Abstract
p63 is a p53 homologue required for cutaneous development that is expressed in immature squamous epithelium and reserve cells of the cervix. Humans with p63 mutations exhibit defects in limb, accessory organ (skin appendage, breast, prostate), and genitourinary development. Because p63 expression patterns imply a strong role of the gene in the female genital tract development, newborn female p63−/−, +/−, and +/+ mice were examined in situ, dissected, and compared. Nuclear p63 protein was localized to the skin, vagina, bladder, urethra, and basal columnar cells of the caudal uterus in p63+/+ and +/− animals. p63−/− mice exhibited abnormal genital morphogenesis with hypoplastic genitalia, a single cloacal opening, and persistence of columnar epithelium at lower genital tract sites that normally undergo squamous and urothelial differentiation. The defects observed support p63-dependent pathways of genital tract development that permit externally, ectodermal basal cell replenishment integral to reciprocal epithelial stromal signaling, urorectal septation, and modeling of the external genitalia; and internally, the emergence of basal epithelial cell populations capable of divergent epithelial cell differentiation in the vagina, cervix, and urinary tract. Defects in the first pathway explain imperforate anus, vaginal septum, genital hypoplasia, and micropenis reported in humans with p63 mutations. The second is necessary for the generation of multipotential reserve cells in the cervix and may be operative in other epithelial stromal interactions integral to the emergence of uterine basal cells later in life.
The human female reproductive tract is a complex multifunction organ system that passes through its most critical developmental stages in the first 12 weeks of gestation. 1 The formation of the urorectal septum subdivides the primitive cloaca and distinguishes the anorectal and urogenital sinus. 2 The Mullerian duct terminates in the urogenital sinus at the Mullerian tubercle and subsequent interactions with the urogenital sinus form the cervix and vagina. 3 The mucosal lining of the latter arises via replacement of urogenital sinus epithelium with squamous mucosa and the vaginal uterine interface becomes the squamocolumnar junction and subsequently, the cervical transformation zone. 4 The caudal cloacal folds evolve into the urogenital folds, which fuse ventrally to form the genital tubercle (clitoris) and are joined by the labial folds to eventually form the labia minora and majora, respectively. 3
Previously, Yang and colleagues 5 cloned a homologue of the tumor suppresser gene p53, designated p63, and identified multiple functions of this gene in vitro that were attributed to two major transcripts encoding both full-length, with p53-like (transactivating) activities, and truncated, with DNA binding (dominant-negative) activities. The major dominant-negative transcript isoform contains a sterile α motif domain at the carboxy terminus, ΔNp63α. Sterile α motifs are protein modules of ∼65 to 70 amino acids found in many diverse proteins whose functions range from signal transduction to transcriptional repression. 6 They localize p63 expression to basal squamous cells and subcolumnar reserve cells of the cervix, breast, salivary gland, and prostate. Subsequent studies in p63-null mice showed that loss of p63 prevented the founder (primitive) epithelial cells from proliferating, obviating basal squamous cell replenishment and resulting in absence of skin development. The latter was associated with absence of mesenchymal growth and differentiation, resulting in facial anomalies and defects in limb and tail morphogenesis. 7 Subsequent studies of human uterine cervical epithelium showed a strong predilection of p63 for reserve or immature squamous cells and parallel studies of the prostate, uterus, and breast have confirmed the relationship between p63 expression and basal/reserve cells in these sites. 7-12 These studies imply a potentially significant role of p63 in the developing female urogenital tract. 7,12,13
This article summarizes the first detailed morphological study of female reproductive anomalies in the p63-null mouse. From this analysis of both p63-null and wild-type heterozygotes a model of urogenital tract morphogenesis is proposed in which epithelial p63 dictates both mesenchymal and epithelial differentiation.
Materials and Methods
Construction of p63-Deficient Mice
Targeted disruption of the murine p63 gene was performed as previously described, and embryonic stem (ES) cell lines heterozygous for the mutation were microinjected into the blastocysts of B57BL/6 and BALB/c mice. Mice heterozygous for the mutation were interbred and the genotype of progeny determined by Southern blotting. 5 The Harvard Medical School is an Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited institution and the mice were cared for in accordance with institutional guidelines.
