Abstract
Rheumatoid arthritis is characterized by progressive synovial inflammation and joint destruction. While matrix metalloproteinases (MMPs) are implicated in the erosion of unmineralized cartilage, bone destruction involves osteoclasts, the specialized cells that resorb calcified bone matrix. RANK ligand (RANKL) expressed by stromal cells and T cells, and its cognate receptor, RANK, were identified as a critical ligand-receptor pair for osteoclast differentiation and survival. A decoy receptor for RANKL, osteoprotegerin, (OPG) impinges on this system and regulates osteoclast numbers and activity. RANKL is also expressed in collagen-induced arthritis (CIA) in which focal collections of osteoclasts are prominent at sites of bone destruction. To determine the role of RANK signaling events in the effector phase of CIA, we investigated effects of Fc-osteoprotegerin fusion protein (Fc-OPG) in CIA. After induction of CIA in Dark Agouti rats, test animals were treated with or without Fc-OPG (3 mg/kg/day) subcutaneously for 5 days, beginning at the onset of disease. Paraffin-embedded joints were then analyzed histologically and the adjacent bone assessed by histomorphometry. Osteoclasts were identified using TRAP staining and expression of the mRNA for OPG and RANKL was identified by in situ hybridization. The results indicated that short-term Fc-OPG effectively prevented joint destruction, even though it had no impact on the inflammatory aspects of CIA. In arthritic joints, Fc-OPG depleted osteoclast numbers by over 75% and diminished bone erosion scores by over 60%. Although cartilage loss was also reduced by Fc-OPG, the effects on cartilage were less striking than those on bone. In arthritic joints OPG mRNA was highly expressed and co-localized with RANK ligand, and treatment with Fc-OPG did not affect the expression of endogenous RANKL or OPG mRNA. These data demonstrate that short term Fc-OPG treatment has powerful anti-erosive effects, principally on bone, even though synovitis is not affected. These findings indicate the potential utility of disrupting RANK signaling to preserve skeletal integrity in inflammatory arthritis.
Rheumatoid arthritis (RA) is characterized by profound focal and generalized bone loss attributable to the cellular action of osteoclasts. 1-3 The control of osteoclast formation, activation, and apoptosis has been elucidated through identification of new members of the tumor necrosis factor (TNF) receptor and TNF ligand families, osteoprotegerin (OPG), and receptor activator of NFκΒ ligand (RANKL), respectively. 4-7 Receptor activator of NFκΒ (RANK) is a membrane- bound TNF receptor homologue that binds RANKL and mediates intracellular signals obligatory for osteoclastogenesis as well as osteoclast activation and survival in vivo. 8 OPG is a secreted TNF receptor member that functions as a decoy receptor for RANKL, thereby inhibiting these processes and accelerating osteoclast apoptosis. 9,10
Consistent with its osteoclast inhibitory effects in vitro, OPG produces hypocalcemia in thyroparathyroidectomized rats treated with parathyroid hormone (PTH) and reduces bone loss occurring after intervention such as ovariectomy, tail suspension, or sciatic nerve crush injury. 11-14 OPG inhibits bone destruction in mouse models of bone metastasis, prevents and reverses hypercalcaemia in animal models of humoral hypercalcemia of malignancy, and prevents hypercalcaemia and osteolysis induced by injections of 1,25 dihydroxyvitamin D3, PTH/PTHrP, TNFα, or interleukin (IL)-1 in normal mice. 15-18 The powerful effects of OPG in vivo were shown by targeted deletion of the OPG gene in mice. These mice exhibited osteoporosis and fragility fractures, 19 while the overexpression of OPG in transgenic mice resulted in osteopetrosis because of a failure of osteoclast formation. 6
RANKL is expressed in adjuvant- 20 and collagen-induced arthritis (CIA) 21 in rodents, as well as rheumatoid arthritis (RA). 22-26 RANKL is derived mainly from osteoblastic-stromal cells, synovial fibroblasts, and activated T lymphocytes. Activated T cells express biologically active RANKL as a membrane-bound peptide, a soluble fragment cleaved from the cell surface or a secreted protein. 20,25 Activated T cells and stromal-osteoblast lineage cells support osteoclast formation in vitro 20,22,24-26 and therefore are implicated in the osteoclastogenic process in erosive arthritis. In addition, the levels of OPG and RANKL in osteoblastic and stromal cells are often reciprocally regulated in vitro and in vivo by bone active cytokines and hormones. 27 Therefore, excessive production of RANKL, and/or deficiency of OPG may contribute to the increased bone resorption typified by focal bone erosions and periarticular bone loss in RA.
Because CIA is a T cell- and TNF-dependent disease that recapitulates several aspects of RA, 21,28 it is a suitable model for analysis of the role of RANK/RANKL receptor-ligand function in arthritis. In this in vivo study, we evaluated the effect of a chimeric OPG fusion protein (Fc-OPG) on synovitis, joint destruction, and osteoclast numbers in rats with CIA. We also sought expression of OPG and RANKL relative to bone erosions. The data show that short-term Fc-OPG injections reduced osteoclast numbers and focal bone erosions, even though treatment had no major effect on inflammatory synovitis or pannus. OPG also reduced cartilage loss, although the effect on cartilage erosion scores was less than the bone protective effects. These data show the potential utility of disrupting RANK signaling and indicate that the phenomena of synovial inflammation and pannus can be dissociated from bony damage in arthritis. They strengthen the concept that down-regulating osteoclast numbers preserves skeletal integrity in inflammatory arthritis.
Materials and Methods
Reagents
Lyophilized native bovine type II collagen (CII) was purchased from Sigma Chemical Company (St. Louis, MO, USA) and dissolved at a concentration of 2 mg/ml in 0.01 mol/L acetic acid at 4°C. Recombinant OPG fusion protein (Fc-OPG) was provided by Amgen (Thousand Oaks, CA). Fc-OPG is a chimeric molecule comprising amino acids 22–194 of human OPG, fused at the N-terminus to the C-terminus of the Fc domain of human IgG1, 6 and is covalently dimerized through the Fc domain. The dose of Fc-OPG was determined by previous dose finding studies in mice, 18 which showed that doses of ∼2 to 3 mg/kg/day abrogated hypercalcemia and increased osteoclast numbers induced by injections of pro-inflammatory cytokines (TNFα or IL-1). Non-immune human IgG1 (κ) was purchased from Sigma.
Animals
Female 10-week-old Dark Agouti (DA) rats (n = 36) were obtained from Monash Animal Services (Clayton, Australia). Six rats served as normal controls and 30 were used to induce CIA. All rats were fed standard rodent chow and given water ad libitum. The animals were weighed and monitored daily for signs of arthritis and all experimental procedures conformed to the National Health and Medical Research Council guidelines and were approved by an institutional animal ethics committee.
