Abstract
Several groups of proteolytic enzymes are able to degrade components of the extracellular matrix. During atherosclerosis, matrix remodeling is believed to influence the migration and proliferation of cells within the plaque. In the present study, gene expression of several proteases and their inhibitors was analyzed during the development of atherosclerosis in apolipoprotein E-deficient (ApoE−/−) mice. Quantitative real-time polymerase chain reaction was used to study gene expression of proteases after 10 and 20 weeks in ApoE−/− and C57BL/6 mice and in atherosclerotic lesions and nonaffected regions of the same ApoE−/− mouse. Some of the differentially expressed proteolytic enzymes were studied by immunohistochemistry. The matrix metalloproteinase (MMP)-9 and its inhibitor TIMP-1 were differentially expressed and the expression increased with time. Urokinase-type plasminogen activator showed no major expression. In contrast, cathepsins B, D, L, and S all showed strong and increased expression in ApoE−/− mice compared to C57BL/6 mice whereas the expression of their inhibitor, cystatin C, did not differ between the two mouse strains. The expression of cathepsins was mainly localized to the lesions and not to nonaffected regions of the aorta of ApoE−/− mice. Furthermore, cathepsin expression was similar to the expression of the macrophage marker macrosialin (CD68) although expression of cathepsins B, D, and L could be demonstrated in healthy C57BL/6 mice and in nonaffected vessel segments of atherosclerotic ApoE−/− mice. Cathepsin S mRNA expression was restricted to lesions of ApoE−/− mice. Furthermore, cathepsin S was the only cathepsin that was expressed in the media and absent in lipid-rich regions. All cathepsins studied showed intimal expression, the degree and localization of which differed between individual cathepsins. In conclusion, increased expression of several cathepsins in atherosclerotic lesions suggests that these proteases may participate in the remodeling of extracellular matrix associated with the atherosclerotic process.
Atherosclerosis is characterized by arterial intimal enlargement with subsequent lipid deposition leading to the formation of a necrotic core. The infiltration of smooth muscle cells (SMCs), T cells, and macrophages into the intima of the inflamed artery contributes in different ways to the formation of complicated atherosclerotic lesions. 1 Macrophages and SMCs produce a large number of proteases, such as serine, cysteine, aspartic, and matrix metalloproteases (MMPs). 2,3 Their presence in the arterial wall might lead to enhanced infiltration of cells and, at a later stage, to plaque rupture as a result of increased matrix degradation. At least three types of proteases have been implicated in the atherosclerotic process although their exact function has not yet been fully understood. The expression of MMPs and serine proteases has been analyzed extensively, 2,4-6 whereas the cysteine and aspartic protease groups, comprising several cathepsins, have been studied to a lesser extent. Lysosomal proteases are considered to be involved in the physiological breakdown of proteins as well as in pathological degradative and invasive processes, 7-9 and some cathepsins have been shown to express increased activity in human atherosclerotic aortas. 10 Most of the cysteine and aspartic proteases have a broad substrate specificity and are found in a wide range of cell types. Even though they have optimal function at acidic pH, studies of cathepsin S have shown that this enzyme is stable and possesses potent proteolytic activity also at neutral pH. 11,12 Furthermore, monocyte-derived macrophages are able to form cathepsin-containing acidic microenvironments outside the cell that would enable these cells to use acidic proteases also outside the lysosome. 13 The ability of macrophages and other cells to mobilize elastolytic cathepsins at or near the cell surface may lead to accelerated extracellular matrix degradation at sites of inflammation. 14 Besides extracellular matrix degradation cathepsins may also exert other effects in atherosclerosis. For instance, cathepsins B and D could be of importance in low-density lipoprotein degradation. 15 In addition, SMCs and macrophages produce cathepsins that have the ability to degrade native but not modified low-density lipoprotein, which could lead to accumulation of modified low-density lipoprotein in the vessel wall, promoting foam cell formation and accelerated atherosclerosis. 16,17
Apolipoprotein E-deficient (ApoE−/−) mice develop severe hypercholesterolemia and accelerated atherosclerosis. Furthermore, disease progression in ApoE−/− mice is similar to human atherosclerosis. 18,19 So far, expression of MMPs and serine proteases has been demonstrated in the arterial wall of ApoE−/− mice whereas the expression profile of cysteine and aspartic proteases has not been studied. 20 Evidence has been obtained for expression of cathepsins S, K, and D in human atherosclerotic lesions, but the expression pattern of the other members of the cathepsin family remains unknown. 8,21 Based on previous array data, 22 we analyzed here the gene expression of several proteases and their inhibitors during the development of atherosclerosis in ApoE−/− mice using quantitative real-time polymerase chain reaction (PCR) and immunohistochemistry.
