Abstract
The immunoregulatory cytokine transforming growth factor (TGF)-β1 is secreted as a biologically inactive complex with latency-associated peptide, which must be modified by local factors to expose the functionally active cytokine. The epithelial integrin αvβ6 mediates local activation of TGF-β1 in the lung and β6−/− mice exhibit exaggerated pulmonary inflammation, but their response to inflammatory stimuli in the gut has not been investigated. We found that both β6 and TGF-β1 are constitutively expressed in the jejunal epithelial compartment in uninfected mice and during infection with the intestinal nematode Nippostrongylus brasiliensis. We also present data showing that β6−/− mice are seriously compromised in their ability to mount a mucosal mast cell response after infection, and there is a significant reduction in the expression and systemic release of the granule chymase, mouse mast cell protease-1. Because in vitro expression of this chymase is regulated by TGF-β1, these data indicate that in the absence of αvβ6 epithelially expressed TGF-β1 may not be activated, with a consequent absence of expression of mouse mast cell protease-1 and down-regulation of the mucosal mast cell response.
The multifunctional cytokine, transforming growth factor (TGF)-β1 plays an important role in the remodeling of tissues during allergic responses and in other TH2-regulated responses in the lung. 1 In the gut, TGF-β1 is produced by enterocytes and mediates epithelial restitution 2 in addition to modulating the severity of inflammatory diseases 3 and microbial infections. 4,5 Secreted TGF-β1 is usually associated with latency-associated peptide to form a biologically inactive complex that must be modified extracellularly to expose the active molecule. 6 Factors activating TGF-β1 latency-associated peptide include plasmin, thrombospondin, and the integrin αvβ6, but the relative contributions of these pathways in vivo have not been fully established. 6 Recently, it has been shown that the expression and activation of TGF-β1 in the lungs may be regulated through a variety of macrophage-dependant pathways that are themselves controlled by interleukin-13. 1
The jejunal epithelium is populated by intraepithelial lymphocytes (IELs) and by large numbers of intraepithelial mucosal mast cells (MMCs) during nematode infection. 7 Recent in vitro studies on murine MMC homologues have shown that expression and secretion of the gut-specific β-chymase, mouse mast cell protease-1 (mMCP-1) is induced by TGF-β1. 8,9 This leads to the possibility that in vivo MMC differentiation and mMCP-1 expression are regulated by activation of TGF-β1 within the epithelial compartment. Experiments with transfected cells have shown effective activation of TGF-β1 in vitro by the integrin αvβ6 through cell surface-associated binding of latency-associated peptide. 6 This integrin is expressed almost exclusively on epithelial cells; 10 is rapidly up-regulated in the lungs and skin in response to injury and inflammation; and is a receptor for the matrix proteins fibronectin, tenascin, and vitronectin. 11,12 Furthermore, transgenic mice (β6−/−) lacking this integrin develop exaggerated inflammation in lungs that is reversed by epithelial expression of the human β6 transgene. 13 These observations indicate that epithelial expression of the integrin β6 is important for local TGF-β1 activation during inflammatory responses.
Since the earliest phase of MMC hyperplasia and expression of mMCP-1 occur within the gut epithelium 14,15 we wished to test the hypotheses that the integrin β6 is expressed by jejunal enterocytes and that it is required for mMCP-1 expression during nematode infection. Our results show that β6 and TGF-β1 transcripts are co-expressed within the epithelial compartment of the gut and that, in the absence of β6, mMCP-1 expression and MMC hyperplasia are severely compromised during enteric nematodiasis.
Materials and Methods
Transgenic Mice, Parasite Infections, and Sample Preparation
Maintenance of and infections with Nippostrongylus brasiliensis (rat strain) were performed as in previous studies. 15 To determine whether the integrin β6 is expressed by murine enterocytes, S129 mice (Becton and Dickinson, Cowley, UK Ltd.) were infected subcutaneously with 500 N. brasiliensis L3 (rat adapted strain) and groups of three to four mice were killed 5, 7, and 9 days after infection together with uninfected controls. Intact sheets of jejunal epithelium that included both villi and crypts were exfoliated by vascular perfusion with ethylenediaminetetraacetic acid as described by Bjerknes and Cheng 16 and modified by Rosbottom and colleagues. 17 Isolated epithelium was allowed to settle in ice-cold phosphate-buffered saline (PBS) before transfer into 4% paraformaldehyde for histological examination and into Tri-reagent for RNA analysis to monitor the expression of MMC proteases, integrin β6, and TGF-β1. Additional S129 mice were killed at day 7 of infection and samples of intact jejunum collected starting 10 cm from the pylorus for integrin β6 immunocytochemistry. Jejunum was rolled outermost on villi a pastette, transferred onto labeled cork disks, covered in OCT, and snap-frozen in dry-ice-cooled isopentane.
Fourteen sex-matched β6+/+ and β6−/− transgenic S129 mice, 8 to 12 weeks old, 18 were each infected subcutaneously with 500 N. brasiliensis L3 (rat adapted strain) and killed on days 5, 7, and 9 after infection (four to five per group) in addition to uninfected controls (four per group). Blood was collected for serum and samples of jejunum were removed starting 10 cm from the pylorus for RNA isolation (0.5 cm), quantification of mMCP-1 by enzyme-linked immunosorbent assay (1 cm), fixation in 4% paraformaldehyde (3 cm) and in Carnoy’s fluid (3 cm), respectively, and the remainder of the small intestine used for adult worm isolation, by modified Baerman’s as described previously. 19 Parasites were enumerated from four to five mice/group per time point, and data were compared using the Mann-Whitney nonparametric test (Instat), with a significance level of P < 0.05. The stomach was opened longitudinally on thick filter paper and small samples of the glandular region removed for RNA isolation and mMCP-1 quantification by enzyme-linked immunosorbent assay, before dividing into two longitudinal sections for fixation in 4% paraformaldehyde and Carnoy’s fluid. All experiments were done in accordance with the United Kingdom’s Animals (Scientific Procedures) Act 1986 and the University of California at San Francisco guidelines on animal care.
