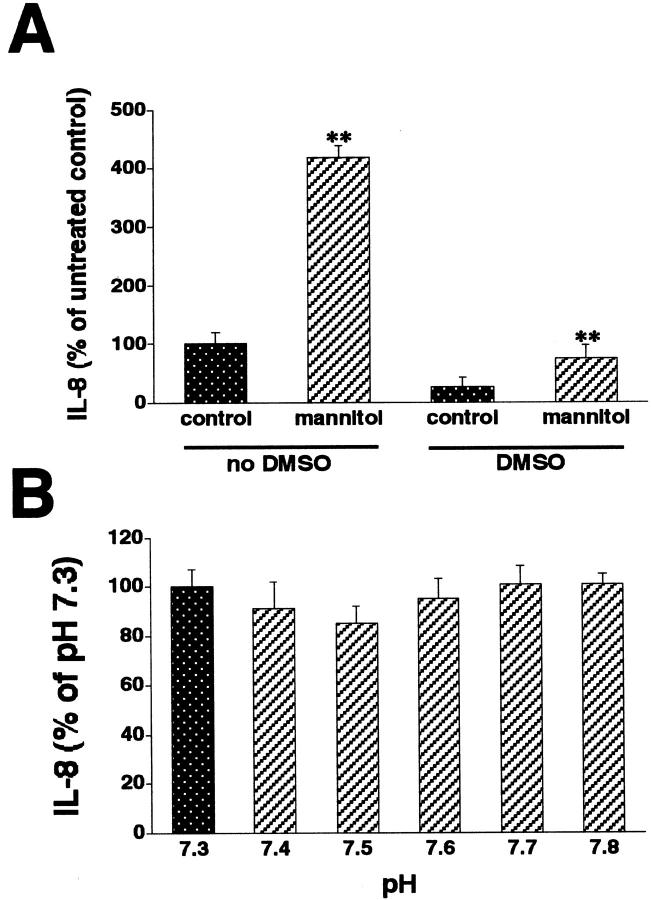
Because NHEs are important membrane proteins that are involved in mediating the intracellular effects of hyperosmolarity, 29-31 we tested the possibility that hyperosmolarity increased IL-8 production via an NHE-dependent mechanism. Inhibition of NHE activation with amiloride, MIA, or EIPA almost completely abrogated the stimulatory effect of mannitol on IL-8 production (Figure 2; A, B, and C) ▶ . Furthermore, the nonselective NHE inhibitors cimetidine, clonidine, or harmaline all blunted the IL-8 response to mannitol (Figure 2; D, E, and F) ▶ . Interestingly, in these studies using NHE inhibitors, IL-8 production was lower than in the studies depicted in Figure 1 ▶ , which was because of the presence of DMSO. That is, because in independent experiments, we found that 0.5% DMSO that was used to dilute the NHE inhibitors caused a substantial decrease in both baseline and hyperosmolarity-stimulated IL-8 levels (Figure 3A) ▶ . DMSO was not toxic to the cells, as determined with the MTT assay. Finally, NHE inhibition also decreased IL-8 production, when it was induced using hyperosmolar NaCl or sucrose (data not shown). The effects of NHE inhibitors were specific, because none of the NHE inhibitors had any effect on cell viability (data not shown).
Figure 2.
Treatment of HT-29 cells with the selective NHE inhibitors amiloride (A), MIA (B), or EIPA (C) suppresses mannitol-induced IL-8 production. Treatment of HT-29 cells with the nonamiloride NHE inhibitors cimetidine (D), clonidine (E), or harmaline (F), reproduces the suppressive effect of selective NHE inhibitors on the production of IL-8 by mannitol-induced HT-29 cells. When harmaline was used, supernatants for IL-8 measurement were taken 4 hours after the hyperosmotic challenge. In the case of amiloride, MIA, EIPA, cimetidine, and clonidine, IL-8 levels were measured from supernatants obtained 18 hours after the hyperosmotic challenge. Hyperosmotic medium prepared by the addition of 100 mmol/L of mannitol to isosmolar growth medium was used for hyperosmotic stimulation. Data are mean ± SEM of n = 6 to 12 wells from two different experiments. *, P < 0.05; **, P < 0.01. Dotted bar, no mannitol; cross-hatched bars, mannitol.
Figure 3.
A: DMSO (0.5%) inhibits both basal and mannitol (100 mmol/L)-stimulated IL-8 production by HT-29 cells. Growth medium on cells was switched with fresh growth medium with or without mannitol, both in the presence or absence of 0.5% DMSO. IL-8 production was measured from supernatants taken 18 hours after the addition of fresh medium. B: Increasing medium pH fails to augment the production of IL-8 by HT-29 cells. Growth medium on cells was switched with fresh growth medium with pH values ranging from 7.3 to 7.8. IL-8 production was measured from supernatants taken 18 hours after the addition of fresh medium. Data are mean ± SEM of n = 6 to 12 wells from two different experiments. **, P < 0.01.
