Abstract
Simian virus 40 (SV40) sequences of the early region coding for the large T antigen (Tag) oncoprotein were investigated in DNA samples from human pleomorphic adenoma (PA) of parotid glands. Specific SV40 sequences were detected, by PCR and filter hybridization with an internal oligoprobe, in 28 of 45 (62%) human PA specimens. None of the DNA samples from 11 normal salivary gland tissues was SV40-positive. DNA sequence analysis, carried out in all PCR amplified products from SV40-positive PA specimens, confirmed the SV40 specificity and indicated that PCR products had a sequence not distinguishable from SV40 DNA wild-type strain 776. SV40 Tag expression was revealed by immunohistochemistry with the specific monoclonal antibody Pab 101 in PA thin sections with a highly sensitive technical approach which retrieved the nuclear viral oncoprotein in 26 out of 28 (93%) samples previously found SV40-positive by PCR. Detection of SV40 sequences and Tag expression in human PA suggests that this oncogenic virus may play a role as a cofactor in the onset and/or progression of this benign neoplasm, or that SV40 DNA could replicate and express the Tag in PA cells.
The pleomorphic adenoma (PA) or mixed tumor is the most common neoplasm of the salivary gland, accounting for about 65% of all salivary gland neoplasms. Usually, PA arises in parotid glands where it represents 65% of parotid gland neoplasms. Even though no specific age range has been observed, PA is most commonly diagnosed in patients between 30 and 50 years. A bimodal distribution of patients stratified according to age with a two peak incidence, at 24 and 51 years, respectively, has recently been demonstrated. 1,2 PA is more common in females, with a male-to-female ratio ranging from 1:3 to 1:4. The clinical presentation of this neoplasm is characterized by a painless, slow growing, firm mass. In the early phase of development PA is usually movable, but following growth the tumor becomes more nodular and more stable. Recurrent PA is multinodular and appears as small nodules that may seem fixed on palpation. With adequate surgical excision, the prognosis is excellent. PA is one of the few benign neoplasms that can undergo malignant transformation, with an incidence of 4.5%. The likelihood of a malignant change in PA increases with the duration of the tumor and with the age of the patient. A carcinoma derived from PA is an aggressive neoplasm. Approximately 40 to 50% of PA patients develop one or more recurrences. The metastatic rate varies from series to series, with up to 71% of these patients developing local or distant metastases. This tumor usually has a capsule-like structure surrounding the mass. Tumors penetrating the capsule have a poor prognosis with 5-year survival rates in the range of 25 to 65%. 3 At present, the best form of therapy is a wide surgical excision with a contiguous lymph node dissection and an adjuvant radiation therapy. 4
In recent years, it has been shown that PA is a genetically heterogeneous neoplasm characterized by different chromosome aberrations, translocations, and gene mutations. 5 Different PA subgroups have been identified, with anomalies mainly involving the chromosomes 3, 8, 9, and 12. 6 Related to the chromosome aberrations, deregulations of specific genes have been detected, such as PLAG1 gene encoding a zinc finger protein related to the control of IGF II expression. 7 It should be noted that approximately 30% of PA does not show chromosome alterations. 8 Little is known about the causes of PA onset/progression, chromosome aberrations, and gene mutations detected in this tumor. Simian virus 40 (SV40), a highly oncogenic DNA tumor virus, was recently found associated with different human tumors, namely brain and bone tumors, malignant pleural mesotheliomas, thyroid carcinomas, pituitary adenomas, and different lymphoproliferative disorders. 9-11 SV40 is a monkey virus, which was believed to be transmitted to humans only under exceptional situations in natural infection. 9 SV40 contaminated vaccines, in particular anti-polio vaccines between 1955 and 1963, were administered to millions of humans worldwide. 9-11 However, the presence of this viral agent in humans, before the introduction of SV40-contaminated vaccines, cannot be discarded. 12 Follow-up of individuals administered with SV40-contaminated vaccines did not show an apparent increase of tumors rate. 13 However, it was reported that among the follow up group a 15-year-old girl developed a PA of the salivary gland. 13 In animal models, the SV40 Tag oncoprotein expression in the submandibular gland of SV40-transgenic mice induces cell transformation and extensive ductal hyperplasia, 14 whereas transgenic mice with the Tag oncoprotein expressed under the control of the submandibular gland secretory protein “b” develop adenocarcinomas of duct origin. 15 Although the histotype of these murine tumors differs from the human PA, it is possible that SV40 exerts its oncogenic potential in different oral glands.