Histochemistry and Immunocytochemistry
Cryostat sections (10 μm) of the snap-frozen jejunum tissue samples were air-dried for 20 minutes, fixed in absolute methanol for 10 minutes at −20°C, and air-dried for 15 minutes. Sections were blocked with PBS [0.5 mol/L NaCl/0.5% Tween-80 (high-salt)] containing 4% bovine serum albumin for 1 hour at 21°C in a humidified container. Nonspecific immunoglobulin interactions were further blocked by a 2-hour incubation with high-salt-containing 10% normal donkey serum at 4°C in a humidified container. Sections were then incubated overnight at 4°C in a humidified container with rabbit anti-integrin β6 IgG (B1) tissue culture supernatant 13 or 0.5 μg/ml of normal rabbit control IgG (Cambridge Bioscience, Cambridge, UK) in high-salt/10% normal donkey serum. After washing with PBS, slides were incubated with Alexa Fluor-488-conjugated polyclonal donkey anti-rabbit IgG (Cambridge Bioscience) at 2 μg/ml in high-salt/10% normal donkey serum for 2 hours at 4°C in a humidified container. Slides were washed in PBS and mounted with Mowiol mounting media (CN Biosciences UK, Nottingham, UK). Fluorescent images were acquired using an MRC-600 confocal laser-scanning microscope (Bio-Rad Laboratories, Hemel Hempstead, UK) mounted on an Axiovert 100 inverted microscope equipped with a ×63 Plan-Apochromat objective lens (Carl Zeiss, Welwyn Garden City, UK). Images were prepared for publication using Object-Image. 20 Object Image is a public domain software package, based on NIH Image 21 developed by Norbert Vischer (The University of Amsterdam, Amsterdam, The Netherlands) and is available via the Internet at http://simon.bio.uva.nl/object-image.html.
For mast cell evaluation, 3-cm lengths of jejunum were rolled villi-outermost using the Swiss-roll technique, and fixed in Carnoy’s fluid or 4% paraformaldehyde before subsequent processing and sectioning. 22 Mast cells were detected by staining sections from Carnoy’s fixed tissue overnight in 0.5% toluidine blue (Merck, Poole, UK) in 0.5 mol/L HCl, pH 0.5, and counterstaining in 0.1% eosin solution (Surgipath, Peterborough, UK) 22 or by staining paraformaldehyde-fixed sections for esterase in Fast Garnet GBC salt and naphthol AS-D chloroacetate. 23 mMCP-1+ve MMCs were detected immunohistochemically with monoclonal antibody RF 6.1 as described previously. 15 In jejunal sections, mast cells were enumerated per 20 villus-crypt units (vcu) both in the mucosa and in the submucosa directly below the 20 vcu 19 and expressed per vcu. In sections of stomach, positively stained mast cells were counted in five adjacent fields in the glandular fundus and enumerated per mm2. 15 Paraformaldehyde-fixed sections of jejunum werestained with Alcian Blue for mucin glycoproteins 24 for enumeration of goblet cells (counted in 20 vcu/sample) and with carbol chromatrope for enumeration of eosinophils 25 [counted at ×500 in 20 adjacent fields (4.8 mm2 total area)]. Intraepithelial lymphocytes were detected by staining with anti-CD3 antibody 26 and counted in 20 vcu/sample. All data were compared using the nonparametric Mann-Whitney test (Instat) with significance levels of P < 0.05.
Quantification of mMCP-1
Tissues for enzyme-linked immunosorbent assay analysis were snap-frozen in dry ice immediately after collection and stored at −70°C. Concentrations of mMCP-1 in tissues (μg/g wet weight) and in serum (ng/ml) were assayed using the rat monoclonal RF 6.1-based enzyme-linked immunosorbent assay with modifications. 15,19
Detection of Transcripts by Semiquantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Samples of jejunum (0.5 cm) were transferred into 0.5 ml of RNA-later reagent (Ambion Inc., Austin, TX) immediately after collection and stored at 4°C for 3 weeks then at −20°C until processing. Stripped epithelium was transferred directly into TriReagent (Sigma, Poole, UK) and processed as described previously. 17 Homogenization of tissues in TriReagent and removal of contaminating DNA using DNA-free DNase (Ambion Inc.) has been described. 19 One μg of RNA was reverse-transcribed as previously described 22 using 2.5 μmol/L of (dT)15 oligonucleotide primers for protease gene amplification or 2.5 μmol/L of random hexamer primers for amplification of integrin β6 or TGF-β1. One-twentieth volume was amplified by PCR using gene-specific primers described below, with equivalent quantities of nonreverse-transcribed RNA as negative controls. Reaction conditions were optimized to ensure the number of thermocycles used correlated with the amplification stage of the PCR, and magnesium concentration optimized as necessary (IgA only). Primers for the protease genes mMCP-1 and mMCP-2 and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) have already been described. 19,22 Amplifications were performed for 1 minute at 94°C, 2 minutes at 63°C, and 3 minutes at 72°C for 26 thermocycles. Primers B6-6F and B6-5R for integrin β6 have been described previously. 18 Amplifications were performed for 40 seconds at 94°C, 40 seconds at 52°C, and 120 seconds at 72°C for 36 thermocycles along with GAPDH controls. Primers for mouse TGF-β1 were purchased from Stratagene (Cambridge, UK) and amplifications performed for 40 seconds at 94°C, 40 seconds at 60°C, and 120 seconds at 72°C for 34 thermocycles along with GAPDH controls. Primers for IgA were used as a control for epithelial purity because B cells are abundant in the lamina propria but absent from the epithelium in the jejunum. Primers for mouse IgA (forward TCTCCTCCTCTTCTTGTCATACGC; reverse GGAGGTAAGTACCACAGGAGCGTTT) were designed from unique regions of the IgA sequence 27 to give a PCR product of 316 bp. Amplifications were performed for 40 seconds at 94°C, 40 seconds at 58°C, and 120 seconds at 72°C for 34 thermocycles in 10 mmol/L of Tris-HCl and 1 mmol/L of MgCl2 along with GAPDH controls. PCR products were visualized on ethidium bromide stained 1.2 to 1.6% agarose gels and images recorded and analyzed by densitometry using a Kodak Digital Science Image Station 440CF and 1D Image Analysis software.
