Abstract
Effector-memory T cells are strategically placed to epithelial tissues to provide frontline immune protection against pathogens. Their detrimental effects, however, have been rarely examined because of difficulty in sampling these T cells in pathological settings. Our previous studies suggested persistence of a similar subset of intraepidermal CD8+ T cells at high frequencies in the lesions of fixed drug eruption, a localized variant of drug-induced dermatoses. In situ activation of this subset resulting in localized epidermal injury can be traced in the lesions after antigen challenge by paired immunohistochemical staining, reverse transcriptase-polymerase chain reaction in situ, and flow cytometry of dispersed cells. Here we show that effector-memory T cells were greatly enriched in these intraepidermal CD8+ T cells, but not dermal and circulating counterparts, and that they constitutively express an early activation marker CD69 even before challenge. Surprisingly, a large proportion of these T cells expressed immediate effector function as evidenced by the rapid production of high levels of interferon-γ in situ with much faster kinetics than their counterparts at the mRNA and protein levels after challenge. This was followed by localized epidermal injury. The intracellular cytokine assay ex vivo shows that the great majority of these dispersed T cells produce interferon-γ. This study provides the first in situ description of the detrimental effects specifically mediated by effector-memory T cells residing at the effector site of immunopathology.
The ability of T cells to accurately migrate into the appropriate tissue depending on their functional phenotype is critical for effective T-cell-mediated immune responses aimed at eliminating pathogens while minimizing their tissue-damaging effects. 1-4 In view of the immediate response of effector T cells to pathogens, it makes good biological sense that they are preferentially distributed to epithelial surfaces, an area subjected to repeated challenge by pathogens. Recently, it has become clear that effector-memory T cells preferentially accumulate in the places where protection against pathogens is needed the most, such as epithelial tissues. 2 Effector-memory CD8+ T cells, also referred to as “effector-type” T cells, are defined by expression of CD45RA, CD11a, and CD11b with concomitant absence of expression of CD27, CD28, and CD62L, whereas memory-type CD8+ T cells, also referred to as “central memory” T cells, express CD45RO, CD95, CD27, and CD28. 5-8 The vast majority of the effector-memory CD8+ T cells are able to produce large amounts of interferon (IFN)-γ and tumor necrosis factor (TNF)-α with rapid kinetics on a per cell basis, but not interleukins (IL)-2 and -4. 3,5,8 These effector-memory CD8+ T cells have a cytolytic machinery such as perforin, granzyme A and B, and Fas ligand. 5,7 Thus, effector-memory T cells retain many of phenotypic and functional features that are traditionally characteristic of cells belonging to the innate arm of the immune system, such as natural killer (NK) cells. Nevertheless, there is no direct evidence to indicate that activation of these T cells could contribute to protection or pathology in a number of diverse physiological and pathological settings.
Recent studies have shown that CD8+ T cells that share some of the phenotypic features with the effector-memory T cells or NK cells are abundantly identified in the blister fluid from patients with toxic epidermal necrolysis, 9 a serious life-threatening drug-induced cutaneous reaction. It is, therefore, likely that the severe pathophysiological changes in the epidermal tissue are mediated by these T cells. However, the investigation into early molecular and cellular events critical for the evolution of epidermal injury in toxic epidermal necrolysis is difficult to perform. Although obtaining homogeneous populations of primed T cells is a necessary prerequisite to analyze and appreciate their pathogenic role, intraepidermal T cell populations thus far obtained in normal and pathological settings showed a more heterogeneous profile with various proportions of CD4+ and CD8+ T cells, and NK T cells. 10-13 In this regard, fixed drug eruption (FDE), which is a distinct, localized variant of drug-induced dermatoses characterized by the relapse in the same location after readministration of the causative drug, 14 would seem an ideal disease model for studying the pathological consequence of inflammation mediated by these T cells. This is because previous studies demonstrated that homogeneous populations of T cells persist at very high frequencies in the lesional epidermis after resolution of inflammatory responses 15,16 and because FDE lesions can be reproduced at the same sites after clinical challenge with the causative drug without causing serious systemic symptoms.
Here we show that CD8+ intraepidermal T cells residing in the FDE lesions retain an effector-memory phenotype and constitutively express an early activation marker even in the resting lesion before clinical challenge, unlike their counterparts in the dermis and peripheral blood. Surprisingly, these intraepidermal T cells, on challenge, displayed the capacity to rapidly produce high levels of IFN-γ with kinetics much faster than their counterparts at the mRNA and protein levels. Such early IFN-γ production in situ was only observed in the intraepidermal T cells resident in the lesions, but not those in the perilesional skin, and subsequently progressed to localized epidermal injury. The in situ findings accorded well with the flow cytometric characteristics of dispersed intraepidermal T cells. Our results indicate that these CD8+ intraepidermal T cells persist in a state of activation in the resting FDE lesions and are capable of acquiring potent cytotoxic activity with the rapid kinetics on clinical challenge; and that early IFN-γ production by effector-memory T cells residing in the lesions is the primary mechanism for the subsequent destructive stages of disease.
Materials and Methods
Patients and Biopsy Characteristics
Five patients with characteristic clinical findings of FDE who had given informed consent participated in this study. Case 1 (YS) was a 67-year-old woman and presented with multiple pruritic erythematous macules with blisters over the entire body. There was an associated high fever. The patient was hospitalized and asked to discontinue all of medications, which led to rapid resolution of the lesions without pigmentation. The patient’s medical history revealed that 24 hours before this presentation she had self-administered mefenamic acid and that similar eruptions had occurred at the same site after taking mefenamic acid. Our presumptive diagnosis was a bullous form of an erythema multiforme or multiple FDE. Six weeks later, an oral challenge with one-tenth of a single mefenamic acid dose was performed. Within 3 hours the same erythematous plaques evolved at exactly the same sites as her former attacks. Skin biopsy specimens were taken from the lesion itself and the adjacent uninvolved perilesional skin before, and 3 hours and 24 hours after challenge. Blood samples were also obtained at the same time points as biopsies.
Case 2 (TT) was a 31-year-old man who presented with a recurrent erythema on his lip, both hands, right elbow, and glans penis after taking a nonprescription cold relief medication containing allylisopropylacetylurea. A diagnosis of FDE was made. The symptoms had resolved completely after avoidance of allylisopropylacetylurea. Ninety days later, an oral challenge test with a single allylisopropylacetylurea dose was performed. Within 2 hours many of the previously involved sites flared. A biopsy specimen was obtained from the lesion on the dorsum of right hand before, and 2 hours and 24 hours after challenge. Blood samples were also obtained at the same time points as biopsies.
Case 3 (MS) was a 45-year-old woman who had a 1-month history of recurrent erythematous macules on her left cheek after taking salicylamide. A diagnosis of FDE was made and an oral challenge test with a single salicylamide dose was performed. Twelve hours after challenge, erythematous lesions appeared on the previously involved site. A skin biopsy specimen was taken from her lesion before and 12 hours after challenge.
Case 4 (MH) was a 51-year-old man who presented with recurrent erythematous eruptions on his lower lip, the dorsum of both hands, right knee, and the glans penis. After admission, an oral challenge test with a single allylisopropylacetylurea dose was performed. One hour later, he experienced a burning in many previously involved pigmented lesions over the extremities and the genital area. A biopsy specimen was taken from his wrist before and 3 hours after challenge.