Altogether these data prompted us to investigate whether SV40 Tag sequences and Tag expression could be detected in human PA. To this purpose, SV40 Tag amino (N)-terminal coding sequences were investigated in PA of salivary gland specimens by PCR followed by filter hybridization with a specific internal oligoprobe. Then, PCR products were DNA sequenced to further assess the SV40 specificity. The presence of the Tag oncoprotein in PA samples was determined by immunohistochemistry with the specific monoclonal antibody (mab) Pab101 16 which recognizes a Tag carboxyl(C)-terminal epitope, using a very sensitive technical approach that allowed the retrieval of the viral nuclear phosphoprotein.
Materials and Methods
Specimens and Nucleic Acid Purification Methods
Forty-five PA of the parotid gland from tumor patients and 11 normal tissues of the salivary gland from patients affected by neoplasms of the neck area, which differ from PA, were obtained from the ENT Clinic, University of Ferrara. Samples were classified according to Seifert’s histological subtypes (Table 1) ▶ . 17 Tissues were fixed in 10% formalin, dehydrated and paraffin-embedded at the Section of Pathology, University of Ferrara. All specimens under analysis had never been investigated before for SV40. Tissue samples were treated with 1% SDS and 500 μg/ml proteinase K. DNA was extracted with a mixture of phenol-chloroform-isoamyl alcohol (25:24:1) and dialyzed for 24 hours with TEN buffer (10 mmol/L Tris, pH 7.5, 1 mmol/L EDTA, 1 mol/L NaCl) and for a further 24-hour period with TE buffer, which is identical to TEN buffer but without NaCl. To verify whether cross-contaminations occurred during DNA extraction procedure, each sample was purified simultaneously with a specimen of salmon sperm DNA and a mock sample lacking DNA, and then all samples were subjected to PCR analysis. 16,18
Table 1.
Human Pleomorphic Adenomas of Salivary Glands Analyzed by PCR and Immunohistochemistry for SV40 Tag Coding Sequences and Tag Oncoprotein
| Sample | Age | Sex | Histological type* | SV40-like DNA | Tag expression |
|---|---|---|---|---|---|
| 1 | 59 | M | 2 | + | + |
| 2 | 72 | M | 1 | + | + |
| 3 | 69 | F | 1 | − | − |
| 4 | 21 | F | 1 | + | + |
| 5 | 72 | F | 3 | + | + |
| 6 | 21 | M | 2 | + | + |
| 7 | 56 | M | 1 | + | + |
| 8 | 17 | M | 1 | + | − |
| 9 | 65 | F | 1 | + | + |
| 10 | 68 | F | 4 | − | − |
| 11 | 33 | F | 1 | + | + |
| 12 | 54 | M | 4 | − | − |
| 13 | 72 | F | 2 | + | + |
| 14 | 48 | M | 1 | + | + |
| 15 | 51 | F | 1 | + | + |
| 16 | 69 | F | 4 | − | − |
| 17 | 16 | F | 1 | + | + |
| 18 | 59 | F | 2 | − | − |
| 19 | 67 | M | 1 | + | + |
| 20 | 51 | F | 2 | + | + |
| 21 | 39 | F | 2 | + | + |
| 22 | 53 | F | 1 | − | − |
| 23 | 34 | F | 2 | − | − |
| 24 | 28 | M | 1 | − | − |
| 25 | 36 | M | 1 | − | − |
| 26 | 15 | F | 1 | − | − |
| 27 | 23 | M | 3 | − | − |
| 28 | 21 | M | 1 | + | + |
| 29 | 52 | F | 4 | + | + |
| 30 | 61 | F | 2 | − | − |
| 31 | 27 | F | 2 | − | − |
| 32 | 21 | M | 1 | + | + |
| 33 | 28 | F | 1 | + | − |
| 34 | 73 | M | 1 | + | + |
| 35 | 62 | F | 1 | + | + |
| 36 | 34 | M | 2 | + | + |
| 37 | 22 | M | 1 | + | + |
| 38 | 39 | F | 2 | + | + |
| 39 | 49 | F | 2 | − | − |
| 40 | 55 | M | 1 | + | + |
| 41 | 38 | M | 3 | + | + |
| 42 | 49 | F | 1 | − | − |
| 43 | 45 | F | 3 | − | − |
| 44 | 42 | F | 1 | + | + |
| 45 | 25 | F | 1 | − | − |
*1, equal proportion between stroma and epithelium; mucoid stroma differentiation, pleomorphic epithelium differentiation; 2, high proportion of stroma (about 80%); pleomorphic stroma and epithelium differentiation; 3, low proportion of stroma (about 25%); pleomorphic stroma and epithelium differentiation; 4, monomorphic epithelium differentiation.