Induction of CIA
Solubilized CII was emulsified 1:1 in incomplete Freund’s adjuvant (Sigma) and arthritis was induced under anesthesia by an intradermal injection at 5 to 6 sites at the base of the tail with 300 μl of emulsified CII. 21,29 With this protocol, 100% of immunized rats developed CIA after ∼14 days. Treatments were initiated immediately after the clinical onset of arthritis. Each rat that exhibited erythema and/or swelling in one or more limbs was randomly assigned to daily subcutaneous injections of either daily phosphate-buffered saline (vehicle), IgG1 (3 mg/kg/day), or Fc-OPG injections (3 mg/kg/day) for 5 days.
Clinical Assessment of Collagen-Induced Arthritis
Arthritis was monitored daily by using a macroscopic scoring system ranging from 0 to 4 for each limb, as previously described by Bakharevski et al, 29 as follows: 0, no arthritis; 1, swelling and/or redness of one to two interphalangeal (IP) joints; 2, involvement of three to four IP joints or one larger joint; 3, more than four joints red/swollen; 4, severe arthritis of an entire paw, yielding a score of 0 to 16 per animal. In addition, the hind footpad width was measured with calipers.
Specimen Preparation
The hindpaws of each rat were dissected and fixed immediately by immersion in 4% paraformaldehyde in DEPC-PBS for 6 hours and decalcified with 15% EDTA in 0.5% paraformaldehyde/PBS at 4°C for 2 to 3 weeks. The decalcifying solution was changed twice each week. Tissue specimens were processed for paraffin wax embedding and multiple 5-μm sections prepared and stained with hematoxylin and eosin (H&E) for general evaluation or toluidine blue for specific evaluation of cartilage. To identify osteoclasts, consecutive sections were stained for tartrate resistant acid phosphatase (TRAP) using an acid phosphatase kit (Sigma).
Histopathological Scoring
Histological sections of inflamed hindpaw joints were scored without knowledge of specific interventions. To allow for the fact that the distal interphalangeal (IP) joints were always the first joints to become inflamed, followed by the proximal IP joints, distal and proximal IP joints were scored separately in addition to the tibiotalar joints. The sections of digits were prepared to ensure that proximal, middle, and distal phalanges with their opposing cortices and cartilage ends were present. At least six H&E sections were scored by two independent observers at low power for inflammation and pannus, and low and high power for bone erosion. Sequential toluidine blue-stained sections were then scored for cartilage damage and proteoglycan loss. Then, for each animal, the mean score for each histopathological parameter was calculated. The pathology was rated 0 to 5 (with 0 being normal and 5 being severely affected) using a semi-quantitative rating scale, as described by Bendele et al 30 with minor modifications. Inflammation was scored 0 to 5 according to the following criteria: 0, normal; 1, minimal inflammatory infiltration; 2, mild infiltration; 3, moderate infiltration with moderate edema; 4, marked infiltration with marked edema; and 5, severe infiltration with edema. Pannus was defined as synovial tissue intimately invading bone and/or cartilage and was scored 0 to 3 as follows: 0, none; 1, minimal; 2, moderate; 3, severe. Cartilage damage was scored 0 to 5 according to the following criteria: 0, normal; 1, minimal (loss of toluidine blue staining only); 2, mild (loss of toluidine blue staining and mild cartilage thinning); 3, moderate (moderate diffuse or multifocal [depth to middle-zone] cartilage loss); 4, marked (marked [depth to deep-zone] diffuse or multifocal cartilage loss); and 5, severe diffuse or multifocal [depth to tidemark] cartilage loss). Bone resorption was scored 0 to 5 according to the following criteria: 0, none; 1, minimal (not readily apparent on low magnification); 2, mild (more numerous areas of resorption, not readily apparent on low magnification; 3, moderate (obvious foci of resorption, numerous osteoclasts); 4, marked (large erosions extending into bone cortices, more numerous osteoclasts); and 5, extensive erosions, markedly disrupted joint architecture). To enumerate osteoclasts, histomorphometric analysis was performed on the metaphysis adjacent to the joints, according to standard procedures, 31 using the Osteomeasure bone analysis system (Osteometrics, Inc., Decatur, GA). Only TRAP-positive osteoclasts in contact with the endosteal surface were included in cell counts. Results were recorded as mean osteoclast surface per unit of bone surface measured (osteoclast/bone surface).
In Situ Hybridization
In situ hybridization was carried out as previously described. 31 Digoxigenin-labeled riboprobes for RANKL were described previously. 32 OPG riboprobes were generated as described for RANKL using pSMR936 plasmid containing a murine OPG sequence (778–1323, 545 bp), amplified by reverse transcriptase-polymerase chain reaction from mouse calvarial osteoblast RNA and cloned into pGEM-T vector (Promega, Madison, WI). Plasmids were linearized with NdeI or SacII (Promega), and transcribed with either T7 or SP6 RNA polymerase (Boehringer Mannheim, Mannheim, Germany) to generate digoxigenin (DIG)-labeled antisense or sense riboprobes, respectively. Sense strand negative controls for each riboprobe were negative and were tested with each set of samples (not shown).