Case 5 (ET) was a 70-year-old woman who was seen with a 1-month history of recurrent erythema on her right arm. Our presumptive diagnosis was FDE, although the causative drug was not determined. Seven months later she was seen again for skin eruption of exactly the same morphological features at the same sites as before. She had taken tosufuloxacin ∼2 hours before onset of the eruptions. Five months after resolution of the lesions a skin biopsy specimen was obtained from her lesion.
Clinical data concerning the type of FDE lesions, involved sites, causative drugs, and time between challenge and lesional onset are summarized in Table 1 ▶ .
Table 1.
Clinical Findings of Patients with FDE
| Case no./age, year/sex | Type of lesions | Involved sites | Causative drug | Time after recent flare* | Lag time, hours† | Systemic manifestation |
|---|---|---|---|---|---|---|
| 1 /67/F | Nonpigmenting Multiple | Face, trunk, arms, legs | Mefenamic acid | 40 days | 3 | + |
| 2/31/M | Multiple | Lip, hands, arms, genitalia | Allylisopropylacetylurea | 90 days | 2 | − |
| 3/45/F | Solitary | Cheek | Salicylamide | 60 days | 12 | − |
| 4/51/F | Multiple | Arms, knee, genitalia | Allylisopropylacetylurea | 15 days | 3 | − |
| 5/70/F | Multiple | Arms, shoulder | Tosufuloxacin | 150 days | 2 | − |
*The interval between recent flares and clinical challenge. Clinical challenge with the causative drug was performed at the indicated time points after recent flares.
†The lag time from clinical challenge to lesional onset of the eruption. Biopsy specimens were obtained before and immediately after lesional onset of the eruption.
Reagents
Monoclonal antibodies (mAbs) against CD3 (Becton Dickinson, Mountain View, CA), CD4 (Nichirei, Inc., Tokyo, Japan), CD8 (DAKO, Glostrup, Denmark), CD45RO (DAKO), CD45RA (Becton Dickinson), TCR-αβ (DAKO), CD11a (Becton Dickinson), CD11b (Serotec, Oxford, UK), CD27 (Becton Dickinson), CD28 (Becton Dickinson), CD56 (DAKO), CD69 (Pharmingen, San Diego, CA), CD158a (Beckman Coulter, Inc., Fullerton, CA), granzyme B (DAKO), perforin (DAKO), αE β7 (Immunotech, Inc., Marseille, France), IFN-γ (Harlan, Sera-Lab, Ltd., Loughborough, UK), and the respective isotype controls were purchased commercially, respectively. HECA-452 mAb was a kind gift from L. Picker (University of Texas, Southwestern Medical Center, Dallas, TX).
Immunohistochemisty
The biopsy specimens were frozen immediately in liquid nitrogen, embedded in ornithine carbamyl transferase compound (Tissue Tek II; Miles Laboratories, Naperville, IL) and stored at −80°C until used. Frozen sections 6-μm thick, were air-dried, fixed in acetone for 10 minutes, and stained by the labeled streptavidin/biotinylated horseradish peroxidase method (LSAB2 kit; DAKO) as described previously. 15 The slides were counterstained with Mayer hematoxylin and mounted for light microscopy.
To more accurately evaluate the presence of different cell subsets in the lesions, double-immunohistochemical staining procedures were performed on selected samples with the following combination of mAbs: CD8/CD45RA or CD8/CD45RO, and CD69/CD3. As in single stainings, sections were first stained with anti-CD8 or anti-CD69 by labeled streptavidin/biotinylated horseradish peroxidase method with red 3-amino-9-ethylcarbazol (AEC) color reaction. Subsequently, sections were washed in 0.1 mol/L of glicyn, and sequentially incubated with a second mAb, CD45RA, CD45RO, or CD3, followed by the alkaline phosphatase method (DAKO) with the Vector blue detection system (Vector Laboratories, Inc., Burlingame, CA). Stained sections were assessed using an Olympus BH-2 microscope (Olympus, Tokyo, Japan) at ×40 magnification in a blind manner by two observers (YM and YY) independently and there was no significant difference between assessment by the two. For each specimen at least three randomly selected fields of areas were assessed under ×400 magnification. Double-stained cells were characterized if both red and blue color could be discerned within one cell.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) in Situ
In situ detection of RT-PCR products for IFN-γ mRNA was performed as previously described, 17,18 with some modifications. Frozen sections 10-μm thick were fixed in 10% formaldehyde overnight at room temperature, and incubated with 0.1 μg/ml of proteinase K at 37°C for 30 minutes. After digestion, slides were washed in phosphate-buffered saline (PBS) and air-dried. For a reverse transcription procedure, the Omniscript RT kit (Qiagen, Hilden, Germany) was used according to the instructions supplied by the manufacturer. The sections were incubated for 60 minutes at 42°C under a coverslip in 65 μl of the following reaction mixture: 1× Omniscript RT buffer (Qiagen), 0.5 mmol/L of dNTP (Amersham Pharmacia Biotech, Buckinghamshire, UK), 1 U/μl of ribonuclease inhibitor (Takara, Otsu Siga, Japan), 1 μmol/L of the anti-sense primer for IFN-γ mRNA: 3′-ctACTGGTCTCGTAGGTT TTCTCAC-5′, and 0.2 U/μl of Omniscript RT (Qiagen).
PCR in situ of anti-sense-primed cDNA was performed by using the In Situ PCR System 1000 (Perkin Elmer, Foster, CA) in 50 μl of the following reaction mixture: 1× PCR Gold buffer (Perkin Elmer), 3 mmol/L MgCl2, 197 μmol/L dNTP (Amersham Pharmacia Biotech), 0.8 μmol/L of IFN-γ primers (sense: 5′-GCATCGTTTTGGGTTCTCTTGG; and anti-sense: 3′-CTACTGGTCTCGTAGGTTTTCTCAC-5′), and 10 U Taq gold polymerase (Perkin Elmer). After initial denaturation, 45 cycles of amplification, each at 95°C for 30 seconds and 60°C for 2 minutes, were performed. In each experiment, sections without treatment with RT were processed for PCR in situ together with each sample studied, as negative controls. The samples were then washed in PBS and air-dried.
PCR products were then hybridized in situ with a digoxigenin-labeled oligonucleotide probe. After washing, slides were hybridized in a solution containing 50% formamide, 1× Denhardt’s solution, 1 mmol/L ethylenediaminetetraacetic acid, 100 μg/ml fish DNA, 100 μg/ml yeast RNA, 4× standard saline citrate (SSC), and 100 ng/ml of digoxigenin-labeled oligonucleotide probe overnight at 37°C. After hybridization, slides were washed as follows: 2× standard saline citrate and 0.03× standard saline citrate at 50°C for 10 minutes each. Bound probe was detected with anti-digoxigenin antibody conjugated with alkali phosphatase (Roche, Basel, Switzerland), and color reaction was performed with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Roche).