Dissection and Analysis of Murine Newborns
p63-null and wild-type newborn mice were fixed in 10% neutral buffered formalin, embedded in paraffin in the sagittal orientation, and serially sectioned. Selected sections were stained with hematoxylin and eosin and sections containing genital tract were culled for comparative histological and immunohistochemical analysis.
Localization of p63 Expression in Murine and Human Genital Tract
Preparation of a monoclonal antibody to p63 was described in detail previously. 5 Briefly, a cDNA fragment containing the N-terminal portion (amino acids 1 to 205) of *Np63 was overexpressed as a glutathione S-transferase fusion protein and used for subsequent monoclonal antibody production. Immunostaining for p63 was performed on deparaffinized sections using horseradish peroxidase-conjugated goat anti-mouse IgG antibody as previously described. 8 For staining of mouse tissues, secondary antibody was diluted 1:2500 to reduce background.
Results
External Phenotype of p63-Competent (p63+/+, p63+/−) and Homozygous Null (p63−/−) Mice
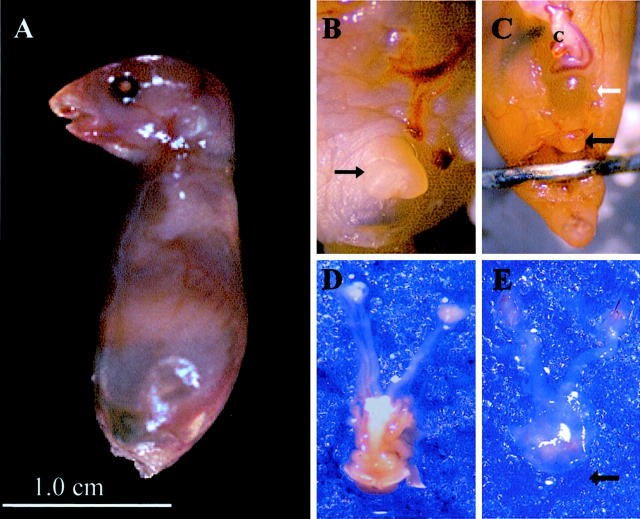
A total of three homozygotes, six heterozygotes, and four p63-null mice were studied. p63 wild-type and heterozygote animals had identical phenotypes and are designated as p63-competent. Comparative urogenital gross anatomy of p63-competent and -null mice is illustrated in Figure 1 ▶ . External characteristics that have been detailed previously in p63-null mice consisted of dysmorphic craniofacial development, absence of protruding limbs and tail, and absence of skin (Figure 1A) ▶ . 7 Relative to wild-type (Figure 1B) ▶ , the genital region of null mice demonstrated clitoral hypoplasia (Figure 1C ▶ , black arrow). In addition, there was attenuation of the suprapubic pelvic wall, exposing the anterior bladder (Figure 1C ▶ , white arrow). Genital tracts of heterozygous and null mice were further dissected, separated en bloc, and examined (Figure 1, D and E) ▶ . Caudal structures in the p63-null animals were markedly hypoplastic (arrow), and accompanied by a reduction in supporting connective tissue (Figure 1E) ▶ . The uterus and upper genital tract was slightly smaller by comparison but structurally identical to heterozygotes and wild-type animals (Figure 1, D and E) ▶ .
Figure 1.
A: External phenotype of p63-null newborn mouse. B: p63-competent (+/−) animal with normal clitoral development (black arrow). C: p63-null newborn exhibits a pelvic wall defect (white arrow) and hypoplastic clitoris (black arrow). Dissected reproductive tract of the p63-competent null mouse shows developed introitus (bottom) in contrast to marked attenuation of supporting tissues and absence of exterior genital mucosa in the null animal (E, arrow).
Whole mount sagitally sectioned caudal regions of p63-competent and -null mice were compared (Figure 2, A and B) ▶ . Anomalies in the homozygous p63−/− mice included structural defects in coordinate epithelial and mesenchymal morphogenesis. The attenuated suprapubic pelvic wall consisted of abdominal musculature only. The principal structural genital anomalies extended from the clitoris to the tail and consisted of a loss of skin and squamous mucosa (Figure 2B) ▶ . Associated with the latter were: 1) marked reduction in size of the clitoris, because of absence of both mature erectile tissue and overlying skin; 2) absence of a recto-vaginal septum resulting in a common cloacal opening; and 3) shortening of the tail (Figure 2B) ▶ . Cephalad to the cloaca, development and orientation of rectum, uterus, and bladder were unremarkable excepting the epithelial alterations detailed below.