In an attempt to identify mechanisms contributing to the NHE-mediated inflammatory response caused by hyperosmolarity, we tested the possibility that an increase in intracellular pH 32 underlies the enhancing effect of hyperosmolarity-induced NHE activation on IL-8 production. To this end, we exposed HT-29 cells to medium with increasing pH (7.3 to 7.8) for 18 hours. The pH value of 7.3 was the starting point because we found that McCoy’s 5A medium with 10% fetal bovine serum had a pH of 7.3 (data not shown). Because hyperosmolarity has been documented to increase intracellular pH by up to 0.5 U, 32 the highest pH studied was 7.8. As shown in Figure 3B ▶ , increasing medium pH failed to enhance IL-8 production, indicating that a change in intracellular pH is unlikely to mediate the augmenting effect of hyperosmolarity-induced NHE activation on IL-8 production.
p38 and p42/44 Activation Contributes to Hyperosmolarity-Induced IL-8 Production in HT-29 Cells
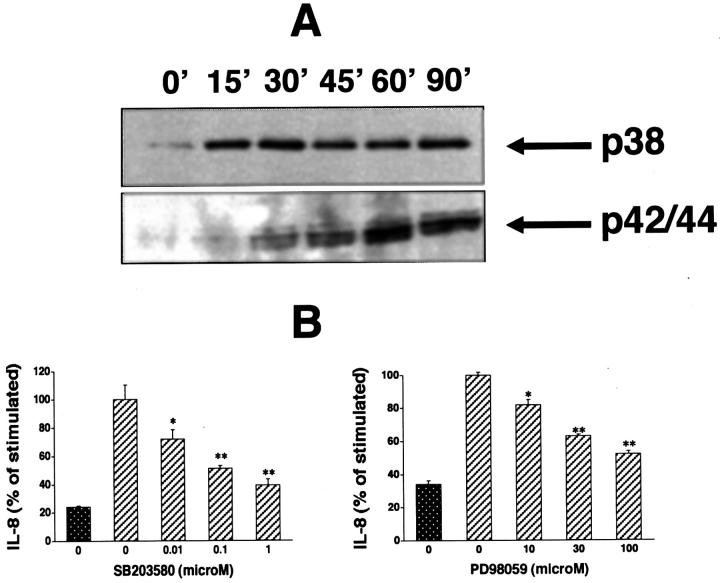
Downstream from NHEs, activation of p38 and p42/44 MAP kinases are well-characterized signaling pathways, whereby extracellular hyperosmolarity exerts it cellular effects. 33 Therefore, we examined whether hyperosmolarity enhanced IL-8 production by a mechanism involving p38 and p42/44. Using Western blotting with antibodies against the active, double-phosphorylated form of p38 and p42/44, we found that hyperosmotic medium containing 100 mmol/L mannitol induced both p38 and p42/44 activation in HT-29 cells (Figure 4A) ▶ . Mannitol induced p38 activation as early as 15 minutes after stimulation, which remained increased during the 90-minute observation period. The mannitol-induced activation of p42/44 was delayed as compared to p38, reaching its maximum 60 minutes after mannitol administration.
Figure 4.
Hyperosmolarity induces p38 and p42/44 activation in HT-29 cells (A). Cells were incubated with hyperosmotic medium prepared by the addition of mannitol (for a final concentration of 100 mmol/L) to isosmolar medium for the indicated time periods. p38 and p42/44 activation was determined using Western blotting using antibodies raised against the active, double-phosphorylated form of p38 and p42/44. This figure is representative of two separate experiments. Treatment of HT-29 cells with the selective p38 inhibitor SB203580 or selective p42/44 inhibitor PD98059 suppresses the hyperosmolarity-induced IL-8 response (B). Hyperosmolarity was achieved by the addition of mannitol (for a final concentration of 100 mmol/L) to isosmolar medium. In control wells, cells were incubated with isosmolar medium. Data are mean ± SEM of n = 12 wells from two separate experiments. *, P < 0.05; **, P < 0.01. Dotted bar, no mannitol; cross-hatched bars, mannitol.
To examine, whether this activation of p38 and p42/44 caused by mannitol contributed to the stimulatory effect of mannitol on IL-8 production, we next investigated whether MAP kinase inhibition decreased mannitol-induced IL-8 production. Treatment of the cells with either the selective p38 inhibitor SB203580 (Figure 4B) ▶ or the selective p42/44 inhibitor PD98059 (Figure 4B) ▶ produced a concentration-dependent blunting of the IL-8 response to mannitol. Furthermore, neither of these inhibitors caused any toxicity, as determined using the MTT assay (data not shown). These data indicate that both p38 and p42/44 activation contributes to the stimulatory effect of mannitol on IL-8 production by IECs.
Hyperosmolarity Stimulates IL-8 mRNA Accumulation in HT-29 Cells
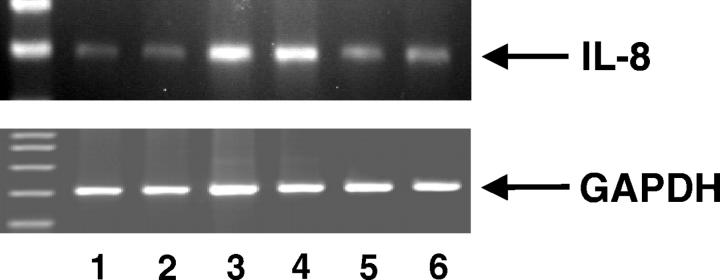
We next examined whether the effect of hyperosmolarity on IL-8 protein synthesis was associated with increased accumulation of IL-8 mRNA. Treatment of HT-29 cells with hyperosmotic medium containing 100 mmol/L of mannitol for 4 hours stimulated the accumulation of IL-8 mRNA as measured by reverse transcriptase-PCR (Figure 5) ▶ . Furthermore, similar to its effect on IL-8 production, amiloride abolished the mannitol-induced increase in IL-8 mRNA levels, confirming that NHEs play a central role in the hyperosmolarity-induced IL-8 response in HT-29 cells (Figure 5) ▶ . The effect of both mannitol and amiloride was selective because none of these agents affected mRNA levels of the housekeeping gene GAPDH.
