Abstract
Large congenital melanocytic nevi (CMN) are at an increased risk of developing melanoma. Several forms of secondary proliferations can arise in congenital nevi on rare occasions. Although some of these closely resemble melanoma both clinically and histologically, metastasis is rare. We used comparative genomic hybridization to analyze chromosomal aberrations in different types of proliferations arising in CMN and compared them to typical congenital nevi, clear-cut melanomas arising in congenital nevi, as well as primary cutaneous melanomas that were not associated with a CMN. Cases of CMN and CMN with secondary proliferations were assigned to six groups according to the predominant histological pattern: group I, bland congenital nevi (n = 6); group II, congenital nevi with foci of increased cellularity (n = 4); group III, CMN with a proliferation simulating superficial spreading melanoma in situ (n = 3); group IV, CMN with a proliferation simulating nodular melanoma (n = 9); group V, proliferating neurocristic hamartoma (n = 1); and group VI, melanoma arising in congenital nevus (n = 6). No aberrations were found in groups I to III, whereas seven of nine cases of group IV, and one of one case of group V, showed aberrations. In group IV six of seven cases with aberrations (86%) showed numerical aberrations of whole chromosomes exclusively. This pattern differed significantly from the findings in melanoma that arose within CMN (n = 6), group VI, or independent of CMN (n = 122) in which only 5% showed numerical changes only. The single case in group V showed aberrations similar to melanoma. The finding of frequent numerical chromosomal aberrations in atypical nodular proliferations arising in CMN identifies these as clonal neoplasms with a genomic instability consistent with a mitotic spindle checkpoint defect. This difference compared to the aberration pattern found in melanoma might explain their more benign clinical behavior and may be of diagnostic value in ambiguous cases.
Patients with congenital melanocytic nevi (CMN) have an increased risk of developing melanoma within lesional skin. Whereas in small (<1.5 cm) and intermediate size (1.5 to 20 cm) CMN the risk seems to be low, 1,2 patients with large CMN (>20 cm) carry a 5 to 15 times increased risk of developing melanoma and, rarely, other neural crest-derived malignancies. 3-10
During the neonatal period several types of melanocytic tumors can develop within CMN, many of which are thought to be distinct from melanoma. 9 These range widely in size, and can grow very fast and ulcerate. 11 Even the most clinically worrisome tumors arising within the neonatal period often have a benign course and tend to stabilize or regress after a period of rapid growth. Because true melanoma can occur in the neonate, the development of any secondary proliferation in a CMN is of great concern. These lesions can be extremely difficult to classify histologically. Four different histological patterns of secondary proliferations in CMN during the neonatal period have been described: 11 1) simulants of superficial spreading melanoma, in which the epidermis and superficial dermis contain large epithelioid melanocytes, sometimes with pagetoid spread in the epidermis; 2) simulants of nodular melanoma with a nodular proliferation of large melanocytes with uniform nuclei in the dermis; 3) cases described as proliferative neurocristic hamartoma, characterized by a deep dermal or subcutaneous proliferation with a variety of forms of neural or mesenchymal differentiation; and 4) true melanoma, most of which show small blast-like melanocytes with hyperchromatic nuclei, scant cytoplasm, and a high mitotic rate. We studied the chromosomal aberrations in 10 atypical nodular proliferations and compared them to conventional congenital nevi, CMN with less alarming secondary proliferative changes as well as CMN in which clear-cut melanoma developed. We show that chromosomal aberrations are common in nodular proliferations, and are absent from conventional congenital nevi. However, in contrast to melanoma in which structural chromosomal aberrations are found in the vast majority of cases, the aberrations in these secondary proliferations are predominantly numerical changes. These findings point toward a qualitatively different type of genomic instability in atypical nodular proliferations in congenital nevi possibly explaining their less aggressive behavior. The different genomic characteristics may also be useful in the classification of histopathologically ambiguous cases.
