Abstract
Cytomegalovirus (CMV) is the most frequent infectious cause of developmental brain disorders in humans. Here we show the role of innate immune responses caused by natural killer (NK) cells and nitric oxide (NO) derived from brain macrophages during murine CMV (MCMV) infection of the developing brain. Viral replication in the brain of newborn mice was significantly enhanced by administration of anti-asialo-GM1 antibody, specific for NK cells, or L-N6-(1-imminoethyl)-lysine, a specific inhibitor of NO synthase 2 (NOS2). These results suggest that NK cells and NO contribute to the viral clearance from the brain. At 3 days postinfection (dpi) MCMV early antigen (Ag)-positive cells were immunohistochemically detected in the periventricular area, where most of the positive cells were macrophages. At 7 dpi MCMV-Ag was found not only in cells of the periventricular area but also in neurons of the hippocampus and cortex. At 11 dpi MCMV-Ag disappeared from the periventricular area, but persisted in neurons. In the periventricular area, NK cells and NOS2-positive macrophages were associated with MCMV-Ag-positive cells. In contrast, there were very few NK cells and NOS2-positive macrophages around the MCMV-Ag-positive neurons. In situ hybridization for MCMV DNA demonstrated that positive signals were found mostly in the periventricular cells, and rarely in neurons. These results suggest that the innate immune responses are restricted to the virus-replicating cells, and do not affect MCMV-infected neurons. Therefore, evasion of the innate immune responses by MCMV-infected neurons may be an important factor in supporting the viral persistence in the developing brain.
Cytomegalovirus (CMV) is the most significant infectious cause of developmental brain disorders during the embryonal and perinatal periods in humans. 1,2 Microcephaly is the most prominent manifestation of the brain in congenital CMV infection, and ventriculoencephalitis is a frequent histopathological finding of the infected brain. 1,3,4 The infection occasionally persists and causes neurological disorders, including sensorineural hearing loss and mental retardation. 4 Because CMV has strict species specificity, we have developed model systems for brain abnormalities induced by the infection of mouse embryos and neonates with murine CMV (MCMV). 5,6 We have shown that MCMV displays neurotropic features and that viral antigen (Ag) in neurons of the hippocampus and cerebral cortex was observed for a prolonged time after infection. 6 These findings suggest that MCMV infection preferentially persists in the cerebral neurons of the developing brain, perhaps via a mechanism involving evasion of host defenses.
Innate immunity caused by natural killer (NK) cells, macrophages, and cytokines acts as the first line of the host defense against viral infection. Subsequently, adaptive immunity works to eliminate virus via specific T cells and antibodies. 7 In addition to their ability to lyse virus-infected cells through perforin, NK cells can secrete interferon (IFN)-γ, which not only has direct antiviral activity but can also act by stimulating expression of nitric oxide synthase type 2 (NOS2) in macrophages. 8,9 NOS2-derived NO appears to be important in the elimination of various viruses, including CMV, in vitro and in vivo. 9-12 Furthermore, innate immunity seems to be primarily responsible for host defenses against CMV infection, especially during the perinatal period, because of the immaturity of adaptive immunity. 13 There have been several reports concerning the innate immune responses during acute CMV infection in the liver, spleen, lung, and salivary glands. 9,12 However, the role of innate immunity to CMV infection of the developing brain and its relation to viral persistence in neurons still remain to be clarified.
In this study we investigated the dynamics of MCMV infection of the developing brain and the role of innate immune responses mediated by NK cells and NO derived from brain macrophages. The results indicated that the innate immune responses are directed against MCMV-infected nonneuronal cells but not against infected neurons, which may contribute to the establishment of viral persistence in the developing brain.
Materials and Methods
Mice
Inbred specific pathogen-free pregnant C57BL/6 mice were obtained from SLC Japan (Hamamatsu, Japan). Newborn mice were maintained by maternal suckling during the postnatal period.
Virus, Cells, and Plaque Assay
The Smith strain of MCMV, which had been passaged in mouse embryonic fibroblasts, was provided by Dr. Y. Minamishima (Miyazaki, Japan). 14 Mouse embryonic fibroblasts were prepared from 12.5-day-old embryos of BALB/c mice (SLC Japan), and were grown in Dulbecco’s modified Eagle’s essential medium containing penicillin (100 U/ml), streptomycin (50 μg/ml), and 10% fetal bovine serum. The viral titer was determined by a plaque assay in mouse embryonic fibroblasts monolayers as described previously. 15 The titer of the virus stock was 4 × 108 plaque-forming units (PFU)/ml.
MCMV Infection
Two days after birth, 4 × 103, 2 × 104, or 1 × 105 PFU of MCMV in 5 μl of minimum essential medium was injected into the right cerebral hemisphere of neonatal mice under cryoanesthesia using a 10-μl Hamilton syringe with a 27-gauge needle from a midpoint between the ear and eye. Control mice were similarly injected with minimum essential medium. At 3, 7, or 11 days postinfection (dpi), mice were killed under anesthesia with ether. The brains were removed, stored at −80°C and used for plaque assays or reverse transcriptase-polymerase chain reaction (RT-PCR) assays. The brains for immunohistochemistry and in situ hybridization were removed, sliced, and quickly frozen in n-hexane at −80°C. Coronal sections were cut at 5 μm using a cryostat, air-dried, and stored at −80°C.
Administration of Anti-Asialo GM1 (aGM1) Antibody
Twelve hours before the inoculation of 2 × 104 PFU of MCMV, 20 μg of rabbit anti-aGM1 polyclonal antibody (pAb) (Wako Chemical, Osaka, Japan) in 50 μl of phosphate-buffered saline (PBS) were injected into the peritoneal cavity. Control mice were similarly injected with 20 μg of normal rabbit IgG. At 6 dpi the brains were removed and stored at −80°C. MCMV titers were determined by plaque assay.
Administration of L-N6-(1-Imminoethyl)-Lysine (L-NIL)
Twelve hours before the inoculation of 2 × 104 PFU of MCMV, 50 μg of L-NIL (Calbiochem, Darmstadt, Germany), a specific inhibitor of NOS2, 16 in 5 μl of PBS was injected into the brain. After MCMV infection, the same dose of L-NIL was administered every other day. Control mice were similarly injected with PBS. At 6 dpi brains were removed and stored. MCMV titers were determined by plaque assay.