Statistical Analysis
Differences between groups were analyzed by one or two way analysis of variance followed by Fisher’s post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Clinical Features of CIA Are Similar in Rats Treated with or without Fc-OPG
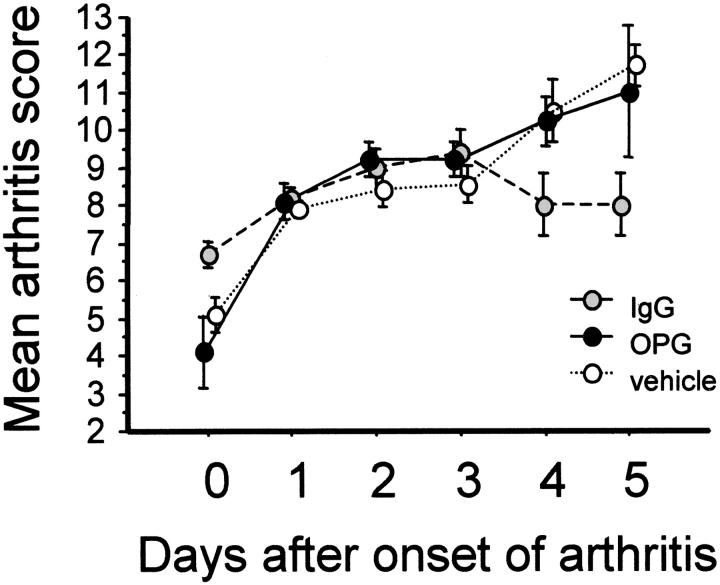
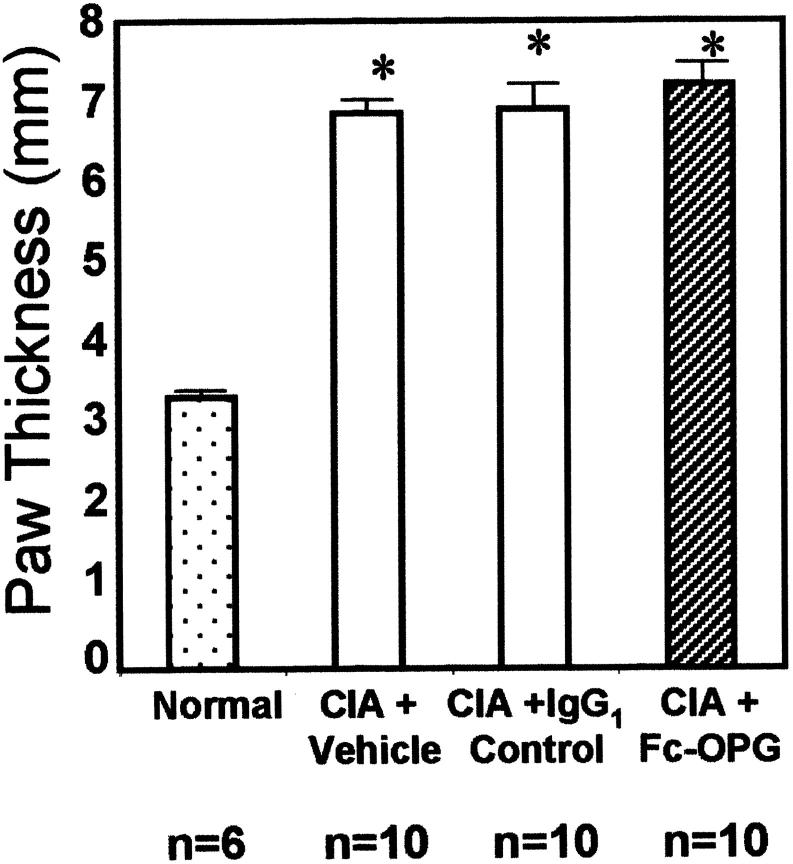
Following immunization, rats developed progressive CIA (time of onset 15 to 20 days after CII inoculation) with 100% incidence. Within the 5-day time interval of experiments, arthritis was confined mainly to the hindpaw joints and was most severe in the IP joints. Inflammatory arthritis was seen first in the distal then proximal IP joints, followed by the metatarsophalangeal and tibiotarsal joints. After onset, CIA progressed similarly in rats treated with and without Fc-OPG, although after 3 days the scores stabilized significantly in the IgG1 control group (Figure 1) ▶ . Overall, there were no important clinical differences between rats treated with or without Fc-OPG. At the end of the experiments, the mean increase in hindpaw thickness of arthritic rats was comparable in all treatment groups (Figure 2) ▶ .
Figure 1.
Kinetics of arthritis scores of CIA in DA rats. Rats were immunized with a single injection of CII and CIA developed in all animals 15 to 20 days later. At the onset of arthritis, they were randomly assigned to treatment groups for 5 days and arthritis was evaluated daily by a clinical score [as described in Ref. 29 ] (maximum score per animal, 16). Results show means (±SEM) for rats in each group.
Figure 2.
Paw swelling in rats with CIA treated with and without Fc-OPG. The hind footpad thickness was assessed by caliper measurement at termination and bars represent the mean (±SEM) values for the animals in each group. (*, P < 0.01)
Histological Features of CIA are Similar in Rats Treated with or without Fc-OPG
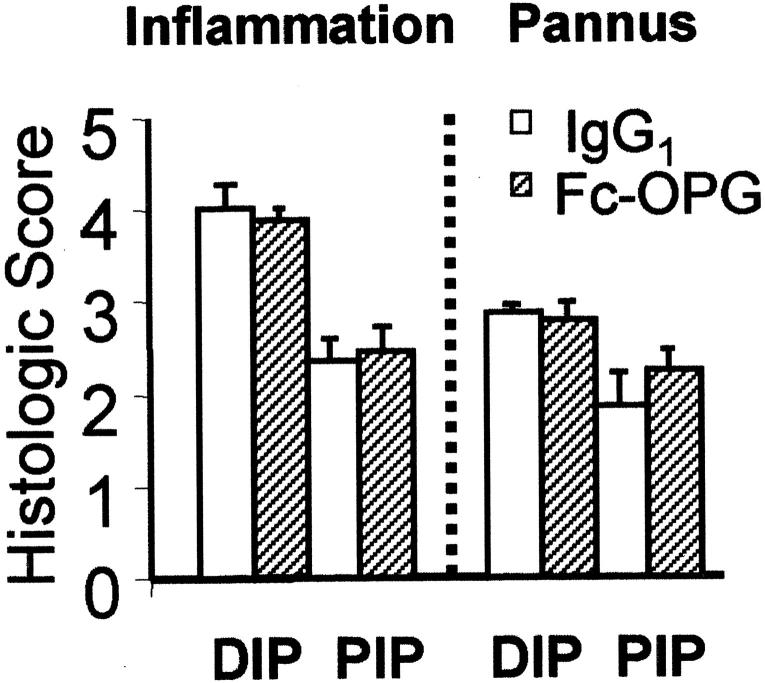
Histopathological changes in the joints 5 days after arthritis onset were characterized by synovial lining hyperplasia, erosion formation at the cartilage-bone-pannus junction, and leukocyte infiltration (30 to 50% neutrophils, 50 to 70% mononuclear cells) of the synovial sublining layer. Although the rats exhibited ankle inflammation, after 5 days, synovial inflammation and joint destruction was most prominent in the interphalangeal (IP) joints. Therefore, to accurately reflect the disease process, the IP joints were histologically scored, both at the distal and proximal IP joints (Figure 3) ▶ . Consistent with the clinical scores and paw swelling, the inflammation and pannus scores were comparable in rats treated with or without Fc-OPG (Figure 3) ▶ .
Figure 3.
Histological evaluation of inflammation and pannus in CIA. The IP joints from arthritic rats (each group, n = 10) treated with human IgG1 or Fc-OPG were scored using a semi-quantitative system [as described in Materials and Methods]. Results are expressed as the mean scores (±SEM) for rats treated without or with OPG. DIP, distal IP joints; PIP, proximal IP joints.
Short-Term Fc-OPG Significantly Reduced Osteoclasts and Focal Bone Erosions
In the context of continued synovial inflammation, bone erosion scores were significantly reduced by over 60% in the joints of arthritic rats treated with daily injections of Fc-OPG (Figure 4) ▶ , P < 0.05. In many animals receiving OPG, there were no identifiable focal bone erosions (Figure 4) ▶ . Thus, short-term Fc-OPG injections started after the onset of CIA effectively uncoupled synovial inflammation from joint destruction. Larger joints (ankles) were not evaluated because the extent of tibio-talar joint destruction was mild (data not shown), and therefore insufficient to evaluate OPG. Recent studies have verified that the multinucleated cells located at sites of bone erosion in CIA are authentic osteoclasts, 21 being positive for TRAP as well as calcitonin receptors. To determine the net effect of Fc-OPG on the number of bone resorbing cells, joint sections were stained with TRAP to identify osteoclasts. In Fc-OPG treated rats, osteoclasts were markedly depleted in juxta-articular bone and there was absence of marginal and subchondral focal bone erosions (Figure 5) ▶ . Histomorphometric analysis of the bones of Fc-OPG treated rats showed at least 75% reduction of trabecular osteoclast surface (Figure 5) ▶ .
