Abstract
Infection of mice with mouse hepatitis virus (MHV), strain JHM, results in acute and chronic demyelination with many similarities to the human disease multiple sclerosis. This pathological process is primarily T cell-mediated and MHV infection of mice lacking B and T cells does not result in demyelination. In apparent contradiction to these results, robust demyelination is detected in MHV-infected young nude (athymic) mice. Herein, we show that demyelination in nude mice was mediated by γδ T cells. These cells, but not conventional CD4 or CD8 αβ T cells, were detected in the central nervous system of MHV-infected nude mice and their depletion with neutralizing antibody resulted in an 80% reduction in demyelination. These results show, for the first time, that γδ T cells can substitute for αβ T cells in a virus model of demyelination and further support a pathological role for γδ T cells in patients with multiple sclerosis.
Demyelination in the human disease multiple sclerosis is immune-mediated but the details of this process are only partly understood. 1,2 The host immune response is also primarily responsible for demyelination in mice infected with mouse hepatitis virus (MHV), Theiler’s murine encephalomyelitis virus (TMEV), or in rodents with experimental autoimmune encephalomyelitis. 3-6 In all of these animal models CD4 or CD8 T cells are critical for the demyelinating process. In mice infected with MHV or TMEV these CD4 or CD8 T cells are primarily specific for viral antigen although myelin-specific T cells may also be involved. 6,7 In the case of experimental autoimmune encephalomyelitis, these cells are specific for myelin proteins. 5,8 Consistent with these results, demyelination does not develop when mice lacking functional T cells [mice with severe combined immunodeficiency (SCID) or with recombination activation gene 1 deficiency (RAG1−/−)] are infected with MHV or TMEV. However, adoptive transfer of virus-specific CD4 or CD8 T cells results in demyelination in these models. 9-11
One exception to this requirement for CD4 or CD8 T cells is that young nude (athymic) mice develop demyelination after infection with MHV, TMEV, or Semliki Forest virus (SFV). 10,12-14 Demyelination in each case is indistinguishable from that observed in immunocompetent mice infected with the same viruses and is characterized by mononuclear cell infiltration into the central nervous system (CNS). Nude mice have near normal numbers of B lymphocytes and normal to elevated numbers of natural killer (NK) cells. Additionally, nude mice have several populations of extrathymically matured T lymphocytes, including γδ T cells. CD4 and CD8 T cells are not detected in the spleen or peripheral lymphoid tissue at 8 weeks of age, although extrathymically matured CD4 and CD8 T cells are present in nude mice beginning at 10 to 12 weeks of age. 15,16 These cells are not present in normal number or ratio. Because demyelination is observed in nude mice at times when CD4 or CD8 T cells are not detectable, another cell type must be able to substitute for these cells in the demyelinating process.
Mice infected with MHV develop either acute or chronic demyelinating diseases. 6 Although nude mice and rats develop demyelination after infection with MHV, transfer of splenocytes from nude mice to MHV-infected SCID mice did not result in demyelination. 10 These results suggested that the cells responsible for demyelination were present in insufficient quantity in the transferred populations to cause disease in recipient SCID mice. B cells are the most numerous cell types in the spleens of nude mice and therefore are unlikely to be responsible for demyelination because most transferred cells should be of this cell type. Furthermore, demyelination was not detected after transfer of B6 splenocytes depleted of both CD4 and CD8 T cells to MHV-infected RAG1−/− mice even though B cells are present in normal quantities in the transferred cells. 9 One caveat is that, in these experiments, splenocytes were harvested from mice at 7 to 8 days after inoculation, the time of maximal MHV-specific T cell, but not antibody, response.
Similarly, NK cells are present in nude, SCID, and RAG1−/− mice, making it unlikely that these cells are responsible for demyelination. In contrast, γδ T cells do not develop in RAG1−/− mice and are present at very low numbers in the spleens of immunocompetent mice (<1% of splenocytes). 17 However, because γδ T cells are present in young nude mice, we reasoned that these cells might function in lieu of αβ T cells to induce demyelination in these animals. γδ T cells have many of the same effector functions as αβ T cells. 18,19 γδ T cells are also involved in the immune response to viruses such as vaccinia virus, herpes simplex virus, Coxsackie B virus, and vesicular stomatitis virus. 20 Herein, we show that they also mediate demyelination in MHV-infected nude mice.
Materials and Methods
Mice
BALB/c-nude and C57BL/6 (B6) mice were obtained from the National Cancer Institute (Bethesda, MD). B6-nude and B6-RAG1−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME). RAG1−/− mice were bred at the University of Iowa (Iowa City, IA). Mice used in all experiments were 6 weeks old. We analyzed 63 BALB/c-nude, 12 B6-nude, 10 BALB/c, 12 B6, and 10 RAG1−/− mice in these experiments. MHV-infected mice were scored for clinical disease using the following scale: 0, asymptomatic; 1, limp tail; 2, wobbly gait; 3, hindlimb paresis; 4, quadriparesis/paralysis; 5, moribund/dead. All animal studies were approved by the University of Iowa Animal Care and Use Committee.
Virus
The neuroattenuated variant of MHV, strain JHM, (strain 2.2-V-1), a gift from Dr. J. Fleming (University of Wisconsin, Madison, WI), was used in all studies. Mice were intracerebrally inoculated with 1000 PFU of MHV. Viruses were titered by plaque reduction assay as previously described. 21
Flow Cytometry
Lymphocytes from the CNS were prepared as previously described. 22 Briefly, the CNS was homogenized and cells were purified using Percoll (Pharmacia, Uppsala, Sweden) and Lympholyte-M (Cedarlane Laboratories, Homby, Ontario, Canada). Cells were stained using phycoerythrin-conjugated anti-γδ-TCR (GL-3) mAb (BD Pharmingen, San Diego, CA), or fluorescein isothiocyanate-conjugated anti-CD19 (a gift of Dr. T. Waldschmidt, University of Iowa), anti-CD4 (GK1.5), or anti-CD8α (Lyt-2) mAb (Pharmingen). In all cases, an isotype-matched fluorescein isothiocyanate- or phycoerythrin-conjugated antibody was used as control. Flow cytometry was conducted on a FACScan (Becton-Dickinson, Mountain View, CA) at the University of Iowa flow cytometry facility.
In Vivo Depletion of γδ T Cells
Mice were given 250 μg of the anti-γδ-TCR monoclonal antibody UC7-13D5 (hybridoma provided by Dr. J. Harty, University of Iowa) by intraperitoneal inoculation 2 days before, the day of, and 2 days after, intracerebral inoculation of MHV. Similar concentrations of this antibody were used previously to deplete γδ T cells in vivo. 23,24 Control mice were sham-depleted with hamster Ig (provided by Dr. T. Waldschmidt) using the same protocol.
