Abstract
Schistosoma mansoni egg-induced lung pathology requires the actions of interleukin (IL)-4 and IL-13. Because receptors for IL-4 and IL-13 share chains, we examined the effect of a fusion protein comprised of IL-13 and Pseudomonas exotoxin (IL13-PE) on the development of pulmonary granulomas in mice. At day 8 after an intravenous injection of live S. mansoni eggs, whole lung samples from IL13-PE-treated mice exhibited significantly lower IL-4 and IL-13 gene expression, smaller granulomas, decreased collagen levels, and increased IL-13 receptor α2 gene expression compared to controls. The therapeutic effects of IL13-PE were also observed at day 16 despite the termination of IL13-PE treatment at day 8. These studies demonstrate that targeting IL-4- and IL-13- responsive cells with IL13-PE effectively arrests S. mansoni egg granuloma formation.
IL-4 and IL-13 are a duo of pleiotropic cytokines that exert similar and divergent effects in immune responses. 1-4 Conserved or overlapping functions of these cytokines include Ig isotype switching to IgE, eosinophilia, regulation of inflammatory responses, and protective immunity against helminth infections. 3,5 The functions of these cytokines diverge in key areas such as Th2 cell development, tissue fibrosis, and endotoxemia. 6,7 The complex roles of IL-4 and IL-13 are further compounded by their interaction with an array of cell surface receptors. IL-4 directly binds the IL-4 receptor α (IL-4Rα) and IL-13 specifically binds the IL-13Rα1 chain, 8 but IL-4Rα and IL-13Rα1 can form a functional receptor complex that binds both ligands. 9 IL-13 also binds with 100-fold higher affinity for IL-13Rα2 than IL-13Rα1, but the IL-13Rα2 chain does not seem to signal and may actually function as an in vivo inhibitor of IL-13 function. 10 IL-4 and IL-13 receptors are expressed on a variety of immune and nonimmune cells including B cells, natural killer cells, monocytes, mast cells, endothelial cells, and fibroblasts, 8,11,12 but naïve and memory T cells do not appear to respond to the extracellular presence of IL-13. 13,14
Previous studies have shown that the granulomatous response elicited by Schistosoma mansoni via infection 15-20 after intravenous injection of live S. mansoni eggs 16,21-26 or after intravenous injection of S. mansoni egg-antigen-coated Sephadex beads 27,28 is dependent on the Th2-dominant cellular events mediated by IL-4 and IL-13. These previous findings were derived from studies that used cytokine immunoneutralization, gene-knockout technology, or receptor decoy strategies designed to target IL-4, IL-13, both cytokines, or their receptors. For example, anti-IL-4 treatment markedly inhibited granuloma formation in the lungs of mice intravenously challenged with eggs. 21 Similarly, IL-4 knockout or deficient (−/−) mice have been observed to generate a modestly smaller granulomatous response to intravenously introduced S. mansoni eggs compared with appropriate wild-type mice. 22 The specific role for IL-13 after S. mansoni infection 17 and pulmonary embolization of S. mansoni eggs 24 was elucidated using a soluble IL-13Rα2-Fc; this IL-13 inhibitor markedly attenuated granuloma development, pulmonary eosinophilia, and pathological hepatic and pulmonary fibrosis. However, the greatest attenuation of the synchronous pulmonary granulomatous response to S. mansoni eggs has been observed in signal transducer and activator of transcription (Stat)-6-deficient mice 16 and in IL-4/IL-13−/− mice. 25 Together, these previous studies highlight that IL-4 and IL-13 play significant roles during S. mansoni-induced granuloma formation.
Given the exclusive and mutual roles of IL-4 and IL-13 during the granulomatous response to S. mansoni eggs, the exploration of therapeutics that targets the action of both cytokines is warranted. In the present study, we examined the effect of IL-13 cytotoxin-PE38QQR (IL13-PE), which is a chimeric molecule containing human IL-13 and a derivative of Pseudomonas exotoxin (BH Joshi and RK Puri, unpublished results) 29,30 during synchronous S. mansoni egg-induced pulmonary granuloma formation. We have previously used IL13-PE to successfully reverse all of the Th2-associated features of Aspergillus fumigatus-induced allergic airway disease including airway hyperreactivity and airway remodeling. 31,32 In addition, IL13-PE markedly reduced or abolished the whole lung expression of IL-4Rα and IL-13Rα1 during Aspergillus-induced allergic airway disease demonstrating that this IL-13 immunotoxin targets cells that are responsive to IL-4 and IL-13. 31,32 The present study showed that when given immediately after or as late as 8 days after the intravenous injection of live S. mansoni eggs, IL13-PE treatment significantly arrested the development of the Th2-mediated pulmonary granulomatous response. Interestingly, the IL13-PE treatment seemed to be mediated through the inhibition of IL-4, IL-13, and a concomitant dramatic increase in IL-13 decoy receptor (ie, IL-13Rα2) expression.
Materials and Methods
S. mansoni Egg Pulmonary Granuloma Model
Primary, synchronous pulmonary granulomas were induced in female, CBA/J mice (6 to 8 weeks old; Jackson Laboratories, Bar Harbor, ME) after the intravenous injection of 3000 live, mature S. mansoni eggs. Live S. mansoni eggs were derived from Swiss-Webster mice heavily infected with S. mansoni (courtesy of Dr. Fred Lewis, Biomedical Research Laboratory, Rockville, MD) according to previously described methods. 33 The primary model of Schistosoma egg-induced lung inflammation has been used extensively to characterize the synchronous pulmonary granulomatous response 34,35 and model the evolution of the Th2 response. 36
IL13-PE Treatment during Pulmonary Granulomatous Responses to Embolized S. mansoni Eggs
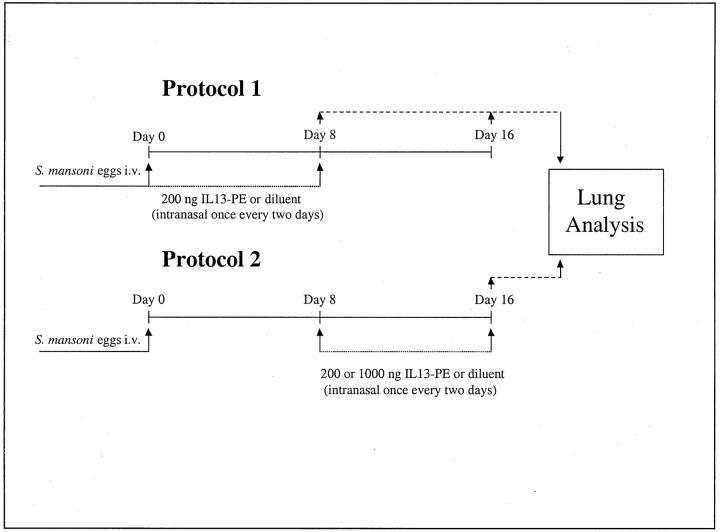
IL13-PE has been previously used in vivo to target and kill tumor cells expressing IL-4Rα, IL-13Rα1, and IL-13Rα2. 37,38 We have previously shown that IL13-PE (at a dose of 200 ng/dose) ameliorated chronic fungal asthma in two independent studies. 31,32 The dosing protocols used in the present study are outlined in Figure 1 ▶ . In the first protocol, groups of 15 mice received a 200 ng/dose of IL13-PE dissolved in 10 μl of phosphate-buffered saline (PBS) containing 0.25% human serum albumin, or diluent, via an intranasal bolus starting immediately after and every other day until day 8 after the S. mansoni egg challenge. Mice were then examined at days 8 and 16 after the induction of the pulmonary granulomatous response. In the second protocol, groups of five mice received a 200 or 1000 ng/dose of IL13-PE, or diluent starting at day 8 and continuing every other day until day 16 after the egg challenge, at which time the whole lungs from each mouse were subjected to lung histological and morphometric analysis (see below). In both protocols, control groups received the appropriate volume of diluent alone throughout the same time course.
Figure 1.
Summary of the treatment protocols used in the present study.
Lung Histological and Morphometric Analysis
Whole lungs from IL13-PE and control groups of mice (n = 10/group) at days 8 and 16 after the S. mansoni egg challenge were fully inflated with 10% formalin, dissected, and placed in fresh formalin for 24 hours. Routine histological techniques were used to paraffin-embed the entire lung, and 5-μm sections of whole lung were stained with hematoxylin and eosin (H&E) or with Masson trichrome. Inflammatory infiltrates and structural alterations were examined around pulmonary granulomas containing a single egg using light microscopy at a magnification of ×200. Morphometric analysis of egg granuloma size and Masson trichrome staining was performed as previously described in detail. 39 A minimum of 15 granulomas per lung section was analyzed for granuloma size and collagen deposition. Once the mean granuloma size in each mouse was statistically analyzed, these measurements were then used for the group mean analysis. Granuloma eosinophils were counted in high-powered fields at a magnification of ×1000.
Culture of Dispersed Lung and Spleen Cells
Whole lungs from IL13-PE and control groups of mice (n = 5/group) were removed at days 8 and 16 after S. mansoni egg embolization. A portion of the right lobe and the spleen from each mouse was then processed according to methods described in detail elsewhere. 40 Briefly, lung and spleen were mechanically dissociated by grinding each organ over fine steel mesh, individual cells were quantified using a hemocytometer, and 2 × 106 dispersed cells were added to each well of six-well tissue culture plates (Corning Inc., Corning, NY). Triplicate wells were left unstimulated or exposed to 5 μg of purified S. mansoni egg antigen. Tissue culture plates containing dispersed lung or spleen cells were then maintained in a 37°C incubator for 48 hours, after which time cell-free supernatants were removed for enzyme-linked immunosorbent assay (ELISA) analysis (see below).
ELISA Analysis
Murine RANTES/CCL5, macrophage-derived chemokine (MDC/CCL22), C10 chemokine/CCL6, macrophage inflammatory protein-1α (MIP-1α/CCL3), and IL-12 levels were measured in 50-μl samples from cell-free supernatants using a standardized sandwich ELISA technique previously described in detail. 41 Each ELISA was screened to ensure antibody specificity and recombinant murine cytokines, and chemokines were used to generate the standard curves from which the concentrations present in the samples were derived. The limit of ELISA detection for each cytokine was consistently more than 50 pg/ml. The cytokine levels in each sample were normalized to total protein levels measured using the Bradford assay.
Serum levels of total IgE, IgG1, and IgG2a in the IL13-PE treatment and control groups were analyzed using complementary capture and detection antibody pairs for IgE, IgG1, and IgG2a according to the manufacturer’s directions (PharMingen, San Diego, CA). Duplicate sera samples were diluted to 1:100 for IgE determination and 1:1000 for determination of IgG1 and IgG2a levels. Immunoglobulin levels were then calculated from optical density readings at 492 nm, and Ig concentrations were calculated from a standard curve generated using recombinant IgE, IgG1, or IgG2a (all three standard curves ranged from 5 to 2000 pg/ml).