Figure 4.
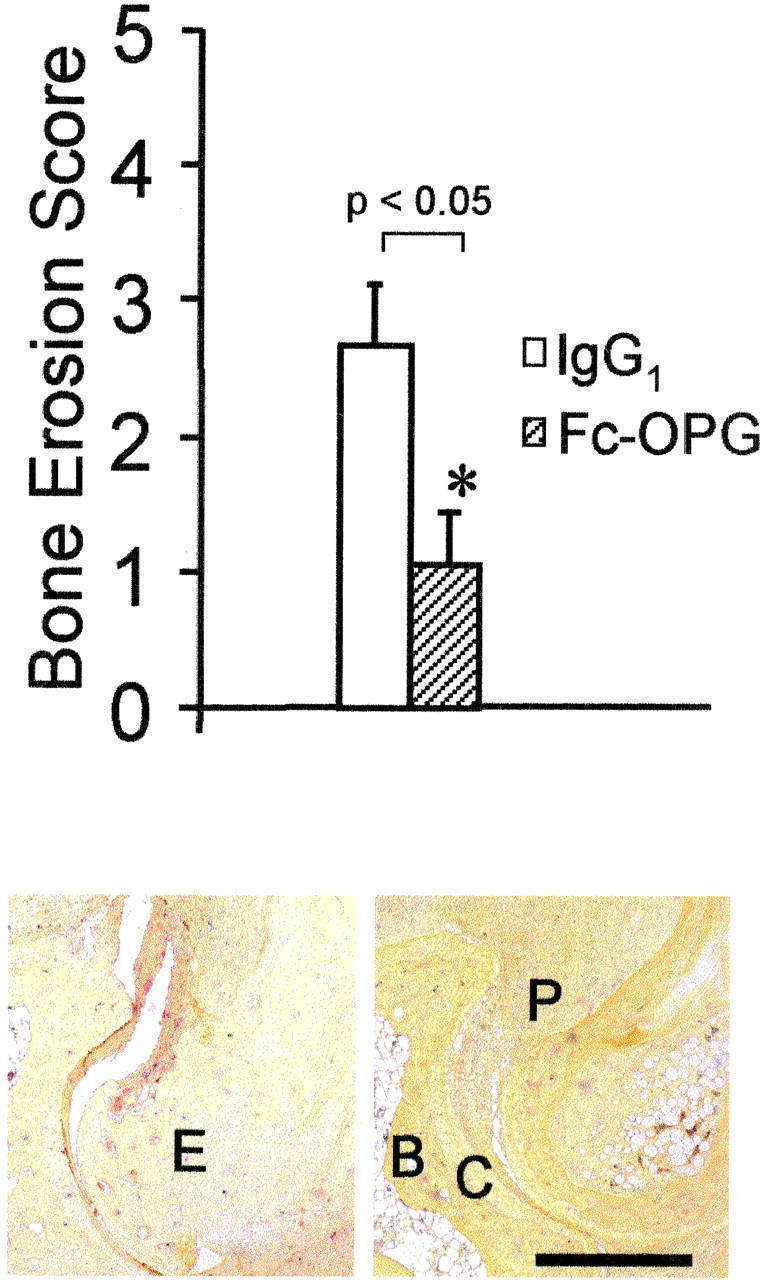
Top: Effect of Fc-OPG on bone erosion scores. The IP joints from all arthritic rats treated without (n = 10) and with (n = 10) Fc-OPG were evaluated for bone erosions using a scoring system [as described in Materials and Methods]. Results are expressed as the mean scores (±SEM) of each group of animals (*, P < 0.05). Bottom: Osteoclasts at erosion sites in CIA (TRAP stain). Left: CIA + IgG1 control. Note TRAP-positive (red) cells eroding marginal and subchondral bone (E, erosion). Right: CIA + Fc-OPG. There is pannus (P) but absence of TRAP-positive osteoclasts or bone erosions. B, subchondral bone; C, cartilage. Bar, 500 μm.
Figure 5.
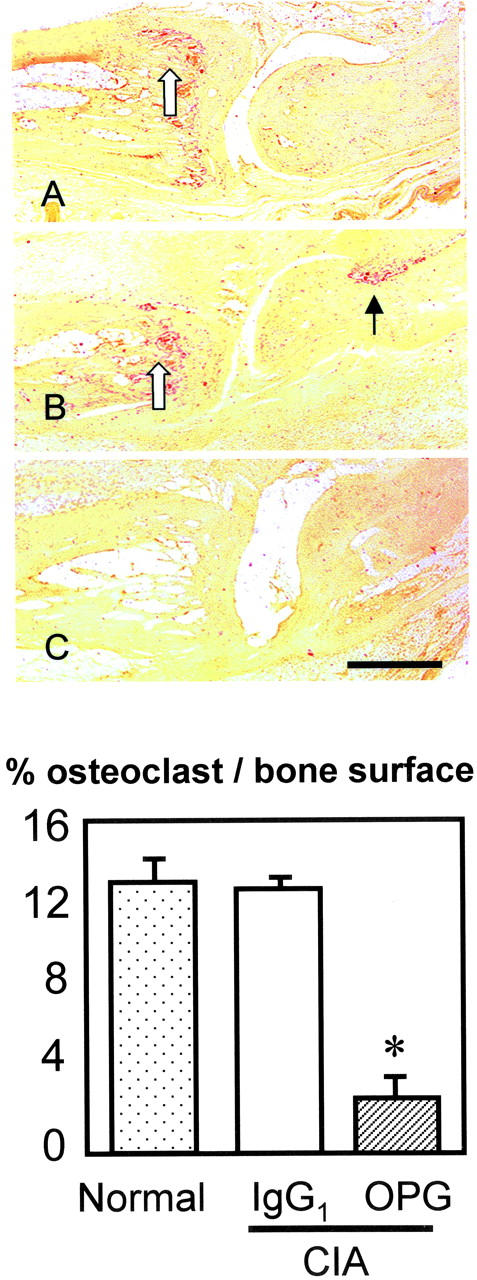
Top: Effect of Fc-OPG on osteoclasts in CIA (TRAP-stain). Sagittal section of IP joint from normal rats (A), CIA rats treated with IgG1 (B) or CIA treated with Fc-OPG (C). Osteoclasts are colored red. Note osteoclasts in metaphysial bone (open arrow) and at focal bone erosion site (black arrow) in CIA controls (B) but not Fc-OPG treated CIA (C). Bar, 500 μm. Bottom: Effect of Fc-OPG on osteoclast surface. The percent osteoclast surface (Ocs/Bs) in the juxta-articular bone of non-arthritic and arthritic rats treated without or with Fc-OPG was determined. The bars represent the means (±SEM) for the animals in each group. (*, P < 0.01).