Figure 5.
Hyperosmolarity induces up-regulation of IL-8 mRNA levels in HT-29 cells. Amiloride pretreatment (300 μmol/L) inhibits hyperosmolarity-induced IL-8 mRNA accumulation. Lanes 1 and 2: Control (isosmolar medium); lanes 3 and 4: hyperosmolarity (100 mmol/L of mannitol); lanes 5 and 6: amiloride and hyperosmolarity. GAPDH levels were not affected by both hyperosmolar and amiloride treatment. IL-8 and GAPDH mRNA levels were quantitated using reverse transcriptase-PCR. This figure is representative of three separate experiments.
Hyperosmolarity Induces Activation of NF-κB in HT-29 Cells
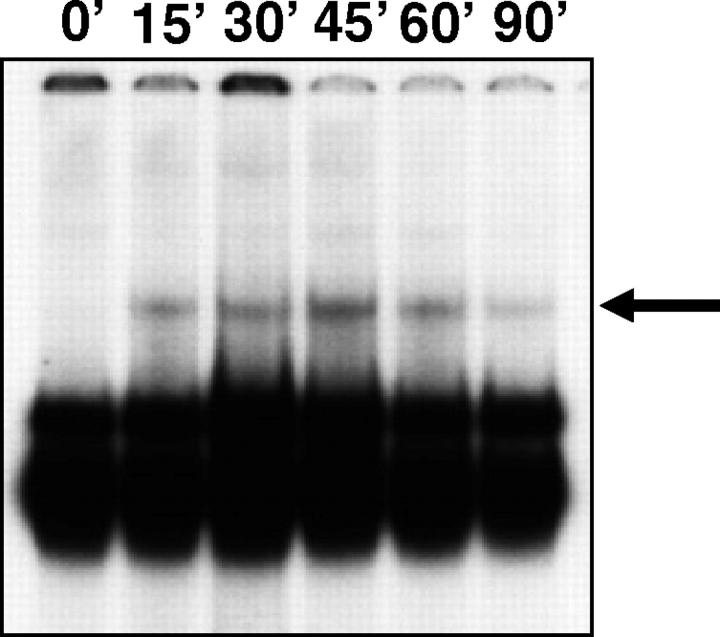
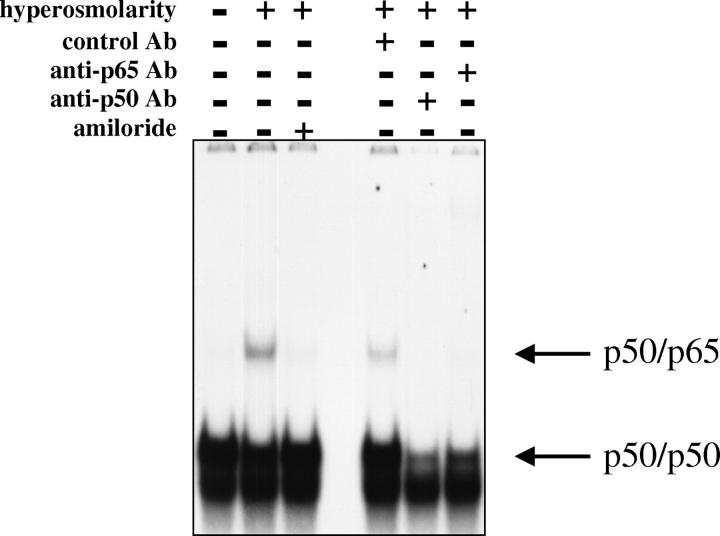
Because NF-κB is the central regulator of IL-8 gene expression in IECs, 4 we examined the effect of hyperosmolarity on NF-κB activation both by measuring NF-κB DNA binding and NF-κB-dependent transcriptional activity. Exposure of HT-29 cells to hyperosmotic medium containing 100 mmol/L of mannitol induced a NF-κB DNA-binding complex that was not seen in unstimulated cells (Figure 6) ▶ . This complex could be detected as early as 15 minutes after hyperosmotic stimulation, became maximal 45 minutes after the stimulus, and had almost completely disappeared 90 minutes after stimulation (Figure 6) ▶ . Supershift studies established that the complex induced by mannitol contained both p65 and p50. That is because both the p65 and p50 antibodies inhibited the formation of this complex (Figure 7) ▶ . Stimulation of the cells with IL-1β induced a complex that was identical in its position to the one induced by mannitol and also contained both p50 and p65 (data not shown). Finally, similar to its action on IL-8 protein synthesis and mRNA expression, NHE inhibition by amiloride caused a near complete disappearance of the mannitol-induced NF-κB DNA-binding complex (Figure 7) ▶ .
Figure 6.
Hyperosmolarity induces NF-κB DNA binding in HT-29 cells. Cells were incubated with hyperosmotic medium prepared by the addition of mannitol (for a final concentration of 100 mmol/L) to isosmolar medium for the indicated time periods. NF-κB DNA binding was assessed using EMSA. The figure is representative of two separate experiments.
Figure 7.
Amiloride pretreatment (300 μmol/L) inhibits hyperosmolarity-induced NF-κB DNA binding in HT-29 cells. NF-κB-specific complexes are indicated by arrows as determined by antibody supershifting. Hyperosmolarity was produced by the addition of mannitol (for a final concentration of 100 mmol/L) to isosmolar medium for 45 minutes. Control cells were treated with isosmolar medium. This figure is representative of three separate experiments.