Isolation of Intraepidermal T Cells from the FDE Lesions and Flow Cytometric Analysis
Intraepidermal T-cell suspensions were prepared from the resting lesions of these FDE patients before challenge, as described previously with some modifications. 19 In brief, subcutaneous adipose tissue was scraped off with a scalpel and the residual biopsy specimen was placed, epidermal side up, in 0.025 mol/L ethylenediaminetetraacetic acid for 2 hours at 37°C. Epidermal sheets were peeled off from the underlying dermis, were placed in RPMI 1640 containing 10 mg/ml of Dispase, and were then incubated in 37°C for 1 hour. Single cell suspensions were prepared by gentle agitation and pipetting. Cells thus prepared were further incubated for 14 to 16 hours at 37°C because this incubation procedure made it possible to detect CD8 and CD4 antigens on these T cells. The epidermal cell suspensions were used for flow cytometric analysis, as described previously. 19 After washing with PBS containing 0.1% bovine serum albumin (BSA) (PBS/BSA), cells were directly stained with cell surface markers for 25 minutes at 4°C. After staining, the cells were washed and resuspended in PBS/BSA for immediate flow cytometric analysis or in 0.5 ml of 1% paraformaldehyde for overnight storage before analysis. The samples were analyzed with a FACS Calibur Flow Cytometer (Becton Dickinson, San Jose, CA). List mode multiparameter data were analyzed using the Paint-a-Gate plus program (Becton Dickinson).
Simultaneous flow-cytometric assessment of T cell phenotype and cytokine synthesis was performed, as previously described. 20 Briefly, isolated epidermal cells (106/ml) were stimulated for 4 hours with 25 ng/ml of phorbor-12-myristate 13-acetate (PMA) plus 1 μg/ml of ionomycin in the presence of 10 μg/ml of Brefeldin A, an inhibitor of protein translocation from the endoplasmic reticulum to the Golgi apparatus. After stimulation, cells were washed twice with PBS/BSA, incubated in 0.5 ml of lysing solution and 0.5 ml of permeabilizing solution (Becton Dickinson) for 10 minutes at room temperature, washed with PBS/BSA, and then incubated for 25 minutes with appropriately titered mAb fluorochrome conjugates. The simultaneous detection of IFN-γ (fluorescein isothiocyanate-conjugated mAb) and IL-4 (phycoerythrin-conjugated mAb) on cells gated on the CD8+ or CD4+ subset was performed by the FACS Calibur flow cytometer.
Statistical Analysis
The data represent mean ± SEM of measurements obtained from four to five specimens. Statistical comparison between groups was performed using the Student’s t-test. Statistical significance was set at P < 0.05.
Results
Phenotypic Analysis of Intraepidermal T Cells before Challenge
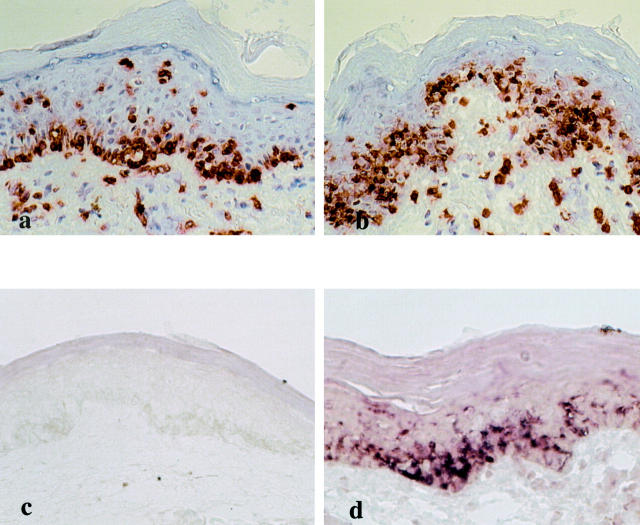
Selected biopsy materials obtained from the resting FDE lesions of the five patients before challenge were used for phenotypic characterization of the intraepidermal T cells by single- and double-label immunohistochemistry. As observed previously, 15 the frequencies of the intraepidermal T cells in individual biopsy samples before challenge varied substantially among the patients and lesions examined: the highest frequency was noted along the epidermal/dermal junction in the FDE lesion of case 1. Similar T-cell distribution patterns were found in other biopsy specimens, although the frequencies were much less. The overwhelming majority (>90%) of T cells in the epidermis expressed CD8 (Figure 1a) ▶ and very few CD4+ T cells were found (data not shown); in contrast, T cells in the dermis were of both CD4 and CD8 phenotype, although the former predominated. These findings indicate a preferential intraepidermal localization of CD8+ T cells. Analysis of serial sections stained with mAbs to TCR-αβ, CLA, and αEβ7 revealed that virtually all of the intraepidermal T cells expressed TCR-αβ, CLA, and αEβ7 (Figure 1; b, c, and d) ▶ and CLA and TCR-αβ expression was also noted in most of the dermal T cells, whereas αEβ7 expression was only observed on the intraepidermal T cells (>90%), but not on the dermal T cells (Figure 1d) ▶ . Flow cytometric analysis demonstrated that there was simultaneous staining for αEβ7 and CLA on the great majority of CD8+ intraepidermal T cells (>80%) but this subset was rarely detected in CD8+ peripheral blood T cells (<1%) of the same patients (Table 2) ▶ . Analysis of double-immunohistochemical staining with the combination of mAbs to CD8 and CD45RA or CD45RO demonstrated that the highest frequency of CD45RA+ T cells (100%) in the CD8+ intraepidermal T cells was noted in cases 2 and 5 (Figure 1g) ▶ . The CD45RA+ cell subset represented 96.6 ± 3.9% of the total CD8+ intraepidermal T cells and 40.4 ± 14.2% of CD8+ dermal T cells (Figure 1, e and g) ▶ . In contrast, CD45RO was expressed on 8.3 ± 6.0% of the CD8+ intraepidermal T cells and 13.3 ± 7.3% of CD8+ dermal T cells (Figure 1f) ▶ .
Figure 1.
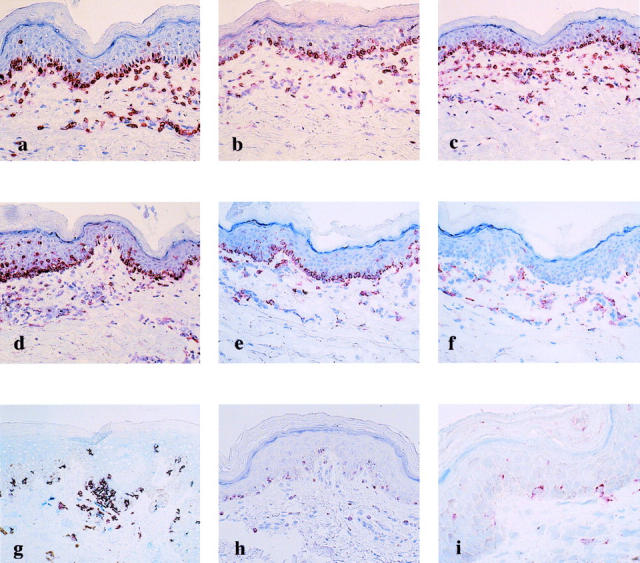
Immunophenotype of intraepidermal T cells residing in the resting FDE lesions before ingestion of the causative drugs. The vast majority of the intraepidermal T cells in case 1 express CD8 (a), TCR-αβ (b), CLA (c), αEβ7 (d), CD45RA (e), and granzyme B (h) but not CD45RO (f). g: Double-label immunohistochemistry for CD8 (red) and CD45RA (blue) in case 5 shows co-expression of both molecules, resulting in purple cells. i: CD158a expression is detected on a significant number of intraepidermal T cells in case 4. Original magnifications: ×66 (a–h); ×132 (i).
