Abstract
The extent to which simian immunodeficiency virus (SIV) replication in lung tissues contributes to the pool of viruses replicating during acute infection is incompletely understood. To address this issue, in situ hybridization was used to examine SIV replication in multiple lobes of lung from rhesus macaques infected with pathogenic SIV. Despite widespread viral replication in lymphoid and intestinal tissues, the lungs during acute infection harbored rare productively infected cells. Simultaneous immunohistochemical staining for the monocytic marker, CD68, revealed that SIV RNA+ cells in lung tissues during acute infection were CD68−, whereas during AIDS they were predominantly CD68+ and localized in large foci in caudal lobes. SIV RNA+ cells in spleen remained CD68− throughout disease. Since CD68 is also expressed by subpopulations of dendritic cells (DC), we also examined pulmonary CD68+ cells for expression of additional DC markers. DC-LAMP mRNA was abundant in lung tissues and expressed predominantly by CD68− cells, whereas DC-SIGN mRNA was expressed in only very rare cells, indicating that SIV RNA+ cells late in disease were most likely macrophages. These studies of SIV/host interactions demonstrate that macaque lung tissues are minimally infected during acute infection, exhibit changes in predominant target cells for infection, and express very little DC-SIGN.
The lungs represent an extremely large interface between the host and environment, with the mucosal/epithelial surface area of an adult human estimated to be 75 m2. 1 Accordingly, among the collection of outcomes comprising AIDS in human immunodeficiency virus type-1 (HIV-1)-infected individuals, pathology within the lungs is a frequent component. In two independent, retrospective examinations of AIDS autopsy cases spanning the years 1984 to 1999, 2,3 75 to 85% of cases demonstrated pathology in lung tissues. The observation that Pneumocystis carinii is one of the most frequent opportunistic pathogens affecting HIV-1-infected individuals also further underscores the importance of the lung as an immunologically impaired environment during HIV-1 infection. 2-7 Maintaining appropriate immune surveillance and effector activities in lung tissues, and at the appropriate levels, is important in combating pathogens encountered through pulmonary routes, and is frequently deficient during HIV-1 infection.
The extent to which local and systemic HIV-1 replication in humans, and simian immunodeficiency virus (SIV) replication in rhesus macaques, contributes to disruption of immune function in the lungs is not known. HIV-1 DNA can be detected in bronchoalveolar lavages (BAL) obtained from infected individuals throughout the course of disease, but different studies have reported variable frequencies and magnitudes of viral replication. 8-10 Both HIV-1 and SIV replicate to differing extents throughout the course of infection with higher viral loads detected during the acute phase and terminal stage (AIDS). 11-14 During the acute phase of SIV infection (2 weeks postinfection [PI]) there is a high level of viral replication within lymphoid tissues 11,13 as well as lymphoid regions of the gastrointestinal tract. 15,16 After resolution of the primary viremia and during clinical latency, lymphoid tissue viral loads are low 11-13,17 as are intestinal and lung tissue viral loads. 11,17-19 However, the extent to which virus replicates productively throughout the lungs during acute infection has not been fully examined. Increased levels of viral replication in the lungs appear during the shift from clinical latency to AIDS, 17 although proviral DNA can be detected throughout the entire course of infection. 10 These issues are of importance because, despite the advent of anti-retroviral drug combinations and their demonstrated efficacy in potent suppression of viral replication with associated immune reconstitution, 20-22 there is a persistent viral reservoir that is long-lived and likely replenished. 23 These viruses may be replicating in cells of the monocyte/macrophage lineage, 24,25 and it is possible that the lungs serve as such a reservoir.
Examination of rhesus macaque lung tissues has demonstrated productive SIV replication predominantly in monocytes/macrophages during AIDS, 18,19 although not in all animals that succumb to the immunodeficiency-inducing effects of the virus. During progression to AIDS, the populations of viral variants in approximately 50% of HIV-1-infected individuals switch from a non-syncitium-inducing phenotype (NSI) to syncitium-inducing phenotype (SI) when examined in in vitro culture systems, 26,27 which correlates with a switch in tropism from primary macrophages to established T cell lines. This has led to the supposition that macrophage-tropic (M-tropic) variants replicate early during the course of infection and T cell tropic (T-tropic) variants replicate later. Viral tropism has been based primarily on examination of the replicating properties of viruses in in vitro assays or, in a more limited way, on examination of the genetic and biochemical properties of the envelope glycoproteins of the viruses. Nevertheless, these important analyses do not examine directly the population of cells that actually serve as targets for productive viral replication within host tissues. Recent in situ hybridization (ISH) studies have identified T-lymphocytes as the predominant target cells for productive infection in lymphoid tissues of HIV-1-infected individuals 28,29 and SIV-infected rhesus macaques 29 early during the course of infection. The relative contributions made by T- versus M-tropic variants of SIV to the local pool of productively replicating viruses in the lungs during different stages of infection have not been examined directly in tissues.
To determine the extent to which SIV/DeltaB670 productively replicates in lung tissues during acute infection, and in which cell types, we have comprehensively examined lung tissues from adult rhesus macaques during acute infection or AIDS. ISH was used to detect and quantitate SIV viral RNA+ (vRNA+) cells in five lobes of lung from each macaque. Despite widespread viral replication in lymphoid and gastrointestinal tissues, the lung was not a major target organ for productive replication early in the course of infection. As virus replication increased in lung tissues during AIDS, there was a tissue-specific change in the populations of productively infected target cells from rare, predominantly CD68− cells during acute infection to predominantly CD68+ cells during AIDS. Although CD68 has been a widely used marker for monocytes/macrophages, it is also expressed by subpopulations of dendritic cells. 30 Therefore, we examined the expression of DC-associated mRNAs and demonstrated that although DC were numerous in parenchymal lung tissue throughout the entire course of disease, they were almost exclusively DC-LAMP+, CD68−, and DC-SIGN− (dendritic-cell-specific ICAM-3 grabbing nonintegrin). DC-SIGN, which can bind HIV and SIV and promote its passage to other susceptible target cells, 31 was expressed in lung tissue only rarely and only late in the disease, whereas it was expressed abundantly in lymphoid and gastrointestinal tissues. These studies provide a comprehensive definition of the virologic events occurring during peak viral replication in SIV-infected macaques within the large pulmonary compartment.
