Abstract
Caveolin-1 (Cav-1) is the principal structural protein of caveolae membranes that are found in most cells types, including mammary epithelial cells. Recently, we mapped the human CAV1 gene to a suspected tumor suppressor locus (7q31.1/D7S522) that is deleted in a variety of human cancers, as well as mammary tumors. In addition, the CAV1 gene is mutated (P132L) in up to ∼16% of human breast cancers. The mechanism by which deletion or mutation of the Cav-1 gene contributes to mammary tumorigenesis remains unknown. To understand the role of the Cav-1 (P132L) mutation in the pathogenesis of human breast cancers, we generated the same mutation in wild-type (WT) Cav-1 and studied its behavior in cultured cells. Interestingly, the P132L mutation leads to formation of misfolded Cav-1 oligomers that are retained within the Golgi complex and are not targeted to caveolae or the plasma membrane. To examine whether the Cav-1 (P132L) mutant behaves in a dominant-negative manner, we next co-transfected cells with Cav-1 (P132L) and WT Cav-1, and evaluated their caveolar targeting. Our results indicate that Cav-1 (P132L) behaves in a dominant-negative manner, causing the mislocalization and intracellular retention of WT Cav-1. Virtually identical results were obtained when Cav-1 (P132L) was stably expressed at physiological levels in a nontransformed human mammary epithelial cell line (hTERT-HME1). These data provide a molecular explanation for why only a single mutated CAV1 allele is found in patients with breast cancer. Thus, we next investigated if functional inactivation of Cav-1 gene expression leads to mammary tumorigenesis in vivo. For this purpose, we performed mammary gland analysis on Cav-1-deficient mice (−/−) that harbor a targeted disruption of the Cav-1 gene (a null mutation). Interestingly, we show that inactivation of Cav-1 gene expression leads to mammary epithelial cell hyperplasia, even in 6-week-old virgin female mice. These data clearly implicate loss of functional Cav-1 in the pathogenesis of mammary epithelial cell hyperplasia, and suggest that Cav-1-null mice represent a novel animal model to study premalignant mammary disease.
During adult life, the mammary gland undergoes multiple cycles of proliferation, lactogenic differentiation, and regression. As such, normal mammary gland development involves complex interactions between growth factors and their receptors, steroidogenic nuclear hormone receptors, and proto-oncogenes, as well as tumor suppressor genes. 1-3
Adult mammary gland development consists of four separate stages: 1) nonpregnant, 2) gestation, 3) lactation, and 4) involution. During gestation, rapid lobulo-alveolar outgrowth occurs. Proliferation and functional differentiation of the secretory epithelium are hallmarks of lactation. Finally, at the end of weaning, lactation is suppressed and leads to involution of the lobulo-alveolar compartment. Involution returns the mammary gland to its nonpregnant state. 4 Thus, dysregulation of the factors that govern this cyclical developmental process can lead to mammary epithelial hyperplasia, and ultimately to mammary tumor formation. 5
Sager and colleagues 6 initially suggested a role for caveolin-1 (Cav-1) in breast cancer development. Using differential display and subtractive hybridization techniques, they identified a number of “candidate tumor suppressor genes,” ie, genes whose mRNAs were down-regulated in human mammary adenocarcinoma-derived cells. By using this screening approach, Cav-1 was independently identified as 1 of 26 gene products down-regulated during mammary tumorigenesis. Furthermore, they reported that Cav-1 expression was severely reduced or absent in several transformed human mammary epithelial cell lines (MT-1, MCF-7, ZR-75-1, T47D, MDA-MB-361, and MDA-MB-474). In striking contrast, Cav-1 mRNA and protein are abundant in normal human mammary epithelial cells. 5-9
Additional evidence has now accumulated supporting the idea that Cav-1 may function as a mammary gland tumor suppressor. Lee and colleagues 10 showed that recombinant expression of Cav-1 in a human breast cancer-derived cell line (T-47D) induces a 50% reduction in cell proliferation and leads to a 15-fold reduction in anchorage-independent growth; and Cav-1 expression also diminishes the metastatic potential of the mammary tumor cell line, MTLn3. 8 MTLn3 cells were originally derived from a metastatic rat mammary adenocarcinoma. MTLn3 cells undergo lamellipodia extension and increased chemotaxis in response to epidermal growth factor (EGF). However, recombinant expression of Cav-1 in MTLn3 cells blocks both EGF-induced lamellipodia extension and cell migration. As such, Cav-1 expression in MTLn3 cells induces a nonmotile phenotype. 8
For more than 5 years now, it has been recognized that a certain locus (D7S522; 7q31.1) is an aphidicolin-induced fragile site in the human genome 11,12 and a hot spot for deletions in a variety of human epithelial cell tumors, including breast cancers. 7,13 Interestingly, the human CAV1 gene maps to 7q31.1, adjacent to the loss of heterozygosity marker D7S522, and as of yet, still remains the closest known gene to this putative tumor suppressor locus. 7
In addition, a recent report indicates that the human Cav-1 gene is heterozygously mutated in up to 16% of human breast cancer samples examined. 14 Recombinant expression of the Cav-1 cDNA harboring this mutation (P132L) was sufficient to transform NIH 3T3 cells. 14 As similar results have been previously obtained using an anti-sense approach to ablate Cav-1 expression, 15 these results suggest that the Cav-1 (P132L) mutation may behave in a dominant-negative manner. However, this hypothesis remains untested. Interestingly, an analogous mutation occurs within the caveolin-3 gene (P104L) in patients with a novel form of autosomal dominant limb-girdle muscular dystrophy (LGMD-1C). 16
To gain a better understanding of the role of Cav-1 mutations (P132L and null) in the pathogenesis of human breast cancers, we studied the phenotypic behavior of Cav-1 (P132L) in cultured cells and we performed mammary gland analysis on Cav-1-deficient mice that harbor a targeted disruption of the Cav-1 gene (a null mutation). Taken together, our results implicate a loss of functional Cav-1 expression in the pathogenesis of mammary epithelial cell hyperplasia, in an in vivo setting.
Materials and Methods
Materials
Antibodies and their sources were as follows: anti-Cav-1 IgG [mouse monoclonal antibody (mAb) 2297 17 for Western blot analysis; gift of Dr. Roberto Campos-Gonzalez, BD Transduction Laboratories]; anti-Cav-1 IgG [rabbit polyclonal antibody (pAb) for immunofluorescence microscopy; Santa Cruz Biotech, Inc., Santa Cruz, CA]; anti-Cab45 IgG (rabbit pAb, 18 gift of Dr. Philipp E. Scherer, Albert Einstein College of Medicine); anti-Myc IgG (mouse mAb 9E10; Santa Cruz Biotech, Inc.); and anti-green fluorescent protein (GFP) (rabbit pAb; Santa Cruz Biotech, Inc.). 4,6-Diamidino-2-phenylindole (DAPI) was obtained from Molecular Probes, Inc. (Eugene, OR) and a 100-μg/ml stock solution was prepared using deionized water. All other biochemicals used were of the highest purity available and were obtained from regular commercial sources.