Materials and Methods
Cases
Formalin-fixed, paraffin-embedded tissues from CMN and benign proliferations arising therein (groups I to V) were retrieved from the archives of the Department of Pathology, University of California at San Francisco. The patients’ ages ranged from 1 day to 20 years, the average and median ages were 3.5 and 0.3 years. All nodular proliferations were from infants 2 days to 4 months old. The cases were assigned to one of five histological groups according to the predominant histological pattern (Tables 1 and 2) ▶ ▶ (Figure 1) ▶ .
Table 1.
Histological Definition of Groups I to VI
| Group I | Congenital nevus, superficial or superficial and deep type |
| Group II | Congenital nevus, superficial or superficial and deep type, with superficial dermal foci of increased cellularity |
| Group III | Congenital nevus, superficial or superficial and deep type, with marked intraepidermal upward scatter and large junctional nests of melanocytes simulating superficial spreading melanoma |
| Group IV | Congenital nevus, superficial or deep type, with nodular proliferations of high cellularity, nuclear atypia, and markedly increased proliferation rate |
| Group V | Features of proliferative neurocristic hamartoma with nests of epitheloid melanocytes surrounded by a loose myxoid stroma with spindle cells |
| Group VI | Melanoma arising in a congenital nevus |
Table 2.
Summary of Clinical, Histological, and Genetic Findings of Cases
| Case | Age | Sex | Clinical | Histology* | CGH | Ki67 | Follow-up |
|---|---|---|---|---|---|---|---|
| CN28A | 5 years | f | Large CMN on right face | I | No changes | 1% | 5.5 years |
| CN25 | 5 months | f | Large CMN on buttock, abdomen, scalp and ear | I | No changes | 1% | 5.5 years |
| CN2 | 20 years | f | Intermediate CMN on lower leg | I | No changes | <1% | N/A |
| CN3 | 15 years | m | Intermediate CMN on upper back | I | No changes | <1% | N/A |
| CN4 | 20 years | f | Small CMN on chin | I | No changes | <1% | N/A |
| CN1 | 13 years | m | Small CMN on left flank | I | No changes | 5% | N/A |
| CN26 | 1 years | f | Intermediate CMN on left hand | II | No changes | 1% | 2 years |
| CN12 | 7 months | m | Large CMN on back, trunk, left arm | II | No changes | 1% | 2.5 years |
| CN17 | 2 years | f | Large CMN on scalp | II | No changes | 1% | 3 years |
| CN11/1 | 3 months | f | Large CMN on trunk, centered over LS spine, extending onto right thigh. Multiple satellites. Grey-blue color, multinodular and infiltrated pattern; congenital erosion | II | No changes | N.D. | 5 years |
| CN19 | 10 months | f | Large CMN with segmental pattern right groin and buttock onto leg; Very dark at birth but lightened, but with marked irregular specks and mottling | III | No changes | 1% | 4 years |
| D122 | 1 days | m | Large, atypical appearing CMN19 × 10 cm centered on R chest. Stellate ∼6.5 × 4 cm “scar like area” in center | III | No changes | 8% | 9 months |
| CN14/1 | 7 months | F | 1) Large CMN involving back, buttock and perineum. Recent growth with perirectal involvement and consecutive obstruction. | III | No changes | 5% | Died of disease probably due to massive pelvic |
| 2) Lymph node metastasis perinodal and capsular involvement by melanocytic cells | dim (4q34-qter,7, 14q13-14q), enh (1q21-1q41, 8q21-qter) | obstruction. | |||||
| CN16 | 4 months | f | Large CMN on scalp with a firm nodule in the central portion | IV | dim (5) | 10% | 4 years |