Histology and Quantification of Demyelination
For toluidine blue staining, spinal cords were fixed in 2.5% glutaraldehyde and embedded in epon. One-μm sections were stained with toluidine blue and visualized by light microscopy. For luxol fast blue staining, mice were perfused with phosphate-buffered saline and spinal cords were fixed in 10% zinc formalin (Labsco, Solon, OH). Eight-μm sections were stained with luxol fast blue and counterstained with hematoxylin and eosin. Entire spinal cords were photographed and digitalized using a Leitz DMB100 microscope (Leica Microscope, Wetzlar, Germany) and an Optronics camera (Optronics, Goleta, CA). Demyelination was quantified using Vtrace software (Image Analysis Facility, University of Iowa) as previously described. 25
Immunohistochemical and Immunofluorescence Assays
Eight-μm sections of zinc formalin-fixed spinal cords were deparaffinized, hydrated, and permeabilized with 0.1% Triton X-100. Sections were blocked with CASBlock (Zymed Laboratories, South San Francisco, CA) and incubated overnight at 4°C with antibody to macrophages/microglia (anti-F4/80 mAb, CI:A3-1; Serotec, Oxford, England) or to the MHV nucleocapsid (N) protein (mAb 5B188.2; provided by Dr. M. Buchmeier, Scripps Research Institute, La Jolla, CA). After washing, sections were incubated with biotinylated goat anti-rat Ig (F4/80) or goat anti-mouse Ig (N) (Jackson ImmunoResearch, West Grove, PA). After washing, streptavidin-horseradish peroxidase was applied. Sections were developed with 3,3′-diaminobenzidine (Sigma, St. Louis, MO). For immunofluorescence assays, sections were incubated overnight with the anti-phosphoneurofilament mAb cocktail SMI-312 (Sternberger Monoclonals, Lutherville, MD). After washing, samples were incubated with fluorescein isothiocyanate-conjugated anti-mouse IgG (Jackson ImmunoResearch) and examined by fluorescent microscopy.
Adoptive Transfer Experiments
B6-nude mice were intraperitoneally immunized with 3 × 105 PFU of wild-type MHV 7 days before transfer. Wild-type MHV was used to maximize the immune response. Four days before transfer, RAG1−/− mice were intracranially inoculated with MHV-2.2-V-1. On the day of transfer, splenocytes were isolated from the immunized nude mice and depleted of erythrocytes. Cells (1 × 107) were transferred to each RAG1−/− mouse. In other experiments, B6 mice were immunized with wild-type MHV 21 days before transfer into MHV-infected RAG1−/− mice.
Results
MHV-Infected Athymic Mice Exhibit Demyelination
Wild-type BALB/c and B6 mice infected with MHV develop an acute demyelinating disease with clearance of virus by 10 to 21 days after inoculation. Most mice survive the acute infection. 10 Nude mice of both species infected with the same virus also developed clinical signs of demyelination, including hindlimb and forelimb paresis, a wobbly gait, and an inability to right themselves by days 7 to 9 after inoculation (Figure 1) ▶ . By 12 days after inoculation, most mice had developed hindlimb paralysis and lethargy, with death occurring by day 13 after inoculation. Consequently, all experimental mice were harvested at day 12 after inoculation. In agreement with previous results, 10 we detected high levels of MHV in nude mice of both strains at this time (Table 1) ▶ . Examination of the spinal cord revealed similar amounts of demyelination in B6 and BALB/c nude mice (Figure 2A ▶ , Table 1 ▶ ). B6-nude mice had, on average, 11.3% demyelination, whereas BALB/c-nude mice had 14.0%. MHV-induced demyelination in immunocompetent BALB/c or B6 mice is characterized by an extensive mononuclear cell infiltration. Similarly, we detected large numbers of macrophages/microglia at sites of demyelination in BALB/c-nude mice (Figure 2B) ▶ and B6-nude mice (data not shown). We also detected abundant viral antigen throughout the gray and white matter, although generally not in areas with extensive demyelination (Figure 2C) ▶ . Consistent with previous reports of MHV infection of immunocompetent mice, 26,27 demyelination was primarily confined to the ventral and lateral funiculi, although occasionally, it was evident in dorsal white matter tracts (Figure 2D) ▶ .
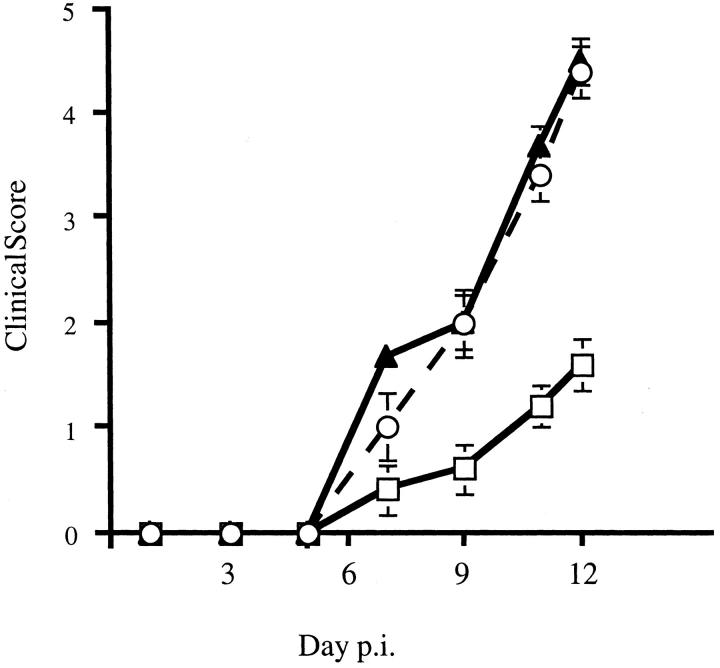
Figure 1.
Clinical disease in MHV-infected nude mice. BALB/c-nude mice infected with MHV were evaluated for clinical disease. Untreated mice (filled triangles) and those treated with hamster Ig (open circles) demonstrated similar disease progression. All untreated mice died by day 13 after inoculation. Mice treated with anti-γδ mAb UC7-13D5 (open squares) exhibited significantly less severe disease. Five mice per group were analyzed. Disease scoring scale: 0, asymptomatic; 1, limp tail; 2, wobbly gait; 3, hindlimb paresis; 4, quadriparesis/paralysis; 5, moribund/dead.
Table 1.