Materials and Methods
Animals and Tissue Processing
All animal studies were performed under the approval and guidance of the University of Pittsburgh Institutional Animal Care and Use Committee. The 12 adult rhesus macaques (Macaca mulatta) used in this study were negative for SIV, simian retrovirus (type D), and simian T-lymphotropic viruses -1, -2, and -3 and have been described. 17 Briefly, all animals were inoculated intravenously (I.V.) and sacrificed either 2 weeks PI during the acute phase of infection or on progression to AIDS. During necropsy, transcardial perfusion was performed with 0.9% saline to remove contaminating blood cells from tissues. Tissue specimens were fixed by immersion in fresh 4% paraformaldehyde (Sigma Co., St. Louis, MO)/phosphate-buffered saline (Biowhittaker, Walkersville, MD) (PF/PBS) and processed as described. 32,33
Plasma Viral Loads
Quantitation of virion-associated RNA in plasma was performed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) on an ABI Prism 7700 (Applied Biosystems, Foster City, CA) as described. 34
In Situ Hybridization
ISHs were performed as described, 17,35,36 except that overnight hybridizations were performed at 50°C. The riboprobes in these studies encompassed sequences from four regions of the SIVmacBK28 molecular clone 34,37 spanning portions of the gag, pol, env, and nef genes, and included positions 47–1130, 1676–3121, 6600–8266, and 8453–9267 (GenBank accession number M19499). Our generation of a rhesus macaque DC-SIGN cDNA (GenBank accession number AF369755) has been described. 38 A rhesus macaque DC-LAMP cDNA was generated from total RNA extracted from snap-frozen lung using Trizol (Life Technologies, Rockville, MD), and RT-PCR was performed using the Access RT-PCR System (Promega Corporation, Madison, WI) and the following cycling parameters: 48°C for 45 minutes; 94°C for 2 minutes; 30 cycles of 94°C for 30 seconds, 54°C for 30 seconds, and 68°C for 2 minutes; and 68°C for 3 minutes. The primer sequences were YKCDCLAMPF1 (5′-ATGCCCCGGCAGCTCAGCGCGGCGG-3′) and YKCDCLAMPR1 (5′-TTAGATTCTCTGGTATCCAGATGA-3′), based on the human sequences. 39 Products were ligated to the pGEM-T vector (Promega) and sequenced completely in both directions using manual and automated strategies (GenBank accession number AF416334).
Simultaneous ISH and Immunohistochemistry (IHC)
Following stringent ISH and washing, tissue sections were equilibrated in 1X PBS for 5 minutes, and blocked for 1 hour at room temperature in 5% nonfat dry milk/1X PBS supplemented with 1.6% horse serum. Excess blocking agent was removed and the anti-CD68 mAb (clone KP1 (1:50 dilution), Dako Corp., Carpinteria, CA) was applied to the tissue and incubated for 45 minutes at room temperature in a humid chamber. The sections were washed twice in 1X PBS for 3 minutes, and antigen-positive cells were detected by the ABC method (Vector Labs, Burlingame, CA) using 3,3′-diaminobenzidine as the substrate. The reaction was stopped after 8 to 10 minutes by rinsing in 1X PBS, and the tissues were dehydrated in graded ethanols containing 0.3 mol/L ammonium acetate. SIV vRNA+ cells were then detected by emulsion autoradiography with exposure times of 2 to 3 days, and DC-LAMP and DC-SIGN mRNA+ cells were detected with exposure times of 7 days.
Image Capture and Analysis
For the quantitation of the numbers of vRNA+ cells in lungs, 10 random microscopic fields from each lung tissue section were captured through a 60X Plan apochromat objective using the Metaview software package (Universal Imaging Corp., West Chester, PA) and a RT Slider Spot camera (Diagnostic Instruments, Inc., Sterling Heights, MI). Each nucleus and productively infected cell was counted from each captured image using the manual counting feature of Metaview. The percentages of SIV vRNA+ cells that expressed CD68 were examined in the same manner, and 100 SIV vRNA+ cells per section were categorized as CD68 positive or negative. The percentages of DC-associated mRNA+ cells that expressed CD68 were examined in the same manner, and 100 DC-LAMP or DC-SIGN mRNA+ cells per section were categorized as CD68 positive or negative.
Results
Minimal Productive SIV Replication in Lung Tissues during Acute Infection, Despite Widespread Systemic Infection
To examine comprehensively the viral and immunological events in lung tissues and their timing throughout the course of infection, 12 adult rhesus macaques (Table 1) ▶ were inoculated intravenously with a characterized stock of SIV/DeltaB670 40 and sacrificed at different times after infection. Of these animals, eight were sacrificed 2 weeks PI, which is when plasma viral loads 41 and lymphoid tissue viral loads 11 reach their maximal levels. The remaining four macaques were maintained until they progressed to AIDS, between 21 and 55 weeks PI.
Table 1.
Rhesus Macaques, SIV in Situ Hybridization Signals, and Clinicopathological Findings
| Animal | Duration infection* (wk) | SIV vRNA+ cells† | SIV vRNA+ cells/nuclei in lung‡ | Clinicopathological findings§ | ||
|---|---|---|---|---|---|---|
| Spleen | AxLN | Jejunum | ||||
| M5299 | 2 | + | + | ++ | 5/11,232 | Mild hypercellularity in LN; gastritis |
| M5499 | 2 | ++ | ++ | ++ | 8/8,490 | Reduced % CD4+ T-lymphocytes |
| M5599 | 2 | +/− | +/− | − | 0/8,983 | None |
| M5699 | 2 | + | ++ | + | 1/6,073 | None |
| M0999 | 2 | + | + | +/− | 1/6,434 | None |
| M5899 | 2 | + | + | − | 0/5,594 | Reduced % CD4+ T-lymphocytes; mild LH; gastritis |
| M5999 | 2 | + | + | ++ | 0/6,469 | Reduced % CD4+ T-lymphocytes |
| M6299 | 2 | + | ++ | ++ | 0/6,718 | Reduced % CD4+ T-lymphocytes |
| M1799 | 21 | + | + | + | 3/9,389 | Weight loss; CD4+ T-lymphocyte loss; LH; Pc |
| M5199 | 24 | + | + | +/− | 5/9,953 | Weight loss; CD4+ T-lymphocyte loss; LH; Pc |
| M6199 | 32 | + | + | + | 5/5,958 | Weight loss; CD4+ T-lymphocyte loss; LH; mild encephalitis |
| M5799 | 55 | + | + | + | 11/8,191 | Weight loss; CD4+ T-lymphocyte loss; LH; B lymphocytic lymphoma |
*All macaques were inoculated intravenously with a characterized stock of pathogenic isolate SIV/DeltaB670, 44 and have been described. 37 Clinicopathological data are presented here again for comparative purposes.