RT-PCR
Total RNA was extracted from the frozen stocks of brains using Isogen (Wako Chemical) as specified by the manufacturer, and the extracted RNA was treated with RNase-free DNase (Boehringer Mannheim, Indianapolis, IN). The extracted RNA was reverse-transcribed with the reverse transcription system (Promega, Madison, WI), and PCR was performed. The HPRT gene was amplified from each sample as a control. The primers used for the detection of the indicated mRNAs were as follows: interleukin (IL)- 12, 5′-ATGGCCATGTGGGAGCTGGAG-3′ and (reverse) 5′-GGACTTCACACTTCGTGGTTT-3′; INF-γ, 5′-CATTGAAAGCCTAGAAAGTCTG-3′ and (reverse) 5′-GCTTTTTCCTACGTAAGGTACTC-3′; HPRT,5′-GTTGGATACAGGCCAGACTTTGTTG-3′ and (reverse) 5′-TCGGTATCCGGTCGGATGGGAG-3′.
Immunohistochemistry
After the fixation with isopropyl alcohol, serial sections were incubated with normal rabbit serum or goat serum for 10 minutes at room temperature. Then immunohistochemical staining was performed using mouse monoclonal antibody (mAb) D5 and rat mAb Q3, which are specific for the MCMV early nuclear Ag and cytoplasmic Ag, respectively, 17,18 rabbit anti-aGM1 pAb (Wako Chemical), rabbit anti-NOS2 pAb (Upstate Biotechnology, Lake Placid, NY), rat mAb F4/80, which is specific for mouse macrophage/microglia (Serotec, Oxford, UK), rabbit anti-glial fibrillary acidic protein (GFAP) pAb (DACO Japan, Kyoto, Japan), rabbit anti-neuron specific enolase pAb (provided by Dr. K. Kato, Aichi Prefectural Colony, Aichi, Japan). The sections were then sequentially incubated with a goat anti-mouse IgG (Pharmingen, San Diego, CA), goat anti-rabbit IgG (Pharmingen), or rabbit anti-rat IgG (Pharmingen) conjugated with alkaline phosphatase or horseradish peroxidase. Alkaline phosphatase and horseradish peroxidase were colored with Fast-blue BB (Sigma, St. Louis, MO) and 3-amino-9-ethylcarbazole (AEC; DACO Japan), resulting in the formation of a blue and a red precipitate, respectively. Immunohistochemical double staining was performed as described previously. 18 One Ag was labeled with alkaline phosphatase-conjugated secondary Ab and colored with Fast-blue BB, and then another Ag was labeled with horseradish peroxidase-labeled secondary Ab and colored with AEC.
Detection of MCMV DNA by in Situ Hybridization
Using the sections fixed with 4% paraformaldehyde adjacent to the sections subjected to immunostaining, in situ DNA-DNA hybridization was performed according to the methods of Pomeroy and colleagues 19 A 30-bp oligonucleotide probe homologous to the sequences in exon 4 of the major immediate-early MCMV gene 1 was labeled with digoxigenin according to the manufacturer’s specifications (Boehringer Mannheim). The base sequence of the probe (bases 1900 to 1871) was 5′-CCAGACTCTCTTTTCTGAGGGCCCTAGATT-3′. After hybridization, the sections were incubated with sheep anti-digoxigenin Fab fragments conjugated with alkaline phosphatase (Boehringer Mannheim), and colored with 5-bromo-4-chloro-3-indoxyl phosphate/nitroblue tetrazolium.
Cell Counts in Tissue Sections
The numbers of cells positive for MCMV early nuclear Ag, in situ hybridization signal, aGM1 or NOS2 were counted in the coronal sections of the right hemispheres of MCMV-infected brains. Right hemispheres were divided into periventricular, hippocampal, and cortical areas. The numbers of the positive cells in each of these areas were counted. The cell counting was blindly performed by two observers (HK and YA). Averaged data from the numbers of cells counted by them were used. The data of three animals at 3, 7, or 11 dpi were averaged.
Results
Survival and Intracerebral Viral Growth
To determine an appropriate dose of MCMV, we investigated the survival and intracerebral growth of MCMV in C57BL6 neonatal mice infected with MCMV at three different doses (Figure 1A) ▶ . All mice infected with 1 × 105 PFU (2.5 times of LD50) of MCMV died by 10 dpi, whereas all mice infected with 4 × 103 PFU (one-tenth of LD50) of MCMV survived. Mice inoculated with 2 × 104 PFU (half of LD50) of MCMV died between 10 and 20 dpi, and the survival rate of the infected mice was 66.7%. The intracerebral viral growth peaked around at 7 dpi in the mice inoculated with all three experimental doses of MCMV (Figure 1B) ▶ . All further experiments were performed by injection of at 2 × 104 PFU of MCMV (half of LD50).
Figure 1.
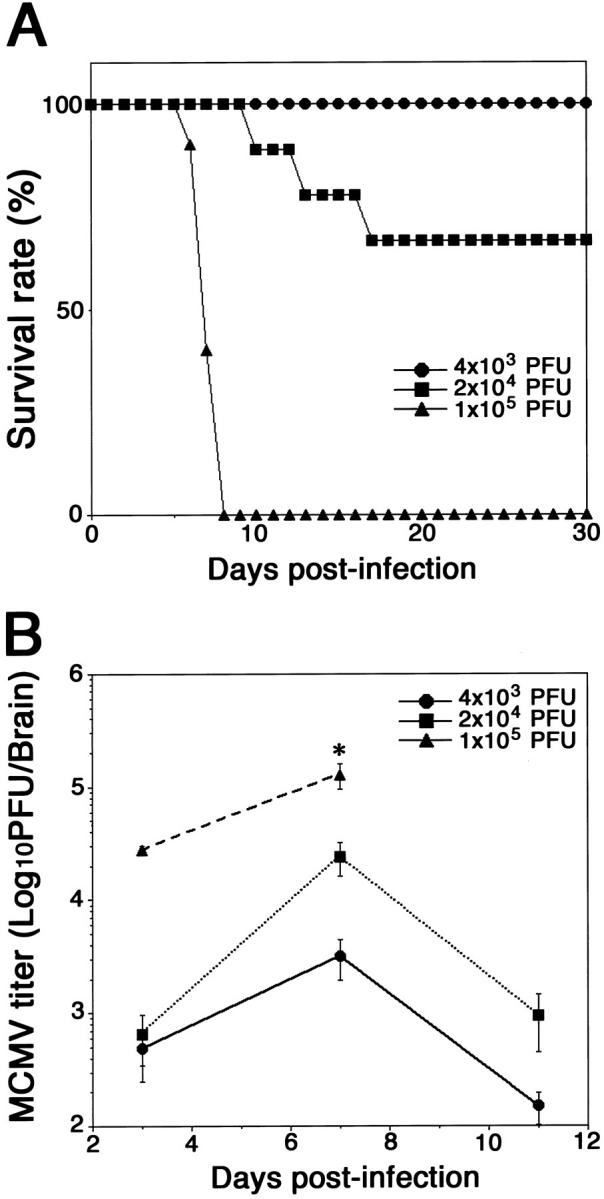
A: Survival of newborn C57BL/6 mice intracerebrally inoculated with 4 × 103 PFU (one-tenth of LD50, •), 2 × 104 PFU (half of LD50, ▪), or 1 × 105 PFU (2.5 times of LD50, ▴) of MCMV 2 days after birth. Nine animals were used for each dose of MCMV. B: Intracerebral MCMV growth. Twenty animals were used for each dose. Averaged titers from five animals at each point are shown. *, n = 2.