Table 2.
Preferential Intraepidermal Localization of CLA+ αEβ7+ CD8+ T Cells
| Intraepidermal CD8+ T cells* | Peripheral blood CD8+ T cells* | |
|---|---|---|
| CLA+ | 87.7 ± 7.7 | 8.7 ± 3.6 |
| αEβ+7 | 91.7 ± 5.9 | 2.6 ± 0.5 |
| CLA+αEβ7+ | 82.0 ± 6.7 | 0.3 ± 0.2 |
*Intraepidermal T cell suspensions and peripheral blood mononuclear cells were prepared from the same patients with FDE before challenge. Flow cytometric analyses were performed as described in Materials and Methods. Numbers give the percentages of CLA+, αEβ+7, and CLA+αEβ+7 cells within the CD8+ T cell subset, respectively.
Furthermore, the expression of CD11a was detected on a significantly higher percentage of the intraepidermal T cells, while the co-stimulatory molecules, CD27 and CD28, and the adhesion molecule, CD62L, were barely or not detectable on these T cells. Based on these findings that the majority of CD8+ CD45RA+ T cells expressed CLA, αEβ7, and CD11a but lacked CD27, CD28, and CD62L expression, we conclude that the intraepidermal T cells share a number of phenotypic features that have been previously reported for effector-type T cells or effector-memory T cells, but not for memory-type or central memory T cells. 5,7,8
Because effector-memory T cells are shown to be unique in the abundant expression of various NK cell receptors, 7,21,22 we next asked whether these T cells could also bear the phenotypic properties of NK cells and NKT cells. Biopsy specimens from the lesional skin were analyzed with mAbs directed against CD56, CD57, granzyme B, and killer-cell immunoglobulin-like inhibitory receptors (KIRs). Although most of the intraepidermal T cells lacked expression of CD56 and CD57 typically present on NK cells, a significant number of these T cells expressed granzyme B (Figure 1h) ▶ and CD158a (Figure 1i) ▶ . In contrast, CD158a reactivity was only detected on <1% of CD8+ peripheral blood T cells obtained from the same patient (data not shown). Interestingly, in case 1, CD158a expression could not be detected. Thus, the intraepidermal T cells share markers with, but are distinct from, both NK cells and NKT cells. In view of the flow cytometric finding that a similar CD8+CD45RA+ CD27− phenotype represented only <1% of CD8+ peripheral blood T cells (data not shown), the CD8+ intraepidermal T cells abundantly observed in the resting lesions of FDE would represent a unique subset that share many if not all characteristics with effector-memory T cells different from circulating central memory T cells.
In Situ Activation of Intraepidermal T Cells after Challenge
To investigate whether these T cells could retain the effector-memory phenotype on in situ activation and how activation of the T cells resulted in destruction of epidermal structures, sequential biopsy specimens were obtained from the lesional skin at various time points after clinical challenge in cases 1 to 4 and were examined immunohistochemically. As shown in Figures 1a and 2a ▶ ▶ , rich alignment of CD8+ T cells was observed along the epidermal basal layer in the FDE lesion of case 1 before challenge. Three hours after challenge, CD8+ intraepidermal T cells that had remained confined to the basal layer before challenge appeared to move to the upper part of the epidermis, and they morphologically became more dendritic and were diffusely distributed at a lower half of the epidermis (Figure 2b) ▶ . At this time yet extensive epidermal damage was not detectable despite activation of the CD8+ intraepidermal T cells; and a perivascular infiltration of CD8+ T cells as well as CD4+ T cells remained confined to the upper dermis: none of the infiltrates had extended into the overlying epidermis. The CD8+ intraepidermal T cells retained the effector-memory phenotype even after in situ activation (data not shown). Destruction of epidermal structures and a dramatic decrease in the number of the CD8+ intraepidermal T cells were observed at later time points (data not shown). Similar findings were also observed in case 2 (Figure 3, a and b) ▶ and other patients (data not shown).
Figure 2.
In situ activation of intraepidermal T cells after clinical challenge and their IFN-γ mRNA expression in case 1. a: Numerous CD8+ intraepidermal T cells are seen along the basal layer in the resting FDE lesion before challenge. b: Three hours after challenge, most of the CD8+ intraepidermal T cells are distributed at the lower half of the epidermis. c: No significant IFN-γ mRNA is detected in the same lesion as a. d: Intense IFN-γ mRNA expression is detected in the same lesion as b. The distribution of IFN-γ mRNA-expressing cells is quite identical to that of CD8+ T cells, indicating that essentially all of the CD8+ intraepidermal T cells produce IFN-γ. Original magnifications, ×66.
Figure 3.
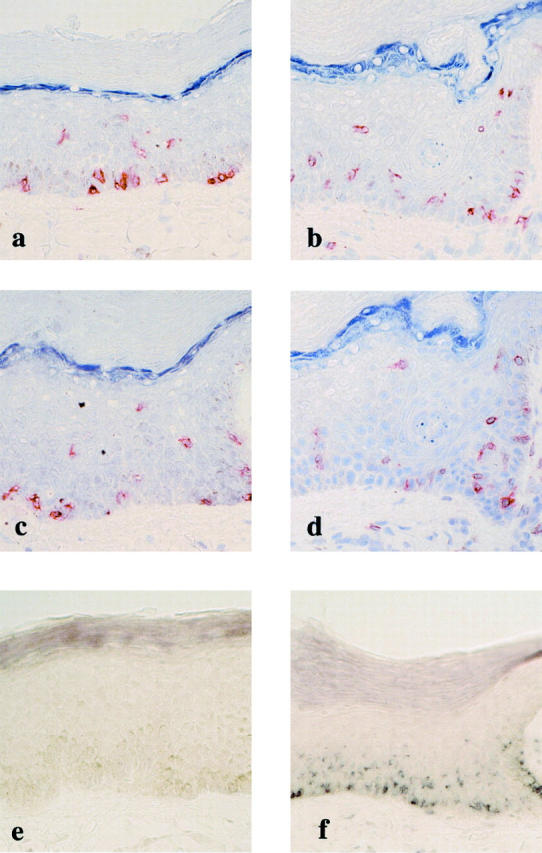
In situ activation of intraepidermal T cells after clinical challenge and their CD69 and IFN-γ mRNA expression in case 2. Before challenge, CD8+ intraepidermal T cells (a) are located along the epidermal/dermal junction and constitutively express CD69 (c). e: IFN-γ mRNA expression is not detected before challenge. Two hours after challenge, a significant number of CD8+ (b), CD69+ (d) intraepidermal T cells appear to move upwards and they are induced to express IFN-γ mRNA (f). Original magnifications, ×66.