Oligonucleotides, PCR, RT-PCR, and Filter Hybridization
DNA extracted from human specimens was first assessed for suitability to PCR analysis by a control reaction designed to amplify β-globin gene sequences, as described before. 16 Then, DNA samples were investigated for SV40 Tag N-terminal coding sequences. To confirm the reproducibility of PCR assays and to avoid possible contaminations, DNAs were extracted with the procedure recommended for PCR investigation in a laboratory equipped with PCR facilities. To verify whether cross-contaminations occurred during the DNA extraction procedure, each sample was purified simultaneously with a specimen of salmon sperm DNA and a mock specimen lacking DNA, and then subjected to PCR analysis. The distinct phases of PCR procedures were performed in separate rooms by different operators at the Section of Histology and Embryology, and Center of Biotechnology (BL3/P3 laboratories), University of Ferrara. SV40 DNA wild-type (wt) strains VA 45–54 A1 10 (our laboratory) and 776 19 (Invitrogen, Milan, Italy) were used as control in PCR amplification and sequence analysis. DNA samples were analyzed in triplicate by three different operators in a blind fashion. The results obtained by the three different operators did not show discrepancy. SV40 Tag N-terminal sequences were investigated by semi-nested PCR (snPCR) 18 using the primer sets SV.for2-SV.rev and SV.for2-PYV.rev, 20 yielding amplification products of 575 bp and 543 bp, respectively. 18 These primers allow amplification of an N-terminal Tag coding sequence, which contains the pRb pocket binding domain and the Tag intron. 18,20 The SV40 specificity of PCR amplified products was assessed by filter hybridization with the internal SV oligoprobe. 18,20
DNA (0.5 μg) was PCR amplified in a total volume of 50 μl containing 10 mmol/L Tris-HCl pH 8.3, 50 mmol/L KCl, 2.5 mmol/L MgCl2, 0.01% gelatin, 150 μmol/L of each dNTP and 25 μmol/L of each primer, 1 unit of Taq-DNA polymerase (Roche, Milan, Italy), together with 1 unit of platinum Taq antibody as indicated by the manufacturer (Invitrogen, Milan, Italy). By adding the Taq antibody, the Taq polymerase activity was blocked up to 94°C, thus avoiding the generation of nonspecific amplification products at room and ramping temperatures. PCR products were migrated in a 1% agarose gel and transferred to a nylon membrane (Amersham Pharmacia Biotech). DNA was cross-linked to filters by UV irradiation for 2 minutes. All filters were hybridized with the SV oligoprobe which is specific for the SV40 Tag sequences analyzed. The SV oligoprobe was previously 3′-end labeled with a tail of dUTP-fluorescein by terminal transferase (Amersham Pharmacia Biotech). Detection of the fluorescent DNA hybrid was carried out with anti-fluorescein horseradish peroxidase (HRP)-conjugated antibody, as indicated by the supplier (Amersham Pharmacia Biotech). The film exposure was at room temperature for 15 minutes to 1 hour. Filter hybridization with the specific SV oligoprobe was used to prove the SV40 specificity of the amplified sequences. 18
DNA Sequencing
To further confirm the SV40 specificity of the amplified sequences, PCR products, 40 μl each, were purified from agarose gel by using a Gel Extraction Kit (QIAquick, Qiagen, Germany) and then directly sequenced by Sanger’s technique, 21,22 or by an automatic DNA sequence apparatus (ABI Prism 377).