Demyelination and Virus Titers in MHV-Infected B6 Nude and BALB/c Nude Mice
| Strain | Demyelination* | Virus titer (PFU/g)* |
|---|---|---|
| BALB/c-nude | 14.0 ± 2.9% (15) | 1.3 ± 0.6 × 105 (9) |
| B6-nude | 11.3 ± 2.2% (9) | 2.0 ± 1.4 × 105 (9) |
*Spinal cords of BALB/c nude and B6 nude mice were harvested and analyzed for demyelination. Brains were homogenized and viral titers determined by plaque assay. The differences between groups are not statistically significant. The number of spinal cords analyzed is shown in parentheses.
Figure 2.
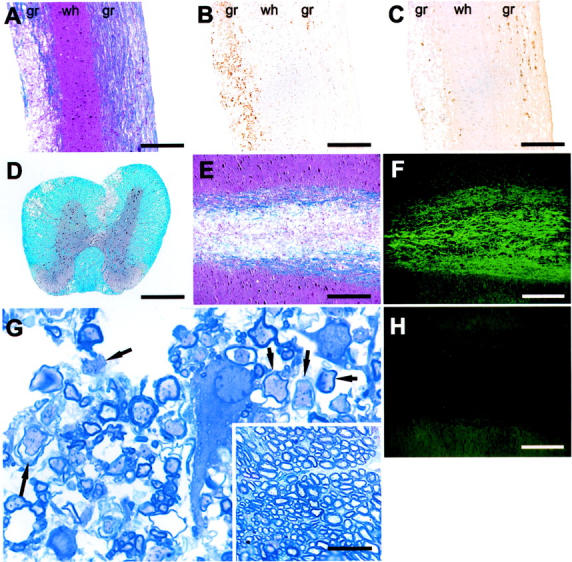
Detection of demyelination in MHV-infected BALB/c-nude mice at 12 days after inoculation. Spinal cords were harvested and 8-μm sagittal sections were stained for demyelination (A), macrophages (B), or viral antigen (C) as described in Materials and Methods. Demyelination was detected (A) in an area of macrophage/microglia infiltration (B). C: Viral antigen was abundant in normal appearing white matter and diminished in areas of frank demyelination. D: Demyelination was localized primarily in the ventral and ventrolateral funiculi with lesser amounts in the dorsal white matter tracts. In areas of gross demyelination (E), axons are preserved (F) as indicated by staining with anti-phosphoneurofilament H Ab. H: No staining was detected in the absence of primary antibody. G: Toluidine blue staining of epon-embedded sections revealed many axons with diminished or absent myelin staining (arrows). The inset in G shows an area of normal myelin from the spinal cord of the same mouse. g, Gray matter; wh, white matter. Scale bars: 100 μm (A–D); 50 μm (E, F, H); 20 μm (G); 40 μm (inset in G).
MHV infection of B6 mice results in primary demyelination with relative preservation of axons. 27 Next, we sought to determine whether MHV-infected nude mice exhibited the same phenotype, using two approaches. First, we stained adjacent 8-μm longitudinal sections with a cocktail of anti-neurofilament antibodies and luxol fast blue. In areas of extensive demyelination as identified by staining with luxol fast blue (Figure 2E) ▶ , we detected abundant neurofilament staining suggesting relative sparing of axons (Figure 2, F and H) ▶ . In a second approach, 1-μm epon-embedded sections were stained for myelin with toluidine blue. Examination of these sections also revealed intact axons with absent or diminished amounts of myelin, consistent with primary demyelination (Figure 2G) ▶ .
Characterization of CNS-Infiltrating Lymphocytes
To begin to characterize the cells responsible for demyelination in nude mice, we determined the phenotype of lymphocytes infiltrating the CNS of MHV-infected mice. We isolated 2.5 ± 0.60 × 105 (n = 7) mononuclear cells from the CNS of infected BALB/c-nude mice. This is ∼25% of the number of cells harvested from the CNS of MHV-infected B6 or BALB/c mice with acute encephalitis. 22,28 We stained these cells with antibodies to CD19, a marker for B cells, γδ TCR, CD4, and CD8α. Fluorescence-activated cell sorting analysis revealed that 40 to 60% of CNS-derived lymphocytes were B cells, and ∼15% were γδ T cells. Notably, there were no T cells expressing CD4 or CD8, including CD8αα cells, in the CNS (Figure 3; A to D) ▶ . The remaining cells in the CNS included NK cells and macrophages/microglia (data not shown). In contrast, lymphocytes harvested from the CNS of wild-type BALB/c mice infected with MHV consisted of ∼60% CD4 and CD8 T cells and 10% B cells. There were no detectable γδ T cells (Figure 3; E to H) ▶ .
Figure 3.
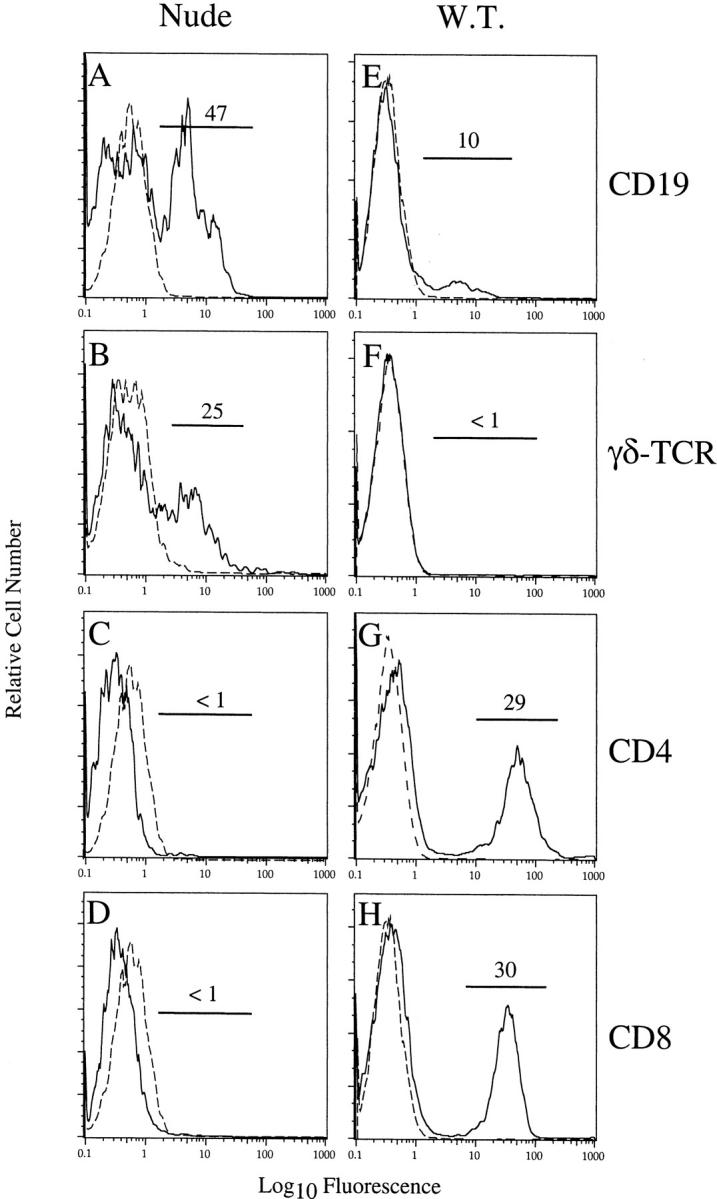
B and γδ T cells, but not CD4 or CD8 T cells, were detected in the CNS of MHV-infected BALB/c-nude mice. Lymphocytes were harvested from the CNS of BALB/c-nude (A–D) or BALB/c (E–H) mice at 12 days after inoculation. Lymphocytes from three BALB/c-nude mice were pooled and stained for flow cytometry as described in Materials and Methods. Cells from individual BALB/c mice were similarly analyzed. Although B cells were present in both samples (A, E), γδ T cells were detected only in the CNS of MHV-infected nude mice (B, F). No CD4 or CD8 T cells, including CD8αα T cells, were present in the CNS of MHV-infected nude mice (C, D). CD4 and CD8 T cells comprised 60% of mononuclear cells identified in the CNS of MHV-infected BALB/c mice (G, H). Dashed lines represent staining with fluorophore-conjugated control antibody. Results shown are representative of three independent experiments.