Expression of Early Activation Marker on Intraepidermal T Cells
Because CD69 is an early activation marker and is constitutively expressed at low levels and up-regulated with rapid kinetics on innate immune cells such as NK cells than acquired immune cells, 23 we investigated whether CD69 could be preferentially and rapidly expressed on the intraepidermal T cells as compared with the counterparts in the dermis and peripheral blood obtained from the same patients. To this end, CD69 expression of the intraepidermal T cells before, and 2 to 3 hours and 24 hours after clinical challenge was compared with that of CD3+ peripheral blood T cells obtained at the same time as the skin biopsy. Surprisingly, analysis of double-immunohistochemical staining with the combination of mAbs to CD69 and CD3 demonstrated that the majority of the intraepidermal T cells were of a highly activated phenotype, characterized by the expression of CD69 even before challenge (Table 3 ▶ and Figure 3c ▶ ); in contrast, CD69 expression was rarely observed in the peripheral blood counterparts. CD69 was also expressed, although much less than intraepidermal T cells, on a significant numbers of dermal counterparts. Two to 3 hours after challenge, the frequencies of CD69 expression on the intraepidermal T cells remained elevated, whereas those on the circulating T cells were not significantly increased as compared with those before challenge. These data indicate that the intraepidermal T cells, unlike the peripheral blood counterparts, normally exist in a state of activation or become activated more easily during a preparation procedure. However, it is apparent that CD69 expression on the majority of intraepidermal T cells in the resting FDE lesions was not a consequence of damage during the tissue sampling and processing because the prevalence of CD69 expression (22.7 ± 3.9%, n = 3) on intraepidermal T cells similarly prepared from the psoriatic lesions was much less than those in the FDE lesions.
Table 3.
D69 Expression on Intraepidermal and Dermal T Cells and Peripheral Blood T Cells in Patients with FDE before and after Challenge
| Time after challenge, hours | Intraepidermal T cell* | Dermal T cell* | Peripheral blood T cell* |
|---|---|---|---|
| 0 | 86.8 ± 9.3†‡ | 33.0 ± 3.2‡ | 1.2 ± 0.2† |
| 2 or 3 | 84.9 ± 5.7†§ | 51.6 ± 7.6§ | 1.6 ± 0.6† |
| 24 | (59.0≈94.1¶) | (13.4≈61.9¶) | 4.0 ± 0.5 |
*The percentages of CD69-positive cells in the indicated T cell populations are determined by double immunohistochemical staining. (n = 4).
†P < 0.001.
‡P < 0.005.
§P < 0.01.
¶At 24 hours biopsy samples were only obtained from the limited number (n = 2) of patients with FDE. The range, therefore, is given instead of the mean ± SEM.
Induction of IFN mRNA Expression in Intraepidermal T Cells after Challenge
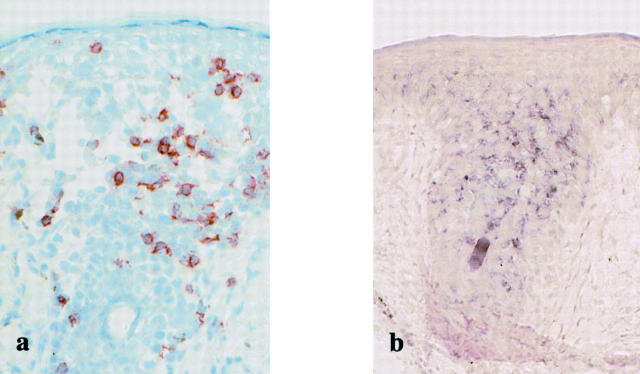
Previous studies demonstrated that effector-type CD8+CD45RA+ T cells can react to antigenic re-exposure by rapid onset of IFN-γ production. 5,8,24 We therefore investigated whether the CD8+ intraepidermal T cells could be induced to express both IFN-γ mRNA and protein with rapid kinetics on in situ activation. To compare the overall time course of IFN-γ induction in the intraepidermal T cells and circulating T cells, in situ RT-PCR studies were performed with biopsy specimens and peripheral blood mononuclear cells isolated before and at various time points after clinical challenge in cases 1 to 3. Biopsy specimens obtained before challenge did not show significant expression of IFN-γ mRNA (Figures 2c and 3e) ▶ ▶ . As shown in Figures 2d and 3f ▶ ▶ , numerous IFN-γ mRNA-positive cells were distributed in a manner suggestive of the intraepidermal T cells throughout the mid to lower layers of the epidermis at 3 hours in case 1 (Figure 2d) ▶ or along the basal epidermal layer (Figure 3f) ▶ at 2 hours in case 2. Expression of IFN-γ mRNA was only observed in the epidermis with the exception of case 2, in which a few dermal cells were found to be positive for IFN-γ mRNA. High staining intensity was seen particularly in the lesional epidermis of case 1 (Figure 2d) ▶ . Compared with those in case 1, the numbers of IFN-γ mRNA-positive cells in cases 2 and 3 were considerably lower. The distribution pattern of IFN-γ mRNA-expressing cells revealed three types of arrangements: in a first pattern, IFN-γ-positive cells were arranged in an array along the epidermal/dermal junction, like case 2 (Figure 3f) ▶ ; in a second pattern, these cells were seen as individual cells distributed diffusely throughout the epidermis, like case 1 (Figure 2d) ▶ . In case 3, IFN-γ mRNA-positive cells appeared to be accentuated in the follicular epithelium and were detected even at 12 hours after challenge (Figure 4b) ▶ . For cellular localization of IFN-γ mRNA in the epidermis, we performed parallel immunohistochemical staining of serial sections for CD8, CD4, and CD3. A large proportion of the IFN-γ mRNA-expressing cells in the epidermis were identified as CD8+ intraepidermal T cells (Figures 2a, 2b, 3a, and 3b) ▶ ▶ indicating that the CD8+ intraepidermal T cells are the main source of IFN-γ at 2 to 12 hours. These results indicate that a strong induction of IFN-γ mRNA occurred specifically in the CD8+ intraepidermal T cells at the earliest time point examined after clinical challenge. In contrast, IFN-γ mRNA was not detectable in biopsy samples obtained at subsequent time points (24 hours) and in all of the peripheral blood mononuclear cell samples obtained at the same time from the same patients when assayed in the same conditions (data not shown). Thus, the IFN-γ response of the T cells would evolve within a short time after challenge and in the relative absence of IFN-γ production by dermal and circulating T cells. Severe epidermal damage was only detected at later time points (ie, 12 hours to 24 hours) in the lesions that specifically retain significant numbers of these CD8+ intraepidermal T cells expressing IFN-γ mRNA (data not shown), but not in the perilesional skin, in which IFN-γ mRNA was never detectable even after challenge. These results indicated that early IFN-γ production by these T cells is the primary mechanism for the later destructive stages of disease.
Figure 4.
IFN-γ mRNA expression on CD8+ intraepidermal T cells (a) 12 hours after clinical challenge in case 3. IFN-γ mRNA-expressing cells (b) are accentuated in the follicular epithelium. Original magnifications, ×66.