Materials and Methods
Animals and Tissue Preparation
C57BL/6 and ApoE−/− mice 6 to 8 weeks of age were obtained from Jackson Laboratories and Bomholtgaard Breeding and Research Center, Denmark, respectively. C57BL/6 mice were maintained on chow diet, containing 4.5% fat by weight (0.02% cholesterol), and ApoE−/− mice were fed a Western-type diet, containing 21% fat by weight (0.15% cholesterol). Mice from each group were sacrificed at 10 and 20 weeks of diet in groups of five mice by exsanguination under carbon dioxide anesthesia on consecutive days, in total 15 from each group on both occasions. After perfusion with ice-cold phosphate-buffered saline (PBS), the heart and the total aorta were dissected out and placed in ice-cold PBS. After further mechanical rinsing under a dissection microscope, the aorta was cut out and either mounted in a block (aortic root) and snap-frozen in n-heptane chilled with liquid nitrogen or used for RNA preparation. In a second experimental set-up, six 18-week-old ApoE−/− mice on chow diet were used. The atherosclerotic lesions and nonaffected aortic regions were isolated within 1 hour under a dissection microscope and frozen immediately in liquid nitrogen for mRNA extraction. The studies were approved by the Animal Ethics Committee of the Karolinska Institute.
Real-Time Polymerase Chain Reaction
The frozen samples were homogenized in a dismembranator (B. Braun Melsungen AG, Germany). Lysing buffer (Dynal, New York) was added to the homogenate, and mRNA isolated on oligo-dT-conjugated magnetic beads (Dynabeads; Dynal AS, Oslo, Norway). The mRNA quantity was estimated using DNA Dip Stick (Invitrogen, Carlsbad, CA). Twenty ng of mRNA was reverse-transcribed using Superscript II according to the manufacturer’s manual (Life Technologies, Inc., Rockville, MD). cDNA (1.5 μl) was amplified by real-time PCR with 1× TaqMan Buffer, 5 mmol/L MgCl2, 200 μmol/L of each dNTP, 200 μmol/L of each primer, 1.25 pmol/L of probe, 0.25 U Amp-Erase uracil N-glycosylase, 1.25 U AmpliTaq Gold (PE Biosystems, Foster City, CA). Primers and probes are given in Table 1 ▶ . For normalization of RNA loading, control samples were run using β-actin. Each sample was analyzed in duplicate using ABI Prism 7700 Sequence Detector (PE Biosystems). The PCR amplification was estimated by comparison with a standard curve. The reactions were performed in MicroAmp optical 96-well reaction plates (PE Biosystems).
Table 1.
Primers and Probes Used for Real-Time PCR
| MMP-9 | |
| Forward primer: | 5-CCAGCTGGCAGAGGCATAC |
| Reverse primer: | 5-GCTTCTCTCCCATCATCTGGG |
| Probe: | 5-CCGCTATGGTTACACCCGGGCC |
| uPA | |
| Forward primer: | 5-CGATTCTGGAGGACCGCTTA |
| Reverse primer: | 5-CCAGCTCACAATCCCACTCA |
| Probe: | 5-CTGTAACATCGAAGGCCGCCCAACT |
| TIMP-1 | |
| Forward primer: | 5-TCATGGAAAGCCTCTGTGGAT |
| Reverse primer: | 5-CGGCCCGTGATGAGAAACT |
| Probe: | 5-CCACAAGTCCCAGAACCGCAGTGAA |
| TIMP-2 | |
| Forward primer: | 5-GCGTTTTGCAATGCAGACG |
| Reverse primer: | 5-ATTCCCGGAATCCACCTCC |
| Probe: | 5-TGATCAGAGCCAAAGCAGTGAGCGA |
| Cathepsin B | |
