Abstract
Nitric oxide (NO) is involved in the modulation of inflammatory responses. In psoriatic skin, NO is highly produced by epidermal keratinocytes in response to interferon-γ and tumor necrosis factor-α. In this study, we investigated whether the NO donors, S-nitrosoglutathione (GS-NO) and NOR-1, could regulate chemokine production by human keratinocytes activated with interferon-γ and tumor necrosis factor-α. In addition, we studied the effects of the topical application of a GS-NO ointment on chemokine expression in lesional psoriatic skin. NO donors diminished in a dose-dependent manner and at both mRNA and protein levels the IP-10, RANTES, and MCP-1 expression in keratinocytes cultured from healthy patients and psoriatic patients. In contrast, constitutive and induced interleukin-8 production was unchanged. GS-NO-treated psoriatic skin showed reduction of IP-10, RANTES, and MCP-1, but not interleukin-8 expression by keratinocytes. Moreover, the number of CD14+ and CD3+ cells infiltrating the epidermis and papillary dermis diminished significantly. NO donors also down-regulated ICAM-1 protein expression without affecting mRNA accumulation in vitro, and suppressed keratinocyte ICAM-1 in vivo. Finally, NO donors inhibited nuclear factor-κB and STAT-1, but not AP-1 activities in transiently transfected keratinocytes. These results define NO donors as negative regulators of chemokine production by keratinocytes.
The skin is a frequent site of T-cell-mediated diseases, such as psoriasis, atopic dermatitis, and allergic contact dermatitis. In these disorders, infiltrating T cells release lymphokines that influence the immune functions of keratinocytes. In particular, cytokine-activated keratinocytes become an important source of chemokines, which direct the recruitment of specific leukocyte populations in the skin. 1,2 Several in vitro and in vivo studies have documented that keratinocytes produce a variety of chemokines in a coordinated manner and with distinct expression profiles according to the inducing stimulus. Interferon (IFN)-γ and tumor necrosis factor (TNF)-α are the cytokines most effective in eliciting chemokine synthesis in keratinocytes, with interleukin (IL)-1, IL-4, and IL-17 also having a modulatory activity. 3,4 Moreover, keratinocytes from patients with psoriasis or atopic dermatitis may have intrinsic defects in chemokine gene expression. In particular, psoriatic keratinocytes produce exaggerated amounts of IL-8 (CXCL8), IP-10 (CXCL10), and MCP-1 (CCL2), 5-8 whereas keratinocytes from patients with atopic dermatitis synthesize higher levels of RANTES (CCL5). 8 These alterations can contribute to the accumulation of different leukocyte subsets in the skin in these diseases. 8,9 Keratinocytes exposed to IFN-γ, TNF-α, and IL-17 also express membrane ICAM-1, which plays a relevant role in the adhesion of lymphocytes to keratinocytes, and in the regulation of lymphocyte effector functions. 3,10,11
Nitric oxide (NO) is a short-lived radical produced from the l-arginine pathway by different isoforms of NO synthase (NOS) that are expressed by various cell types residing in the skin. Increasing evidence indicates that NO is involved in the maintenance of skin homeostasis as well as in the modulation of inflammatory reactions. High levels of NO have been measured in the skin affected with psoriasis, atopic dermatitis, or allergic contact dermatitis. 12-15 In these conditions, proinflammatory cytokines stimulate keratinocytes to express inducible NOS (iNOS), which in turn catalyzes NO production. Fibroblasts and dendritic cells also become iNOS-positive after exposure to bacterial endotoxin and IFN-γ, and endothelial cells express iNOS after activation with IL-1β. 14-16 The role of NO in the regulation of inflammatory responses has been extensively investigated. Depending on the concentration, the cell type, and its state of activation, as well as the presence of other inflammatory mediators, NO can either block or stimulate inflammatory responses. 17 A novel function of NO is its ability to modulate chemokine expression, as already assessed in leukocytes and glomerular cells. 18,19 In particular, IFN-γ- and TNF-α-induced IP-10 and Mig (CXCL9) expression decreased in resident glomerular cells of kidneys on NO treatment through inhibition of nuclear factor (NF)-κB activity. 19 Moreover, the production of MCP-1 and RANTES by the human keratinocyte cell line HaCaT could be reduced by NO donors. 20,21 Finally, NO donors down-regulated endothelial cell expression of various adhesion molecules including ICAM-1, VCAM-1, and E-selectin. 22,23
In this study we tested whether synthetic NO donors could modulate the expression of chemokines and ICAM-1 in keratinocyte primary cultures established from healthy patients and patients with psoriasis. In addition, the expression of chemokines and ICAM-1 on keratinocytes as well as the amount and quality of inflammatory infiltrate were investigated in psoriatic skin before and after application of a NO-releasing cream.
Materials and Methods
Chemicals
Glutathione (GS-H), S-nitrosoglutathione (GS-NO) and (±)-(E)-methyl-2-((E)-hydroxyimino)-5-nitro-6-methoxy-3-hexenamide (NOR-1) were purchased from Calbiochem (Darmstadt, Germany).
Keratinocyte Cultures
Keratinocyte cultures were prepared from skin biopsies taken from healthy patients (n = 3, two females and one male; ages 28 to 37 years) and normal-appearing skin of patients with psoriasis vulgaris (n = 3, two males and one female; ages 25 to 42 years). Biopsies were disaggregated to single-cell suspensions using 0.25% trypsin (Biochrom, Berlin, Germany). Primary cultures were prepared by seeding cell suspensions on a feeder layer of irradiated 3T3/J2 mouse fibroblasts, and cultured according to an optimized Rheinwald and Green culture technique. 24 Second or third passage keratinocytes were used in all experiments, with cells cultured in six-well plates in serum-free medium (Keratinocyte Growth Medium; Clonetics, San Diego, CA) for at least 3 to 5 days before performing experiments. Keratinocytes were stimulated with 100 U/ml of IFN-γ and 50 ng/ml of TNF-α (R&D Systems, Abingdon, Oxon, UK) for 16 or 24 hours. These time points were chosen because they were optimal for studying the expression of most inflammatory genes in keratinocytes in response to cytokines. 1-4,24 Treatments with NO donors and/or cytokines were performed in medium devoid of hydrocortisone and bovine pituitary extract, but supplemented with 0.1% bovine serum albumin (Sigma-Aldrich, Milan, Italy).
Enzyme-Linked Immunosorbent Assay (ELISA)
Cell-free supernatants from resting or stimulated keratinocyte cultures were tested for RANTES content using the antibody (Ab) pair, rabbit polyclonal 20581D for coating and 20582D for detection (BD PharMingen, San Diego, CA). IP-10 was assayed using the purified 4D5/A7/C5 and the biotinylated 6D4/D6/G2 anti-human IP-10 monoclonal antibodies (mAbs) (BD PharMingen). IL-8 and MCP-1 were measured with OptEIA kits (BD PharMingen), as per the manufacturer’s protocol. Soluble ICAM-1 was detected with an ELISA kit from Bender MedSystems (Vienna, Austria). The plates were analyzed in an ELISA reader (model 3550 UV; Bio-Rad, Hercules, CA). Keratinocyte cultures were performed in triplicate for each condition. Results are given as mean ng/106 cells ± SD.