Cartilage Destruction in CIA Is Similar in Rats Treated with or without Fc-OPG
Although joints in several OPG-treated rats showed nearly normal cartilage thickness and intensity of toluidine blue staining compared to IgG controls (Figure 6) ▶ , cartilage scoring revealed that mild cartilage loss was present in many animals, particularly at sites in contact with pannus (Figure 6) ▶ . Consistent with the short duration of arthritis, cartilage loss was mild, and there was significantly less cartilage damage in Fc-OPG treated rats at proximal IP joints (P < 0.05) where inflammation scores were lower than those at the distal IP joints (Figures 3 and 6) ▶ ▶ . In contrast, no difference was noted in distal IP joints (Figure 6) ▶ , where inflammation scores were high.
Figure 6.
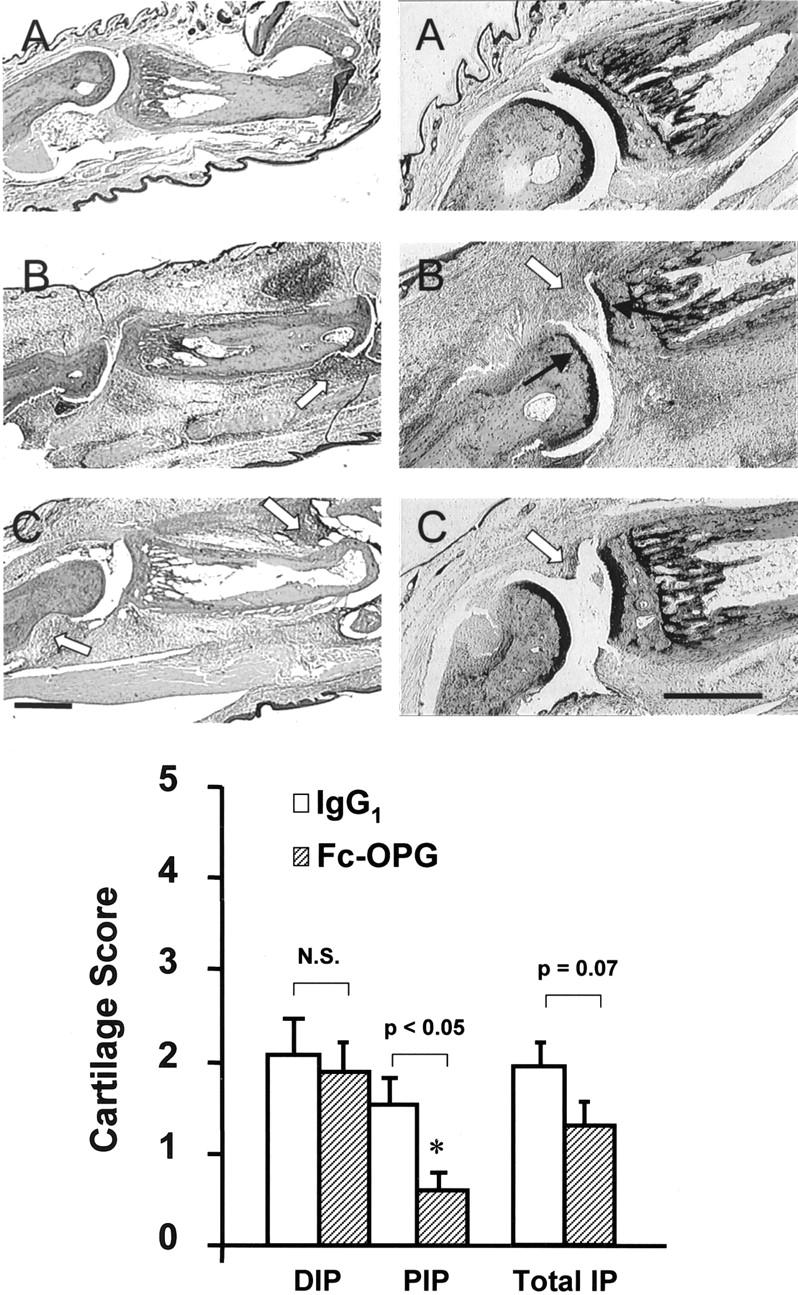
Top: Effect of Fc-OPG on cartilage. Consecutive sections show representative IP joints from normals (A), CIA treated with IgG1 (B) or CIA treated with Fc-OPG (C) for 5 days. Left: H&E stain. Right: Toluidine blue stains for cartilage. Note digital edema and synovial inflammation (arrowheads) in arthritic rats treated without or with Fc-OPG and cartilage loss (black arrows) in IgG1 controls. Bar, 500 μm. Bottom: Effect of Fc-OPG on cartilage erosion scores. The IP joints from arthritic rats treated without (n = 10) and with (n = 10) Fc-OPG were evaluated for cartilage erosions using a scoring system [as described in Materials and Methods]. Results are expressed as the mean (±SEM) scores for distal IP (DIP), proximal IP (PIP), or total IP joints of each intervention group. (*, P < 0.05).
RANKL and OPG Are Expressed at Sites of Inflammation and Bone Destruction in CIA
To identify the precise location of cells expressing RANKL or OPG, and their spatial relationship to osteoclasts and focal bone erosions, arthritic joints were analyzed by TRAP staining and in situ hybridization for RANKL or OPG mRNA. While focal bone erosions were never seen in normal joints, in CIA major foci of erosion were located at the synovial reflections at cartilage-bone junctions (Figures 4 and 5) ▶ ▶ . Although some TRAP-positive cells were present away from bone surfaces, most TRAP-positive cells were multinucleated osteoclasts present at the synovial/bone margin lying within resorptive pits in the bone. In normal joints, RANKL mRNA was observed in osteoblasts lining the trabecular bone surfaces, and weakly or not at all by osteoclasts, which were sparse (Figure 7) ▶ . In normal joints, OPG mRNA was detected in articular chondrocytes and osteoblasts at periosteal and endosteal sites, as well as osteoclasts (Figure 7) ▶ . RANKL mRNA was expressed in the synovial infiltrate adjacent to bone erosions in CIA, especially in infiltrating synovial cells, osteoblasts, osteoclasts, as well as chondrocytes. In CIA, OPG expression was essentially identical to RANKL, and concentrated at sites of erosion (Figure 7) ▶ . Finally, although Fc-OPG administration reduced bone erosions in CIA, the pattern of endogenous RANKL or OPG mRNA expression in the inflamed joints was not different to CIA controls (Figure 7) ▶ .
Figure 7.