| Forward primer: | 5-GCCCGACCATTGGACAGAT |
| Reverse primer: | 5-GCCCCAAATGCCCAACA |
| Probe: | 5-AGAGACCAGGGCTCCTGCGGCT |
| Cathepsin D | |
| Forward primer: | 5-GTGCACATGGACCAGTTGGA |
| Reverse primer: | 5-CAATAGCCTCACAGCCTCCC |
| Probe: | 5-TGGGCAATGAGCTGACCCTGTGC |
| Cathepsin L | |
| Forward primer: | 5-GACCGGGACAACCACTGTG |
| Reverse primer: | 5-CCCATCAATTCACGACAGGAT |
| Probe: | 5-TTGCCACCGCGGCCAGC |
| Cathepsin S | |
| Forward primer: | 5-AAGCGGTGTCTATGACGACCC |
| Reverse primer: | 5-GAGTCCCATAGCCAACCACAA |
| Probe: | 5-TCCTGTACGGGCAATGTGAATCATGGT |
| Cystatin C | |
| Forward primer: | 5-CATCTGATGAGGAAGGCACTCTG |
| Reverse primer: | 5-TGTCAGGGAGTGTGTGCCTTT |
| Probe: | 5-TCCTTCCAGATCTACAGCGTGCCCTG |
| Macrosialin (CD68) | |
| Forward primer: | 5-CAAGGTCCAGGGAGGTTGTG |
| Reverse primer: | 5-CCAAAGGTAAGCTGTCCATAAGGA |
| Probe: | 5-CGGTACCCATCCCCACCTGTCTCTCTC |
| β-actin | |
| Forward primer: | 5-AGAGGGAAATCGTGCGTGAC |
| Reverse primer: | 5-CAATAGTGATGACCTGGCCGT |
| Probe: | 5-CACTGCCGCATCCTCTTCCTCCC |
Immunohistochemistry
Serial cryostat sections after fixing in acetone for 10 minutes were washed and then incubated with PBS/5% blocking serum (horse or goat depending on the secondary antibody used) for 30 minutes. Excess blocking serum was blotted from the coverslip, and the sections were then exposed to primary antibodies against α-actin, Mac-1, CD3, and cathepsins B, D, L, and S overnight at 4°C in a humidifying chamber. Controls included use of nonimmune sera instead of primary antibody as well as omission of the primary antibody. After washing three times in PBS for 5 minutes each time, the sections were incubated with biotinylated horse anti-goat or mouse anti-hamster IgG for 30 minutes, washed three times (5 minutes each time) in PBS, and stained with an avidin DH/biotinylated peroxidase complex for 30 minutes (ABC kit). The reactions were visualized using diaminobenzidine tetrahydrochloride for 3 minutes. Sections were counterstained with Harris hematoxylin solution.
Polyclonal antibodies against cathepsins B, D, L, and S were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyclonal antibodies against CD3 and Mac-1 were supplied by Pharmingen (San Diego, CA) and horseradish peroxidase-labeled smooth muscle α-actin IgG antibodies were supplied by Sigma. Biotinylated horse anti-goat IgG (H+L) secondary antibody came from Vector Laboratories (Burlingame, CA). Avidin DH/biotinylated peroxidase complex (Vectastain ABC-kit) was obtained from Vector Laboratories as well as the diaminobenzidine tetrahydrochloride.
Data Analysis
Data are shown as mean ± SD. Differences in gene expression between atherosclerotic and nonaffected arterial segments were compared using a paired Student’s t-test. A value of P ≤ 0.05 was considered significant.
Results
Aortic Gene Expression during the Progression of Atherosclerosis in ApoE−/− Mice
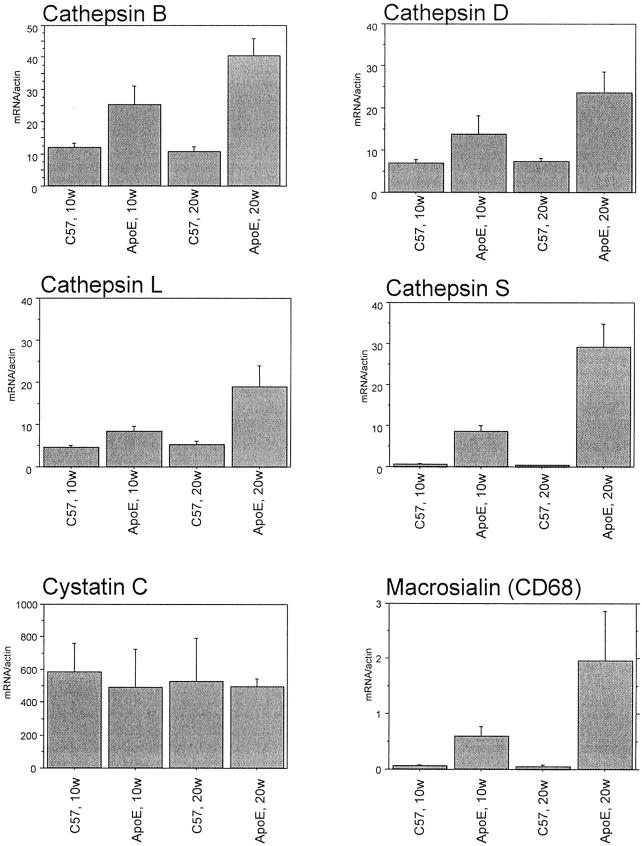
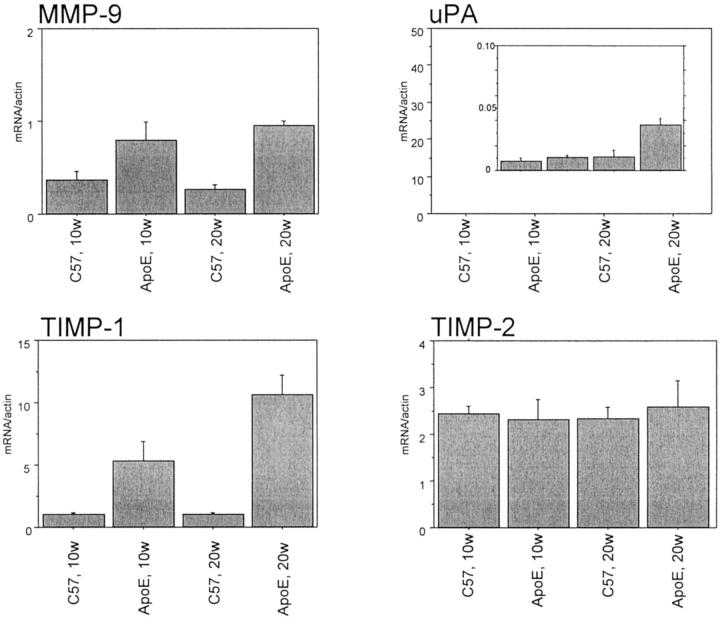