Construction of Myc-Tagged WT and P132L Cav-1
The cDNA encoding wild-type (WT) C-terminally Myc-tagged Cav-1 (WT Cav-1; 17,19 ) was cloned into the pCAGGS expression vector (gift of Dr. Armin Rehm, Ploegh Laboratory, Harvard Medical School, Boston, MA). C-terminally Myc-tagged Cav-1 (P132L) was generated by polymerase chain reaction amplification using appropriate internal primers and subcloned into the pCAGGS expression vector. The correctness of intended base substitutions and the absence of unwanted mutations were verified by DNA sequencing. The cDNA encoding GFP-Cav-1-WT was as we extensively characterized and described previously. 20
Cell Culture and Transient Transfection
Human embryonic kidney 293T and COS-7 cells were propagated in Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum, 2 mmol/L glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. HEK-293T cells were transfected by calcium phosphate precipitation, whereas COS-7 cells were transfected with the Effectene reagent (Qiagen, Valencia, CA).
Velocity Gradient Centrifugation
Samples were dissociated in MES-buffered saline containing 60 mmol/L octyl-glucoside. Solubilized material was loaded atop a 5 to 40% linear sucrose gradient and centrifuged at 50,000 rpm (34,000 × g) for 10 hours in a SW60 rotor (Beckman Instruments, Fullerton, CA). 21-24 Gradient fractions were collected from above and subjected to immunoblot analysis. Molecular mass standards for velocity gradient centrifugation were as described previously. 21-24
Preparation of Caveolae-Enriched Membrane Fractions
Transfected cells were scraped into 2 ml of MES-buffered saline (MBS; 25 mmol/L MES, pH 6.5, 0.15 mol/L NaCl) containing 1% (v/v) Triton X-100. 17,19,25-33 Homogenization was performed with 10 strokes of a loose-fitting Dounce homogenizer. The homogenate was adjusted to 40% sucrose by the addition of 2 ml of 80% sucrose prepared in MBS and placed at the bottom of an ultracentrifuge tube. A 5 to 30% linear sucrose gradient was formed above the homogenate and centrifuged at 39,000 rpm for 16 to 20 hours in a SW41 rotor (Beckman Instruments). A light-scattering band confined to the 15 to 20% sucrose region was observed that contained Cav-1, but excluded most of the other cellular proteins. From the top of each gradient, 1-ml gradient fractions were collected to yield a total of 12 fractions. An equal volume from each gradient fraction was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to immunoblot analysis.
Immunoblot Analysis
Cellular proteins were resolved by SDS-PAGE (12.5% acrylamide) and transferred to nitrocellulose membranes. Blots were incubated for 2 hours in TBST (10 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 0.2% Tween 20) containing 2% powdered skim milk and 1% bovine serum albumin. After three washes with TBST, membranes were incubated for 2 hours with the primary antibody (∼1000-fold diluted in TBST) and for 1 hour with horseradish peroxidase-conjugated goat anti-rabbit/mouse IgG (∼5000-fold diluted). Proteins were detected using the ECL detection kit (Amersham, Arlington Heights, IL).
Immunofluorescence Microscopy
Transfected cells grown on glass coverslips were washed three times with phosphate-buffered saline (PBS) and fixed for 30 minutes at room temperature with 2% paraformaldehyde in PBS. Fixed cells were rinsed with PBS and permeabilized with 0.1% Triton X-100, 0.2% bovine serum albumin for 10 minutes. Then cells were treated with 25 mmol/L of NH4Cl in PBS for 10 minutes at room temperature to quench free aldehyde groups. Cells were rinsed with PBS and incubated with the primary antibodies for 1 hour at room temperature. After three washes with PBS (10 minutes each), cells were incubated with the secondary antibody for 1 hour at room temperature: lissamine rhodamine B sulfonyl chloride-conjugated goat anti-rabbit antibody (5 μg/ml) and fluorescein isothiocyanate-conjugated goat anti-mouse antibody (5 μg/ml). Finally, cells were washed three times with PBS (10 minutes each wash), slides were mounted with slow-fade anti-fade reagent (Molecular Probes) and observed under a Bio-Rad MR 600 confocal microscope (Bio-Rad, Richmond, CA.
Co-Expression of WT Cav-1 and Cav-1 (P132L)
Cos-7 cells were transiently co-transfected with Myc-tagged Cav-1 (P132L) and GFP-tagged WT Cav-1. Cells were also co-transfected with Myc-tagged and GFP-tagged forms of WT Cav-1 for comparison. Cells grown on glass coverslips were then fixed and immunostained to visualize the cellular distribution of Cav-1, using double labeling with antibodies directed against the Myc- and GFP-epitope tags.
Origin and Cell Culture Conditions for hTERT-HME1 Cells
hTERT-HME1 cells (human telomerase-immortalized human mammary epithelial cells) were purchased from Clontech, Inc., Palo Alto, CA (catalog no. C4002-1). hTERT-HME1 cells were grown in mammary epithelium basal medium (catalog no. CC-3151; Clonetics, San Diego, CA) supplemented with an MEGM bullet kit (containing 52 μg/ml bovine pituitary extract, 0.5 μg/ml hydrocortisone, 10 ng/ml hEGF, 5 μg/ml insulin, and 50 μg/ml gentamicin; catalog no. CC-4136, Clonetics).
Derivation of hTERT-HME1 Cells Stably Expressing Cav-1 (WT and P132L)
The cDNAs encoding C-terminally Myc-tagged Cav-1 (WT and P132L) were subcloned into the pBABE-retroviral expression vector (using the MCS: BamHI/EcoRI), that contains a puromycin resistance marker. Phoenix cells (a packaging cell line) were then transiently transfected with the pBABE-Cav-1 vectors, using a modified calcium-phosphate precipitation protocol. Forty-eight hours after transfection, the culture media containing retrovirus was collected and filtered using a 0.45-μm filter. Retrovirus containing culture media was then mixed with polybrene (at a final concentration of 4 μg/ml) and used to infect hTERT-HME1 cells. Forty-eight hours after infection, hTERT-HME1 cells were switched to selection media [mammary epithelium basal medium containing puromycin (2.5 μg/ml)]. As a consequence, a stable pool was selected with >90% of the cells expressing either WT Cav-1 or Cav-1 (P132L).
Generation and Maintenance of Cav-1-Deficient Mice
The strategy used to target the Cav-1 locus and generate Cav-1 null mice was as previously described. 34,35 All animals used in these studies (mice homozygous null for the Cav-1 gene and their WT littermates) were in a C57BL/6 genetic background and were genotyped by polymerase chain reaction, as previously described. 34,35 WT and Cav-1 null mice were generated through heterozygous matings. Housing and maintenance was provided by the Albert Einstein College of Medicine barrier facility; mice were kept on a 12-hour light/dark cycle and had ad libitum access to chow (Picolab 20; PMI Nutrition International) and water. All animal protocols used in this study were preapproved by the Albert Einstein College of Medicine Institute for Animal Studies.
Nuclear Staining of Paraffin Sections from Murine Mammary Glands
Tissue sections derived from WT and Cav-1 null mice were deparaffinized in xylene for 4 minutes and rehydrated through a graded series of ethanol and placed in PBS. For the nuclear staining, DAPI (1 μg/ml in PBS) was added for 15 minutes at room temperature. The sections were then washed in PBS for 15 minutes. Slow-fade anti-fade reagent was added to prevent bleaching. Samples were then imaged with an Olympus inverted microscope.