Depletion of γδ T Cells Abrogates Demyelination in Nude Mice
To determine whether γδ T cells mediate demyelination in MHV-infected nude mice, we depleted these cells in vivo using the anti-γδ TCR antibody UC7-13D5. This antibody has been shown previously to effectively deplete γδ T cells in mice. 23,24 At 12 days after inoculation, mice treated with anti-γδ TCR antibody exhibited substantially different clinical signs when compared to mice sham-depleted with hamster Ig. Mice treated with hamster Ig developed signs characteristic of demyelination, including limb paresis and difficulty righting and were indistinguishable clinically from mice not treated with antibody. Mice treated with anti-γδ TCR mAb did not develop limb paresis but rather were mildly lethargic and wobbly at the time of harvest (Figure 1) ▶ .
To extend these clinical observations, we examined the spinal cords from these mice after staining for myelin. γδ T cell-depleted mice exhibited little demyelination (4.0 ± 1.3%) whereas hamster Ig-treated mice retained wild-type levels (17.3 ± 5.2%) (Table 2) ▶ . Flow cytometric analysis of CNS-derived lymphocytes from anti-γδ TCR mAb-treated mice revealed no detectable γδ T cells with an unchanged CD19+ B cell infiltrate (Figure 4) ▶ . A γδ T cell infiltrate into the CNS was still detected after treatment with hamster Ig. CD4 and CD8 T cells were not present in either mouse population (data not shown). To determine whether γδ T cells also contributed to demyelination in immunocompetent mice, we depleted wild-type BALB/c mice of γδ cells using the same protocol as for BALB/c-nude mice. Anti-γδ-TCR mAb- and hamster Ig-treated BALB/c mice exhibited similar amounts of demyelination on MHV inoculation (Table 2) ▶ .
Table 2.
Effect of γδ T Cell Depletion on Demyelination and Macrophage Localization in Nude and Immunocompetent BALB/c Mice
| Mouse | Depletion | Demyelination† | Macrophages per 1.25-mm-wide section* | ||
|---|---|---|---|---|---|
| Gray matter | White matter | Total | |||
| BALB/c nude | Anti-γδ-TCR | 4.0 ± 1.3%‡ (7) | 35.3 ± 1.6§ | 25.1 ± 2.2§ | 60.4 ± 3.0§ |
| Hamster Ig | 17.3 ± 5.2% (6) | 50.7 ± 4.9 | 64.7 ± 4.7 | 115.3 ± 8.2 | |
| BALB/c | Anti-γδ-TCR | 10.2 ± 5.5% (6) | n.d.¶ | n.d. | n.d. |
| Hamster Ig | 12.0 ± 3.6% (4) | n.d. | n.d. | n.d. | |
*Midsagittal spinal cord sections stained with anti-F4/80 antibody were examined at ×20 magnification using a Leitz DMB microscope. Eight 1.25-mm-wide crosssections spaced by ≈2.5 mm were analyzed from each cord. Three spinal cords were examined for each data point.
†The number of spinal cords analyzed for demyelination is shown in parentheses.
‡P < 0.01 as compared to hamster Ig control.
§P < 0.005 as compared to hamster Ig control.
¶n.d.: not determined.
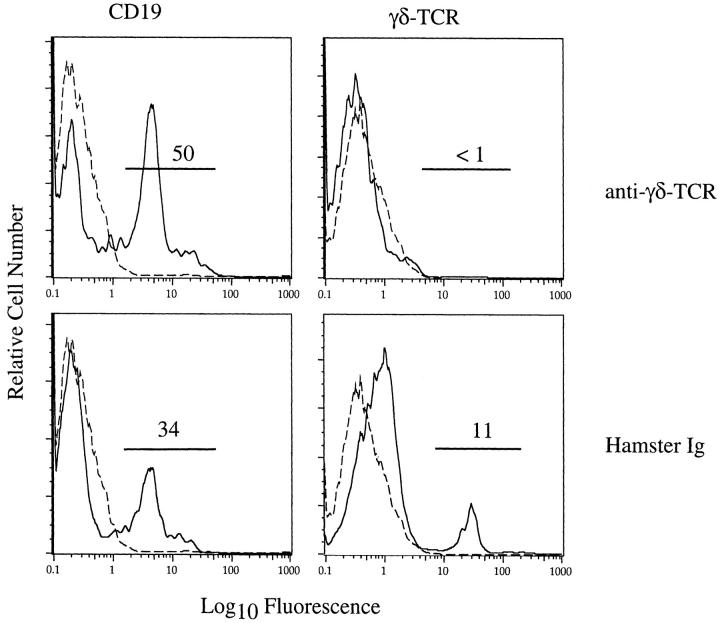
Figure 4.
Depletion of γδ T cells from infected BALB/c-nude mice. Nude mice were treated with anti-γδ-TCR mAb UC7-13D5 or hamster Ig as a control as described in Materials and Methods. These mice were then infected intracranially with MHV and 12 days later lymphocytes were harvested from the CNS and examined by flow cytometry. No γδ T cells were detected in the CNS after treatment with anti-γδ-TCR mAb but were readily detectable after hamster Ig treatment. Treatment with either antibody did not substantially affect the frequency of B cells in the infected CNS. Dashed lines represent staining with fluorophore-conjugated Ig. The results shown are representative of three independent experiments.