Figure 2.
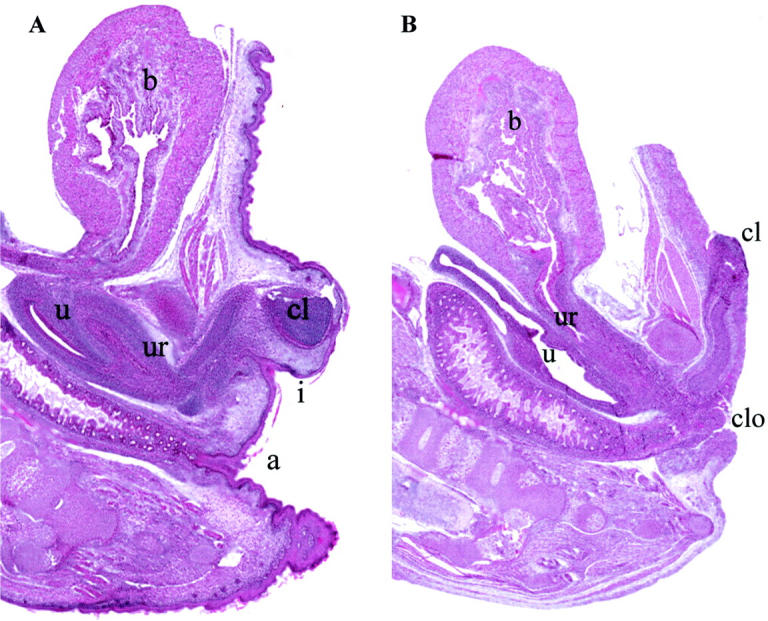
A: Sagittal section of p63+/− newborn mouse revealing normal bladder (b), clitoral (cl), uterine (u), and urethral (ur) development. The vaginal introitus (i) is distinct from the anus (a). B: p63-null mouse with absent skin, clitoral hypoplasia, and absence of perineal rectovaginal septation with a common cloacal (clo) opening.
Histopathological Findings and p63 Immunohistochemistry in Urogenital Tracts of p63-Competent Mice
Reproductive tracts of p63-competent and -null animals are compared in greater detail in Figure 3 ▶ . In this description, epithelia are defined as squamous (vaginal), cervical (Mullerian epithelium from the nonbifid portion of the uterus), and uterine horn. A low-stratified vaginal squamous mucosa formed a sharp transition with the cervical epithelium (Figure 3A ▶ , arrowhead). The cervix was lined by a pseudo-stratified tall columnar epithelium (Figure 3, A and E) ▶ that was continuous with the uterine horns (not shown). p63 expression was prominent in both the vaginal squamous and cervical columnar epithelia, concentrating in the basal and parabasal cells in both (Figure 3B) ▶ . Just caudal to the squamocolumnar junction, p63 distinguished basal transitional cells of the urethra from the vaginal squamous mucosa (Figure 3C) ▶ . The urethral, bladder, and ureteral mucosa was p63-positive (Figure 3D) ▶ . p63-positive basal nuclei were found in lower frequency in the cervix cephalad to the squamocolumnar junction (Figure 3F) ▶ diminishing to occasional nuclei in the uterine horns (not shown).
Figure 3.