Enhancement of MCMV Growth by the Administration of Anti-aGM1 Antibody and L-NIL
Newborn mice were inoculated intracerebrally with MCMV at 2 × 104 PFU. The cerebral MCMV titer of mice injected intraperitoneally with anti-aGM1 antibody 12 hours before infection was increased approximately five times as compared with that of mice injected with normal rabbit serum (Figure 2A) ▶ . The cerebral MCMV titer of mice injected intracerebrally with L-NIL, a specific inhibitor of NOS2, was also enhanced approximately five times (Figure 2B) ▶ . These results suggest that NK cells and NO contribute to viral clearance from the brain.
Figure 2.
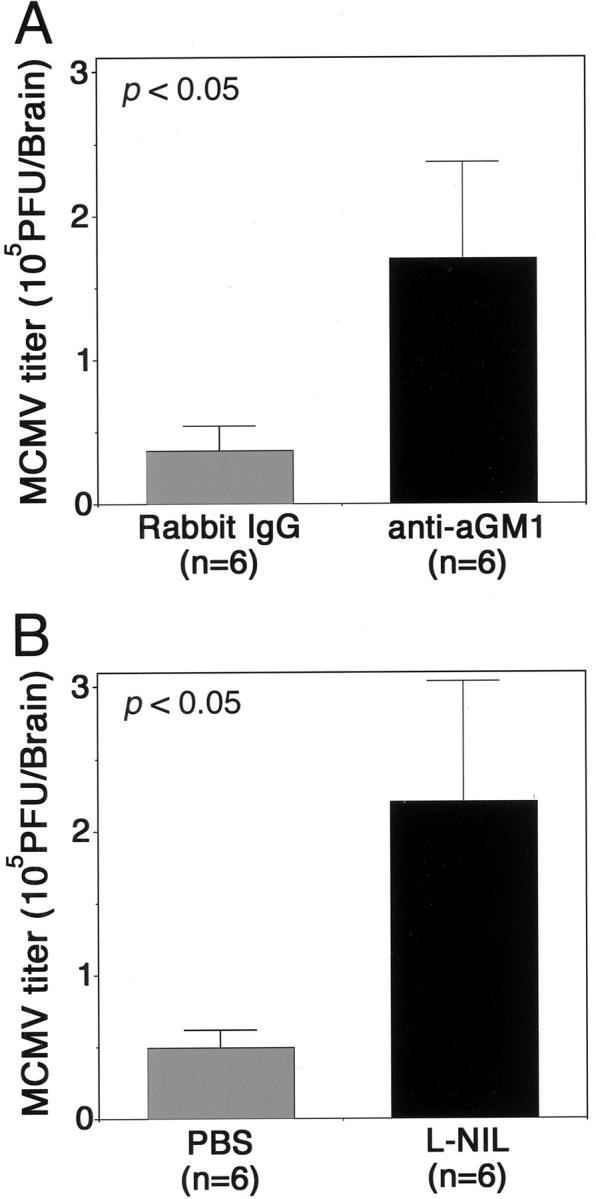
The enhancement of the intracerebral MCMV growth by injection of either anti-aGM1 Ab (A) or L-NIL (B). A: Twelve hours before inoculation of 2 × 104 PFU of MCMV, 20 μg of rabbit anti-aGM1 pAb was injected into the peritoneal cavity. Control mice were similarly injected with normal rabbit IgG. At 6 dpi the brains were removed and MCMV titers were determined by plaque assays. B: Twelve hours before MCMV inoculation, 50 μg of L-NIL was injected into the brain. After MCMV infection the same dose of L-NIL was administered every other day. Control mice were similarly injected with PBS. At 6 dpi the brains were removed and MCMV titers were determined by plaque assays. Averaged titers per brain from six animals are shown. *, P < 0.05 versus control mice by Student’s t-test.
Cytokine Expression in the MCMV-Infected Brains
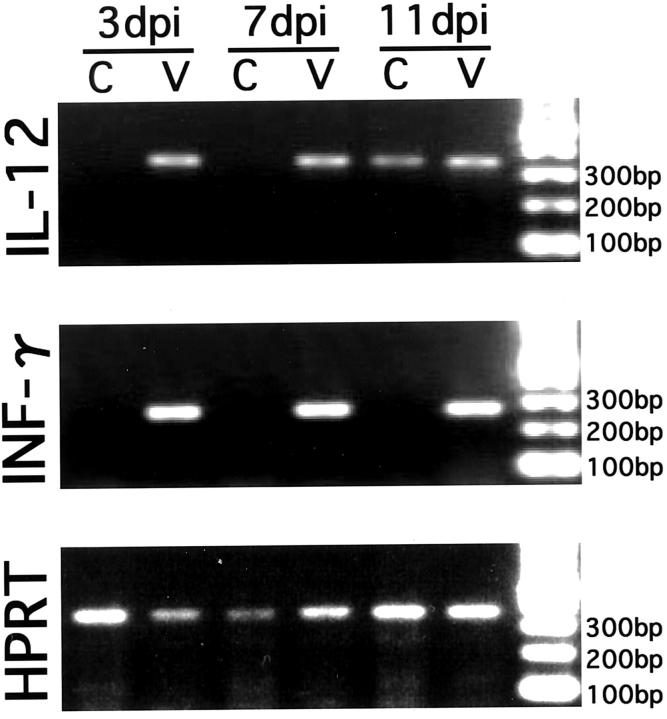
Using RT-PCR, we investigated the mRNA transcription of IL-12 and INF-γ in the brain. These are representative cytokines in the innate immune response and are closely related to the activation of NK cells and macrophages. 20 Throughout the experimental observation period, the RT-PCR signals of both cytokines were definitely detected in the MCMV-infected brains but not in the uninfected brains, except for a weakly positive signal of IL-12 mRNA in some brains of the uninfected mice at 11 dpi (Figure 3) ▶ . These results suggest that IL-12 and INF-γ may contribute to the innate immune response to intracerebral MCMV infection, and that the expression of these cytokines may occur from the early phase of infection through the phase when viral replication is significantly decreased.
Figure 3.
Induction of expression of IL-12 and INF-γ mRNAs in the brain by MCMV infection. Cytokine mRNAs in the whole brain were detected by RT-PCR at 3, 7, and 11 dpi. As a control, the RT-PCR product of constitutively expressed HPRT was analyzed. Representative data of control and MCMV-infected brains at each time point are shown. C, control brain; V, MCMV-infected brain.