Pulmonary Fibroblast Culture
The left lung from the IL13-PE-treated and control groups (n = 5/group) at days 8 and 16 after the S. mansoni egg challenge was prepared as previously described 42 to obtain pure primary cultures of lung fibroblasts. In brief, the whole lung sample from each group was finely dispersed on steel mesh and the dispersed cells were then placed into 150-cm2 cell culture flasks (Corning Inc.). These cultured lung cells were serially passaged a total of five times to yield pure populations of lung fibroblasts. 42 The purified fibroblasts were then added to 24-well (at 1 × 103 cells/well) or 6-well (at 1 × 105 cells/well) tissue culture plates. To triplicate wells (in the six-well plates) the following was added: medium alone, or medium plus IL13-PE (0, 10, 200, or 1000 ng/ml). To other triplicate wells (in the 24-well plates) the following were added: 10 ng/ml of cytokine [IL-4, interferon (IFN)-γ, IL-10, IL-12, or IL-13] for 48 hours with the addition of 200 ng/ml of IL13-PE at 24 hours. Fibroblast proliferation was assessed in 24-well plates via [3H]thymidine incorporation (10 μCi/well) and RNA was isolated from lung fibroblasts in the six-well tissue culture plates.
Preparation of cDNA and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Amplification
Total RNA was prepared from the right lung samples removed from mice in the IL13-PE-treated and control groups (n = 3/group) at days 8 and 16 after the egg challenge, and from the cultured pulmonary fibroblasts grown from the same groups at day 16 after the S. mansoni egg challenge. RNA was isolated using TRIzol Reagent according to the manufacturer’s (Life Technologies, Inc., Rockville, MD) directions. The purified RNA was subsequently reverse-transcribed into cDNA using a BRL reverse transcription kit and oligo (dT) 12-18 primers. The amplification buffer contained 50 mmol/L KCl, 10 mmol/L Tris-HCl, pH 8.3, and 2.5 mmol/L MgCl2). Specific oligonucleotide primers were added (200 ng/sample) to the buffer, along with 1 μl of reverse-transcribed cDNA sample. The following murine oligonucleotide primers (5′ to 3′ sequences) were used for RT-PCR analysis: IL-13 receptor α1 sense, GAATTTGAGCGTCTCTGTCGAA; IL-13 receptor α1 anti-sense, GGTTATGCCAAATGCACTTGAG; IL-13 receptor α2 sense, ATGGCTTTTGTGCATATCAGAT; IL-13 receptor α2 anti-sense, CAGGTGTGCTCCATTTCATTCT; procollagen I sense, TCGTGACCGTGACCTTGCG; procollagen I anti-sense, GAGGCACAGACGGCTGAGTAG; procollagen III sense, AGCCACCTTGGTCAGTCCTA; procollagen III anti-sense, TTCCTCCCACTCCAGACTTG.
These mixtures were then first incubated for 4 minutes at 94°C and amplified using the following cycling parameters: IL-4Rα: procollagen I, and procollagen III cycled 38 times at 94°C for 30 seconds, 55°C for 45 seconds, and elongated at 72°C for 60 seconds; IL-13α1R: cycled 38 times at 94°C for 30 seconds, 66°C for 60 seconds, and elongated at 72°C for 60 seconds; and IL-13α2R: cycled 38 times at 94°C for 30 seconds, 66°C for 60 seconds, and elongated at 72°C for 60 seconds. After amplification the samples were separated on a 2% agarose gel containing 0.3 μg/ml of ethidium bromide and bands visualized and photographed using a translucent UV source.
Real-Time TaqMan PCR Analysis
Total RNA was isolated from right lobes of the lung (n = 5/group) and cultured pulmonary fibroblast samples at days 0, 8, and 16 after the S. mansoni egg challenge as described above. A total of 0.5 μg of total RNA was reverse-transcribed to yield cDNA, and IL-13Rα2, IL-4, IL-13, IFN-γ, tumor necrosis factor (TNF)-α, IL-5, MCP-1/CCL2, and MIP-1α gene expression was analyzed by real-time quantitative RT-PCR procedure using an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). GAPDH was analyzed as an internal control. All primers and probes were purchased from Applied Biosystems. Gene expression was normalized to GAPDH before the fold change in gene expression was calculated. The fold increases in receptor, cytokine, and chemokine gene expression were calculated via the comparison of gene expression of these cytokines and cytokine receptor during the granulomatous response to that detected before the intravenous injection of eggs (ie, T = 0). All mRNA levels before egg embolization were assigned an arbitrary value of 1.
Statistical Analysis
All results are expressed as mean ± SEM (SE). A one-way analysis of variance and a Dunnett’s multiple comparisons test or a Student’s t-test were used to reveal statistical differences between the control group and the IL13-PE treatment groups before and at days 8 and 16 after the S. mansoni egg challenge; P < 0.05 was considered statistically significant.
Results
IL13-PE Treatment Arrested S. mansoni Egg-Induced Pulmonary Granuloma Formation
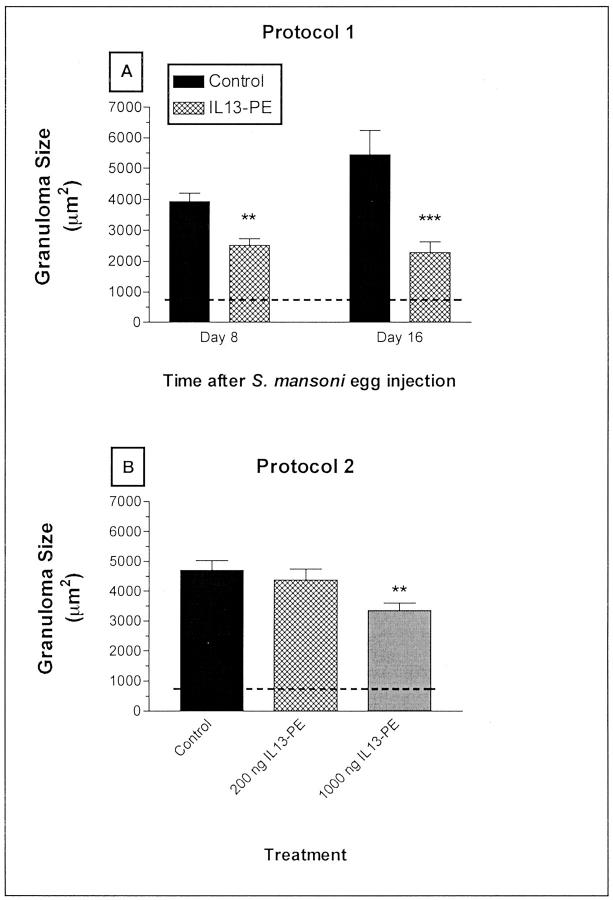
Morphometric analysis of pulmonary granulomas area is summarized in Figure 2 ▶ , A (protocol 1) and B (protocol 2). After treatment according to protocol 1, pulmonary granulomas in IL13-PE-treated mice were significantly (P < 0.01) smaller at day 8 (the final day of the 200 ng/dose-IL13-PE treatment) and (P < 0.001) at day 16 compared with control-treated mice at the same times after the S. mansoni egg challenge (Figure 2A) ▶ . Specifically, the day 8 and day 16 granulomas were 44% and 67% smaller, respectively, in the IL13-PE treatment groups compared with the control groups at the same time after the egg challenge. Mice treated with IL13-PE according to protocol 2, also exhibited significantly (P < 0.01) smaller granulomas, but this effect was only observed in the group of S. mansoni egg-challenged mice that received the 1000 ng/dose of IL13-PE every other day from day 8 to day 16 (Figure 2B) ▶ . Mice that received the higher dose of IL13-PE in protocol 2 had lung granulomas that were 33% smaller than the granulomas in the control group. Thus, IL13-PE treatment using protocol 1 markedly attenuated and appeared to interfere with the ongoing development of pulmonary granulomas around S. mansoni ova in naïve mice. Given that IL13-PE seemed to have a greater preventive (ie, protocol 1) than therapeutic (ie, protocol 2) effect on S. mansoni egg granuloma development we focused the remainder of our study on protocol 1.
Figure 2.
Morphometric analysis of pulmonary granuloma areas. Cohorts of 10 mice/group were injected intravenously with 3000 live S. mansoni eggs. A: S. mansoni egg-challenged mice then received diluent or 200 ng/dose of IL13-PE every other day starting at day 0 and concluding at day 8 after the egg challenge (see protocol 1 in Figure 1 ▶ ). B: In other cohorts of S. mansoni egg-challenged mice, diluent or IL13-PE was administered once every other day from days 8 to 16 after the egg challenge (see protocol 2 in Figure 1 ▶ ). At least 10 granulomas/mouse were measured per group. The dashed line demarcates the average area of an egg. Values are expressed as mean ± SE; n = 5 mice/group. **, P ≤ 0.01; ***, P ≤ 0.001 compared with the granuloma size in the control group at the same time after S. mansoni egg challenge.
IL13-PE Treatment Significantly Increased Whole Lung Gene Expression of IL-13Rα2
RT-PCR analysis revealed that the expression of IL-13Rα1, a receptor chain that binds both IL-4 and IL-13, 9 was constitutively present in whole lung samples from naïve mice (Figure 3A) ▶ . After S. mansoni egg embolization, IL-13Rα1 gene expression was marginally increased at days 8 and 16 in the PBS control groups (densitometry analysis is shown in Figure 3B ▶ ). In contrast, gene expression for IL-13Rα1 gene was not increased at either day after the egg challenge in the IL13-PE-treated groups (Figure 3, A and B) ▶ . At day 16 after the egg challenge, the level of gene expression for IL-13Rα1 was significantly decreased in the IL13-PE-treated group compared with the control group. Because we found that RT-PCR analysis was not sufficiently sensitive enough to detect IL-13Rα2 expression in whole lung samples, we used quantitative TaqMan PCR analysis to examine temporal changes in this decoy receptor for IL-13. 43 As shown in Figure 3C ▶ , whole lung expression of IL-13Rα2 was not altered in the control group during the course of the S. mansoni egg challenge in the control group. Conversely, IL-13Rα2 gene expression was increased at day 8 and statistically significantly increased at day 16 when compared to the control group at the same time after the egg embolization. Taken together, these data suggested that IL13-PE targeted cells expressing a receptor that can recognize IL-4 and IL-13 while concomitantly increasing the pulmonary expression of an IL-13-specific decoy receptor.
Figure 3.