Induction of IFN-γ Expression at Protein Level
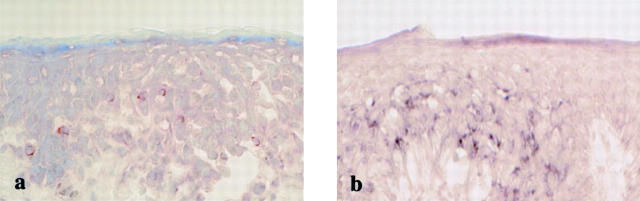
To determine whether the observed induction in IFN-γ production was also detected at the protein level, we performed immunohistochemistry with a mAb specific for IFN-γ. When reaction products were diffusely detected in their cytoplasm, the staining was considered positive. The staining intensity of IFN-γ differed considerably among individual cases examined. IFN-γ immunoreactivity was detected in the lesional epidermis at 3 to 12 hours in cases 1, 3, and 4: a considerable proportion of the intraepidermal T cells displayed IFN-γ immunoreactivity, whereas most T cells located in the dermis were IFN-γ-negative. The highest IFN-γ expression was observed in skin samples at 12 hours after challenge in case 3 (Figure 5a) ▶ : significant epidermal damage was only detectable in the lesion retaining the IFN-γ-positive cells. Similar but less intense reactivities were obtained in cases 1 and 4: in case 1, the staining reaction included the intraepidermal T cells and intercellular spaces, most probably because of the existence of the secreted cytokine (data not shown). IFN-γ expression at the protein level was detected even at 24 hours in case 1 (data not shown), at which time the mRNA expression was no longer detected. On serial sections, the intraepidermal IFN-γ-positive cells were identified as CD8+ T cells (data not shown). These results clearly showed a strong and exclusive induction of IFN-γ mRNA and protein in the CD8+ intraepidermal T cells at very early time points after challenge.
Figure 5.
IFN-γ expression in intraepidermal T cells at both mRNA (b) and protein levels (a) in case 3. IFN-γ expression at the protein level is also induced in intraepidermal T cells 12 hours after clinical challenge. Original magnifications, ×66.
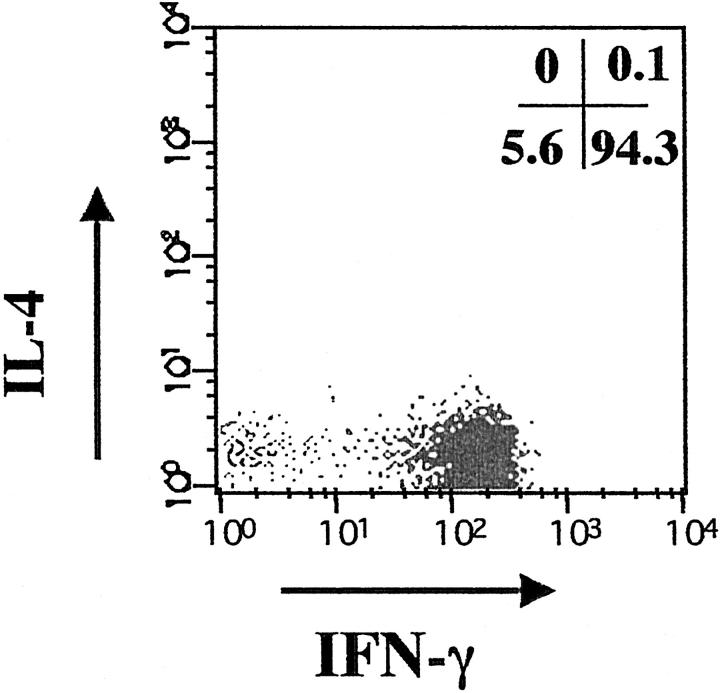
To further demonstrate the cellular source of IFN-γ, epidermal cells freshly isolated from the resting FDE lesions and peripheral blood mononuclear cells prepared from the same patients were stimulated for 4 hours with PMA and ionomycin, and the frequencies of cytokine-producing T cells were determined by flow cytometry after mAb staining for surface CD8 and CD4, and intracellular IFN-γ and IL-4. Cytokines were not constitutively expressed by either cell population. The maximum frequencies of cytokine-producing T cells detected by intracellular IFN-γ and IL-4 were attained 4 to 6 hours after stimulation. In all cases examined, the vast majority of CD8+ intraepidermal T cells produced IFN-γ. In contrast, the proportions of CD8+ intraepidermal T cells producing IL-4 were very low (<1%). Representative results are shown in Figure 6 ▶ . The frequencies of CD4+ intraepidermal and peripheral blood T cells that produced IFN-γ were much less than those of CD8+ counterparts (data not shown). These results clearly indicate that the ability to rapidly produce IFN-γ almost exclusively resides within the CD8+ intraepidermal T cell fraction.
Figure 6.
Flow cytometric detection of intracellular IFN-γ and IL-4 production by CD8+ intraepidermal T cells. The cells gated on CD8+ cells were analyzed for their expression of IFN-γ (x axis) and IL-4 (y axis). The number in the lower right quadrant represents the percentage of total CD8+ intraepidermal T cells expressing the IFN-γ+ IL-4− phenotype on ex vivo stimulation with PMA+ ionomycin, and the number in the upper left quadrant represent the percentage of total CD8+ intraepidermal T cells expressing the IFN-γ− IL-4+ phenotype.
Discussion
Previous analyses of T cells present in the human epidermis have revealed several features that differ from those identified in peripheral blood and other organs, but resemble more effector-memory T cells or innate immune cells such as NK cells. 9,25 In the present study, we have shown that the lesional epidermis harbors remarkably increased numbers of CD8+ T cells with a CD8+CD45RA+CD11a+CD27−CD56− phenotype that most closely resemble effector-type T cells 7 or effector-memory T cells (Table 4) ▶ . 1,3,8 The CD8+CD45RA+ subset constituted the vast majority of total intraepidermal T cells present in the FDE lesion. Such overwhelming predominance of the effector-memory CD8+ T cells in epithelial tissues has never been reported previously and previous studies demonstrated that there is substantial heterogeneity among populations of intraepidermal T cells with respect to phenotype. T cell populations isolated from normal epidermis showed a more heterogeneous profile with variable proportions of conventional CD4+ T cells, CD8+ T cells, CD8αα+ T cells, and CD4−8− cells, with minor populations of TCR γδ+ cells 10 and that those in the psoriatic epidermis also consisted of variable ratio of CD4+ and CD8+ T cells and some expressed natural killer receptors (NKRs) typically confined to NK cells. 11,25 Another remarkable difference between our results and previous studies was found in the frequencies of CD45RA+ T cells: in normal epidermis CD45RO+ T cells were the most abundant population of intraepidermal T cells. 10 These results imply that although T cells of various phenotypes are present at low numbers in normal epidermis, effector-memory CD8+ T cells are greatly enriched in the lesional epidermis of FDE. Because such enrichment for effector-memory CD8+ T cells within the epidermis was specifically observed in the resting FDE lesions but not in other chronic inflammatory diseases such as psoriasis, this is not a general phenomenon associated with chronic inflammation.
Table 4.
Phenotypic and Functional Properties of CD8+ Memory T Cell Subsets
| Intraepidermal | Effector memory* | Central memory* | |
|---|---|---|---|
| CD45RA | + | + | − |
| CD45RO | − | − | + |
| CD27 | − | − | + |
| CD28 | − | − | + |
| CD11a | + | ++ | + |
| CD62L | − | − | +/− |
| CD158a | +/− | + | N.D.† |
| Granzyme B | + | ++ | +/− |
| IFN-γ | ++ | ++ | +/− |
Why effector-memory CD8+ T cells are selectively retained in the FDE lesions while those of other phenotypes and CD4+ T cells should disappear might relate to the ability of effector-memory CD8+ T cells to be efficiently retained in the epidermis. When considering the observations that CD4+ T cells as well as CD8+ T cells were abundantly observed within the epidermis during the evolution phase even in the FDE lesions and the frequencies of CD4+ T cells declined rapidly after the resolution of the lesion (unpublished data), CD4+ T cells would be destined to undergo apoptosis within the epidermis or eventually migrate out of the epidermis. This possibility is likely, because our recent unpublished observations have demonstrated that CD8+ T cells, but not CD4+ T cells, can persist in the epidermis because CD8+ T cells express much higher levels of αEβ7, a molecule required for retention within the epidermis (unpublished data). Thus, we reason that preferentially expressed αEβ7 on CD8+ T cells may act to selectively retain effector-memory CD8+ T cells within the lesional epidermis.