Kinetics of MCMV-Ag-Positive Cells in the Infected Brains
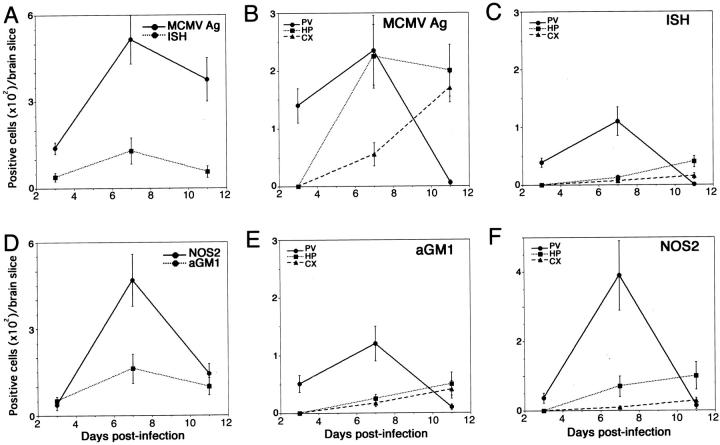
At 3 dpi, MCMV-Ag-positive cells were mostly found in the periventricular area, extending from the ventricular surface, including the choroid plexus, to the subventricular zone, while few were found in the hippocampus and cortex (Figure 4, A and B) ▶ . Even in the high-dose infection, the MCMV-Ag-positive cells were restricted to the periventricular area (data not shown). At 7 dpi, the MCMV-Ag-positive cells were found not only in the periventricular area, but also in the pyramidal cell layer of the hippocampus, with few positive cells in the cortex (Figure 4, D and E) ▶ . The number of MCMV-Ag-positive cells in the cerebrum peaked at this time, and the number of positive cells in the periventricular area was nearly equal to that in the hippocampus (Figure 5, A and B) ▶ . With the increase in the number of MCMV-Ag-positive cells, inflammatory cells, including mono- and polymorphonuclear leukocytes, markedly infiltrated the periventricular area, resulting in ventriculoencephalitis (Figure 4D ▶ and Figure 7A ▶ ). However, there was no prominent inflammatory reaction in the hippocampus. At 11 dpi, the number of MCMV-Ag-positive cells in the cerebrum was decreased (Figure 4, G and H ▶ , and Figure 5A ▶ ). The positive cells disappeared from the periventricular area (Figure 4H ▶ and Figure 5B ▶ ). The ventriculoencephalitis subsided with the dilatation of the lateral ventricles (Figure 4G) ▶ . In contrast, MCMV-Ag-positive cells, most of which were neurons, remained in the hippocampus and cortex (Figure 4H ▶ and Figure 5B ▶ ).
Figure 4.
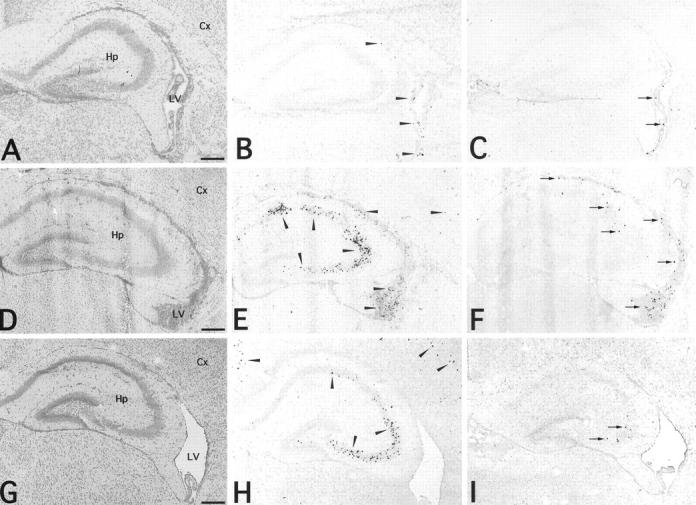
Histology (A, D, G), immune staining for MCMV early nuclear Ag (B, E, H) and in situ hybridization for viral DNA (C, F, I) in the brains of neonatal C57BL/6 mice infected with 2 × 104 PFU of MCMV. MCMV-Ag (arrowheads) was detected by mAb D5 followed by reaction with goat anti-mouse IgG conjugated with horseradish peroxidase and colored with AEC. MCMV DNA (arrows) was detected by DNA-DNA in situ hybridization. A–C, 3 dpi; D–F, 7 dpi; G–I, 11 dpi. Hp, hippocampus; Cx, cortex; LV, lateral ventricle. Scale bars, 300 μm.
Figure 5.
Kinetics of MCMV-Ag-, in situ hybridization signal-, aGM1- or NOS2-positive cells in the coronal sections of the right hemisphere of MCMV-infected brains (A and D). Right hemispheres were divided into periventricular, hippocampal, and cortical areas. The numbers of positive cells in each area were counted (B, C, E, and F). Data of three animals were averaged at each point. The numbers of MCMV-Ag- or in situ hybridization signal-positive cells (A) and aGM1- or NOS2-positive cells (D) were counted in coronal sections of the right hemisphere. The numbers of positive cells in the periventricular area (PV, •), hippocampus (HP, ▪), and cortex (CX, ▴) are shown in B (MCMV-Ag), C (in situ hybridization signal), E (aGM1), and F (NOS2).
Figure 7.
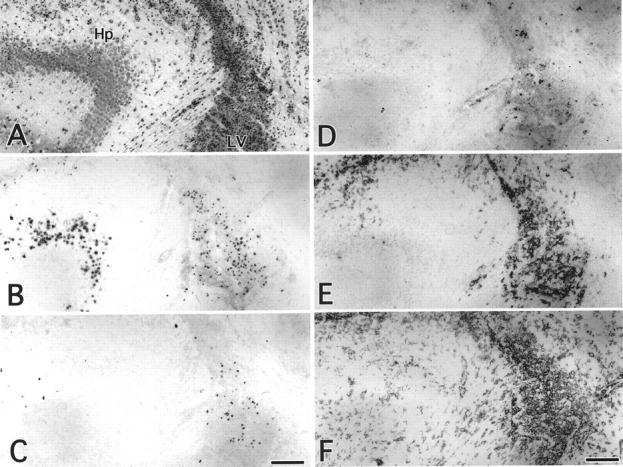
Comparison of histology, immune staining for MCMV early-Ag and cell markers, and in situ hybridization for MCMV DNA in serial sections of MCMV-infected brain at 7 dpi. A, HE; B, MCMV-Ag; C, in situ hybridization; D, aGM1; E, NOS2; F, F4/80-Ag. Hp, hippocampus; LV, lateral ventricle. Scale bars, 150 μm.
