Abstract
Injured muscle can initiate regeneration promptly by activating myogenic cells that proliferate and differentiate into myotubes and myofibers. However, the recovery of the injured skeletal muscle often is hindered by the development of fibrosis. We hypothesized that the early-appearing myogenic cells in the injured area differentiate into myofibroblasts and eventually contribute to the development of fibrosis. To investigate this, we transplanted a genetically engineered clonal population of muscle-derived stem cells (MC13 cells) into the skeletal muscle of immunodeficient SCID mice, which were lacerated 4 weeks after transplantation. The MC13 cells regenerated numerous myofibers in the nonlacerated muscle and these myogenic cells were gradually replaced by myofibroblastic cells in the injured muscle. Our results suggest that the release of local environmental stimuli after muscle injury triggers the differentiation of myogenic cells (including MC13 cells) into fibrotic cells. These results demonstrate the potential of muscle-derived stem cells to differentiate into different lineages and illustrate the importance of controlling the local environment within the injured tissue to optimize tissue regeneration via the transplantation of stem cells.
Growth and repair of skeletal muscle is usually initiated by the activation of a population of muscle precursors, called satellite cells, located beneath the basement membrane of muscle fibers. 1,2 Based on their ability to repair injured or damaged muscle fibers in the postnatal stage, satellite cells were proposed as a population of muscle stem cells. 2,3 However, there is evidence that satellite cells are heterogeneous in nature because they behave differently in vitro and in vivo. 3,4 At least two populations of satellite cells have been isolated from human skeletal muscle tissue: fusing and nonfusing satellite cells. 5 In addition, a series of recent studies reported that a subpopulation of cells extracted from skeletal muscle displays a stem cell phenotype 6-8 and can differentiate into various lineages. 8-10
After muscle injuries, myogenic precursor cells are released and activated early in the healing process. Once activated, the myogenic cells rapidly regenerate the injured skeletal muscle either by fusing with the local myofibers or by generating new myofibers. 1,2 Although this prompt regeneration seems to contribute to muscle healing, the functional recovery of the injured muscle often is hindered by the development of scar tissue. 11,12 The development of scar tissue after injury is usually associated with the overproduction of extracellular matrix. 13,14 Even when regeneration is enhanced by different growth factors, fibrosis still takes place and inhibits functional muscle recovery. 11,15 Our research team has shown that the use of anti-fibrosis agents (eg, decorin) that inactivate transforming growth factor-β1 (TGF-β1) can reduce muscle fibrosis and significantly improve muscle healing after injuries. 16 It is therefore apparent that fibrosis interferes with the functional recovery of injured skeletal muscle.
We hypothesize that the early-appearing myogenic cells, which probably include muscle-derived stem cells, can differentiate into another cell lineage (eg, myofibroblasts) because of the influence of environmental stimuli released at the injured area. The differentiation of myogenic cells toward the fibroblastic lineage may explain the rapid development of large scar tissue after injury. To validate this hypothesis, we designed a set of experiments involving the transplantation of a clonal population of muscle-derived stem cells (MC13 cells) 8 into skeletal muscles that were subsequently injured by laceration. Our results suggest that myogenic precursor cells can differentiate into myofibroblasts after muscle injury and consequently contribute to the development of fibrosis. These results may prove extremely important in the development of approaches to prevent muscle fibrosis after injury.
Materials and Methods
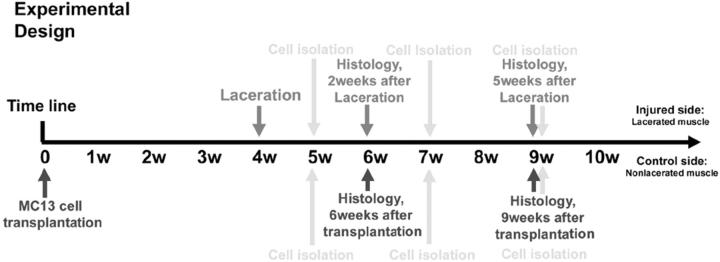
Experimental Design
A population of muscle-derived stem cells (MC13 cells) was transplanted into skeletal muscles that were subsequently injured by laceration using a protocol previously described. 12,15,16 MC13 cells were injected into both the left and right gastrocnemius muscles (GMs) of SCID mice (C57BL6J/SJ, 4 to 6 weeks of age). Forty immunodeficient SCID mice were separated into five groups for this experiment (five mice were used for histology and three mice were used for the preplate technique analysis to isolate primary cell culture). Four weeks after implantation (experimental design in Figure 1 ▶ ), the GMs in the left legs were lacerated and the right leg muscles were kept as controls (ie, nonlacerated). The muscle tissue was either used to prepare primary cell cultures (Figure 2A) ▶ at 1 to 5 weeks after injury or cryostat-sectioned for histological assessment (Figure 1) ▶ . All animal procedures were performed in accordance with the guidelines approved by Children’s Hospital and the University of Pittsburgh Animal Care Committee (protocol no. 2/98).
Figure 1.
Schematic representation of the experimental design.
Figure 2.
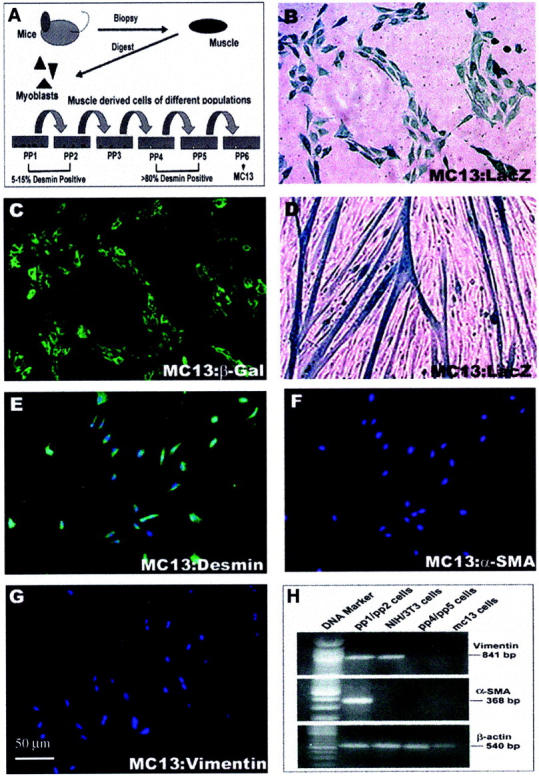
Illustration of the isolation of primary muscle cells (pp1-pp6) via the preplate technique (A). MC13 cells purified from pp6 are positive for β-galactosidase (B, C) and desmin (E). Even after differentiation, the MC13 cells remain positive for LacZ (D). The MC13 cells are negative for α-SMA and vimentin (F, G). These results were confirmed by RT-PCR (H). The pp1/pp2 cell population was positive for both vimentin and α-SMA, but the NIH/3T3 cell population was positive only for vimentin. MC13 cells and pp6 cell populations were both negative for vimentin and α-SMA. β-Actin was used as an external control for the RT-PCR experiment.