RNase Protection Assay and Northern Blot Analysis
Total RNA was extracted from cultured keratinocytes using the Trizol reagent (Invitrogen Italia, Milan, Italy). The multiprobe template set hCK5 and the complete kit for RNase protection assay were purchased from BD PharMingen. [α32P]-labeled anti-sense riboprobes were generated from DNA corresponding to RANTES, IP-10, MIP-1α (CCL3), MIP-1β (CCL4), MCP-1, IL-8, and I-309 (CCL1), as well as the housekeeping genes, L32 and GAPDH. Ten μg of each RNA sample were used in the assays and processed as per the manufacturer’s protocol. Two separate experiments with keratinocytes from different donors were performed with similar results. For Northern blot experiments, 5 μg of total RNA were fractionated on 1% formaldehyde-agarose gel, blotted to nylon membranes (Amersham-Pharmacia-Biotech, Milan, Italy), and fixed by UV irradiation. The ICAM-1 probe (accession no. M83071) was obtained by reverse transcriptase-polymerase chain reaction performed on RNA isolated from keratinocyte cultures stimulated with IFN-γ plus TNF-α. ICAM-1 probe was labeled with [32P] dCTP, and used for hybridization performed for 1 hour at 68°C in Quickhyb solution (Stratagene, La Jolla, CA). Blots were washed under highly stringent conditions and subjected to autoradiography. Equal loading and integrity of RNA were assessed either by ethidium bromide staining of the gels or hybridizing the membrane with a probe specific for 28S rRNA.
Flow Cytometry Analysis
Keratinocyte expression of membrane ICAM-1 and HLA-DR was evaluated using fluorescein isothiocyanate-conjugated anti-CD54 (clone 84H10; Immunotech, Marseille, France) and anti-HLA-DR (clone L243, BD PharMingen) mAbs. In control samples, staining was performed using isotype-matched control Abs. Apoptosis and necrosis of keratinocytes were evaluated using the Genzyme TACS Annexin V apoptosis detection kit (R&D Systems). Cells were analyzed with a FACScan equipped with Cell Quest software (Becton Dickinson, Mountain View, CA). Results are expressed as net mean fluorescence intensity, which represents the mean fluorescence intensity subtracted of the fluorescence of isotype-matched control Ab.
Immunohistochemistry
Three patients (two females and one male, ages 29 to 39 years) with chronic plaque psoriasis underwent treatment with an ointment containing 1% GS-NO or vehicle alone applied for 2 weeks (two applications/day) on two similar lesions. Scales were formerly removed from the index lesions by a 3-day treatment with 5% salicylic acid in petrolatum. Patients were not receiving any systemic or topical therapy for at least 2 weeks before testing. Informed consent was obtained from the patients, and the local ethical committee approved the study. Four-mm punch biopsies were taken from both vehicle- and GS-NO-treated psoriatic skin and snap-frozen in OCT compound. Cryostat sections were fixed with 4% paraformaldehyde, treated with 0.3% hydrogen peroxide, and normal horse serum, and finally permeabilized with 0.1% Triton X-100. Staining was performed using the following Abs: goat polyclonal anti-RANTES (2 μg/ml), anti-MCP-1 (2 μg/ml), and anti-IL-8 (5 μg/ml) (R&D Systems), mouse mAbs anti-IP-10 (1:20) (kindly provided by M. G. Uguccioni, Institute for Research in Biomedicine, Bellinzona, Switzerland), anti-ICAM-1 (1:20), anti-CD14 (1:10), anti-CD3 (1:10) (BD PharMingen), and anti-Ki67 (1:40) (DAKO, Glostrup, Denmark). Immunoreactivity was revealed using avidin-biotin-peroxidase system and 3-amino-9-ethylcarbazole as chromogen. Sections were counterstained with Mayer’s hematoxylin. As negative controls, primary Abs were omitted or replaced with isotype-matched Ig. Slides were analyzed blind by two observers. CD14+ and CD3+ cells as well as Ki67+ keratinocytes were counted on two different sections with an eyepiece graticule at a magnification of 200 in 10 adjacent fields.
Transient Transfection of Cultured Keratinocytes
Keratinocytes from healthy patients and patients with psoriasis were transiently transfected in duplicate using Lipofectin reagent (Invitrogen). Typically, 2 to 2.5 × 105 cells were seeded in six-well plates 24 to 48 hours before transfection (60 to 80% confluence), and co-transfected with 1.0 μg of pCMV.SPORT-β-gal plasmid (Invitrogen) and 1.0 μg of pNF-κB-Luc, pGAS-Luc or pAP-1-Luc vectors (Stratagene). The latter plasmids contain the luciferase reporter gene driven by a basic promoter element (TATA box) joined to tandem repeats of prototypical NF-κB-, STAT1-, and AP-1-binding sites. After a 6-hour transfection, culture medium was removed, and keratinocytes were stimulated for 24 hours with IFN-γ plus TNF-α in the presence or the absence of 2.5 mmol/L of GS-H, GS-NO, or NOR-1. β-Galactosidase and luciferase activities were then measured in keratinocyte lysates using the β-Gal ELISA kit (Boehringer Mannheim, Mannheim, Germany) and the luciferase assay system (Promega, Madison, WI), respectively. The luciferase activity of each sample was normalized to the β-galactosidase activity.
Statistical Analysis
Wilcoxon’s signed rank test was used (SigmaStat; Jandel, San Rafael, CA) to compare differences in chemokine release, cell apoptosis/necrosis, luciferase activities of transiently transfected keratinocytes, and CD14+, CD3+, and Ki67+ cells in psoriatic skin sections. P values ≤0.05 were considered significant.
Results
NO Donors Down-Regulate Keratinocyte Expression of IP-10, MCP-1, and RANTES Induced by IFN-γ and TNF-α
In the first set of experiments we sought to determine whether NO donors could regulate the expression of chemokines in activated keratinocyte cultures prepared from healthy patients and patients with psoriasis. Untreated keratinocyte cultures released spontaneously only moderate amounts of IL-8, with psoriasis keratinocytes producing more chemokine than healthy cells (data not shown). 5,8 Keratinocytes treated with IFN-γ and TNF-α secreted high levels of chemokines with psoriasis keratinocytes releasing much higher amounts of IP-10, MCP-1, and IL-8 compared to keratinocytes from healthy donors (Figure 1) ▶ . Keratinocytes stimulated with IFN-γ and TNF-α in the presence of GS-NO showed a markedly inhibited production of IP-10, MCP-1, and RANTES. This effect was dose-dependent and was not shared by GS-H. Moreover, NOR-1, which releases NO with a more rapid kinetics compared to GS-NO, 25 resulted more efficacious than GS-NO in blocking IP-10, MCP-1, and RANTES release. In contrast, both NOR-1 and GS-NO were ineffective in reducing IL-8 secretion (Figure 1) ▶ . The effects of NO donors on chemokine expression were also examined at the mRNA level (Figure 2) ▶ . IP-10, MCP-1, and IL-8 mRNA signals induced by IFN-γ plus TNF-α were more represented in keratinocytes from patients with psoriasis than in keratinocytes from control patients, as previously described. 8 On the other hand, RANTES and I-309 (CCL1) mRNA signals were similar in the two keratinocyte types. GS-NO or NOR-1 decreased IFN-γ/TNF-α-induced RANTES, IP-10, MCP-1, and I-309 mRNA expression in both healthy and psoriasis keratinocytes, whereas IL-8 mRNA was not affected (Figure 2) ▶ . IFN-γ and TNF-α induced a modest increase in keratinocyte apoptosis, as assessed by fluorescence-activated cell-sorting analysis after staining with propidium iodide and anti-annexin V antibody (Table 1) ▶ . GS-NO or NOR-1 did not significantly elicit apoptosis nor necrosis of keratinocytes, and did not augment IFN-γ/TNF-α-induced apoptosis.