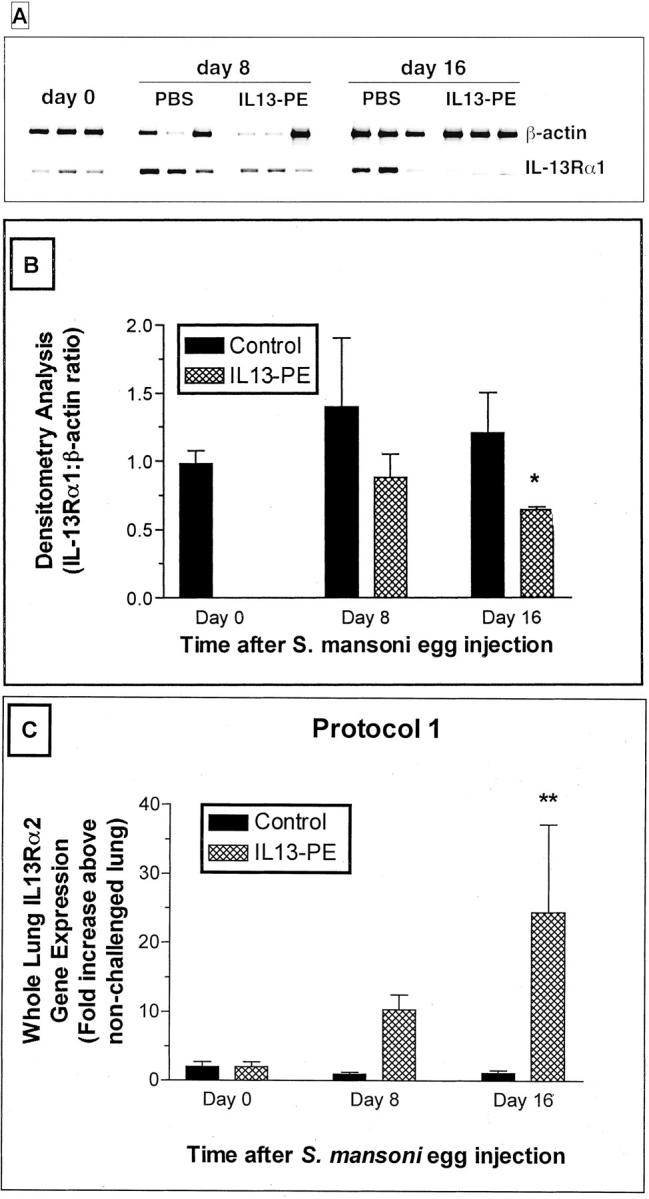
A: Representative RT-PCR analysis of β-actin and IL-13 receptor α1 (IL-13Rα1) in whole lung samples from S. mansoni egg-challenged mice at days 0, 8, and 16 after the egg challenge. S. mansoni egg-challenged mice then received diluent or a 200 ng/dose of IL13-PE every other day starting at day 0 and concluding at day 8 after the egg challenge (see protocol 1 in Figure 1 ▶ ). B: Densitometry analysis was used to determine the ratio of IL-13Rα1:β-actin mRNA in the treatment groups. These ratios confirmed that the mRNA levels for IL-13Rα1 were significantly reduced in the IL13-PE treatment group compared with the control group (at day 16 after the egg challenge). *, P ≤ 0.05. Values are expressed as mean ± SEM; n = 3 mice/group. C: Because IL-13Rα2 mRNA was expressed in minuscule quantities in the whole lung samples using RT-PCR analysis, we used quantitative TaqMan PCR to monitor changes in IL-13Rα2 expression. Temporal significant changes in IL-13Rα2 gene expression were noted in the IL13-PE-treated groups but not in the control groups after the egg challenge. Values are expressed as mean ± SE; n = 5 mice/group. **, P ≤ 0.01 compared with IL-13Rα2 gene expression in the control group at the same time after the egg challenge.
IL13-PE Treatment Did Not Significantly Reduce Circulating Levels of IgE, IgG1, or IgG2a
Serum levels of total IgE, IgG1, and IgG2a are summarized in Table 1 ▶ . When administered according to protocol 1, IL13-PE treatment from day 0 to day 8 after S. mansoni egg embolization did not significantly affect circulating levels of IgE, IgG1, and IgG2a. Serum levels of IgG1 were significantly increased at day 16 after egg embolization in the IL13-PE-treated group compared with the control group at the same time. Thus, these data suggested that IL13-PE did not specifically target Ig-producing cells during S. mansoni egg challenge.
Table 1.
Serum Levels of IgE, IgG1, and IgG2a at days 0, 8, and 16 after S. mansoni egg injection
| Ig isotype (μg/ml) | Time after S. mansoni Egg Injection | ||||
|---|---|---|---|---|---|
| Day 0 | Day 8 (control) | Day 8 (+IL13-PE) | Day 16 (control) | Day 16 (+IL13-PE) | |
| IgE | N.D. | N.D. | N.D. | 6.5 ± 0.2 | 8.3 ± 1.4 |
| IgG1 | 11.4 ± 1.8 | 21 ± 3.9 | 18.3 ± 3.7 | 16.8 ± 0.6 | 29 ± 0.6* |
| IgG2a | 34 ± 5.8 | 88.7 ± 15 | 78.2 ± 2.2 | 69.2 ± 5.1 | 90 ± 24 |
N.D., none detected.
* P ≤0.05 compared with serum IgG1 levels measured in the control group at day 16.
Mice were treated with IL13-PE or diluent according to protocol 1. Values are expressed as mean ± SE; n = 5 mice/group.
The IL13-PE Treatment Significantly Reduced the Presence of Eosinophils and Collagen in Pulmonary Granulomas
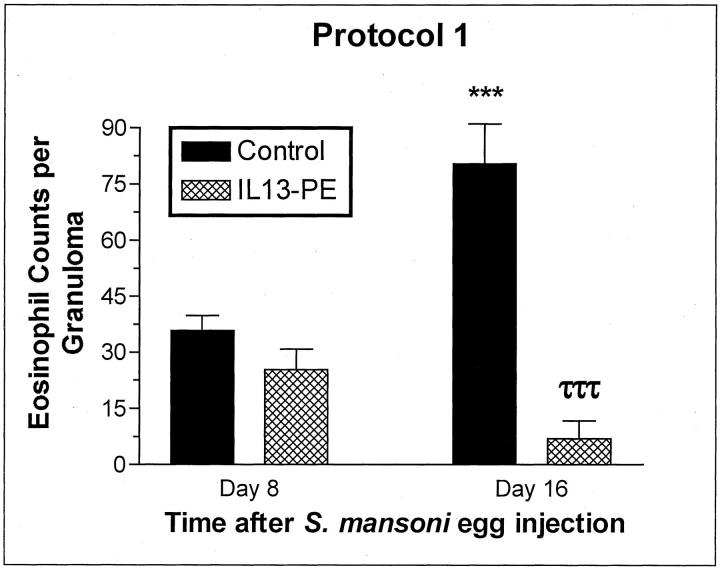
S. mansoni egg granulomas are composed of a number of immune and nonimmune cells including eosinophils, macrophages, lymphocytes, neutrophils, mast cells, and fibroblasts. 15 Given the strong Th2-driven response invoked by the S. mansoni egg, eosinophils typically comprise between 50 to 70% of the cells present in the circumoval granulomas. 24 Representative H&E-stained histological sections from the control and IL13-PE treatment groups included in protocol 1 are shown in Figure 4 ▶ (A and B represent day 8; C and D represent day 16). The average pulmonary granuloma size was markedly reduced at both times after the egg challenge in the IL13-PE treatment groups (Figure 4, B and D ▶ , respectively). However, despite the reduced size of granulomas at day 8 in the IL13-PE-treated group, eosinophils were prominent around S. mansoni eggs at this time. Quantification of eosinophils around pulmonary granulomas confirmed that similar numbers of eosinophils were present in both groups at day 8, despite the significant differences in the size of the granulomas (Figure 5) ▶ . The diminution of granuloma size in the IL13-PE-treated groups appeared to be because of the marked absence of mononuclear cells in these lesions. However, significantly fewer eosinophils were detected in pulmonary granulomas from the IL13-PE treatment group at day 16 after the S. mansoni ova challenge (Figure 5) ▶ .
Figure 4.
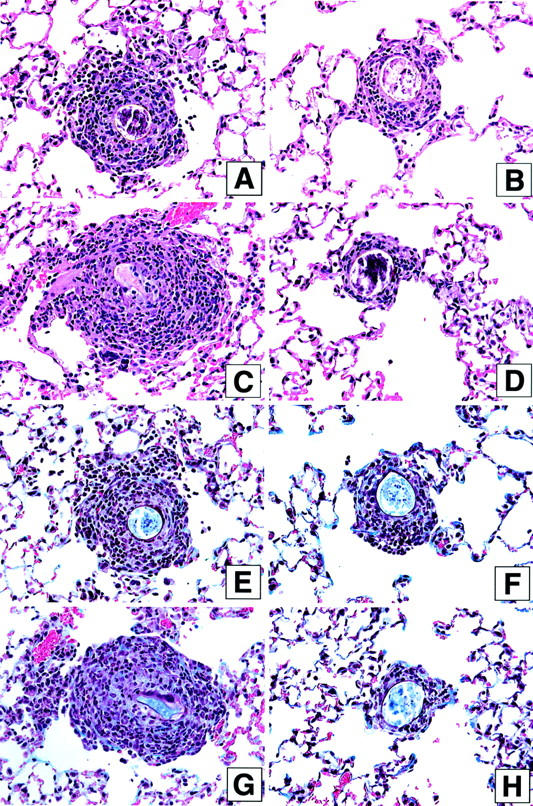
Representative photomicrographs of H&E-stained (A–D) and Masson trichrome-stained (E–H) whole lung sections at days 8 and 16 after S. mansoni egg injection in the control (human serum albumin-PBS vehicle or diluent) and IL-13-PE38QQR (IL13-PE) treatment groups. S. mansoni egg-challenged mice received diluent or a 200 ng/dose of IL13-PE every other day starting at day 0 and concluding at day 8 after the egg challenge (see protocol 1 in Figure 1 ▶ ). Histological analysis of whole lung samples from both groups at days 8 and 16 after the egg challenge was conducted as described in Materials and Methods. A marked accumulation of inflammatory leukocytes was apparent around egg granulomas at days 8 (A) and 16 (C) in naïve mice that received diluent alone from days 0 to 8 after the egg challenge. In contrast, fewer leukocytes were apparent around egg granulomas in IL13-PE-treated mice at days 8 (B) and 16 (D) corresponding to the smaller sized granulomas measured in these mice (see Figure 3 ▶ ). Collagen (light blue-stained material) was prominent around S. mansoni eggs at days 8 (E) and 16 (G) in the control groups. Collagen was also detected around S. mansoni eggs in IL13-PE-treated mice at day 8 (F), but markedly less collagen was observed around granulomas in this group at day 16 (H), presumably because of the much smaller size of the granulomas measured in these mice (see Figure 3 ▶ ). Original magnifications, ×200.
Figure 5.
Eosinophil counts in individual granulomas at days 8 and 16 after S. mansoni egg injection in the control (human serum albumin-PBS vehicle or diluent) and IL-13-PE38QQR (IL13-PE) treatment groups. S. mansoni egg-challenged mice received diluent or a 200 ng/dose of IL13-PE every other day starting at day 0 and concluding at day 8 after the egg challenge (see protocol 1 in Figure 1 ▶ ). Histological analysis of whole lung samples from both groups at days 8 and 16 after the egg challenge was conducted as described in Materials and Methods. Values are expressed as mean ± SE; n = 5 mice/group. ***, P ≤ 0.001 compared with eosinophils counted at day 8 in the control group. τττ, P ≤ 0.001 compared with eosinophils counted at day 16 in the control group.