The finding that the large proportion of the intraepidermal T cells express high levels of the early activation marker CD69 before challenge was somewhat surprising, because CD69 was weakly expressed on only a small proportion of the CD8+ T cells in the peripheral blood of the same patients. Similar to the CD8+ intraepidermal T cells in the resting FDE lesions, unstimulated NK cells belonging to innate immune cells in the blood also have been shown to constitutively express low levels of CD69. 23 These findings are reminiscent of intrahepatic hepatitis C virus-specific CD8+ T cells: those were all CD69+, whereas circulating counterparts were predominantly negative. 26 These results may indicate that intraepidermal T cells residing in the FDE lesions normally exist in a state of activation, like innate immune cells and intrahepatic CD8+ T cells. Constitutive expression of CD69 on intraepidermal T cells in the FDE lesions could be interpreted as an indication that intraepidermal T cells are associated with the innate immune system, because the gene encoding CD69 is located in the NK complex region that encodes numerous surface receptors on NK cells. 27
Our study was initially designed to provide direct evidence for the localization of the disease-causing T cells at an effector site of FDE. Although our previous studies have established the importance of intraepidermal CD8+ T cells in the effector phase of epidermal injury in FDE, 15,16,19 the mechanism underlying CD8+ T cell-mediated injury remained speculative. Given that despite the selective retention of large numbers of effector-memory CD8+ T cells within the lesional epidermis no epidermal injury is evident before challenge, it can be assumed that on antigenic stimulation these T cells are activated to rapidly release large amounts of cytokines at early time points, before they cause epidermal injury. The demonstration that rapid production of IFN-γ by these intraepidermal T cells clearly preceded the epidermal invasion of recruited T cells (at 2 to 3 hours after challenge) indicates that CD8+ effector-memory T cells indigenously residing in the lesional epidermis constitute a major cell type primarily responsible for epidermal injury in FDE lesions through the rapid production of IFN-γ. To our knowledge, this is the first report of IFN-γ production in situ at the single cell level by effector-memory T cells that are already resident at the site of immunopathology. Our results provide direct evidence to indicate that effector-memory T cells are fully functional on activation in situ. Almost all of these T cells could be induced to express IFN-γ within 2 to 3 hours of stimulation, although they did not express this cytokine constitutively. Of special interest was our finding that rapid production of large amounts of IFN-γ at an early time point (< 2 to 3 hours) was solely observed in the subset residing in the epidermis but not their counterparts in the dermis and blood in most if not all of the cases examined. Nevertheless, it would be premature to conclude that local production of IFN-γ alone is sufficient to effect T cell-mediated epidermal injury, because severe epidermal injury was only evident at later time points. A role for effector-memory type CD8+ T cells as effectors in tissue injury has been implicated by their ability not only to respond rapidly to antigen through rapid production of cytokines but also to lyse target cells. 3 Indeed, intraepidermal T cells have the cytolytic machinery. Thus, granule exocytosis is also likely the important pathway of cytotoxicity mediated by intraepidermal CD8+ T cells. The most probable explanation for the late appearance of severe epidermal injury is that IFN-γ can, in conjunction with other cytokines such as TNF-α, cause extensive epidermal damage by inducing the expression of apoptosis-associated proteins such as Fas and TNF-related apoptosis-inducing ligand (TRAIL). 28-30 Intraepidermal T cells, through production of early bursts of IFN-γ, could prime other immune cells, such as macrophages, to produce large amounts of TNF-α, as suggested in γδ T cells producing IFN-γ. 31 Indeed, the importance of TNF-α produced by skin resident cells in pathophysiology of FDE has been demonstrated in our previous studies, 15 and TNF-α as well as IFN-γ was found to be one of the first cytokines to appear in the bloodstream, followed by IL-6 in a patient with multiple FDE after clinical challenge (unpublished data). In summary, a deleterious effect of intraepidermal T cells is likely to be mediated via a collaboration with cells of the innate immune system.
In light of such potent ability of the intraepidermal T cells to rapidly produce large amounts of IFN-γ, it is critical to carefully control the activation of these T cells to avoid unwanted tissue injury. Interestingly, our study shows that a substantial population of these T cells expressed KIRs, which have been originally described on NK cells and can transduce inhibitory signals for cellular cytotoxicity after binding to MHC class I molecule. 31-34 Probably, KIR expression on these T cells may serve to play a physiological role in preventing these T cells from cross-reacting with surrounding keratinocytes expressing MHC class I. Thus, KIR expression on these T cells may be a means to prevent uncontrolled effector activation, which could in itself lead to greater pathological consequence.
In conclusion, this study provides direct evidence that activation of effector-memory T cells residing in the lesional epidermis occurs early in the course of disease and suggests that the degree of activation early in clinical illness is related to ultimate disease severity. Effector-memory T-cell populations residing in epithelial tissues should be viewed not only as favorite protectors against pathogens but also as potentially hostile predators.
Footnotes
Address reprint requests to Dr. Yoshiko Mizukawa, Department of Dermatology, Kyorin University School of Medicine, 6-20-2 Shinkawa, Mitaka, Tokyo 181-8611, Japan. E-mail: tpshio@kyorin-u.ac.jp.
Supported in part by grants from the Ministry of Education, Sports, Science, and Culture of Japan (to T. S. and Y. M.) and from the Ministry of Health, Labor, and Welfare of Japan (to T. S.).