Results
Recombinant Expression of Cav-1 (P132L) in Cultured Cells
To begin to understand the role of the Cav-1 (P132L) mutation in the pathogenesis of human breast cancers, we generated the same mutation in WT Cav-1 subcloned into the mammalian expression vector pCAGGS (Figure 1A) ▶ . A Myc epitope-tag was placed at its extreme C-terminus, to allow us to distinguish Cav-1 (P132L) expression from endogenous Cav-1. For comparison, a pCAGGS vector expressing C-terminally Myc-tagged WT Cav-1 was constructed as well. It has been previously well-documented that the placement of a Myc-epitope tag at the N- or C-terminus of Cav-1 does not interfere with its correct functioning, oligomerization, or proper targeting to caveolae membrane domains. 17,36-39
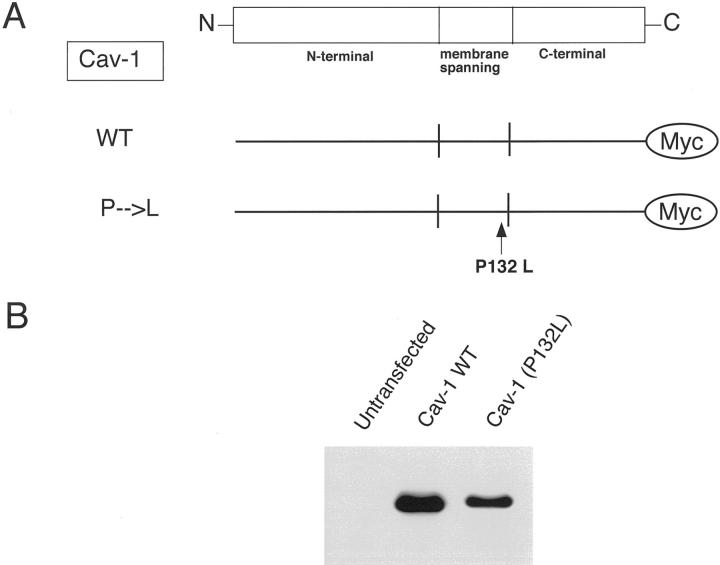
Figure 1.
Construction and expression of Cav-1 (P132L), a mutant found in human breast cancers. A: Schematic diagram. The Cav-1 (P132L) mutation is as indicated. Note that the mutation occurs within the membrane-spanning domain of Cav-1. Both WT and mutant forms contain a C-terminal Myc-epitope tag. B: Western blot analysis. WT Cav-1 and Cav-1 (P132L), subcloned into the pCAGGS expression vector, were transiently expressed in HEK-293T cells, and their expression level was assessed by immunoblotting with a specific mAb probe directed against the Myc-epitope tag (mAb 9E10). Note that Cav-1 (P132L) is expressed at slightly lower levels than WT Cav-1, ie, at ∼85% of WT levels.
We next transiently transfected this Cav-1 (P132L) mutant into HEK-293T cells and assessed its expression relative to WT Cav-1 by Western blot analysis with a specific mAb probe directed against the Myc-epitope tag (mAb 9E10) (Figure 1B) ▶ . Our results indicate that both WT and mutant forms of Cav-1 are well expressed, but that the Cav-1 (P132L) mutant is expressed at slightly lower levels than achieved with WT Cav-1 (∼85% of WT levels); similar results were obtained with Cos-7 cells (data not shown). However, this did not impede our analysis of the phenotypic behavior of this Cav-1 mutant in cultured cells.
Oligomeric State and Caveolar Targeting of Cav-1 (P132L)
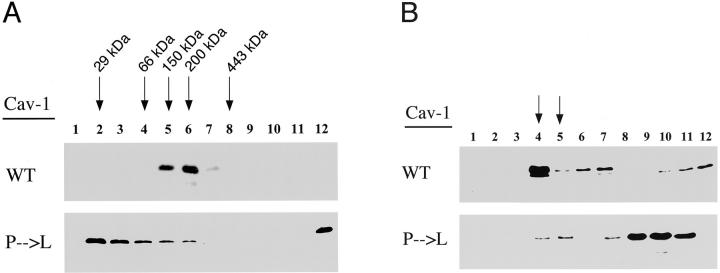
We next investigated the oligomeric state of Cav-1 (P132L). For this purpose, we used an established velocity gradient system developed previously to study the oligomeric state of Cav-1, -2, and -3. 21,22,24 Figure 2A ▶ shows that WT Cav-1 behaved as a high-molecular mass complex, migrating between the 150- and 200/443-kd molecular mass standards (peak fractions 5 and 6). In contrast, Cav-1 (P132L) migrated predominantly as a monomer/dimer and as a high-molecular mass oligomer of >443 kd, forming high-molecular mass aggregates. Our results dramatically show that the Cav-1 (P132L) mutation adversely affects the oligomerization process.
Figure 2.
Oligomeric state and caveolar targeting of Cav-1 (P132L). A: Velocity gradient centrifugation analysis of the oligomeric state of Cav-1 (P132L). HEK-293T cells were transiently transfected with WT Cav-1 or Cav-1 (P132L). After extraction in a buffer containing 60 mmol/L of octyl-glucoside, the solubilized material was loaded atop a 5 to 40% linear sucrose gradient and centrifuged. Gradient fractions were collected from above and subjected to immunoblot analysis with a specific mAb probe directed against the Myc-epitope tag (mAb 9E10). Note that WT Cav-1 behaved as a high-molecular mass complex, migrating between the 150- and 443-kd molecular mass standards (peak fractions 5 and 6). In contrast, Cav-1 (P132L) migrated predominantly as a monomer/dimer (fractions 2 to 3) and as a high-molecular mass aggregate (fraction 12). Molecular mass standards for velocity gradient centrifugation are as indicated. B: Cav-1 (P132L) is excluded from caveolae-enriched membrane fractions. HEK-293T cells were transiently transfected with either WT Cav-1 or Cav-1 (P132L). To separate membranes enriched in lipid rafts/caveolae from the bulk of cellular membranes and cytosolic proteins, an established equilibrium sucrose density gradient system was used. In this fractionation scheme, immunoblotting with anti-Cav-1 IgG can be used to track the position of caveolae-derived membranes within these bottom-loaded sucrose gradients. Gradient fractions were collected from above and subjected to immunoblot analysis with a specific mAb probe directed against the Myc-epitope tag (mAb 9E10). Note that WT Cav-1 is correctly targeted to these low-density Triton-insoluble membranes (fractions 4 and 5; arrows) that are enriched in caveolae. In contrast, Cav-1 (P132L) is predominantly excluded from these caveolae-enriched fractions.
To separate membranes enriched in caveolae from the bulk of cellular membranes and cytosolic proteins, an established equilibrium sucrose density gradient system was used. 17,19,25-33 In this fractionation scheme, immunoblotting with anti-Cav-1 IgG can be used to track the position of caveolae-derived membranes within these bottom-loaded sucrose gradients.