Cells
MC13 cells were isolated from primary muscle cells (pp6) of mdx mice and transfected with a plasmid encoding for the β-galactosidase (by human cytomegalovirus; HCMV promoter), minidystrophin (by chicken β-actin; CAG promoter), and neomycin resistance genes (by phosphoglycerate PKG promoter). 8,17 The primary muscle cells isolated at different preplates (pp1 to pp6, see below in Isolation of Donor-Derived Muscle Cells from the Lacerated and Nonlacerated Skeletal Muscle) were obtained using the preplate technique as previously described. 8,18-20 The fibroblast cell line NIH/3T3 was used as the control for the reverse transcriptase-polymerase chain reaction (RT-PCR) experiments.
RT-PCR
Equal numbers (1 × 105) of four different types of cells [ie, the MC13, NIH/3T3, and primary muscle-derived cells (pp1/pp2, pp6)] were seeded into 75-cm2 flasks (Falcon, Becton Dickinson Laboratory, Franklin Lakes, NJ) and cultured for 48 hours. Then the cells’ total RNA was extracted using a monophasic solution of phenol and guanidine isothiocyanate (TRIzol, 10 cm2/ml; Life Technologies, Inc., Grand Island, NY). Reverse transcription was performed with Superscript II (Gibco BRL, Life Technologies, Inc., Rockville, MD), and cDNA was amplified by PCR with primers specific for vimentin 5′-TCAGCTCACCAACGACAAGG-3′ and 3′-GGAGTGTTCTTTTTGAGTGGG-5′; α-smooth muscle actin (α-SMA), 5′-CTGGAGAAGAGCTACGAACTGC-3′ and 3′-CTGATCCACATCTGCTGGAAGG-5′; β-actin, 5′-GTGGG-CCGCTCTAGGCACCAA-3′ and 3′-CTCTTTGATGTCACGCACGATTTC-5′.
Detection of α-SMA and Vimentin by Western Blot
Two hundred thousand MC13 cells were seeded into 25-cm2 flasks in Dulbecco’s modified Eagle’s medium containing different concentrations of TGF-β1 (0.01 ng/ml, 0.1 ng/ml, 1.0 ng/ml, and 10 ng/ml). Twelve hours later, the cells were lysed and separated on a 12.5% electrophoresis sodium dodecyl sulfate-polyacrylamide gel. Equal aliquots (25 μl) of samples diluted in Laemmli sample buffer (Bio-Rad, Richmond, CA) were boiled for 5 minutes before loading. After 12 hours of electrophoresis at 30 V, the separated proteins were transferred to a nitrocellulose membrane (60 V overnight). Membranes were blocked with 1% nonfat dry milk and 2% horse serum in phosphate-buffered saline (PBS) for 1 hour at room temperature. The primary antibodies used for these experiments were mouse anti-α-SMA (1:1000) and goat anti-vimentin (1:2000) applied to the membranes for 2 hours at room temperature. The secondary antibodies used were anti-mouse IgG-horseradish peroxidase (1:5000) and anti-goat IgG-horseradish peroxidase (1:5000) incubated with the membranes for 1 hour. The blots were developed using SuperSignal West Pico Chemiluminescent substrate (Pierce, Rockford, IL), and the positive bands were visualized on X-ray film.
Cell Transplantation and Development of Muscle Injury Model (Laceration)
Forty immunodeficient SCID mice were separated into five groups. After receiving anesthesia, 1 × 106 MC13 cells were injected intramuscularly into the right and left GMs of the mice. Four weeks later, the injected muscles from the left legs were injured by laceration using a protocol previously described. 12,15,16 The GM was lacerated at the largest diameter through 50% of its width and 100% of its thickness using a protocol previously described. 12,15,16 The injected muscles of the right legs were not lacerated and served as controls. At different time points after transplantation and laceration injury, the animals were sacrificed and the GMs were isolated to be prepared for primary muscle cell culture via the preplate technique and/or to be assessed by histology and immunohistochemistry for the expression of β-galactosidase, α-SMA, and vimentin (experimental design in Figure 1 ▶ ).
LacZ Staining
The muscle cryosections (10 μm) and culture cells (MC13, pp1/pp2, pp4/pp5) were fixed with 1% glutaraldehyde (Sigma Chemical Co., St. Louis, MO), and then were incubated with LacZ staining solution (0.5 mmol/L K4Fe(CN)6, 0.5 mmol/L K3Fe(CN)6, 1.0 mmol/L MgCl2) for 2 hours at 37°C as previously described. 8 The sections were subsequently stained with eosin, while the cultured cells were visualized under a light microscope after LacZ staining. All results were visualized by regular microscopy (Nikon Diaphot 300, Nikon Eclipse E-800; Nikon, Tokyo, Japan).
Quantitation of LacZ-Positive Myofibers at Different Time Points after Injection
Northern Eclipse software (Empix Imaging, Inc., North Tonawanda, NY) was used to count the number of LacZ-positive myofibers in both injured and control skeletal muscles at different time points after transplantation. Tissue sections were stained with LacZ and eosin as described above and 10 sections were selected for counting from each group of mice at different time points (two sections from each mouse). The results were analyzed by Student’s t-test and P < 0.05 was considered significant.
Immunostaining
The injected sites (LacZ-positive) were also stained by immunohistochemistry. For β-galactosidase, the first antibody (biotin-conjugated anti-β-galactosidase, diluted to 1:100 in PBS; Sigma) was applied to the sections overnight at room temperature, and this was followed by streptavidin-conjugated Alexa Fluor 488 (1:1000 in PBS; Molecular Probes, Eugene, OR) used for 1 hour at room temperature. For α-SMA, a primary mouse antibody anti-α-SMA (1:400 in PBS for 2 hours at room temperature; Sigma) was used first, followed by a biotin-conjugated anti-mouse IgG (1:250 in PBS for 1 hour; Boehringer Mannheim, Indianapolis, IN) and a streptavidin-conjugated Cy3 (1:100 in PBS for 1 hour at room temperature; Sigma). Vimentin was detected using a goat anti-vimentin antibody-conjugated Cy3 (1:100 in PBS; Sigma) for 1 hour at room temperature. All immunofluorescence was visualized by fluorescent microscopy (Nikon Eclipse E-800).
Co-Localization of α-SMA and LacZ in Injured Skeletal Muscle
The biotinylated anti-β-galactosidase antibody was incubated overnight at room temperature (1:100 in PBS). Next, the secondary antibody, a streptavidin conjugated-Alexa Fluor 488 (1:1000), was incubated with the section for 1 hour. The mouse anti-α-SMA antibody (1:200) was incubated for 1 hour at room temperature followed by an anti-mouse-Cy3 antibody (1:150) for 45 minutes. The sections were subsequently incubated with 4,6-diamidino-2-phenylindole (5 minutes) to visualize the nuclei. The results were observed using fluorescence microscopy as described above.