Immunohistochemistry
Thin sections, 5 μm each, were deparaffinized in xylol and rehydrated with ethanol solutions. To retrieve the SV40 Tag, a nuclear phosphoprotein which binds the products of the tumor suppressor genes p53 and pRb family, and co-activator products p300 and p400, thin sections were treated in 50 mmol/L Tris-HCl, pH 10.0, for 20 minutes in a microwave oven at 600 W. Then, specimens were incubated at room temperature for 15 minutes in 3% H2O2 in PBS to block endogenous peroxidase. Slides were incubated overnight at 4°C with the mouse anti-SV40 Tag monoclonal antibody Pab 101 (Santa Cruz Biotechnology, Santa Cruz, CA) and with biotinylated anti-mouse and anti-rabbit immunoglobulins (CytoScan HRP Detection System, Cell Marque, Hot Springs, AR). Tissue sections of PA and normal tissues from parotid glands were developed with standard routine streptavidin-biotin immunohistochemical methods and counterstained with Mayer’s hematoxylin. SV40-transformed human fibroblasts, WI 38–13A, expressing SV40 Tag and normal human fibroblasts, WI 38, purchased from the American Type Culture Collection (Manassas, VA), were pelleted, formalin-fixed, paraffin-embedded, processed as tissues, and then used as SV40-positive and SV40-negative controls in each experiment.
Results
PCR Analysis of SV40 Tag Coding Sequences in Human PA of Salivary Glands
In this study, human PA of salivary gland specimens and normal salivary gland tissues used as control were analyzed by snPCR for SV40 Tag coding sequences, followed by filter hybridization with the SV internal specific oligoprobe (Table 1) ▶ . DNA samples were analyzed for the conserved SV40 Tag N-terminal coding sequences by oligonucleotide pairs which efficiently amplify these sequences. 14,18,20 The sensitivity and specificity of our snPCR approach followed by filter hybridization were described before. 18 Briefly, in reconstruction experiments with serial dilution of high-purified SV40 DNA, from 100 ng to 1 ag, in a background of 500 ng of genomic DNA from human placenta, the N-terminal Tag coding sequences of 543 bp, amplified by snPCR, was detected in gel stained by ethidium bromide till 10 fg, whereas these SV40 sequences were detected till 10 ag by filter hybridization with the SV probe (data not shown).
The prevalence of SV40 Tag N-terminal region in primary PA samples was 28/45 (62%), whereas 11 normal salivary gland tissue samples were all negative (Table 1 ▶ ; Figure 1 ▶ ). These data indicate that, under our DNA extraction and PCR conditions, human PA of salivary gland specimens carry at high prevalence DNA sequences coding for SV40 Tag oncoprotein.
Figure 1.
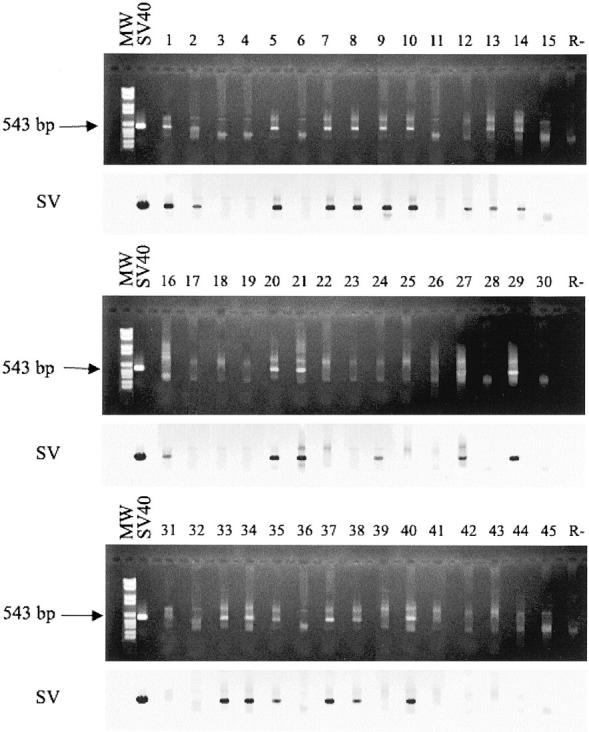
Agarose gels stained with ethidium bromide (dark top panels) and film images (light bottom panels) of DNA samples from human PA analyzed by snPCR and filter hybridization. MW, molecular weight markers (marker VI, Roche Diagnostics, Milan, Italy). SV40 is the PCR positive control. 1–45 are the DNA samples from PA specimens. Lane R is the negative control of the PCR reaction without DNA template. Top: PCR analysis for the SV40 Tag N-terminal sequences. Arrows indicate the product size obtained by snPCR (543 bp). Bottom: film images of snPCR amplified products of the top panels hybridized to the SV40 specific internal SV probe.