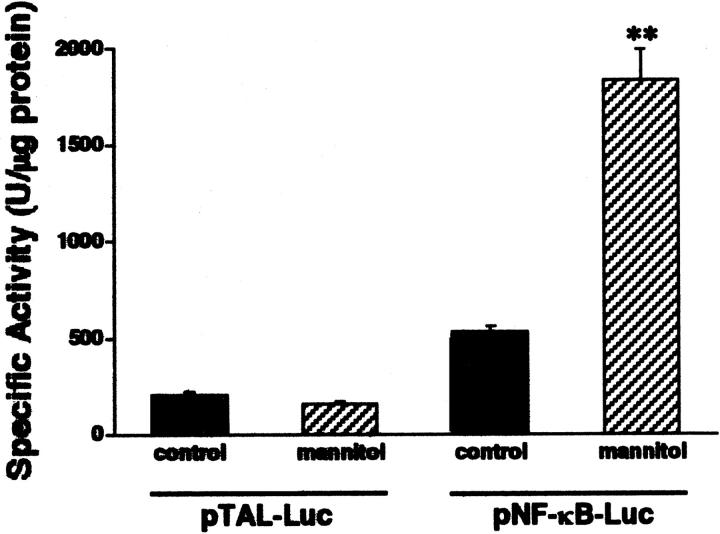
We next investigated whether the mannitol-elicited increase in NF-κB DNA binding corresponded with an increase in NF-κB-dependent gene transcription. To this end, HT-29 cells were transiently transfected with a NF-κB-luciferase reporter construct or the empty vector. Exposure of HT-29 cells to hyperosmotic medium containing 100 mmol/L of mannitol for 16 hours induced a substantial increase in luciferase activity in cells transfected with the NF-κB-luciferase reporter construct but not the empty vector (Figure 8) ▶ . Therefore, we can conclude that hyperosmolarity activates NF-κB-dependent transcriptional activity in HT-29 cells.
Figure 8.
Hyperosmolar stimulation increases NF-κB-dependent transcriptional activity as compared to isosmolar stimulation. HT-29 cells were transiently transfected with a NF-κB-luciferase promoter construct, after which the cells were incubated for 16 hours with either hyperosmolar (100 mmol/L of mannitol) or isosmolar (control) medium. NF-κB-dependent transcriptional activity was determined using the luciferase assay (pNF-κB-Luc). This figure also shows that hyperosmolarity does not influence luciferase activity in cells transfected with an enhancerless construct (pTAL-Luc). Data are mean ± SEM of n = 14 to 16 wells from two separate experiments. **, P < 0.01.
Discussion
In this study, we show that osmotic stress increases IL-8 gene expression and protein secretion in the human intestinal epithelial cell lines HT-29 and Caco-2. The regulation of cell function by osmotic stress requires osmosensing structures, which can sense these volume changes, and osmosignaling pathways that can transmit the signal toward the effector sites of regulation.
NHEs are well-described osmosensing structures that could contribute to the hyperosmolarity-induced enhancement of IL-8 production in IECs. 29-31,34 Recent studies have documented that NHEs are a gene family from which seven mammalian isoforms (NHE1, NHE2, NHE3, NHE4, NHE5, NHE6, and NHE7) have been cloned and sequenced. 35-38 IECs are unique in that in contrast to most other cell types that express only one NHE isoform. IECs have been found to express NHE1 to NHE4, although the expression of these isotypes varies even between different cell clones. 39-44 NHEs are reversibly inhibited by amiloride, MIA, and EIPA, as well as by a variety of nonrelated drugs, including cimetidine, clonidine, and harmaline. 37,38 Because NHE inhibition with these agents produced a near complete prevention of the hyperosmolarity-induced enhancement of IL-8 production, it can be suggested that NHEs play a central role in the hyperosmolarity-induced signal cascade of IECs resulting in IL-8 production. Although a role for NHEs in the regulation of inflammatory cytokine production has been reported previously, 45-47 our study is the first one to demonstrate that NHEs regulates a hyperosmolarity-induced inflammatory response. In this respect, it is also important to mention that we recently found that lithium, an agent known to stimulate NHEs, induced the intestinal epithelial inflammatory response. 48 This lithium-induced IEC inflammatory response was suppressed by NHE inhibitors in the same way as the response triggered by hyperosmolarity. 48 Thus, these data with lithium further highlight the importance of NHEs in the regulation of the IEC inflammatory response. At this point, it would be difficult to speculate which NHE isoform(s) participate in the regulation of IL-8 production and other inflammatory processes. Because NHE inhibition yielded a similar suppression of cytokine production in macrophages 45 and human umbilical vein endothelial cells, 49 both of which express only NHE1, 50,51 it is possible that NHE1 is the most important isoform regulating cellular inflammatory events.
Recent evidence suggests that NHEs and the intestinal epithelial inflammatory response are interconnected on yet another level. That is, an interesting study by the group of Rocha and colleagues 52 showed that chronic treatment with the inflammatory cytokine interferon-γ down-regulated the expression of NHEs in IECs. Taken together, based on the above evidence, we speculate that the mechanism of NHE promotion of inflammatory processes may have evolved as a positive feedback signal during IEC activation. On the other hand, the down-regulation of NHEs by inflammatory signals may serve as a protective mechanism against the NHE-amplified inflammatory response during chronic inflammatory states.
