Abstract
Retinal neovascularization is a leading cause of human blindness. However, little is known concerning the molecular mechanisms controlling retinal neovascularization in vivo. Here we provide evidence that exposure of a collagen type IV cryptic epitope detected by monoclonal antibody (mAb) HUIV26, delineates sites of vascular bud formation and represents one of the earliest structural remodeling events required before vessel out-growth. Exposure of these cryptic sites was inhibited in matrix metalloproteinase (MMP)-9-deficient but not MMP-2-deficient mice implicating MMP-9 in their exposure. Retinal endothelial cell interactions with the HUIV26 epitopes induced endothelial cell migration, which was blocked by mAb HUIV26. Importantly, subcutaneous administration of mAb HUIV26 potently inhibited retinal angiogenesis in vivo. Taken together, these findings suggest a novel mechanism in which MMP-9 facilitates exposure of HUIV26 cryptic sites, thereby promoting retinal endothelial cell migration and neovascularization in vivo.
A leading cause of human blindness associated with diabetic retinopathy, retinopathy of prematurity, and macular degeneration results from aberrant retinal neovascularization. 1-3 Clinical intervention aimed at blocking neovascularization within specific ocular microenvironments such as the retina, may have a significant impact on loss of vision. Moreover, because much of the retinal damage associated with these neovascular eye diseases are irreversible, early detection of retinal neovascularization is likely to be of critical importance in initiating effective treatments to limit vision loss. Thus, early detection of vascular changes associated with angiogenesis in these diseases and an in-depth understanding of the molecular and biochemical mechanisms that control retinal neovascularization are of great importance.
A primary focus of recent anti-angiogenic strategies has been directed to growth factors and their receptors, cell adhesion molecules, and proteolytic enzymes. 4-7 A variety of growth factors and cytokines have been shown to contribute to retinal neovascularization, including vascular endothelial growth factor (VEGF) and insulin-like growth factor-1. 8,9 These factors function in conjunction with cell adhesion molecules and matrix-altering enzymes to create a permissive microenvironment for new vessel growth. 1-10 Although cellular interactions with the extracellular matrix (ECM) contribute to nearly all of the biochemical processes that regulate angiogenesis, little attention has been directed toward the ECM as an important regulator of retinal neovascularization. It is important to note that although cellular interactions with native ECM components are essential for homeostasis, interactions with denatured forms of ECM proteins are likely to be of critical importance in disease processes. In fact, proteolytic remodeling may play a vital role in retinal angiogenesis because protease antagonists inhibit neovascularization in animal models. 11,12 Although the mechanisms by which proteolytic activity contributes to retinal angiogenesis is not clear, it is possible that this activity may act to selectively expose matrix-immobilized cryptic regulatory elements that are normally inaccessible within native ECM molecules. Cellular interactions with these cryptic sites may initiate unique signaling cascades required for new blood vessel development. If these assumptions prove correct, then exposure of unique cryptic ECM regulatory elements may represent some of the earliest structural remodeling events that are crucial for the initiation of angiogenesis. More importantly, exposure of these cryptic ECM sites may not only provide critical new information concerning the molecular control mechanisms regulating neovascularization, but may provide a unique opportunity for the early detection, targeting, and treatment of neovascular eye diseases.
To this end, we developed a panel of monoclonal antibodies (mAbs) directed to cryptic matrix epitopes that are exposed after proteolysis. 13 One of these cryptic epitopes (HUIV26) was detected within basement membrane collagen type IV of tumor-associated blood vessels. 13,14 Importantly, this mAb is directed to a cryptic epitope that plays a functional role in angiogenesis and tumor growth because systemic administration of this mAb potently inhibited these processes in vivo. 14 Interestingly, the ECM of retinal vessels and the intraocular levels of matrix-altering enzymes in patients diagnosed with proliferative diabetic retinopathy are altered as compared to normal patients. 15-17 In fact, the composition and molecular integrity of the ECM surrounding different vascular beds may be distinct. Interestingly, studies have suggested that certain molecules that are known to regulate tumor angiogenesis in vivo, have only little if any effect in models of retinal angiogenesis. 18,19 These findings suggest that angiogenesis occurring in different vascular beds may be controlled by distinct mechanisms. Therefore, it is critical that we understand the mechanisms that regulate angiogenesis associated with distinct diseases so that we might develop new approaches for their treatment.
Materials and Methods
Chemicals, Reagents, and Antibodies
Purified ECM protein collagen type I and collagen type IV were obtained from Sigma (St. Louis, MO). Mounting medium and fluorescein isothiocyanate (FITC)-Lycopersicon esculentum lectin were obtained from Vector Laboratories (Burlingame, CA). L. esculentum lectin-coated magnetic beads were obtained from Dynal (Oslo, Norway). FITC-dextran (2 × 106) was obtained from Sigma. Polyclonal antibody AB769 that recognizes both triple-helical and denatured collagen type IV was obtained from Chemicon (Temecula, CA), mAb HUIV26 has been described previously, 13,14 mAb to VEGF was obtained from Santa Cruz Biotechnology (Santa Cruz, CA,) and polyclonal antibodies to matrix metalloproteinase (MMP)-2 and MMP-9 were obtained from Chemicon (Temecula, CA).
Cells and Cell Culture
Retinal endothelial cells were prepared from bovine sensory retina by mechanical dissection and filtration through nylon mesh and concentrated with L. esculentum lectin-coated magnetic beads (Dynal). The cells were grown in endothelial growth medium, obtained from Clonetics (San Diego, CA), supplemented with 10% fetal bovine serum, bovine brain extract (12 μg/ml), human epidermal growth factor (10 ng/ml), and hydrocortisone (1 μg/ml). The identity of microvascular endothelial cells was confirmed by their cobblestone morphology on phase-contrast microscopy and the purity was determined by strong positive immunoreactivity to von Willebrand factor and incorporation of dil-acetylated low-density lipoprotein (Biomedical Technologies, Stoughton, MA). Only cultures that had >98% von Willebrand factor- and dil-acetylated low-density lipoprotein-positive cells were used for analysis.