DNA Sequence Analysis
To confirm the specificity of SV40 footprints detected in human PA, all PCR products were subjected to DNA sequence analysis. All SV40 PCR amplified products, ie, the Tag N-terminal region of 543 bp, were sequenced. 18 DNA sequence analysis showed that the viral sequence of our samples was not distinguishable from the SV40 wt 776 strain (data not shown). These data are in agreement with the results obtained by several investigators who detected SV40 776 sequences in different human samples. 11,18 This result does not seem to be a consequence of laboratory contamination, because we used as control two SV40 strains, 776 and VA 45–54-A1 (GenBank accession numbers AF3161397 and AF15619 for strain 776 and VA 45–54-A1, respectively). Indeed, none of the viral fragments amplified by snPCR from specimens under analysis exhibited the DNA sequences of the SV40 strain VA 45–54-A1.
Immunohistochemistry Analysis of the Tag Oncoprotein
Immunohistochemical staining of routine histological sections by the PAP technique resulted in a dark brown reaction product in antigen-containing cells, whereas the background appeared pale blue owing to the counterstain. The SV40 Tag transformed human fibroblasts WI 38 13A, which represent the positive control, were immunoreactive, whereas the normal human fibroblasts WI 38, the negative control, were all negative (Figure 2, A and B) ▶ . Nuclear staining was found in 26 of 28 (93%) of PA tissue specimens previously found SV40-positive by PCR. The most consistent morphological sign of the virus footprint was a bright finely granular nuclear staining either in the epithelial component or in the stromal component. SV40 Tag oncoprotein was detected as a fine particulate or dense homogeneous pattern into the nucleus. SV40 Tag protein was detected in the solid, trabecular, tubular, or cystic structures consisting of epithelial cells of varying differentiation both of the tumor and normal cells near the PA sample (Figure 2, D–F) ▶ . In addition, SV40 Tag was found in the mucoid, chondroid or mixed mucoid-chondroid stromal areas. No nuclear signal for SV40 Tag was revealed in acinar cells, in endothelial and myoepithelial cells of the normal parotid gland (Figure 2C) ▶ .
Figure 2.
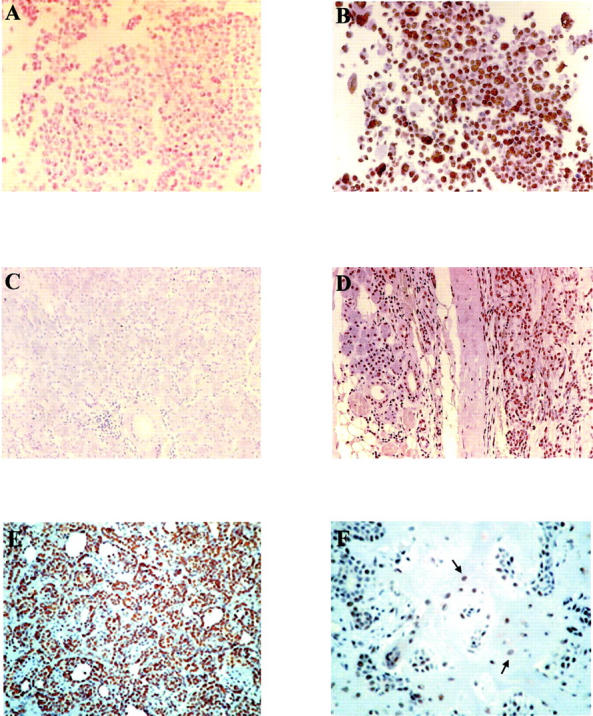
Histological staining of pleomorphic adenomas for SV40 Tag oncoprotein. A (×200) and B (×320) show normal (WI 38) and SV40 Tag-transformed human fibroblasts (WI 38–13A), used as negative and positive controls, respectively. Immunoreactive cells are present only in the SV40 Tag-transformed cells. C (×140) shows cells from a normal salivary gland tissue, whereas D (×50) shows a PA of a parotid gland and the normal tissue surrounding the tumor. None of cells of the normal salivary gland is immunoreactive (C), while positive staining is present both in the transformed and normal cells of the PA sample. (D). E (×140), and F (×200) represent two different morphology, the trabecular-tubular and mixoid-stromal aspect of the PA; the positive staining appears as a dense homogeneous pattern into the nucleus of the trabecular-tubular component (E), and in the mixoid-stromal dispersed cells (F, arrows).