Two of the best-characterized osmosignaling structures in mammalian cells are p38 and p42/44 MAP kinases. 32,33 We found that activation of both the p38 and p42/44 MAP kinases is involved in the IL-8 response of IECs to hyperosmolarity. Evidence supporting the notion that both the p38 and p42/44 MAP kinases mediate the effect of hyperosmolarity on IEC IL-8 production is twofold. First, hyperosmolarity stimulated the activation of both p38 and p42/44. Second, selective pharmacological inhibition of both p38 and p42/44 activation decreased the hyperosmolarity-induced production of IL-8.
NF-κB is a key determinant of the intestinal epithelial inflammatory cascade and occupies a central role in the transcriptional activation of the IL-8 gene in IECs. 4,53-59 Recent evidence has implicated NF-κB as an important osmosignaling molecule that is activated in response to hyperosmolarity in both renal medullary interstitial and endothelial cells. 24,60 Furthermore, it was recently shown that the hyperosmolarity-induced activation of NF-κB is crucial in the initiation of cyclooxygenase-2 gene expression in renal epithelial cells. 24 Interestingly, a very high concentration of mannitol (300 mmol/L) has recently been reported to increase intracellular IL-18 by ∼20% in Caco-2 cells, 61 however, the role of NF-κB in this response has not been investigated. Similarly, although hyperosmolarity has been documented to produce an inflammatory response in other, nonintestinal cell types, the role of NF-κB in those processes has not been characterized. 62-66 Our results demonstrating that hyperosmolarity induces both NF-κB DNA binding and NF-κB-dependent transcriptional activity in IECs, provide evidence that NF-κB is one of the central osmosignaling factors that mediate the production of IL-8 by gut epithelial cells in response to hyperosmolarity. In addition to NF-κB, a variety of transcription factors has been shown to convey the effects of extracellular hyperosmolarity on gene expression. These include AP-1, 67 NF-AT, 68 CREB, 69 and tonicity-responsive enhancer-binding protein. 70 It will require further study to characterize, which of these or other transcription factors may also contribute to the enhanced inflammatory response of IECs caused by hyperosmotic stress.
It is important to mention that IL-8 production was induced when osmotically active agents that are limited in their ability to cross the plasma membrane were used. However, when hyperosmolar solutions of urea, an agent that readily crosses the plasma membrane were used, no enhancement in IL-8 production was seen. Because osmotic agents that are membrane impermeable cause cell shrinkage but urea does not, it can be speculated that hyperosmolarity stimulates IL-8 production in IECs by a mechanism involving cell shrinkage.
The intestinal mucosa is a semipermeable membrane across which osmotic equilibration occurs. This is most evident in the very proximal small bowel, wherein both hyper- and hypotonic meals are rendered isosmotic. Subsequently, isotonicity is maintained during distal progression of a meal until it reaches the distal bowel. Although fasting colonic contents are isotonic, 71 because bacterial metabolism of carbohydrates generates poorly absorbed solutes, the osmolarity of intraluminal contents after meal intake is increased in the colon. 72 There are several implications arising from our results. We speculate that the inflammatory response to hyperosmolarity in the gut may have developed as a protective mechanism against pathogens ingested with food. Furthermore, we hypothesize that although a limited inflammatory process caused by a transient increase in osmolarity may be beneficial, long-lasting substantial hyperosmolarity may have deleterious effects. For example, because patients with Crohn’s disease have dramatically higher colonic osmolarity than healthy patients, 21,22 it is possible that this high colonic osmolarity may contribute to the chronic inflammatory activity of the colonic mucosa in Crohn’s colitis. In fact, the degree of hyperosmolarity observed in Crohn’s colitis (∼100 to 110 mosmol/L 21,22 ) is similar to the extent of hyperosmolarity caused by 100 mmol/L of mannitol or 50 mmol/L of NaCl, which induced a substantial inflammatory response in the HT-29 or Caco-2 cells in our study. Similar mechanisms may operate in neonatal necrotizing colitis, because hyperosmotic feeding has been shown to be associated with an increased incidence of this disease in low-birth-weight human infants. 23
In summary, the present study provides evidence that hyperosmolarity stimulates the IEC inflammatory cascade, a process that involves activation of MAP kinases, NHEs, as well as NF-κB, and results in the production of IL-8 and GRO-α.
Footnotes
Address reprint requests to Dr. György Haskó, Department of Surgery, UMD-New Jersey Medical School, 185 South Orange Ave., University Heights, Newark, NJ 07103. E-mail: haskoge@umdnj.edu.