In the periventricular area, double immunohistochemistry for MCMV-Ag and cell markers demonstrated that most of MCMV-Ag-positive cells were F4/80-positive macrophages/microglia (Figure 6A) ▶ . The rest of the MCMV-Ag-positive cells were GFAP-positive glia (Figure 6B) ▶ , ependymal cells, and choroid plexus epithelium. MCMV-Ag-positive macrophages were prominent in the ventricular surface (Figure 6A) ▶ , whereas the virus-infected cells in the subventricular zone were GFAP-positive glia (Figure 6B) ▶ . The aGM1-poitive and NOS2-positive cells were associated with MCMV-Ag-positive cells (Figure 6, C and D) ▶ , as described below. In the hippocampus and cortex, most of MCMV-Ag-positive cells were neurons (Figure 6G) ▶ and not GFAP-positive glia (Figure 6F) ▶ , as described previously. 21 Viral Ag was rarely found in F4/80-positive macrophages/microglia (Figure 6E) ▶ .
Figure 6.
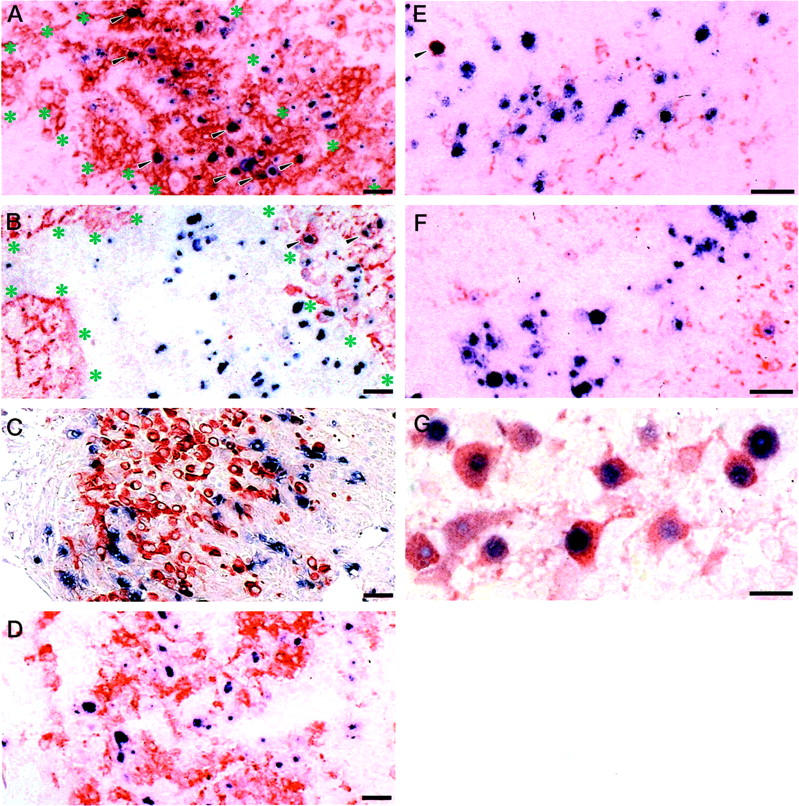
Double immunostaining for MCMV-Ag and cell markers in the periventricular area (A–D) and hippocampus (E–G) of MCMV-infected brains at 7 dpi. Immunostaining for F4/80-Ag (A, E); GFAP (B, F); aGM1 (C); NOS2 (D); neuron-specific enolase (G). MCMV early nuclear Ag was colored with Fast blue BB (blue), while cell markers were colored with AEC (red), except in C (in which MCMV cytoplasmic Ag was colored red, and aGM1 was colored blue). A: In the ventricular surface, MCMV-Ag (blue) was mostly expressed in the nuclei of F4/80-Ag-positive macrophages (red) (arrowheads). B: In the subventricular zone, MCMV-Ag (blue) was expressed in GFAP-positive glia (red) (arrowheads). C: aGM1-positive cells (blue) were found around the virus-infected cells (red). MCMV-Ag was not expressed in aGM1-positive cells. D: MCMV Ag-positive cells (blue) were surrounded by NOS2-positive macrophages (red). NOS2 was not expressed in the cytoplasm of the MCMV-infected cells. E–G: In the hippocampus, MCMV-Ag (blue) was mostly found in neuron-specific enolase-positive neurons (red) (G), rarely found in F4/80-Ag-positive macrophage (E, arrowhead), but not in GFAP-positive glia (red) (F). Asterisks show interface between ventricular surface and subventricular zone in A and B. Scale bar, 30 μm.
In Situ Hybridization for MCMV DNA
In situ hybridization was performed for the detection of MCMV DNA (Figure 4; C, F, and I) ▶ . Although the number of in situ hybridization signal-positive cells changed in parallel with that of the MCMV-Ag-positive cells, the number of in situ hybridization signal-positive cells was lower than that of MCMV-Ag-positive cells (Figure 5, A and C) ▶ . At 3 dpi, the in situ hybridization signal-positive cells were restricted to the periventricular area (Figure 4C) ▶ . At 7 dpi, the in situ hybridization signal-positive cells were still found in the periventricular area, but were rarely found in the hippocampus where MCMV-Ag was markedly expressed in neurons (Figure 4F) ▶ . At 11 dpi, the in situ hybridization signal-positive cells disappeared from the periventricular area, whereas a few positive cells were found in the hippocampus and cortex (Figure 4I) ▶ .
Localization of aGM1-Positive Cells
In the uninfected brains, very few aGM1-positive cells were found. In the MCMV-infected brains, at 3 dpi aGM1-positive cells appeared around the viral MCMV-Ag-positive cells in the periventricular area (Figure 5, D and E) ▶ . At 7 dpi the number of aGM1-positive cells was increased in this area (Figure 5, D and E) ▶ , but such cells were rarely found in the hippocampus (Figure 5E ▶ and Figure 7D ▶ ). At 11 dpi, there were no aGM1-positive cells in the periventricular area (Figure 5E) ▶ . Although aGM1-positive cells were found around the virus-infected cells, MCMV-Ag was not expressed in aGM1-positive cells (Figure 6C) ▶ . The localization of aGM1-positive cells was similar to that of in situ hybridization signal-positive cells (Figure 5, C and E ▶ , and Figure 7, C and D ▶ ).
Localization of Macrophages and NOS2 Expression
In the uninfected brains, F4/80-positive-macrophages were scattered to some degree around the periventricular and meningeal area, as described previously. 22 The staining intensity of F4/80 was markedly enhanced by MCMV infection. F4/80-Ag was strongly expressed in macrophages not only in the infectious foci of the periventricular area, but also in the uninfected area (Figure 7F) ▶ .