Discussion
A new wave of investigations to test the presence of SV40 in human tumors has arisen since the advent of the PCR technology. 5,7 SV40 footprints were detected in tumor samples and normal tissues from children and adults, both exposed and not exposed to SV40-contaminated vaccines. 7 In this study, more than 35% of the patients with SV40-positive pleomorphic adenoma of the salivary gland was probably not administered with SV40-contaminated antipolio vaccines being older than 33 in 1955 or born after 1965 (Table 1) ▶ . These observations further support the hypothesis that SV40 is now present in the human population, and is probably a human virus. However, it is possible that this viral agent was already present in humans before the production and administration of SV40-contaminated vaccines. 8
In our study, carried out by PCR and filter hybridization, SV40 Tag coding sequences were detected with high prevalence in human PA, but not in normal salivary gland tissues. The use of this sensitive technique disclosed the presence of SV40 sequences in human PA. DNA sequence analysis indicated that SV40 sequences, amplified by PCR, belong to SV40 wild-type and not to other simian, human or recombinant polyomaviruses. These results indicate that PA is the seventh human tumor type found SV40-positive. Indeed, in previous reports brain and bone tumors, pleural malignant mesotheliomas, thyroid carcinomas, pituitary adenomas and different lymphoproliferative disorders were found SV40-positive. 11 SV40 Tag analyzed by immunohistochemistry revealed that 93% of the samples, found SV40-positive by PCR, expressed the Tag oncoprotein. It is of interest to note that in PA samples which stain positive for SV40 Tag, the viral oncoprotein was detected only in cells of epithelial origin. In PA samples SV40 Tag was also detected, by immunohistochemistry, in normal cells near the tumor. It is possible that the viral infection may spread from transformed cells to normal cells surrounding the tumor.
The results of this investigation suggest that SV40 may play a role in the onset/progression of the PA, which is a benign neoplasm. It is also possible that PA transformed cells represent an occasional milieu for SV40, while the corresponding normal tissue does not allow SV40 multiplication. However, the detection of the Tag viral oncoprotein expression suggests that SV40 is not a passenger virus. Indeed, with its Tag oncoprotein SV40 may exert its tumorigenic potential in PA tissues. Since previous studies reported SV40 sequences in PBMC of tumor patients 23 and in PBMC of normal individuals, 16,18,23 it is possible that leukocytes may transfer SV40 DNA to different tissues of the host, where SV40 could persist for a long period in a few cells of a given tissue. The association of SV40 with PA suggests some hypotheses. SV40 has a number of characteristics indicating that it may cooperate as a cofactor to the development or progression of human tumors. It has been proposed that the classical Koch’s postulates, cannot be applied to latent viruses with oncogenic potential. 9,24 New rules should be considered for these viruses to establish their oncogenic role 9,24 : 1) presence and persistence of the virus or its nucleic acid in tumor cells; 2) cell immortalization or neoplastic transformation after transfection of the viral genome or its subgenomic fragments; 3) demonstration that the transformed phenotype of the primary tumor and the modifications induced by transfection of cultured cells depend on specific functions expressed by the viral genome; 4) epidemiological and clinical evidence that viral infection represents a risk factor for tumor development.
SV40 fulfils the first three criteria, suggesting that SV40 may cooperate as a cofactor to the development or progression of human pleomorphic adenomas and other neoplasms. Indeed, SV40 DNA is present and expressed in human tumors. SV40 is oncogenic in rodents and in humans, and it is mutagenic and clastogenic in animal and human cells. SV40 Tag is a transforming protein which behaves like an activated oncogene. It binds and inactivates the products of tumor suppressor genes p53 and pRb family and co-activator factors p300 and p400 abolishing their functions. 9,10 SV40 cooperating with the c-ras activated oncogene and the catalytic subunit of the telomerase transforms human fibroblasts 25 and astrocytes in vitro. 26 Transgenic mice expressing SV40 Tag develop adenocarcinomas in submandibular glands. 14,15 It should be noted that SV40 lytic activity in most human cells is minimal, while its transforming properties may operate during a long period of latent/persistence infection. Under these conditions SV40 oncogenicity may act inducing immortalization/transformation or proliferation of clonal neoplastic cells in a population of SV40 latently infected cells. The assessment of the fourth postulate is at present under investigation both in the United States 27 and Italy (our laboratories).