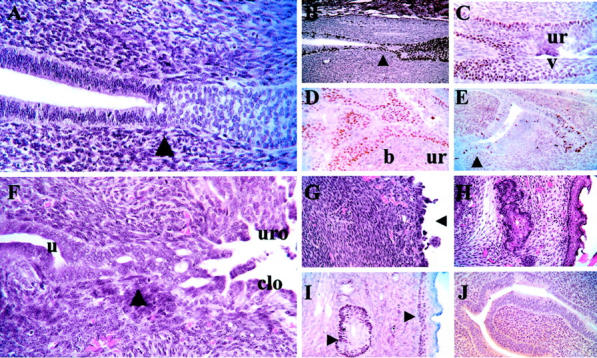
A: The vaginal-cervical squamocolumnar junction (arrow) joins tangentially sectioned vaginal squamous epithelium (right) with cervical Mullerian epithelium (left). B: In a p63-competent mouse, nuclear p63 expression highlights the vaginal squamous mucosa (right) and extends into the basal cells of the cervix (left). p63 expression at the junction of urethra (top) and vagina (bottom) is induced in basal urethral (ur) and squamous (s) cells, respectively (C), and extends into the bladder (b) mucosa (D). E: Staining is present in individual cells and cell groups in the uterus. F: The uterine (u) Mullerian epithelium in the p63-null mouse is contiguous with vaginal mucosa composed of both urogenital (uro) epithelium (top) and cloacal (clo) epithelium (bottom). G: Externally, the latter paves a highly cellular (predifferentiated) perineal mesenchyme (arrow). Normal stroma with mucosa and adnexal glands (H) expressing p63 (I, arrows). Cervical epithelium is unchanged relative to competent animal but is p63-negative (J).
Histopathology of the Urogenital Tracts of p63-Null Mice
Stratified squamous epithelium was absent on the external genitalia, introitus, and vagina. A variable stratified low-columnar vaginal mucosa was formed by a confluence of the urogenital (urethral) epithelium and a variably stratified low-columnar cloacal mucosa (Figure 3F) ▶ . This epithelium extended from its junction with the Mullerian epithelium to the cloacal opening and onto the external genitalia to the base of the clitoris (Figure 3G) ▶ .
This region was also devoid of adnexal glands and exhibited a highly cellular and vascular stroma suggesting uninduced predifferentiated mesenchyme (Figure 3; G, H, and I) ▶ . To exclude the possibility that the cloacal epithelium was present elsewhere but was sloughed after birth, external surfaces from earlier gestational ages was examined. The cloacal mucosa was limited to the genital region.
Epithelia of the cervix (Figure 3J) ▶ and uterine horns were not distinguished from the p63-competent mice, except for the absence of p63 staining. Cervical and uterine horn epithelia from both p63-competent and -null mice showed abundant mitotic activity and apoptotic cells in the epithelium and stroma. The ovaries were normal in appearance, with numerous germ cells. Investing adventitia of the horns and ovaries was slightly diminished, in keeping with the appearance on macroscopic examination (Figure 1E) ▶ .
Discussion
This report demonstrates for the first time that expression of p63 in the basal regenerative cells and their differentiated squamous products profoundly influences both the development of major structural components in the urogenital tract and the emergence of basal epithelial cells capable of divergent (squamous, urothelial) differentiation. The range of anomalies underscores the functional diversity of p63 in the female urogenital tract (Table 1) ▶ . p63 is essential to cloacal septation and external genital modeling, presumably via regenerative replication of basal squamous cells, which in a process of reciprocal signaling promotes proliferation and differentiation of the subjacent perineal mesenchyme. 7,13 In this scenario, p63-expression initiates in the primitive ectoderm early in development and is a permanent fixture in basal epithelial cells. It is critical to maintaining epithelial cells responsible for the induction of underlying mesenchyme, remodeling of the caudal (perineal) urorectal septum, and development of the genital folds and clitoris. In the absence of functioning p63, this terminal epithelial-dependent stromal induction does not take place and the vagina and anus terminate in a common opening (cloaca) with adjacent genital hypoplasia. Further morphological evidence that stromal induction and differentiation are required for this process is the presence of a highly cellular stroma on the genitalia and clitoris, characteristic of an uninduced (predifferentiated) mesenchyme. Defects in urorectal septum development in humans (cloaca) are typically accompanied by abnormalities of the genital folds and genital tubercle development, similar to that observed in the p63-null mouse. 14-16 The restriction of the cloacal abnormality in the p63-null mouse to the perineum implies that the principal defect is in perineal anogenital remodeling.