Detection of TGF-β1 in the Injured Skeletal Muscle
Twenty-four SCID mice were separated into two groups. In group 1, 10 μl (10 μg/ml) of cardiotoxin were injected into the left GMs of the mice, while the right GMs were injected with 10 μl of PBS and served as controls. In group 2, the left GMs of the mice were lacerated, while the right GMs remained nonlacerated as controls. All mice were sacrificed at different time points after injury (3 days, 7 days, and 14 days). The GM tissues were prepared as above, and then stained by immunohistochemistry. The first antibody used was a rat anti-TGF-β1 IgG (Novocastra Laboratories, Newcastle, UK), which was diluted 1:100 and incubated with the sections for 2 hours (room temperature). The secondary antibody, an anti-mouse-IgG-Cy3 conjugated antibody (1:200, Sigma), was incubated with the section for 1 hour (room temperature). Finally, the sections were incubated with 4,6-diamidino-2-phenylindole (5 minutes) to visualize the nuclei.
Isolation of Donor-Derived Muscle Cells from the Lacerated and Nonlacerated Skeletal Muscle
We isolated the injected GMs from both the nonlacerated and lacerated muscles at different time points after injury using a technique previously described. 8,18-20 The isolated cells were suspended in medium (Dulbecco’s modified Eagle’s medium plus 20% fetal bovine serum) and were seeded to collagen-coated flasks. One hour later, the supernatant was transferred into a fresh collagen-coated flask. The cells that quickly adhered to the flask were called pp1. The supernatant was replated in new flasks after 12 hours, and the cells that adhered to the flask during this 12-hour period were called pp2. The other preplate populations (pp3 to pp6) were obtained at intervals of 24 hours. Normally, the pp1 and pp2 populations of cells comprise mostly fibroblasts, because they are 5 to 15% desmin-positive 18 and some of them express α-SMA and vimentin (Figure 1H) ▶ . In contrast, the pp4 and pp5 fractions of cells are highly enriched for desmin-positive cells (>80%). 18 The muscle stem cells (MC13) were purified from the pp6 cell population 8,18 (Figure 2A) ▶ .
Various muscle-derived cell populations (pp1-pp6) were isolated from the lacerated and nonlacerated skeletal muscle at different time points after injury. However, we have combined the pp1 and pp2 (pp1/pp2) populations to form a representative fraction of low desmin-expressing cells (fibroblastic cell population) and the pp4 and pp5 (pp4/pp5) to form a representative fraction of high desmin-positive cells (myogenic cell population). The isolated cells (pp1/pp2 and pp4/pp5) from both lacerated and nonlacerated skeletal muscles were stained for β-galactosidase. For each population of cells (pp1/pp2 and pp4/pp5), isolated from lacerated and nonlacerated skeletal muscles, 1 × 104 cells were analyzed for LacZ expression. Statistical significance was assessed by a Student’s t-test; P < 0.05 was considered significant.
Results
Muscle-Derived Stem Cells Display the Expression of Myogenic and Stem Cell Markers but Are Negative for Vimentin and α-SMA
We and others have reported the isolation of various populations of muscle-derived cells (Figure 2A) ▶ through the preplate technique, a technique used to enrich for myogenic cells. 18-20 The MC13 cells 8 used in this study represent a clonal population of muscle-derived cells isolated from the preplate 6 (pp6) cell population as shown in Figure 2A ▶ . The genetically engineered MC13 cells expressed β-galactosidase in the nondifferentiated state (Figure 2, B and C) ▶ , as well as in differentiated myotubes, when cultured in fusion medium in vitro (Figure 2D) ▶ . We also have reported recently that the MC13 cells expressed early myogenic and stem cell markers, and are capable of differentiating into myogenic and osteogenic lineages. 8 Undifferentiated MC13 cells were also positive for desmin (Figure 2E) ▶ and other myogenic proteins, including myogenin and MyoD, 8 but they lacked the expression of myofibroblastic markers such as vimentin and α-SMA 21,22 (Figure 2; F, G, and H) ▶ . The pp4/pp5 fraction of muscle-derived cells also lacked vimentin and α-SMA expression (Figure 2H) ▶ . In contrast, the combined pp1/pp2 cell population expressed both vimentin and α-SMA (Figure 2H) ▶ . Many of the pp1/pp2 cells were desmin-negative and were likely to be fibroblastic in nature. 18-20 We used NIH/3T3 cells, a well-known fibroblastic cell line, as a control cell population for the RT-PCR experiments. The NIH/3T3 cells express vimentin, but lack α-SMA expression (Figure 2H) ▶ .
MC13 Cells Differentiate into Myofibers after Transplantation in Nonlacerated Skeletal Muscle
We first investigated the fate of the injected MC13 cells in nonlacerated skeletal muscles at 6 and 9 weeks after transplantation (experimental design Figure 1 ▶ ). The injected skeletal muscles were stained for LacZ, and the co-expression of α-SMA and vimentin was investigated by immunohistochemistry. As expected, the injection of MC13 cells within skeletal muscles resulted in the regeneration of many myofibers at 6 weeks after transplantation. In fact, many MC13 cells had fused into myotubes and myofibers expressing β-galactosidase at 6 weeks after injection (Figure 3; A to D) ▶ . Immunohistochemical staining for α-SMA (Figure 3, E and F) ▶ and vimentin (Figure 3, G and H) ▶ revealed that cells at the injected site expressed α-SMA but lacked vimentin expression at 6 weeks after injection. At 9 weeks after implantation, the injected site contained numerous large myofibers expressing the β-galactosidase reporter gene (Figure 3; I to L) ▶ . This finding demonstrates that the regenerated myofibers present at 6 weeks after injection had persisted and matured at 9 weeks after injection. However, a complete absence of α-SMA (Figure 3, M and N) ▶ and vimentin expression (Figure 3, O and P) ▶ was observed in the injured site. These results show that the injection of MC13 cells into a nonlacerated skeletal muscle contributed to the regeneration of myofibers at the injected site (LacZ-expressing myofibers) without the development of muscle fibrosis at 9 weeks after implantation.
Figure 3.
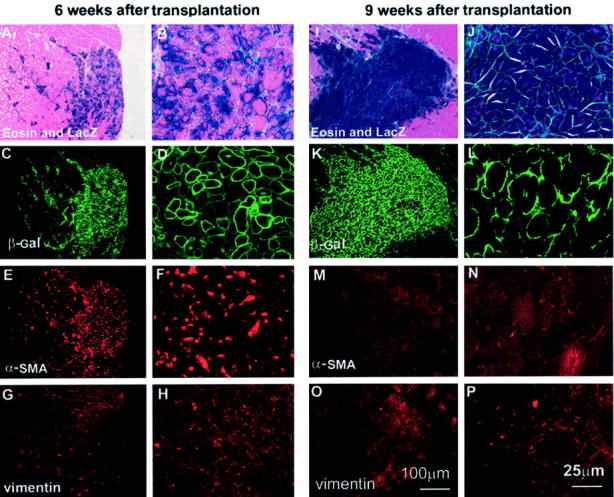
Many MC13 cells had fused into myotubes and myofibers at 6 weeks after transplantation in the nonlacerated muscle (A–D). The injected sites were positive for α-SMA (E, F), but negative for vimentin (G, H). At 9 weeks after transplantation, most of the β-galactosidase-expressing myofibers had matured and become larger than at 6 weeks after transplantation (I–L). However, the injected sites were negative for both α-SMA (M, N) and vimentin (O, P).