Figure 2B ▶ illustrates that in this fractionation scheme WT Cav-1 is correctly targeted to these low-density Triton-insoluble membranes (fractions 4 and 5) that are enriched in caveolae membranes. In contrast, Cav-1 (P132L) was predominantly excluded from these caveolae-enriched fractions. These results indicate that the Cav-1 (P132L) mutation clearly prevents the incorporation of Cav-1 into caveolae membranes.
Immunolocalization of the Cav-1 (P132L) Mutant to a Perinuclear Intracellular Compartment
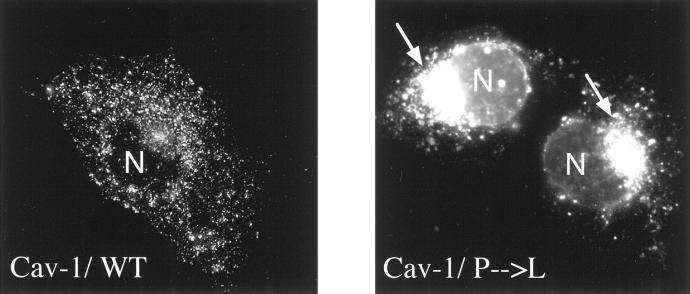
As the Cav-1 (P132L) mutant was excluded from caveolae membranes, we next determined its subcellular localization by immunofluorescence using confocal microscopy. Figure 3 ▶ shows the localization of Cav-1 (P132L). The distribution of WT Cav-1 is shown for comparison. Note that Cav-1 (P132L) is primarily retained intracellularly in a perinuclear compartment and did not reach the plasma membrane. We identified this perinuclear compartment as the Golgi complex (data not shown) by performing double-labeling experiments with antibodies directed against the resident Golgi marker protein, Cab45, that is endogenously expressed. 18 In contrast, WT Cav-1 was efficiently targeted to the plasma membrane under these conditions.
Figure 3.
Cav-1 (P132L) is retained intracellularly in a perinuclear compartment. Cos-7 cells were transiently transfected with either WT Cav-1 or Cav-1 (P132L). Cells grown on glass coverslips were then fixed and immunostained with anti-Cav-1 IgG (pAb; N-20) to visualize the cellular distribution of Cav-1. Note that Cav-1 (P132L) is primarily retained intracellularly in a perinuclear compartment and did not reach the plasma membrane. In contrast, WT Cav-1 was efficiently targeted to the plasma membrane.
The Cav-1 (P132L) Mutant Behaves in a Dominant-Negative Manner
Expression of Cav-1 (P132L) Prevents the Correct Targeting of WT Cav-1 to Caveolae Membrane Domains
To examine whether the Cav-1 (P132L) mutant behaves in a dominant-negative manner, we next co-transfected HEK-293T cells with Myc-tagged Cav-1 (P132L) and untagged WT Cav-1 and evaluated their caveolar targeting. It should be noted that addition of the Myc-tag allowed us to distinguish between tagged and untagged forms of Cav-1, as the Myc-tagged form of Cav-1 migrates at a slightly higher molecular weight that is distinguishable by SDS-PAGE. 40 Cells were also co-transfected with Myc-tagged and untagged forms of WT Cav-1 for comparison.
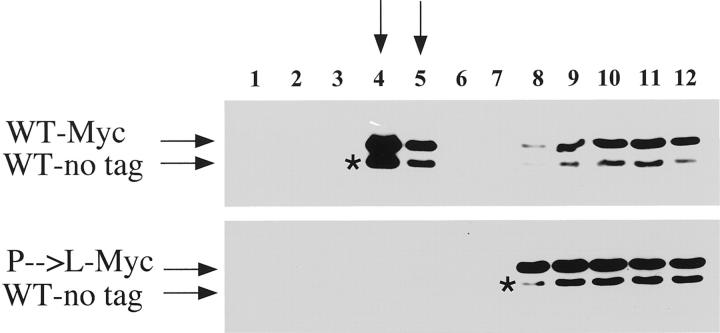
Figure 4 ▶ shows that in cells co-expressing Myc-tagged Cav-1 (P132L) and untagged WT Cav-1, both are excluded from caveolae-membrane domains (Figure 4 ▶ , bottom). In contrast, in cells co-expressing Myc-tagged and untagged forms of WT Cav-1, both are properly targeted to caveolae membrane domains (Figure 4 ▶ , top). These results clearly indicate that the Cav-1 (P132L) mutation behaves in a dominant-negative manner, causing the mistargeting of WT Cav-1.
Figure 4.
Expression of Cav-1 (P132L) prevents the correct targeting of WT Cav-1 to caveolae membrane domains. HEK-293T cells were transiently co-transfected with Myc-tagged Cav-1 (P132L) and untagged WT Cav-1. Cells were also co-transfected with Myc-tagged and untagged forms of WT Cav-1 for comparison. To separate membranes enriched in lipid rafts/caveolae from the bulk of cellular membranes and cytosolic proteins, an established equilibrium sucrose density gradient system was used. In this fractionation scheme, immunoblotting with anti-Cav-1 IgG (pAb; N-20) can be used to track the position of caveolae-derived membranes within these bottom-loaded sucrose gradients. Note that in cells co-expressing Myc-tagged Cav-1 (P132L) and untagged WT Cav-1, both forms are excluded from caveolae-membrane domains. In contrast, in cells co-expressing Myc-tagged and untagged forms of WT Cav-1, both are properly targeted to caveolae membrane domains. The position of untagged WT Cav-1 is indicated by an asterisk (*).
Expression of Cav-1 (P132L) Causes the Intracellular Retention of WT Cav-1
Complementary experiments using co-expression of Cav-1 (P132L) and WT Cav-1 within a single cell show that both forms are retained intracellularly—at the level of the Golgi complex (Figure 5) ▶ .
Figure 5.
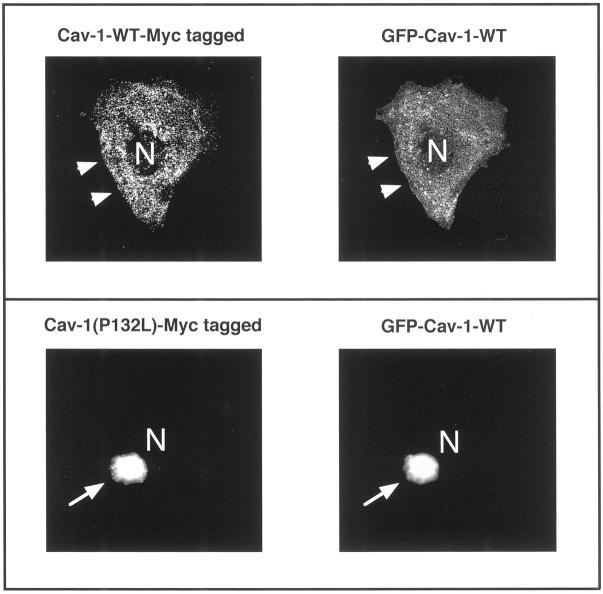
Expression of Cav-1 (P132L) causes the intracellular retention of WT Cav-1. Cos-7 cells were transiently co-transfected with Myc-tagged and GFP-tagged forms of Cav-1. Cells grown on glass coverslips were then fixed and immunostained to visualize the cellular distribution of Cav-1, using double labeling with antibodies directed against the Myc- and GFP-epitope tags. Top: Myc-tagged Cav-1-WT and GFP-tagged Cav-1-WT. Note that in cells co-expressing Myc-tagged and GFP-tagged forms of WT Cav-1, both are properly targeted to the plasma membrane (arrowheads). N, Nucleus. Bottom: Myc-tagged Cav-1 (P132L) and GFP-tagged Cav-1-WT. Note that in cells co-expressing Myc-tagged Cav-1 (P132L) and GFP-tagged Cav-1-WT, both forms are retained intracellularly in a perinuclear Golgi compartment (arrows). N, Nucleus.