Table 1.
p63-Associated Epithelial Phenotypes of the Female Genital Tract
| Site | p63 Expression in wild-type mouse | Gestation | Cell fate | Anomalies in p63-null mouse |
|---|---|---|---|---|
| Uterine horn | Focal basal | Late | Multi-potential | None |
| Cervix | Extensive basal | Late | Multi-potential | Persistent Mullerian columnar epithelium |
| Vagina | Diffuse | Late | Squamous | Persistent Mullerian and cloacogenic epithelium |
| Introitus | Diffuse basal | Early | Squamous | Incomplete urorectal septation; persistence of cloacal epithelium |
| Genital skin | Diffuse basal | Early | Squamous | Absence of epidermis and dermis; clitoral hypoplasia; |
| Truncal skin | Diffuse basal | Early | Squamous | Absence of epidermis, dermis |
Inducible, expression occurs later in development, is conditional and varies as a function of epithelial-stromal interactions; constitutive, expression occurs early in development with the emerging ectoderm and is permanent.
The precise molecular interactions that must be perturbed to produce the anomalies seen in the p63 mouse have yet to be elucidated. The roles of p63 are diverse and include involvement in proliferation and cell-cycle arrest, positive and negative regulation of apoptosis, replicative senescence, and development. 17-22 Many of these contrasting functions may be attributed to the unique capacity of p63 to transactivate p53 target genes, inhibit (via DNA binding) p53 functions, and effect protein-protein interactions via the sterile α motif region peculiar to the major ΔNp63 α isoform. 6 Recently, a range of human syndromes characterized by ectodermal dysplasia, cleft palate, limb anomalies (ectrodactyly), and frequently urogenital malformations, have been linked to a diversity of heterozygous (dominant) p63 mutations. The ectrodactyly, ectodermal dysplasia, and facial clefts syndrome is characterized by mutations in the DNA-binding domain of ΔNp63α. 23 In contrast, the acro-dermato-ungual-lacrimal-tooth syndrome is associated with gain of function mutations in the DNA-binding domain of ΔNp63α that do not alter DNA binding per se, but are associated with increased expression of the δNp63γ isoform. 24 In Hay-Wells syndrome, also known as ankyloblepharon-ectodermal dysplasia-clefting, the DNA-binding region is unaltered, but amino acid substitutions are present in the sterile α motif domain of ΔNp63α that is associated with protein-protein interactions. 25 Presumably, the effects mediated by these mutations stem from dominant-negative and gain-of-function mechanisms rather than loss of a functioning p63 allele. 26 This hypothesis is supported further by the normal appearance of the murine heterozygotes (p63+/−) despite complete inactivation of one p63 allele. 7
A consistent defect in all of the above syndromes is ectodermal dysplasia, which is characterized by epidermal thinning, diminished hair follicles, abnormal dental development, and hypoplastic sweat, salivary, and mammary glands. Studies of animal models indicate that disturbances of both ectoderm regenerative capacity and differentiation, even in intact epithelium, will adversely influence growth and patterning of underlying mesenchyme. 27 These studies and the p63-null mouse phenotype suggest multiple functions of p63, including both the maintenance of epithelial replenishment (proliferation) and direct participation in signaling across the cutaneous-stromal interface. The former is supported indirectly by absent basal cell replenishment in p63-null animals and more directly by a recent study in zebra fish embryos that linked δNp63 to proliferation of epidermal cells by inhibiting p53 activity. 22 The role of p63 in epithelial-stromal interactions is less clear, but p63-null mice do not express msx1, a mesenchymal protein dependent on specific epithelial stromal interactions. 13,27,28