Macrophages or microglia are the terminal effector cells of demyelination induced by MHV. 6 We reasoned that decreased activation of macrophages/microglia or diminished migration into the spinal cord after treatment with anti-γδ TCR mAb or hamster Ig could account for the differences in demyelination. To address this question, we counted the number of F4/80+ cells in midsagittal sections of spinal cords. The total number of macrophages/microglia present in mice treated with anti-γδ TCR mAb was significantly lower than in mice treated with hamster Ig (Table 2 ▶ , 48% reduction). Also, we observed a change in the ratio of gray matter to white matter macrophages in anti-γδ TCR mAb-treated mice as compared to hamster Ig-treated animals. A greater proportion of macrophages/microglia were detected in the gray matter of γδ T cell-depleted mice, when compared to hamster Ig-treated animals (Figure 5, B and E) ▶ even in areas with little demyelination (Figure 5, A and D) ▶ and similar amounts of virus antigen (Figure 5, C and F) ▶ . The percentage of macrophages/microglia in the white matter was 42% in γδ T cell-depleted mice compared to 56% in hamster Ig-treated animals. Thus, these results suggest that γδ T cells were involved in recruitment or activation, or both, of macrophages/microglia and in their migration into the white matter.
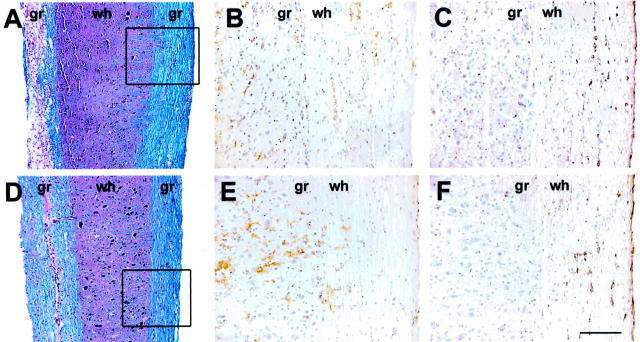
Figure 5.
Depletion of γδ T cells altered the number and location of macrophages/microglia in the spinal cord. Serial sections from spinal cords of hamster Ig-treated (A–C) or anti-γδ-TCR mAb-treated (D–F) BALB/c-nude mice were stained for myelin (A, D), macrophages/microglia (B, E), or viral antigen (C, F). Extensive demyelination was observed only in hamster Ig-treated sections (A, D). Distribution of macrophages/microglia and virus antigen was examined in an area of intact white matter with minimal demyelination (boxes in A and D). Macrophages/microglia infiltrated the white matter of hamster Ig-treated mice (B), whereas in anti-γδ-TCR mAb-treated mice these cells were predominately in the gray matter (E), despite the presence of equivalent amounts of virus in both spinal cords (C, F). gr, Gray matter; wh, white matter. Scale bars: 100 μm (A, D); 50 μm (B, C, E, F).
B Cells Do Not Mediate Demyelination in MHV-Infected Mice
Demyelination was not induced in RAG1−/− mice by transfer of T cell-depleted MHV-immune splenocytes from B6 mice. 9 However, these experiments were performed with splenocytes harvested at day 7 after inoculation, at a time when the anti-MHV B cell response is not maximal. We repeated these experiments using CD4 and CD8 T cell-depleted B6 splenocytes harvested 21 days after intraperitoneal inoculation with MHV. By 21 days after inoculation, plasma cells secreting anti-MHV antibody were readily detectable in the spleen (data not shown). After adoptive transfer, we detected no significant demyelination in MHV-infected RAG1−/− recipients, showing that B cells, in the absence of T cells, did not mediate demyelination, even at times when a robust anti-MHV B cell response had developed (data not shown).
Finally, we re-examined the capacity of splenocytes from nude mice to mediate demyelination on adoptive transfer into MHV-infected RAG1−/− mice. In a previous study, splenocytes transferred from B6-nude mice were unable to mediate demyelination in MHV-infected SCID mice after adoptive transfer. However, nude mice were not immunized with MHV in those experiments. 10 To determine whether previous immunization with MHV changed the outcome, we infected B6-nude mice intraperitoneally with MHV 7 days before transfer to MHV-infected B6 RAG1−/− mice. At the time of harvest, the vast majority of donor splenocytes were CD19+ (Figure 6) ▶ . This large B cell response may reflect the role that αβ TCR T cells have in B cell homeostasis, because exaggerated numbers of B cells have also been observed in TCR-α−/− mice. 29 Spinal cords were harvested at 11 to 12 days after transfer. We detected no significant demyelination (<1.0%) in six of seven recipients. In the remaining mouse, we observed that 20% of the spinal cord was demyelinated, a value comparable to the level of demyelination detected in MHV-infected nude mice (Table 1) ▶ . We do not know why demyelination was observed in this single animal. However, these results collectively indicated that B cells did not mediate demyelination in nude mice.
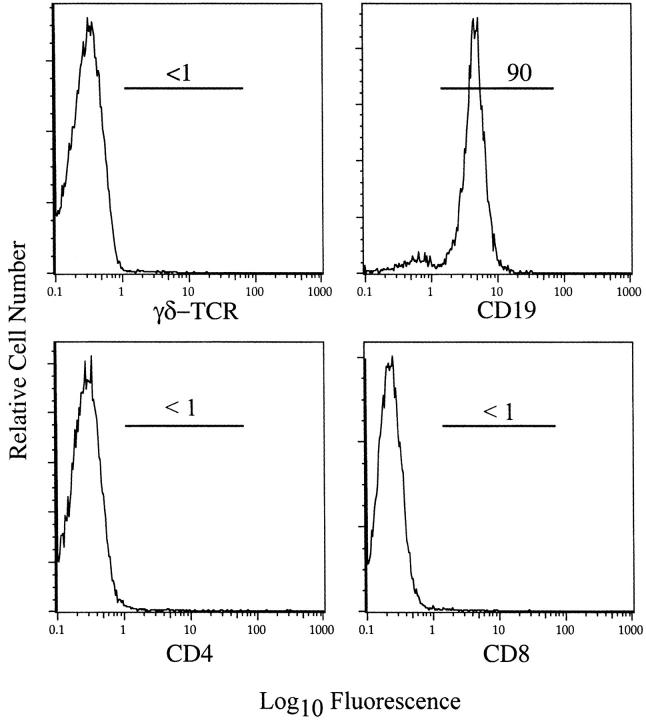
Figure 6.