Briefly, Cos-7 cells were transiently co-transfected with Myc-tagged Cav-1 (P132L) and GFP-tagged WT Cav-1. Cells were also co-transfected with Myc-tagged and GFP-tagged forms of WT Cav-1 for comparison. Cells grown on glass coverslips were then fixed and immunostained to visualize the cellular distribution of Cav-1, using double labeling with antibodies directed against the Myc- and GFP-epitope tags.
Interestingly, in cells co-expressing Myc-tagged Cav-1 (P132L) and GFP-tagged WT Cav-1, both forms are retained intracellularly in a perinuclear Golgi compartment (Figure 5 ▶ ; bottom, arrows). In contrast, in cells co-expressing Myc-tagged and GFP-tagged forms of WT Cav-1, both are properly targeted to the plasma membrane (Figure 5 ▶ ; top, arrowheads).
Recombinant Expression of Cav-1 (P132L) in Normal Human Mammary Epithelial Cells Causes the Intracellular Retention of Endogenous Cav-1
To further study the properties of the Cav-1 (P132L) mutation in the context of a mammary epithelial cell, we stably expressed Myc-tagged forms of WT Cav-1 or Cav-1 (P132L) in hTERT-HME1 cells, a normal (nontransformed) human mammary epithelial cell line that has been immortalized with human telomerase.
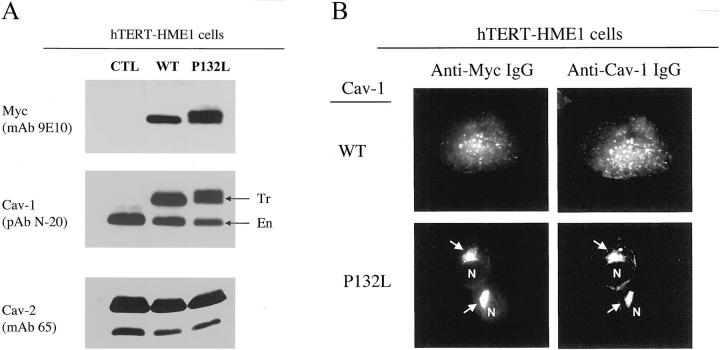
The cDNAs encoding C-terminally Myc-tagged Cav-1 (WT and P132L) were subcloned into the pBABE-retroviral expression vector, containing a puromycin resistance marker. Culture media containing retrovirus was then used to infect hTERT-HME1 cells. After selection in media containing puromycin, a stable cellular pool was selected with >90% of the cells expressing either WT Cav-1 or Cav-1 (P132L). These recombinant Cav-1 proteins were expressed at the same level as endogenous Cav-1, as seen by Western blot analysis (Figure 6A) ▶ ; also, expression of Cav-1 (P132L) did not affect the expression levels of endogenous Cav-1 and Cav-2, as compared with WT Cav-1.
Figure 6.
Recombinant stable expression of Cav-1 (P132L) in human mammary epithelial cells causes the intracellular retention of endogenous Cav-1. hTERT-HME1 cells were stably-transfected with Myc-tagged WT Cav-1 or Cav-1 (P132L). A: Western blot analysis. Cell lysates were prepared, separated by SDS-PAGE, and transferred to nitrocellulose. Blots were then probed with 1) anti-Myc IgG (mAb 9E10) to detect epitope-tagged forms of Cav-1 (WT and P132L); 2) anti-Cav-1 IgG (pAb N-20) to detect both endogenous (En) and transfected (Tr) forms of Cav-1 (arrows); and 3) anti-Cav-2 IgG (mAb 65) to detect endogenous Cav-2. As expected, Myc-tagged Cav-1 migrates slightly slower than endogenous Cav-1. Note that these recombinant Cav-1 (WT and P132L) proteins were expressed at the same levels as endogenous Cav-1; also, expression of Myc-tagged Cav-1 (P132L) did not affect the expression levels of endogenous Cav-1 and Cav-2, as compared with Myc-tagged WT Cav-1. B: Immunolocalization. Cells grown on glass coverslips were fixed and immunostained to visualize the cellular distribution of Cav-1, using double labeling with antibodies directed against the Myc-epitope (mAb 9E10) and Cav-1 (pAb N-20). Because these cell lines stably express Myc-tagged Cav-1 (WT and P132L) at the same level as endogenous Cav-1 (see panel A), ∼50% of the signal observed with anti-Cav-1 IgG must be because of the localization of endogenous Cav-1. Note that in cells expressing Myc-tagged Cav-1 (P132L), virtually all of the Cav-1 signal appears to be retained intracellularly in a perinuclear Golgi compartment (arrows). In contrast, in cells expressing Myc-tagged WT Cav-1, virtually all of the Cav-1 signal appears to be properly targeted to the plasma membrane. N, Nucleus.
Most importantly, Cav-1 (P132L) was retained intracellularly at the level of the Golgi complex, along with the bulk of endogenous Cav-1 (Figure 6B ▶ , bottom), consistent with the notion that Cav-1 (P132L) also behaves in a dominant-negative manner in the context of a mammary epithelial cell. However, expression of WT Cav-1 did not affect the distribution of endogenous Cav-1 (Figure 6B ▶ , top) and both remained associated with the plasma membrane.
Functional Inactivation of Cav-1 Gene Expression Leads to Mammary Epithelial Cell Hyperplasia in Cav-1-Null Mice in Vivo
Our results indicate that Cav-1 (P132L) behaves in a dominant-negative manner, causing the mislocalization and intracellular retention of WT Cav-1. These data provide a molecular explanation for why only a single mutated Cav-1 allele is found in patients with breast cancer. 14 Thus, we next investigated if functional inactivation of Cav-1 gene expression leads to mammary tumorigenesis in vivo. For this purpose, we performed mammary gland analysis on Cav-1-deficient mice 34,35 that harbor a targeted disruption of the Cav-1 gene (a null mutation).