Results
Expression of the Integrin β6 and TGF-β1 in Normal and Parasitized Jejunal Epithelium
To determine whether the integrin β6 was expressed within the epithelial compartment and whether expression was altered during nematode infection, jejunal epithelium was separated from the lamina propria by vascular perfusion with ethylenediaminetetraacetic acid 16,17 in normal uninfected 129S mice and on days 5, 7, and 9 after infection with 500 N brasiliensis L3 (n = 3/group/day). The samples of exfoliated epithelium were checked histologically to confirm that they comprised >95% epithelium with little or no contamination from the lamina propria (Figure 1a) ▶ as described previously. 16,17 Similarly the presence of IgA transcripts was obvious in RNA prepared from whole jejunum samples but very low or undetectable in all of the epithelial samples tested (Figure 1d) ▶ , suggesting lamina propria cells are unlikely to contribute significantly to RT-PCR signals observed. Transcripts coding for β6 were detected in all jejunal epithelial samples and PCR products were generally of greater intensity than that from RNA prepared from intact jejunum amplified under the same conditions (Figure 1d) ▶ . Expression of β6 by gut epithelium was also confirmed by immunocytochemistry on frozen sections of jejunum using rabbit anti-integrin β6 IgG 13 (Figure 1, b and c) ▶ . Importantly, integrin β6 shows a circumferential localization on enterocytes within the crypt epithelium (Figure 1b) ▶ . Transcription of TGF-β1 was detected in all epithelial samples and expression appeared to be constitutive (Figure 1d) ▶ , which is consistent with previous findings in inflamed and normal lung tissue. 6
Figure 1.
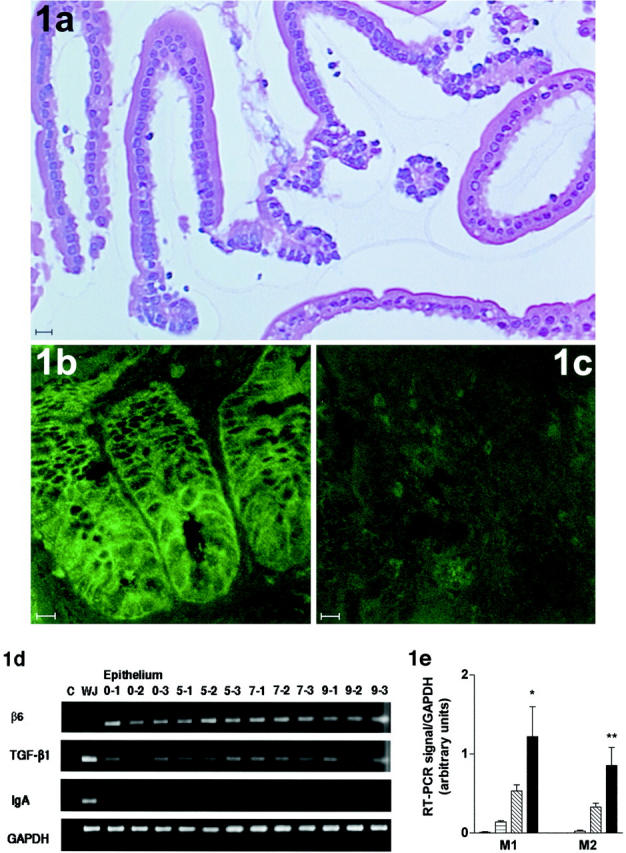
a: H&E-stained exfoliated jejunal epithelium from S129 mice, showing the relative absence of contaminating cells from the lamina propria (horizontal bar, 20 μm). b and c: Sections of jejunum from an S129 mouse taken on day 7 after infection with N. brasiliensis, stained with rabbit anti-integrin β6 IgG B1, specific for integrin β6 (b) or normal rabbit control IgG (c) (horizontal bar, 10 μm). Note the predominantly circumferential immunofluorescence in the crypt epithelium. Confocal images were acquired in fast photon-counting mode (300 accumulated frames) and have not been subjected to contrast enhancement of any form. d: RT-PCR products for TGF-β1 (405 bp), integrin β6 (340 bp), IgA (316 bp), and the housekeeping gene GAPDH (430 bp) from total RNA extracted from whole jejunum on day 7 after challenge with N. brasiliensis (WJ), and from isolated epithelium taken on day 0 (0-1 to 0-3), day 5 (5-1 to 5-3), day 7 (7-1 to 7-3), and day 9 (9-1 to 9-3) after infection with N. brasiliensis and a negative control with nonreverse-transcribed RNA only (c). Note that transcripts for β6 are apparently less abundant in whole jejunum than in isolated epithelium and that, conversely, the intensity of RT-PCR signals for TGF-β1 and for IgA are stronger in samples of whole jejunum (WJ) with little or no signal for IgA in isolated epithelium. e: Relative abundance of mast cell protease transcripts in isolated epithelium from S129 mice. The graphs show the intensity of each PCR product as a proportion of corresponding GAPDH product from the same sample. Data are shown from samples taken on day 0 (uninfected) (open columns), day 5 (horizontally lined columns), day 7 (hexagonally lined columns), and day 9 (filled columns) after infection with N. brasiliensis. Primers were for mMCP-1 (M1) and mMCP-2 (M2). (dOvsd9: ∗p < 0.05, ∗∗p < 0.01).