B cells comprised the great majority of cells in the spleen of nude mice 7 days after intraperitoneal inoculation with MHV. Splenocytes were prepared from MHV-infected B6-nude mice and analyzed by flow cytometry after staining for CD4, CD8, CD19, and γδ TCR. The vast majority of cells were CD19+ B cells with few γδ, CD4, or CD8 T cells detected.
Discussion
In the present work, we demonstrated that γδ T cells were the major effectors of demyelination in nude mice infected with MHV. Nude mice infected with TMEV or SFV develop demyelination 10,12-14 and it is also likely that γδ T cells mediate demyelination in mice infected with these other viruses. The nude mice used in the present experiments were 6 to 7 weeks old at the time of infection. These mice lack extrathymically matured CD4 and CD8 T cells (Figure 3) ▶ , and have severe deficiencies in their ability to mount T cell-dependent antibody responses. 15,16 Throughout time, nude mice develop extrathymically matured CD4 and CD8 T cells that can act in a similar manner to traditional, thymically matured T cells. 30,31 As such, analysis at this early age was critical for identifying γδ T cells as effector cells in the demyelinating process.
Although our results are the first to show that demyelination was nearly completely abrogated in the absence of γδ T cells, γδ T cells have also been implicated in the pathological process in mice with experimental autoimmune encephalomyelitis, in humans with multiple sclerosis, and in other autoimmune diseases. 32-34 γδ T cells were identified in demyelinating lesions and in cerebrospinal fluid in humans with multiple sclerosis 35-38 and in the inflamed synovium of patients with rheumatoid arthritis 39,40 although their role in these pathogenic processes has not been established. In mice with experimental autoimmune encephalomyelitis, CD4 or CD8 αβ T cells are the major effectors of demyelination 5,8 but depletion of γδ T cells resulted in a reduction in inflammation and demyelination. 41,42 Furthermore, γδ T cell-depleted mice had reduced levels of proinflammatory cytokines and chemokines including interferon-γ, tumor necrosis factor-α, interleukin-6, interleukin-1β, CCL3/MIP-1α, and CCL5/RANTES, primarily at disease onset. 17,41 These data suggest that, in the presence of αβ T cells, γδ T cells function early in the disease process in the development of a maximal proinflammatory immune response, although they are not necessary for demyelination to occur. Our results extend these data by demonstrating that γδ T cells infiltrated the CNS of MHV-infected nude mice and were able to substitute for αβ T cells in the demyelinating process. γδ T cell-mediated demyelination in nude mice was characterized by myelin destruction with relative sparing of axons and mononuclear cell infiltration, and was indistinguishable from the pathology observed in immunocompetent mice (Figure 1) ▶ .
A subsequent objective was to determine the spatial relationship of γδ T cells to areas of demyelination and macrophage infiltration in MHV-infected nude mice. However, we were unable to detect these cells by immunohistochemistry using an antibody to CD3 antigen, present on all T cells. Using this antibody, we labeled T cells in the CNS of MHV-infected wild-type mice, suggesting that the number of γδ T cells was insufficient to be detected by this method. Consistent with this, there are 5- to 10-fold more T cells in the CNS of MHV-infected B6 mice than are present in the CNS of infected nude mice. 43 Of note, there may not be a close association between the presence of γδ T cells and areas of demyelination in nude mice because, in MHV-infected B6 mice, T cells are found predominantly in areas of virus antigen and not in demyelinating lesions. 44,45
An outstanding issue is the identification of the target recognized by γδ T cells in MHV-infected mice. Targets of γδ T cells include both protein and nonpeptide antigens. 18-20 The majority of targets identified thus far are proteins expressed during stress responses and are not microbial in origin. For example, in a recent report, cutaneous γδ T cells were shown to recognize Rae-1 and H60, two major histocompatibility complex class I-related molecules, via interactions with NKG2d, a receptor also expressed on NK cells. 46 However, in one case, γδ T cell recognition of a viral protein was described. 47,48 In mice infected with herpes simplex virus type 1, some γδ T cells recognize the amino terminus of glycoprotein I in a nonmajor histocompatibility complex-restricted manner. Recognition occurred even when the protein was immobilized in a tissue culture well. Specificity for MHV or CNS antigen may not be required for γδ T cell-induced demyelination since we recently showed that activated CD8 T cells not specific for either type of antigen were able to induce demyelination in MHV-infected mice. In these experiments, two strains of αβ TCR transgenic RAG2−/− mice were infected with MHV and transgenic cells were activated with cognate peptide peripherally. Demyelination was not detected in MHV-infected mice in the absence of specific activation of the transgenic CD8 T cells or in uninfected mice treated with cognate peptide (unpublished data). By analogy, activated γδ T cells may induce demyelination in the absence of cognate antigen in the CNS.
A second important issue is to define the mechanism of action of γδ T cells in demyelination. γδ T cells have cytotoxic function and also secrete proinflammatory cytokines. 19,20 In mice infected with Listeria monocytogenes, secretion of macrophage chemoattractants by γδ T cells was critical for induction of a protective immune response. 49 Secretion of proinflammatory cytokines by γδ T cells was also important in the immune response to vaccinia virus 50 and to herpes simplex virus type 1. 20 In mice infected with the latter virus, γδ T cells were able to effect virus clearance in the absence of αβ T cells. 47 In addition, γδ T cells may function by direct lysis of virus-infected cells in mice infected with either virus.
In MHV-infected mice, γδ T cells may also function by lysing infected target cells in the CNS. However, it is more likely that γδ T cells, by secreting proinflammatory cytokines or chemokines, induce the activation of macrophages or microglia and their migration into the white matter (Table 2) ▶ . In other models of MHV-induced demyelination, CCL5/RANTES and interferon-γ were critical for the pathological process and demyelination was significantly diminished in their absence. 51,52 In both instances, the absence of a cytokine or chemokine correlated with a decrease in the number of macrophages/microglia in the spinal cord, especially in the white matter.
Our results further argue against a role for B cells in MHV-induced demyelination. B cells may contribute to virus clearance from astrocytes and microglia by an antibody-independent mechanism 53 or by direct lysis of MHV-infected target cells. 54,55 B cells may also contribute to an inflammatory milieu via cytokine secretion. 56 However, depletion of γδ T cells significantly reduced demyelination in nude mice in the setting of an intact B cell infiltrate into the CNS, suggesting that production of proinflammatory cytokines or chemokines by B cells or B cell-mediated virus clearance is not sufficient for demyelination to occur.