| CN22 | 3 months | f | Large CMN on upper back with a papular growth | IV | enh (8, 15, 20, 21) dim (10) | 5% | N/A |
| CN24 | 2 days | m | Large CMN on entire lower back | IV | No changes | N/A | |
| D140 | 1.5 months | f | Large CMN covering trunk, groin and thighs, with satellites, irregular colors. Multiple papules and vascular-nodules at time of biopsy | IV | enh (10, 11, 16, 20, 22) dim (12) | 5% | 1 year |
| D32 | 2 days | f | Large CMN with bathing suit like distribution | IV | dim (3, 5, 7, 9, 11) enh (20) | 8% | 2.5 years |
| D62 | 2 months | f | CMN with occipital scalp | IV | No changes | 5% | N/A |
| D146 | 4 months | m | Large CMN on abdomen | IV | dim (7, 10) enh (8) | 10% | N/A |
| CN29 | 3 months | f | Large CMN on left leg extending onto perineum and buttocks with multiple satellite lesions | IV | dim (7) | 5% | 1.5 years |
| Clinically extreme variability with flesh colored and reddish nodules, black macules on leg | |||||||
| CN30 ½ | 16 days | f | Large CMN on buttocks with recent bleeding pigmented plaque on right buttock and proliferating nodules inguinal area | IV | enh (6p) | 7% | 2 months |
| CN10 | 3 days | f | Large CMN on posterior trunk | V | dim (3, 4, 5p, 9pter-9q21, 18) enh (2pter-p13, 2q32-qter, 6p, 6q24-qter, 7, 8, 15q21-15qter, 16q21-qter, 20) | 7% | 15 years |
| D168 | 59 years | m | Nodule arising in a small CNM | VI | dim (4pter-4qter, 9, 12q14-qter) enh (7, 8, 9q34-qter, 11q13-11q13, 19, 20) | N.D. | N/A |
| CN31 | 42 years | f | Nodule arising in a large CNM on buttocks | VI | dim (1p, 2, 5cen-q32, 6cen-q22, 9, 13) | N.D. | Died of metastatic melanoma |
| CN35 | 65 years | f | Nodule in a congenital nevus on the buttock | VI | Dim (1p32-cen, 5q31-33, 6q21-23, 9pter-p13, 14q21-qter, Xq23-qter | N.D. | Positive sentinel lymph node biopsy |
| CN38 | 22 months | m | Metastatic melanoma to the brain in a child with a large congenital nevus on the trunk and neurocutaneous melanosis 25 | VI | Dim (4p, 8p, 11q23-qter, Xq) enh (6p, 8q, 20q12-qter, 22) amp (11q13-14) | N.D. | Died of disease at age of 24 months |
| CN40 | 8 years | m | Ulcerated and bleeding nodule within a large CMN on posterior trunk | VI | dim (5, 7, 10, 15q14-21, 16q), enh (1q, 8, 11q13, 16p, 19, 20, 21q22) | N.D. | N/A |
| CN41 | 18 months | f | Left shoulder mass arising in a partially excised large CMN | VI | dim (4q, 5q, 6q, 13q21-22), enh (1q, 6p, 7, 8, 20, 22) | N.D. | Lymph node metastasis, alive 4 years after surgery |
Figure 1.
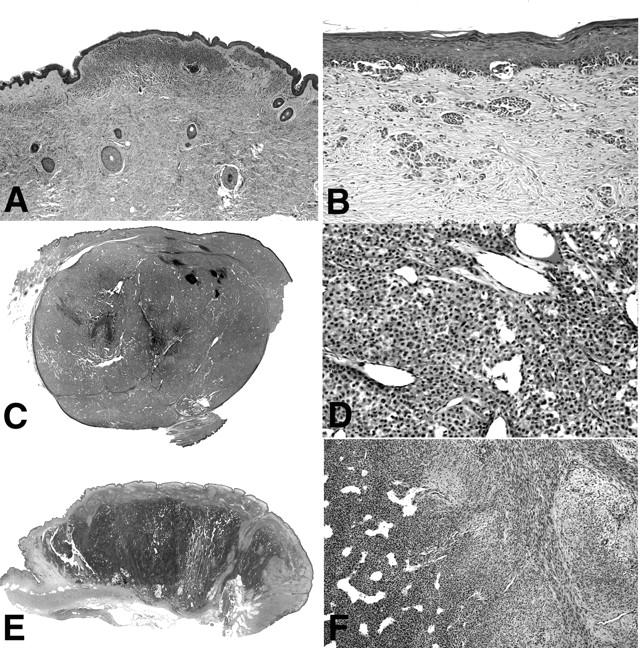
Histopathology of cases in groups II to V. A: Photomicrograph of case CN12, representative for cases of group II. In the center of the superficial dermis there is focus of increased cellularity in an otherwise bland superficial congenital nevus. B: Case CN14, representative of group III, showing a junctional melanocytic proliferation with melanocytes disposed in irregular nests and solitary units, simulating superficial spreading melanoma. This case additionally shows marked desmoplasia and irregularly configured nests of melanocytes in the dermis. Scanning magnification and close up of case D62, representative of cases in group IV (C, D), and case CN10, the only case in group V (E, F). The images show a large, sharply demarcated dermal nodule (C) with increased cellularity and marked angiogenesis (D). A large dermal nodule (E) with areas of increased cellularity and a hemangiopericytoma-like vascular pattern (F, left), and areas with spindle-shaped cells in a myxoid stroma (F, right).