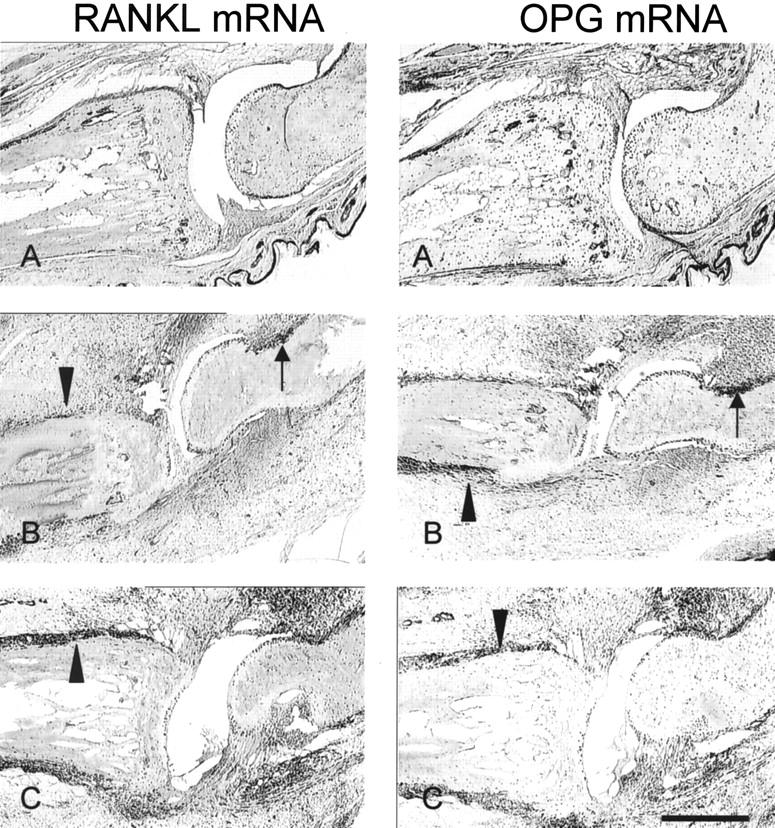
In situ localization of RANKL and OPG mRNA in CIA. Consecutive IP joint sections were processed for DIG-labeled in situ hybridization as described in Methods. A, Normal; B, CIA + IgG1; C, CIA + Fc-OPG. Positive signal for the respective mRNAs is seen as dark purple staining. Note the high RANKL and OPG mRNA expression at the sites of bone erosion (black arrows) and periosteal inflammation (arrowheads). Bar, 500 μm.
Discussion
Collagen-induced arthritis in rats is a useful model of autoimmunity as well as focal bone erosions, and juxta-articular and generalized osteoporosis in RA. 21,28,33 Using this arthritis model, we have shown that short-term Fc-OPG injections reduce bone erosion scores significantly, indicating major contingency of bone resorption on RANK signaling. The likely mode of action of Fc-OPG is that it binds RANKL, preventing its engagement with RANK, an essential signaling receptor for osteoclast formation. 8 Moreover, RANK signaling is vital for osteoclast activation and survival. 9,10
In agreement with studies using a similar OPG treatment regimen in adjuvant-induced arthritis (AIA) in the rat, 20 we noted that OPG reduced osteoclast numbers and bone erosions in CIA in the context of continuing inflammation, emphasizing the concept of dissociation between inflammation and bone destruction. Both AIA and CIA models are widely used for pre-clinical testing of anti-arthritic therapeutic agents. 30 Rat AIA is typified by polyarticular inflammation, striking bone resorption as an early feature, and periosteal inflammation. Cartilage destruction occurs in AIA but is disproportionately mild in comparison with the inflammation and massive bone destruction. CIA is produced by immunization against homologous or heterologous type II collagen. The polyarthritis in CIA is characterized by severe cartilage destruction that is associated with immune complex deposition on articular surfaces, focal bone erosions, and periosteal proliferation and moderate to severe synovitis and periarticular inflammation. Of note, the lesions in CIA are considered more analogous to those seen in human RA. 30
The preservation of juxta-articular bone despite persistent inflammation in both AIA and CIA supports the concept that bone loss in inflammatory arthritis is carried out chiefly by osteoclasts, proving the principle that OPG protects bone by modulating osteoclast numbers. Osteoprotegerin is known to have at least two roles: an effect on reducing osteoclast formation and activity and promotion of apoptosis of mature osteoclasts. 7,9,10 The relative contribution of these two pathways in the CIA and AIA models is unclear. However, OPG treatment of osteoclasts in vitro produces massive apoptosis of these cells within 48 hours. 10 Feige et al. 34 also reported the effect of timed delivery of OPG in the AIA model over 7 days. Daily OPG injections for only 3 days after arthritis onset reduced osteoclast numbers over 50%, while injections for the entire 7-day period essentially eliminated osteoclasts. Short-term (3 days) OPG injections reduced focal bone erosions and periarticular bone loss as effectively as continuous OPG for 7 days. Even when OPG injections were delayed until 4 days after the onset of arthritis, osteoclasts were reduced by 50%, focal erosions did not progress and bone mineral loss was stabilized. These reports using the AIA model emphasize that effects of OPG on bone pathology are evident even after short-term administration, as observed in our study of collagen-induced arthritis.
Besides osteoclast differentiation, RANK mediates dendritic cell survival, T- and B-cell development, and lymph node organogenesis. 4,35 It is possible that autoreactive T cell/dendritic cell-to-cell interactions involving RANK are important for initiation and/or perpetuation of inflammatory synovitis. In the present study, Fc-OPG had no impact on synovial inflammation when administered at the onset of CIA. This suggests that any putative RANK signaling between dendritic cells and T cells is insignificant or redundant in the effector phase of CIA. Further studies of prophylactic administration of OPG may clarify the role of RANK-RANKL interaction on the pre-effector immune mechanisms that precede clinical expression of erosive arthritis.
In comparison to the marked effects of Fc-OPG on bone erosions, we found that OPG had only a modest effect on cartilage in the context of severe synovitis in the CIA model. In contradistinction, in the AIA model, Kong et al 20 reported that OPG largely preserved articular cartilage, except at marginal sites where cartilage was in direct contact with pannus. In this study, we provide semi-quantitative analysis of cartilage damage while Kong et al 20 did not provide such an analysis. Our data therefore offer detailed insight into cartilage damage in relation to inflammation and bone erosion scores in different joints. We noted significant chondroprotection by Fc-OPG in mildly inflamed joints and no significant protection in more severely inflamed joints in those rats treated with OPG. These findings suggest that OPG may have chondroprotective properties in the early, effector phase of CIA, however once synovitis is established and severe, OPG fails to provide chondroprotection. Therefore, although bone erosion scores were clearly reduced, cartilage loss continued in these rats. Similarly, in a serum transfer model of arthritis, RANKL-deficient mice exhibited cartilage loss despite protection from bone erosions, although there was a trend for less cartilage destruction. 36
Collectively, these findings are consistent with different mechanisms of cartilage damage in these arthritis models. One major difference between AIA and CIA models is that in AIA there is early and massive subchondral bone loss (often before synovial swelling is clinically evident) and cartilage destruction appears to depend significantly on the loss of this subchondral bone. 20,30 In CIA, cartilage erosion may be contingent on deposition of immune complexes and direct invasion by adjacent pannus. When taken together with our results, the findings by Kong et al. 20 support the notion that, compared to AIA, cartilage destruction in CIA is less dependent on RANK signaling events such as osteoclastic bone destruction. Consequently, interventions that are primarily bone protective may offer significantly more chondroprotection in adjuvant arthritis compared to collagen-induced arthritis. These observations in the two arthritis models are clearly relevant to the disease process occurring at the synovial-cartilage-bone nexus in RA.