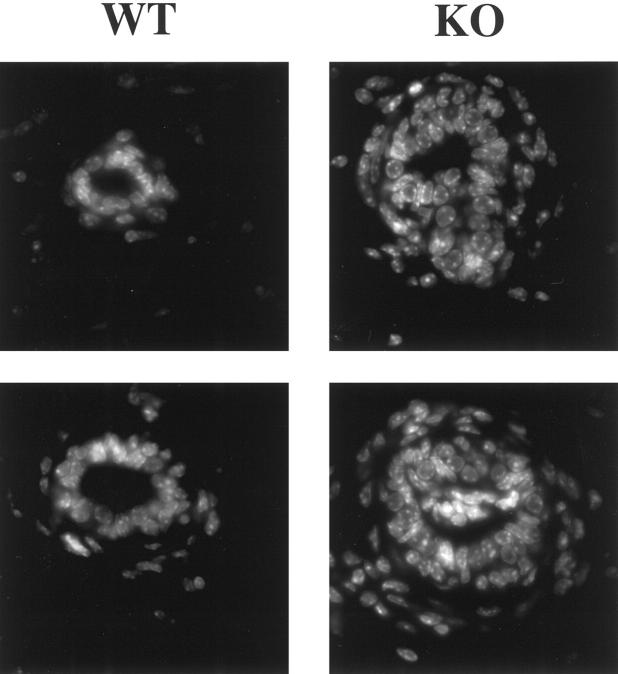
Mammary glands (no. 4) were harvested from virgin WT and Cav-1 knockout mice, paraffin embedded, and stained with hematoxylin and eosin. Interestingly, inactivation of Cav-1 gene expression leads to wide-spread mammary epithelial cell hyperplasia (a premalignant mammary lesion), even in 6-week-old virgin female mice (Figure 7) ▶ . These Cav-1 null mice show clear intraductal hyperplasia, ie, the epithelial cell layer is now at least approximately three to four cells thick (Figure 7) ▶ . This multilayering of the ductal epithelia in Cav-1 knockout mice was better appreciated when paraffin sections were treated with the nuclear stain, DAPI, which emits blue fluorescence on binding to AT-rich DNA regions (Figure 8) ▶ .
Figure 7.
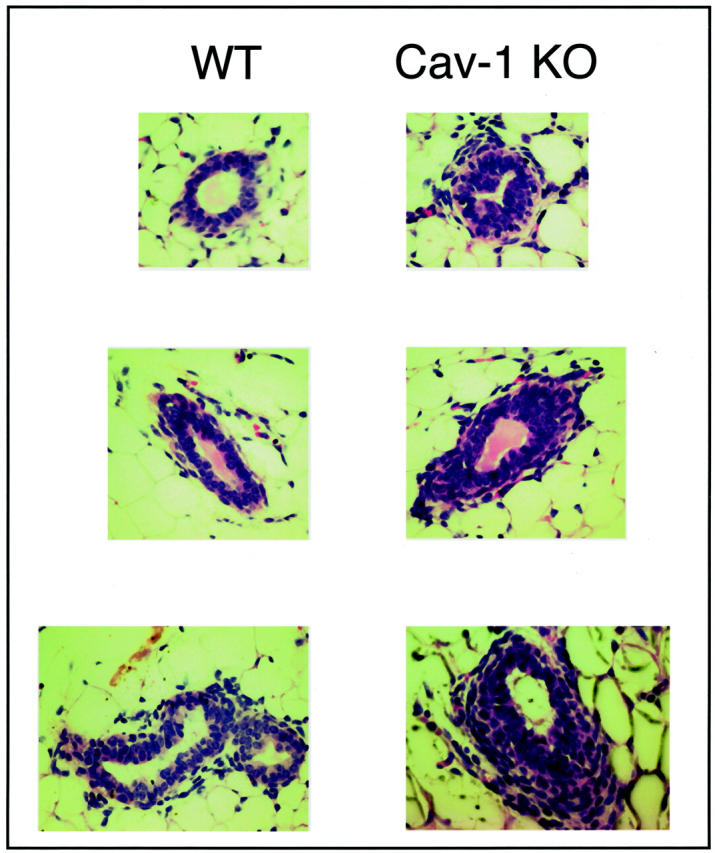
Cav-1-deficient mice show significant mammary epithelial cell hyperplasia. Mammary glands (no. 4) were harvested from 6-week-old virgin WT and Cav-1 knockout mice, paraffin-embedded, and stained with H&E. Note that Cav-1-null mice show intraductal hyperplasia, ie, the epithelial cell layer is now at least approximately three to four cells thick. Three different duct sizes are shown for each genotype.
Figure 8.
Intraductal hyperplasia in Cav-1-deficient mice as seen by DAPI staining. Mammary glands (no. 4) were harvested from 6-week-old virgin WT and Cav-1 knockout mice and paraffin-embedded. Sections were treated with a nuclear stain, DAPI, which emits blue fluorescence upon binding to AT-rich DNA regions. Note the intraductal hyperplasia with multilayering. Two examples are shown for each genotype.
Interestingly, Cav-1 null mice fail to spontaneously develop mammary tumors, even at up to 9 months of age. However, at this age, mammary glands from Cav-1 null mice (Figure 9, A and B) ▶ show 1) pronounced lobular development with numerous acini (up to 20) per terminal ductal lobular unit, 2) hyperplasia of the mammary epithelial lining, and 3) dramatic fibrosis. In contrast, age-matched WT mice (Figure 9A) ▶ show normal lobular development. Thus, loss of Cav-1 expression is sufficient to induce mammary epithelial cell hyperplasia, but not overt mammary tumorigenesis in mice.
Figure 9.
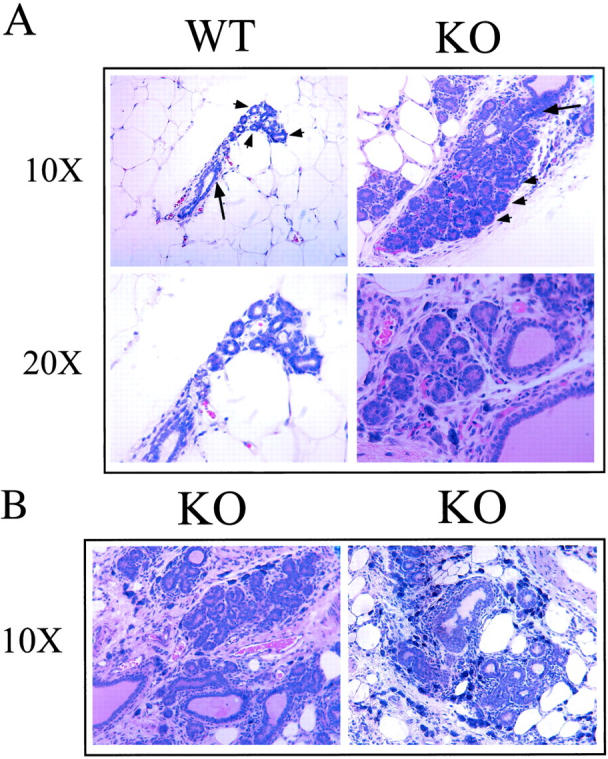
Mammary gland ductal morphology in 9-month-old Cav-1-deficient mice. Mammary glands (no. 4) were harvested from 9-month-old virgin WT and Cav-1 knockout mice, paraffin-embedded, and stained with H&E. A: In Cav-1 knockout mice, note the pronounced lobular development with numerous acini (up to 20) per terminal ductal lobular unit, and the hyperplasia of the epithelial lining. In contrast, WT mice show normal lobular development. Top: Arrows point at the duct and arrowheads point at the acini. Images taken with ×10 and ×20 objectives are shown, as indicated. B: Additional views of H&E sections derived from Cav-1 knockout mammary gland tissue. Images were taken with a ×10 objective.