The expression of NOS2 was restricted to the macrophages around MCMV-Ag-positive cells in the periventricular area (Figure 7E) ▶ . The number of NOS2-positive macrophages peaked at 7 dpi (Figure 5D) ▶ . However, NOS2 was rarely expressed in the macrophages around the MCMV-Ag-positive neurons in the hippocampus (Figure 7E) ▶ . The localization of the NOS2-positive macrophages was similar to that of in situ hybridization signal-positive cells (Figure 5, C and F ▶ , and Figure 7, C and E ▶ ).
Double immunohistochemistry for MCMV-Ag and NOS2 demonstrated that MCMV-Ag-positive cells were surrounded by NOS2-positive macrophages (Figure 6D) ▶ . Interestingly, NOS2 was not expressed in the cytoplasm of the MCMV-infected cells in which MCMV-Ag was expressed in the nucleus. This result indicates that the expression of NOS2 in the MCMV-infected macrophages may be blocked by viral infection.
Discussion
CMV is the most significant infectious cause of brain disorders caused by intrauterine infection in humans with an average incidence of ∼1% of all live births. 2 It is estimated that ∼10% of infected infants have severe brain damage at birth such as microcephaly and periventricular calcification and usually die soon after birth. 2-4 Of the other infected infants nearly 10% have subclinical congenital CMV infection and will subsequently develop brain disorders including mental retardation and epilepsy. 23 Our experimental model focused on the transitional state from the acute brain damage to the chronic infection.
It has been reported that CMV causes ventriculoencephalitis in the developing brain in the acute phase, and that the infection then becomes persistent and results in neurological disorders. 24 The present study demonstrated that MCMV infection in the developing mouse brain began in the periventricular area and subsequently extended into the cerebral parenchyma. The periventricular area is the most vulnerable site for MCMV replication and the accompanying inflammatory reactions and tissue damage, resulting in ventriculoencephalitis, as shown in previous clinical reports about CMV infection in the brain. 3,24,25
In the present study, during acute infection, MCMV tended to infect F4/80-positive macrophages and GFAP-positive glial cells in the periventricular area, and thereafter the infection shifted to the pyramidal neurons in the hippocampus. Innate immune responses mediated by NK cells and NO derived from macrophages seem tocontribute to the viral elimination from the brain, as they have been shown in other organs such as spleen and liver. 9,12 It is possible that NK cells and NOS2-positive macrophages play a crucial role in excluding MCMV-infected cells from the periventricular area but not MCMV-infected neurons. The viral DNA detected by in situ hybridization was prominent in the periventricular cells but not in the pyramidal neurons, although the viral early Ag was expressed in both of these types of cells. It is possible that the amount of viral DNA in the Ag-positive neurons was under the detection limit of the in situ hybridization used in this study. This suggests that MCMV replication may be slow or may occur at a very low level in the MCMV-infected neurons as compared with infected macrophages and other brain cells. This in turn suggests that innate immune responses may be preferentially directed against the virus-replicating cells in the periventricular area.
Lines of evidences suggesting the preferential permissiveness for MCMV replication in the cells of the periventricular area have been reported. Recent advances in neuroscience have indicated that GFAP-positive glial cells in the subventricular zone are glial progenitor cells or radial glia, which are abundant in the subventricular area of the developing brain. 26 The permissiveness for CMV replication in these glial cells was demonstrated in a previous report 27 and in our previous studies using an experimental model of intrauterine MCMV infection. 21,28 Furthermore, we showed that CNS stem/progenitor cells were susceptible to MCMV infection 29 and that activation of the MCMV-immediate early promoter was observed in the glial progenitor cells of transgenic mice. 30
Macrophages, including microglia, are also well known to be major target cells for CMV infection, and to contribute to the systemic dissemination of infective virus. 31,32 Furthermore, because the choroid plexus is a highly vascularized area containing fenestrated endothelial cells, unlike the parenchymal endothelial cells associated with the tight blood-brain barrier, 33 blood-borne monocytes/macrophages permissive for MCMV may be recruited and support the viral replication in the periventricular area.
In contrast to the periventricular area, in the cerebral parenchyma, neurons were major target cells for MCMV infection. 6,34 The present study showed that infection pattern of cerebral neurons was distinctly different from that of nonneuronal cells in the periventricular area. The features of MCMV infection in cerebral neurons were as follows: 1) delayed expression of viral Ag, 2) prolonged expression of the viral early Ag even after disappearance of the productive viral infection in the periventricular area, 3) very low production of infectious virus despite expression of the viral Ag, and 4) absence of innate immune responses caused by NK cells and macrophages. These findings seem to support the concept of MCMV persistence in neurons of the developmental brain suggested in our previous reports. 6
It has been reported that many neurotropic viruses cause persistent neuronal infection without cytopathicity. 35,36 Although the exact mechanisms of the viral persistence in neurons are still not known, these studies showed that the intracellular milieu of neurons seems to restrict the level of viral gene expression and replication as compared with those in nonneuronal cells that are fully permissive for viral replication. Our previous in vivo studies demonstrated that expression of the MCMV IE1 protein essential for MCMV replication was suppressed in neurons of the developing brain despite the sufficient expression of the MCMV early Ag. 21 Furthermore, the promoter activity of the immediate-early gene was not expressed in neurons in transgenic mice. 30,37 It was reported that MCMV replication in primary cultured neurons was delayed and very low compared with that in primary glial cells. 27 In addition, the blockade of apoptosis in virus-infected cells is considered to be an important factor for persistent viral infections. 38 We have previously demonstrated that apoptosis of neurons was inhibited by MCMV infection in the developing brain. 18 At this moment, despite suggestive data, we could not show the obvious evidence of persistent infection in neurons of the developing brain.
Concerning adaptive immunity to virus infected neurons, Joly and colleagues 39,40 reported that neurons are deficient in major histocompatibility complex class I molecules for the presentation of viral Ag to specific cytotoxic T lymphocytes and suggested that viral infection would be persistent in these cells. The results of the present study suggest that the absence of the innate immune responses mediated by NK cells and macrophages against MCMV-infected neurons also drives these cells toward persistent infection. The blood-brain barrier is well known to be an important anatomical factor contributing to the immune privilege of CNS neurons. 41 It has been reported that the innate immune responses begin with the recognition of MCMV-infected cells by NK cells via the interaction between the Ly49H molecule of NK cells and the m157 protein of MCMV on virus-infected cells. 42,43 It seems that expression of m157 proteins as ligands for Ly49H receptors of NK cells may be insufficient in MCMV-infected neurons to induce the innate immune response.