References
- 1.Fiocchi C: Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Physiol 1997, 273:G769-G775 [DOI] [PubMed] [Google Scholar]
- 2.Hecht G, Savkovic SD: Effector role of epithelia in inflammation—interaction with bacteria. Aliment Pharmacol Ther 1997, 3:S64-S68 [DOI] [PubMed] [Google Scholar]
- 3.Schmid RM, Adler G: NF-kappaB/rel/IkappaB: implications in gastrointestinal diseases. Gastroenterology 2000, 118:1208-1228 [DOI] [PubMed] [Google Scholar]
- 4.Jobin C, Sartor RB: The Iκ B/NF-κB system: a key determinant of mucosal inflammation and protection. Am J Physiol 2000, 278:C451-C462 [DOI] [PubMed] [Google Scholar]
- 5.Madara JL: Pathobiology of neutrophil interactions with intestinal epithelia. Aliment Pharmacol Ther 1997, 3:S57-S62 [DOI] [PubMed] [Google Scholar]
- 6.Podolsky DK: Mucosal immunity and inflammation. V Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol 1999, 277:G495-G499 [DOI] [PubMed] [Google Scholar]
- 7.Yang SK, Eckmann L, Panja A, Kagnoff MF: Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology 1997, 113:1214-1223 [DOI] [PubMed] [Google Scholar]
- 8.Gerwirtz AT, Simon PO, Jr, Schmitt CK, Taylor LJ, Hagedorn CH, O’Brien AD, Neish AS, Madara JL: Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest 2001, 107:99-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papadakis KA, Targan SR: The role of chemokines and chemokine receptors in mucosal inflammation. Inflamm Bowel Dis 2000, 6:303-313 [DOI] [PubMed] [Google Scholar]
- 10.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF: A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 1995, 95:55-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick BA, Colgan SP, Delp-Archer C, Miller SI, Madara JL: Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signaling to subepithelial neutrophils. J Cell Biol 1993, 123:895-907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cominelli F, Pizarro TT: Interleukin-1 and interleukin-1 receptor antagonist in inflammatory bowel disease. Aliment Pharmacol Ther 1996, 10 2:S49-S53 [DOI] [PubMed] [Google Scholar]
- 13.Xu DZ, Lu Q, Kubicka R, Deitch EA: The effect of hypoxia/reoxygenation on the cellular function of intestinal epithelial cells. J Trauma 1999, 46:280-285 [DOI] [PubMed] [Google Scholar]
- 14.Taylor CT, Fueki N, Agah A, Hershberg RM, Colgan SP: Critical role of cAMP response element binding protein expression in hypoxia-elicited induction of epithelial tumor necrosis factor-alpha. J Biol Chem 1999, 274:19447-19454 [DOI] [PubMed] [Google Scholar]
- 15.Taylor CT, Dzus AL, Colgan SP: Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology 1998, 114:657-668 [DOI] [PubMed] [Google Scholar]
- 16.Deitch EA: Role of the gut lymphatic system in multiple organ failure. Curr Opin Crit Care 2001, 7:92-98 [DOI] [PubMed] [Google Scholar]
- 17.Caplan MS, Sun XM, Hsueh W: Hypoxia causes ischemic bowel necrosis in rats: the role of platelet-activating factor (PAF-acether). Gastroenterology 1990, 99:979-986 [DOI] [PubMed] [Google Scholar]
- 18.Gewirtz AT, Rao AS, Simon PO, Jr, Merlin D, Carnes D, Madara JL, Neish AS: Salmonella typhimurium induces epithelial IL-8 expression via Ca2+-mediated activation of the NF-κB pathway. J Clin Invest 2000, 105:79-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao D, Keates AC, Kuhnt-Moore S, Moyer MP, Kelly CP, Pothoulakis C: Signal transduction pathways mediating neurotensin-stimulated interleukin-8 expression in human colonocytes. J Biol Chem 2001, 276:44464-44471 [DOI] [PubMed] [Google Scholar]
- 20.Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL: Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest 2001, 107:861-869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vernia P, Gnaedinger A, Hauck W, Breuer RI: Organic anions and the diarrhea of inflammatory bowel disease. Dig Dis Sci 1988, 33:1353-1358 [DOI] [PubMed] [Google Scholar]
- 22.Schilli R, Breuer RI, Klein F, Dunn K, Gnaedinger A, Bernstein J, Paige M, Kaufman M: Comparison of the composition of faecal fluid in Crohn’s disease and ulcerative colitis. Gut 1982, 23:326-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheromcha DP, Hyman PE: Neonatal necrotizing enterocolitis. Inflammatory bowel disease of the newborn. Dig Dis Sci 1988, 33:78S-84S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao CM, Yull F, Blackwell T, Komhoff M, Davis LS, Breyer MD: Dehydration activates an NF-κB-driven, COX2-dependent survival mechanism in renal medullary interstitial cells. J Clin Invest 2000, 106:973-982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsen EM, Johansen F-E, Jahnsen FL, Lundin KEA, Scholz T, Brandtzaeg P, Haraldsen G: Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut 1998, 42:635-642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haskó G, Szabó C, Németh ZH, Kvetan V, Pastores SM, Vizi ES: Adenosine receptor agonists differentially regulate IL-10, TNF-α, and nitric oxide production in RAW 2647 macrophages and in endotoxemic mice. J Immunol 1996, 157:4634-4640 [PubMed] [Google Scholar]