Figure 1.
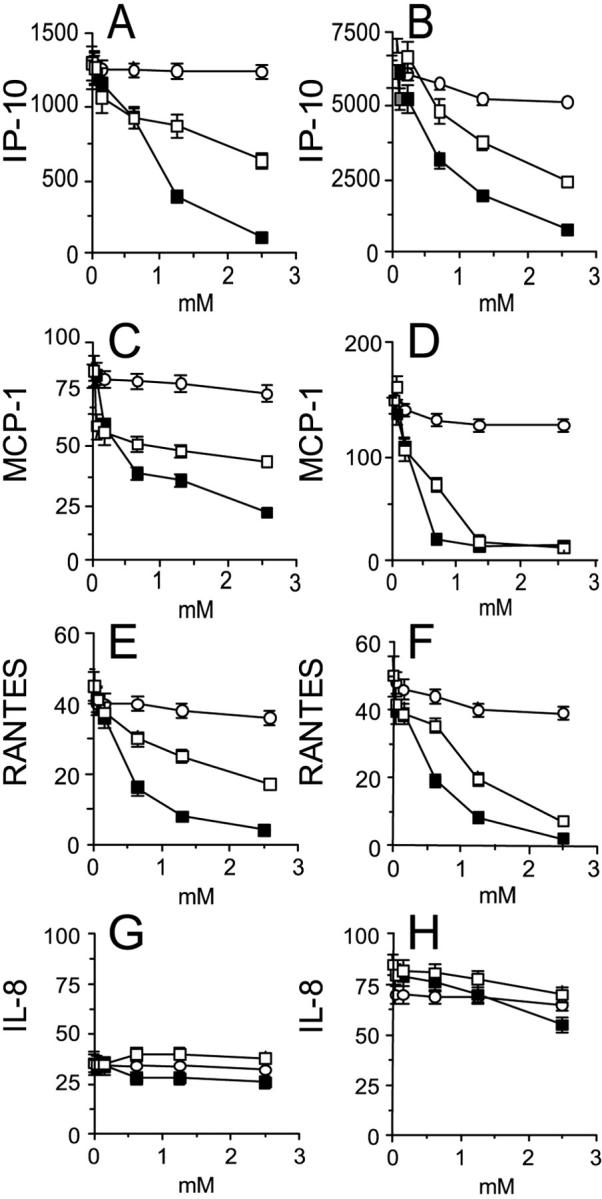
NO donors dose dependently decrease keratinocyte release of IP-10, MCP-1, and RANTES, but not IL-8. Keratinocyte cultures were established from healthy patients (A, C, E, G) and patients with psoriasis (B, D, F, H). Keratinocytes were stimulated for 24 hours with 100 U/ml of IFN-γ and 50 ng/ml of TNF-α in the presence of different doses of GS-H (○), GS-NO (□), or NOR-1 (▪). Chemokine release was evaluated in the supernatants by ELISA. Data are expressed as mean ng/106 cells ± SD of triplicate cultures. Similar results were observed in keratinocyte cultures prepared from three healthy patients and three psoriatic patients.
Figure 2.
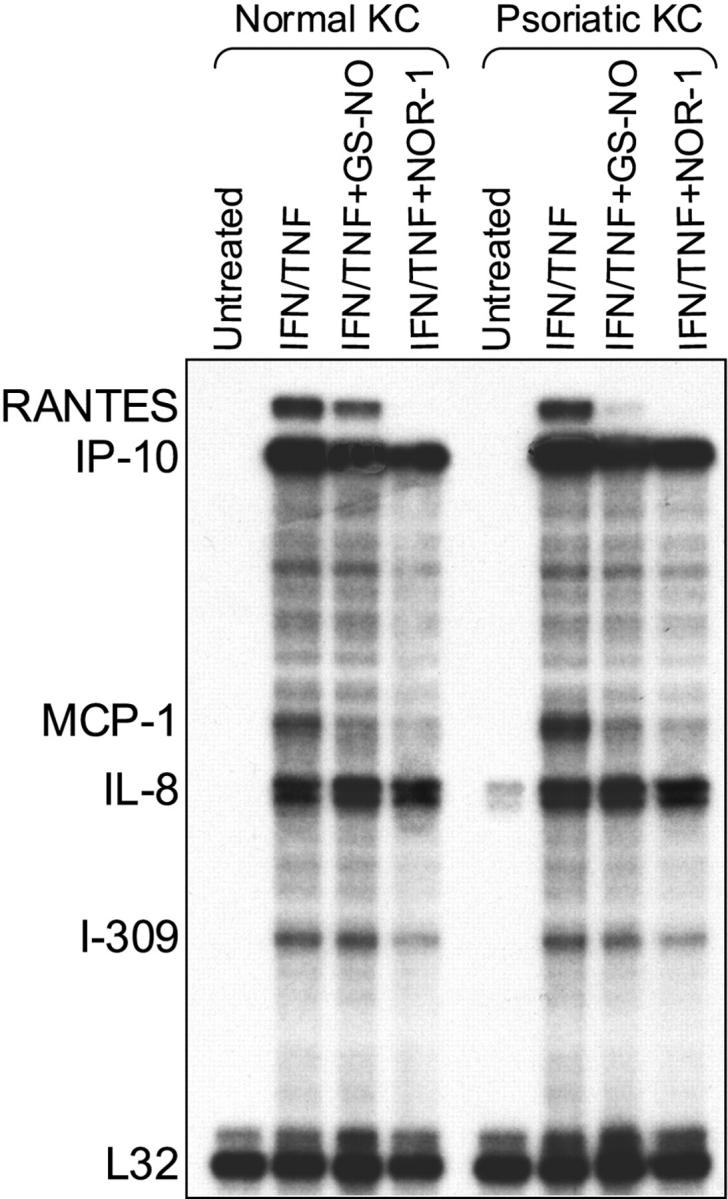
IFN-γ/TNF-α-induced IP-10, MCP-1, and RANTES mRNA expression in keratinocytes is down-regulated by NO donors. Normal and psoriatic keratinocytes were stimulated with IFN-γ and TNF-α in the presence or not of GS-NO (2.5 mmol/L) or NOR-1 (2.5 mmol/L). After 16 hours, total RNA was extracted and subjected to RNase protection assay using a human CK5 multiprobe template. Films were exposed for 8 hours.
Table 1.