In summary, our data show that γδ T cells can substitute for αβ T cells in mediating demyelination, most likely by inducing the activation of macrophages/microglia and their migration into the white matter. It remains to be determined whether each type of T cell effects this process by the same mechanism.
Acknowledgments
We thank Dr. Steven Moore for assistance with the histopathology; and Dr. John Harty, Dr. Craig Morita, Dr. Jodie Haring, and Taeg Kim for critical review of the manuscript.
Footnotes
Address reprint requests to Dr. Stanley Perlman, Department of Pediatrics, University of Iowa, 2042 Medical Laboratories, Iowa City, IA 52242. E-mail: stanley-perlman@uiowa.edu.
Supported in part by grants from the National Institutes of Health [grant NS40438, institutional training grant AI07485 (to A. D.), and an individual predoctoral NIH National Research Service Award NS42981 (to A. D.)] and the National Multiple Sclerosis Society (grant RG2864).
References
- 1.Hemmer B, Archelos JJ, Hartung HP: New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci 2002, 3:291-301 [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG: Multiple sclerosis. N Engl J Med 2000, 343:938-952 [DOI] [PubMed] [Google Scholar]
- 3.Murray PD, Pavelko KD, Leibowitz J, Lin X, Rodriguez M: CD4(+) and CD8(+) T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple sclerosis. J Virol 1998, 72:7320-7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsunoda I, Fujinami RS: Two models of multiple sclerosis: experimental allergic encephalomyelitis and Theiler’s murine encephalomyelitis virus. J Neuropathol Exp Neurol 1996, 55:672-686 [DOI] [PubMed] [Google Scholar]
- 5.Steinman L: Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron 1999, 24:511-514 [DOI] [PubMed] [Google Scholar]
- 6.Stohlman SA, Bergmann CC, Perlman S: Mouse hepatitis virus. Ahmed R Chen I eds. Persistent Viral Infections. 1998:pp 537-557 John Wiley & Sons, Ltd., New York
- 7.Miller SD, Vanderlugt C, Begolka W, Pao W, Yauch R, Neville K, Katz-Levy Y, Carrizosa A, Kim B: Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med 1997, 3:1133-1136 [DOI] [PubMed] [Google Scholar]
- 8.Steinman L: Myelin-specific CD8 T cells in the pathogenesis of experimental allergic encephalitis and multiple sclerosis. J Exp Med 2001, 194:F27-F30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu GF, Dandekar AA, Pewe L, Perlman S: CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J Immunol 2000, 165:2278-2286 [DOI] [PubMed] [Google Scholar]
- 10.Houtman JJ, Fleming JO: Dissociation of demyelination and viral clearance in congenitally immunodeficient mice infected with murine coronavirus JHM. J Neurovirol 1996, 2:101-110 [DOI] [PubMed] [Google Scholar]
- 11.Njenga MK, Asakura K, Hunter SF, Wettstein P, Pease LR, Rodriguez M: The immune system preferentially clears Theiler’s virus from the gray matter of the central nervous system. J Virol 1997, 71:8592-8601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorensen O, Saravani A, Dales S: In vivo and in vitro models of demyelinating disease, XVII. The infectious process in athymic rats inoculated with JHM virus. Microb Pathog 1987, 2:79-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gates MC, Sheahan BJ, Atkins GJ: The pathogenicity of the M9 mutant of Semliki Forest virus in immune-compromised mice. J Gen Virol 1984, 65:73-80 [DOI] [PubMed] [Google Scholar]
- 14.Roos RP, Wollmann R: DA strain of Theiler’s murine encephalomyelitis virus induces demyelination in nude mice. Ann Neurol 1984, 15:494-499 [DOI] [PubMed] [Google Scholar]
- 15.Benveniste P, Chadwick BS, Miller RG, Reimann J: Characterization of cells with T cell markers in athymic nude bone marrow and of their in vitro-derived clonal progeny. Comparison with euthymic bone marrow. J Immunol 1990, 144:411-419 [PubMed] [Google Scholar]
- 16.Kennedy JD, Pierce CW, Lake JP: Extrathymic T cell maturation. Phenotypic analysis of T cell subsets in nude mice as a function of age. J Immunol 1992, 148:1620-1629 [PubMed] [Google Scholar]
- 17.Rajan AJ, Asensio VC, Campbell IL, Brosnan CF: Experimental autoimmune encephalomyelitis on the SJL mouse: effect of γδ T cell depletion on chemokine and chemokine receptor expression in the central nervous system. J Immunol 2000, 164:2120-2130 [DOI] [PubMed] [Google Scholar]
- 18.Morita CT, Mariuzza RA, Brenner MB: Antigen recognition by human γδ T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol 2000, 22:191-217 [DOI] [PubMed] [Google Scholar]
- 19.Hayday AC: γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 2000, 18:975-1026 [DOI] [PubMed] [Google Scholar]
- 20.Sciammas R, Bluestone JA: TCRγδ cells and viruses. Microbes Infect 1999, 1:203-212 [DOI] [PubMed] [Google Scholar]
- 21.Perlman S, Schelper R, Bolger E, Ries D: Late onset, symptomatic, demyelinating encephalomyelitis in mice infected with MHV-JHM in the presence of maternal antibody. Microb Pathog 1987, 2:185-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pewe L, Heard SB, Bergmann CC, Dailey MO, Perlman S: Selection of CTL escape mutants in mice infected with a neurotropic coronavirus: quantitative estimate of TCR diversity in the infected CNS. J Immunol 1999, 163:6106-6113 [PubMed] [Google Scholar]
- 23.Peterman GM, Spencer C, Sperling AI, Bluestone JA: Role of γδ T cells in murine collagen-induced arthritis. J Immunol 1993, 151:6546-6558 [PubMed] [Google Scholar]
- 24.Mukasa A, Hiromatsu K, Matsuzaki G, O’Brien R, Born W, Nomoto K: Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of αβ and γδ T cells. J Immunol 1995, 155:2047-2056 [PubMed] [Google Scholar]
- 25.Xue S, Sun N, van Rooijen N, Perlman S: Depletion of blood-borne macrophages does not reduce demyelination in mice infected with a neurotropic coronavirus. J Virol 1999, 73:6327-6334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perlman S, Jacobsen G, Olson AL, Afifi A: Identification of the spinal cord as a major site of persistence during chronic infection with a murine coronavirus. Virology 1990, 175:418-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang F-I, Hinton D, Gilmore W, Trousdale M, Fleming JO: Sequential infection of glial cells by the murine hepatitis virus JHM strain (MHV-4) leads to a characteristic distribution of demyelination. Lab Invest 1992, 66:744-754 [PubMed] [Google Scholar]