†SIV vRNA+ cells in spleen, axillary lymph node (AxLN) and jejunum were hybridized with SIV-specific riboprobes: +/−, <5 vRNA+ cells/mm2; +, 5–50 vRNA+ cells/mm2; ++, 51–100 vRNA+ cells/mm2; +++, >100 vRNA+ cells/mm2.
‡Number of vRNA+ cells/total nuclei in 10 random fields in lung sections hybridized with SIV-specific riboprobes.
§LN, lymph node; LH, lymphoid hyperplasia; Pc, Pneumocystis carinii infection.
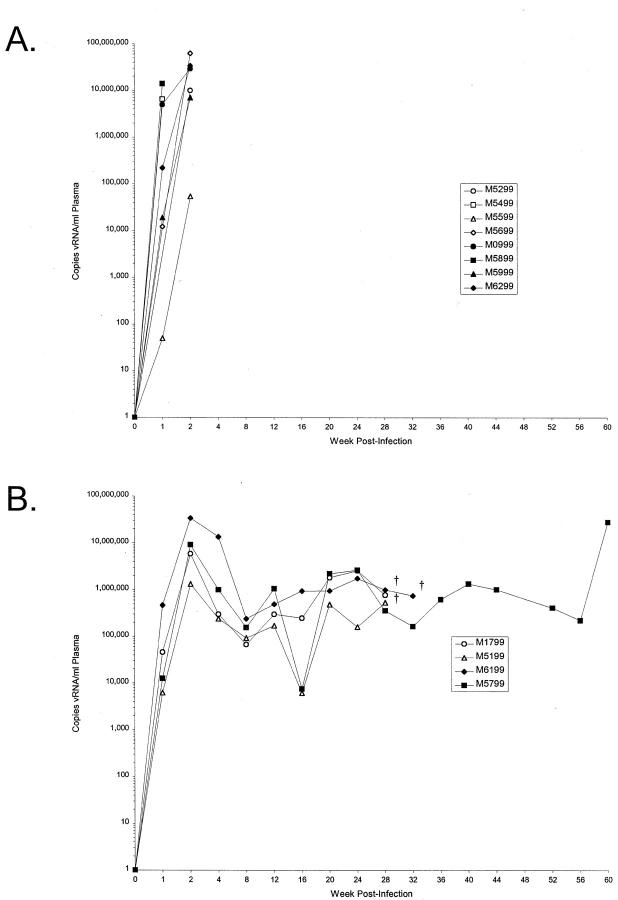
The extent of systemic SIV replication in each animal was examined by real-time RT-PCR quantitation of viral RNA in plasma (Figure 1) ▶ and ISH detection of SIV vRNA+ cells in lymphoid tissues (Table 1) ▶ . At necropsy, the plasma RNA levels ranged from 5.4 × 104 to 1.0 × 108 copies/ml with a geometric mean of 1.2 × 107 copies/ml for the acute phase samples available. The plasma RNA levels at necropsy for the macaques that progressed to AIDS ranged from 5.4 × 105 to 2.8 × 107 copies/ml with a geometric mean of 1.8 × 106 copies/ml. The extents of SIV replication in lymphoid tissues, as determined by ISH, were concordant with the plasma viral RNA loads. The numbers of vRNA+ cells in spleen tissue sections from each animal sacrificed during the acute phase of infection ranged from 2.2 to 57.0 vRNA+ cells/mm2, with animal M5599 harboring the fewest vRNA+ cells (Table 1) ▶ . M5599 also had the lowest plasma viral RNA loads among all of the animals sacrificed 2 weeks PI. Parallel ISH analyses of axillary lymph nodes and intestinal tissues (Table 1) ▶ revealed that three of the eight animals harbored abundant vRNA+ cells in lymph node, whereas four animals had abundant vRNA+ cells in the jejunal lamina propria. These findings provided further evidence of widely disseminated SIV replication during the acute phase of infection. In comparison, the numbers of vRNA+ cells in spleen tissue sections from animals that progressed to AIDS at rapid (M1799 and M5199) or intermediate (M6199 and M5799) rates were lower than in the animals sacrificed 2 weeks PI (Table 1) ▶ . These data indicated that in all animals except one there was extensive systemic viral replication during acute infection and AIDS.
Figure 1.
Plasma viral loads in rhesus macaques infected with SIV/DeltaB670. Plasma viral loads of SIV-infected rhesus macaques were determined by real-time (Taqman) RT-PCR. The plasma viral loads of the macaques sacrificed at 2 weeks PI (A) and the macaques sacrificed during AIDS (B) are shown as copies of vRNA/ml of plasma. Endpoints (†) indicate that the animal became moribund and was humanely sacrificed.
To determine the extent to which SIV was productively replicating in lung tissues during acute infection and AIDS, we used ISH to identify SIV vRNA+ cells in tissue sections from each of five lobes of lung in each macaque. The numbers of vRNA+ cells in 10 random, high power microscopic fields were manually counted after capturing digital images. To account for the inherent acellularity of lung tissues, variably sized alveolar spaces, and/or compression during tissue sectioning, the total numbers of nuclei present in each field also were counted. In this way, the numbers of vRNA+ cells per captured field were normalized for the total number of cells in that field. Using this sampling strategy, vRNA+ cells could be observed, but were rare in all lobes of lung from all 12 macaques in this study, regardless of disease state at necropsy (Table 1) ▶ . Examination of 600 random high power fields and 93,484 total cells revealed that there were only 39 vRNA+ cells, 24 of which were observed in the lung tissues from the four animals with AIDS. These values were not significantly different between the acute infection and AIDS groups. Therefore, despite widespread, systemic infection in these macaques (Figure 1 ▶ and Table 1 ▶ ), there was a paucity of vRNA+ cells in lung tissues.
The sampling strategy we used provided an estimation of the vRNA+ cell burden, but assumed productively infected cells were evenly distributed throughout the tissue. Complete examination of each tissue section revealed the presence of lobe-specific foci of vRNA+ cells in the lungs of all animals with AIDS (Figure 2B) ▶ . The focal collections of vRNA+ cells were large, consisting of approximately 40 to 500 vRNA+ cells in a single 14-μm section, and were detected in the caudal lobes of lung from all four macaques with AIDS, but not in any of the lung tissues from the animals sacrificed during acute infection. In animals M1799, M5199, and M5799, the left caudal lobes of lung contained a large focus of vRNA+ cells, whereas only the right caudal lobe of animal M6199 contained such a focus. Although not fully reflected in the random sampling of 10 high power fields, the viral burden in the lungs from macaque M5799 was the highest among the 12 macaques in this study. Notably, M5799 was also infected with SIV for the longest duration (55 weeks). These data demonstrated that during the acute phase of infection with SIV/DeltaB670, lung tissues were not a major target organ for productive viral replication, and that during AIDS, this anatomical compartment did not necessarily universally harbor large numbers of productively infected cells, although small numbers of large focal collections were present in all animals with AIDS.