NO Donors Do Not Induce Keratinocyte Apoptosis or Necrosis in Vitro
| Treatment* | % Annexin V+ cells | % PI+ cells | % Annexin V+/PI+ cells |
|---|---|---|---|
| None | 0.6 ± 0.4 | 6.1 ± 2.5 | 0.9 ± 0.3 |
| IFN-γ/TNF-α | 2.6 ± 0.8† | 6.6 ± 1.4 | 5.1 ± 0.4† |
| GS-NO | 0.8 ± 0.3 | 7.6 ± 0.9 | 1.6 ± 0.5 |
| NOR-1 | 1.1 ± 0.4 | 7.3 ± 0.6 | 2.6 ± 0.6 |
| IFN-γ/TNF-α + GS-NO | 3.6 ± 1.4† | 8.6 ± 1.2 | 6.8 ± 1.1† |
| IFN-γ/TNF-α + NOR-1 | 3.8 ± 0.7† | 8.2 ± 0.4 | 6.6 ± 0.5† |
*Keratinocyte cultures were left untreated or stimulated with 100 U/ml of IFN-γ and 50 ng/ml of TNF-α and/or 2.5 mmol/L of GS-NO or NOR-1. After 24 hours, cells were stained with propidium iodide and anti-annexin V antibody, and then analyzed by flow cytometry. Results are expressed as mean percentage ± SD of positive cells from three independent experiments.
†P < 0.05 compared to untreated keratinocytes.
NO Donors Decrease Keratinocyte Expression of ICAM-1 Induced by IFN-γ and TNF-α
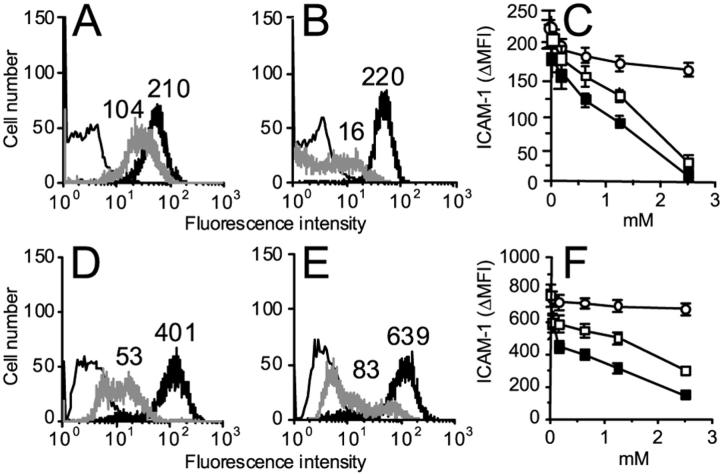
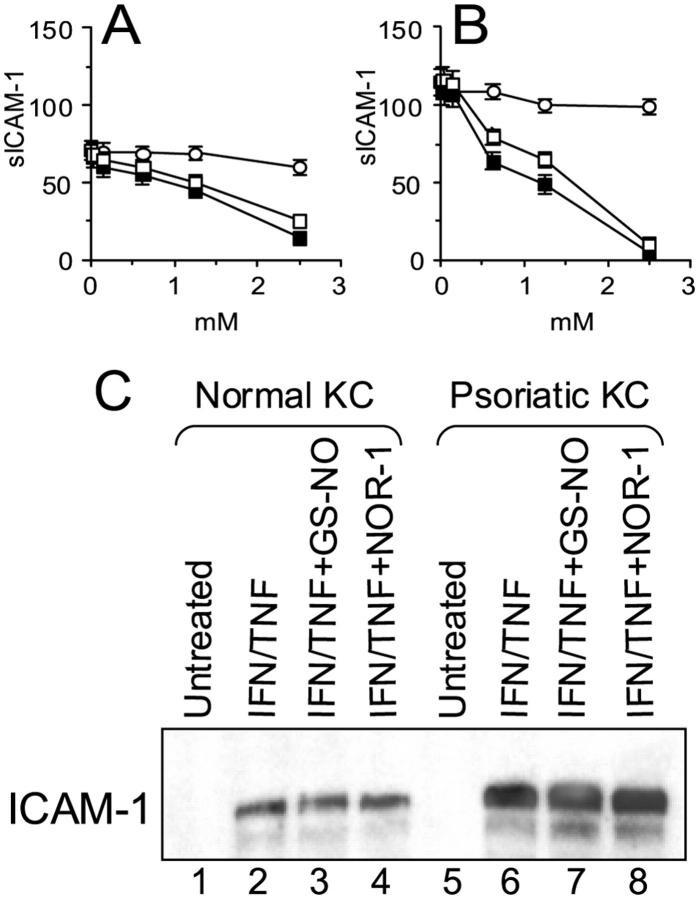
Resting keratinocytes do not express ICAM-1 or MHC class II molecules, but they do so after activation with IFN-γ and/or TNF-α. 3 ICAM-1 provides a major adhesion pathway for the retention of T lymphocytes in the epidermis. Moreover, ICAM-1 serves as an important co-stimulatory molecule for the cytotoxic activity of CD4+ and some CD8+ T lymphocytes against keratinocytes. 11 Therefore, we next examined whether NO donors could modulate ICAM-1 and HLA-DR expression on activated keratinocytes. Keratinocytes exposed to IFN-γ plus TNF-α showed high ICAM-1 expression, with psoriatic keratinocytes expressing higher levels of ICAM-1 compared to healthy cells (Figure 3) ▶ . When activation was performed in the presence of GS-NO or NOR-1, but not GS-H, a markedly and dose-dependent reduction of membrane ICAM-1 was observed (Figure 3) ▶ . In contrast, the IFN-γ/TNF-α-induced expression HLA-DR did not vary in keratinocytes co-treated with NO donors (data not shown). In the following experiments, we tested whether the decreased membrane ICAM-1 promoted by NO donors was associated with changes in the release of sICAM-1. As shown in Figure 4, A and B ▶ , addition of GS-NO or NOR-1, but not GS-H, strongly and dose dependently reduced the IFN-γ/TNF-α-induced ICAM-1 content in supernatants from both healthy and psoriasis keratinocyte cultures. Northern blot analysis revealed that psoriatic keratinocytes treated with IFN-γ and TNF-α had ICAM-1 mRNA levels fourfold higher compared to normal keratinocytes. Surprisingly, GS-NO or NOR-1 did not alter ICAM-1 mRNA accumulation (Figure 4C) ▶ , suggesting that these drugs alter ICAM-1 expression at a posttranscriptional level.
Figure 3.
GS-NO and NOR-1 dose dependently down-regulate membrane ICAM-1 expression induced by IFN-γ and TNF-α in keratinocytes. Keratinocytes from either healthy individuals (A–C) and psoriatic patients (D–F) were activated with IFN-γ and TNF-α in the presence or not of GSH, GS-NO, or NOR-1. After 24 hours, cells were detached and analyzed by flow cytometry using a fluorescein isothiocyanate-conjugated anti-ICAM-1 mAb. In A, B, D, and E, black bold lines represent keratinocytes treated with IFN-γ/TNF-α and GSH (2.5 mmol/L). Gray lines show cells treated with 2.5 mmol/L of GS-NO (A, D) or 2.5 mmol/L of NOR-1 (B, E). Thin lines represent ICAM-1 expression of unstimulated keratinocytes. In C and F, ICAM-1 expression was evaluated on IFN-γ/TNF-α-stimulated keratinocytes co-treated with grading doses of GS-H (○), GS-NO (□), or NOR-1 (▪). Numbers indicate the net mean fluorescence intensity. Similar results were confirmed in keratinocytes from two healthy patients and three psoriatic patients.