MC13 Cells Differentiate into Myofibroblasts in Lacerated Skeletal Muscle
We next proceeded to investigate the fate of the MC13 cells injected into the skeletal muscles that were lacerated at 4 weeks after transplantation. The fate of the injected cells was observed as described above at 2 and 5 weeks after laceration (6 and 9 weeks after transplantation, respectively) (see Figure 1 ▶ for experimental design). Like the nonlacerated muscles, the injected, lacerated muscles were also assessed by LacZ staining in combination with immunohistochemistry for β-galactosidase, α-SMA, and vimentin expression. As observed in the nonlacerated skeletal muscle, many LacZ-expressing myofibers were found in the injured sites at 2 weeks after laceration (Figure 4; A to D) ▶ . However, the expression of α-SMA (Figure 4, E and F) ▶ and vimentin (Figure 4, G and H) ▶ was also observed at the injured site (Figure 4; A to D) ▶ . These results suggest that as early as 2 weeks after injury, some of the injected MC13 cells may have differentiated into myofibroblastic cells in the injured site. At 5 weeks after laceration, a large scar tissue had developed in the injured skeletal muscle. Nearly all of the LacZ-expressing myofibers found at 2 weeks after laceration (Figure 4; A to D) ▶ had disappeared by 5 weeks after injury (Figure 4; I to L) ▶ . Surprisingly, most of the LacZ-positive cells were found in the scar tissue, suggesting that many of the injected MC13 cells had differentiated into fibrotic cells after injury (Figure 4; I to L) ▶ . Indeed, immunohistochemical staining revealed that the β-galactosidase-positive scar tissue area (Figure 4, K and L) ▶ co-localized with α-SMA (Figure 4, M and N) ▶ and vimentin expression (Figure 4, O and P) ▶ . These results suggest that on muscle injury, the injected MC13 cells potentially differentiated toward myofibroblastic lineages, which further contributed to the development of fibrosis within the injured skeletal muscle.
Figure 4.
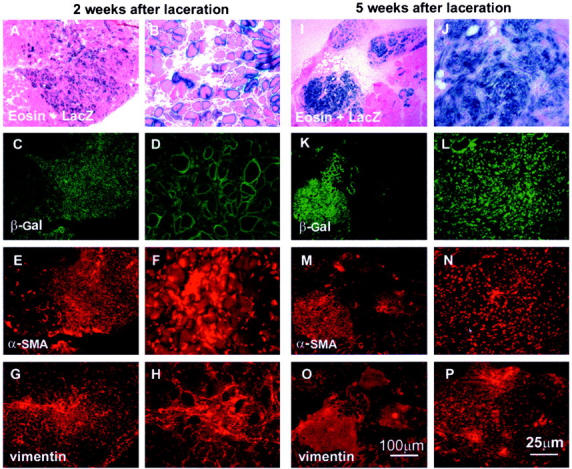
The MC13 cells had differentiated into myotubes and myofibers in the injured skeletal muscle at 2 weeks after injury (A–D). However, the injected sites were highly positive for both α-SMA (E, F) and vimentin (G, H) at 2 weeks after injury. At 5 weeks after injury, the regenerating myofibers present at 2 weeks after injury had disappeared and been replaced by scar tissue (I–L). Indeed, most of the β-galactosidase-expressing injected cells were now found in the scar tissue (I–L) because the injected sites were highly positive for both α-SMA (M, N) and vimentin (O, P).
We have used immunohistochemistry to further co-localize the expression of β-galactosidase-expressing cells with α-SMA-positive cells in the injured skeletal muscle at 1, 3, and 5 weeks after injury (Figure 5) ▶ . At 1 week after injury, we have observed occasional myofibers expressing β-galactosidase (Figure 5A ▶ , asterisks) that co-localized with myofibers expressing α-SMA (Figure 5, B and C ▶ , asterisks), but the vast majority of the β-galactosidase-expressing myofibers lacked the α-SMA expression (Figure 5, B and C ▶ , arrowheads). However, many α-SMA-expressing cells were found between myofibers (Figure 5, B and C ▶ , arrows). At 3 weeks after laceration, the majority of the large β-galactosidase-expressing myofibers was found negative for α-SMA (Figure 5; D, E, and F) ▶ , whereas a few small β-galactosidase-expressing myofibers expressed α-SMA (Figure 5; D, E, and F ▶ , asterisks).
Figure 5.
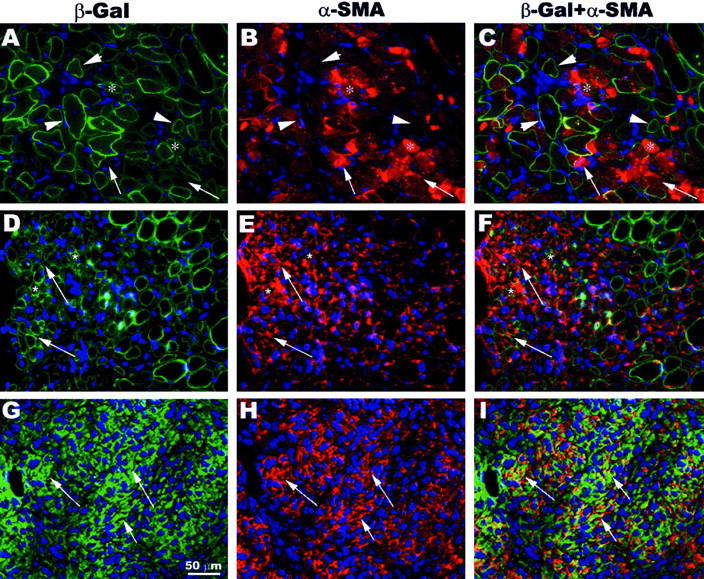
Co-localization of β-galactosidase-expressing cells with α-SMA-positive cells in the injured skeletal muscle. α-SMA was expressed in β-galactosidase-expressing myofibers at 1 week after laceration (A–C, asterisks). The expression of α-SMA also was found in the interstitial tissue between the myofibers (A–C, arrows). The vast majority of β-galactosidase-expressing myofibers was negative for α-SMA (A–C, arrowheads). However, the expression of α-SMA was mainly observed in interstitial tissue at 3 weeks after laceration (D–F, arrows). There were a few small myofibers in the lacerated area co-expressing β-galactosidase and α-SMA (D–F, asterisks). At 5 weeks after laceration, a complete absence of β-galactosidase-expressing myofibers was observed in the injected site, and the expression of α-SMA was found exclusively in interstitial tissue containing numerous β-galactosidase-expressing cells (G–I, arrows).