Footnotes
Address reprint requests to Mauro Tognon, Ph.D., Department of Morphology and Embryology, Section of Histology and Embryology, School of Medicine, University of Ferrara, Via Fossato di Mortara 64/B, 44100 Ferrara, Italy. E-mail: tgm@unife.it.
Supported in part by grants from Associazione Italiana per la Ricerca sul Cancro (to M.T.), C.N.R. Target Project “Biotechnology” (to M.T.), and M.I.U.R. PRIN (to M.T.), and Local Projects (to M.T. and F.C.). M.M. was supported by a fellowship from the Fondazione Italiana per la Ricerca sul Cancro (Milano).
M.M. and F.M. contributed equally to this work.
References
- 1.Farina A, Pelucchi S, Carinci F: Evidence of bimodal distribution of age in patients affected by pleomorphic adenoma of the parotid gland. Oral Oncol 1997, 33:288-289 [DOI] [PubMed] [Google Scholar]
- 2.Farina A, Pelucchi S, Grandi E, Carinci F: Histological subtypes of pleomorphic adenoma and age-frequency distribution. Br J Oral Maxillofac Surg 1998, 37:154-155 [PubMed] [Google Scholar]
- 3.Carinci F, Farina A, Pelucchi S, Calearo C, Fini-Storchi O, Merlo R, Pastore A: Parotid gland carcinoma: 1987 and 1997 UICC T classifications compared for prognostic accuracy at 5 years. Eur Arch Otorhinolaryngol 2001, 258:150-154 [DOI] [PubMed] [Google Scholar]
- 4.Carinci F, Farina A, Pelucchi S, Calearo C, Pastore A: Parotid gland carcinoma: surgical strategy based on local risk factors. J Craniofac Surg 2001, 12:434-437 [DOI] [PubMed] [Google Scholar]
- 5.Toida M, Balazs M, Mori T, Ishimaru JI, Ichihara H, Fujitsuka H, Hyodo I, Yokoyama K, Tatematsu N, Adany R: Analysis of genetic alterations in salivary gland tumors by comparative genomic hybridization. Cancer Genet Cytogenet 2001, 127:34-37 [DOI] [PubMed] [Google Scholar]
- 6.Roijer E, Kas K, Klawitz I, Bullerdiek J, Van de Ven W, Stenman G: Identification of a yeast artificial chromosome spanning the 8q12 translocation breakpoint in pleomorphic adenomas with t(3;8)(p21;q12). Genes Chromosomes Cancer 1996, 17:166-171 [DOI] [PubMed] [Google Scholar]
- 7.Voz ML, Agten NS, Van de Ven WJ, Kas K: PLAG1, the main translocation target in pleomorphic adenoma of the salivary glands, is a positive regulator of IGF-II. Cancer Res 2000, 60:106-113 [PubMed] [Google Scholar]
- 8.Jin C, Martins C, Jin Y, Wiegant J, Wennerberg J, Dictor M, Gisselsson D, Strombeck B, Fonseca I, Mitelman F, Tanke HJ, Hoglund M, Mertens F: Characterization of chromosome aberrations in salivary gland tumors by FISH, including multicolor COBRA-FISH. Genes Chromosomes Cancer 2001, 30:161-167 [PubMed] [Google Scholar]
- 9.Barbanti-Brodano G, Martini F, De Mattei M, Lazzarin L, Corallini A, Tognon M: BK and JC human polyomaviruses and simian virus 40: natural history of infection in humans, experimental oncogenicity and association with human tumors. Adv Virus Res 1998, 50:69-99 [DOI] [PubMed] [Google Scholar]
- 10.Butel JS, Lednicky JA: Cell and molecular biology of simian virus 40: implications for human infections and disease. J Natl Cancer Inst 1999, 91:119-134 [DOI] [PubMed] [Google Scholar]
- 11.Jasani B, Cristaudo A, Emri SA, Gazdar AF, Gibbs A, Krynska B, Miller C, Mutti L, Radu C, Tognon M, Procopio A: Association of SV40 with human tumors. Semin Cancer Biol 2001, 11:49-61 [DOI] [PubMed] [Google Scholar]