Murine Model of Ischemia-Induced Retinal Neovascularization
The murine ischemia-induced retinal neovascularization model was performed essentially as described. 20 Briefly, 7-day-old pups and their nursing mothers (C57BL/6J; Jackson Laboratory, Bar Harbor, ME) housed in an air-tight chamber were exposed to 75% O2 for 5 days and then returned to normoxic conditions (room air) for an additional 5 days. Retinal neovascularization occurred in 100% of the animals with a maximum angiogenic response noted at P17. In antibody inhibition experiments, purified mAbs or isotype-matched controls were injected subcutaneously twice a day (1.0 μg to 100 μg/mouse/injection) from P12 to P17. Quantification of retinal neovascularization was performed as previously described. 20 At day 17 mice were sacrificed and eyes were enucleated, fixed in 4% paraformaldehyde at 4°C, and embedded in paraffin. Step axial sections (6 μm, every 18 μm) were obtained, starting at the edge of the optic nerve head and passing from nasal to temporal. Tissue sections were stained with periodic acid-Schiff reagent and hematoxylin and up to 20 sections were analyzed over a span of 480 μm. Neovascularization was quantified by counting the number of retinal vascular cell nuclei internal to the inner limiting membrane. No vascular cell nuclei internal to the inner limiting membrane were seen in normal animals. Retinal neovascularization was also visualized by either staining for collagen type IV, intracardiac perfusion (1.5 ml) of 5% (w/v) FITC-dextran (2 × 106) in 4% paraformaldehyde, or staining by intracardiac perfusion of FITC-L. esculentum lectin. All of the animal studies adhered to the “Association for Research in Vision and Ophthalmology (ARVO) Statement on the Use of Animals in Ophthalmic and Vision Research.”
MMP-Null Mouse Studies
The MMP-2-null mouse line was a generous gift from Dr. Shigeyoshi Itohara (Brain Science Institute, Riken, Japan). 21 The MMP-9-null mouse line has been described previously. 22 Neovascularization was initiated in litters from heterozygous parents. Genotyping of each pup was performed after sacrifice by polymerase chain reaction. MMP-2−/−: primers for endogenous MMP-2 gene, sense: 5′-caa cga tgg agg cac gag tg-3′; anti-sense: 5′-gcc ggg gaa ctt gat gat gg-3′, expected fragment size, 120 bp; primers for pgk-neo gene cassette: sense: 5′-ctt ggg tgg aga ggc tat tc-3′; anti-sense: 5′-agg tga gat gac agg aga tc-3′, expected fragment size, 280 bp; MMP-9−/−: primers for endogenous MMP-9 gene, sense: 5′-tgc ccc atg tca ctt tcc ct-3′; anti-sense: 5′-cag gaa gac gaa ggg gaa ga-3′, expected fragment size, 370 bp; primers for the target gene, sense: 5′-caa cct cac gga cac cca gc-3′; anti-sense: 5′-tct tga cga gtt ctt ctg ag-3′, expected fragment size, 900 bp. Homozygous and wild pups from the same heterozygous parents were used for analysis. Data were derived from at least three independent experiments.
Whole-Mount Immunofluorescence Studies
For whole-mount preparations, eyes were enucleated and the retina was exposed by removing the cornea, lens, and the vitreous. The eyes were fixed with 4% (w/v) paraformaldehyde at 4°C overnight. After washing with phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 1% (w/v) Triton X-100 (PBS-bovine serum albumin) for 20 minutes three times, the eyes were incubated at 4°C overnight with a primary antibody and washed for 20 minutes three times. Next, the tissues were incubated at 4°C overnight in a fluorescent-labeled secondary antibody and washed 20 minutes three times. Radial cuts were then made in peripheral retina to allow flat mounting on a glass slide in mounting medium for viewing by fluorescence microscopy. The flat-mounted retina was viewed on a Nikon TE300 microscope equipped with epifluoresence filters. Images were collected with a slide book digital-imaging software (Intelligent Imaging Innovations, Inc., Denver, CO) on a Power Macintosh computer G3 linked to a high-resolution digital video camera Sensi Cam, (Cooke Co., NY) mounted on a Nikon Diaphot inverted fluorescence microscope (TE300).
Quantification of HUIV26-Positive Sites on Whole-Mount Retinas
Quantification of HUIV26 sites were performed on whole-mount retinas from mice in which vessels were stained by perfusion of FITC-L. esculentum lectin and co-stained with mAb HUIV26. HUIV26-positive sites on the pre-existing vessels were manually counted throughout the retina in a fully masked protocol. Twelve retinas were evaluated for each condition. Student’s t-test was used in data populations with normal distributions and equal variance. Data were analyzed with Mann-Whitney rank sum test for populations with nonnormal distributions and unequal variance.
Gelatin Zymography
Cultures of subconfluent endothelial cells were washed and serum-free media containing VEGF at concentrations ranging from 10 to 500 ng/ml was added. The cells were allowed to incubate for 24 hours at which time the conditioned medium was removed and concentrated. Endothelial whole-cell lysates were prepared in lysis buffer containing 1.0% TX-100, 50 mmol/L Tris, 300 mmol/L NaCl, pH 7.5. Twenty μg of total cell lysate or equal volume of concentrated conditioned medium were electrophoreses through a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel polymerized with 0.2% gelatin. Gels were washed 3 times for 1 hour each with 2.5% TX-100 and incubated for 16 hours at 37°C in collagenase buffer containing 50 mmol/L Tris, 200 mmol/L NaCl, and 10 mmol/L CaCl2, pH 7.5. Gelatinolytic activity was visualized by staining with 0.5% Coomassie blue.