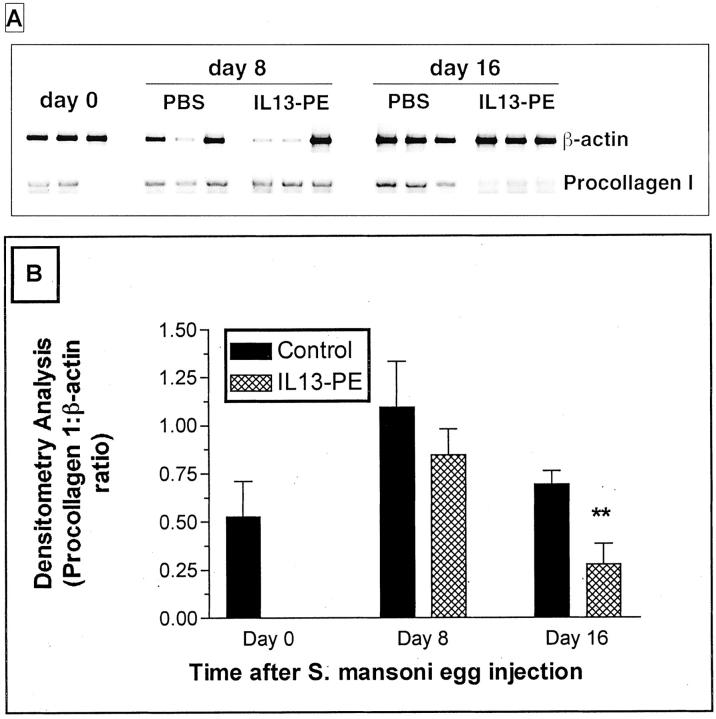
Previous studies have shown that IL-13 is a major mediator of fibroblast activation and tissue fibrosis. 17,44,45 In the present study, the peribronchial distribution of extracellular matrix and fibroblasts, revealed by the Masson trichrome stain, was pronounced in the granulomas of the control groups at days 8 and 16 after the S. mansoni egg challenge (Figure 4, E and G) ▶ . In contrast, Masson trichrome staining appeared to be much less prominent in granulomas in the IL13-PE treatment groups particularly at day 16 after the egg challenge given the markedly smaller granulomas present in these mice (Figure 4, F and H) ▶ . RT-PCR confirmation of this observation is shown in Figure 6, A and B ▶ , in which the levels of procollagen I mRNA expression were significantly lower in the IL13-PE treatment groups at day 16 after the egg challenge. Taken together, these data suggest that targeting lung cells that recognize IL-4 and IL-13 significantly reduced the degree of lung fibrosis associated with S. mansoni egg-induced pulmonary granulomas.
Figure 6.
Whole lung procollagen I mRNA expression (A) and percentage of collagen content at days 8 and 16 after S. mansoni egg injection in the control (human serum albumin-PBS vehicle or diluent) and IL-13-PE38QQR (IL13-PE) treatment groups (B). Densitometry analysis was used to determine the ratios of procollagen I:β-actin mRNA in the treatment groups. Values are expressed as mean ± SE; n = 3 mice/group. **, P ≤ 0.01 compared with the densitometry analysis of the control group at day 16 after the egg challenge.
IL13-PE Treatment Prevented the Significant Increase in Whole Lung Gene Expression of IL-13 and IL-4 after S. mansoni Egg Challenge
We next examined by TaqMan real-time PCR whether the IL13-PE treatment used in protocol 1 affected the gene expression of IL-13, IL-4, and IFN-γ in whole lung samples at days 0, 8, and 16 after S. mansoni egg challenge. At day 8 after the egg challenge in the control group, IL-13 and IL-4 gene expressions were increased 70- and 125-fold, respectively, compared with levels of these genes in whole lung samples at day 0 (Figure 7, A and B) ▶ . In addition, whole lung gene expression of IL-13 was significantly lower in the IL13-PE-treated group compared with the control group at this time. At day 16 after the egg challenge, IL-4 and IL-13 gene expressions were significantly increased in the control group above levels measured at day 0, and gene expression for both Th2 cytokines was significantly decreased compared with the control group at this time after the egg challenge. Because IL-4/IL-13−/− mice exhibit a marked increase in IFN-γ levels during intrapulmonary S. mansoni egg challenge, 25 we determined whether a similar shift in IFN-γ levels occurred in IL13-PE-treated mice. As shown in Figure 7C ▶ , significantly (P = 0.0329) less whole lung IFN-γ gene expression was observed in the IL13-PE-treated group compared with the control group at day 8 after the egg challenge. At day 16, both groups exhibited similar gene expression for IFN-γ. These data showed that IL13-PE targeted cells that generated IL-13 and IL-4, but this same treatment also negatively affected IFN-γ-expressing cells in the lung during S. mansoni egg challenge.
Figure 7.
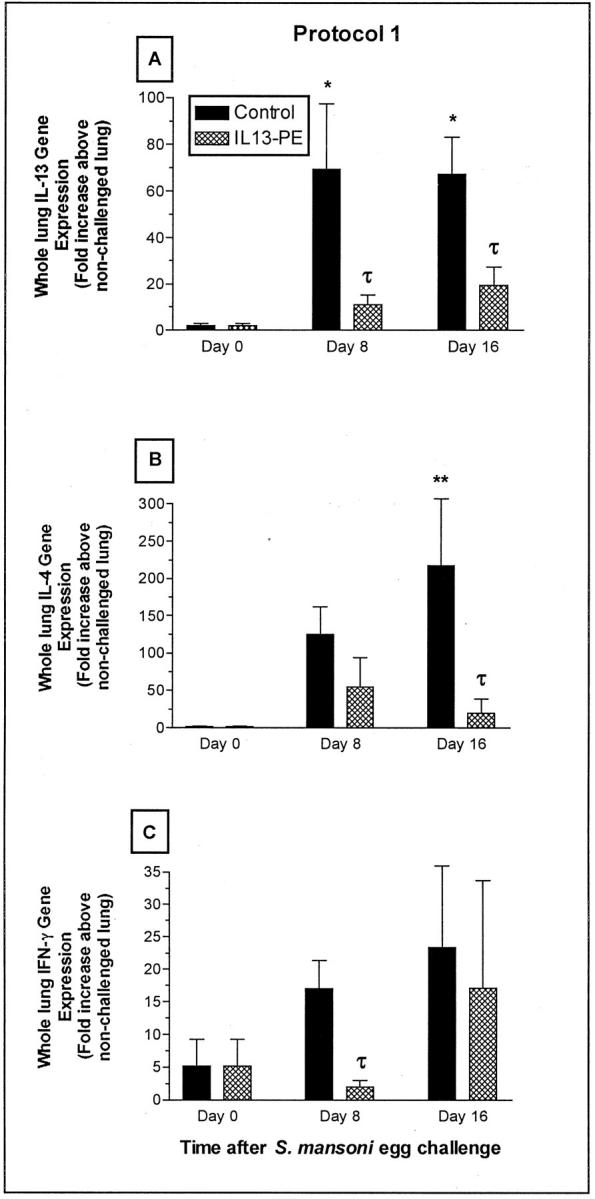
Quantitative TaqMan PCR analysis of whole lung gene expression of IL-13 (A), IL-4 (B), and IFN-γ (C) at days 0, 8, and 16 after S. mansoni egg challenge in naïve mice. RNA was isolated from whole lung samples and cytokine gene expression was quantified as described in Materials and Methods. Values are expressed as mean ± SE; n = 5 mice/group. *, P ≤ 0.05; **, P ≤ 0.01 compared with cytokine gene expression at day 0. τ, P ≤ 0.05 compared with cytokine gene expression in the control group at day 8 or day 16 after the egg challenge.
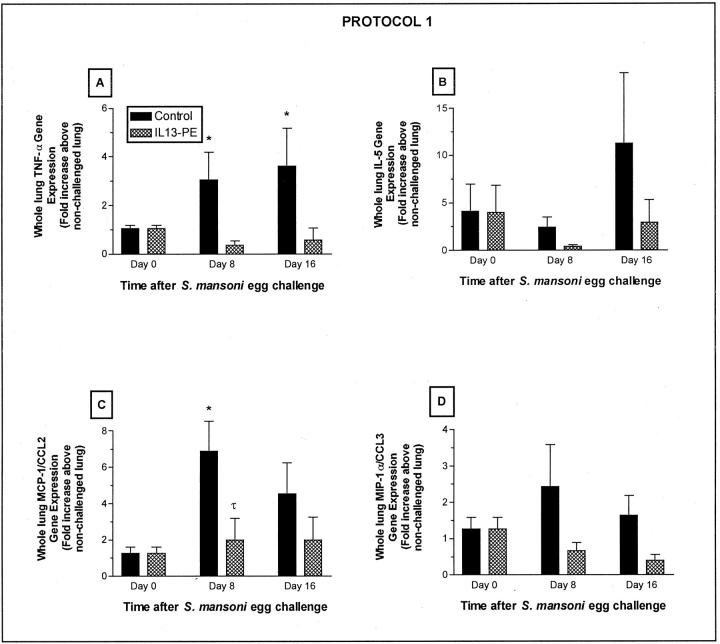
IL13-PE Treatment Prevented the Significant Increase in Whole Lung Gene Expression of TNF-α and MCP-1/CCL2 after S. mansoni Egg Challenge
TNF-α, IL-5, MCP-1/CCL2, and MIP-1α/CCL3 gene expression were examined in whole lung samples using TaqMan real-time PCR (Figure 8; A to D) ▶ . TNF-α (Figure 8A) ▶ and MCP-1/CCL2 (Figure 8C) ▶ gene expression was significantly increased in whole lung samples in the control group at day 8 compared with corresponding gene expression at day 0. Similar significant increases in TNF-α and MCP-1/CCL2 were not detected in IL13-PE-treated mice at either day 8 or 16 after the egg challenge. In addition, elevations in gene expression for IL-5 (Figure 8B) ▶ and MIP-1α/CCL3 (Figure 8D) ▶ were detected in the control groups at both days after the egg challenge, but these increases were not statistically different from gene expression measured at day 0. Whole lung gene expression for these two mediators was also lower in the IL13-PE-treated groups at days 8 and 16 after S. mansoni egg challenge. These data suggested that the IL13-PE treatment also targeted cells with the capacity to generate cytokines and chemokines that have been previously shown to contribute to the granulomatous response to S. mansoni eggs. 35,46
Figure 8.
Quantitative TaqMan PCR analysis of whole lung gene expression of TNF-α (A), IL-5 (B), MCP-1/CCL2 (C), and MIP-1α/CCL3 (D) at days 0, 8, and 16 after S. mansoni egg challenge in naïve mice. RNA was isolated from whole lung samples and cytokine gene expression was quantified as described in Materials and Methods. Values are expressed as mean ± SE; n = 5 mice/group. *, P ≤ 0.05 compared with cytokine gene expression at day 0; τ, P ≤ 0.05 compared with cytokine gene expression in control group at day 8.