A critical determinant of limb growth during embryogenesis is the formation of the apical ectodermal ridge, which is induced by the underlying mesenchyme and arises via p63-dependent replication of basal cells. 23,29 Absence of p63 or excision of the apical ectodermal ridge nullifies limb development, suggesting that the increased concentration of p63-expressing cells in apical ectodermal ridge facilitates mesenchymal signaling. Stratification of epithelium is also seen in other areas of coordinated mesenchymal growth, including the tail and to a lesser degree, the epithelial surfaces of the brachial arches. 7,30 Predictably, the squamous epithelial cells in the early human embryo between the clitoris and anus are stratified relative to those in the extra-genital truncal ectoderm, evidence that the modeling of genital soft tissue and completion of the urorectal septum, like the limbs and palate, are coordinated with (and induced by) p63-expressing proliferating epithelial cells (GL Mutter, unpublished data). 6 A range of urogenital anomalies associated with human p63 mutations described above (genital hypoplasia, micropenis, imperforate anus, transverse vaginal septa, and anal atresia) indirectly validates the significance of this association. 31
Traditional models of reproductive tract development propose that vaginal epithelialization occurs via cephalad migration of introital squamous mucosa with replacement of cloacogenic and distal Mullerian epithelium. 4 More recent models have proposed induction of epithelial differentiation via interactions with underlying stroma. 32,33 The latter are based on cross culturing of murine newborn vaginal and uterine epithelia with uterine and vaginal stroma, resulting in epithelial phenotypes specific for underlying stroma. 34 In this context the principal role of p63 is in the induction and perpetuation of basal cells capable of spawning squamous, reserve cells and urothelial differentiation within the Mullerian and urogenital sinus epithelium. 12,32 The default epithelia remaining in the absence of this induction include the Mullerian vaginal (cephalad), urogenital sinus (superior), and cloacal (inferior) epithelium, the latter present because of the previous defect in urorectal septation (Figure 3F) ▶ . The contribution of p63 to Mullerian differentiation includes both vaginal squamous and cervical reserve cells with restricted and variably restricted cell fates. 32 In the adult human vagina and ectocervix, basal keratinocytes co-express bcl-2, p63, and cytokeratin 14, the latter characterizing commitment to squamous differentiation. 8,32 Reserve cells in the uterine cervix are associated with both squamous and columnar epithelium, comprise both CK14-positive and -negative populations, and accordingly, support both squamous and columnar cell differentiation. 8,35 Bi-directional (squamocolumnar) differentiation in both human and murine cervix is linked to hormonal (estrogen) factors. 36 A scenario in which hormones disrupt mesenchymal induction of p63 has been recently proposed to explain the effects of diethyl-stilbestrol during embryogenesis. 31
The gradient of p63 expression in the mouse cervix and uterine horns parallels that seen in humans, in which p63-positive cervical reserve cells diminish in number cephalad and are not present in concentrated numbers in the endometrium and fallopian tubes during reproductive life. 9 However, recent studies have established that a p63-positive basal cell phenotype may reoccur in adult human endometrium, a phenomenon most commonly associated with alterations in the underlying stroma (including regeneration and polyps). 9 p63 expression thus appears conditional on unique epithelial-stromal interactions that ultimately influence uterine epithelial differentiation. 9,37
It is not yet known whether humans with heterozygous p63 mutations harbor alterations in the cervical epithelium. However, dysplastic bladder epithelium has been described in the ectrodactyly, ectodermal dysplasia, and facial clefts syndrome, similar to that seen in the p63-null animals. 38 Appropriate histological studies of such individuals conceivably could reveal alterations in reserve cell development and differentiation capacity that would provide further insights into the role of p63 in the genesis and maintenance of the cervical transformation zone.
Footnotes
Address reprint requests to Christopher P. Crum, M.D., Department of Pathology, Brigham and Women’s Hospital, 75 Francis St., Boston, MA 02115. E-mail: cpcrum@rics.bwh.harvard.edu.
Supported by grants from the HMS/Affiliated Hospital Collaborative Seed Grant Program (F.D.M. and C.P.C.), Aventis Pharmaceuticals, (F.D.M.), NIH R03 CA91287 (C.P.C.) and NIH K08 CA092013 (T.A.I.).