Cell Migration Assay
Cell migration assays were performed essentially as described. 23,24 Briefly, membranes from transwell migration chambers were coated with either triple-helical or denatured collagen type I or IV (25 μg/ml). Bovine retinal endothelial cells (BRECs) were resuspended in migration buffer (RPMI 1640 medium containing 1.0 mmol/L MgCl2, 0.2 mmol/L MnCl2, and 0.5% bovine serum albumin) in the presence or absence of mAb HUIV26 or control antibody (100 μg/ml). Cells were allowed to migrate to the underside of the coated membranes for 3 hours. BRECs remaining on the top side were removed and cells that had migrated to the underside were stained with crystal violet. Migration was quantified by measuring the optical density of the eluted dye at a wavelength 600 nm.
Statistical Analysis
Statistical analysis was performed using Student’s t-test with data populations with normal distributions and equal variance. P values <0.05 were considered significant. Data were analyzed with Mann-Whitney rank sum test for populations with nonnormal distributions and unequal variance.
Results
Expression of Collagen Type IV, VEGF, and MMP-9 in the Ischemic Retina
Based on our previous findings, we hypothesized that exposure of the HUIV26 cryptic epitope may be one of the earliest structural remodeling events that occurs within the retinal vascular basement membrane required for initiation of angiogenesis. To evaluate this possibility, we studied the exposure and functional activity of the cryptic HUIV26 epitope after ischemia-induced retinal neovascularization in mice. Postnatal day 7 mice (P7) were subjected to hyperoxic conditions for 5 days, which induces extensive capillary dropout. 20 Mice were then returned to normoxic conditions for an additional 5 days during which time VEGF levels increased primarily because of retinal ischemia. 20 VEGF contributes to angiogenesis by initiating a cascade of signaling events leading to stimulation of endothelial cell proliferation, enhancing the expression of matrix-degrading enzymes and promoting cellular migration. 25-27 To understand the temporal, spatial, and functional relationships between angiogenesis regulatory molecules, and structural changes that may occur within the ECM, we prepared retinal tissues from mice at 0, 1, 2, 3, and 5 (P12 to P17) days after ischemia. Retinas were examined for the expression of VEGF, collagen type IV, and MMP-9 and for exposure of the HUIV26 cryptic epitopes. Collagen type IV delineates all retinal vascular beds including capillaries, arteries, and veins at all time points examined (Figure 1A) ▶ . The conventional antibodies directed to collagen type IV could not distinguish between triple-helical and denatured collagen and thus no significant changes in integrity were noted. However, exposure of the HUIV26 cryptic epitope of collagen type IV was detected at discrete sites along pre-existing vessels and occurred in a time-dependent manner (Figure 1, A and C) ▶ . The expression of VEGF and MMP-9 were also increased in association with ECM of retinal vessels (Figure 1B) ▶ . Previous studies have suggested that secreted growth factors such as VEGF can regulate expression of MMPs. 25 Therefore we examined the effects of VEGF on endothelial cell MMP expression in vitro. Our results indicated little if any change in endothelial cell MMP expression after VEGF stimulation (10 to 500 ng/ml) as assessed by gelatin zymography (data not shown). Although in vitro findings do not necessarily correlate with in vivo events, our results suggest the possibility that the MMP-9 co-localized with the HUIV26 epitope may be produced from nonendothelial sources.
Figure 1.
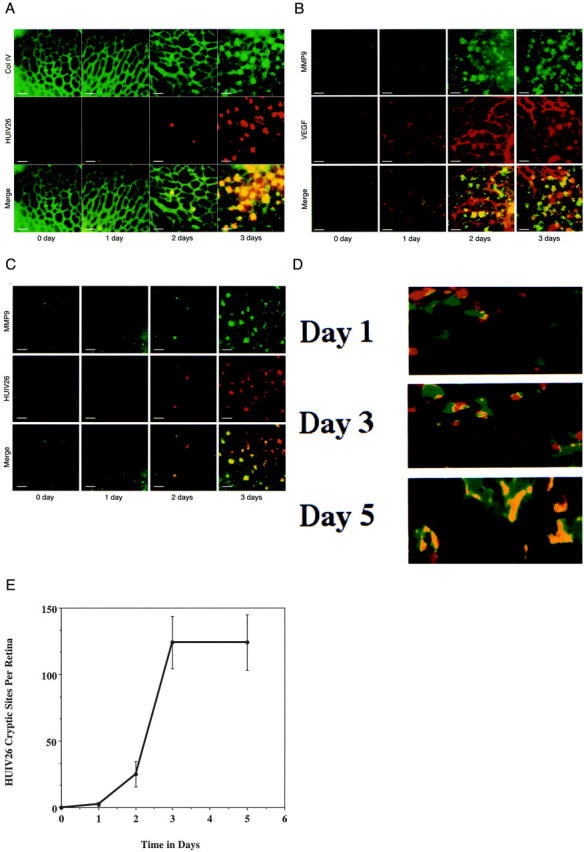
Time course of expression of collagen type IV, VEGF, and MMP-9 in the ischemic retina. Ischemia-induced retinal neovascularization was initiated and retinas subjected to hyperoxia were obtained at 0, 1, 2, 3, and 5 days (P12, P13, P14, P15, and P17) after their return to normoxic conditions. A: Retinas were co-stained with a polyclonal antibody directed to collagen type IV (green) and biotinylated mAb HUIV26 (red). B: Retinas were co-stained with a polyclonal antibody directed to MMP-9 (green) and a mAb directed to VEGF (red). C: Retinas were co-stained with a polyclonal antibody directed to MMP-9 (green) and biotinylated mAb HUIV26 (red). D: Retinas were co-stained with a polyclonal antibody directed to MMP-2 (green) and biotinylated mAb HUIV26 (red). Flat mount retinas were analyzed on a Nikon TE300 microscope with epifluoresence filters and images were captured with a charge-couple device camera for analysis. Co-localization is indicated in yellow. White bars equal 50 μm. E: Quantitation of the number of HUIV26 cryptic sites (spots) per retina. The total number of HUIV26 cryptic sites (spots) was manually counted within the total area of each retina and 18 retinas were counted per time point. Data bars represent the mean ± SD.