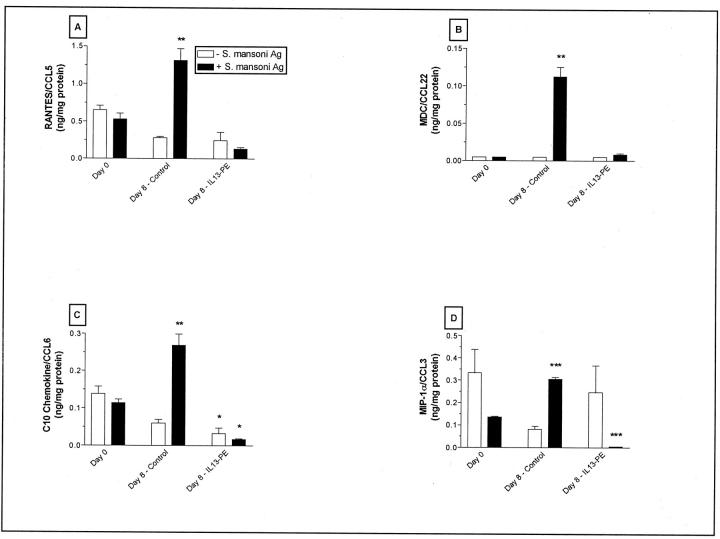
IL13-PE Treatment Significantly Reduced S. mansoni Antigen-Induced Chemokine Synthesis by Cells Cultured from Whole Lung Samples
A number of previous studies have shown that the granulomatous response to S. mansoni eggs and antigens requires the concerted actions of chemotactic cytokines or chemokines. 47 The analysis of chemokine generation in cultures of dispersed lung cells is summarized in Figure 9 ▶ . These experiments addressed whether IL13-PE targeted cells in the lung that could respond to S. mansoni egg antigen and subsequently generate chemokines. The incubation of dispersed lung cells from control mice at day 8 after egg challenge with S. mansoni egg antigen resulted in a significant elevation in RANTES/CCL5 (Figure 9A) ▶ , MDC/CCL22 (Figure 9B) ▶ , C10/CCL6 (Figure 9C) ▶ , and MIP-1α/CCL6 (Figure 9D) ▶ in cell-free supernatants compared with supernatants from similarly challenged and equivalent numbers (2 × 106 cells/well) of dispersed lung cells from naïve mice (ie, day 0). Two million S. mansoni egg antigen-challenged lung cells from IL13-PE-treated mice at day 8 after egg challenge did not exhibit similar increases in chemokine generation, and C10/CCL6 and MIP-1α/CCL3 levels were actually significantly decreased compared with S. mansoni egg antigen-challenged lung cells from naïve mice. These data suggested that IL13-PE targeted S. mansoni egg antigen-responsive cells typically associated with this pulmonary granulomatous response.
Figure 9.
Chemokine generation by cultured lung cells with or without S. mansoni egg antigen rechallenge. Lung cells were dispersed from whole lung samples removed at day 0 or day 8 after S. mansoni egg challenge, cultured for 48 hours in the presence or absence of S. mansoni egg antigen, and cell-free supernatants were removed for ELISA analysis. Values are expressed as mean ± SE; n = 5 mice/group. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 compared with levels measured at day 0 (ie, before S. mansoni egg injection).
IL13-PE Significantly Increased IL-12 Production by Cells Cultured from Whole Lung and Spleen Samples
Previous studies have shown that IL-12 is a potent inhibitor of the granulomatous and Th2 responses evoked by S. mansoni. 18,48-51 The effect of IL13-PE on IL-12 synthesis by dispersed lung cells and spleen cells is shown in Figure 10 ▶ , top and bottom panels, respectively. Dispersed lung cells (2 × 106 cells/well) from the control and IL13-PE-treated groups at day 8 after the egg challenge produced significantly higher amounts of IL-12 on S. mansoni egg antigen challenge compared with similar number of cells from naïve mice activated in the same manner. However, it was noteworthy that unstimulated cultures of dispersed lung cells from IL13-PE-treated mice also released significantly increased amounts of IL-12 compared with naïve lung cells. The effect of IL13-PE on IL-12 synthesis appeared to be limited to the lung because no significant differences were detected among the spleen cell cultures examined (Figure 10 ▶ , bottom). These data suggested that IL13-PE significantly altered the generation of IL-12 in the lung, and this effect may have partly accounted for the effect of this chimeric protein on the granulomatous response to S. mansoni eggs.
Figure 10.
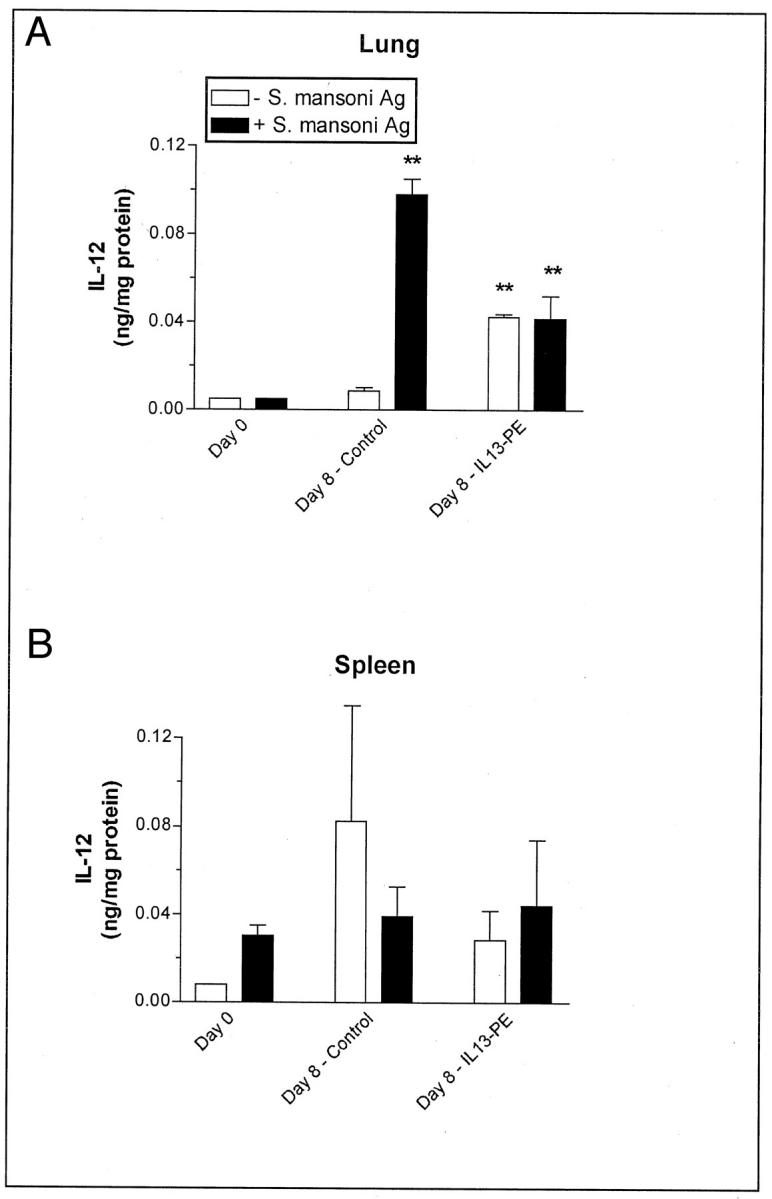
IL-12 levels in cultures of dispersed cells derived from whole lung homogenates (A) and whole spleens (B) at day 8 after S. mansoni egg challenge in the control (human serum albumin-PBS vehicle or diluent) and IL-13-PE38QQR (IL13-PE) treatment groups. S. mansoni egg-challenged mice received diluent or a 200 ng/dose of IL13-PE every other day starting at day 0 and concluding at day 8 after embolization of eggs (see Materials and Methods). Immunoreactive levels of IL-12 in cell-free supernatants were measured using a specific ELISA as described in the Materials and Methods section. Values are expressed as mean ± SE; n = 5 mice/group. **, P ≤ 0.01 compared with levels measured at day 0 (ie, before S. mansoni egg injection).
IL13-PE Inhibits Proliferation and Procollagen III Expression in Cultured Pulmonary Fibroblasts Derived from S. mansoni Egg Granulomas
The direct effect of IL13-PE on cultured pulmonary fibroblast proliferation and receptor expression is summarized in Figure 11 ▶ . Pulmonary fibroblasts were purified from whole lung samples removed from untreated mice at day 16 after the S. mansoni egg embolization. These cultured pulmonary fibroblasts exhibited aggressive proliferative characteristics, but as shown in Figure 11A ▶ the exposure of these cultured fibroblasts to 200 or 1000 ng/ml of IL13-PE significantly reduced their proliferation as determined by [3H]thymidine incorporation. RT-PCR analysis of mRNA from cultured pulmonary fibroblasts after their exposure to IL13-PE revealed that this chimeric protein also significantly reduced the gene expression of procollagen III; the greatest inhibitory effect on procollagen III gene expression was observed when these cells were exposed to 1000 ng/ml of IL13-PE (Figure 11B) ▶ . Thus, these findings demonstrated that IL13-PE targeted fibroblasts and markedly inhibited the proliferative and procollagen III synthesis responses by these cells.
Figure 11.
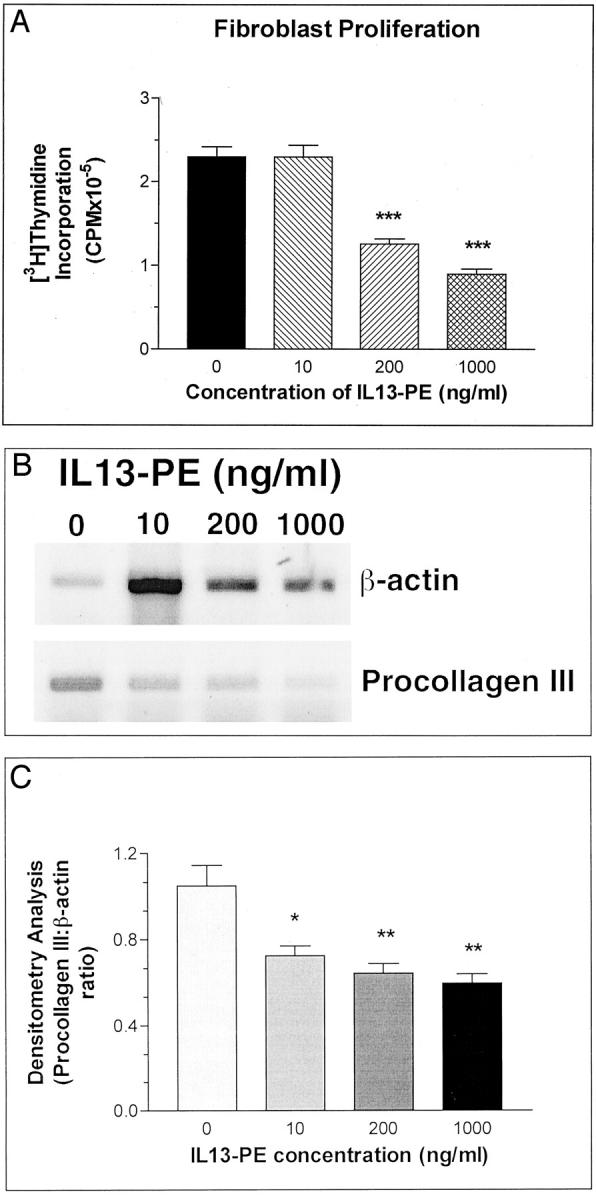
Fibroblast proliferation (A) and procollagen III gene expression (B) in cultured lung fibroblasts. Densitometry analysis was used to determine the ratios of procollagen III:β-actin mRNA in the treatment groups (C). Pulmonary fibroblasts were isolated from untreated mice at day 16 after egg embolization and exposed to increasing (0 to 1000 ng/ml) amounts of IL13-PE for 48 hours. After this time, [3H]thymidine incorporation was monitored in some cultures whereas others were used to isolate mRNA for RT-PCR analysis. Values are expressed as mean ± SE; n = 5 mice/treatment. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 compared with proliferation measured in control or untreated fibroblasts.