Figure 2.
In situ hybridization detection of SIV vRNA+ cells and simultaneous detection of SIV and CD68 in rhesus macaque tissues. ISH was performed on lung tissues (M5499, A; M1799, B) with a pool of SIV-specific anti-sense riboprobes and representative fields are presented. The arrows in (A) denote SIV vRNA+ cells. Simultaneous ISH for SIV and IHC for CD68 was performed on lung tissue (M5299, C; M1799, E) and spleen (M5299, D; M5799, F). Productively infected cells are identified by the overlying collection of silver grains and CD68+ cells are identified by the brown precipitate. The arrows in C and D denote SIV vRNA+/CD68− cells. Parallel hybridizations with control sense riboprobes provided no autoradiographic signal. The bar in A represents 100 μm (A and B), whereas the bar in C represents 20 μm (C–F). Original magnifications, ×200 (A and B) and ×600 (C–F).
Changes in Populations of Target Cells for SIV Infection in Lung Tissues during Progression to AIDS
Productive SIV replication in lung tissues, as observed in animals with AIDS and in AIDS patients, has been associated with selective replication in cells of the monocyte/macrophage lineage, 19,42 such as alveolar macrophages (AMs). To determine the extent to which actively replicating SIV variants in lung tissues were preferentially replicating in monocytes/macrophages during the different stages of disease, we simultaneously performed ISH for SIV RNA and IHC for the monocyte/macrophage lineage marker, CD68 (Figure 2, C–F) ▶ . In the lung tissues from animals sacrificed during acute infection, only a small proportion (4.0%) of the rare vRNA+ cells were of the monocyte/macrophage lineage (ie, CD68+; Figure 2C ▶ and Figure 3 ▶ ). In contrast, 85.9% of vRNA+ cells in the lung tissues obtained from animals with AIDS were CD68+ (Figure 2E ▶ and Figure 3 ▶ ). This increase in the proportion of vRNA+ cells that were CD68+ was not due to an influx of CD68+ cells, because the proportion of total cells that were CD68+ was very similar during acute infection (4%) and AIDS (7%). Parallel analysis of spleen tissues from these same animals indicated that in this anatomical compartment there was no change in the predominant target cells for the replicating SIV variants. Only 3.1% and 4.4% of vRNA+ cells were CD68+ in the spleens during acute infection and AIDS, respectively (Figure 2, D and F ▶ , and Figure 3 ▶ ). The productively infected CD68− cells were likely CD3+ T-lymphocytes, but attempts to stain for CD3ε by IHC, while simultaneously performing ISH for SIV RNA, were unsuccessful. In summary, there was a pulmonary-specific change in the predominantly infected target cells, from CD68− to CD68+, between the acute phase of infection and AIDS.
Figure 3.
Proportions of vRNA+ cells that were CD68+ in lung and spleen tissues from SIV-infected rhesus macaques. ISH for SIV RNA and IHC for CD68 were applied simultaneously to identify productively infected cells that were CD68+ monocytes/macrophages. Presented are the percentages of vRNA+ cells that were also CD68+. Whenever possible >100 vRNA+ cells were examined from each animal for each tissue. The data from all five lobes of lung have been combined for each animal. Lung tissues examined that contained no vRNA+/CD68+ cells are indicated (*).
To characterize more fully the pulmonary CD68+ cells, which were targets for infection during AIDS, we performed ISH with probes for the DC-associated mRNAs, DC-SIGN, and DC-LAMP. DC are professional antigen-presenting cells that are present in both the conducting airways and in the parenchyma of the lungs, 43 and subpopulations of DC have been reported to express CD68. 30 DC-SIGN is expressed more intensely by immature DC than by mature DC 44,45 and can serve as a viral attachment factor that enhances HIV and SIV replication in trans 45 and in cis-, 46 whereas DC-LAMP is a DC-specific lysosome-associated glycoprotein expressed predominantly by mature DC. 39,44 In the lungs of all 12 SIV-infected animals, as well as two uninfected controls, DC-LAMP mRNA+ cells were dispersed throughout the lung parenchyma (Figure 4A) ▶ with the most prominent populations of DC-LAMP mRNA+ cells localized in alveolar septal walls. DC-LAMP mRNA+ cells comprised between 10.8 ± 1.6% to 11.1 ± 2.8% of the total nuclei in the lung sections regardless of disease state and were predominantly CD68− (98.2%). Despite the presence of abundant DC in lung tissues, DC-SIGN mRNA+ cells were extremely rare in this compartment in all uninfected and acutely infected animals, and in nearly all animals with AIDS. Rare DC-SIGN mRNA+ cells were observed in alveolar spaces and within alveolar walls in macaque M5799, which developed AIDS and had higher levels of local SIV replication in the lungs. The DC-SIGN mRNA+ cells in lung tissues of this animal were predominantly CD68+ (85.9%) (eg, Figure 4B ▶ ). The paucity of DC-SIGN mRNA+ cells in lung tissues was in striking contrast to the abundant expression of DC-SIGN in intestinal and lymphoid tissues (eg, Figure 4, C and D ▶ ), which generally harbored abundant vRNA+ cells during acute infection (Table 1) ▶ . In summary, the pulmonary CD68+ cells productively infected with SIV during AIDS were most likely macrophages and not DC.
Figure 4.