- 28.Bergmann CC, Altman JD, Hinton D, Stohlman SA: Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J Immunol 1999, 163:3379-3387 [PubMed] [Google Scholar]
- 29.Wen L, Roberts SJ, Viney JL, Wong FS, Mallick C, Findly RC, Peng Q, Craft JE, Owen MJ, Hayday AC: Immunoglobulin synthesis and generalized autoimmunity in mice congenitally deficient in αβ(+) T cells. Nature 1994, 369:654-658 [DOI] [PubMed] [Google Scholar]
- 30.MacDonald HR, Lees RK, Sordat B, Zaech P, Maryanski JL, Bron C: Age-associated increase in expression of the T cell surface markers Thy-1, Lyt-1, and Lyt-2 in congenitally athymic (nu/nu) mice: analysis by flow microfluorometry. J Immunol 1981, 126:865-870 [PubMed] [Google Scholar]
- 31.Maleckar JR, Sherman LA: The composition of the T cell receptor repertoire in nude mice. J Immunol 1987, 138:3873-3876 [PubMed] [Google Scholar]
- 32.Salerno A, Dieli F: Role of γδ T lymphocytes in immune response in humans and mice. Crit Rev Immunol 1998, 18:327-357 [DOI] [PubMed] [Google Scholar]
- 33.Wildner G, Hunig T, Thurau SR: Orally induced, peptide-specific γ/δ TCR+ cells suppress experimental autoimmune uveitis. Eur J Immunol 1996, 26:2140-2148 [DOI] [PubMed] [Google Scholar]
- 34.Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K: Aerosol insulin induces regulatory CD8 γδ T cells that prevent murine insulin-dependent diabetes. J Exp Med 1996, 184:2167-2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battistini L, Selmaj K, Kowal C, Ohmen J, Modlin RL, Raine CS, Brosnan CF: Multiple sclerosis: limited diversity of the Vδ2-Jδ3 T-cell receptor in chronic active lesions. Ann Neurol 1995, 37:198-203 [DOI] [PubMed] [Google Scholar]
- 36.Hvas J, Oksenberg JR, Fernando R, Steinman L, Bernard CC: γδ T cell receptor repertoire in brain lesions of patients with multiple sclerosis. J Neuroimmunol 1993, 46:225-234 [DOI] [PubMed] [Google Scholar]
- 37.Shimonkevitz R, Colburn C, Burnham JA, Murray RS, Kotzin BL: Clonal expansions of activated γ/δ T cells in recent-onset multiple sclerosis. Proc Natl Acad Sci USA 1993, 90:923-927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA: γδ T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci USA 1992, 89:4588-4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kageyama Y, Koide Y, Miyamoto S, Inoue T, Yoshida TO: The biased Vγ gene usage in the synovial fluid of patients with rheumatoid arthritis. Eur J Immunol 1994, 24:1122-1129 [DOI] [PubMed] [Google Scholar]
- 40.Chomarat P, Kjeldsen-Kragh J, Quayle AJ, Natvig JB, Miossec P: Different cytokine production profiles of γδ T cell clones: relation to inflammatory arthritis. Eur J Immunol 1994, 24:2087-2091 [DOI] [PubMed] [Google Scholar]
- 41.Spahn TW, Issazadah S, Salvin AJ, Weiner HL: Decreased severity of myelin oligodendrocyte glycoprotein peptide 33-35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR δ chain gene. Eur J Immunol 1999, 29:4060-4071 [DOI] [PubMed] [Google Scholar]
- 42.Rajan AJ, Gao YL, Raine CS, Brosnan CF: A pathogenic role for γδ T cells in relapsing-remitting experimental allergic encephalomyelitis in the SJL mouse. J Immunol 1996, 157:941-949 [PubMed] [Google Scholar]
- 43.Haring JS, Pewe LL, Perlman S: High magnitude, virus-specific CD4 T-cell response in the central nervous system of coronavirus-infected mice. J Virol 2001, 75:3043-3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stohlman SA, Bergmann CC, Lin MT, Cua DJ, Hinton DR: CTL effector function within the central nervous system requires CD4+ T cells. J Immunol 1998, 160:2896-2904 [PubMed] [Google Scholar]
- 45.Castro RF, Evans GD, Jaszewski A, Perlman S: Coronavirus-induced demyelination occurs in the presence of virus-specific cytotoxic T cells. Virology 1994, 200:733-743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC: Regulation of cutaneous malignancy by γδ T cells. Science 2001, 294:605-60911567106 [Google Scholar]
- 47.Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA: T cell receptor-γ/δ cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med 1997, 185:1969-1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sciammas R, Bluestone JA: HSV-1 glycoprotein I-reactive TCR γδ cells directly recognize the peptide backbone in a conformationally dependent manner. J Immunol 1998, 161:5187-5192 [PubMed] [Google Scholar]
- 49.Hiromatsu K, Yoshikai Y, Matsuzaki G, Ohga S, Muramori K, Matsumoto K, Bluestone JA, Nomoto K: A protective role of γ/δ T cells in primary infection with Listeria monocytogenes in mice. J Exp Med 1992, 175:49-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selin LK, Santolucito PA, Pinto AK, Szomolanyi-Tsuda E, Welsh RM: Innate immunity to viruses: control of vaccinia virus infection by γδ T cells. J Immunol 2001, 166:6784-6794 [DOI] [PubMed] [Google Scholar]
- 51.Pewe LL, Perlman S: CD8 T cell-mediated demyelination is IFN-γ dependent in mice infected with a neurotropic coronavirus. J Immunol 2002, 168:1547-1551 [DOI] [PubMed] [Google Scholar]
- 52.Lane TE, Liu MT, Chen BP, Asensio VC, Samawi RM, Paoletti AD, Campbell IL, Kunkel SL, Fox HS, Buchmeier MJ: A central role for CD4+ T-cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol 2000, 74:1415-1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramakrishna C, Stohlman SA, Atkinson RD, Shlomchik MJ, Bergmann CC: Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J Immunol 2002, 168:1204-1211 [DOI] [PubMed] [Google Scholar]
- 54.Wysocka M, Korngold R, Yewdell J, Bennink J: Target and effector cell fusion accounts for B lymphocyte-mediated lysis of mouse hepatitis virus-infected cells. J Gen Virol 1989, 70:1465-1472 [DOI] [PubMed] [Google Scholar]
- 55.Morales S, Parra B, Ramakrishna C, Blau DM, Stohlman SA: B-cell-mediated lysis of cells infected with the neurotropic JHM strain of mouse hepatitis virus. Virology 2001, 286:160-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE: Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 2000, 1:475-482 [DOI] [PubMed] [Google Scholar]