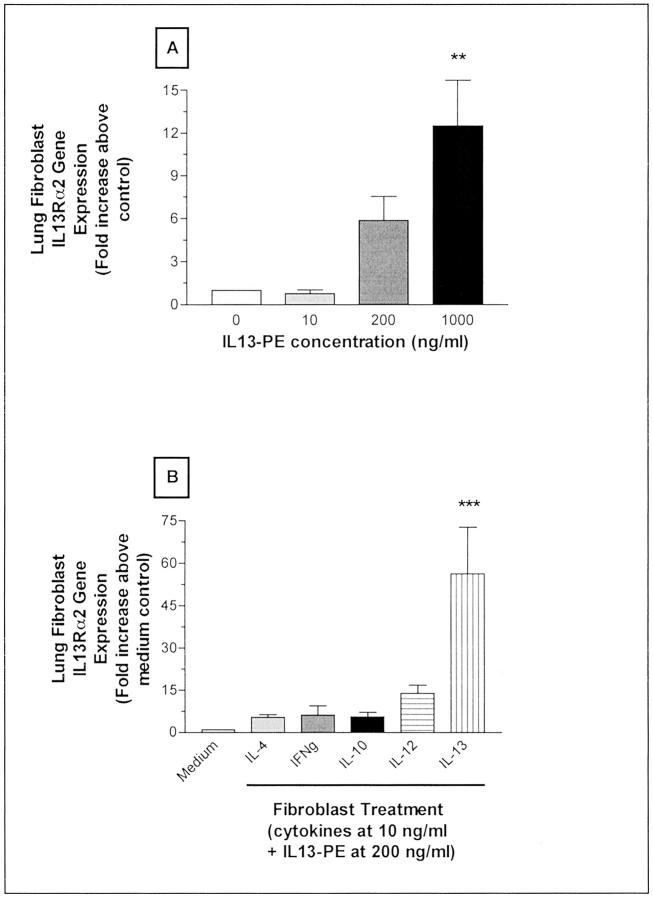
IL13-PE Induces IL-13Rα2 Gene Expression in Cultured Pulmonary Fibroblasts Derived from S. mansoni Egg Granulomas
The intriguing finding that IL13-PE treatment significantly increased IL-13Rα2 expression in whole lung samples led us to examine whether this effect was related to changes in pulmonary fibroblasts. We therefore examined the effect of IL13-PE on the proliferation of cultured lung fibroblasts and the ability of these cells to express IL-13Rα2 after their exposure to IL13-PE. Of some surprise was the finding that those fibroblasts that remained after IL13-PE exposure displayed a significant, dose-related increase in IL-13Rα2 gene expression (Figure 12, A and B) ▶ . This increase may have been related to an effect mediated by IL-13 in this fusion protein considering that recombinant IL-13 was a potent inducer of IL-13Rα2 gene expression in pulmonary fibroblasts when IL13-PE (200 ng/ml) was present in the culture (Figure 12C) ▶ . Together, these data provided evidence that IL13-PE targeted pulmonary fibroblasts (presumably accounting for the significant decrease in fibroblast proliferation) and induced IL-13Rα2 gene expression in surviving fibroblasts (presumably accounting for the marked increase in IL-13Rα2 in whole lung samples from IL13-PE-treated mice).
Figure 12.
Quantitative TaqMan PCR analysis of IL-13Rα2 gene expression in cultured pulmonary fibroblasts. Pulmonary fibroblasts were cultured and treated as detailed in Materials and Methods. IL13-PE treatment alone induced the expression of IL-13Rα2 in pulmonary fibroblasts. A significant increase in IL-13Rα2 gene expression was observed in pulmonary fibroblasts exposed to 1000 ng/ml of IL13-PE (A). Pulmonary fibroblasts also exhibited a significant increase in IL-13Rα2 gene expression after exposure to IL-13 for 48 hours and the addition of 200 ng/ml of IL13-PE at 24 hours. Values are expressed as mean ± SE. **, P ≤ 0.01; ***, P ≤ 0.001 compared with levels measured in cultures of fibroblasts exposed to medium alone.
Discussion
Experimental S. mansoni egg embolization in the lung initiates a dynamic Th2 cytokine-mediated pathological process that involves the concerted actions of IL-4 and IL-13. 3 Previous studies have shown that the simultaneous disruption of IL-4 and IL-13 in mice through a single vector gene targeting strategy abrogates the development of pulmonary granulomas around intravenously injected S. mansoni eggs, whereas IL-4−/− and IL-13−/− mice retained the ability to mount a limited Th2 response. 25 These findings and additional observations in STAT-6−/− mice 16 were our impetus to explore the effect of a novel chimeric protein, IL13-PE38QQR or IL13-PE, containing human IL-13 and Pseudomonas exotoxin on the aggressive granulomatous and Th2 responses driven by intravenously introduced S. mansoni eggs. 52 IL13-PE 30 has been previously used to successfully target human cancer cells expressing functional IL-4 and IL-13 receptors in vitro 29,53-55 and in vivo. 37,38,56,57 The utility of this molecule was also recently described in two independent studies of a model of chronic allergic airway disease induced by A. fumigatus. 31,32 Because IL13-PE targeted IL-4Rα- and IL-13Rα1-positive cells in the allergic lung 31,32 and in the lungs of mice challenged with live S. mansoni eggs (observed in the present study), this chimeric protein provided an effective treatment for attenuating the granulomatous and Th2 responses invoked by S. mansoni eggs. The data shown herein demonstrate that the intranasal administration of IL13-PE, whether given immediately after (according to protocol 1) or at day 8 (according to protocol 2) after S. mansoni egg injection, significantly reduced the S. mansoni egg-induced granuloma formation. It was apparent that IL13-PE treatment had a pronounced inhibitory effect on the developmental stage of the egg granuloma (ie, from days 0 to 8), and a more modest therapeutic effect in this model once the egg granuloma was established (ie, after day 8). One explanation for the diminished therapeutic effect of IL13-PE may be that this chimeric protein was unable to effectively penetrate and target the leukocytes in the established egg granuloma. Nevertheless, when administered according to protocol 1, IL13-PE significantly reduced most of the major Th2-mediated features of this model including enhanced Th2 cytokine generation, eosinophil persistence in the granuloma, and the fibrotic response around S. mansoni eggs. More importantly, these Th2-associated features did not reappear on the cessation of IL13-PE treatment suggesting that IL13-PE effectively targets the effector cells that modulate the ova-induced granulomatous response. Also of major interest, was the finding that IL13-PE up-regulated the expression of the decoy IL-13 receptor (IL-13Rα2) in whole lung samples and in cultured fibroblasts. Although it remains to be confirmed whether the increase in IL-13Rα2 gene expression represents the entire gene or the extracellular domain of IL-13Rα2 chain, these findings demonstrate that IL13-PE not only inhibits the proliferation of IL-13-responsive fibroblasts, it also induces IL-13Rα2 chain on these cells. The IL-13Rα2 chain has been shown to effectively block the effects of IL-13. Taken together these findings demonstrate that IL13-PE significantly attenuates the effects of IL-4 and IL-13 during the immune response to S. mansoni ova.
Considerable research attention has been directed at identifying the specific temporal roles of several inflammatory and immune mediators in the development of synchronous S. mansoni-induced egg granulomas in the lung. Early studies pointed to distinct roles for arachidonic acid metabolites such as prostaglandins 58 and leukotrienes 59 in the pulmonary granulomatous response. More recently, attention has turned to cytokines and gene analysis revealed that the introduction of S. mansoni eggs into mice initiated a unique pattern of cytokine expression dominated by IFN-γ, IL-1β, and IL-6 at day 1; IL-2, IL-4, and IL-10 at day 3; and TNF-α and IL-5 at day 6. 60 When S. mansoni ova-challenged mice received either anti-IL-2 or anti-IL-4 antibodies, the size of the egg granulomas were significantly reduced in both groups of treated mice. 60 Additional studies highlighted that IL-1β 61 and TNF-α (via intercellular adhesion molecule-1) 62 both contribute to the development of S. mansoni egg granulomas in the lung. A number of recent studies have addressed the relative importance of the Th2 response to the pulmonary granulomatous response. These studies have revealed that Th2 cells 60 and eosinophils, 63 which generate IL-4, strongly promote the development of granulomas whereas natural killer cells presumably generating IFN-γ and IL-12 suppress the formation of this lesion. 48 Further studies in cytokine gene-deficient mice outlined the relative importance of individual as well as synergistic actions of these cytokines in the pulmonary granulomatous response: IL-10−/− mice exhibited a mixed Th1 and Th2 response with reduced granulomas; IL-4−/− mice exhibited diminished Th2 response and modest reductions in granuloma size; IL-4/IL-10−/− mice exhibited a completely defective pulmonary granuloma formation process and a Th1 cytokine response; IL-12/IL-10−/− mice exhibited an exaggerated Th2 cytokine response. 64 The granulomatous and Th2 response to S. mansoni eggs has also been shown to be dependent on the contribution of B7-2 (a CD28/CTLA4 ligand), 23 Stat-6−/−, 16 and IL-13. 24 Ultimately, it was the finding that IL-4/IL-13−/− mice fail to develop any evidence of a granulomatous or Th2 response to S. mansoni eggs that clearly demonstrated the unequivocal role of both Th2 cytokines in this response. 25
Clearly, the data derived from gene knockout mice show that IL-4 and IL-13 act in concert to promote an aggressive response to S. mansoni eggs revealing the need to explore therapeutics that target these cytokines or the cells they activate. 16,25 The recent studies by Chiaramonte and colleagues 24 also highlighted this fact in showing that a soluble IL-13Rα2-Fc fusion protein administered by intraperitoneal injection significantly reduced serum IgE levels and the size of egg granulomas, but this soluble IL-13 antagonist only attenuated the Th2 cytokine response, eosinophilia, and the fibrotic response when it was administered to IL-4−/− mice after S. mansoni egg challenge. In the present study, IL13-PE treatment appeared to target or modulate the function of IL-4- and IL-13-responsive cells because IL-13Rα1 gene expression was markedly reduced in mice that received this chimeric protein. At this point the characteristics of the targeted cells are unknown, but considering that IL-4 and IL-13 gene expression were all markedly reduced at days 8 and 16 after the egg challenge it is probable that CD4+ T cells were targeted by IL13-PE. Previous studies have shown that the production of both cytokines is primarily dependent on the presence of CD4+ T cells. 24 In addition, we have previously observed that IL13-PE treatment significantly reduced the numbers of CD4+ T cells in the lung during chronic fungal asthma (CM Hosgaboam, unpublished findings). However, other cells that concurrently express IL-4 and/or IL-13 receptors, and generate these cytokines include mast cells, basophils, natural killer cells, B cells, and monocytes. 14 The targeting of these other cells may also explain the reduction in whole lung gene expression of TNF-α, and in chemokines such as macrophage inflammatory protein-1α (MIP-1α/CCL3) and monocyte chemoattractant protein-1 (MCP-1/CCL2). The latter two chemokines contribute to the development of synchronous pulmonary granulomas after S. mansoni egg challenge. 33,65,66 Other deficiencies in chemokine generation were noted in experiments designed to assess the antigen recall of mixed cultures of lung cells. These studies showed that lung cells from day 8 (after egg challenge) lungs exposed to IL13-PE had a markedly reduced capacity to generate RANTES/CCL5, MDC/CCL22, C10/CCL6, and MIP-1α/CCL3 compared with day 8 lungs cells from the control group. The lack of chemokine generation in the IL13-PE-treated group may explain the diminished number of leukocytes that were present in the granulomas of these treated mice. Specifically, the diminished numbers of eosinophils in the granulomas of IL13-PE-treated mice may have reflected the diminished levels of major eosinophils chemoattractants such as C10/CCL6, RANTES/CCL5, and MIP-1α/CCL3. 67
Because naïve Stat-6−/− 16 and IL-4/IL-13−/− 25 mice appear to develop a S. mansoni egg antigen-specific Th1 response when intravenously challenged with S. mansoni eggs, we examined whether IL13-PE treatment promoted a similar Th1 response. Our previous studies using IL13-PE treatment in a model of chronic fungal asthma showed that targeting IL-4 and IL-13-responsive cells resulted in significantly increased levels of IFN-γ in the lung. 31 In the present study, examination of whole lung samples revealed that IFN-γ gene expression levels were significantly decreased (at day 8) by the IL13-PE treatment used in protocol 1. However, analysis of dispersed cells from whole lungs of IL13-PE-treated mice at day after S. mansoni egg challenge revealed enhanced S. mansoni egg antigen-induced IL-12 production. This finding is particularly interesting in light of the potent down-regulatory effect of IL-12 on the development of S. mansoni egg-induced granulomatous responses. 49,68,69 However, these findings are somewhat tempered by recent findings from Stat-4−/− mice, which lack IL-12-mediated immune responses, 70 in which pulmonary granuloma formation was modestly inhibited during S. mansoni infection. 16 Regardless of the precise role invoked by Th1-type cytokines during S. mansoni egg granuloma formation, it was apparent from the present study that IL13-PE treatment did not appear to promote an aggressive Th1 cytokine response as a result of its diminution of the Th2 cytokine response.