To enable monitoring of potential changes in protease expression with time and severity of atherosclerosis, mRNA was obtained from C57BL/6 mice and atherosclerotic ApoE−/− mice at different time points. The model has been described in detail elsewhere and differential gene expression has been demonstrated using a gene expression array. 22 In the present study, gene expression of proteases and their inhibitors was further studied in the mRNA prepared from pooled aortas (n = 3 × 5) using quantitative real-time PCR (Figures 1 and 2) ▶ ▶ . Cathepsins B, D, L, and S were strongly expressed with a higher expression in ApoE−/− mice after 20 weeks (Figure 1) ▶ . The expression of cathepsin S was very low in C57BL/6 whereas the other cathepsins showed a substantial expression also in the C57BL/6 mice. In contrast, the expression of the cathepsin inhibitor cystatin C did not differ between C57BL/6 and ApoE−/− mice (Figure 1) ▶ . MMP-9 had a low but increased gene expression in ApoE−/− mice (Figure 2) ▶ . The MMP-inhibitor TIMP-1 expression was higher in ApoE−/− mice than in C57BL/6 at 10 and 20 weeks of diet whereas TIMP-2 expression did not differ (Figure 2) ▶ . Urokinase-type plasminogen activator had a very low level of expression, however it was higher in ApoE−/− than C57BL/6 mice after 20 weeks (Figure 2) ▶ . Macrophage accumulation was restricted to ApoE−/− mice as demonstrated by the quantification of macrosialin (Figure 1) ▶ .
Figure 1.
Quantitative real-time PCR analysis of cathepsins, cystatin C, and macrosialin mRNA of aortas of ApoE−/− and C57BL/6 mice after 10 and 20 weeks. Mean ± SD of ratio between macrosialin and β-actin of three pooled samples each containing five mice for each time point are shown.
Figure 2.
Quantitative real-time PCR analysis of MMP-9, Urokinase-type plasminogen activator, and TIMP mRNA of aortas of ApoE−/− and C57BL/6 mice after 10 and 20 weeks. Mean ± SD of ratio between investigated mRNA and β-actin of three pooled samples each containing five mice for each time point are shown.
Gene Expression in Atherosclerotic and Nonatherosclerotic Areas of ApoE−/− Mice
In an additional set of experiments, atherosclerotic as well as nonatherosclerotic vessel segments from 18-week-old ApoE−/− mice on normal chow diet were analyzed and compared pairwise. As shown in Figure 3 ▶ , macrosialin expression was restricted to the lesions, indicating that the macroscopically normal nonatherosclerotic tissue was indeed unaffected. Furthermore, cathepsin S expression was restricted to the lesions whereas cathepsins B, D, and L were expressed in both lesions and nonatherosclerotic areas (Figure 3) ▶ . However, the expressions of cathepsin B and cathepsin D were found to be significantly increased in atherosclerotic lesions in a paired comparison between lesions and unaffected areas. The expression of the cathepsin inhibitor cystatin C did not differ in the two areas. There was no significant difference in MMP-9 expression between the healthy and atherosclerotic areas. However, in some of the samples, the expression of MMP-9 was too low to be measured accurately. The expression of TIMP-1 was increased in the lesion area.
Figure 3.