Figure 4.
NO donors dose dependently inhibit sCAM-1 release from activated keratinocytes but do not influence ICAM-1 mRNA. ELISA for sICAM-1 was performed on supernatants from normal (A) and psoriatic (B) keratinocytes treated with IFN-γ/TNF-α plus GS-H (○), GS-NO (□), or NOR-1 (▪). Data are expressed as mean ng/106 cells ± SD of triplicate cultures. C: Total mRNA was extracted from keratinocytes cultured for 16 hours with IFN-γ/TNF-α and 2.5 mmol/L of GS-NO or NOR-1. Northern blot analysis was performed using an ICAM-1-specific probe. Results were confirmed in three normal and psoriatic keratinocyte strains.
NO Donors Inhibit Chemokine and ICAM-1 Expression in Keratinocytes in Vivo
To evaluate the effects of NO donors in vivo, patients with chronic plaque psoriasis underwent treatment with a 1% GS-NO ointment on a selected lesion. As control, a similar distant lesion was treated with vehicle alone. After 14 days, biopsies from both sites were analyzed by immunohistochemistry. Psoriatic epidermis showed a diffuse and intense cytoplasmic staining for IP-10, MCP-1, and RANTES more evident in the basal and suprabasal epidermal layers. Keratinocyte immunoreactivity for IP-10, MCP-1, and RANTES prominently diminished after topical treatment with the NO-releasing preparation compared to skin treated with vehicle alone (Figure 5) ▶ . In contrast, no significant difference in IL-8 reactivity was noted between vehicle- and NO-treated psoriatic skin. GS-NO did not seem to affect chemokine expression in the leukocytes infiltrating the dermis of psoriatic skin. Immunohistochemical analysis revealed also a marked decrease of ICAM-1 expression on basal keratinocytes but not in infiltrating leukocytes or endothelial cells of GS-NO-treated skin (Figure 6) ▶ . A substantial reduction of Ki67-positive proliferating keratinocytes was also observed in NO-treated psoriatic epidermis compared to control skin (190 ± 39 versus 110 ± 30; mean ± SD, n = 3, P = 0.02). Finally, in psoriatic lesions treated with GS-NO there were significantly fewer CD14+ (63 ± 10 versus 24 ± 5; mean ± SD, n = 3, P = 0.01) and CD3+ (90 ± 18 versus 42 ± 9; mean ± SD, n = 3, P = 0.01) cells localized in the epidermis, in proximity of the epidermis and in the papillary dermis, whereas their number did not vary in the mid dermis.
Figure 5.
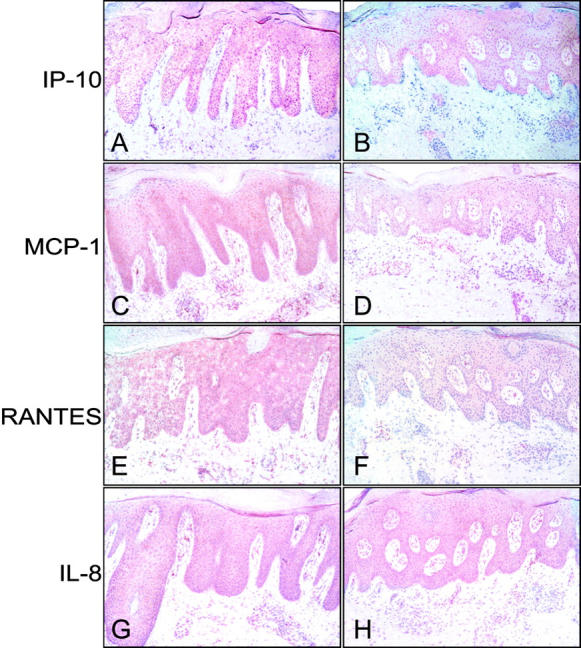
IP-10, MCP-1, and RANTES, but not IL-8 expression is significantly reduced in psoriatic skin treated with a GS-NO ointment. Vehicle alone (A, C, E, G) or 1% GS-NO ointment (B, D, F, H) were applied on two distinct psoriasis plaques. Skin biopsies were taken after 14 days and processed for immunohistochemistry on frozen sections. Reactivity was revealed by using avidin-biotin-peroxidase complex and amino-ethylcarbazole. Sections were counterstained with Mayer’s hematoxylin. Results are representative of staining performed on biopsies from three patients. Original magnifications, ×100.
Figure 6.
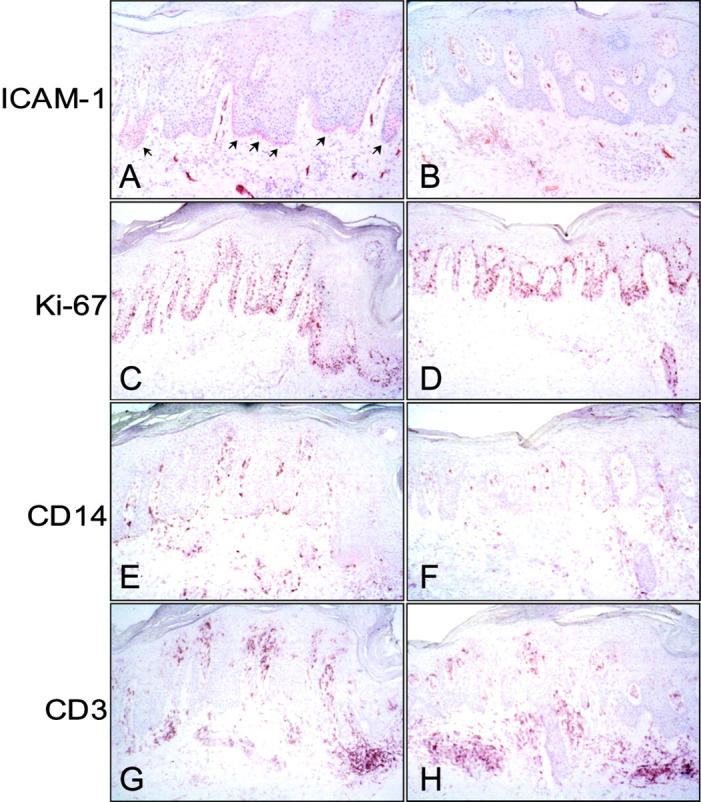
Treatment of psoriatic lesions with GS-NO diminishes ICAM-1 and Ki-67 expression in basal keratinocytes and the number of CD14+ and CD3+ cells located in proximity of the epidermis. Sections from untreated (A, C, E, G) and GS-NO-treated (B, D, F, H) psoriatic skin were processed for immunohistochemistry using mAbs against the indicated markers. Arrows in A indicate ICAM-1 expression in basal keratinocytes. Original magnifications, ×100.