In addition, we also studied six cases of bona fide melanoma that arose in congenital nevi, that were retrieved from the Department of Pathology, University of California at San Francisco; the Department of Pathology, Memorial Sloan Kettering Cancer Center, New York; and the Department of Pathology, Children’s Memorial Hospital, Northwestern University, Chicago. These cases were assigned to group VI (see Table 2 ▶ for clinical information).
As a comparison we used a group of 122 primary cutaneous melanomas that had been previously analyzed by CGH, some of which have been previously published. 12,13 The average tumor thickness in this group was 3.7 mm.
Comparative Genomic Hybridization (CGH)
DNA Extraction
Tissue used for CGH analysis was selectively microdissected from regions of highest cellular density that were most representative of the particular histological group. Tumor-bearing tissue was microdissected from 30-μm sections (2 to 20 per tumor) using hematoxylin and eosin-stained sections as guidance. DNA extraction and labeling was performed as published earlier. 12
CGH
All measurements were performed in duplicates: once with 1 μg of tumor DNA labeled with fluorescein-12-dUTP (Dupont Inc., Boston, MA), and 200 ng of Texas Red-5-dUTP-labeled reference DNA (standard labeling), and a second time with the labeling reversed.
Controls and Threshold Definitions
Normal DNA and DNA from tumor cell lines with known aberrations were used as negative and positive controls for CGH, respectively. We regarded a region as aberrant when either the standard labeling or the reverse labeling resulted in a tumor/reference fluorescent ratio <0.80 or >1.2, or both the standard and the reverse labeling resulted in a tumor:reference fluorescent ratio <0.85 or >1.15. 12
Immunohistochemistry
Proliferation was assessed using an antibody against Ki-67 (Mib-1, dilution 1:500; Beckman Coulter, Fullerton, CA) according to the manufacturer’s instructions. Immunoreactivity was assessed using the ×20 objective and only cells that showed definitive nuclear staining were counted.
Fluorescence in Situ Hybridization (FISH)
Dual-color FISH was performed on tissue sections of the array as described previously. 14 The probes for chromosome 9p21 labeled with Spectrum Orange and 9q34.1 labeled with Spectrum Green were provided by Vysis Inc. (Downers Grove, IL).
Results
None of the 13 cases (0%) of groups I to III showed any chromosomal aberrations by CGH whereas seven of nine (78%) cases of group IV, one of one (100%) case of group V, and six of six (100%) cases of group VI showed aberrations. The predominant pattern in the cases of group IV was gain or loss of entire chromosomes only. This differs from the aberration pattern observed in melanoma, 12,13 in which most cases have aberrations involving only partial chromosomes (Table 3) ▶ . In a total of 122 melanomas that we have studied by CGH we found aberrations in 116 cases (95%). Of these 116 melanomas, 111 (96%) showed at least one aberration that involved a fraction of a chromosome. Only five melanomas (4%) showed gains or losses of entire chromosomes exclusively. In contrast, of seven lesions of group IV that had any aberration, six (86%) cases had changes involving entire chromosomes only. Only one case had a change involving a partial chromosome, which was gain of chromosome 6p.
Table 3.