At 5 weeks after laceration, there was a high expression of α-SMA in muscles at the injured site, whereas no β-galactosidase-expressing myofibers were observed (Figure 5; G, H, and I) ▶ . Indeed, at 5 weeks after injury, a large number of mononucleated cells that co-expressed β-galactosidase and α-SMA was found in the injured area (Figure 5; G, H, and I ▶ , arrows).
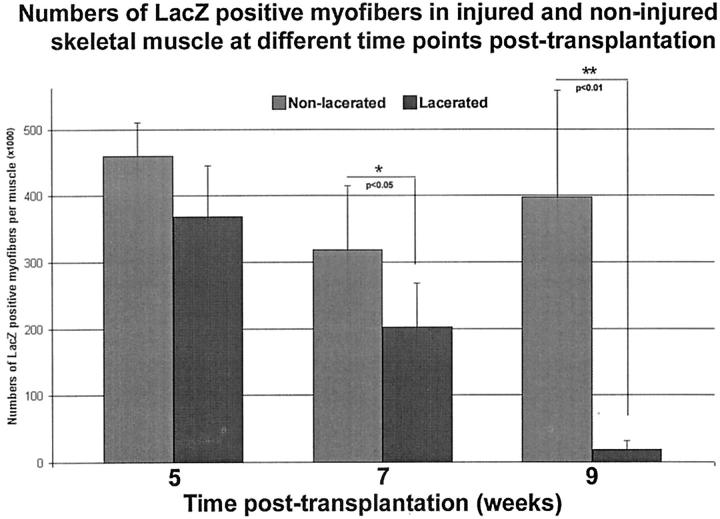
We have quantitated the number of β-galactosidase-expressing myofibers present in the lacerated skeletal muscle at different time points after injury, and the results were compared with the nonlacerated skeletal muscle. The number of LacZ-positive myofibers in the lacerated skeletal muscle was significantly lower than in the nonlacerated skeletal muscle at 7 and 9 weeks after transplantation (Figure 6) ▶ . Although the number of β-galactosidase-expressing myofibers within the lacerated skeletal muscle at 1 week after injury was no different from in the nonlacerated skeletal muscle, these LacZ-positive myofibers in the lacerated skeletal muscle disappeared almost entirely at 5 weeks after injury (Figure 6) ▶ .
Figure 6.
The quantitation of β-galactosidase-expressing myofibers in both injured and nonlacerated skeletal muscle revealed that: 1) similar numbers of LacZ-expressing myofibers were found in injured (1 week after injury) and nonlacerated muscle at 5 weeks after transplantation; and 2) a significant decrease in LacZ-expressing myofibers was found in the injured muscle (3 and 5 weeks after injury) when compared to nonlacerated muscle at 7 and 9 weeks after transplantation. At 5 weeks after injury (9 weeks after transplantation), there was a near complete absence of LacZ-expressing myofibers in the injured skeletal muscle.
Isolation of Donor-Derived Cells with a Myofibroblastic Phenotype from the Injured Skeletal Muscle
To confirm the differentiation of MC13 cells into a myofibroblastic lineage following muscle injury, the injected skeletal muscles were used to isolate a pp1/pp2 fibroblastic fraction and a pp4/pp5 myogenic fraction of muscle-derived cells via the preplate technique (Figure 2A) ▶ at different time points after injury. Various cells expressing β-galactosidase (Figure 7, A and C) ▶ that co-localized with α-SMA (Figure 7B) ▶ and vimentin (Figure 7D) ▶ were found in the pp1/pp2 culture derived from the injured skeletal muscle at 2 weeks after injury. These results suggest that MC13 cells, identified by the LacZ staining (Figure 7, A and C) ▶ , survived in the injected skeletal muscle, and following injury they differentiated toward myofibroblastic lineage and hence expressed vimentin and α-SMA (Figure 7, B and D) ▶ . The MC13 cells were found negative for these myofibroblast markers before implantation (Figure 2; F, G, and H) ▶ . We also observed that the MC13 cells isolated from the lacerated skeletal muscles lost their capacity to differentiate into myotubes in vitro (not shown), unlike the MC13 cells before injection (Figure 2D) ▶ . This co-localization of LacZ-positive cells with vimentin and α-SMA was found primarily in the injured skeletal muscle at 2, 3, and 5 weeks after injury, and was not observed in the nonlacerated muscle controls. Although some of the LacZ-positive MC13 cells that co-localized with α-SMA and vimentin could be found in the injured muscle at 1 week after laceration, their numbers were significantly lower than at 2, 3, and 5 weeks after injury. These results confirm the histological assessment (Figures 4 to 6) ▶ ▶ ▶ and suggest that the MC13 cells gradually differentiated into myofibroblastic cells in a time-dependent manner after injury.
Figure 7.

The LacZ-positive cells isolated within the pp1/pp2 fraction from the injured muscle at 2 weeks after laceration (A, C) were also positive for α-SMA (B) and vimentin (D). The percentage of LacZ-positive cells isolated within the pp1/pp2 and pp4/pp5 fractions from the injured and control skeletal muscles at 5, 7, and 9 weeks after implantation revealed that: 1) a significantly higher percentage of LacZ-positive cells was found in the pp4/pp5 fraction than in the pp1/pp2 fraction from the injured muscle at 1 week after injury; 2) a similar percentage of pp1/pp2 and pp4/pp5 cells were LacZ-positive at 3 weeks after injury; and 3) the percentage of LacZ-positive cells was significantly higher in the pp1/pp2 fraction than in the pp4/pp5 fraction at 5 weeks after injury. In contrast, a significantly higher percentage of LacZ-positive cells were consistently found in the pp4/pp5 fraction than in the pp1/pp2 fraction at all time points tested in the control muscle (5, 7, and 9 weeks after transplantation). **, P < 0.01.
Gradual Differentiation of MC13 Cells toward Myofibroblasts at Different Time Points after Injury
To further confirm a gradual differentiation of the MC13 cells toward myofibroblastic lineage after injury, we analyzed whether the number of LacZ-positive cells (pp1/pp2 fibroblastic fraction versus pp4/pp5 myogenic fraction) isolated from the lacerated skeletal muscle would vary in a time-dependent manner after injury. A similar experiment was performed in the control nonlacerated muscles. The LacZ-positive cells derived from the injured skeletal muscles at 1 week after injury (5 weeks after transplantation) were mostly isolated from the pp4/pp5 myogenic fraction (Figure 7E) ▶ . Interestingly, at 3 weeks after injury (7 weeks after transplantation) the number of LacZ-positive cells in the pp4/pp5 myogenic fraction was similar to that in the pp1/pp2 fibroblastic fraction. At 5 weeks after injury (9 weeks after transplantation) most of the LacZ-positive cells were found in the pp1/pp2 fibroblastic fraction (Figure 7E) ▶ . In contrast to the observations at 1 week after injury, the number of LacZ-positive cells found in the pp1/pp2 fibroblastic fraction was significantly higher than the number found in the pp4/pp5 myogenic fraction at 5 weeks after injury. We observed that most of the LacZ-positive cells derived from the nonlacerated muscle at 5, 7, and 9 weeks after transplantation were always isolated in the pp4/pp5 myogenic fraction (Figure 7E) ▶ . In fact, there were significantly more LacZ-positive cells in the pp4/pp5 myogenic fraction than in the pp1/pp2 fibroblastic fraction at every tested time point after transplantation (Figure 7E) ▶ .