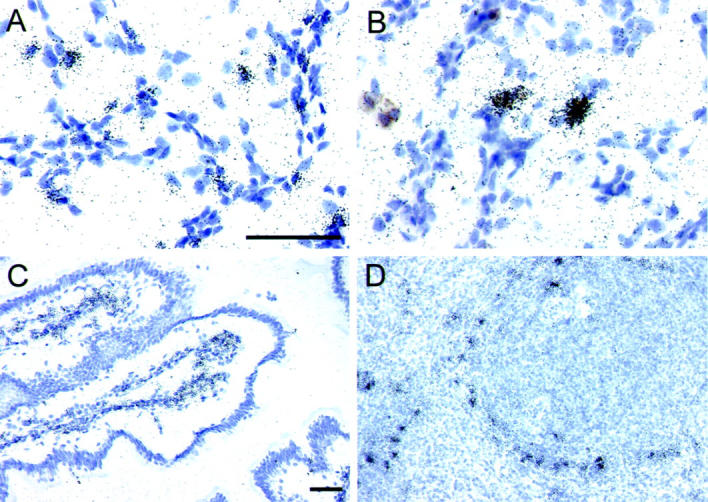
In situ hybridization detection of DC-LAMP+ and DC-SIGN+ cells in rhesus macaque lung tissues. ISH with DC-LAMP- or DC-SIGN-specific riboprobes was performed on lung, intestinal and lymphoid tissues and representative fields are presented. The DC-LAMP anti-sense riboprobe was hybridized to lung tissue from an uninfected rhesus macaque (A). Simultaneous ISH for DC-SIGN and IHC for CD68 was performed on lung tissues from a macaque with AIDS (M5799, B). The DC-SIGN anti-sense riboprobe was also hybridized to jejunal (C) and spleen (D) tissues from an uninfected macaque. Parallel hybridizations with control sense riboprobes provided no autoradiographic signal. The bars in A and C represent 50 μm and apply to (A and B) and (C and D), respectively. Original magnifications, ×600 (A and B) and ×200 (C and D).
Discussion
We have presented evidence here that the lungs are not a major target organ for productive viral replication during the acute phase of infection, through comprehensive examination of tissue sections from five lobes of lung from each of eight SIV-infected rhesus macaques. This is in contrast to the high level of viral replication occurring in the lymphoid and intestinal tissues. We also have demonstrated that the rare SIV vRNA+ cells present in the lungs during acute infection are CD68− and most likely T-lymphocytes, whereas, the more abundant vRNA+ cells in the lungs during AIDS are predominantly CD68+ cells, which morphologically and microanatomically appear to be AMs. Although, CD68 is also expressed by a subpopulation of DC, 30 we determined that >98% of the DC in the macaque lung were CD68−. Therefore, the vRNA+/CD68+ cells in macaque lung tissues were most likely macrophages.
SIV productively infected cells can be very abundant in lung tissues during AIDS, 11-13,18,19 but are extremely rare during clinical latency. 18,19 The data presented here represent the most comprehensive examination to date of productive SIV replication in lung tissues during the acute phase of infection when viral replication reaches a high peak. 11,41 By examining tissue sections from each of five lobes of lung obtained from eight rhesus macaques during acute infection following intravenous infection with the pathogenic SIV/DeltaB670 isolate, we have shown that despite widespread systemic replication of SIV in lymphoid and intestinal tissues (Table 1) ▶ and peripheral blood (Figure 1) ▶ , there was a paucity of SIV vRNA+ cells in all lung tissues examined. Therefore, lung tissues are not a target organ that is seeded to the same extent during acute infection, as are lymphoid and intestinal tissues. If the lungs are a persistent viral reservoir throughout the course of infection, this reservoir is comprised of only a small pool of locally replicating viruses during acute infection (Table 1) ▶ and clinical latency. 18,19 The four macaques in this study that progressed to AIDS also had low numbers of vRNA+ cells in lung tissues, which may be a function of the duration of infection. This is supported by the observation that the largest numbers of vRNA+ cells were detected in lung tissues from the animal (M5799) infected for the greatest duration. Despite only minimal local viral replication, two of these animals (M1799 and M5199) had evidence of active infection with P. carinii in the lung. 47 These observations are consistent with findings by Mankowski et al, 19 and provide support that local immunological dysfunction in lung tissues is a consequence of systemic viral replication and its immunopathological consequences, rather than a consequence only of local viral replication within the lungs. 19
Although viral replication in the lung tissues from the animals with AIDS was not widespread, we consider the cell-associated viral burdens to have been higher than in the acutely infected animals, due to discrete focal collections of vRNA+ cells (eg, Figure 2B ▶ ). These focal collections of productively infected cells were large (>40 vRNA+ cells per 14-μm section) and were detected exclusively in the caudal lobes in three of the four animals with AIDS, and in the middle and caudal lobes in the one animal that was infected for the longest duration. It is not clear why the caudal lobes were a microanatomic site in which SIV preferentially replicated and the reasons for this are most likely multifactorial. One possibility is that there are distinct populations of cells in this specific microenvironment that are more susceptible to infection by SIV. There are examples of distinct populations of cells in different pulmonary microenvironments, as demonstrated for DC in dorsal and ventral pulmonary epithelia, 48 and this might be true for subpopulations of macrophages as well. Alternatively, trafficking of specific cell populations to this microanatomic region, whether under normal or diseased conditions, might be different from other regions. Additionally, distinct and possibly changing cytokine environments in the lower respiratory regions might provide suitable niches that allow specific viral variants to replicate (discussed below).
It is unclear why viral replication in lung tissues is limited during acute infection, as this anatomical compartment is highly vascularized and continually exposed to plasma-associated virus and large numbers of trafficking cells, including T-lymphocytes. The restriction of viral replication during acute infection and the expansion of vRNA+ cells during AIDS could be explained in part due to the selection of viral variants that preferentially replicate in cells of the monocyte/macrophage lineage. There is evidence for this from studies of molecularly cloned SIVs, such as SIVmac239, that are categorized as T-tropic but expand their tropism to include monocytes/macrophages as infection progresses in rhesus macaques. 24,49,50 The data we have presented here are consistent with such an interpretation. The rare vRNA+ cells detected in lung tissues from SIV/DeltaB670-infected rhesus macaques during the acute phase of infection were predominantly CD68−, whereas during AIDS the vRNA+ cells were predominantly CD68+. Although we have not examined the env nucleotide sequences or the co-receptor usage of the viruses present in the lung tissues in our animals, we argue that direct examination of the types of cells that are productively infected in the host tissues is an equally rigorous determination of tropism. Our findings suggest that in lung tissues the predominant target cells for productive infection are initially T-lymphocytes, but on progression to AIDS, they are monocytes/macrophages. We cannot rule out the possibilities that the early CD68− target cells are not T-lymphocytes, and that the late CD68+ target cells were not monocytes/macrophages. However, by simultaneously examining pulmonary CD68+ cells for DC-LAMP and DC-SIGN expression, we have demonstrated that >98% of the CD68+ cells are DC-LAMP− and DC-SIGN−. Therefore, these CD68+ cells are most likely macrophages. Importantly, the predominant target cells for SIV and HIV-1 infection in lymphoid tissues early after exposure to virus have been previously determined to be CD3+ T-lymphocytes. 28,29
The causes of the differences in the cellular targets of the productively replicating SIVs in lung tissues during acute infection versus AIDS are likely multifactorial. Progressive erosion of the collective immune responses against SIV might allow additional variants to eventually grow, particularly if they are provided with an appropriate niche. Before fulminant immunodeficiency, strong and effective immune responses might force the evolution of viral variants that have expanded cellular tropisms that are advantageous to the virus. Such advantages could include replication in cells that are less efficiently recognized and killed by virus-specific CTL, such as macrophages, 51 or the ability to replicate via a CD4-independent mechanism. 52 Interestingly, selection for replication in CD68+ target cells late in disease is not completely systemic, since the vast majority of vRNA+ cells in the spleen tissues from rhesus macaques with AIDS were predominantly CD68− (Figure 3) ▶ . This could be due to different cytokine environments in lymphoid and lung tissues. There is evidence that a type 1 cytokine environment induced by complete Freund’s adjuvant and consisting of IFN-γ is inhibitory to viral replication in macrophages in SHIV-infected rhesus macaques and in in vitro cultures. 53 In contrast, a type 2 cytokine environment induced by Schistosoma mansonii eggs and consisting of IL-4 is supportive of viral replication in macrophages. 53 We 33 and others 54-57 have demonstrated that IFN-γ levels are elevated in lymphoid tissues from SIV-infected rhesus macaques and HIV-1-infected individuals, indicating that there is a predominantly type 1 environment in lymphoid tissues during infection. A sustained type 1 environment in lymphoid tissues might therefore favor continued and ongoing replication selectively in T-lymphocytes.