Comparison of Chromosomal Aberrations in Proliferative Nodules Arising in CMN (Group IV) and Those in Primary Cutaneous Melanoma
| n | Loss of 9 | Loss of 10 | Loss of 7 | No aberrations | Whole chromosomes only | Whole and partial chromosomes | Partial chromosomes only | |
|---|---|---|---|---|---|---|---|---|
| Proliferative nodules in CMN | 10 | 2 (20%) | 2 (20%) | 3 (30%) | 2 (22%) | 6 (66%) | 0 (0%) | 1 (11%) |
| Primary cutaneous melanoma | 122 | 73 (60%) | 71 (58%) | 0 (0%) | 6 (5%) | 5 (4%) | 72 (59%) | 45 (37%) |
In addition to the difference in aberration pattern there were also differences in the specific chromosomes involved. In the melanomas with aberrations involving whole chromosomes only (5 of 122), chromosomes 9 or 10 were always involved. Indeed, losses involving these chromosomes are the most frequent cytogenetic change in primary cutaneous melanoma, occurring in ∼60% of all cases. In the cases of group IV, however, losses of chromosomes 9 and 10 were less frequent. Only one case (14%) had loss of 9, and two (20%) cases had loss of 10. Gains of 10 were never observed in any of the cases in group VI or the 122 melanomas. Loss of chromosome 7 was seen in three cases of group I, but this change was not observed in any of the122 melanomas. Interestingly, one of the melanomas arising in congenital nevi (group VI) showed this aberration.
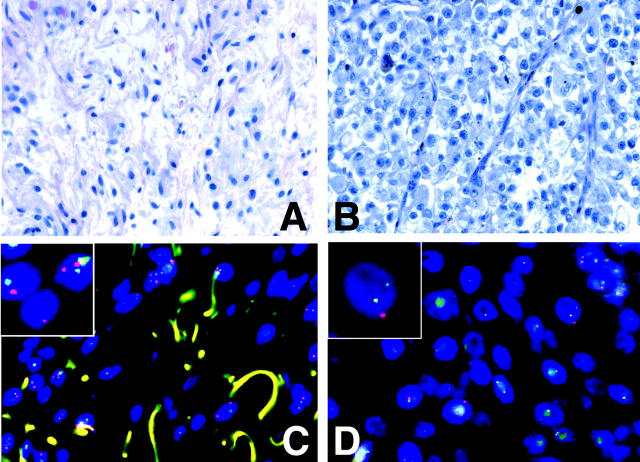
The case showing features previously described as proliferative neurocristic hamartoma (group V) 11 had an aberration pattern similar to melanoma. All six cases of melanomas arising in CMN (group VI) showed multiple cytogenetic aberrations in a pattern indistinguishable from that of the melanomas not associated with congenital nevus (Table 2) ▶ . There were multiple aberrations that frequently involved chromosomal fragments. Moreover, loss of chromosome 9 was present in all three cases. In one case (CN35) there was sufficient tissue available to allow a separate CGH analysis of the nevus part. No aberrations were found. In an additional two cases we performed FISH to look for aberrations in the nevus that CGH detected in the melanoma part. Both cases showed loss of chromosome 9, which is a frequent and probably early event in melanoma. 12 FISH confirmed the loss of chromosome 9p in the melanoma part, no copy number changes of chromosome 9 were detected in the nevus part (Figure 2) ▶ .
Figure 2.
Copy number changes in a congenital nevus (A, C) and a melanoma arising therein (B, D). Top: Higher power fields of the nevus part and the melanoma of case CN31. Bottom: A dual-color FISH with probes for chromosomes 9p21 (red) and chromosome 9q34.1 (green). The melanocytes in the nevus (C) have equal copy numbers of red and green signals whereas the cells in the melanoma part (D) have lost one or both copies of 9p21.