Expression of Mast Cell Proteases in Jejunal Epithelium
Because MMCs in parasitized mice are predominantly intraepithelial, 7,15 their presence in the isolated epithelium preparations was further evaluated by RT-PCR. Transcripts for the MMC-specific genes mMCP-1 and mMCP-2 28 were abundant in gut epithelium isolated fromS129 mice infected with N. brasiliensis compared with uninfected controls (day 0 versus day 9; P < 0.05) (Figure 1e) ▶ . This was consistent with epithelial infiltration by mMCP-1-expressing MMCs that is detectable by immunohistochemistry 15 and previous observations in isolated epithelium from BALB/c mice. 17
Mice Lacking the Integrin β6 Tolerate Infection with N. Brasiliensis and Expel the Worms with Normal Kinetics
To investigate whether the expression of β6 by enterocytes is of functional importance in the immune rejection of nematodes, β6−/− and β6+/+ S129 mice (n = 4 to 5 mice/group/time point) were infected subcutaneously with 500 N. brasiliensis larvae and the course of infection was followed until day 9. Mice in the two groups carried comparable worm burdens at all stages of infection and immune rejection of the parasite was complete by day 9 (Table 1) ▶ . This suggested that the rejection process was not affected by the absence of β6.
Table 1.
Mean Worm Burdens (±SE) from β6+/+ and β6−/− Mice 5, 7, and 9 Days after Infection with 500 N. brasiliensis (Rat Adapted) L3
| Worm burdens | ||
|---|---|---|
| β6+/+ | β6−/− | |
| Day 5 | 62.5 ± 37.1 | 102.3 ± 44.8 |
| Day 7 | 9 ± 5.0 | 3.4 ± 1.1 |
| Day 9 | 0.2 ± 0.2 | 0.5 ± 0.3 |
MMC Hyperplasia Is Compromised in Mice Lacking the Integrin β6
The number of MMCs detected by toluidine blue staining increased significantly above uninfected control levels in infected β6+/+ mice (P < 0.05), but MMCs were virtually absent in both control and infected β6−/− mice except on day 9, when the count was ∼10% of that seen in the β6+/+ mice at the same time point (β6+/+ versus β6−/− on day 5, P < 0.05; and on days 7 and 9, P < 0.01) (Figure 2a) ▶ . The majority of MMCs were intraepithelial in the infected β6+/+mice [91% (SE ± 1.4) on day 9] (Figure 2c) ▶ . MMCs in β6−/− mice were rare (Figure 2d) ▶ and only 39% (SE ± 4.4) were intraepithelial. The lack of MMCs in β6−/− mice was confirmed by staining for esterase; the numbers of esterase+ve MMCs increased significantly above uninfected levels in the β6+/+ mice late in infection [day 0, 0.1 to 0.4 MMCs/vcu (SE ± 0.4); day 9, 0.4 to 3.7 MMCs/vcu (SE ± 0.8) (P < 0.05)] whereas esterase+ve MMCs in the β6−/− mice were absent in most samples [day 9, 0 to 0.05 MMCs vcu (SE ± 0.01); β6−/− versus β6+/+; P < 0.01]. This was in contrast with the staining with toluidine blue in which some mast cells were found in the β6−/− jejunum on day 9 of infection (Figure 2, a and d) ▶ . Toluidine blue+ve and esterase+ve mast cells in the submucosa were rare in both groups (0.05 to 0.25 toluidine blue+ MMCs per vcu in both groups on day 9). The lack of esterase staining is consistent with studies in mMCP-1−/− mice, suggesting that in the absence of this chymase (see below), toluidine blue+ve MMCs are esterase−ve. 22 Interestingly, numbers of toluidine blue-stained mast cells in the gastric mucosa (glandular region) of the stomach were significantly greater (P < 0.05) in uninfected β6−/− mice when compared with β6+/+controls (Figure 2b) ▶ . Although there was a trend toward increasing numbers of gastric MMCs in the β6−/− mice during infection, this was not significantly different from uninfected levels or from infected β6+/+ mice.
Figure 2.