There seem to be two types of responses of macrophages to MCMV infection in the brain. First, a broad and nonspecific response was observed in macrophages not only in the infectious foci but also in the uninfected area as indicated by the detection of up-regulated expression of F4/80 Ag, which has been reported to contribute to the phagocytic function of macrophages and to be constitutively expressed in them. 44 This type of response may be induced by the blood-borne signals from the systemic circulation, such as cytokines and lipopolysaccharides. 45 Second, the specific response restricted to the infectious foci was shown by the inducible expression of NOS2 with the recruitment of NK cells. IFN-γ, which is secreted by NK cells activated by contacting MCMV-infected cells, is considered to be the most potent factor for induction of NOS2 in macrophages. 8 In turn, MCMV infection has been reported to induce relatively high levels of IL-12, which enhances secretion of IFN-γ from the activated NK cells. 20 It is evident that NOS2-derived NO is essential for the activation of NK cells via the IL-12 signaling pathway. 46 Therefore, NK cells and macrophages are intimately influenced by each other and cooperatively regulate MCMV infection. It is thought that the expression of NOS2 in macrophages is strictly regulated by the cell-to-cell interactions between MCMV-infected cells, NK cells, and macrophages. Few such interactions may occur with MCMV-infected neurons.
It is worth noting that NOS2 was expressed in uninfected macrophages but not in MCMV-infected macrophages. The inhibition of NOS2 expression has also been shown in the HCMV-infected retinal pigmented epithelial cells. 47 It seems that MCMV infection may inhibit IFN-γ-mediated induction of NOS2 in macrophages in a manner similar to the blockade of IFN-γ-induced expression of major histocompatibility complex class II molecules in bone marrow-derived macrophages by MCMV, 48 because NOS2 has been reported to be an important IFN-γ-inducible gene product, as are major histocompatibility complex class II molecules. 49
In conclusion, the present study suggests that control of CMV infection in the developing brain depends on the innate immune responses mediated by NK cells and macrophages. The responses are preferentially directed against MCMV-infected cells of the periventricular area, which is the site of marked viral replication. The escape of MCMV-infected cerebral neurons from the innate immune responses may be an important factor in establishment of viral persistence in the developing brain. However, it needs to be investigated in future studies whether or not CMV persistence in the developing brain causes the functional disturbances in the virus-infected neurons, as reported previously. 50
Footnotes
Address reprint requests to Isao Kosugi, M.D., Ph.D., Department of Pathology, Hamamatsu University School of Medicine, 1-20-1 Handayama, Hamamatsu, 431-3192, Japan. E-mail: kos180@tm.hama-med.ac.jp.
Supported in part by the Ministry of Education, Science, Culture, and Technology, Japan (grant no. 13670210).
References
- 1.Weller TH: The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med 1971, 285:203-214 [DOI] [PubMed] [Google Scholar]
- 2.Ho M: Ho M eds. Congenital and Perinatal Human Cytomegalovirus Infection. 1991:pp 205-277 Plenum, New York
- 3.Becroft DM: Prenatal cytomegalovirus infection: epidemiology, pathology and pathogenesis. Perspect Pediatr Pathol 1981, 6:203-241 [PubMed] [Google Scholar]
- 4.Bale JF, Jr: Human cytomegalovirus infection and disorders of the nervous system. Arch Neurol 1984, 41:310-320 [DOI] [PubMed] [Google Scholar]
- 5.Tsutsui Y, Kashiwai A, Kawamura N, Kadota C: Microphthalmia and cerebral atrophy induced in mouse embryos by infection with murine cytomegalovirus in midgestation. Am J Pathol 1993, 143:804-813 [PMC free article] [PubMed] [Google Scholar]
- 6.Tsutsui Y, Kashiwai A, Kawamura N, Aiba-Masago S, Kosugi I: Prolonged infection of mouse brain neurons with murine cytomegalovirus after pre- and perinatal infection. Arch Virol 1995, 140:1725-1736 [DOI] [PubMed] [Google Scholar]
- 7.Biron CA, Brossay L: NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol 2001, 13:458-464 [DOI] [PubMed] [Google Scholar]
- 8.Ding AH, Nathan CF, Stuehr DJ: Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol 1988, 141:2407-2412 [PubMed] [Google Scholar]
- 9.Tay CH, Welsh RM: Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol 1997, 71:267-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris N, Buller RM, Karupiah G: Gamma interferon-induced, nitric oxide-mediated inhibition of vaccinia virus replication. J Virol 1995, 69:910-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiss CS, Komatsu T: Does nitric oxide play a critical role in viral infections? J Virol 1998, 72:4547-4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noda S, Tanaka K, Sawamura S, Sasaki M, Matsumoto T, Mikami K, Aiba Y, Hasegawa H, Kawabe N, Koga Y: Role of nitric oxide synthase type 2 in acute infection with murine cytomegalovirus. J Immunol 2001, 166:3533-3541 [DOI] [PubMed] [Google Scholar]
- 13.Nesin M, Cunningham-Rundles S: Cytokines and neonates. Am J Perinatol 2000, 17:393-404 [DOI] [PubMed] [Google Scholar]
- 14.Ebihara K, Minamishima Y: Protective effect of biological response modifiers on murine cytomegalovirus infection. J Virol 1984, 51:117-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wentworth BB, French L: Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med 1970, 135:253-258 [DOI] [PubMed] [Google Scholar]
- 16.Moore WM, Webber RK, Fok KF, Jerome GM, Connor JR, Manning PT, Wyatt PS, Misko TP, Tjoeng FS, Currie MG: 2-Iminopiperidine and other 2-iminoazaheterocycles as potent inhibitors of human nitric oxide synthase isoforms. J Med Chem 1996, 39:669-672 [DOI] [PubMed] [Google Scholar]
- 17.Tsutsui Y, Naruse I: Murine cytomegalovirus infection of cultured mouse embryos. Am J Pathol 1987, 127:262-270 [PMC free article] [PubMed] [Google Scholar]
- 18.Kosugi I, Shinmura Y, Li RY, Aiba-Masago S, Baba S, Miura K, Tsutsui Y: Murine cytomegalovirus induces apoptosis in non-infected cells of the developing mouse brain and blocks apoptosis in primary neuronal culture. Acta Neuropathol (Berl) 1998, 96:239-247 [DOI] [PubMed] [Google Scholar]