GS-NO Reduces IFN-γ/TNF-α-Induced Luciferase Activity Driven by NF-κB and STAT1, But Not by AP-1 in Transiently Transfected Human Keratinocytes
The majority of inflammatory molecules are transcriptionally regulated by NF-κB, AP-1, and STAT-1. 26-28 To identify the mechanisms through which NO donors exerted inhibitory effects on keratinocytes, we performed transient transfections with plasmids carrying luciferase gene under NF-κB-, STAT-1-, or AP-1-dependent promoters (pNF-κB-Luc, pGAS-Luc, or pAP-1-Luc). Normal and psoriatic keratinocytes were first transfected, and then treated with IFN-γ/TNF-α in the presence or the absence of NO donors, and finally analyzed for luciferase activity (Figure 7) ▶ . Normal and psoriatic keratinocytes transfected with the pNF-κB-Luc plasmid and stimulated with IFN-γ/TNF-α showed a 60-fold and a 110-fold increase in luciferase activity, respectively, compared to basal condition. Co-treatment with GS-NO or NOR-1, but not with GS-H, decreased very efficiently the luciferase activity of pNF-κB-Luc plasmid. Luciferase activity relative to the pGAS-Luc was enhanced in IFN-γ/TNF-α-stimulated normal and psoriatic keratinocytes, with an increase of 6-fold and 35-fold, respectively, compared to resting cells. Keratinocytes treated with GS-NO or NOR-1 showed a reduced luciferase activity of pGAS-Luc suggesting an effective interference of NO donors also in the STAT-1 pathway. Interestingly, pGAS-Luc activity was inhibited more markedly in psoriatic keratinocytes than in control cells. In contrast, GS-NO and NOR-1 were not able to influence the AP-1-dependent luciferase activity promoted by IFN-γ and TNF-α in both healthy and psoriatic keratinocytes.
Figure 7.
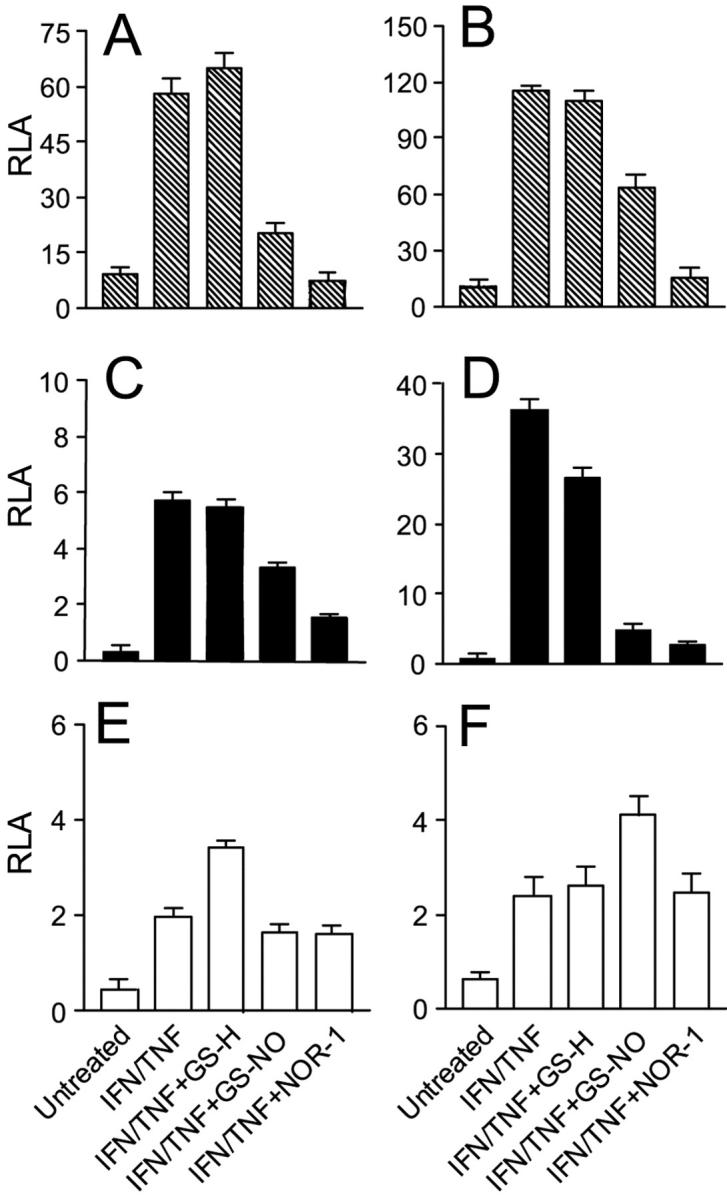
NO donors reduce luciferase expression driven by NF-κB and STAT-1 transcription factors in transiently transfected keratinocytes activated with IFN-γ and TNF-α. Keratinocytes obtained from healthy patients (A, C, E) and from psoriatic patients (B, D, F) were transfected with 1 μg of pNF-κB-Luc (A, B), pGAS-Luc (C, D), or pAP-1-Luc (E, F) plasmids, and then treated as indicated. After 24 hours keratinocytes were lysed to determine β-galactosidase and luciferase activities. Results are expressed as relative luciferase activity (RLA), which represents the luciferase activity normalized to the β-galactosidase activity. Values are the mean ± SD of four replicates for each experimental condition.
Discussion
Psoriasis is a genetically determined skin disease characterized by aberrant proliferation and differentiation of keratinocytes as well as cutaneous inflammation. T cell-mediated immune mechanisms have a primary role in the pathogenesis of psoriasis. 29-32 In particular, activated Th1 cells releasing IFN-γ and TNF-α stimulate keratinocytes to produce cytokines, chemokines, and adhesion molecules, which further amplify the inflammatory response. Keratinocyte production of chemokines contributes relevantly to the establishment of the inflammatory infiltrate. Specifically, IL-8 and related chemokines are responsible for the intraepidermal collection of neutrophils. 5 MCP-1, RANTES, IP-10, and other CXCR3 ligands attract predominantly monocytes and Th1 cells, 6-8 whereas MIP-3α (CCL20) recruits Langerhans cells and dendritic cells. 33,34 Moreover, psoriatic keratinocytes may have intrinsic defects leading to exaggerated synthesis of certain chemokines such as IL-8, MCP-1, and IP-10. 5,8 Here we confirmed that keratinocytes cultured from patients with psoriasis produce spontaneously higher levels of IL-8, and on activation with IFN-γ and TNF-α higher amounts of IL-8, MCP-1, and IP-10. NO can be made by several cell types residing in the skin including both genuine immune-system cells as well as endothelial cells, fibroblasts, and keratinocytes. NO actively participates in the modulation of inflammatory reactions and the trafficking of leukocytes. 17,35 In particular, NO and NO donors inhibit the expression of adhesion molecules (eg, ICAM-1, VCAM-1, E-selectin) on endothelial cells, and impede the rolling, firm adherence and/or transmigration of monocytes and granulocytes. 22,23 Moreover, NO can interfere with the activity of chemokines by several mechanisms. NO as well as NO donors can inhibit the production of IP-10, Mig, RANTES, and MCP-1. 19,20,21 Additionally, NO can reduce of activity of chemokines (such as IL-8) through peroxynitrite-dependent tyrosine nitration 36 and it can function as an intracellular messenger in chemokine signaling pathways. 37
In this study, we have shown that NO donors reduce in a dose-dependent manner the synthesis and release of IP-10, RANTES, and MCP-1 from keratinocytes cultured from healthy individuals and patients with psoriasis. In contrast, IL-8 production was not affected by NO donors. ICAM-1 provides a major adhesion mechanism by which T cells and neutrophils bind to keratinocytes and are thus retained in the epidermis. Psoriatic keratinocytes activated with IFN-γ and TNF-α showed a ICAM-1 induction higher than normal keratinocytes. NO donors could efficiently and dose dependently down-regulate membrane and soluble ICAM-1 expression induced by IFN-γ and TNF-α in both normal and psoriatic keratinocytes. This activity was specific because membrane HLA-DR expression was not changed. In contrast to what observed for chemokines, ICAM-1 mRNA was not affected by NO donors, suggesting a posttranscriptional regulation. To see whether NO donors could affect chemokine and ICAM-1 expression also in vivo, a GS-NO-releasing ointment was applied on psoriatic lesions for 14 days. The results showed that GS-NO could markedly reduce IP-10, RANTES, and MCP-1, but not IL-8 immunoreactivity in keratinocytes, paralleling the in vitro results. In line with the reduced production of chemokines by keratinocytes, the skin treated with the GS-NO ointment showed diminished keratinocyte ICAM-1 expression and lower numbers of T cells and monocytes within and in proximity of the epidermis as well as in the upper dermis. In contrast, the number of T cells and monocytes in the deeper dermis was not changed.