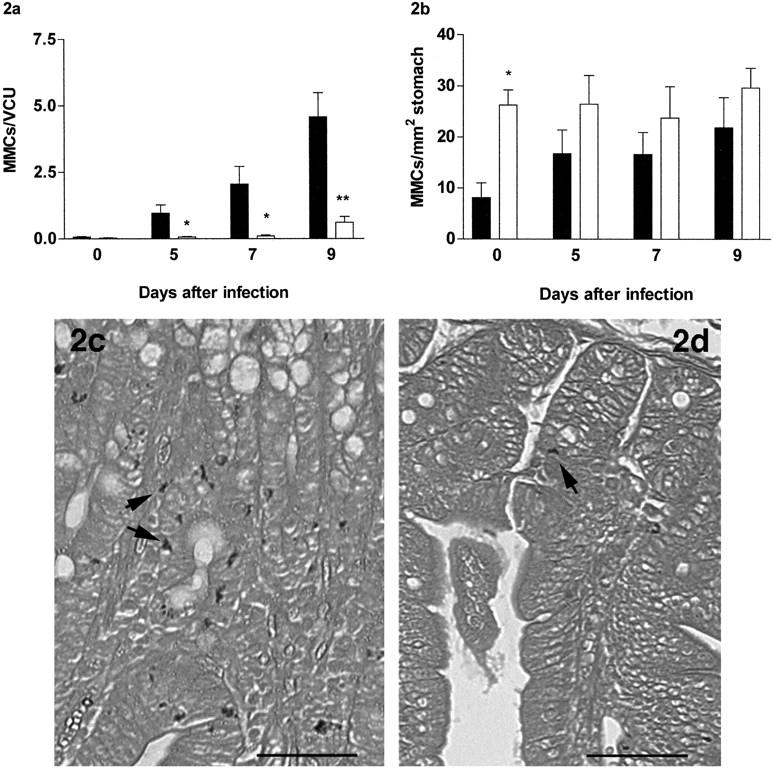
a: Mucosal mast cells quantified per villus/crypt unit (vcu) (±SE) in the jejunum from β6+/+ (filled columns) and β6−/− (open columns) S129 mice on day 0 (uninfected), and days 5, 7, and 9 after N. brasiliensis infection. b: Mast cells (±SE) per mm2 in the stomach (glandular region) from β6+/+ and β6−/− S129 mice on day 0 (uninfected), and days 5, 7, and 9 after N. brasiliensis infection. (β6+/+ vs. β6−/−; ∗p < 0.05, ∗∗p < 0.01). c and d: Toluidine blue/eosin-stained jejunum from β6+/+ (c) and β6−/− (d) S129 mice 9 days after infection with N. brasiliensis (horizontal bar, 50 μm). Arrows indicate the presence of toluidine blue-stained MMCs.
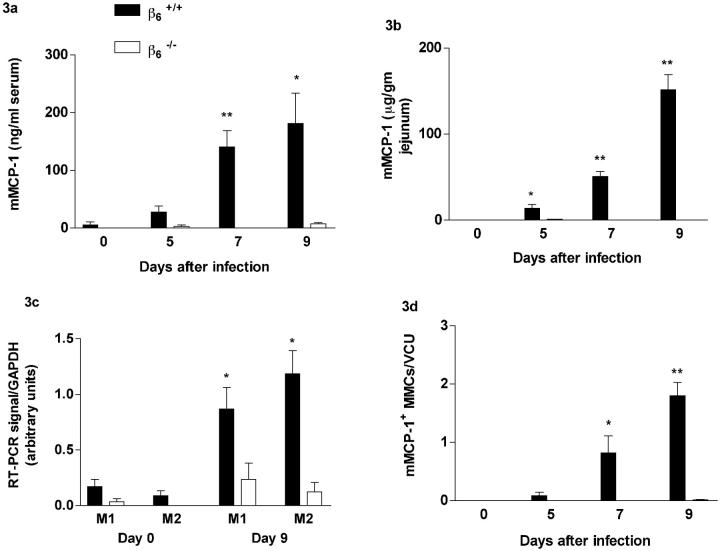
Expression of the Mucosal Mast Cell-Specific Chymase mMCP-1 Is Reduced in β6−/− Mice
The kinetics of the systemic release of mMCP-1 into the circulation (Figure 3a) ▶ and of mMCP-1 expression in the jejunum (Figure 3b) ▶ in β6+/+ controls after infection with N. brasiliensis were as described previously for the rat-passaged strain of the parasite. 15 In contrast, mMCP-1 was virtually undetectable in β6−/− mice at any stage of infection (β6−/− versus β6+/+controls on day 5, P < 0.05; and on days 7 and 9, P < 0.01) (Figure 3b) ▶ . RT-PCR analysis of jejunal RNA from β6+/+ mice showed significant up-regulation of transcription of the MMC proteases mMCP-1 and mMCP-2 in infected compared to uninfected mice (day 0 versus day 9; P < 0.05) (Figure 3c) ▶ . mMCP-1 and mMCP-2 transcripts remained low or were undetectable in β6−/− gut samples (β6−/− versus β6+/+controls on day 9, P < 0.05) (Figure 3c) ▶ . Immunohistochemical staining showed that mMCP-1+ve MMCs were significantly increased in infected β6+/+ mice (P < 0.05) but were virtually absent in the β6−/− mice (Figure 3d) ▶ , which was consistent with the absence of esterase staining in these mice. mMCP-1+ve cells in infected β6+/+ jejunum were again predominantly intraepithelial. mMCP-1+ve cells in the stomach of infected β6+/+ mice were very rare, and none were detected in infected β6−/− mice (data not shown). Integrin β6 transcripts were expressed in β6+/+ jejunum and stomach, but were not altered during infection (data not shown). No β6 transcripts were detected in β6−/− tissues. TGF-β1 transcripts were constitutively expressed and appeared to be unaltered in both infected and uninfected β6+/+ and β6−/− jejunum (data not shown) in accordance with previous data that TGF-β1 synthesis is unaffected by β6 deletion. 6
Figure 3.
a and b: Concentrations of mMCP-1 in serum (μg/ml) (a) and jejunal homogenates (μg/g wet wt) (b) from β6+/+ (filled columns) and β6−/− (open columns) S129 mice on day 0 (uninfected), and days 5, 7, and 9 after infection. c: Abundance of protease gene transcripts in jejunal RNA. The data shows the intensity of each PCR product as a proportion of the corresponding GAPDH product from the same sample. Data are shown from samples taken from β6+/+ and β6−/− 129 mice on days 0 and 9 after infection with N. brasiliensis as indicated. Primers were for mMCP-1 (M1) and mMCP-2 (M2) as indicated. d: mMCP-1+ve MMC counts (±SE) in the jejunum from β6+/+ and β6−/− S129 mice on day 0 (uninfected), and days 5, 7, and 9 after N. brasiliensis infection after staining with mAb RF6.1. Cell counts are shown per villus/crypt unit (vcu). (β6+/+ vs. β6−/−: ∗p < 0.05, ∗∗p < 0.01).