- 27.Haskó G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabó C: Adenosine inhibits IL-12 and TNF-α production via adenosine. A2a receptor-dependent and independent mechanisms. EMBO J 2000, 14:2065-2074 [DOI] [PubMed] [Google Scholar]
- 28.Haskó G, Kuhel DG, Németh ZH, Mabley JG, Stachlewitz RF, Virág L, Lohinai Z, Southan GJ, Salzman AL, Szabó C: Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol 2000, 164:1013-1019 [DOI] [PubMed] [Google Scholar]
- 29.Haussinger D, Schliess F: Osmotic induction of signaling cascades: role in regulation of cell function. Biochem Biophys Res Commun 1999, 255:551-555 [DOI] [PubMed] [Google Scholar]
- 30.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D: Functional significance of cell volume regulatory mechanisms. Physiol Rev 1998, 78:247-306 [DOI] [PubMed] [Google Scholar]
- 31.Haussinger D: Liver fibrosis and Na+/H+ exchange. Gastroenterology 2001, 120:572-575 [DOI] [PubMed] [Google Scholar]
- 32.Krump E, Nikitas K, Grinstein S: Induction of tyrosine phosphorylation and Na+/H+ exchanger activation during shrinkage of human neutrophils. J Biol Chem 1997, 272:17303-17311 [DOI] [PubMed] [Google Scholar]
- 33.Lee JC, Young PR: Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol 1996, 59:152-157 [DOI] [PubMed] [Google Scholar]
- 34.Demaurex N, Grinstein S: Na+/H+ antiport: modulation by ATP and role in cell volume regulation. J Exp Biol 1994, 196:389-404 [DOI] [PubMed] [Google Scholar]
- 35.Donowitz M, Khurana S, Tse CM, Yun CH: G protein-coupled receptors in gastrointestinal physiology. III Asymmetry in plasma membrane signal transduction: lessons from brush-border Na+/H+ exchangers. Am J Physiol 1998, 274:G971-G977 [DOI] [PubMed] [Google Scholar]
- 36.Numata M, Orlowski J: Molecular cloning and characterization of a novel (Na+, K+)/H+ exchanger localized to the trans-Golgi network. J Biol Chem 2001, 276:17387-17394 [DOI] [PubMed] [Google Scholar]
- 37.Szabo EZ, Numata M, Shull GE, Orlowski J: Kinetic and pharmacological properties of human brain Na(+)/H(+) exchanger isoform 5 stably expressed in Chinese hamster ovary cells. J Biol Chem 2000, 275:6302-6307 [DOI] [PubMed] [Google Scholar]
- 38.Yu FH, Shull GE, Orlowski J: Functional properties of the rat Na/H exchanger NHE-2 isoform expressed in Na/H exchanger-deficient Chinese hamster ovary cells. J Biol Chem 1993, 268:25536-25541 [PubMed] [Google Scholar]
- 39.Gonda T, Maouyo D, Rees SE, Montrose MH: Regulation of intracellular pH gradients by identified Na/H exchanger isoforms and a short-chain fatty acid. Am J Physiol 1999, 276:G259-G270 [DOI] [PubMed] [Google Scholar]
- 40.Janecki AJ, Montrose MH, Tse CM, de Medina FS, Zweibaum A, Donowitz M: Development of an endogenous epithelial Na(+)/H(+) exchanger (NHE3) in three clones of caco-2 cells. Am J Physiol 1999, 277:G292-G305 [DOI] [PubMed] [Google Scholar]
- 41.Maouyo D, Chu S, Montrose MH: pH heterogeneity at intracellular and extracellular plasma membrane sites in HT29–C1 cell monolayers. Am J Physiol 2000, 278:C973-C981 [DOI] [PubMed] [Google Scholar]
- 42.Turner JR, Black ED, Ward J, Tse CM, Uchwat FA, Alli HA, Donowitz M, Madara JL, Angle JM: Transepithelial resistance can be regulated by the intestinal brush-border Na+/H+ exchanger NHE3. Am J Physiol 2000, 279:C1918-C1924 [DOI] [PubMed] [Google Scholar]
- 43.Ghishan FK, Knobel SM, Summar M: Molecular cloning, sequencing, chromosomal localization, and tissue distribution of the human Na+/H+ exchanger (SLC9A2). Genomics 1995, 30:25-30 [DOI] [PubMed] [Google Scholar]
- 44.Dudeja PK, Rao DD, Syed I, Joshi V, Dahdal RY, Gardner C, Risk MC, Schmidt L, Bavishi D, Kim KE, Harig JM, Goldstein JL, Layden TJ, Ramaswamy K: Intestinal distribution of human Na+/H+ exchanger isoforms NHE-1, NHE-2, and NHE-3 mRNA. Am J Physiol 1996, 271:G483-G493 [DOI] [PubMed] [Google Scholar]
- 45.Nemeth ZH, Mabley JG, Deitch EA, Szabo C, Hasko G: Inhibition of the Na+/H+ antiporter suppresses IL-12 p40 production by mouse macrophages. Biochim Biophys Acta 2001, 1539:233-242 [DOI] [PubMed] [Google Scholar]
- 46.Haddad JJ, Land SC: Amiloride blockades lipopolysaccharide-induced proinflammatory cytokine biosynthesis in an Iκ B-alpha/NF-κB-dependent mechanism. Evidence for the amplification of an antiinflammatory pathway in the alveolar epithelium. Am J Respir Cell Mol Biol 2002, 26:114-126 [DOI] [PubMed] [Google Scholar]
- 47.Rolfe MW, Kunkel SL, Rowens B, Standiford TJ, Cragoe EJ, Jr, Strieter RM: Suppression of human alveolar macrophage-derived cytokines by amiloride. Am J Respir Cell Mol Biol 1992, 6:576-582 [DOI] [PubMed] [Google Scholar]
- 48.Németh ZH, Deitch EA, Szabó C, Fekete Z, Hauser CJ, Haskó G: Lithium induces NF-κB activation and IL-8 production in human intestinal epithelial cells. J Biol Chem 2002, 277:7713-7719 [DOI] [PubMed] [Google Scholar]
- 49.Németh ZH, Deitch EA, Lu Q, Szabó C, Haskó G: Na+/H+ exchanger blockade inhibits chemokine production and NF-κB activation in immunostimulated endothelial cells. Am J Physiol 2002, 283:C396-C403 [DOI] [PubMed] [Google Scholar]