Remarkably, the HUIV26 cryptic epitope was detected at discrete sites along pre-existing retinal blood vessels and the number of these cryptic sites continued to increase until day 3 when they reached a maximum (Figure 1E) ▶ . These cryptic sites seemed to delineate early sites of vascular bud formation because they co-localized with endothelial cells from pre-existing vessels. Interestingly, these HUIV26 cryptic sites co-localized with MMP-9 (Figure 1C) ▶ and were associated with the basement membrane of pre-existing retinal vessels, before new angiogenic vessels were detected. The lack of new angiogenic vessels associated with the HUIV26 cryptic sites at these early time points was confirmed by lack of discrete endothelial-lined lumens and lack of pooled FITC-dextran after perfusion (data not shown). These results suggest that exposure of the HUIV26 cryptic site is an early structural modification of subendothelial collagen type IV that occurs before vessel outgrowth and may be facilitated by up-regulation of MMP-9. Although MMP-9 co-localization with the HUIV26 cryptic sites could be detected as early as 2 to 3 day after return to normoxic conditions (P14 to P15), MMP-2 was constitutively expressed and showed little co-localization at early (day 1 or 3) time points (Figure 1D) ▶ . However, at latter times points (day 5 or P17) MMP-2 did exhibit extensive co-localization with angiogenic vessels (Figure 1D) ▶ . These findings suggest that whereas both MMP-2 and MMP-9 can cleave triple-helical collagen type IV, MMP-9 may be more important in the early exposure of the HUIV26 cryptic sites within the ischemic retina.
Exposure of HUIV26 Cryptic Sites in MMP-Deficient Mice
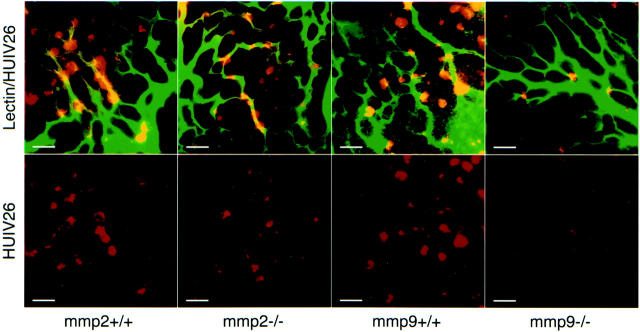
To examine whether unmasking of the HUIV26 cryptic sites requires expression of these MMPs, we studied the exposure of the HUIV26 cryptic sites during early phases of retinal neovascularization (P15) in both MMP-2- and MMP-9-null mice. 21,22 Exposure of the HUIV26 cryptic sites were quantified 3 days after mice were returned to normal oxygen conditions (P15) so that the angiogenic cascade could be initiated yet few if any angiogenic vessels would be formed. Neovascularization was initiated in litters from heterozygous parents and the number of exposed HUIV26 cryptic sites were then compared between homozygous and wild-type pups. Vessels were stained by perfusion with FITC-L. esculentum lectin to mark the pre-existing vasculature and isolated whole retinas were co-stained with mAb HUIV26 (Figure 2) ▶ . The number of exposed HUIV26 cryptic sites were then quantified. Exposure of HUIV26 cryptic sites within the retina of MMP-9-null mice were decreased by ∼75% (P < 0.001) as compared to wild-type mice, whereas little reduction was detected in MMP-2-deficient mice (data not shown). Importantly, there was no significant difference in the total avascular area formed or in VEGF mRNA or protein levels after the return to normoxic conditions (data not shown). These findings indicate that the reduction in the exposure of the HUIV26 cryptic sites within MMP-9-null mice was not associated with alterations in the total avascular area induced by hyperoxia or VEGF. Taken together, these results suggest that MMP-9, but not MMP-2, plays an essential role in the initial exposure of the HUIV26 cryptic site within the ischemic retina.
Figure 2.
Exposure of HUIV26 cryptic sites in MMP-deficient mice. Retinal angiogenesis was induced in MMP-deficient mice and wild-type controls. Retinas from P15 mice were co-stained with biotinylated mAb HUIV26 and either FITC-L. esculentum lectin or polyclonal antibody to collagen type IV. The total number of HUIV26 cryptic sites (spots) was manually counted. Examples of co-stained retinas from MMP-2-null (MMP-2−/−) and wild-type (MMP-2+/+) mice, as well as MMP-9-null (MMP-9−/−) and wild-type (MMP-9+/+) mice. Red indicates exposure of the HUIV26 cryptic sites and green indicates endothelial cell staining with FITC-L. esculentum lectin. Yellow indicates co-localization. White bars represent 50 μm.
Reduced Retinal Neovascularization in MMP-Deficient Mice
Next, we examined the relationship between the exposure of the HUIV26 cryptic sites and retinal neovascularization in MMP-2- and MMP-9-null mice. Retinal neovascularization could be detected in wild-type mice but was significantly reduced by 35 to 45% (P < 0.01) in MMP-2- and MMP-9-deficient mice, respectively (data not shown). These findings indicate that both MMP-2 and MMP-9 contribute to ischemia-induced retinal neovascularization. However, expression of MMP-9 rather than MMP-2, may be more important in the initial exposure of the HUIV26 cryptic sites and the subsequent vascular bud formation. These findings suggest that specific members of the MMP family may play distinct roles at different times during the retinal angiogenic cascade.