Discussion
Deletions in the q31.1 region of human chromosome 7 have been implicated in the pathogenesis of many types of cancers. 11,12,41-55 These deletions were identified using loss of heterozygosity analysis, by using microsatellite markers that map to the 7q31.1 region, including D7S522. Deletions in the 7q31.1 region are closely situated near the D7S522 locus, thereby defining a smallest common deleted region of ∼1 Mb. 46,47,51,52,56 Loss of the D7S522 locus occurs in multiple epithelial tumors, including primary breast, 47 prostate, 12,46 ovarian, 57 colon, 51 and renal cell carcinomas. 11 D7S522 also spans a common fragile site, known as FRA7G. 11,12 Given the association of 7q31.1 and D7S522 loss of heterozygosity with the malignant tumorigenic phenotype, it has been proposed by a number of investigators that an unknown tumor suppressor gene may lie within the 7q31.1 region. Using fluorescent in situ hybridization analysis, we have now shown that the human genes encoding CAV1 and CAV2 co-localize to the 7q31.1 chromosomal region. 13,58,59 Specifically, DNA sequencing revealed that D7S522 is located ∼67 kb upstream of the Cav-2 gene and that the Cav-2 gene precedes the Cav-1 gene by ∼19 kb. 7
As the Cav-1 protein possesses transformation suppressor activity, 8,10,15,60-64 we and others have proposed that the CAV1 gene represents a novel tumor suppressor located at the D7S522 locus on human chromosome 7. 7,13,58,59,65 Several lines of evidence directly support this assertion: 1) Cav-1 mRNA and protein are down-regulated during oncogenic transformation of cultured NIH 3T3 cells, in tumors derived from mouse models of breast cancer, and in cell lines isolated from human breast cancers; 5-8,60,61,64 2) recombinant expression of Cav-1 in transformed NIH 3T3 cells or breast cancer cell lines suppresses their anchorage-independent growth; 8,10,61,64 and 3) targeted down-regulation of Cav-1 (using an anti-sense cDNA vector) promotes anchorage-independent cell growth, drives tumorigenesis in nude mice, and hyperactivates the p42/44 MAP kinase cascade in NIH 3T3 cells. 15 Importantly, transformation induced using the Cav-1 anti-sense vector is reversible, ie, when Cav-1 protein levels are restored to normal, the transformed phenotype is reverted. 15
Neu (c-ErbB2) is a proto-oncogene that encodes a receptor tyrosine kinase, most closely related to the EGF-receptor. At least two mechanisms have been implicated in Neu-mediated transformation of cultured fibroblasts and in human breast cancers in vivo; these include gene amplification of WT c-Neu and mutational activation of Neu (Neu T). Furthermore, mutational activation of c-Neu selectively down-regulates Cav-1 protein expression by approximately fourfold, both in NIH 3T3 and Rat 1a cells. 5 Also, dramatic reductions in Cav-1 expression occur in mammary tumors derived from MMTV-Neu/ErbB2 transgenic mice, as well as other transgenic lines that express down-stream effectors of Neu-mediated signaling [such as MMTV-Ras(G12V), MMTV-Myc, and MMTV-Src mice]; however, Neu-mediated transformation does not completely eliminate Cav-1 expression. 5 Importantly, recombinant expression of Cav-1 effectively blocks Neu-mediated signal transduction in cultured cells. These results directly demonstrate that a negative reciprocal relationship exists between c-Neu tyrosine kinase activity and Cav-1 protein expression. In addition, the caveolin-scaffolding domain (a 20-amino acid peptide derived from Cav-1; residues 82 to 101) dramatically inhibited Neu-autophosphorylation, as measured using an in vitro kinase assay. 5 Interestingly, Herceptin (Genentech, Inc.), an antibody that blocks the function of c-Neu (also known as HER2), is an effective treatment for human breast cancers. As Cav-1 expression also inhibits c-Neu signal transduction, Cav-1-based therapeutic approaches may provide a fruitful and independent avenue for the treatment of breast cancer in humans.
To further investigate the possible clinical significance of Cav-1 in mammary tumorigenesis, Hayashi and colleagues 14 screened 92 human breast cancer samples for a mutation in the CAV1 gene. Their results identified a mutation at residue 132 (P132L) in ∼16% of the samples tested. This P132L mutation most closely correlated with invasive scirrhous breast carcinomas. 14 NIH 3T3 cells stably expressing the Cav-1 (P132L) mutant demonstrated increased growth in soft agar, as well as an altered cellular morphology because of disruption of the actin cytoskeleton. These results emphasize the importance of WT Cav-1 expression in the normal regulation of mammary epithelial cell growth and differentiation.
From the above studies, it is clear that Cav-1 assumes a dynamic role in regulating mammary epithelial cell proliferation. To understand the role of Cav-1 mutations (P132L and Null) in the pathogenesis of human breast cancers we studied the phenotypic behavior of Cav-1 (P132L) in cultured cells and we performed mammary gland analysis on Cav-1-deficient mice that harbor a targeted-disruption of the Cav-1 gene (−/−; a null mutation). Here, we clearly demonstrate that the Cav-1 (P132L) mutation behaves in a dominant-negative manner, causing the mislocalization and intracellular retention of WT Cav-1. These data provide a molecular explanation for why only a single mutated CAV1 allele is found in patients with breast cancer. Interestingly, we also show that inactivation of Cav-1 gene expression in mice is sufficient to induce mammary epithelial cell hyperplasia (a premalignant lesion), but not overt mammary tumorigenesis. Taken together, our data implicate loss of functional Cav-1 protein expression (by deletion or mutation) in the pathogenesis of mammary epithelial cell hyperplasia. Our results also suggest that Cav-1 null mice may represent a novel animal model to study premalignant mammary disease.
Recently, it has recently been shown that Cav-1 protein expression is dramatically down-regulated in a number of other human tumors, such as colon carcinomas, ovarian carcinomas, malignant dermal vascular tumors, and sarcomas. 66-70 Similarly, Cav-1 expression is down-regulated in certain prostate cancer-derived cell lines and the CAV1 gene promoter is heavily methylated in human prostate cancer samples. 71,72 Thus, it is likely that dominant-negative mutations in the CAV1 gene will soon be identified in other forms of human cancer.
Acknowledgments
We thank Dr. Roberto Campos-Gonzalez (BD Transduction Laboratories, Lexington, KY) for donating mAbs directed against caveolin-1, caveolin-2, and caveolin-3.
Footnotes
Address reprint requests to Michael P. Lisanti, M.D., Ph.D., Department of Molecular Pharmacology, Albert Einstein College of Medicine, Golding Bldg., Room 202, 1300 Morris Park Ave., Bronx, NY 10461. E-mail: lisanti@aecom.yu.edu.