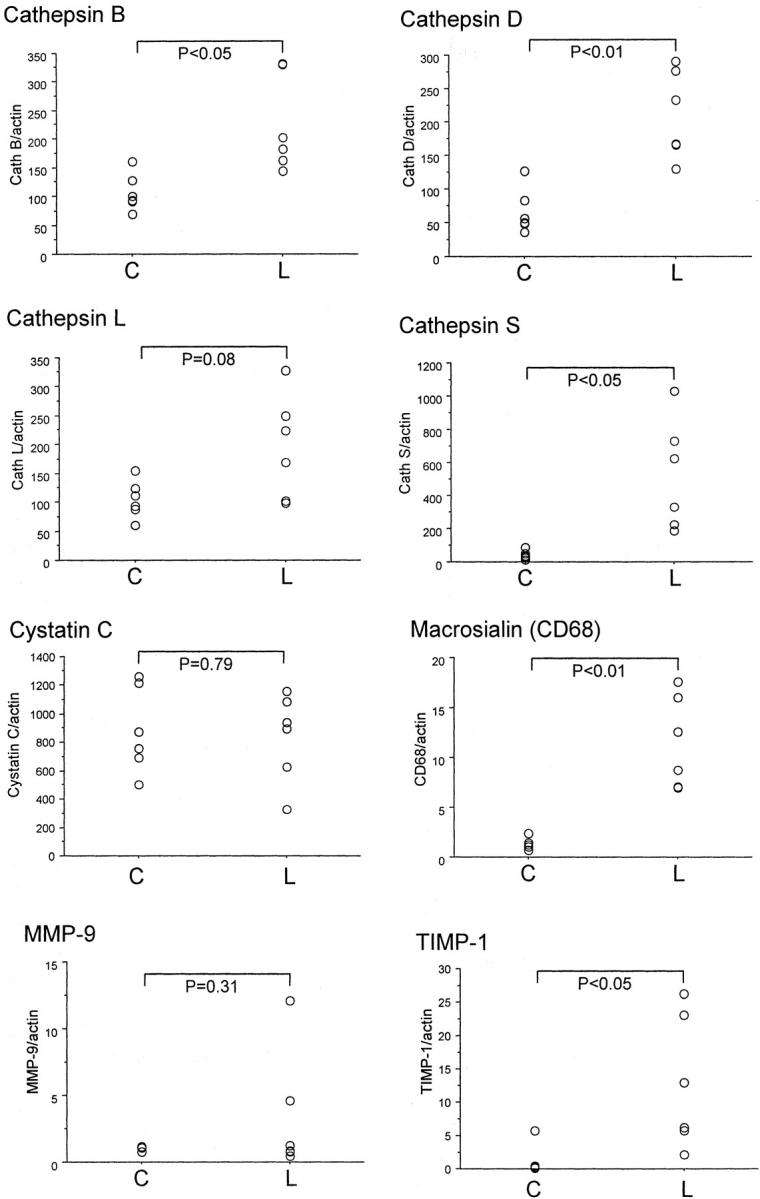
Quantitative real-time PCR analysis of protease mRNA of atherosclerotic lesions and nonaffected regions of ApoE−/− mice. Six mice (18 weeks of age) were sacrificed and the atherosclerotic lesions were dissected out, mRNA isolated, and analyzed by quantitative real-time PCR. Visible nonaffected aortic regions from the same mouse served as control. Mean ± SD of ratio between investigated mRNA and β-actin are shown. Differences in gene expression were tested using a paired t-test. C and L denote nonaffected aorta and lesion, respectively.
Localization of Protease Expression in ApoE−/− Mice Using Immunohistochemistry
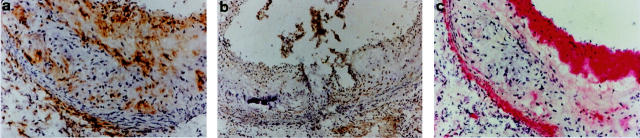
Microscopical examination of ApoE−/− mice sacrificed at 20 weeks of age showed severe atherosclerosis consisting of intimal enlargement, fibrous plaques with necrotic cores, and lipid deposits. In contrast, C57BL/6 control mice showed no signs of atherosclerosis (Figures 4 and 5) ▶ ▶ . Macrophages that stained positively for Mac-1 were mostly located in the fibrous cap and in the lipid core whereas SMCs, which stained positively for α-actin, were found predominantly in the fibrous cap and the media (Figure 6) ▶ . CD3-positive T cells were found in low numbers spread across the sections (Figure 6) ▶ . Controls without primary antibodies were all negative.