Most of the LacZ-positive cells were found in the pp4/pp5 myogenic fraction, which is where most of the myogenic cells are usually found after the preplate technique. 18-20 These results suggest that the MC13 cells retain their myogenic phenotype after transplantation into nonlacerated skeletal muscle. The LacZ-positive cells derived from the injured skeletal muscle at 1 week after injury were primarily myogenic and, therefore, could also be isolated in the pp4/pp5 myogenic fraction. However, at 5 weeks after injury the MC13 cells had differentiated toward a myofibroblastic lineage and, consequently, could be isolated from the pp1/pp2 fibroblastic fraction where mostly nonmyogenic cells (desmin-negative) are isolated. 18-20 These results further confirm that upon muscle injury, the MC13 cells gradually differentiate from a myogenic lineage toward a myofibroblastic one. Because the percentage of LacZ-positive cells (pp1/pp2 and pp4/pp5) obtained from the nonlacerated and lacerated skeletal muscles at different time points after injection was similar, it is likely that the loss of MC13 cells following intramuscular injection was equivalent between the injured and control skeletal muscles.
The Role of TGF-β1 in the Development of Muscle Fibrosis after Muscle Injury
A time-dependent expression of TGF-β1 was detected in the injured skeletal muscle (cardiotoxin and laceration). Our results show that TGF-β1 is highly expressed in both mononucleated cells (arrows) and myofibers (asterisks) at 3 days after injury (Figure 8, A and B) ▶ . However, the expression of TGF-β1 declines at 7 and 14 days after injury (laceration). These results suggest that high levels of TGF-β1 may play a role in the subsequent development of fibrosis after muscle injury. To further validate this hypothesis, we have tested whether the stimulation of MC13 cells with TGF-β1 will induce the expression of myofibroblastic markers, including α-SMA and vimentin. Indeed, we have used both RT-PCR (Figure 8E) ▶ and Western blot (Figure 8F) ▶ to observe that the expression of α-SMA and vimentin by the MC13 cells can be induced by TGF-β1 stimulation in a dose-dependent manner. These results further validate the hypothesis that TGF-β1 triggers the differentiation of these MC13 cells toward a myofibroblastic lineage, which consequently contributes to the development of muscle fibrosis.
Figure 8.
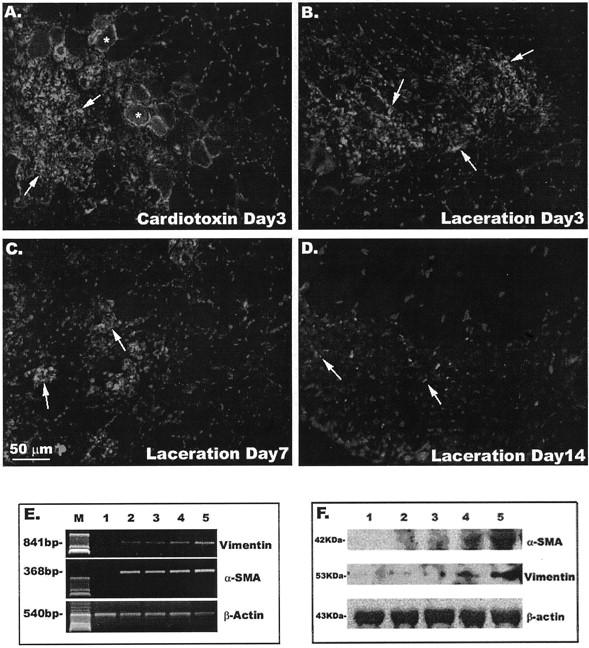
Implication of TGF-β1 in the development of scar tissue within the injured skeletal muscle. There is expression of TGF-β1 in injured skeletal muscle (asterisks indicate myofibers and arrows indicate interstitial tissue) at 3 days after injury (cardiotoxin, A; laceration, B). This TGF-β1 expression decreased in the lacerated injured skeletal muscle at 7 days (C) and 14 days (D). RT-PCR (E) and Western blot (F) indicated an induction of α-SMA and vimentin expression in MC13 cells stimulated with TGF-β1 in a dose-dependent manner. For both RT-PCR and Western blot, lane 1 represents nonstimulated MC13 cells and lanes 2 to 5 represent MC13 cells stimulated with 0.01, 0.1, 1, and 5 ng/ml of TGF-β1, respectively. In E, the M indicates marker DNA ladder control.
Discussion
It is widely accepted that myogenic precursor cells released at the site of a muscle injury will differentiate into satellite cells, which progressively differentiate into myoblasts and fuse into myotubes and myofibers. 1-4 However, some of these cells can differentiate not only into myogenic cells, but also into a variety of lineages, such as osteogenic and hematopoietic cells. 6-8,10 This multidifferentiation capability has been noted in stem cells derived from different adult tissues (eg, bone marrow, liver, and nerve). 10,23-27 Recently, cells derived from bone marrow and brain were found capable of differentiating into myogenic cells under appropriate stimuli. 10,23,24 It seems likely that appropriate stimulation is required for the differentiation of stem cells into different tissues. 28-31 The factor(s) that triggers the differentiation of stem cells into various lineages remains unknown, but growth factors and cytokines are likely molecular candidates. A change in the biochemical environment resulting from muscle injury also could prove to be an important trigger that influences the differentiation events. 29,31
In muscle injuries the release of growth factors at the injured site is an important step in the initiation of the healing process. These growth factors can stimulate the growth and differentiation of various muscle-derived cells. 1,16,21,22,32 Growth factors are known to promote myoblast proliferation and differentiation, which can eventually lead to muscle regeneration and healing after injury. 11,12,15,16 However, some growth factors (eg, TGF-β1 and platelet-derived growth factor) are highly expressed at the injured site and are likely to be involved in the development of muscle fibrosis. 32-34 TGF-β1 has been considered a key factor during hepatic stellate cell differentiation into myofibroblast. 35,36 It also has been postulated that platelet-derived growth factor is closely associated with chronic liver fibrosis. 37 These growth factors could potentially trigger the differentiation of the MC13 cells toward a myofibroblastic lineage after injury. The gradual differentiation process after an injury may be influenced by relative levels of expression of various growth factors present in the injured skeletal muscle, such as TGF-β1 and platelet-derived growth factor.