Subcutaneous Administration of mAb HUIV26 Inhibits Retinal Neovascularization in Vivo
To determine whether ischemia-induced retinal neovascularization requires exposure of the HUIV26 cryptic sites, antibody inhibition studies were performed. Retinal angiogenesis was induced in wild-type mice. Mice were then treated twice a day by subcutaneous injections of purified mAb HUIV26 or an isotype-matched control. Subcutaneous administration of mAb HUIV26 inhibited retinal angiogenesis as indicated by a dramatic reduction in the area of neovessel growth (Figure 3A ▶ , arrows) as compared to controls. Quantification of retinal neovascularization in cross sections demonstrated that mAb HUIV26 inhibited neovascularization in a dose-dependent manner with a maximum inhibition of ∼70% (P < 0.001) at the highest concentration tested (Figure 3, B and C) ▶ . These findings suggest that exposure of the HUIV26 cryptic epitope plays an essential role in retinal neovascularization in vivo.
Figure 3.
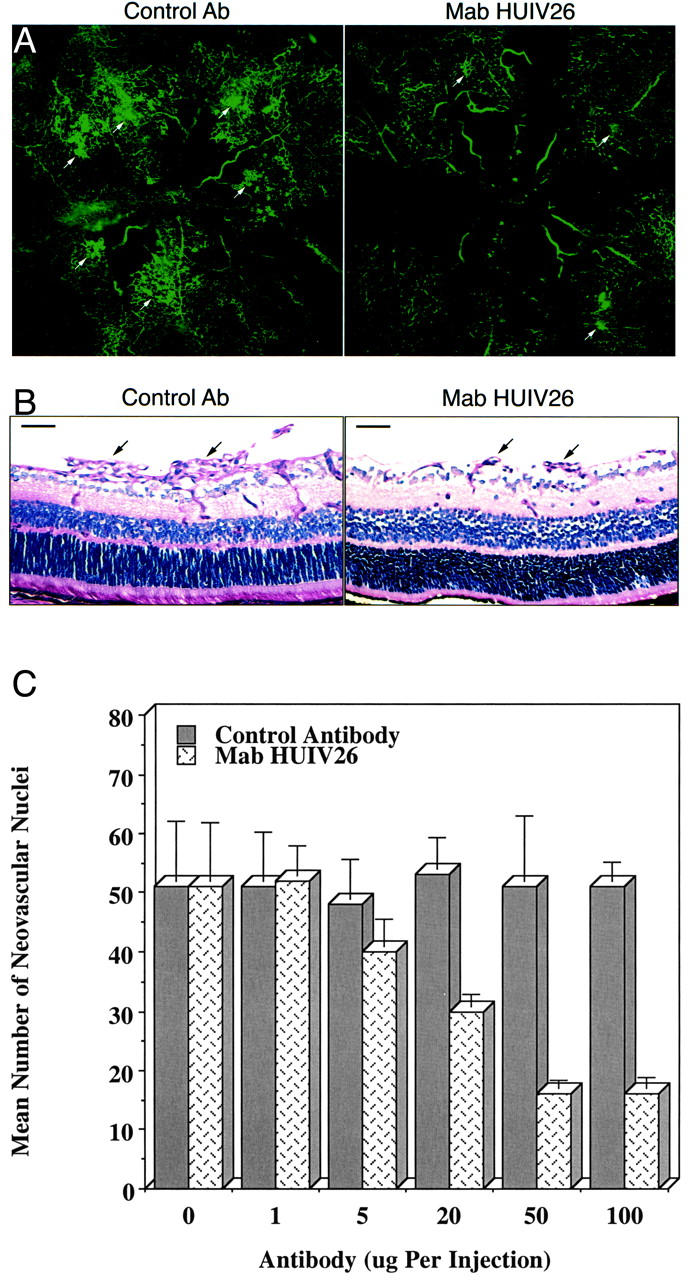
Effects of mAb HUIV26 on retinal neovascularization. Retinal angiogenesis was induced as described previously. Mice were untreated or injected twice daily (P12 through P17) with mAb HUIV26 or an isotype-matched control. A: Representative whole mounts of retinas from mAb HUIV26 and control-treated mice after FITC-dextran perfusion. Arrows indicate areas of enhanced retinal neovascularization. B: Cross sections of retinas from mice treated with either control antibody or mAb HUIV26. Arrows indicate neovessels that have grown internal to the inner limiting membrane. Black bars represent 50 μm. C: Quantification of retinal angiogenesis after a dose-response study of daily subcutaneous injections of mAb HUIV26 or control antibody. Data bars represent mean ± SEM of the number of neovascular nuclei on the vitreal side of the inner limiting membrane.
Exposure of the HUIV26 Cryptic Epitope Promotes Retinal Endothelial Cell Migration
Although it is known that integrin-mediated cellular interactions with ECM components can regulate migration, little is known concerning the migratory ability of retinal endothelial cell on denatured collagen. Because endothelial cell invasion is an early event in retinal neovascularization, coupled with the fact that exposure of the HUIV26 cryptic site occurs as an early event after induction of ischemia, we hypothesized that endothelial cell interactions with the HUIV26 site may facilitate angiogenesis by regulating retinal endothelial cell migration. To evaluate this possibility, we examined retinal endothelial cell migration on triple helical and denatured collagen. Retinal endothelial cells showed little difference in their capacity to migrate on triple helical or denatured collagen type I (Figure 4A) ▶ . In contrast, migration was enhanced by threefold on denatured collagen type IV as compared to triple-helical collagen type IV (Figure 4A) ▶ . This increase in migration was dependent on the exposure of the HUIV26 cryptic element because the enhanced migration could be completely inhibited in the presence of mAb HUIV26 (Figure 4B) ▶ . These findings suggest that exposure of the HUIV26 cryptic epitope may contribute to retinal angiogenesis by promoting endothelial cell migration.