CD68 is not expressed solely by monocytes/macrophages, but also by a subpopulation of DC. 30 For this reason, we examined the expression levels and patterns of the DC-associated mRNAs, DC-SIGN and DC-LAMP, directly in lung tissues. DC-LAMP mRNA+ cells were abundant in lung tissues, comprising approximately 11% of the total cells, regardless of disease state, although only 2% of the DC-LAMP mRNA+ cells were also CD68+. However, DC-SIGN mRNA+ cells were rare in lung tissues from all macaques regardless of disease state, except in one macaque with AIDS (M5799) in which SIV had disseminated somewhat in the lungs. The association between the general absence of local DC-SIGN expression and the limited extent of virus replication suggests that the paucity of local DC-SIGN might play a role in restricting virus replication within lung tissues. Consistent with this interpretation are our findings (eg, Figure 4 ▶ ) and those of others, 58 that DC-SIGN is abundantly expressed in lymphoid and intestinal tissues, in which SIV generally replicates to high levels early (Table 1) ▶ and throughout infection. 11,41 DC-SIGN binds to the surface glycoproteins of HIV-1 and SIV, increases the half-life of bound virus, and maintains the virus in a form that is infectious for other susceptible target cells such as CD4+ T-lymphocytes, 59,60 although the necessity for DC-SIGN in these DC/T-cell/virus interactions is not yet entirely clear. 61 In addition, expression of DC-SIGN on cells that also express viral receptors can increase the susceptibility of the cells to infection. 46 Further analyses will be required to determine precisely what role DC-SIGN is playing in the replication of SIV in lung and other tissues.
In summary, these studies demonstrate that during acute infection lung tissues harbor only rare productively infected cells that are CD68−, but as the infection progresses SIV replicates predominantly in CD68+ cells of the monocyte/macrophage lineage. In addition, the predominant target cells for infection in lymphoid and lung tissues are different during AIDS. Although our data suggest that the very limited expression of DC-SIGN in lung tissues compared to lymphoid and intestinal tissues is associated with restricted local SIV replication, additional studies will be necessary to further evaluate this. These studies underscore the ongoing need for systematic and coordinated examination of the in vivo target tropisms of replicating viruses and of the local immune environment directly in tissues from each anatomical compartment during different stages of disease to better understand overall virus/host interactions and ensuing pathogenesis.
Acknowledgments
We thank Dawn McClemens-McBride, Melanie O’Malley, and Shane Ritchea for assistance with project coordination and animal care, Dr. Ed Klein for assistance in the pathological reviews, Dr. Carey Balaban for advice and assistance with transcardial perfusion, and Matt Delp, Brandy Mlechick, and Melanie Pfeifer for technical assistance.
Footnotes
Address reprint requests to Todd A. Reinhart, Department of Infectious Diseases and Microbiology, Graduate School of Public Health, University of Pittsburgh, 130 DeSoto St., Pittsburgh, PA 15261. E-mail: reinhar@pitt.edu.
Supported by a grant from the National Institute of Health (RO1 HL62056 to T.A.R.).
Present address for Saverio Capuano, III is the Department of Obstetrics, Gynecology and Reproductive Sciences, Magee-Womens Research Institute, Pittsburgh, PA.