Although chondrocytes express both OPG and RANK, human articular chondrocytes do not have an intact RANK signaling apparatus. 37 Therefore, the issue of whether OPG impinges directly on chondrocyte metabolism in arthritis, independent of its effects on osteoclastic bone erosion or RANK signaling, requires further investigation.
Although we did not study RANK expression in CIA, studies in normal rats show that mRNA for RANK is localized mainly in the myeloid lineage cells, including osteoclast precursors, as well as mature osteoclasts. 38 In the present study, the pattern of RANKL or OPG mRNA in synovial tissue as assessed by in situ hybridization was not altered by Fc-OPG. Reduced RANKL expression was reported after OPG therapy in a mouse model of osteolytic tumor metastasis, 17 and modulation of RANKL and/or OPG expression in CIA by Fc-OPG cannot be excluded. OPG mRNA was expressed and co-localized with RANKL mRNA in CIA. The presence of bone loss in CIA, contrasting with reduced osteoclasts and bone erosion after OPG indicates that a RANKL/OPG disequilibrium exists in the joint microenvironment that strongly favors bone resorption. Cytokines such as TNFα, IL-17, or IL-1 likely synergize with RANKL to promote this bone loss. 39-42 Indeed, TNFα stimulates osteoclast differentiation of precursors primed by <1% of the amount of RANKL normally required to induce osteoclast formation. 40 TNFα induces IL-1 production, and IL-1 and RANKL function as survival and activation factors for nascent osteoclasts. 27,42 Consequently, in autoimmune inflammatory arthritis TNFα and IL-1 likely amplify the effects of RANKL, promoting massive bone loss. This is exemplified in transgenic mice overexpressing the human TNFα gene. These transgenic mice exhibit an erosive arthritis, which is improved by administration of OPG, neutralizing anti-TNFα antibodies, or the bisphosphonate pamidronate. 43
Like OPG, combined anti-cytokine therapy with the pegulated soluble TNF receptor-I (which neutralizes TNFα) and IL-1ra (which neutralizes IL-1α and IL-1β), also prevents bone erosions by regulating osteoclast numbers. However, in contrast to OPG, anti-TNF therapy also clearly suppressed inflammatory synovitis and cartilage destruction. 42,44 Similarly, overexpression of IL-4 suppresses IL-17 as well as RANKL mRNA production, reducing inflammation, as well as bone and cartilage damage in CIA. 45
In conclusion, Fc-OPG reduces bone erosions in CIA, highlighting a central role of osteoclasts in bone destruction and dependence of this process on RANKL-RANK interaction. In rheumatoid arthritis, it is well established that blockade of cytokines such as TNFα and/or IL-1 improves inflammatory synovitis and retards joint destruction. 46 While Fc-OPG has no substantial or consistent effects on synovitis or cartilage damage in the CIA model, Fc-OPG or other inhibitors of RANK signaling offer a powerful new opportunity to mitigate bone loss in rheumatoid arthritis.
Acknowledgments
We thank Hui Ling He for preparing tissue samples and Dr. Olga Bakharevski for technical assistance with the CIA model.
Footnotes
Address reprint requests to Evan Romas, MBBS, Ph.D. FRACP, The University of Melbourne, Department of Medicine, St. Vincent’s Hospital, 41 Victoria Parade, Fitzroy, Victoria, 3065, Australia. E-mail: romase@svhm.org.au.
Supported by NHMRC Program Grant (003211) and the Arthritis Foundation of Australia. Dr. E. Romas is an NHMRC Post-Doctoral Fellow and Dr. N. Sims is supported by an R. J. Gleghorn Fellowship.
References
- 1.Goldring SR: The final pathogenetic steps in focal bone erosions in rheumatoid arthritis. Ann Rheum Dis 2000, 59(Suppl 1):i72-i74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gough A, Sambrook P, Devlin J, Huissoon A, Njeh C, Robbins S, Nguyen T, Emery P: Osteoclastic activation is the principal mechanism leading to secondary osteoporosis in rheumatoid arthritis. J Rheumatol 1998, 25:1282-1289 [PubMed] [Google Scholar]
- 3.Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR: Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 1998, 152:943-951 [PMC free article] [PubMed] [Google Scholar]
- 4.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM: OPGL is a key regulator of osteoclastogenesis, lymphocyte development, and lymph-node organogenesis. Nature 1999, 397:315-323 [DOI] [PubMed] [Google Scholar]
- 5.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ: Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93:165-176 [DOI] [PubMed] [Google Scholar]
- 6.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Boyle WJ: Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997, 89:309-319 [DOI] [PubMed] [Google Scholar]
- 7.Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ: Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 1999, 96:3540-3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ: RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA 2000, 97:1566-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, Hu S, Lacey DL: The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 1999, 145:527-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, Rattan A, Scully S, Fletcher F, Juan T, Kelley M, Burgess TL, Boyle WJ, Polverino AJ: Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 2000, 157:435-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto M, Murakami T, Nishikawa M, Tsuda E, Mochizuki S, Higashio K, Akatsu T, Motoyoshi K, Nagata N: Hypocalcemic effect of osteoclastogenesis inhibitory factor/osteoprotegerin in the thyroparathyroidectomized rat. Endocrinology 1998, 139:4012-4015 [DOI] [PubMed] [Google Scholar]
- 12.Bolon B, Carter C, Daris M, Morony S, Capparelli C, Hsieh A, Mao M, Kostenuik P, Dunstan CR, Lacey DL, Sheng JZ: Adenoviral delivery of osteoprotegerin ameliorates bone resorption in a mouse ovariectomy model of osteoporosis. Mol Ther 2001, 3:197-205 [DOI] [PubMed] [Google Scholar]
- 13.Bateman TA, Dunstan CR, Ferguson VL, Lacey DL, Ayers RA, Simske SJ: Osteoprotegerin mitigates tail suspension-induced osteopenia. Bone 2000, 26:443-449 [DOI] [PubMed] [Google Scholar]
- 14.Bateman TA, Dunstan CR, Lacey DL, Ferguson VL, Ayers RA, Simske SJ: Osteoprotegerin ameliorates sciatic nerve crush induced bone loss. J Orthop Res 2001, 19:518-523 [DOI] [PubMed] [Google Scholar]
- 15.Capparelli C, Kostenuik PJ, Morony S, Starnes C, Weimann B, Van G, Scully S, Qi M, Lacey DL, Dunstan CR: Osteoprotegerin prevents and reverses hypercalcemia in a murine model of humoral hypercalcemia of malignancy. Cancer Res 2000, 60:783-787 [PubMed] [Google Scholar]