- 19.Pomeroy C, Hilleren PJ, Jordan MC: Latent murine cytomegalovirus DNA in splenic stromal cells of mice. J Virol 1991, 65:3330-3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orange JS, Wang B, Terhorst C, Biron CA: Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med 1995, 182:1045-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinmura Y, Aiba-Masago S, Kosugi I, Li RY, Baba S, Tsutsui Y: Differential expression of the immediate-early and early antigens in neuronal and glial cells of developing mouse brains infected with murine cytomegalovirus. Am J Pathol 1997, 151:1331-1340 [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson PB, Perry VH, Gordon S: Intracerebral injection of proinflammatory cytokines or leukocyte chemotaxins induces minimal myelomonocytic cell recruitment to the parenchyma of the central nervous system. J Exp Med 1992, 176:255-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pass RF, Stagno S, Myers GJ, Alford CA: Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics 1980, 66:758-762 [PubMed] [Google Scholar]
- 24.Perlman JM, Argyle C: Lethal cytomegalovirus infection in preterm infants: clinical, radiological, and neuropathological findings. Ann Neurol 1992, 31:64-68 [DOI] [PubMed] [Google Scholar]
- 25.Arribas JR, Storch GA, Clifford DB, Tselis AC: Cytomegalovirus encephalitis. Ann Intern Med 1996, 125:577-587 [DOI] [PubMed] [Google Scholar]
- 26.Hartfuss E, Galli R, Heins N, Gotz M: Characterization of CNS precursor subtypes and radial glia. Dev Biol 2001, 229:15-30 [DOI] [PubMed] [Google Scholar]
- 27.van Den Pol AN, Mocarski E, Saederup N, Vieira J, Meier TJ: Cytomegalovirus cell tropism, replication, and gene transfer in brain. J Neurosci 1999, 19:10948-10965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li RY, Tsutsui Y: Growth retardation and microcephaly induced in mice by placental infection with murine cytomegalovirus. Teratology 2000, 62:79-85 [DOI] [PubMed] [Google Scholar]
- 29.Kosugi I, Shinmura Y, Kawasaki H, Arai Y, Li RY, Baba S, Tsutsui Y: Cytomegalovirus infection of the central nervous system stem cells from mouse embryo: a model for developmental brain disorders induced by cytomegalovirus. Lab Invest 2000, 80:1373-1383 [DOI] [PubMed] [Google Scholar]
- 30.Li RY, Baba S, Kosugi I, Arai Y, Kawasaki H, Shinmura Y, Sakakibara SI, Okano H, Tsutsui Y: Activation of murine cytomegalovirus immediate-early promoter in cerebral ventricular zone and glial progenitor cells in transgenic mice. Glia 2001, 35:41-52 [DOI] [PubMed] [Google Scholar]
- 31.Pulliam L: Cytomegalovirus preferentially infects a monocyte derived macrophage/microglial cell in human brain cultures: neuropathology differs between strains. J Neuropathol Exp Neurol 1991, 50:432-440 [DOI] [PubMed] [Google Scholar]
- 32.Zhuravskaya T, Maciejewski JP, Netski DM, Bruening E, Mackintosh FR, St Jeor S: Spread of human cytomegalovirus (HCMV) after infection of human hematopoietic progenitor cells: model of HCMV latency. Blood 1997, 90:2482-2491 [PubMed] [Google Scholar]
- 33.Prats N, Briones V, Blanco MM, Altimira J, Ramos JA, Dominguez L, Marco A: Choroiditis and meningitis in experimental murine infection with Listeria monocytogenes. Eur J Clin Microbiol Infect Dis 1992, 11:744-747 [DOI] [PubMed] [Google Scholar]
- 34.Tsutsui Y, Kashiwai A, Kawamura N, Nagahama M, Mizutani A, Naruse I: Susceptibility of brain cells to murine cytomegalovirus infection in the developing mouse brain. Acta Neuropathol (Berl) 1989, 79:262-270 [DOI] [PubMed] [Google Scholar]
- 35.Sharpe AH, Fields BN: Pathogenesis of viral infections. Basic concepts derived from the reovirus model. N Engl J Med 1985, 312:486-497 [DOI] [PubMed] [Google Scholar]
- 36.Oldstone MB: Viruses can cause disease in the absence of morphological evidence of cell injury: implication for uncovering new diseases in the future. J Infect Dis 1989, 159:384-389 [DOI] [PubMed] [Google Scholar]
- 37.Aiba-Masago S, Baba S, Li RY, Shinmura Y, Kosugi I, Arai Y, Nishimura M, Tsutsui Y: Murine cytomegalovirus immediate-early promoter directs astrocyte-specific expression in transgenic mice. Am J Pathol 1999, 154:735-743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roulston A, Marcellus RC, Branton PE: Viruses and apoptosis. Annu Rev Microbiol 1999, 53:577-628 [DOI] [PubMed] [Google Scholar]
- 39.Joly E, Mucke L, Oldstone MB: Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science 1991, 253:1283-1285 [DOI] [PubMed] [Google Scholar]
- 40.Joly E, Oldstone MB: Neuronal cells are deficient in loading peptides onto MHC class I molecules. Neuron 1992, 8:1185-1190 [DOI] [PubMed] [Google Scholar]
- 41.Bradbury MW: The blood-brain barrier. Exp Physiol 1993, 78:453-472 [DOI] [PubMed] [Google Scholar]
- 42.Daniels KA, Devora G, Lai WC, O’Donnell CL, Bennett M, Welsh RM: Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to ly49h. J Exp Med 2001, 194:29-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL: Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 2002, 296:1323-1326 [DOI] [PubMed] [Google Scholar]
- 44.Gordon S: Macrophage-restricted molecules: role in differentiation and activation. Immunol Lett 1999, 65:5-8 [DOI] [PubMed] [Google Scholar]
- 45.Perry VH, Andersson PB, Gordon S: Macrophages and inflammation in the central nervous system. Trends Neurosci 1993, 16:268-273 [DOI] [PubMed] [Google Scholar]
- 46.Diefenbach A, Schindler H, Rollinghoff M, Yokoyama WM, Bogdan C: Requirement for type 2 NO synthase for IL-12 signaling in innate immunity. Science 1999, 284:951-955 [DOI] [PubMed] [Google Scholar]
- 47.Bodaghi B, Goureau O, Zipeto D, Laurent L, Virelizier JL, Michelson S: Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J Immunol 1999, 162:957-964 [PubMed] [Google Scholar]
- 48.Miller DM, Cebulla CM, Rahill BM, Sedmak DD: Cytomegalovirus and transcriptional down-regulation of major histocompatibility complex class II expression. Semin Immunol 2001, 13:11-18 [DOI] [PubMed] [Google Scholar]
- 49.Xaus J, Comalada M, Barrachina M, Herrero C, Gonalons E, Soler C, Lloberas J, Celada A: The expression of MHC class II genes in macrophages is cell cycle dependent. J Immunol 2000, 165:6364-6371 [DOI] [PubMed] [Google Scholar]
- 50.de la Torre JC, Mallory M, Brot M, Gold L, Koob G, Oldstone MB, Masliah E: Viral persistence in neurons alters synaptic plasticity and cognitive functions without destruction of brain cells. Virology 1996, 220:508-515 s [DOI] [PubMed] [Google Scholar]