β6−/− Mice Develop Normal Jejunal IEL Recruitment, Goblet Cell Hyperplasia, and Eosinophilia in Response to Infection
It was important to establish whether, in the absence of β6, other potential effector responses such as goblet cell hyperplasia, tissue eosinophilia, or recruitment of CD3+ IELs were altered after infection. Uninfected β6−/− mice tended to show higher baseline numbers of goblet cells than β6+/+ mice, but this was not significant (Table 2) ▶ . Both β6+/+ and β6−/− mice developed goblet cell hyperplasia on infection with no significant differences between the two groups, although this was more pronounced in β6+/+mice (day 0 versus days 5, 7, and 9; P <0.05) (Table 2) ▶ . Numbers of eosinophils were significantly greater in the jejunum of uninfected β6−/− mice when compared with β6+/+ mice (P < 0.05) but numbers increased in both groups during infection and were not significantly different (Table 2) ▶ . Eosinophils were detected in the stomach in both groups, but were not significantly different between β6+/+ and β6−/− mice [β6+/+: day 0, 0.4 to 1.5 eosinophils/mm2 (SE ± 0.3); day 9, 0.2 to 4.2 eosinophils/mm2 (SE ± 0.7); β6−/−: day 0, 0.6 to 3.1 eosinophils/mm2 (SE ± 0.5); day 9, 0.8 to 4.7 eosinophils/mm2 (SE ± 1.1)]. The numbers of CD3+ve IELs in the jejunum were variable but significantly higher in uninfected β6−/− compared to β6+/+ mice (P < 0.05) (Table 2) ▶ . There was no overall increase in numbers of CD3+ve IELs during infection in either group (Table 2) ▶ .
Table 2.
Goblet Cells Per Villus/Crypt Unit (vcu) (±SE), Eosinophils per mm2 (±SE), and CD3+ve Intraepithelial Lymphocytes (IELs) per vcu (±SE) in the Jejunum from β6+/+ and β6−/− on Day 0 (Uninfected) and Days 5, 7, and 9 after Infection with N. Brasiliensis
| Goblet cells/vcu | Eosinophils/mm2 | CD3+ve IELs/vcu | ||||
|---|---|---|---|---|---|---|
| β6+/+ | β6−/− | β6+/+ | β6−/− | β6+/+ | β6−/− | |
| Day 0 | 7.6 ± 0.8 | 11.7 ± 1.7 | 7.3 ± 1.2 | 14.5 ± 1.7 | 4.3 ± 0.3 | 7.2 ± 1.0 |
| Day 5 | 11.1 ± 0.2 | 12.2 ± 1.0 | 20.2 ± 5.3 | 27 ± 3.1 | 4.1 ± 0.9 | 4.7 ± 0.5 |
| Day 7 | 14.1 ± 0.6 | 12.3 ± 2.8 | 22.5 ± 7.1 | 23.8 ± 2.1 | 4.1 ± 0.7 | 5.6 ± 0.8 |
| Day 9 | 15.7 ± 0.7 | 13.5 ± 1.7 | 19.3 ± 2.7 | 24.5 ± 1.2 | 5.2 ± 0.7 | 5.4 ± 1.1 |
*, P < 0.05 according to Mann-Whitney nonparametric test, as referred to in text.
Discussion
Although there is considerable evidence that integrin αVβ6 mediates lung inflammatory responses through TGF-β1 activation, 6,12,13 the role of this integrin in inflammatory responses in the intestine has not been previously investigated. Our data strongly suggests, for the first time, that integrin αVβ6 and TGF-β1 are constitutively co-expressed in the epithelial compartment of the murine jejunum, and that the integrin αVβ6 is primarily confined to the crypts, the main site of mast cell recruitment and mMCP-1 expression. The responses to intestinal nematode infection in β6−/− mice were marked by a virtual absence of mMCP-1+ve and esterase+ve MMCs and a highly significant reduction in MMC hyperplasia. Our previous in vitro observations showing mMCP-1 is a TGF-β1-inducible gene 8,9 indicates that presentation of mature TGF-β1 at the cell surface of the epithelium to differentiating MMCs would be expected to induce mMCP-1 expression in vivo. The lack of mMCP-1 expression in β6−/− mice lends weight to our hypothesis that the integrin αvβ6 is required for epithelial processing of latent TGF-β1 latency-associated peptide in the intestine. Although numerous pathways for TGF-β1 activation have been identified in the lung apart from integrin αvβ6, many of these are not confined to the epithelium 29 or are macrophage-dependent 1,30,31 and so are unlikely to be important in the intestinal epithelium where macrophages are rare. Our observations are in agreement with the growing body of evidence that activation of latent TGF-β1 is tissue-restricted and mediated by localized factors. 32 The concurrent substantial reduction in intestinal MMCs, but not gastric MMC hyperplasia in β6−/− mice is somewhat surprising. However, gastric MMCs in BALB/c mice, and apparently in S129 mice (PA Knight, unpublished observations) are phenotypically distinct from intestinal MMCs and do not normally express mMCP-1, 14,15 and therefore may be regulated or recruited by a different mechanism. Mast cell precursors increase in number in the jejunal epithelium during the early phase of nematode infection, 33,34 and the most likely explanation for the failure of the MMC response in parasitized β6−/− mice is either that mature TGF-β1 is an essential differentiation or chemotactic factor for the precursors that are recruited to the intestinal epithelium, or that it regulates the local epithelial expression of other MMC-specific differentiation factors that have yet to be identified.