The decrease in procollagen I gene levels in whole lung samples from IL13-PE-treated mice compared with their appropriate controls suggests that this chimeric protein targets the survival and actions of pulmonary fibroblasts. Although the fibrotic response was significantly decreased in IL13-PE-treated mice (corresponding to the significantly smaller granulomas in these mice), the fibrotic response did not appear completely inhibited by the targeting of IL-4- and IL-13-responsive lung cells with this chimeric protein. Examination of the relative contribution of collagen to the cross-sectional area of the egg granulomas confirmed that collagen remained a major component of the diminished granuloma in IL13-PE-treated mice. This finding is clearly important in light of the finding that IL-4−/− and IL-4/IL-13−/− mice develop severe liver pathology and die after infection by S. mansoni and this susceptibility appeared to be related to the lack of containment of the egg antigens by a fibrotic matrix. 20 Thus, any successful treatment directed at IL-4 and IL-13 during a S. mansoni egg challenge should limit but not completely inhibit the fibrotic response; IL13-PE appears to meet this criterion in the context of a pulmonary S. mansoni egg challenge.
Lung fibroblasts express receptors that recognize IL-4 and IL-13 12,44,71 and both cytokines promote the proliferation and synthetic activities of these cells. In this study, pulmonary fibroblasts cultured from day 16 lungs (ie, day 16 after egg embolization) exhibited a baseline aggressive proliferative profile. However, IL13-PE at 200 and 1000 ng/ml effectively targeted the proliferation of these cells as evidenced by a decrease in [3H]thymidine incorporation. IL13-PE also effectively inhibited the gene expression of procollagen III, a major procollagen produced by pulmonary fibroblasts during clinical pulmonary fibrosis. 72 Of particular interest was the observation that IL13-PE induced the expression of IL-13Rα2 in the surviving cells.
In conclusion, the data presented herein demonstrate the beneficial effect of targeting IL-4R- and IL-13R-expressing cells with a chimeric protein comprised of human IL-13 and Pseudomonas exotoxin during S. mansoni egg-induced pulmonary granuloma formation. IL13-PE effectively arrested the pulmonary granulomatous and the corresponding Th2 response adding further weight to the contention that both IL-4 and IL-13 are important targets in this model. These results also hold promise in the clinical treatment of chronic S. mansoni egg pathology with the goal of preventing the development of severe disease.
Footnotes
Address reprint requests to Cory M. Hogaboam, Ph.D., Department of Pathology, University of Michigan Medical School, 301 Catherine Rd., Ann Arbor MI, 48109-0602. E-mail: hogaboam@med.umich.edu.
Supported by the National Institutes of Health and the Biomedical Research Council of the University of Michigan Medical School.
References
- 1.Lucey DR, Clerici M, Shearer GM: Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev 1996, 9:532-562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL: Signaling and transcription in T helper development. Annu Rev Immunol 2000, 18:451-494 [DOI] [PubMed] [Google Scholar]
- 3.McKenzie AN: Regulation of T helper type 2 cell immunity by interleukin-4 and interleukin-13. Pharmacol Ther 2000, 88:143-151 [DOI] [PubMed] [Google Scholar]
- 4.Corry DB: IL-13 in allergy: home at last. Curr Opin Immunol 1999, 11:610-614 [DOI] [PubMed] [Google Scholar]
- 5.de Vries JE: The role of IL-13 and its receptor in allergy and inflammatory responses. J Allergy Clin Immunol 1998, 102:165-169 [DOI] [PubMed] [Google Scholar]
- 6.Chomarat P, Banchereau J: Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol 1998, 17:1-52 [DOI] [PubMed] [Google Scholar]
- 7.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN: Impaired development of Th2 cells in IL-13-deficient mice. Immunity 1998, 9:423-432 [DOI] [PubMed] [Google Scholar]
- 8.Murata T, Obiri NI, Debinski W, Puri RK: Structure of IL-13 receptor: analysis of subunit composition in cancer and immune cells. Biochem Biophys Res Commun 1997, 238:90-94 [DOI] [PubMed] [Google Scholar]
- 9.Murata T, Obiri NI, Puri RK: Structure of and signal transduction through interleukin-4 and interleukin-13 receptors (review). Int J Mol Med 1998, 1:551-557 [DOI] [PubMed] [Google Scholar]
- 10.Kawakami K, Taguchi J, Murata T, Puri RK: The interleukin-13 receptor alpha2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood 2001, 97:2673-2679 [DOI] [PubMed] [Google Scholar]
- 11.Toru H, Pawankar R, Ra C, Yata J, Nakahata T: Human mast cells produce IL-13 by high-affinity IgE receptor cross-linking: enhanced IL-13 production by IL-4-primed human mast cells. J Allergy Clin Immunol 1998, 102:491-502 [DOI] [PubMed] [Google Scholar]
- 12.Murata T, Husain SR, Mohri H, Puri RK: Two different IL-13 receptor chains are expressed in normal human skin fibroblasts, and IL-4 and IL-13 mediate signal transduction through a common pathway. Int Immunol 1998, 10:1103-1110 [DOI] [PubMed] [Google Scholar]
- 13.Graber P, Gretener D, Herren S, Aubry JP, Elson G, Poudrier J, Lecoanet-Henchoz S, Alouani S, Losberger C, Bonnefoy JY, Kosco-Vilbois MH, Gauchat JF: The distribution of IL-13 receptor alpha1 expression on B cells, T cells and monocytes and its regulation by IL-13 and IL-4. Eur J Immunol 1998, 28:4286-4298 [DOI] [PubMed] [Google Scholar]
- 14.Brombacher F: The role of interleukin-13 in infectious diseases and allergy. Bioessays 2000, 22:646-656 [DOI] [PubMed] [Google Scholar]
- 15.Metwali A, Elliott D, Blum AM, Li J, Sandor M, Lynch R, Noben-Trauth N, Weinstock JV: The granulomatous response in murine Schistosomiasis mansoni does not switch to Th1 in IL-4-deficient C57BL/6 mice. J Immunol 1996, 157:4546-4553 [PubMed] [Google Scholar]
- 16.Kaplan MH, Whitfield JR, Boros DL, Grusby MJ: Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol 1998, 160:1850-1856 [PubMed] [Google Scholar]
- 17.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA: An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest 1999, 104:777-785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boros DL, Whitfield JR: Enhanced Th1 and dampened Th2 responses synergize to inhibit acute granulomatous and fibrotic responses in murine schistosomiasis mansoni. Infect Immun 1999, 67:1187-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Ward JM, Cheever AW, Paul WE, Sher A: Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J Immunol 1999, 163:337-342 [PubMed] [Google Scholar]
- 20.Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN: Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol 2000, 164:2585-2591 [DOI] [PubMed] [Google Scholar]
- 21.Cheever AW, Williams ME, Wynn TA, Finkelman FD, Seder RA, Cox TM, Hieny S, Caspar P, Sher A: Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol 1994, 153:753-759 [PubMed] [Google Scholar]
- 22.Pearce EJ, Cheever A, Leonard S, Covalesky M, Fernandez-Botran R, Kohler G, Kopf M: Schistosoma mansoni in IL-4-deficient mice. Int Immunol 1996, 8:435-444 [DOI] [PubMed] [Google Scholar]
- 23.Subramanian G, Kazura JW, Pearlman E, Jia X, Malhotra I, King CL: B7–2 requirement for helminth-induced granuloma formation and CD4 type 2 T helper cell cytokine expression. J Immunol 1997, 158:5914-5920 [PubMed] [Google Scholar]
- 24.Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA: IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J Immunol 1999, 162:920-930 [PubMed] [Google Scholar]
- 25.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN: Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med 1999, 189:1565-1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN: T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med 2000, 191:1069-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chensue SW, Terebuh PD, Warmington KS, Hershey SD, Evanoff HL, Kunkel SL, Higashi GI: Role of IL-4 and IFN in Schistosoma mansoni egg-induced hypersensitivity granuloma formation. Orchestration, relative contribution, and relationship to macrophage function. J Immunol 1992, 148:900-906 [PubMed] [Google Scholar]
- 28.Ruth JH, Warmington KS, Shang X, Lincoln P, Evanoff H, Kunkel SL, Chensue SW: Interleukin 4 and 13 participation in mycobacterial (type-1) and schistosomal (type-2) antigen-elicited pulmonary granuloma formation: multiparameter analysis of cellular recruitment, chemokine expression and cytokine networks. Cytokine 2000, 12:432-444 [DOI] [PubMed] [Google Scholar]
- 29.Puri RK, Leland P, Obiri NI, Husain SR, Kreitman RJ, Haas GP, Pastan I, Debinski W: Targeting of interleukin-13 receptor on human renal cell carcinoma cells by a recombinant chimeric protein composed of interleukin-13 and a truncated form of Pseudomonas exotoxin A (PE38QQR). Blood 1996, 87:4333-4339 [PubMed] [Google Scholar]
- 30.Debinski W, Obiri NI, Pastan I, Puri RK: A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem 1995, 270:16775-16780 [DOI] [PubMed] [Google Scholar]
- 31.Blease K, Jakubzick C, Schuh JM, Joshi BH, Puri RK, Hogaboam CM: IL-13 fusion cytotoxin ameliorates chronic fungal-induced allergic airway disease in mice. J Immunol 2001, 167:6583-6592 [DOI] [PubMed] [Google Scholar]