In our current experiment, we have observed that muscle-derived stem cells likely begin their differentiation toward the myofibroblastic lineage as early as 1 to 2 weeks after laceration. At this time point, the majority of the LacZ-positive cells derived from the injured skeletal muscle was still found in the myogenic cell (pp4/pp5) population rather than in the fibroblastic cell (pp1/pp2) population. This suggests that only a few of the MC13 cells had differentiated toward a myofibroblastic lineage at this time point after injury whereas the majority of the injected cells had differentiated into the myogenic lineage, leading to a large number of regenerating myofibers. At 3 weeks after laceration, we found that similar numbers of LacZ-positive cells (MC13 cells) could be isolated in the fibroblastic (pp1/pp2) and myogenic (pp4/pp5) populations. These results suggest that approximately half of the LacZ-positive cells derived from MC13 cells had differentiated into myofibroblastic cells at 3 weeks after injury. At 5 weeks after injury the vast majority of the isolated LacZ-positive MC13 cells was found in the fibroblastic (pp1/pp2) population, suggesting that most of the injected cells had differentiated into the myofibroblastic lineage by this time. These results validate our histological studies in vivo, in which a large number of regenerating myofibers present in the MC13-injected muscles at 2 weeks after laceration were replaced by scar tissue at 5 weeks after injury. These results also suggest that the injected MC13 cells could differentiate into fibrotic cells in the injured skeletal muscle.
Because TGF-β1 has been considered a key factor in the development of fibrosis in various tissues, 32-36 we have tested the role of TGF-β1 in the development of scar tissue after muscle injuries. We have observed that TGF-β1 is highly expressed in the injured skeletal muscle (cardiotoxin and laceration) at 3 days after injury. The expression of TGF-β1 is transient; a decline of expression is observed at 14 days after injury. In addition, our in vitro results suggest that TGF-β1 is capable of inducing the expression of myofibroblastic markers, such as α-SMA and vimentin, in MC13 cells in a dose-dependent manner. Although we cannot exclude the participation of other growth factors in the development of muscle fibrosis, it seems possible based on these results that TGF-β1, the key factor involved in the fibrosis of various tissues, also plays a role in the development of scar tissue formation after muscle injury. Furthermore, TGF-β1 probably is involved in the differentiation of MC13 cells toward myofibroblast lineage, which likely also contributes to the development of fibrosis.
The regenerated myofibers that appeared shortly after muscle injury subsequently disappeared at 5 weeks after injury. This is an unexpected finding and difficult to reconcile with normal persistence of regenerated muscles. The loss of these myofibers (and replacement by fibrosis) is hard to explain but might be because of a lack of reinnervation of the new MC13 myofibers, as immune rejection seems unlikely in the SCID host mouse. Furthermore the fate of these regenerated myofibers that disappeared is unclear. Recently, it has been shown that multinucleated myotubes could dedifferentiate in vitro and give rise to mononucleated cells that can differentiate into other lineages. 38-40 Based on these new results, it is possible that after injury myofibers differentiate into other cell lineages, including myofibroblastic lineage, although this has not yet been demonstrated for mammalian myotubes in vivo. The disappearance of the β-galactosidase-expressing myofibers and the presence of numerous β-galactosidase-expressing mononucleated cells co-expressing α-SMA and vimentin at 5 weeks after injury might be because of this phenomenon in these skeletal muscles after injury. Clearly, validation of this hypothesis will require additional experimentation. Alternatively, the myofibroblasts may have arisen independently from proliferation of persisting mononucleated (myogenic) MC13 stem cells in the environment of the degenerating myofibers.
Fibrosis can occur in a variety of tissues, including liver, lung, kidney, skin, nerve, and muscle. It was thought that the fibrotic event that occurred shortly after tissue injury was a preventive response from the host, and that this process could be beneficial to tissue repair. 13,41,42 However, additional stimulation induced by the release of local stimuli at the injured site can further promote the fibrotic response. 41,42 It is believed that tissue injury frequently stimulates the cells of the extracellular matrix, promoting its activation and growth, as well as the overproduction of local collagens. 16,21,22,43,44 It has been reported that bone marrow cells and a population of circulating cells with fibroblast properties can infiltrate the site of the injured tissue and enhance scar tissue formation. 43,44 Other studies in tissue culture suggest that various cell types, including kidney epithelial cells and liver satellite cells, can transdifferentiate into myofibroblasts. 36,45,46 Thus the differentiation of various cell types toward a fibroblastic lineage also may play an integral role in the development of tissue fibrosis.
We report here that myogenic cells, including muscle-derived stem cells that are present at an injured site, can differentiate into fibrotic cells upon stimulation because of muscle injury. However, the loss of numerous regenerating myofibers after muscle injury suggests that not only early myogenic precursors, but also differentiated muscle cells might be triggered to differentiate into a fibrotic lineage. Therefore, it is possible that the fibrotic process includes both the overproduction of extracellular matrix after injury and the stimulation of functional cells, such as myogenic cells that differentiate into fibrotic cells. It would be interesting to further characterize the mechanism of this differentiation and the cytokines involved in that process. We have provided evidence in this study that TGF-β1, whose involvement in the fibrosis of other tissues has already been established, 32-36 is potentially involved in muscle fibrosis as well. These results could help to explain the process of scar tissue formation, which is often associated with diseases such as Duchenne muscular dystrophy. 47 These observations also may help to shed light on the process by which fibrosis develops after muscle injury, and eventually to provide insight into the design of biological approaches to block muscle fibrosis.
Acknowledgments
We thank William Foster, Marcelle Pellerin, James Cummins, Thomas Payne, and Ryan Pruchnic for their assistance in this research; Dr. Zhuqing Qu, Dr. Baohong Cao, Dr. Makoto Ikezawa, and Ms. Bridget Deasy for their critical comments about the manuscript; and Lauren Rudick and Ryan Sauder for their assistance in preparing the manuscript.
Footnotes
Address reprint requests to Johnny Huard, Ph.D., Growth and Development Laboratory, Children’s Hospital of Pittsburgh, 4151 Rangos Research Center, 3705 Fifth Ave., Pittsburgh, PA 15213-2583. E-mail: jhuard+@pitt.edu.
Supported by project grants from National Institutes of Health (1R01 AR 47973-01 to J. H.) and the William F. and Jean W. Donaldson Chair at Children’s Hospital of Pittsburgh.