The signal transduction initiated by IFN-γ and TNF-α involves principally a cooperation between STAT-1 and NF-κB transcription factors. 26,28 In contrast, AP-1 is known to be less important in the signaling elicited by these cytokines. We have shown that in transiently transfected keratinocytes, IFN-γ and TNF-α induced a strong NF-κB and STAT-1-binding activity, whereas the induction of AP-1 function was less evident. Interestingly enough, psoriatic keratinocytes exhibited a more prominent NF-κB and STAT-1, but not AP-1 activity compared to control keratinocytes. Indeed, perturbation in signal transduction pathways and in the activation of transcription factors have been implicated in this dysregulated functions of psoriatic keratinocytes. 38,39 When keratinocyte stimulation was performed in the presence of NO donors an impaired NF-κB and STAT-1, but not AP-1, activation was observed. Inhibition of NF-κB and STAT-1 activity may in part explain the capacity of NO donors to reduce the synthesis of IP-10, RANTES, and MCP-1. The inability of NO donors to influence IL-8 production may be partially because of the lack of effects on AP-1, which is critical for IFN-γ/TNF-α-induced IL-8 gene expression. 40 NO donors have been shown to inactivate keratinocyte differentiation markers such as transglutaminase 1, loricrin, and involucrin by interfering with AP-1 activity. 41 In this study, however, keratinocyte AP-1 transactivation was induced by phorbol esters.
A variety of studies suggest that endogenous NO contributes to the formation of psoriatic lesion. Nonetheless, NO may have regulatory effects on diverse aspects of inflammation. Our results indicate that NO donors efficiently suppress IFN-γ/TNF-α-induced keratinocyte activation both in vitro and in vivo. In particular, NO donors were potent inhibitors of the expression of chemokines and adhesion molecules relevant to the generation of the inflammatory infiltrate during psoriasis. Based on these observations, NO donors seem interesting therapeutic candidates for psoriasis and other chronic inflammatory skin diseases.
Footnotes
Address reprint requests to Giampiero Girolomoni, Istituto Dermopatico dell’Immacolata, Via Monti di Creta 104, 00167 Rome, Italy. E-mail: giro@idi.it.
Supported by the Italian Ministry of Health.
References
- 1.Albanesi C, Scarponi C, Sebastiani S, Cavani A, Federici M, Sozzani S, Girolomoni G: A cytokine-to-chemokine axis between T lymphocytes and keratinocytes can favor Th1 cell accumulation in chronic inflammatory skin diseases. J Leukoc Biol 2001, 70:617-623 [PubMed] [Google Scholar]
- 2.Sebastiani S, Albanesi C, De Pità O, Puddu P, Cavani A, Girolomoni G: The role of chemokines in allergic contact dermatitis. Arch Dermatol Res 2002, 293:552-559 [DOI] [PubMed] [Google Scholar]
- 3.Albanesi C, Cavani A, Girolomoni G: IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokines production in human keratinocytes: synergistic or antagonistic effects with IFN-γ and TNF-α. J Immunol 1999, 162:494-502 [PubMed] [Google Scholar]
- 4.Albanesi C, Scarponi C, Sebastiani S, Cavani A, Federici M, De Pità O, Puddu P, Girolomoni G: IL-4 enhances keratinocytes expression of CXCR3 agonistic chemokines. J Immunol 2000, 165:1395-1402 [DOI] [PubMed] [Google Scholar]
- 5.Nickoloff BJ, Mitra RS, Varani J, Dixit VM, Polverini PJ: Aberrant production of interleukin-8 and thrombospondin-1 by psoriatic keratinocytes mediates angiogenesis. Am J Pathol 1994, 144:820-828 [PMC free article] [PubMed] [Google Scholar]
- 6.Gillitzer R, Wolff K, Tong D, Müller C, Yoshimura T, Hartmann AA, Stingl G, Berger R: MCP-1 mRNA expression in basal keratinocytes of psoriatic lesions. J Invest Dermatol 1993, 101:127-131 [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb AB, Luster AD, Posnett DN, Carter DM: Detection of a γ interferon-induced protein IP-10 in psoriatic plaques. J Exp Med 1998, 168:941-948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giustizieri ML, Mascia F, Frezzolini A, De Pità O, Chinni LM, Giannetti A, Girolomoni G, Pastore S: Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokines production profile in response to T cell-derived cytokines. J Allergy Clin Immunol 2001, 107:871-877 [DOI] [PubMed] [Google Scholar]
- 9.Sallusto F, Mackay CR, Lanzavecchia A: The role of chemokine receptors in primary, effector, and memory immune response. Annu Rev Immunol 2000, 18:593-620 [DOI] [PubMed] [Google Scholar]
- 10.Barker JN, Sarma V, Mitra RS, Dixit VM, Nickoloff BJ: Marked synergism between tumor necrosis factor factor-alpha and interferon-gamma in regulation of keratinocyte-derived adhesion molecules and chemotactic factors. J Clin Invest 1990, 85:605-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traidl C, Sebastiani S, Albanesi C, Merk HF, Puddu P, Girolomoni G, Cavani A: Disparate cytotoxic activity of nickel-specific CD8+ and CD4+ T cell subset against keratinocytes. J Immunol 2000, 165:3058-3064 [DOI] [PubMed] [Google Scholar]
- 12.Ormerod AD, Weller R, Copeland P, Benjamin N, Ralston SH, Grabowksi P, Herriot R: Detection of nitric oxide and nitric oxide synthases in psoriasis. Arch Dermatol Res 1998, 290:3-8 [DOI] [PubMed] [Google Scholar]
- 13.Ormerod AD, Dwyer CM, Reid CM, Copeland P, Thomson WD: Inducible nitric oxide synthase demonstrated in irritant and allergic contact dermatitis. Acta Derm Venereol (Stockh) 1997, 77:436-440 [DOI] [PubMed] [Google Scholar]
- 14.Rowe A, Farrel AM, Bunker CB: Constitutive endothelial and inducible nitric oxide synthase in inflammatory dermatoses. Br J Dermatol 1997, 136:18-23 [PubMed] [Google Scholar]
- 15.Sirsjo A, Karlsson M, Gidlof A, Rollman O, Torma H: Increased expression of inducibile nitric oxide synthase in psoriatic skin and cytokine-stimulated cultured keratinocytes. Br J Dermatol 1996, 134:643-648 [DOI] [PubMed] [Google Scholar]