References
- 1.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A: Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401:708-712 [DOI] [PubMed] [Google Scholar]
- 2.Masopust D, Vezys V, Marzo AL, Lefrancois L: Preferential localization of effector memory cells in nonlymphoid tissue. Science 2001, 291:2413-2417 [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Mackay CR, Lanzavecchia A: The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol 2000, 18:593-620 [DOI] [PubMed] [Google Scholar]
- 4.Butcher E, Picker LJ: Lymphocyte homing and homeostasis. Science 1996, 272:60-66 [DOI] [PubMed] [Google Scholar]
- 5.Hamann D, Baars PA, Rep MHG, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RAW: Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med 1997, 186:1407-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamann D, Kostense S, Wolthers KC, Otto SA, Baars PA, Miedema F, van Lier RAW: Evidence that human CD8+CD45RA+CD27− cells are induced by antigen and evolve through extensive rounds of division. Int Immunol 1999, 11:1027-1033 [DOI] [PubMed] [Google Scholar]
- 7.Baars PA, Riberio do Couto LM, Leusen JHW, Hooibrink B, Kuijpers TW, Lens SMA, van Lier RAW: Cytolytic mechanisms and expression of activation-regulating receptors on effector-type CD8+CD45RA+CD27− human T cells. J Immunol 2000, 165:1910-1917 [DOI] [PubMed] [Google Scholar]
- 8.Tussey L, Speller S, Gallimore A, Vessey R: Functionally distinct CD8+ memory T cell subsets in persistent EBV infection are differentiated by migratory receptor expression. Eur J Immunol 2000, 30:1823-1829 [DOI] [PubMed] [Google Scholar]
- 9.Cleach LL, Delaire S, Boumsell L, Bagot M, Bourgault-Villada I, Bensussan A, Roujeau JC: Blister fluid T lymphocytes during toxic epidermal necrolysis functional cytotoxic cells which express human natural killer (NK) inhibitory receptors. Clin Exp Immunol 2000, 119:225-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spetz AL, Strominger J, Groh-Spies V: T cell subsets in normal human epidermis. Am J Pathol 1996, 149:665-674 [PMC free article] [PubMed] [Google Scholar]
- 11.Ferenczi K, Burack L, Pope M, Krueger JG, Austin LM: CD69, HLA-DR and the IL-2R identify persistently activated T cells in psoriasis vulgaris lesional skin: blood and skin comparisons by flow cytometry. J Autoimmunity 2000, 14:63-78 [DOI] [PubMed] [Google Scholar]
- 12.Bonish B, Jullien D, Dutronc Y, Huang BB, Modlin R, Spada FM, Porcelli SA, Nickoloff BJ: Overexpression of CD 1d by keratinocytes in psoriasis and CD 1d-dependent IFN-γ production by NK-T cells. J Immunol 2000, 165:4076-4085 [DOI] [PubMed] [Google Scholar]
- 13.Nickoloff BJ, Bonish B, Huang BB, Porcelli SA: Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J Dermatol Sci 2001, 24:212-225 [DOI] [PubMed] [Google Scholar]
- 14.Shiohara T: What is new in fixed drug eruption? Dermatology 1995, 191:185-187 [DOI] [PubMed] [Google Scholar]
- 15.Teraki Y, Moriya N, Shiohara T: Drug-induced expression of intracellular adhesion molecule-1 on lesional keratinocytes in fixed drug eruption. Am J Pathol 1994, 145:550-560 [PMC free article] [PubMed] [Google Scholar]
- 16.Shiohara T, Moriya N: Epidermal T cells: their functional role and disease relevance for dermatologists. J Invest Dermatol 1997, 109:271-275 [DOI] [PubMed] [Google Scholar]
- 17.Nuovo GJ, MacConnell P, Forda A, Delvenne P: Detection of human papillomavirus DNA in formalin-fixed tissues by in situ hybridization after amplification by polymerase chain reaction. Am J Pathol 1991, 139:847-854 [PMC free article] [PubMed] [Google Scholar]
- 18.Nuovo M, Nuovo G: Utility of HHV8 RNA detection for differentiating Kaposi’s sarcoma from its mimics. J Cutan Pathol 2001, 28:248-255 [DOI] [PubMed] [Google Scholar]
- 19.Komatsu T, Moriya N, Shiohara T: T cell receptor (TCR) repertoire and function of human epidermal T cells: restricted TCR V α-V β genes are utilized by T cells residing in the lesional epidermis in fixed drug eruption. Clin Exp Immunol 1996, 104:343-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teraki Y, Picker LJ: Independent regulation of cutaneous lymphocyte-associated antigen expression and cytokine synthesis phenotype during human CD4+ memory T cell differentiation. J Immunol 1997, 159:6018-6029 [PubMed] [Google Scholar]
- 21.Assarsson E, Kambayashi T, Sandberg JK, Hong S, Taniguchi M, van Kaer L, Ljunggren HG, Chambers BJ: CD8+ T cells rapidly acquire NK1.1 and NK cell-associated molecules upon stimulation in vitro and in vivo. J Immunol 2000, 165:3673-3679 [DOI] [PubMed] [Google Scholar]
- 22.Kambayashi T, Assarsson E, Michaelsson J, Berglund P, Diehl AD, Chambers BJ, Ljunggren HG: Emergence of CD8+ T cells expressing NK cell receptors in influenza A virus-infected mice. J Immunol 2000, 165:4964-4969 [DOI] [PubMed] [Google Scholar]
- 23.Carnaud D, Lee D, Donnars O, Park S-H, Beavis A, Koezuka Y, Bendelac A: Cross-talk between cells of the innate immune system: NK T cells rapidly activate NK cells. J Immunol 1999, 163:4647-4650 [PubMed] [Google Scholar]
- 24.Faint JM, Amnels NE, Curnow SJ, Shields P, Pilling D, Hislop AD, Wu L, Akbar AN, Buckley CD, Moss PAH, Adams D, Rickinson AB, Salmon M: Memory T cells constitute a subset of the human CD8+ CD45RA+ pool with distinct phenotypic and migratory characteristics. J Immunol 2001, 167:212-220 [DOI] [PubMed] [Google Scholar]
- 25.Nickoloff BJ, Wrone-Smith T: Injection of pre-psoriatic skin with CD4+ T cells induces psoriasis. Am J Pathol 1999, 155:145-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He XS, Rehermann B, Lopez-Labrador FX, Boisvert J, Cheung R, Mumm J, Wedemeyer H, Berenguer M, Wright TL, Davis MM, Greenberg HB: Quantitative analysis of hepatitis C virus-specific CD8+ T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA 1999, 96:5692-5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira RA, Scalzo A, Simmons A: A NK complex-linked locus governs acute versus latent herpes simplex virus infection of neurons. J Immunol 2001, 166:5869-5873 [DOI] [PubMed] [Google Scholar]
- 28.Suk K, Kim S, Jim YH, Kim KA, Chang I, Yagita H, Shong M, Lee MS: IFN-γ/TNF-α synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J Immunol 2001, 166:4481-4489 [DOI] [PubMed] [Google Scholar]
- 29.Riccioli A, Starace D, D’Alessio A, Starace G, Padula F, De Cesaris P, Filippini A, Ziparo E: TNF-α and IFN-γ regulate expression and function of the Fas system in the seminiferous epithelium. J Immunol 2000, 165:743-749 [DOI] [PubMed] [Google Scholar]
- 30.Smyth MJ, Cretney E, Takeda K, Wiltrout R, Sedger LM, Kayagaki N, Yagit H, Okumura K: Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon γ-dependent natural killer cell protection from tumor metastasis. J Exp Med 2001, 193:661-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura H, Emoto M, Hiromatsu K, Yamamoto S, Matsuura K, Gomi H, Ikeda T, Itohara S, Yoshikai Y: The role of γδ T cells in priming macrophages to produce tumor necrosis factor-α. Eur J Immunol 1995, 25:1465-1468 [DOI] [PubMed] [Google Scholar]
- 32.Mingari MC, Moretta A, Moretta L: Regulation of KIR expression in human T cells: a safety mechanism that may impair protective T-cell responses. Immunol Today 1998, 19:153-157 [DOI] [PubMed] [Google Scholar]
- 33.Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A: Human NK-cell receptors. Immunol Today 2000, 21:420-422 [DOI] [PubMed] [Google Scholar]
- 34.Lanier LL: On guard—activating NK cell receptors. Nat Immun 2001, 2:23-27 [DOI] [PubMed] [Google Scholar]