References
- 1.McFadden DE, Pantzar JT: Genital system. Dimmick JE Kalousek DK eds. Developmental Pathology of the Embryo and Fetus. 1992:pp 605-610 JB Lippincott, Philadelphia
- 2.Paidas CN, Morreale RF, Holoski KM, Lund RE, Hutchins GM: Septation and differentiation of the embryonic human cloaca. J Pediatr Surg 1999, 34:877-884 [DOI] [PubMed] [Google Scholar]
- 3.Warwick R, Williams PL: Grays Anatomy ed 35 1973:pp 174-177, 186187, 191193 WB Saunders Co., Philadelphia
- 4.Geschickter CF, Fernandez F: Epidermidalization of the cervix. Ann NY Acad Sci 1962, 97:638-652 [DOI] [PubMed] [Google Scholar]
- 5.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F: p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 1998, 2:305-316 [DOI] [PubMed] [Google Scholar]
- 6.Thanos CD, Bowie JU: p53 family members p63 and p73 are SAM domain-containing proteins. Protein Sci 1999, 8:1708-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F: p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999, 398:714-718 [DOI] [PubMed] [Google Scholar]
- 8.Quade BJ, Yang A, Wang Y, Sun D, Park JJ, Sheets EE, Cviko A, Peters R, Federschneider J, McKeon FD, Crum CP: Expression of the p53 homologue p63 in early cervical neoplasia. Gynecol Oncol 2001, 80:24-29 [DOI] [PubMed] [Google Scholar]
- 9.O’Connell JT, Mutter GL, Cviko A, Nucci M, Quade BJ, Sun D, Yang A, McKeon FD, Crum CP: Identification of a “reserve cell” immunophenotype in benign and neoplastic endometrium: a study with the p53 homologue p63. Gynecol Oncol 2001, 80:30-36 [DOI] [PubMed] [Google Scholar]
- 10.Wang TY, Chen BF, Yang YC, Chen H, Wang Y, Cviko A, Quade BJ, Sun D, Yang A, McKeon FD, Crum CP: Histologic and immunophenotypic classification of cervical carcinomas by expression of the p53 homologue p63: a study of 250 cases. Hum Pathol 2001, 32:479-486 [DOI] [PubMed] [Google Scholar]
- 11.Barbareschi M, Pecciarini L, Cangi MG, Macri E, Rizzo A, Viale G, Doglioni C: p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol 2001, 25:1054-1060 [DOI] [PubMed] [Google Scholar]
- 12.Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F, Loda M: p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol 2000, 157:1769-1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A: p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999, 398:708-713 [DOI] [PubMed] [Google Scholar]
- 14.Allen TD, Husmann DA: Cloacal anomalies and other urorectal septal defects in female patients: a spectrum of anatomical abnormalities. J Urol 1991, 145:1034-1039 [DOI] [PubMed] [Google Scholar]
- 15.Wheeler PG, Weaver DD: Partial urorectal septum malformation sequence: a report of 25 cases. Am J Med Genet 2001, 103:99-105 [DOI] [PubMed] [Google Scholar]
- 16.Liang X, Ioffe OB, Sun D: Cloacal dysgenesis sequence: observations in four patients including three fetuses of second trimester gestation. Pediatr Dev Pathol 1998, 1:281-288 [DOI] [PubMed] [Google Scholar]
- 17.Liefer KM, Koster MI, Wang XJ, Yang A, McKeon F, Roop DR: Down-regulation of p63 is required for epidermal UV-B-induced apoptosis. Cancer Res 2000, 60:4016-4020 [PubMed] [Google Scholar]
- 18.Dohn M, Zhang S, Chen X: p63alpha and DeltaNp63alpha can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene 2001, 25:3193-3205 [DOI] [PubMed] [Google Scholar]
- 19.Sasaki Y, Morimoto I, Ishida S, Yamashita T, Imai K, Tokino T: Adenovirus-mediated transfer of the p53 family genes, p73 and p51/p63 induces cell cycle arrest and apoptosis in colorectal cancer cell lines: potential application to gene therapy of colorectal cancer. Gene Ther 2001, 8:1401-1408 [DOI] [PubMed] [Google Scholar]
- 20.Jung MS, Yun J, Chae HD, Kim JM, Kim SC, Choi TS, Shin DY: p53 and its homologues, p63 and p73, induce a replicative senescence through inactivation of NF-Y transcription factor. Oncogene 2001, 41:5818-5825 [DOI] [PubMed] [Google Scholar]