- 16.Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB, O’Keefe PF, Ramnaraine ML, Clohisy DR, Mantyh PW: Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain, and pain-related neurochemical reorganization of the spinal cord. Nat Med 2000, 6:521-528 [DOI] [PubMed] [Google Scholar]
- 17.Morony S, Capparelli C, Sarosi I, Lacey DL, Dunstan CR, Kostenuik PJ: Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res 2001, 61:4432-4436 [PubMed] [Google Scholar]
- 18.Morony S, Capparelli C, Lee R, Shimamoto G, Boone T, Lacey DL, Dunstan CR: A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1β, TNF-α PTH, PTHrP, and 1, 25(OH)2D3. J Bone Miner Res 1999, 14:1478-1485 [DOI] [PubMed] [Google Scholar]
- 19.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS: Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 1998, 12:1260-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM: Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999, 402:304-309 [DOI] [PubMed] [Google Scholar]
- 21.Romas E, Bakharevski O, Hards DK, Kartsogiannis V, Quinn JMW, Ryan PFJ, Martin TJ, Gillespie MT: Expression of osteoclast differentiation factor at sites of bone erosion in collagen-induced arthritis. Arthritis Rheum 2000, 43:821-826 [DOI] [PubMed] [Google Scholar]
- 22.Horwood NJ, Kartsogiannis V, Quinn JMW, Romas E, Martin TJ, Gillespie MT: Activated T cells support osteoclast formation in vitro. Biochem Biophys Res Commun 1999, 265:144-150 [DOI] [PubMed] [Google Scholar]
- 23.Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, Goldring SR: Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum 2000, 43:250-258 [DOI] [PubMed] [Google Scholar]
- 24.Shigeyama Y, Pap T, Kunzler P, Simmen BR, Gay RE, Gay S: Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum 2000, 43:2523-2530 [DOI] [PubMed] [Google Scholar]
- 25.Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, Takahashi K, Furuya T, Ishiyama S, Kim KJ, Saito S, Nishikawa T, Takahashi N, Togari A, Tomatsu T, Suda T, Kamatani N: Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum 2001, 44:1003-1012 [DOI] [PubMed] [Google Scholar]
- 26.Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S: Involvement of receptor activator of nuclear factorκB/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 2000, 43:259-269 [DOI] [PubMed] [Google Scholar]
- 27.Ross FP: RANKing the importance of measles virus in Paget’s disease. J Clin Invest 2000, 105:555-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanyu T, Chotanaphuti T, Arai K, Tanaka T, Takahashi HE: Histomorphometric assessment of bone changes in rats with type II collagen-induced arthritis. Bone 1999, 24:485-490 [DOI] [PubMed] [Google Scholar]
- 29.Bakharevski O, Stein-Oakley AN, Thomson NM, Ryan PFJ: Collagen-induced arthritis in rats: contrasting effect of subcutaneous versus intradermal inoculation of type II collagen. J Rheumatol 1998, 25:1945-1952 [PubMed] [Google Scholar]
- 30.Bendele A, McAbee T, Sennello G, Frazier J, Chlipala E, McCabe D: Efficacy of sustained levels of interleukin-1 receptor antagonist in animal models of arthritis. Arthritits Rheum 1999, 43:498-506 [DOI] [PubMed] [Google Scholar]
- 31.Sims NA, White CP, Sunn KL, Thomas GP, Drummond ML, Morrison NA, Eisman JA, Gardiner EM: Human and murine osteocalcin gene expression: conserved tissue restricted expression and divergent responses to 1,25 dihydroxyvitamin D3 in vivo. Mol Endocrinol 1997, 11:1695-1708 [DOI] [PubMed] [Google Scholar]
- 32.Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JM, Niforas P, Ng KW, Martin TJ, Gillespie MT: Localization of RANKL mRNA and protein in skeletal and extra-skeletal tissues. Bone 1999, 25:525-534 [DOI] [PubMed] [Google Scholar]
- 33.Myers LK, Rosloniec EF, Cremer MA, Kang AH: Collagen-induced arthritis, an animal model of autoimmunity. Life Sci 1997, 61:1861-1872 [DOI] [PubMed] [Google Scholar]
- 34.Feige U, Campagnuolo G, Bolon B: Recombinant osteoprotegerin regulates osteoclast numbers in a schedule dependent manner in joints of male Lewis rats with adjuvant arthritis. Ann Rheum Dis 2001, 60(Suppl)Abstr OP0061
- 35.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L: A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997, 390:175-179 [DOI] [PubMed] [Google Scholar]
- 36.Pettit AR, Ji H, von Stechow D, Muller R, Goldring SR, Choi Y, Benoist C, Gravallese E: TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis Am J Pathol 2001, 159:1689-1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komuro H, Olee T, Kuhn K, Quach J, Brinson DC, Shikhman A, Valbracht J, Creighton-Achermann L, Lotz M: The osteoprotegerin/receptor activator of nuclear factor κB/receptor activator of nuclear factor κB ligand system in cartilage Arthritis Rheum 2001, 44:2768-2776 [DOI] [PubMed] [Google Scholar]
- 38.Ikeda T, Utsuyama M, Hirokawa K: Expression profiles of receptor activator of nuclear factor κB ligand, receptor activator of nuclear factor κB, and osteoprotegerin messenger RNA in aged and ovariectomized rat bones. J Bone Miner Res 2000, 16:1416-1425 [DOI] [PubMed] [Google Scholar]
- 39.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T: IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 1999, 103:1345-1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL: TNFα induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000, 106:1481-1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Am Y: Tumor necrosis factor-α (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem 2001, 276:563-568 [DOI] [PubMed] [Google Scholar]
- 42.Feige U, Hu YL, Gasser J, Campagnuolo G, Munyakazi L, Bolon B: Anti-interleukin-1 and anti-tumor necrosis factor-α synergistically inhibit adjuvant arthritis in Lewis rats. Cell Mol Life Sci 2000, 57:1457-1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redlich K, Maier A, Hayer S, Kollias G, Dunstan CR, Lang S, Woloszczuk W, Steiner G, Smolen J, Schett G: Effect of osteoprotegerin and pamidronate treatment in transgenic mice overexpressing human TNFα. Ann Rheum Dis 2001, 60(Suppl)Abstr FRI0010
- 44.Feige U, Hu YL, Morony S, Campagnuolo G, Duryea D, Bolon B: Osteoclast numbers in joints of rats with adjuvant arthritis are decreased by treatment with IL-1ra and/or pegulated sTNF-RI. Ann Rheum Dis 2001, 60(Suppl)Abstr OP0016
- 45.Lubberts E, Joosten LAB, Chabaud M, van den Bersselaer L, Oppers B, Coenen-De Roo CJ, Richards CD, Miossec P, van Den Berg WB: IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosions. J Clin Invest 2000, 105:1697-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arend WP: Cytokines and cellular interactions in inflammatory arthritis. J Clin Invest 2001, 107:1081-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]