Figure 4.
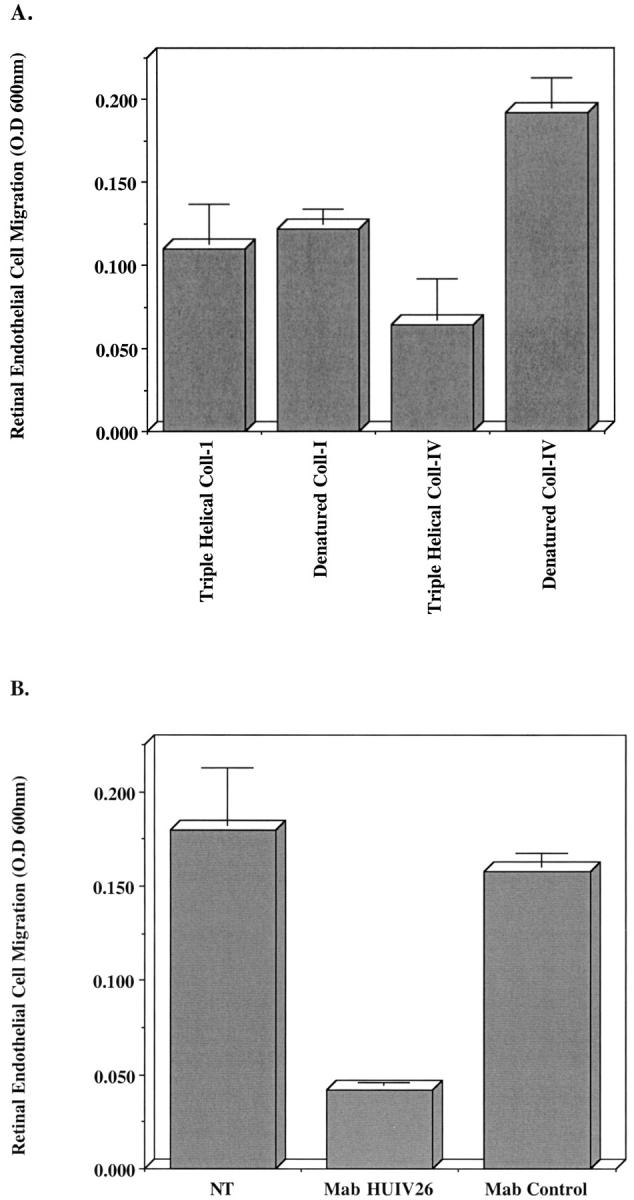
Retinal endothelial cell migration on triple helical and denatured collagen. Transwell migration chambers were coated with triple helical or denatured collagen type I or type IV (25 μg/ml). A: Retinal endothelial cells were resuspended in migration buffer and allowed to migrate for 3 hours. B: Retinal endothelial cells were resuspended in migration buffer in the presence or absence of mAb HUIV26 or an isotype-matched control and were allowed to migrate for 3 hours. Migration was quantified by measuring the optical density of eluted dye from cells that had migrated to the underside of the coated membranes. Data bars represent the mean optical density ± SD from triplicate wells. All assays were completed two to four times with similar results.
Discussion
Clinical intervention aimed at blocking neovascularization within specific ocular microenvironments such as the retina, may have a significant impact on the loss of vision. Moreover, because much of the damage associated with these neovascular eye diseases occur in a time-dependent and progressive manner, early detection of retinal neovascularization is likely to be of critical importance in initiating effective treatments to limit vision loss.
Accumulating evidence suggests that the molecular mechanisms that regulate angiogenesis within distinct vascular beds differ. 18,19,27-31 For example, numerous molecules have been identified that appear to discriminate between distinct vascular beds. 27,28,31 In studies using phage display techniques, investigators have identified distinct protein sequences that selectively bind to retinal vasculature. 27 In other studies, distinct integrin-dependent signaling pathways that regulate angiogenesis have been identified. 5 Interestingly, in αv knockout mice, vascular development within certain tissue compartments appeared normal, however, extensive vascular defects were noted within brain and intestinal tissues. 32 These findings might be explained by different angiogenesis regulatory mechanisms that operate within distinct tissue microenvironments. Moreover, although the angiogenic growth factor, basic fibroblast growth factor, is known to play a role in tumor-induced angiogenesis, little if any effect on retinal neovascularization in basic fibroblast growth factor-deficient mice was observed in a model of ischemia-induced retinopathy or choroidal angiogenesis. 18,19 Taken together, these studies demonstrate the critical importance of an in-depth understanding of the molecular mechanisms that regulate angiogenesis within distinct tissue microenvironments.
In this regard, we have extended our previous studies on cryptic regions of ECM components and angiogenesis by studying the physiological relevance of the unique HUIV26 cryptic site in retinal neovascularization. Our studies indicate for the first time that the HUIV26 cryptic epitopes are specifically associated with retinal blood vessels after ischemia-induced angiogenesis. Surprisingly, these HUIV26 cryptic sites were exposed in a time-dependent manner at discrete locations and co-localized with pre-existing retinal vessels. Exposure of these unique cryptic elements was detected early within the initiation phase of angiogenesis, before any new functional neovessels were observed. These findings suggest that exposure of the HUIV26 cryptic epitope may represent one of the earliest structural remodeling events that occur before retinal neovascularization. Thus, the early detection of the HUIV26 cryptic epitope may provide a clinically important and highly specific method to localize and image regions within the retina in which damage may occur as a result of aberrant neovascularization. In addition, the exposure of HUIV26 epitopes may also represent a highly specific method of targeting anti-angiogenic therapy to specific sites within the ischemic retina.