One patient (CN14), a 7-month-old girl, deserves special comment. She had a giant congenital nevus involving 50% of her back, buttocks, and perineum, which was very unusual in its clinical and histological presentation. The entire nevus appeared inflamed with diffuse erythema and woody induration. No discrete proliferation was notable. A biopsy from the nevus showed a marked desmoplastic stroma reaction surrounding the cytologically bland melanocytes (Figure 1B) ▶ . The patient had a severe failure to thrive and deteriorated throughout the following months. She developed a pelvic mass that led to complete pelvic obstruction requiring colostomy and she died at the age of 1.5 years. Pathology specimens from the pelvic mass and pelvic lymph nodes were suggestive of melanoma. We analyzed tumor tissue from the initial punch biopsy from the nevus (CN14/1), which did not show any chromosomal aberrations. A second biopsy from a lymph node infiltrate of nevoid melanocytes (CN14/2) showed multiple aberrations, similar to melanoma (Table 2) ▶ .
Clinical follow-up information of five cases of group IV (mean follow-up time 1.6 years) did not show any progression to melanoma (Table 2) ▶ . Two patients of group VI died of metastatic melanoma, two had subsequent metastasis, and for the remaining two no follow-up information was available.
We studied the proliferation rate by immunohistochemical stains for Ki-67 (Table 2) ▶ . Eight of nine group IV cases (89%) had a proliferative index of 5% or more, whereas only 1 of 10 cases of groups I and II had a labeling rate >1%. The results seem to correlate the overall higher mitotic rate the cases of groups IV and V than groups I and II.
Discussion
In the current study we report frequent chromosomal aberrations in atypical nodular proliferations arising in congenital nevi. Our results differ from a previous report of two nodular proliferations, which did not reveal any aberrations by conventional cytogenetic analysis. 15 This may be because of the necessity of subculturing for cytogenetic analysis with the potential risk of expanding an irrelevant clone, or simply the small number of cases in that study. The ability of CGH to detect these changes suggests that identical aberrations were present in the majority of cells of the lesions, and implies that these lesions were clonal.
In contrast to the frequent aberrations found in cases of group IV, the congenital nevi in groups I to III did not show any chromosomal aberrations. This is similar to our findings in the majority of Spitz nevi 14 and blue nevi, including cellular and atypical variants (BC Bastian and colleagues, unpublished data), which do not show any chromosomal aberrations at the level of CGH resolution. Only a subset of Spitz nevi has an isolated gain of chromosome 11p. 14
The pattern of the aberrations that we found in the atypical nodular proliferations of group IV differs from those found in primary cutaneous melanoma. 12,13 Six of seven (86%) of the atypical nodular proliferations in group IV with aberrations detected by CGH had involvement of whole chromosomes only, whereas this pattern was seen in only 5% of melanomas. By contrast, 95% of melanomas, including those arising in congenital nevi, showed one or several gains or losses of chromosomal fragments. These findings indicate that the type of genomic instability in atypical nodular proliferations might differ from that predominating in melanoma. The comparison of the nevus and melanoma portions demonstrates that the chromosomal alterations are acquired during the transition to melanoma.
Genomic instability is considered as a significant factor in the pathogenesis of cancer. 16 There are several patterns of genomic instability, which are reflections of different functional defects in the cancer cells. Genetic alterations can affect the structure of chromosomes, their number, or their nucleotide sequence. An example of the last pattern is microsatellite instability in hereditary nonpolyposis colorectal cancer, in which defects in the mismatch repair machinery result in changes of nucleotide sequence. However, numerical or structural chromosomal aberrations are typically lacking in this cancer. 17 This is in strong contrast to other solid cancers, which virtually all show chromosomal aberrations. 18 Aberrations that only affect chromosome number are thought to be because of defects of the mitotic chromosome segregation apparatus, 19 whereas intra- and interchromosomal aberrations seem to be related to the generation or the repair of DNA breaks. 20 Recent data from mutator mutants in yeast have shown that genes involved in S-phase checkpoint functions, recombination, and telomere addition at double-strand breaks are essential for control of chromosomal integrity. 21,22 Future studies are required to determine the relevant factors in mammalian systems.