As might be predicted, the kinetics of worm expulsion was similar in both groups because expulsion of N brasiliensis is influenced by goblet-cell mucins but independent of mast cell responses in the mouse, 35 and there were no significant differences in goblet cell responses between infected β6−/− and β6+/+ mice. Uninfected β6−/− mice had significantly higher levels of CD3+ve IELs and tissue eosinophilia than β6+/+ mice, which is consistent with observations of higher baseline inflammatory responses in the skin and lungs. 13,18 However, eosinophil and IEL responses were similar in both groups during infection. It is surprising that IEL numbers were unaffected in β6−/− mice because they express αEβ7 36 and, as is the case for mast cells, 9 expression of this integrin by T cells is regulated by TGF-β1 in vitro. 37 Nevertheless, in a recent study in which parasitized mice were treated systemically with anti-αE or -β7 antibodies, there was selective depletion of MMCs but not IELs 38 indicating TGF-β1-induced expression of αEβ7 could be critical for recruitment and survival of MMCs but not of IELs within the epithelium.
In conclusion, MMC hyperplasia and granule expression of mMCP-1 during nematode infection require the expression of αVβ6 by jejunal epithelium. Future studies will be directed toward determining whether the attenuation of MMC hyperplasia is a consequence of a defect in the recruitment of mast cell precursors to the epithelium or whether, having entered the epithelial compartment early in infection, 33 the precursors are unable to differentiate in the absence of mature TGF-β1.
Acknowledgments
We thank Dr. Richard Locksley and his group at the University of California, San Francisco, for lending us laboratory facilities and assistance in performing the nematode work with β6−/− mice; Mike Wilkinson (GlaxoSmithKline) for the donation of the Bio-Rad MRC-600 confocal microscope; Dr. Cheryl Scudamore for helpful advice; Liz Thornton and Margaret McPhee for their technical assistance; Neil MacIntyre and others in the Department of Veterinary Pathology for histological processing; and Eileen Duncan, Liz Moore, and Judith Pate for technical support.
Footnotes
Address reprint requests to P. A. Knight, Dept. of Veterinary Clinical Studies, University of Edinburgh, Easter Bush Veterinary Centre, Easter Bush Roslin, Midlothian, UK EH25 9RG. E-mail: pam.knight@ed.ac.uk.
Supported by the Wellcome Trust (grant no. 060312) and the Biotechnology and Biological Science Research Council (grant no. 15/S10130).
References
- 1.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA: Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta (1). J Exp Med 2001, 194:809-822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Podolsky DK: Healing the epithelium: solving the problem from two sides. J Gastroenterol 1997, 32:122-126 [DOI] [PubMed] [Google Scholar]
- 3.MacDonald TT: Effector and regulatory lymphoid cells and cytokines in mucosal sites. Curr Top Microbiol Immunol 1999, 236:113-135 [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C: Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J 1999, 18:1280-1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roche JK, Martins CA, Cosme R, Fayer R, Guerrant RL: Transforming growth factor beta1 ameliorates intestinal epithelial barrier disruption by Cryptosporidium parvum in vitro in the absence of mucosal T lymphocytes. Infect Immun 2000, 68:5635-5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D: The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999, 96:319-328 [DOI] [PubMed] [Google Scholar]
- 7.Miller HR: Mucosal mast cells and the allergic response against nematode parasites. Vet Immunol Immunopathol 1996, 54:331-336 [DOI] [PubMed] [Google Scholar]
- 8.Miller HR, Wright SH, Knight PA, Thornton EM: A novel function for transforming growth factor-beta1: upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific beta-chymase, mouse mast cell protease-1. Blood 1999, 93:3473-3486 [PubMed] [Google Scholar]
- 9.Wright SH, Brown J, Knight PA, Thornton EM, Kilshaw PJ, Miller HRP: Transforming growth factor-beta1 mediates coexpression of the integrin subunit alphaE and the chymase mouse mast cell protease-1 during the early differentiation of bone marrow-derived mucosal mast cell homologues. Clin Exp Allergy 2002, 32:315-324 [DOI] [PubMed] [Google Scholar]
- 10.Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R: Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem 1993, 41:1521-1527 [DOI] [PubMed] [Google Scholar]
- 11.Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, Gillet N, Sheppard D, Matthay MA, Albelda SM, Kramer RH, Pytela R: Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 1995, 108:2241-2251 [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Wu J, Spong S, Sheppard D: The integrin alphavbeta6 is critical for keratinocyte migration on both its known ligand, fibronectin, and on vitronectin. J Cell Sci 1998, 111:2189-2195 [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Wu J, Zhu W, Pytela R, Sheppard D: Expression of the human integrin beta6 subunit in alveolar type II cells and bronchiolar epithelial cells reverses lung inflammation in beta6 knockout mice. Am J Respir Cell Mol Biol 1998, 19:636-642 [DOI] [PubMed] [Google Scholar]