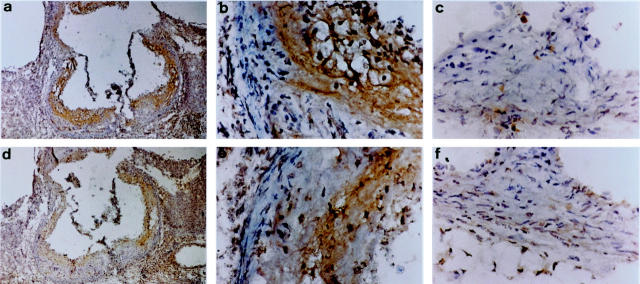
Figure 4.
Immunohistochemical analysis of aortic roots from ApoE−/− and C57BL/6 mice. Staining for cathepsin B in ApoE−/− mice (a and b) and in C57BL/6 mice (c). Staining for cathepsin D in ApoE−/− mice (d and e) and in C57BL/6 mice (f).
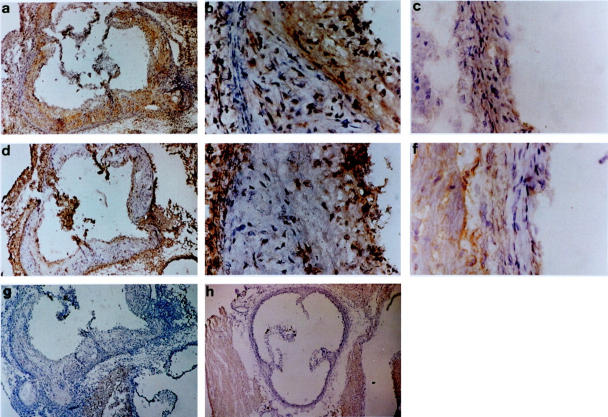
Figure 5.
Immunohistochemical analysis of aortic roots from ApoE−/− and C57BL/6 mice. Staining for cathepsin L in ApoE−/− mice (a and b) and in C57BL/6 mice (c). Staining for cathepsin S in ApoE−/− mice (d and e) and in C57BL/6 mice (f). Negative control for sections taken from aortic roots of ApoE−/− mice (g) and C57BL/6 mice (h).
Figure 6.
Immunohistochemical analysis of cell-specific markers in aortic roots from ApoE−/− mice. Sections were stained for Mac-1 (CD11b/CD18) (a), CD3 (b), and smooth muscle α-actin (c).
A selection of proteases was studied by immunohistochemistry. We focused on cathepsins B, D, L, and S because these proteases showed the greatest differences in gene expression between ApoE−/− and C57BL/6 mice and because there is very limited information on cathepsin expression in the developing atheroma. When staining for cathepsins we observed different patterns of expression for the different proteases. All cathepsins (B, D, L, and S) showed strong protein expression in the ApoE−/− mice sacrificed after 20 weeks (Figures 4 and 5) ▶ ▶ whereas the aortic roots of the C57BL/6 mice showed significantly lower staining for these cathepsins (Figures 4 and 5) ▶ ▶ . Cathepsin S was the only cathepsin to show positive staining in the media. All cathepsins, including cathepsin S, were detected in the intima and the fibrous cap although the exact localization and degree of staining differed between individual cathepsins. All cathepsins, except cathepsin S, were present in lipid-rich areas. Cathepsin L had the most widespread localization in the lipid-rich and nonlipid regions of the intima (Figure 5) ▶ . Cathepsin D had a quite similar expression pattern, with more intracellular intima expression than cathepsin B (Figure 4) ▶ . In summary, cathepsin S was the only cysteine protease that was expressed in the media and absent in lipid-rich regions. All cathepsins studied showed intimal expression, the degree and localization of which differed between individual cathepsins.
Discussion
The extracellular matrix components collagen and elastin are two major building blocks that confer important functional characteristics to the arterial wall. Increased degradation of extracellular matrix molecules may lead to increased infiltration of SMCs and an accelerated atherosclerotic process. There is accumulating evidence that MMPs and serine proteases are important mediators of extracellular matrix degradation. Cysteine and aspartic proteases comprise another set of molecules that has recently been implicated in the process of vascular remodeling. Because of their elastolytic, collagenolytic, and gelatinolytic activities, these proteases can also function as potent degraders of the extracellular matrix, would they be expressed in atherosclerotic lesions.