References
- 1.Dunnill MS: Postnatal growth of the lung. Thorax 1967, 17:329-333 [Google Scholar]
- 2.Jellinger KA, Setinek U, Drlicek M, Bohm G, Steurer A, Lintner F: Neuropathology and general autopsy findings in AIDS during the last 15 years. Acta Neuropathol (Berl) 2000, 100:213-220 [DOI] [PubMed] [Google Scholar]
- 3.Masliah E, DeTeresa RM, Mallory ME, Hansen LA: Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS 2000, 14:69-74 [DOI] [PubMed] [Google Scholar]
- 4.Aviram G, Fishman JE, Sagar M: Cavitary lung disease in AIDS: etiologies and correlation with immune status. AIDS Patient Care STDS 2001, 15:353-361 [DOI] [PubMed] [Google Scholar]
- 5.Graham SM, Coulter JB, Gilks CF: Pulmonary disease in HIV-infected African children. Int J Tuberc Lung Dis 2001, 5:12-23 [PubMed] [Google Scholar]
- 6.Nathoo KJ, Gondo M, Gwanzura L, Mhlanga BR, Mavetera T, Mason PR: Fatal Pneumocystis carinii pneumonia in HIV-seropositive infants in Harare, Zimbabwe. Trans R Soc Trop Med Hyg 2001, 95:37-39 [DOI] [PubMed] [Google Scholar]
- 7.Yparraguirre IT, Sant’Anna CC, Lopes VG, Madi K: Necroscopic study of 14 children with AIDS and pneumonia. Rev Assoc Med Bras 2001, 47:129-136 [DOI] [PubMed] [Google Scholar]
- 8.Koziel H, Kim S, Reardon C, Li X, Garland R, Pinkston P, Kornfeld H: Enhanced in vivo human immunodeficiency virus-1 replication in the lungs of human immunodeficiency virus-infected persons with Pneumocystis carinii pneumonia. Am J Respir Crit Care Med 1999, 160:2048-2055 [DOI] [PubMed] [Google Scholar]
- 9.Lu W, Israel-Biet D: Virion concentration in bronchoalveolar lavage fluids of HIV-infected patients. Lancet 1993, 342:298. [DOI] [PubMed] [Google Scholar]
- 10.Nakata K, Weiden M, Harkin T, Ho D, Rom WN: Low copy number and limited variability of proviral DNA in alveolar macrophages from HIV-1-infected patients: evidence for genetic differences in HIV-1 between lung and blood macrophage populations. Mol Med 1995, 1:744-757 [PMC free article] [PubMed] [Google Scholar]
- 11.Reimann KA, Tenner-Racz K, Racz P, Montefiori DC, Yasutomi Y, Lin W, Ransil BJ, Letvin NL: Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Virol 1994, 68:2362-2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lackner AA, Vogel P, Ramos RA, Kluge JD, Marthas M: Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am J Pathol 1994, 145:428-439 [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabarti L, Isola P, Cumont MC, Claessens-Maire MA, Hurtrel M, Montagnier L, Hurtrel B: Early stages of simian immunodeficiency virus infection in lymph nodes: evidence for high viral load and successive populations of target cells Am J Pathol 1994, 144:1226-1237 [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch VM, Zack PM, Vogel AP, Johnson PR: Simian immunodeficiency virus infection of macaques: end-stage disease is characterized by widespread distribution of proviral DNA in tissues. J Infect Dis 1991, 163:976-988 [DOI] [PubMed] [Google Scholar]
- 15.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA: Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998, 280:427-431 [DOI] [PubMed] [Google Scholar]
- 16.Mattapallil JJ, Smit-McBride Z, Dandekar S: Gastrointestinal epithelium is an early extrathymic site for increased prevalence of CD34(+) progenitor cells in contrast to the thymus during primary simian immunodeficiency virus infection. J Virol 1999, 73:4518-4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhart TA, Rogan MJ, Huddleston D, Rausch DM, Eiden LE, Haase AT: Simian immunodeficiency virus burden in tissues and cellular compartments during clinical latency and AIDS. J Infect Dis 1997, 176:1198-1208 [DOI] [PubMed] [Google Scholar]
- 18.Baskin GB, Murphey-Corb M, Martin LN, Soike KF, Hu FS, Kuebler D: Lentivirus-induced pulmonary lesions in rhesus monkeys (Macaca mulatta) infected with simian immunodeficiency virus. Vet Pathol 1991, 28:506-513 [DOI] [PubMed] [Google Scholar]
- 19.Mankowski JL, Carter DL, Spelman JP, Nealen ML, Maughan KR, Kirstein LM, Didier PJ, Adams RJ, Murphey-Corb M, Zink MC: Pathogenesis of simian immunodeficiency virus pneumonia: an immunopathological response to virus. Am J Pathol 1998, 153:1123-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucy RP, Hockett RD, Derdeyn CA, Saag MS, Squires K, Sillers M, Mitsuyasu RT, Kilby JM: Initial increase in blood CD4(+) lymphocytes after HIV anti-retroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest 1999, 103:1391-1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M: Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 1995, 373:123-126 [DOI] [PubMed] [Google Scholar]
- 22.Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH: Viral dynamics in human immunodeficiency virus type 1 infection. Nature 1995, 373:117-122 [DOI] [PubMed] [Google Scholar]
- 23.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF: Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 1999, 5:512-517 [DOI] [PubMed] [Google Scholar]
- 24.Babas T, Vieler E, Hauer DA, Adams RJ, Tarwater PM, Fox K, Clements JE, Zink MC: Pathogenesis of SIV pneumonia: selective replication of viral genotypes in the lung. Virology 2001, 287:371-381 [DOI] [PubMed] [Google Scholar]
- 25.Sopper S, Demuth M, Stahl-Hennig C, Hunsmann G, Plesker R, Coulibaly C, Czub S, Ceska M, Koutsilieri E, Riederer P, Brinkmann R, Katz M, ter Meulen V: The effect of simian immunodeficiency virus infection in vitro and in vivo on the cytokine production of isolated microglia and peripheral macrophages from rhesus monkey. Virology 1996, 220:320-329 [DOI] [PubMed] [Google Scholar]
- 26.Schuitemaker H, Koot M, Kootstra NA, Dereksen MW, de Goede RE, van Steenwijk RP, Lange JM, Schattenkerk JK, Miedema F, Tersmette M: Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol 1992, 66:1354-1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tersmette M, Gruters RA, de Wolf F, de Goede RE, Lange JM, Schellekens PT, Goudsmit J, Huisman HG, Miedema F: Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol 1989, 63:2118-2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schacker T, Little S, Connick E, Gebhard K, Zhang ZQ, Krieger J, Pryor J, Havlir D, Wong JK, Schooley RT, Richman D, Corey L, Haase AT: Productive infection of T cells in lymphoid tissues during primary and early human immunodeficiency virus infection. J Infect Dis 2001, 183:555-562 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT: Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 1999, 286:1353-1357 [DOI] [PubMed] [Google Scholar]
- 30.Summers KL, Hock BD, McKenzie JL, Hart DN: Phenotypic characterization of five dendritic cell subsets in human tonsils. Am J Pathol 2001, 159:285-295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR: DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 2002, 16:135-144 [DOI] [PubMed] [Google Scholar]
- 32.Fallert BA, Reinhart TA: Improved detection of simian immunodeficiency virus RNA by in situ hybridization in fixed tissue sections: combined effects of temperatures for tissue fixation and probe hybridization. J Virol Methods 2002, 99:23-32 [DOI] [PubMed] [Google Scholar]