- 50.Demaurex N, Orlowski J, Brisseau G, Woodside M, Grinstein S: The mammalian Na+/H+ antiporters NHE-1, NHE-2, and NHE-3 are electroneutral and voltage independent, but can couple to an H+ conductance. J Gen Physiol 1995, 106:85-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zerbini G, Roth T, Podesta F, Cagliero E, Doria A, Canessa M, Lorenzi M: Activity and expression of the Na+/H+ exchanger in human endothelial cells cultured in high glucose. Diabetologia 1995, 38:785-791 [DOI] [PubMed] [Google Scholar]
- 52.Rocha F, Musch MW, Lishanskiy L, Bookstein C, Sugi K, Xie Y, Chang EB: IFN-γ downregulates expression of Na(+)/H(+) exchangers NHE2 and NHE3 in rat intestine and human Caco-2/bbe cells. Am J Physiol 2001, 280:C1224-C1232 [DOI] [PubMed] [Google Scholar]
- 53.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M: Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol 2001, 167:1609-1616 [DOI] [PubMed] [Google Scholar]
- 54.Savkovic SD, Koutsouris A, Hecht G: Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol 1997, 273:C1160-C1167 [DOI] [PubMed] [Google Scholar]
- 55.Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner DA, Sartor RB: Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J Immunol 1998, 160:410-418 [PubMed] [Google Scholar]
- 56.Jobin C, Holt L, Bradham CA, Streetz K, Brenner DA, Sartor RB: TNF receptor-associated factor-2 is involved in both IL-1 beta and TNF-alpha signaling cascades leading to NF-kappa B activation and IL-8 expression in human intestinal epithelial cells. J Immunol 1999, 162:4447-4454 [PubMed] [Google Scholar]
- 57.Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C: The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology 2000, 118:724-734 [DOI] [PubMed] [Google Scholar]
- 58.Bocker U, Schottelius A, Watson JM, Holt L, Licato LL, Brenner DA, Sartor RB, Jobin C: Cellular differentiation causes a selective down-regulation of interleukin (IL)-1 beta-mediated NF-κB activation and IL-8 gene expression in intestinal epithelial cells. J Biol Chem 2000, 275:12207-12213 [DOI] [PubMed] [Google Scholar]
- 59.Parikh AA, Salzman AL, Kane CD, Fischer JE, Hasselgren PO: IL-6 production in human intestinal epithelial cells following stimulation with IL-1 beta is associated with activation of the transcription factor NF-κB. J Surg Res 1997, 69:139-144 [DOI] [PubMed] [Google Scholar]
- 60.Yorek MA, Dunlap JA, Liu W, Lowe WL, Jr: Normalization of hyperosmotic-induced inositol uptake by renal and endothelial cells is regulated by NF-κB. Am J Physiol 2000, 278:C1011-C1018 [DOI] [PubMed] [Google Scholar]
- 61.Takeuchi M, Nishizaki Y, Sano O, Ohta T, Ideda M, Kurimoto M: Immunohistochemical and immuno-electron-microscopic detection of interferon-gamma-inducing factor (“interleukin-18”) in mouse intestinal epithelial cells. Cell Tissue Res 1997, 289:499-503 [DOI] [PubMed] [Google Scholar]
- 62.Shapiro L, Dinarello CA: Hyperosmotic stress as a stimulant for proinflammatory cytokine production. Exp Cell Res 1997, 231:354-362 [DOI] [PubMed] [Google Scholar]
- 63.Shapiro L, Dinarello CA: Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci USA 1995, 92:12230-12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hashimoto S, Matsumoto K, Gon Y, Nakayama T, Takeshita I, Horie T: Hyperosmolarity-induced interleukin-8 expression in human bronchial epithelial cells through p38 mitogen-activated protein kinase. Am J Respir Crit Care Med 1999, 159:634-640 [DOI] [PubMed] [Google Scholar]
- 65.Hashimoto S, Gon Y, Matsumoto K, Takeshita I, Maruoka S, Horie T: Inhalant corticosteroids inhibit hyperosmolarity-induced, and cooling and rewarming-induced interleukin-8 and RANTES production by human bronchial epithelial cells. Am J Respir Crit Care Med 2000, 162:1075-1080 [DOI] [PubMed] [Google Scholar]
- 66.Loitsch SM, von Mallinckrodt C, Kippenberger S, Steinhilber D, Wagner TO, Bargon J: Reactive oxygen intermediates are involved in IL-8 production induced by hyperosmotic stress in human bronchial epithelial cells. Biochem Biophys Res Commun 2000, 276:571-578 [DOI] [PubMed] [Google Scholar]
- 67.Wilmer WA, Dixon CL, Hebert C: Chronic exposure of human mesangial cells to high glucose environments activates the p38 MAPK pathway. Kidney Int 2001, 60:858-871 [DOI] [PubMed] [Google Scholar]
- 68.Lopez-Rodriguez C, Aramburu J, Jin L, Rakeman AS, Michino M, Rao A: Bridging the NFAT and NF-κB families: nFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity 2001, 15:47-58 [DOI] [PubMed] [Google Scholar]
- 69.Borsook D, Konradi C, Falkowski O, Comb M, Hyman SE: Molecular mechanisms of stress-induced proenkephalin gene regulation: cREB interacts with the proenkephalin gene in the mouse hypothalamus and is phosphorylated in response to hyperosmolar stress. Mol Endocrinol 1994, 8:240-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woo SK, Maouyo D, Handler JS, Kwon HM: Nuclear redistribution of tonicity-responsive enhancer binding protein requires proteasome activity. Am J Physiol 2000, 278:C323-C330 [DOI] [PubMed] [Google Scholar]
- 71.Phillips SF: Diarrhea: a current view of the pathophysiology. Gastroenterology 1972, 63:495-518 [PubMed] [Google Scholar]
- 72.Keusch GT, Donowitz M: Pathophysiological mechanisms of diarrhoeal diseases: diverse aetiologies and common mechanisms. Scand J Gastroenterol 1983, 84:3S3-S43 [PubMed] [Google Scholar]