Our previous studies indicated that the HUIV26 cryptic epitope could be exposed by proteolytic activity, because inhibitors of both serine proteases and MMPs inhibited the exposure of this epitope in vitro and in vivo. 13,14 Although MMP-2 was constitutively expressed within the retina, MMP-9 was up-regulated after hypoxia-induced VEGF expression. The HUIV26 cryptic epitope was shown to co-localize with MMP-9 during the early initiation phase of neovascularization. Interestingly, although MMP-2 co-localized with the HUIV26 epitope in tumor-associated vessels, 14 it showed little co-localization with the HUIV26 cryptic epitope during the early phases of retinal neovascularization. Moreover, the number of HUIV26 cryptic sites detected within the retina of MMP-9-deficient mice was reduced by >75% after induction of retinal neovascularization, whereas little change was noted in MMP-2-deficient mice. Taken together, these novel findings are consistent with the hypothesis that MMP-9, rather than MMP-2, plays a more important role in initial exposure of the HUIV26 regulatory element during retinal neovascularization. These results also suggest that specific MMPs may play distinct roles during the angiogenic cascade and their relative contribution to angiogenesis may depend on the microenvironment where the neovascular response occurs. Thus, inhibiting specific MMPs may disrupt distinct processes in a temporal manner during retinal angiogenesis and depending on which MMPs are targeted, may result in differential effects on neovascularization depending on the particular vascular bed in question.
Previous studies have suggested that cellular interactions with the ECM components such as collagen can regulate cellular behavior. 33,34 Interestingly, MMP-2-mediated cleavage of laminin-5 caused enhanced breast tumor cell migration in vitro, suggesting that proteolytic cleavage may result in exposure of cryptic regulatory information that is normally hidden within the three dimensional structure of ECM proteins. 35 Consistent with this possibility, exposure of the HUIV26 cryptic regulatory elements resulted in a threefold increase in retinal endothelial cell migration whereas cell interactions with denatured collagen type I resulted in little if any change. This increase in retinal endothelial cell migratory capacity was dependent on interactions with the HUIV26 cryptic site, because mAb HUIV26 completely inhibited the enhanced migration. Taken together, these results suggest that crucial angiogenesis regulatory sites may be hidden within the three dimensional structure of specific ECM molecules and that exposure of these migratory control elements may play an important role in retinal neovascularization.
Consistent with this hypothesis, subcutaneous administration of purified mAb HUIV26 potently inhibited retinal neovascularization in a dose-dependent manner, suggesting that early exposure of the HUIV26 cryptic element plays a critical role in retinal neovascularization in vivo. Taken together, our studies provide the first evidence for a novel cooperative mechanism between MMP-9 and collagen type IV in regulating retinal angiogenesis. The exposure of a cryptic migratory control element within immobilized three-dimensional collagen type IV defines an essential remodeling event that facilitates cellular access to critical protein residues required to promote retinal endothelial cell migration and subsequent vascular bud formation. Our studies suggest that a wealth of critical angiogenic regulatory information is hidden within the three dimensional structure of ECM molecules and that specific targeting of these unique solid-state cryptic elements represents a highly specific and potentially powerful new strategy for the treatment of neovascular eye disease.
Acknowledgments
We thank Kathryn Carner for expert help in preparing the manuscript and Dr. Daniel Broek for his critical reading and helpful suggestions.
Footnotes
Address reprint requests to Peter C. Brooks Ph.D., New York University School of Medicine, Departments of Radiation Oncology and Cell Biology, The Kaplan Cancer Center, Rusk Building Rm. 806, 400 East 34th St., New York, NY 10016. E-mail: peter.brooks@med.nyu.edu.
Supported in part by grants from the National Institutes of Health [CA74132 and CA91645 (to P. C. B.), EY01545 and EY03040 (to S. J. R.)], the Ruth and Milton Steinbach Foundation to (to P. C. B. and Z. W.), the Kyoto University Foundation (to M. H.), the Japan National Society for the Prevention of Blindness (to M. H.), and the Nippon Eye Bank Association (to M. H.).