It seems logical to assume that defects that permit numerical chromosomal aberrations result in a less malignant phenotype, as compared those that promote structural aberrations, because whole chromosomes are likely to harbor some genes that provide a growth advantage along with others that provide a disadvantage for growth. In contrast, cancers bearing only the fragment carrying the advantageous genes are freed from the normally present growth-inhibiting genes on the same chromosome and would be likely to grow faster and acquire a malignant phenotype. The degree of genomic instability would increase the plasticity of the genome and permit a rapid evolution of the hallmarks of cancer. 23 Tumors with a more rigid genome, such as those whose defect only permits a change in chromosome numbers, would undergo a slower progression or may not be able to acquire all features of malignancy before they undergo replicative senescence and halt progression.
The numerical aberrations in the atypical nodular proliferations position them in the spectrum between benign melanocytic nevi that tend to have no chromosomal aberrations and outright melanoma in which both numerical and structural aberrations are the rule. The aberration pattern in the atypical nodular proliferations points toward a malfunction in chromosomal segregation, possibly within the mitotic spindle checkpoint. 24 Future studies are required to prove this hypothesis.
In contrast, the only case with histological features described as proliferative neurocristic hamartoma showed multiple chromosomal aberrations that involved chromosome fragments, and was genomically indistinguishable from melanoma. However, this patient was alive and free of any signs of malignancy after 15 years. Our data suggests that these lesions may in fact be neoplasms rather than hamartomas. However, more cases of this extremely rare entity need to be studied to place it within the spectrum of tumors associated with congenital nevi.
In summary our data show frequent chromosomal aberrations in atypical nodular proliferations arising in congenital nevi. These aberrations differ from those seen in melanoma in the type of aberrations (numerical aberrations in atypical nodular proliferations versus structural aberrations in melanoma) and the pattern of chromosomes involved (losses of chromosome 7 in atypical nodular proliferations, and frequent losses of chromosomes 9 and 10 in melanoma). These data indicate fundamental differences compared with melanoma, consistent with the generally benign behavior of these lesions. Genomic analysis may help in the classification of ambiguous cases.
Footnotes
Address reprint requests to Dr. Boris C. Bastian, Comprehensive Cancer Center, University of California, San Francisco, Box 0808, San Francisco, CA 94143-0808. E-mail: bastian@cc.ucsf.edu.
Supported by the Marvin and Roma Auerback Melanoma Fund.
References
- 1.Rhodes AR: Melanocytic precursors of cutaneous melanoma. Estimated risks and guidelines for management. Med Clin North Am 1986, 70:3-37 [DOI] [PubMed] [Google Scholar]
- 2.Sahin S, Levin L, Kopf AW, Rao BK, Triola M, Koenig K, Huang C, Bart R: Risk of melanoma in medium-sized congenital melanocytic nevi: a follow-up study. J Am Acad Dermatol 1998, 39:428-433 [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow AJ, English JS, Qiao Z: The risk of melanoma in patients with congenital nevi: a cohort study. J Am Acad Dermatol 1995, 32:595-599 [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Maldonado R, Tamayo L, Laterza AM, Duran C: Giant pigmented nevi: clinical, histopathologic, and therapeutic considerations. J Pediatr 1992, 120:906-911 [DOI] [PubMed] [Google Scholar]
- 5.Quaba AA, Wallace AF: The incidence of malignant melanoma (0 to 15 years of age) arising in “large” congenital nevocellular nevi. Plast Reconstr Surg 1986, 78:174-181 [DOI] [PubMed] [Google Scholar]