References
- 1.Schultz E, Jaryszak DL, Valliere CR: Response of satellite cells to focal skeletal muscle injury. Muscle Nerve 1985, 8:217-222 [DOI] [PubMed] [Google Scholar]
- 2.Bischoff R: Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol 1986, 115:129-139 [DOI] [PubMed] [Google Scholar]
- 3.Stockdale FE: The myogenic lineage: evidence for multiple cellular precursors during avian limb development. Proc Soc Exp Biol Med 1990, 194:71-75 [DOI] [PubMed] [Google Scholar]
- 4.Molnar G, Ho ML, Schroedl NA: Evidence for multiple satellite cell populations and a non-myogenic cell type that is regulated differently in regenerating and growing skeletal muscle. Tissue Cell 1996, 28:547-556 [DOI] [PubMed] [Google Scholar]
- 5.Baroffio A, Hamann M, Bernheim L, Bochaton-Piallat ML, Gabbiani G, Bader CR: Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation 1996, 60:47-57 [DOI] [PubMed] [Google Scholar]
- 6.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA: Pax7 is required for the specification of myogenic satellite cells. Cell 2000, 102:777-786 [DOI] [PubMed] [Google Scholar]
- 7.Beauchamp JR, Morgan JE, Pagel CN, Partridge TA: Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol 1999, 144:1113-1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, Gates C, Robbins P, Wernig A, Huard J: Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol 2000, 150:1085-1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson K, Mi AT, Goodell MA: Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA 1999, 96:14482-14484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC: Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999, 23:390-394 [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Cummins J, Huard J: Muscle injury and repair. Curr Opin Orthop 2001, 12:409-415 [Google Scholar]
- 12.Menetrey J, Kasemkijwattana C, Fu FH, Moreland MS, Huard J: Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am J Sports Med 1999, 27:222-229 [DOI] [PubMed] [Google Scholar]
- 13.Simeon A, Monier F, Emonard H, Wegrowski Y, Bellon G, Monboisse JC, Gillery P, Hornebeck W, Maquart FX: Fibroblast-cytokine-extracellular matrix interactions in wound repair. Curr Top Pathol 1999, 93:95-101 [DOI] [PubMed] [Google Scholar]
- 14.Mutsaers SE, Bishop JE, McGrouther G, Laurent GJ: Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol 1997, 29:5-17 [DOI] [PubMed] [Google Scholar]
- 15.Menetrey J, Kasemkijwattana C, Day CS, Bosch P, Vogt M, Fu FH, Moreland MS, Huard J: Growth factors improve muscle healing in vivo. J Bone Joint Surg 2000, 82:131-137 [DOI] [PubMed] [Google Scholar]
- 16.Fukushima K, Badlani N, Usas A, Riano F, Fu FH, Huard J: The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med 2001, 29:394-402 [DOI] [PubMed] [Google Scholar]
- 17.Kimura S, Ikezawa M, Pruchnic R, Balkir L, Qu Z, Lowenstein J, Takeda S, Gates C, Cao B, Miike T, Huard J: Persistent gene transfer to skeletal muscle mediated by stably transfected early myogenic progenitor cells. Basic Appl Myol 2000, 10:237-248 [Google Scholar]
- 18.Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J: Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol 1998, 142:1257-1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rando TA, Blau HM: Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 1994, 125:1275-1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richler C, Yaffe D: The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol 1970, 23:1-22 [DOI] [PubMed] [Google Scholar]
- 21.Sappino AP, Schurch W, Gabbiani WG: Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest 1990, 63:144-161 [PubMed] [Google Scholar]
- 22.Badid C, Mounier N, Costa AMA, Desmouliere A: Role of myofibroblasts during normal tissue repair and excessive scarring: interest of their assessment in nephropathies. Histol Histopathol 2000, 15:269-280 [DOI] [PubMed] [Google Scholar]
- 23.Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, Coletta M, Mora M, De Angelis MG, Fiocco R, Cossu G, Vescovi AL: Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci 2000, 3:986-991 [DOI] [PubMed] [Google Scholar]
- 24.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F: Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998, 279:1528-1530 [DOI] [PubMed] [Google Scholar]
- 25.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP: Bone marrow as a potential source of hepatic oval cells. Science 1999, 284:1168-1170 [DOI] [PubMed] [Google Scholar]
- 26.Eglitis MA, Mezey E: Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci USA 1997, 94:4080-4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogel G: Harnessing the power of stem cells. Science 1999, 283:1432-1434 [DOI] [PubMed] [Google Scholar]
- 28.Cossu G, Mavilio F: Myogenic stem cells for the therapy of primary myopathies: wishful thinking or therapeutic perspective? J Clin Invest 2000, 105:1669-1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blau HM, Brazelton TR, Weimann JM: The evolving concept of a stem cell: entity or function? Cell 2001, 105:829-841 [DOI] [PubMed] [Google Scholar]
- 30.Slack JM, Tosh D: Transdifferentiation and metaplasia—switching cell types. Curr Opin Genet Dev 2001, 1:581-586 [DOI] [PubMed] [Google Scholar]
- 31.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J: Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol 2002, 157:851-864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Border WA, Noble NA: Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994, 10:1286-1292 [DOI] [PubMed] [Google Scholar]
- 33.Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R: Expression of transforming growth factor-β1 in dystrophic patient muscles correlates with fibrosis. J Clin Invest 1995, 96:1137-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cofalonieri P, Bernasconi P, Cornelio F, Mantegazza R: Transforming growth factor-β1 in polymyositis and dermatomyositis correlates with fibrosis not with mononuclear cell infiltrate. J Neuropathol Exp Neurol 1997, 56:479-484 [DOI] [PubMed] [Google Scholar]
- 35.Bachem MG, Meyer D, Schafer W, Riess U, Melchior R, Sell KM, Gressner AM: The response of rat liver perisinusoidal lipocytes to polypeptide growth regulator changes with their transdifferentiation into myofibroblast-like cells in culture. J Hepatol 1993, 18:40-52 [DOI] [PubMed] [Google Scholar]
- 36.Dooley S, Delvoux B, Lahme B, Mangasser-Stephan K, Gressner AM: Modulation of transforming growth factor beta response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts. Hepatology 2000, 31:1094-1106 [DOI] [PubMed] [Google Scholar]
- 37.Ikura Y, Morimoto H, Ogami M, Jomura H, Ikeoka N, Sakurai M: Expression of platelet-derived growth factor and its receptor in livers of patients with chronic liver disease. J Gastroenterol 1997, 32:496-501 [DOI] [PubMed] [Google Scholar]
- 38.Brockes JP: Amphibian limb regeneration: rebuilding a complex structure. Science 1997, 276:81-87 [DOI] [PubMed] [Google Scholar]
- 39.Odelberg SJ, Kollhoff A, Keating MT: Dedifferentiation of mammalian myotubes induced by msx1. Cell 2000, 103:1099-1109 [DOI] [PubMed] [Google Scholar]
- 40.McGann CJ, Odelberg SJ, Keating MT: Mammalian myotube dedifferentiation induced by newt regeneration extract. Proc Natl Acad Sci USA 2001, 98:13699-13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bissell DM: Hepatic fibrosis as wound repair: a progress report. J Gastroenterol 1998, 33:295-302 [DOI] [PubMed] [Google Scholar]
- 42.Sottile J, Hocking DC, Swiatek PJ: Fibronectin matrix assembly enhances adhesion-dependent cell growth. J Cell Sci 1998, 111:2933-2943 [DOI] [PubMed] [Google Scholar]
- 43.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A: Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994, 1:71-81 [PMC free article] [PubMed] [Google Scholar]
- 44.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN: Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001, 166:7556-7562 [DOI] [PubMed] [Google Scholar]
- 45.Strutz F, Muller GA, Neilson EG: Transdifferentiation: a new angle on renal fibrosis. Exp Nephrol 1996, 4:267-270 [PubMed] [Google Scholar]
- 46.Gressner AM: Transdifferentiation of hepatic stellate cells (Ito cells) to myofibroblasts: a key event in hepatic fibrogenesis. Kidney Int 1996, 54:S39-S45 [PubMed] [Google Scholar]
- 47.Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R: Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis: pathogenetic role of a fibrogenic cytokine. J Clin Invest 1995, 96:1137-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]