References
- 1.Adamis AP, Aiello LP, D’Amato RA: Angiogenesis and ophthalmic disease. Angiogenesis 1999, 3:9-14 [DOI] [PubMed] [Google Scholar]
- 2.Campochiaro PA: Retinal and choroidal neovascularization. J Cell Physiol 2000, 18:301-310 [DOI] [PubMed] [Google Scholar]
- 3.Campochiaro PA, Soloway P, Ryan SJ, Miller JW: The pathogenesis of choroidal neovascularization in patients with age-related macular degeneration. Mol Vis 1999, 5:34-38 [PubMed] [Google Scholar]
- 4.Ozaki H, Seo MS, Ozaki K, Yamada E, Okamoto N, Hofmann F, Wood F, Campochiaro PA: Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol 2000, 156:697-707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA: Definition of two angiogenic pathways by distinct αv integrins. Science 1995, 270:1500-1502 [DOI] [PubMed] [Google Scholar]
- 6.Das A, McLamore A, Song W, McGuire PG: Retinal neovascularization is suppressed with a matrix metalloproteinase inhibitor. Arch Ophthamol 1999, 117:498-503 [DOI] [PubMed] [Google Scholar]
- 7.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D: Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000, 2:737-744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith LE, Shen W, Perruzzi C, Stoker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, Schaeffer JM, Senger DR: Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med 1999, 5:1390-1395 [DOI] [PubMed] [Google Scholar]
- 9.Ferrar N, Alitalo K: Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999, 5:1359-1364 [DOI] [PubMed] [Google Scholar]
- 10.Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA: Disruption of angiogenesis by PEX, a non catalytic metalloproteinase fragment with integrin binding activity. Cell 1998, 92:1157-1164 [DOI] [PubMed] [Google Scholar]
- 11.Salzmann J, Limb GA, Khaw PT, Gregor ZJ, Webster L, Chignell AH, Charteris DG: Matrix metalloproteinases and their natural inhibitors in fibrovascular membranes of proliferative diabetic retinopathy. Br J Ophthalmol 2000, 84:1091-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozerdem U, Mach-Hofacre B, Chen L, Chaidhawangul S, Keefe K, McDermott CD, Bergeron-Lynn G, Appelt K, Freeman WR: The effect of prinomastat (AG3340), a potent inhibitor of matrix metalloproteinases, on a subacute model of proliferative vitroretinopathy. Curr Eye Res 2000, 6:447-453 [PubMed] [Google Scholar]
- 13.Xu J, Rodriguez D, Kim JJ, Brooks PC: Generation of monoclonal antibodies to cryptic collagen sites by using subtractive immunization. Hybridoma 2000, 19:375-385 [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, Davis GE, Brooks PC: Proteolytic exposure of a cryptic site within collagen type-IV is required for angiogenesis and tumor growth in vivo. J Cell Biol 2001, 154:1069-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, Sado Y, Huang ZS, Nesgurn AB, Kenney MC: Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem 1996, 44:1469-1479 [DOI] [PubMed] [Google Scholar]
- 16.Spirin KS, Saghizadeh M, Lewin SL, Zardi L, Kenney MC, Ljubimov AV: Basement membrane and growth factor gene expression in normal and diabetic human retinas. Curr Eye Res 1999, 6:490-499 [DOI] [PubMed] [Google Scholar]
- 17.Kon CH, Asari RH, Occleston NL, Khaw PT, Aylward GW: A prospective study of matrix metalloproteinases in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1998, 8:1524-1629 [PubMed] [Google Scholar]
- 18.Ozaki H, Okamoto N, Ortega S, Chang M, Ozaki K, Sadda S, Vinores MA, Derevjanik N, Zack DJ, Basilioco C, Campoichiaro PA: Basic fibroblast growth factor is neither necessary nor sufficient for the development of retinal neovascularization. Am J Pathol 1998, 153:757-765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjjanik NL, Vinores SA, Basilico C, Campochiaro PA: Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol 1998, 153:1641-1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amato PA: Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994, 35:101-111 [PubMed] [Google Scholar]
- 21.Itoh T, Tanioka M, Yoshida T, Nishimoto H, Itohara S: Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res 1998, 58:1048-1051 [PubMed] [Google Scholar]
- 22.Vu TH, Shipley JM, Bergers G, Berges JE, Helms JA, Hanahan D, Sharpio SD, Senior RM, Werb Z: MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93:411-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks PC, Strombald S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA: Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 1996, 85:683-693 [DOI] [PubMed] [Google Scholar]
- 24.Brooks PC, Klemke RL, Schon S, Lewis JM, Schwartz MA, Cheresh DA: Insulin-like growth factor receptor cooperates with integrin alpha v beta 5 to promote tumor cell dissemination in vivo. J Clin Invest 1997, 99:1390-1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z: Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999, 13:9-22 [PubMed] [Google Scholar]
- 26.Suzuma K, Naruse K, Suzuman I, Akahara N, Ueki K, Aiello LP, King GL: Vascular endothelial growth factor induces expression of connective tissue growth factor via KDR, Flt1 and phosphatidylinositol 3-kinase-Akt-dependent pathways in retinal vascular cells. J Biol Chem 2000, 275:40725-40731 [DOI] [PubMed] [Google Scholar]
- 27.Rajotte D, Arap W, Hagedorn M, Koivunem E, Pasqualini R, Rouslahti E: Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest 1998, 102:430-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St Croix B, Ragom C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW: Genes expressed in human tumor endothelium. Science 2000, 289:1197-1202 [DOI] [PubMed] [Google Scholar]
- 29.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG: Heterogeneity of angiogenesis and blood vessel maturation in human tumor: implications for anti-angiogenic tumor therapies. Cancer Res 2000, 60:1388-1393 [PubMed] [Google Scholar]
- 30.Vinores SA, Derevjanik NL, Vinores MA, Okamoto N, Campoichiaro PA: Sensitivity of different vascular beds in the eye to neovascularization and blood-retinal barrier breakdown in VEGF transgenic mice. Adv Exp Med Biol 2000, 476:129-138 [DOI] [PubMed] [Google Scholar]
- 31.Aird WC, Edelber JM, Weiler-Guettler H, Simmons WW, Smith TW, Rosenberg RD: Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J Cell Biol 1997, 138:1117-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bader BL, Rayburn H, Crowley D, Hynes RO: Extensive vascularization, angiogenesis, organogenesis precede lethality in mice lacking all αv integrins. Cell 1998, 95:507-519 [DOI] [PubMed] [Google Scholar]
- 33.Petitclerc E, Stromblad S, von chalscha TL, Mitjans F, Piulats J, Montgomery AMP, Brooks PC: Integrin αvβ3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res 1999, 59:2724-2730 [PubMed] [Google Scholar]
- 34.Petitclerc E, Boutaud A, Prestakyo A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Hudson BG, Brooks PC: New functions for noncollagenous domains of human collagen type-IV: novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem 2000, 275:8051-8061 [DOI] [PubMed] [Google Scholar]
- 35.Giannelli G, Flak-Marillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V: Induction of cell migration by matrix metalloprotinase-2 cleavage of laminin-5. Science 1997, 277:225-228 [DOI] [PubMed] [Google Scholar]