- 16.Qureshi AA, Hosoi J, Xu S, Takashima A, Granstein RD, Lerner EA: Langerhans cells express inducible nitric oxide synthase and produce nitric oxide. J Invest Dermatol 1996, 107:815-821 [DOI] [PubMed] [Google Scholar]
- 17.Bodgan C: Nitric oxide and the immune response. Nat Immunol 2001, 2:907-916 [DOI] [PubMed] [Google Scholar]
- 18.Zouki C, Jozsef L, Ouellet S, Paquette Y, Filep JG: Peroxynitrite mediates cytokine-induced IL-8 gene expression and production by human leukocytes. J Leukoc Biol 2001, 69:815-824 [PubMed] [Google Scholar]
- 19.Romagnani P, Lazzeri E, Lasagni L, Mavilia C, Beltrame C, Francalanci M, Rotondi M, Annunziato F, Maurenzig L, Coami L, Galli G, Salvadori M, Maggi E, Seri M: IP-10 and Mig production by glomerular cells in human proliferative glomerulonephritis and regulation by nitric oxide. J Am Soc Nephrol 2002, 13:53-64 [DOI] [PubMed] [Google Scholar]
- 20.Wetzler C, Kämpfer H, Pfeilschifter J, Frank S: Keratinocyte-derived chemotactic cytokines: expressional modulation by nitric oxide in vitro and during cutaneous wound repair in vivo. Biochem Biophys Res Commun 2000, 274:689-696 [DOI] [PubMed] [Google Scholar]
- 21.Frank S, Kämpfer H, Wetzler C, Stallmeyer B, Pfeilschifter J: Large induction of the chemotactic cytokine RANTES during cutaneous wound repair: a regulatory role for nitric oxide in keratinocyte-derived RANTES expression. Biochem J 2000, 347:265-273 [PMC free article] [PubMed] [Google Scholar]
- 22.Spiecker M, Darius H, Kaboth K, Hübner F, Liao JK: Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J Leukoc Biol 1998, 63:732-739 [PubMed] [Google Scholar]
- 23.Grisham MB, Granger DN, Lefer DJ: Modulation of leukocyte-endothelial interactions by reactive metabolites of oxygen and nitrogen: relevance to ischemic heart disease. Free Radic Biol Med 1998, 25:404-433 [DOI] [PubMed] [Google Scholar]
- 24.Pastore S, Fanales-Belasio E, Albanesi C, Chinni LM, Giannetti A, Girolomoni G: Granulocyte macrophage colony-stimulating factor is overproduced by keratinocytes in atopic dermatitis. Implications for sustained dendritic cell activation in the skin. J Clin Invest 1997, 99:3009-3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Percival MD, Ouellet M, Campagnolo C, Claveau D, Li C: Inhibition of cathepsin K by nitric oxide donors: evidence for the formation of mixed disulfides and a sulfenic acid. Biochemistry 1999, 38:13574-13583 [DOI] [PubMed] [Google Scholar]
- 26.Gosh S, May MJ, Kopp EB: NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Cell Biol 1998, 16:225-260 [DOI] [PubMed] [Google Scholar]
- 27.Foletta VC, Segal DH, Cohen DR: Transcriptional regulation in the immune system: all roads lead to AP1. J Leukoc Biol 1997, 63:139-152 [DOI] [PubMed] [Google Scholar]
- 28.Horwarth CM, Darnell JE: The state of the STATS: recent developments in the study of signal transduction to the nucleus. Curr Opin Cell Biol 1997, 9:233-239 [DOI] [PubMed] [Google Scholar]
- 29.Bos JD, De Rie MA: The pathogenesis of psoriasis: immunological facts and speculations. Immunol Today 1999, 20:40-46 [DOI] [PubMed] [Google Scholar]
- 30.Nickoloff BJ: Skin innate immune system in psoriasis: friend or foe? J Clin Invest 1999, 104:1161-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickoloff BJ, Schröder JM, von den Driesch P, Raychaudhuri SP, Farber EM, Boehncke WH, Morhenn VB, Rosenberg EW, Schön MP, Holick MF: Is psoriasis a T-cell disease? Exp Dermatol 2000, 9:359-375 [DOI] [PubMed] [Google Scholar]
- 32.Asadullah K, Volk H-D, Sterry W: Novel immunotherapies for psoriasis. Trends Immunol 2002, 23:47-53 [DOI] [PubMed] [Google Scholar]
- 33.Dieu-Nosjean MC, Massacrier C, Homey B, Vanbervliet B, Pin JJ, Vicari A, Lebecque S, Dezutter-Dambuyant C, Schmitt D, Zlotnik A, Caux C: Macrophage inflammatory protein 3α is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med 2000, 191:705-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, Catron D, Buchanan ME, Muller A, deWaal Malefyt R, Deng G, Orozco R, Ruzicka T, Lehmann P, Lebecque S, Caux C, Zlotnik A: Up-regulation of macrophage inflammatory protein-3 α/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol 2000, 164:6621-6632 [DOI] [PubMed] [Google Scholar]
- 35.Laroux FS, Pavlick KP, Hines IN, Kawachi S, Harada H, Bharwani S, Hoffman JM, Grisham MB: Role of nitric oxide in inflammation. Acta Physiol Scand 2001, 173:113-118 [DOI] [PubMed] [Google Scholar]
- 36.Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA: Reactive nitrogen and oxygen species attenuate interleukin-8-induced neutrophil chemotactic activity in vitro. J Biol Chem 2000, 275:10826-10830 [DOI] [PubMed] [Google Scholar]
- 37.Cherta RP, Ganu RK: Stromal cell-derived factor 1α-induced chemotaxis in T cells is mediated by nitric oxide signaling pathways. J Immunol 2001, 166:3067-3074 [DOI] [PubMed] [Google Scholar]
- 38.Karvonen SL, Korkiamaki T, Yla-Outinen H, Nissinen M, Teerikangas H, Pummi K, Karvonen J, Peltonen J: Psoriasis and altered calcium metabolism: downregulated capacitative calcium influx and defective calcium-mediated cell signaling in cultured psoriatic keratinocytes. J Invest Dermatol 2000, 114:693-700 [DOI] [PubMed] [Google Scholar]
- 39.Haase I, Hobbs RM, Romero MR, Broad S, Watt FM: A role for mitogen-activated protein-kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest 2001, 108:527-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K: Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-κB-like binding sites of the interleukin 8 gene. J Biol Chem 1992, 267:22506-22511 [PubMed] [Google Scholar]
- 41.Rossi A, Catani MV, Candi E, Bernassola F, Puddu P, Melino G: Nitric oxide inhibits cornified envelope formation in human keratinocytes by inactivating transglutaminases and activating protein 1. J Invest Dermatol 2000, 115:731-739 [DOI] [PubMed] [Google Scholar]