- 6.Gari LM, Rivers JK, Kopf AW: Melanomas arising in large congenital nevocytic nevi: a prospective study. Pediatr Dermatol 1988, 5:151-158 [DOI] [PubMed] [Google Scholar]
- 7.Egan CL, Oliveria SA, Elenitsas R, Hanson J, Halpern AC: Cutaneous melanoma risk and phenotypic changes in large congenital nevi: a follow-up study of 46 patients. J Am Acad Dermatol 1998, 39:923-932 [DOI] [PubMed] [Google Scholar]
- 8.Bittencourt FV, Marghoob AA, Kopf AW, Koenig KL, Bart RS: Large congenital melanocytic nevi and the risk for development of malignant melanoma and neurocutaneous melanocytosis. Pediatrics 2000, 106:736-741 [DOI] [PubMed] [Google Scholar]
- 9.DeDavid M, Orlow SJ, Provost N, Marghoob AA, Rao BK, Huang CL, Wasti Q, Kopf AW, Bart RS: A study of large congenital melanocytic nevi and associated malignant melanomas: review of cases in the New York University Registry and the world literature. J Am Acad Dermatol 1997, 36:409-416 [DOI] [PubMed] [Google Scholar]
- 10.Marghoob AA, Schoenbach SP, Kopf AW, Orlow SJ, Nossa R, Bart RS: Large congenital melanocytic nevi and the risk for the development of malignant melanoma. A prospective study. Arch Dermatol 1996, 132:170-175 [PubMed] [Google Scholar]
- 11.Clark WH, Elder DE, Guerry D: Dysplastic nevi and malignant melanoma. Farmer ER Hood AF eds. Pathology of the Skin ed 1 1990:pp 729-735 McGraw-Hill, New York
- 12.Bastian BC, LeBoit PE, Hamm H, Bröcker EB, Pinkel D: Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res 1998, 58:2170-2175 [PubMed] [Google Scholar]
- 13.Bastian BC, Kashani-Sabet M, Hamm H, Godfrey T, Moore DH, II, Bröcker EB, LeBoit PE, Pinkel D: Gene amplifications characterize acral melanoma and permit the detection of occult cells in the surrounding skin. Cancer Res 2000, 60:1968-1973 [PubMed] [Google Scholar]
- 14.Bastian BC, Wesselmann U, Pinkel D, LeBoit PE: Molecular cytogenetic analysis of Spitz nevi show clear differences to melanoma. J Invest Dermatol 1999, 113:1065-1069 [DOI] [PubMed] [Google Scholar]
- 15.Mancianti ML, Clark WH, Hayes FA, Herlyn M: Malignant melanoma simulants arising in congenital melanocytic nevi do not show experimental evidence for a malignant phenotype. Am J Pathol 1990, 136:817-829 [PMC free article] [PubMed] [Google Scholar]
- 16.Lengauer C, Kinzler KW, Vogelstein G: Genetic instabilities in human cancers. Nature 1998, 396:643-649 [DOI] [PubMed] [Google Scholar]
- 17.Lengauer C, Kinzler KW, Vogelstein B: Genetic instability in colorectal cancers. Nature 1997, 386:623-627 [DOI] [PubMed] [Google Scholar]
- 18.Mitelman F: Catalog of Chromosome Aberrations in Cancer ed 5 1994. Wiley-Liss, New York
- 19.Shah JV, Cleveland DW: Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell 2000, 103:997-1000 [DOI] [PubMed] [Google Scholar]
- 20.Schar P: Spontaneous DNA damage, genome instability, and cancer—when DNA replication escapes control. Cell 2001, 104:329-332 [DOI] [PubMed] [Google Scholar]
- 21.Myung K, Chen C, Kolodner RD: Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 2001, 411:1073-1076 [DOI] [PubMed] [Google Scholar]
- 22.Myung K, Datta A, Chen C, Kolodner RD: SGS1, the Saccharomyces cerevisiae homologue of BLM and WRN, suppresses genome instability and homeologous recombination. Nat Genet 2001, 27:113-116 [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA: The hallmarks of cancer. Cell 2000, 100:57-70 [DOI] [PubMed] [Google Scholar]
- 24.Amon A: The spindle checkpoint. Curr Opin Genet Dev 1999, 9:69-75 [DOI] [PubMed] [Google Scholar]
- 25.Kadonaga JN, Barkovich AJ, Edwards MS, Frieden IJ: Neurocutaneous melanosis in association with the Dandy-Walker complex. Pediatr Dermatol 1992, 9:37-43 [DOI] [PubMed] [Google Scholar]
- 26.: ISCN (1995): Report of the Standing Commitee on Human Cytogenetic Nomenclature. An International System for Human Cytogenetic Nomenclature. 1995. S. Karger, Basel [DOI] [PubMed]