- 12.Geissler E, Konzer P, Scherneck S, Zimmerman W: Sera collected before introduction of contaminated polio vaccine contain antibodies against SV40. Acta Virol 1985, 29:420-423 [PubMed] [Google Scholar]
- 13.Mortimer EA, Lepow ML, Gold E, Robbins FC, Burton GJ, Fraumeni JF: Long-term follow-up of persons inadvertently inoculated with SV40 as neonates. N Engl J Med 1981, 305:1517-1518 [DOI] [PubMed] [Google Scholar]
- 14.Ewald D, Li M, Efrat S, Auer G, Wall RJ, Furth PA, Hennighausen L: Time-sensitive reversal of hyperplasia in transgenic mice expressing SV40 T antigen. Science 1996, 273:1384-1386 [DOI] [PubMed] [Google Scholar]
- 15.Dardick I, Ho J, Paulus M, Mellon PL, Mirels L: Submandibular gland adenocarcinoma of intercalated duct origin in Smgb-Tag mice. Lab Invest 2000, 80:1657-1670 [DOI] [PubMed] [Google Scholar]
- 16.Martini F, Iaccheri L, Lazzarin L, Carinci P, Corallini A, Gerosa M, Iuzzolino P, Barbanti-Brodano G, Tognon M: Simian virus 40 early region and large T antigen in human brain tumors, peripheral blood cells and sperm fluids from healthy individuals. Cancer Res 1996, 56:4820-4825 [PubMed] [Google Scholar]
- 17.Seifert G, Brocheriou C, Cardesa A, Eveson JW: WHO international histological classification of tumors: tentative histological classification of salivary gland tumor. Pathol Res Pract 1990, 186:555-581 [DOI] [PubMed] [Google Scholar]
- 18.Martini F, Lazzarin L, Iaccheri L, Vignocchi B, Finocchiaro G, Magnani I, Serra M, Scotlandi K, Barbanti-Brodano G, Tognon M: Different simian virus 40 genomic regions and sequences homologous to SV40 large T antigen in DNA of human brain and bone tumors and of leukocytes from blood donors. Cancer 2002, 94:1-13 [PubMed] [Google Scholar]
- 19.Fiers W, Contreras R, Haegemann G, Rogiers R, Van de Voorde A, Van Heuverswyn H, van Herreweghe J, Volckaert G, Ysebaert M: Complete nucleotide sequence of SV40 DNA. Nature 1978, 273:113-120 [DOI] [PubMed] [Google Scholar]
- 20.Bergsagel DJ, Finegold MJ, Butel JS, Kupsky WJ, Garcea RL: DNA sequences similar to those of simian virus SV40 in ependymomas and choroid plexus tumors of childhood. N Engl J Med 1992, 326:988-993 [DOI] [PubMed] [Google Scholar]
- 21.Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 1977, 74:5463-5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chozick BS, Weicker ME, Pezzullo JC, Jackson CL, Finkelstein SD, Ambler MW, Epstein MH, Finch PW: Pattern of mutant p53 expression in human astrocytomas suggests the existence of alternate pathways of tumorigenesis. Cancer 1994, 73:406-415 [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto H, Nakayama T, Murakami H, Hosaka T, Nakamata T, Tsuboyama T, Oka M, Nakamura T, Toguchida J: High incidence of SV40-like sequences detection in tumour and peripheral blood cells of Japanese osteosarcoma patients. Br J Cancer 2000, 82:1677-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.zur Hausen H: Oncogenic DNA viruses. Oncogene 2001, 20:7820-7823 [DOI] [PubMed] [Google Scholar]
- 25.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA: Creation of human tumour cells with defined genetic elements. Nature 1999, 400:464-468 [DOI] [PubMed] [Google Scholar]
- 26.Rich JN, Chuanhai G, McLendon Re, Bigner DD, Wang XF, Counter CM: A genetically tractable model of human glioma formation. Cancer Res 2001, 61:3556-3560 [PubMed] [Google Scholar]
- 27.Ferber D: Monkey virus link to cancer grows stronger. Science 2002, 296:1012-1015 [DOI] [PubMed] [Google Scholar]