- 14.Wastling JM, Scudamore CL, Thornton EM, Newlands GF, Miller HR: Constitutive expression of mouse mast cell protease-1 in normal BALB/c mice and its up-regulation during intestinal nematode infection. Immunology 1997, 90:308-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scudamore CL, McMillan L, Thornton EM, Wright SH, Newlands GF, Miller HR: Mast cell heterogeneity in the gastrointestinal tract: variable expression of mouse mast cell protease-1 (mMCP-1) in intraepithelial mucosal mast cells in nematode-infected and normal BALB/c mice. Am J Pathol 1997, 150:1661-1672 [PMC free article] [PubMed] [Google Scholar]
- 16.Bjerknes M, Cheng H: Methods for the isolation of intact epithelium from the mouse intestine. Anat Rec 1981, 199:565-574 [DOI] [PubMed] [Google Scholar]
- 17.Rosbottom A, Knight PA, McLachlan G, Thornton EM, Wright SW, Miller HR, Scudamore CL: Chemokine and cytokine expression in murine intestinal epithelium following Nippostrongylus brasiliensis infection. Parasite Immunol 2002, 24:67-75 [DOI] [PubMed] [Google Scholar]
- 18.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr, Sheppard D: Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 1996, 133:921-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR: Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med 2000, 192:1849-1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vischer NOE, Huls PG, Woldringh CL: Object-image: an interactive image analysis program using structured point collection. Binary 1994, 6:160-166 [Google Scholar]
- 21.Rasband WS, Bright DS: NIH image: a public domain image processing program for the Macintosh. Microbeam Analysis 1995, 4:137-149 [Google Scholar]
- 22.Wastling JM, Knight P, Ure J, Wright S, Thornton EM, Scudamore CL, Mason J, Smith A, Miller HR: Histochemical and ultrastructural modification of mucosal mast cell granules in parasitized mice lacking the beta-chymase, mouse mast cell protease-1. Am J Pathol 1998, 153:491-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huntley JF, Newlands GF, Gibson S, Ferguson A, Miller HR: Histochemical demonstration of chymotrypsin like serine esterases in mucosal mast cells in four species including man. J Clin Pathol 1985, 38:375-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomita M, Kobayashi T, Itoh H, Onitsuka T, Nawa Y: Goblet cell hyperplasia in the airway of Nippostrongylus brasiliensis-infected rats. Respiration 2000, 67:565-569 [DOI] [PubMed] [Google Scholar]
- 25.Gauvreau GM, O’Byrne PM, Moqbel R, Velazquez J, Watson RM, Howie KJ, Denburg JA: Enhanced expression of GM-CSF in differentiating eosinophils of atopic and atopic asthmatic subjects. Am J Respir Cell Mol Biol 1998, 19:55-62 [DOI] [PubMed] [Google Scholar]
- 26.Anderson C, Rezuke WN, Kosciol CM, Pastuszak WT, Cartun RW: Methods in pathology. Identification of T-cell lymphomas in paraffin-embedded tissues using polyclonal anti-CD3 antibody: comparison with frozen section immunophenotyping and genotypic analysis. Mod Pathol 1991, 4:358-362 [PubMed] [Google Scholar]
- 27.Kashiwamura S, Koyama T, Matsuo T, Steinmetz M, Kimoto M, Sakaguchi N: Structure of the murine mb-1 gene encoding a putative sIgM-associated molecule. J Immunol 1990, 145:337-343 [PubMed] [Google Scholar]
- 28.Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL: Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol 1996, 135:279-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bornstein P: Thrombospondins as matricellular modulators of cell function. J Clin Invest 2001, 107:929-934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalil N, Corne S, Whitman C, Yacyshyn H: Plasmin regulates the activation of cell-associated latent TGF-beta 1 secreted by rat alveolar macrophages after in vivo bleomycin injury. Am J Respir Cell Mol Biol 1996, 15:252-259 [DOI] [PubMed] [Google Scholar]
- 31.Matrat M, Lardot C, Huaux F, Broeckaert F, Lison D: Role of urokinase in the activation of macrophage-associated TGF-beta in silica-induced lung fibrosis. J Toxicol Environ Health A 1998, 55:359-371 [DOI] [PubMed] [Google Scholar]
- 32.Koli K, Saharinen J, Hyytiainen M, Penttinen C, Keski-Oja J: Latency, activation, and binding proteins of TGF-beta. Microsc Res Tech 2001, 52:354-362 [DOI] [PubMed] [Google Scholar]
- 33.Dillon SB, MacDonald TT: Limit dilution analysis of mast cell precursor frequency in the gut epithelium of normal and Trichinella spiralis infected mice. Parasite Immunol 1986, 8:503-511 [DOI] [PubMed] [Google Scholar]
- 34.Tegoshi T, Okada M, Nishida M, Arizono N: Early increase of gut intraepithelial mast cell precursors following Strongyloides venezuelensis infection in mice. Parasitology 1997, 114:181-187 [DOI] [PubMed] [Google Scholar]
- 35.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF, Jr: Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol 1997, 15:505-533 [DOI] [PubMed] [Google Scholar]
- 36.Andrew DP, Rott LS, Kilshaw PJ, Butcher EC: Distribution of alpha 4 beta 7 and alpha E beta 7 integrins on thymocytes, intestinal epithelial lymphocytes and peripheral lymphocytes. Eur J Immunol 1996, 26:897-905 [DOI] [PubMed] [Google Scholar]
- 37.Kilshaw PJ, Murant SJ: Expression and regulation of beta 7 (beta p) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur J Immunol 1991, 21:2591-2597 [DOI] [PubMed] [Google Scholar]
- 38.McDermott JR, Grencis RK, Else KJ: Leucocyte recruitment during enteric nematode infection. Immunology 2001, 103:505-510 [DOI] [PMC free article] [PubMed] [Google Scholar]