- 21.Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G: The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci 2000, 113:1661-1670 [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Kimelman D: A dominant-negative form of p63 is required for epidermal proliferation in Zebrafish. Dev Cell 2002, 5:607-616 [DOI] [PubMed] [Google Scholar]
- 23.Celli J, Duijf P, Hamel BC, Bamshad M, Kramer B, Smits AP, Newbury-Ecob R, Hennekam RC, Van Buggenhout G, van Haeringen A, Woods CG, van Essen AJ, de Waal R, Vriend G, Haber DA, Yang A, McKeon F, Brunner HG, van Bokhoven H: Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell 1999, 2:143-153 [DOI] [PubMed] [Google Scholar]
- 24.Duijf PH, Vanmolkot KR, Propping P, Friedl W, Krieger E, McKeon F, Dotsch V, Brunner HG, van Bokhoven H: Gain-of-function mutation in ADULT syndrome reveals the presence of a second transactivation domain in p63. Hum Mol Genet 2002, 11:799-804 [DOI] [PubMed] [Google Scholar]
- 25.McGrath JA, Duijf PH, Doetsch V, Irvine AD, de Waal R, Vanmolkot KR, Wessagowit V, Kelly A, Atherton DJ, Griffiths WA, Orlow SJ, van Haeringen A, Ausems MG, Yang A, McKeon F, Bamshad MA, Brunner HG, Hamel BC, van Bokhoven H: Hay-Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63. Hum Mol Genet 2001, 10:221-229 [DOI] [PubMed] [Google Scholar]
- 26.van Bokhoven H, McKeon F: Mutations in the p53 homolog p63: allele-specific developmental syndromes in humans. Trends Mol Med 2002, 8:133-139 [DOI] [PubMed] [Google Scholar]
- 27.Priolo M, Silego M, Lerone M, Ravazzolo R: Ectodermal dysplasias: not only “skin” deep. Clin Genet 2000, 58:415-430 [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Sassoon D: Ectoderm-mesenchyme and mesenchyme-mesenchyme interactions regulate Msx-1 expression and cellular differentiation in the murine limb bud. Dev Biol 1995, 2:374-382 [DOI] [PubMed] [Google Scholar]
- 29.Innis JW, Mortlock DP: Limb development: molecular dysmorphology at hand! Clin Genet 1999, 53:337-348 [DOI] [PubMed] [Google Scholar]
- 30.Kaufman MH: The Atlas of Mouse Development. 1992:pp 121-156 Academic Press, London
- 31.Rollnick BR, Hoo JJ: Genitourinary anomalies are a component manifestation in the ectodermal dysplasia, ectrodactyly, cleft lip/palate (EEC) syndrome. Am J Med Genet 1988, 29:131-136 [DOI] [PubMed] [Google Scholar]
- 32.Kurita T, Cunha GR: Roles of p63 in differentiation of Mullerian duct epithelial cells. Ann NY Acad Sci 2001, 948:9-12 [DOI] [PubMed] [Google Scholar]
- 33.Kurita T, Cooke PS, Cunha GR: Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Dev Biol 2001, 240:194-211 [DOI] [PubMed] [Google Scholar]
- 34.Cooke PS, Uchima FD, Fujii DK, Bern HA, Cunha GR: Restoration of normal morphology and estrogen responsiveness in cultured vaginal and uterine epithelia transplanted with stroma. Proc Natl Acad Sci USA 1986, 83:2109-2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smedts F, Ramaekers F, Troyanovsky S, Pruszczynski M, Robben H, Lane B, Leight I, Plantema F, Vooijs P: Basal-cell keratins in cervical reserve cells and a comparison to their expression in cervical intraepithelial neoplasia. Am J Pathol 1992, 140:601-612 [PMC free article] [PubMed] [Google Scholar]
- 36.Elson DA, Riley RR, Lacey A, Thordarson G, Talamantes FJ, Arbeit JM: Sensitivity of the cervical transformation zone to estrogen-induced squamous carcinogenesis. Cancer Res 2000, 60:1267-1275 [PubMed] [Google Scholar]
- 37.Schlemmer SR, Kaufman DG: Endometrial stromal cells regulate gap-junction function in normal human endometrial epithelial cells but not in endometrial carcinoma cells. Mol Carcinog 2000, 28:70-75 [PubMed] [Google Scholar]
- 38.Maas SM, de Jong TP, Buss P, Hennekam RC: EEC syndrome and genitourinary anomalies: an update. Am J Med Genet 1996, 3:472-478 [DOI] [PubMed] [Google Scholar]