The present report, based on quantitative real-time PCR and immunohistochemistry analyses, demonstrates that atherosclerotic aortas from ApoE−/− mice express increased mRNA and protein levels of several cathepsins and that the expression is mainly localized to the atherosclerotic lesion. An inflammatory infiltration of macrophages, SMCs, and T cells was seen, consistent with previous reports. 19 Similar to earlier findings in atherosclerotic arteries we could also detect increased mRNA levels of several adhesion molecules. 22
All cathepsins included in the mRNA analysis that were found to be up-regulated in the lesions of ApoE−/− mice were further investigated by immunohistochemistry. Although cathepsin B showed the most abundant staining pericellularly in the lipid core, cathepsin S was not expressed at this location. Instead, cathepsin S was found intracellularly in the media and in the fibrous cap. Thus, cathepsin S had a unique expression pattern. Earlier studies on plaques from human coronary and carotid arteries have demonstrated cathepsin S expression in the expanding intima and in the subjacent medial SMC in less fibrous and nonocclusive lesions. 8 In contrast, medial SMC in more advanced plaques expressed less cathepsin S than they did in less fibrous lesions. Thus, cell-specific expression will most likely vary between different stages of atherosclerosis, depending on the degree of inflammation and cellular infiltration. Cathepsins B, D, and L were all found to be expressed by macrophage-derived foam cells localized in the necrotic cores of plaques. This has been shown earlier for cathepsin D in human atherosclerotic lesions. 21 Positive staining for all cathepsins (B, D, L, and S) was present in the fibrous cap, which implies that these cathepsins may play a role in the process of plaque rupture.
The mRNA expression of cathepsins B, D, and L was high also outside the plaque and in C57BL/6 mice. Based on the quantification of macrosialin, accumulation of macrophages was restricted to the lesions of ApoE−/− mice, thus suggesting that there are other sources for the expression of these cathepsins than macrophages. Furthermore, these findings suggest that cathepsins B, D, and L play a role in the normal and healthy vessels. In contrast, cathepsin S expression was restricted to the atherosclerotic lesion area of the ApoE−/− mice with no mRNA expression in C57BL/6 or in unaffected regions of ApoE−/− mice. The expression profile followed the expression of macrosialin suggesting that macrophages could be the main source of cathepsin S. However, the immunohistochemistry analysis showed that cathepsin S protein expression was localized to SMCs. Thus, it could be hypothesized that cytokine expression associated with the developing plaque is responsible for SMC expression of cathepsin S and important for the migration of these cells. This notion is supported by the finding that interferon-γ and interleukin-1β can induce cathepsin S expression by SMCs in cell culture. 8
Cathepsins function intracellularly but also extracellularly near the cell surface after mobilization of the enzyme to pericellular acidic compartments. 13 Although all members of the cathepsin family have acidic pH optimum, they also function at neutral pH. The proteolytic activity of cathepsins is regulated by several mechanisms. Different receptors, such as the mannose-6-phosphate receptors that are responsible for trafficking of lysosomal enzymes inside the cell, have been suggested to mediate induction of cathepsin secretion as well as membrane binding and stabilization of cathepsins. 23,24 Furthermore, proteolytic activation by removal of propeptides is executed by cathepsins C and D. 25 Co-localization of different cathepsins could therefore be of importance for their function. This autoactivation is accelerated in the presence of glycosaminoglycans present in the atherosclerotic plaque. Of note, up-regulation of both cathepsins C and D expression and activity has been reported in aneurysms. 26
In summary, increased and differential expression of several cathepsins in atherosclerotic plaques suggests that these proteases may participate in atherogenesis and increase the risk of plaque rupture. In particular, cathepsin S expression was associated with atherosclerosis with an extensive expression in the fibrous plaque and medial SMC layer. However, the inducers of the atherosclerosis-mediated medial SMC release of cathepsin S remain to be elucidated.
Acknowledgments
We thank Inger Bodin for excellent technical assistance.
Footnotes
Address reprint requests to Per Eriksson, King Gustaf V Research Institute, Karolinska Hospital, M:1, S-171 76 Stockholm, Sweden. E-mail: per.eriksson@medks.ki.se.
Supported by grants from the Swedish Medical Research Council (grant 12660), the Swedish Heart-Lung Foundation, the King Gustaf V and Queen Victorias Foundation, the Torsten and Ragnar Söderberg Foundation, the Foundation for Old Servants, and the Professor Nanna Svartz Foundation.