- 33.Reinhart TA, Fallert BA, Pfeifer ME, Sanghavi S, Capuano S, III, Rajakumar P, Murphey-Corb M, Day R, Fuller CL, Schaefer TM: Increased expression of the inflammatory chemokine CXC chemokine ligand 9/monokine induced by interferon-γ in lymphoid tissues of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrome. Blood 2002, 99:3119-3128 [DOI] [PubMed] [Google Scholar]
- 34.Fuller DH, Rajakumar PA, Wilson LA, Trichel AM, Fuller JT, Shipley T, Wu MS, Weis K, Rinaldo CR, Haynes JR, Murphey-Corb M: Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J Virol 2002, 76:3309-3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozden S, Aubert C, Gonzalez-Dunia D, Brahic M: Simultaneous in situ detection of two mRNAs in the same cell using riboprobes labeled with biotin and 35S. J Histochem Cytochem 1990, 38:917-922 [DOI] [PubMed] [Google Scholar]
- 36.Staskus KA, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson DJ, Ganem D, Haase AT: Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol 1997, 71:715-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kornfeld H, Riedel N, Viglianti GA, Hirsch V, Mullins JI: Cloning of HTLV-4 and its relation to simian and human immunodeficiency viruses. Nature 1987, 326:610-613 [DOI] [PubMed] [Google Scholar]
- 38.Baribaud F, Pohlmann S, Sparwasser T, Kimata MT, Choi YK, Haggarty BS, Ahmad N, Macfarlan T, Edwards TG, Leslie GJ, Arnason J, Reinhart TA, Kimata JT, Littman DR, Hoxie JA, Doms RW: Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J Virol 2001, 75:10281-10289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin JJ, Ait-Yahia S, Patel S, Mattei MG, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S: A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity 1998, 9:325-336 [DOI] [PubMed] [Google Scholar]
- 40.Amedee AM, Lacour N, Gierman JL, Martin LN, Clements JE, Bohm R, Jr, Harrison RM, Murphey-Corb M: Genotypic selection of simian immunodeficiency virus in macaque infants infected transplacentally. J Virol 1995, 69:7982-7990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker RA, Regan MM, Reimann KA: Variability of viral load in plasma of rhesus monkeys inoculated with simian immunodeficiency virus or simian-human immunodeficiency virus: implications for using non-human primate AIDS models to test vaccines and therapeutics. J Virol 2001, 75:11234-11238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baskerville A, Ramsay AD, Addis BJ, Dennis MJ, Cook RW, Cranage MP, Greenaway PJ: Interstitial pneumonia in simian immunodeficiency virus infection. J Pathol 1992, 167:241-247 [DOI] [PubMed] [Google Scholar]
- 43.Holt PG: Antigen presentation in the lung. Am J Respir Crit Care Med 2000, 162:S151-S156 [DOI] [PubMed] [Google Scholar]
- 44.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K: Immunobiology of dendritic cells. Annu Rev Immunol 2000, 18:767-811 [DOI] [PubMed] [Google Scholar]
- 45.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y: DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 2000, 100:587-597 [DOI] [PubMed] [Google Scholar]
- 46.Lee B, Leslie G, Soilleux E, O’Doherty U, Baik S, Levroney E, Flummerfelt K, Swiggard W, Coleman N, Malim M, Doms RW: cis Expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J Virol 2001, 75:12028-12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Croix DA, Board K, Capuano S, III, Murphey-Corb M, Haidaris CG, Flynn JL, Reinhart T, Norris KA: Alterations in T lymphocyte profiles of bronchoalveolar lavage fluid from SIV- and Pneumocystis carinii-coinfected rhesus macaques. AIDS Res Hum Retroviruses 2002, 18:391-401 [DOI] [PubMed] [Google Scholar]
- 48.Schon-Hegrad MA, Oliver J, McMenamin PG, Holt PG: Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med 1991, 173:1345-1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desrosiers RC, Hansen-Moosa A, Mori K, Bouvier DP, King NW, Daniel MD, Ringler DJ: Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am J Pathol 1991, 139:29-35 [PMC free article] [PubMed] [Google Scholar]
- 50.Kodama T, Mori K, Kawahara T, Ringler DJ, Desrosiers RC: Analysis of simian immunodeficiency virus sequence variation in tissues of rhesus macaques with simian AIDS. J Virol 1993, 67:6522-6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schutten M, van Baalen CA, Guillon C, Huisman RC, Boers PH, Sintnicolaas K, Gruters RA, Osterhaus AD: Macrophage tropism of human immunodeficiency virus type 1 facilitates in vivo escape from cytotoxic T-lymphocyte pressure. J Virol 2001, 75:2706-2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mori K, Rosenzweig M, Desrosiers RC: Mechanisms for adaptation of simian immunodeficiency virus to replication in alveolar macrophages. J Virol 2000, 74:10852-10859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buch S, Pinson D, King CL, Raghavan R, Hou Y, Li Z, Adany I, Hicks A, Villinger F, Kumar A, Narayan O: Inhibitory and enhancing effects of IFN-γ and IL-4 on SHIV(KU) replication in rhesus macaque macrophages: correlation between Th2 cytokines and productive infection in tissue macrophages during late-stage infection. Cytokine 2001, 13:295-304 [DOI] [PubMed] [Google Scholar]
- 54.Orandle MS, Williams KC, MacLean AG, Westmoreland SV, Lackner AA: Macaques with rapid disease progression and simian immunodeficiency virus encephalitis have a unique cytokine profile in peripheral lymphoid tissues. J Virol 2001, 75:4448-4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khatissian E, Chakrabarti L, Hurtrel B: Cytokine patterns and viral load in lymph nodes during the early stages of SIV infection. Res Virol 1996, 147:181-189 [DOI] [PubMed] [Google Scholar]
- 56.Cheret A, Le Grand R, Caufour P, Neildez O, Matheux F, Theodoro F, Vaslin B, Dormont D: RANTES, IFN-γ, CCR1, and CCR5 mRNA expression in peripheral blood, lymph node, and bronchoalveolar lavage mononuclear cells during primary simian immunodeficiency virus infection of macaques. Virology 1999, 255:285-293 [DOI] [PubMed] [Google Scholar]
- 57.Boyle MJ, Berger MF, Tschuchnigg M, Valentine JE, Kennedy BG, Divjak M, Cooper DA, Turner JJ, Penny R, Sewell WA: Increased expression of interferon-γ in hyperplastic lymph nodes from HIV-infected patients. Clin Exp Immunol 1993, 92:100-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, Iwasaki A: Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol 2002, 76:1866-1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feinberg H, Mitchell DA, Drickamer K, Weis WI: Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 2001, 294:2163-2166 [DOI] [PubMed] [Google Scholar]
- 60.Clapham PR, McKnight A: HIV-1 receptors and cell tropism. Br Med Bull 2001, 58:43-59 [DOI] [PubMed] [Google Scholar]
- 61.Wu L, Bashirova AA, Martin TD, Villamide L, Mehlhop E, Chertov AO, Unutmaz D, Pope M, Carrington M, KewalRamani VN: Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc Natl Acad Sci USA 2002, 99:1568-1573 [DOI] [PMC free article] [PubMed] [Google Scholar]