- 32.Blease K, Schuh J, Jakubzick C, Lukacs NW, Kunkel SL, Joshi BH, Puri RK, Kaplan MH, Hogaboam CM: Stat6-deficient mice develop airway hyperresponsiveness and peribronchial fibrosis during chronic fungal asthma. Am J Pathol 2002, 160:481-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukacs NW, Kunkel SL, Strieter RM, Warmington K, Chensue SW: The role of macrophage inflammatory protein 1 alpha in Schistosoma mansoni egg-induced granulomatous inflammation. J Exp Med 1993, 177:1551-1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren KS, Domingo EO: Granuloma formation around Schistosoma mansoni, S haematobium, and S japonicum eggs. Am J Trop Med Hyg 1970, 19:291-304 [DOI] [PubMed] [Google Scholar]
- 35.Boros DL: The role of cytokines in the formation of the schistosome egg granuloma. Immunobiology 1994, 191:441-450 [DOI] [PubMed] [Google Scholar]
- 36.Lohning M, Grogan JL, Coyle AJ, Yazdanbakhsh M, Meisel C, Gutierrez-Ramos JC, Radbruch A, Kamradt T: T1/ST2 expression is enhanced on CD4+ T cells from schistosome egg-induced granulomas: analysis of Th cell cytokine coexpression ex vivo. J Immunol 1999, 162:3882-3889 [PubMed] [Google Scholar]
- 37.Husain SR, Puri RK: Interleukin-13 fusion cytotoxin as a potent targeted agent for AIDS-Kaposi’s sarcoma xenograft. Blood 2000, 95:3506-3513 [PubMed] [Google Scholar]
- 38.Kawakami K, Joshi BH, Puri RK: Sensitization of cancer cells to interleukin 13-pseudomonas exotoxin-induced cell death by gene transfer of interleukin 13 receptor alpha chain. Hum Gene Ther 2000, 11:1829-1835 [DOI] [PubMed] [Google Scholar]
- 39.Hogaboam CM, Gallinat CS, Bone-Larson C, Chensue SW, Lukacs NW, Strieter RM, Kunkel SL: Collagen deposition in a non-fibrotic lung granuloma model after nitric oxide inhibition. Am J Pathol 1998, 153:1861-1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hogaboam CM, Chensue SW, Steinhauser ML, Huffnagle GB, Lukacs NW, Strieter RM, Kunkel SL: Alteration of the cytokine phenotype in an experimental lung granuloma model by inhibiting nitric oxide. J Immunol 1997, 159:5585-5593 [PubMed] [Google Scholar]
- 41.Evanoff H, Burdick MD, Moore SA, Kunkel SL, Strieter RM: A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest 1992, 21:39-49 [DOI] [PubMed] [Google Scholar]
- 42.Hogaboam CM, Bone-Larson CL, Lipinski S, Lukacs NW, Chensue SW, Strieter RM, Kunkel SL: Differential monocyte chemoattractant protein-1 and chemokine receptor 2 expression by murine lung fibroblasts derived from Th1- and Th2-type pulmonary granuloma models. J Immunol 1999, 163:2193-2201 [PubMed] [Google Scholar]
- 43.Donaldson DD, Whitters MJ, Fitz LJ, Neben TY, Finnerty H, Henderson SL, O’Hara RM, Jr, Beier DR, Turner KJ, Wood CR, Collins M: The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol 1998, 161:2317-2324 [PubMed] [Google Scholar]
- 44.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Jasmin C, Azzarone B: Interleukin (IL) 4 and IL-13 act on human lung fibroblasts. Implication in asthma. J Clin Invest 1998, 101:2129-2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oriente A, Fedarko NS, Pacocha SE, Huang SK, Lichtenstein LM, Essayan DM: Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther 2000, 292:988-994 [PubMed] [Google Scholar]
- 46.Matsukawa A, Lukacs NW, Hogaboam CM, Chensue SW, Kunkel SL, III: Chemokines and other mediators, 8. Chemokines and their receptors in cell-mediated immune responses in the lung. Microsc Res Tech 2001, 53:298-306 [DOI] [PubMed] [Google Scholar]
- 47.Lukacs NW, Hogaboam C, Campbell E, Kunkel SL: Chemokines: function, regulation and alteration of inflammatory responses. Chem Immunol 1999, 72:102-120 [DOI] [PubMed] [Google Scholar]
- 48.Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A: Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med 1994, 179:1551-1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wynn TA, Cheever AW, Jankovic D, Poindexter RW, Caspar P, Lewis FA, Sher A: An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature 1995, 376:594-596 [DOI] [PubMed] [Google Scholar]
- 50.Hoffmann KF, Caspar P, Cheever AW, Wynn TA: IFN-gamma, IL-12, and TNF-alpha are required to maintain reduced liver pathology in mice vaccinated with Schistosoma mansoni eggs and IL-12. J Immunol 1998, 161:4201-4210 [PubMed] [Google Scholar]
- 51.Mountford AP, Coulson PS, Cheever AW, Sher A, Wilson RA, Wynn TA: Interleukin-12 can directly induce T-helper 1 responses in interferon-gamma (IFN-gamma) receptor-deficient mice, but requires IFN-gamma signalling to downregulate T-helper 2 responses. Immunology 1999, 97:588-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grzych JM, Pearce E, Cheever A, Caulada ZA, Caspar P, Heiny S, Lewis F, Sher A: Egg deposition is the major stimulus for the production of Th2 cytokines in murine Schistosomiasis mansoni. J Immunol 1991, 146:1322-1327 [PubMed] [Google Scholar]
- 53.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK: Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin. Clin Cancer Res 1995, 1:1253-1258 [PubMed] [Google Scholar]
- 54.Husain SR, Obiri NI, Gill P, Zheng T, Pastan I, Debinski W, Puri RK: Receptor for interleukin 13 on AIDS-associated Kaposi’s sarcoma cells serves as a new target for a potent Pseudomonas exotoxin-based chimeric toxin protein. Clin Cancer Res 1997, 3:151-156 [PubMed] [Google Scholar]
- 55.Debinski W, Gibo DM, Puri RK: Novel way to increase targeting specificity to a human glioblastoma-associated receptor for interleukin 13. Int J Cancer 1998, 76:547-551 [DOI] [PubMed] [Google Scholar]
- 56.Kawakami K, Kawakami M, Joshi BH, Puri RK: Interleukin-13 receptor-targeted cancer therapy in an immunodeficient animal model of human head and neck cancer. Cancer Res 2001, 61:6194-6200 [PubMed] [Google Scholar]
- 57.Husain SR, Joshi BH, Puri RK: IL-13 receptor directed therapy of human malignant glioma in xenograft model. Int J Cancer 2001, 92:168-175 [DOI] [PubMed] [Google Scholar]
- 58.Chensue SW, Kunkel SL, Ward PA, Higashi GI: Exogenously administered prostaglandins modulate pulmonary granulomas induced by Schistosoma mansoni eggs. Am J Pathol 1983, 111:78-87 [PMC free article] [PubMed] [Google Scholar]
- 59.Kunkel SL, Chensue SW, Mouton C, Higashi GI: Role of lipoxygenase products in murine pulmonary granuloma formation. J Clin Invest 1984, 74:514-524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wynn TA, Eltoum I, Cheever AW, Lewis FA, Gause WC, Sher A: Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol 1993, 151:1430-1440 [PubMed] [Google Scholar]
- 61.Chensue SW, Bienkowski M, Eessalu TE, Warmington KS, Hershey SD, Lukacs NW, Kunkel SL: Endogenous IL-1 receptor antagonist protein (IRAP) regulates schistosome egg granuloma formation and the regional lymphoid response. J Immunol 1993, 151:3654-3662 [PubMed] [Google Scholar]
- 62.Lukacs NW, Chensue SW, Strieter RM, Warmington K, Kunkel SL: Inflammatory granuloma formation is mediated by TNF-alpha-inducible intercellular adhesion molecule-1. J Immunol 1994, 152:5883-5889 [PubMed] [Google Scholar]
- 63.Rumbley CA, Sugaya H, Zekavat SA, El Refaei M, Perrin PJ, Phillips SM: Activated eosinophils are the major source of Th2-associated cytokines in the schistosome granuloma. J Immunol 1999, 162:1003-1009 [PubMed] [Google Scholar]
- 64.Wynn TA, Morawetz R, Scharton-Kersten T, Hieny S, Morse HC, III, Kuhn R, Muller W, Cheever AW, Sher A: Analysis of granuloma formation in double cytokine-deficient mice reveals a central role for IL-10 in polarizing both T helper cell 1- and T helper cell 2-type cytokine responses in vivo. J Immunol 1997, 159:5014-5023 [PubMed] [Google Scholar]
- 65.Chensue SW, Warmington KS, Lukacs NW, Lincoln PM, Burdick MD, Strieter RM, Kunkel SL: Monocyte chemotactic protein expression during schistosome egg granuloma formation. Sequence of production, localization, contribution, and regulation Am J Pathol 1995, 146:130-138 [PMC free article] [PubMed] [Google Scholar]
- 66.Gao JL, Wynn TA, Chang Y, Lee EJ, Broxmeyer HE, Cooper S, Tiffany HL, Westphal H, Kwon-Chung J, Murphy PM: Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med 1997, 185:1959-1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conti P, Barbacane RC, Reale M: Chemokines in inflammatory states. Allergy Asthma Proc 1999, 20:205-208 [DOI] [PubMed] [Google Scholar]
- 68.Wynn TA, Jankovic D, Hieny S, Cheever AW, Sher A: IL-12 enhances vaccine-induced immunity to Schistosoma mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J Immunol 1995, 154:4701-4709 [PubMed] [Google Scholar]
- 69.Todt JC, Whitfield JR, Ivard SR, Boros DL: Down-regulation of interleukin-12, interleukin-12R expression/activity mediates the switch from Th1 to Th2 granuloma response during murine Schistosomiasis mansoni. Scand J Immunol 2000, 52:385-392 [DOI] [PubMed] [Google Scholar]
- 70.Kaplan MH, Sun YL, Hoey T, Grusby MJ: Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature 1996, 382:174-177 [DOI] [PubMed] [Google Scholar]
- 71.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Jasmin C, Canonica GW, Azzarone B: IL-4 and IL-13 specifically increase adhesion molecule and inflammatory cytokine expression in human lung fibroblasts. Int Immunol 1998, 10:1421-1433 [DOI] [PubMed] [Google Scholar]
- 72.Weissler JC: Idiopathic pulmonary fibrosis: cellular and molecular pathogenesis. Am J Med Sci 1989, 297:91-104 [DOI] [PubMed] [Google Scholar]