References
- 1.Ward MR, Pasterkamp G, Yeung AC, Borst C: Arterial remodeling. Mechanisms and clinical implications. Circulation 2000, 102:1186-1191 [DOI] [PubMed] [Google Scholar]
- 2.Leake DS, Hornebeck W, Brechemier D, Robert L, Peters TJ: Properties and subcellular localization of elastase-like activities of arterial smooth muscle cells in culture. Biochim Biophys Acta 1983, 761:41-47 [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D: Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet 1997, 17:439-444 [DOI] [PubMed] [Google Scholar]
- 4.Henney AM, Wakeley PR, Davies MJ, Foster K, Hembry R, Murphy G, Humphries S: Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci USA 1991, 88:8154-8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galis ZS, Sukhova GK, Lark MW, Libby P: Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994, 94:2493-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galis ZS, Sukhova GK, Libby P: Microscopic localization of active proteases by in situ zymography: detection of matrix metalloproteinase activity in vascular tissue. EMBO J 1995, 9:974-980 [DOI] [PubMed] [Google Scholar]
- 7.Riese RJ, Mitchell RN, Villadangos JA, Shi GP, Palmer JT, Karp ER, De Sanctis GT, Ploegh HL, Chapman HA: Cathepsin S activity regulates antigen presentation and immunity. J Clin Invest 1998, 101:2351-2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P: Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest 1998, 102:576-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frosch BA, Berquin I, Emmert-Buck MR, Moin K, Sloane BF: Molecular regulation, membrane association and secretion of tumor cathepsin B. Apmis 1999, 107:28-37 [DOI] [PubMed] [Google Scholar]
- 10.Miller BF, Kothari HV: Increased activity of lysosomal enzymes in human atherosclerotic aortas. Exp Mol Pathol 1969, 10:288-294 [DOI] [PubMed] [Google Scholar]
- 11.Xin XQ, Gunesekera B, Mason RW: The specificity and elastinolytic activities of bovine cathepsins S and H. Arch Biochem Biophys 1992, 299:334-339 [DOI] [PubMed] [Google Scholar]
- 12.Bromme D, Bonneau PR, Lachance P, Wiederanders B, Kirschke H, Peters C, Thomas DY, Storer AC, Vernet T: Functional expression of human cathepsin S in Saccharomyces cerevisiae. Purification and characterization of the recombinant enzyme. J Biol Chem 1993, 268:4832-4838 [PubMed] [Google Scholar]
- 13.Reddy VY, Zhang QY, Weiss SJ: Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci USA 1995, 92:3849-3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman HA, Riese RJ, Shi GP: Emerging roles for cysteine proteases in human biology. Annu Rev Physiol 1997, 59:63-88 [DOI] [PubMed] [Google Scholar]
- 15.Leake DS, Peters TJ: Proteolytic degradation of low density lipoproteins by arterial smooth muscle cells: the role of individual cathepsins. Biochim Biophys Acta 1981, 664:108-116 [DOI] [PubMed] [Google Scholar]
- 16.Tertov VV, Orekhov AN: Metabolism of native and naturally occurring multiple modified low density lipoprotein in smooth muscle cells of human aortic intima. Exp Mol Pathol 1997, 64:127-145 [DOI] [PubMed] [Google Scholar]
- 17.Li W, Yuan XM, Olsson AG, Brunk UT: Uptake of oxidized LDL by macrophages results in partial lysosomal enzyme inactivation and relocation. Arterioscler Thromb Vasc Biol 1998, 18:177-184 [DOI] [PubMed] [Google Scholar]
- 18.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R: ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb 1994, 14:133-140 [DOI] [PubMed] [Google Scholar]
- 19.Reddick RL, Zhang SH, Maeda N: Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression [published erratum appears in Arterioscler Thromb 1994 May;14:839].Arterioscler Thromb 1994, 14:141-147 [DOI] [PubMed] [Google Scholar]
- 20.Jeng AY, Chou M, Sawyer WK, Caplan SL, Von Linden-Reed J, Jeune M, Prescott MF: Enhanced expression of matrix metalloproteinase-3, -12, and -13 mRNAs in the aortas of apolipoprotein E-deficient mice with advanced atherosclerosis. Ann NY Acad Sci 1999, 878:555-558 [DOI] [PubMed] [Google Scholar]
- 21.Kaesberg B, Harrach B, Dieplinger H, Robenek H: In situ immunolocalization of lipoproteins in human arteriosclerotic tissue. Arterioscler Thromb 1993, 13:133-146 [DOI] [PubMed] [Google Scholar]
- 22.Wuttge DM, Sirsjö A, Eriksson P, Stemme S: Gene expression in atherosclerotic lesion of apoE deficient mice. Mol Med 2001, 7:383-392 [PMC free article] [PubMed] [Google Scholar]
- 23.Sloane B: Biochemistry and Molecular Aspects of Selected Cancers. 1994:pp 411-466 Academic Press, San Diego
- 24.Lloyd J: Biology of the Lysosome. 1996. Plenum Press, New York
- 25.Rowan AD, Mason P, Mach L, Mort JS: Rat procathepsin B. Proteolytic processing to the mature form in vitro. J Biol Chem 1992, 267:15993-15999 [PubMed] [Google Scholar]
- 26.Gacko M, Glowinski S: Cathepsin D and cathepsin L activities in aortic aneurysm wall and parietal thrombus. Clin Chem Lab Med 1998, 36:449-452 [DOI] [PubMed] [Google Scholar]