Supported by grants from the National Institutes of Health (NIH), the Muscular Dystrophy Association (MDA), the American Heart Association (AHA), and the Komen Breast Cancer Foundation, as well as a Hirschl/Weil-Caulier Career Scientist Award (all to M.P.L.). H.L. was supported by an NIH Graduate Training Program Grant (T32-DK07513). D.S.P. was supported by an NIH Graduate Training Program Grant (TG-CA09475). R.G.P. was supported by grants from the NIH (R01-CA70897, R01-CA86072, and R01-CA75503), the Komen Breast Cancer Foundation, and the Department of Defense. R.G.P. is the recipient of a Hirschl/Weil-Caulier Career Scientist Award.
References
- 1.Gallego MI, Binart N, Robinson GW, Okagaki R, Coschigano KT, Perry J, Kopchick JJ, Oka T, Kelly PA, Hennighausen L: Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol 2001, 229:163-175 [DOI] [PubMed] [Google Scholar]
- 2.Wennbo H, Tornell J: The role of prolactin and growth hormone in breast cancer. Oncogene 2000, 19:1072-1076 [DOI] [PubMed] [Google Scholar]
- 3.Wennbo H, Gebre-Medhin M, Gritli-Linde A, Ohlsson C, Isaksson OG, Tornell J: Activation of the prolactin receptor but not the growth hormone receptor is important for induction of mammary tumors in transgenic mice. J Clin Invest 1997, 100:2744-2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hennighausen L, Robinson GW: Think globally, act locally: the making of a mouse mammary gland. Genes Dev 1998, 12:449-455 [DOI] [PubMed] [Google Scholar]
- 5.Engelman JA, Lee RJ, Karnezis A, Bearss DJ, Webster M, Siegel P, Muller WJ, Windle JJ, Pestell RG, Lisanti MP: Reciprocal regulation of Neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for mammary tumorigenesis. J Biol Chem 1998, 273:20448-20455 [DOI] [PubMed] [Google Scholar]
- 6.Sager R, Sheng S, Anisowicz A, Sotiropoulou G, Zou Z, Stenman G, Swisshelm K, Chen Z, Hendrix MJC, Pemberton P, Rafidi K, Ryan K: RNA genetics of breast cancer: maspin as a paradigm. Cold Spring Harbor Sym Quant Biol 1994, LIX:537-546 [DOI] [PubMed] [Google Scholar]
- 7.Engelman JA, Zhang XL, Lisanti MP: Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1). Methylation of a CpG island in the 5′ promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett 1999, 448:221-230 [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Razani B, Altschuler Y, Bouzahzah B, Mostov KE, Pestell RG, Lisanti MP: Caveolin-1 inhibits epidermal growth factor-stimulated lamellipod extension and cell migration in metastatic mammary adenocarcinoma cells (MTLn3). Transformation suppressor effects of adenovirus-mediated gene delivery of caveolin-1. J Biol Chem 2000, 275:20717-20725 [DOI] [PubMed] [Google Scholar]
- 9.Park DS, Lee H, Riedel C, Hulit J, Scherer PE, Pestell RG, Lisanti MP: Prolactin negatively regulates caveolin-1 gene expression in the mammary gland during lactation, via a Ras-dependent mechanism. J Biol Chem 2001, 276:48389-48397 [DOI] [PubMed] [Google Scholar]
- 10.Lee SW, Reimer CL, Oh P, Campbel LDB, Schnitzer JE: Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene 1998, 16:1391-1397 [DOI] [PubMed] [Google Scholar]
- 11.Shridhar V, Sun QC, Miller OJ, Kalemkerian GP, Petros J, Smith DI: Loss of heterozygosity on the long arm of human chromosome 7 in sporadic renal cell carcinomas. Oncogene 1997, 15:2727-2733 [DOI] [PubMed] [Google Scholar]
- 12.Jenkins RB, Qian J, Lee HK, Huang H, Hirasawa K, Bostwick DG, Proffitt J, Wilber K, Lieber MM, Liu W, Smith DI: A molecular cytogenetic analysis of 7q31 in prostate cancer. Cancer Res 1998, 58:759-766 [PubMed] [Google Scholar]
- 13.Engelman JA, Zhang XL, Galbiati F, Volonte D, Sotgia F, Pestell RG, Minetti C, Scherer PE, Okamoto T, Lisanti MP: Molecular genetics of the caveolin gene family: implications for human cancers, diabetes, Alzheimer’s disease, and muscular dystrophy. Am J Hum Genet 1998, 63:1578-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi K, Matsuda S, Machida K, Yamamoto T, Fukuda Y, Nimura Y, Hayakawa T, Hamaguchi M: Invasion activating caveolin-1 mutation in human scirrhous breast cancers. Cancer Res 2001, 61:2361-2364 [PubMed] [Google Scholar]
- 15.Galbiati F, Volonté D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP: Targeted down-regulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J 1998, 17:6633-6648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minetti C, Sotgia F, Bruno C, Scartezzini P, Broda P, Bado M, Masetti E, Mazzocco P, Egeo A, Donati MA, Volonte D, Galbiati F, Cordone G, Bricarelli FD, Lisanti MP, Zara F: Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet 1998, 18:365-368 [DOI] [PubMed] [Google Scholar]
- 17.Scherer PE, Tang Z-L, Chun MC, Sargiacomo M, Lodish HF, Lisanti MP: Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution: identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem 1995, 270:16395-16401 [DOI] [PubMed] [Google Scholar]
- 18.Scherer PE, Lederkremer GZ, Williams S, Fogliano M, Baldini G, Lodish HF: Cab45, a novel (Ca2+)-binding protein localized to the Golgi lumen. J Cell Biol 1996, 133:257-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sargiacomo M, Sudol M, Tang Z-L, Lisanti MP: Signal transducing molecules and GPI-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol 1993, 122:789-807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volonte D, Galbiati F, Lisanti MP: Visualization of caveolin-1, a caveolar marker protein, in living cells using green fluorescent protein (GFP) chimeras: the subcellular distribution of caveolin-1 is modulated by cell-cell contact. FEBS Lett 1999, 445:431-439 [DOI] [PubMed] [Google Scholar]
- 21.Sargiacomo M, Scherer PE, Tang ZL, Kubler E, Song KS, Sanders MC, Lisanti MP: Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci USA 1995, 92:9407-9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP: Identification, sequence and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA 1996, 93:131-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP: Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem 1997, 272:29337-29346 [DOI] [PubMed] [Google Scholar]
- 24.Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP: Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem 1996, 271:2255-2261 [DOI] [PubMed] [Google Scholar]
- 25.Sargiacomo M, Scherer PE, Tang Z-L, Casanova JE, Lisanti MP: In vitro phosphorylation of caveolin-rich membrane domains: identification of an associated serine kinase activity as a casein kinase II-like enzyme. Oncogene 1994, 9:2589-2595 [PubMed] [Google Scholar]
- 26.Smart E, Ying Y-S, Conrad P, Anderson RGW: Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol 1994, 127:1185-1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M: Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol 1994, 126:111-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherer PE, Lisanti MP, Baldini G, Sargiacomo M, Corley-Mastick C, Lodish HF: Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J Cell Biol 1994, 127:1233-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisanti MP, Tang Z-T, Scherer P, Sargiacomo M: Caveolae purification and GPI-linked protein sorting in polarized epithelia. Methods Enzymol 1995, 250:655-668 [DOI] [PubMed] [Google Scholar]
- 30.Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP: Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem 1995, 270:15693-15701 [DOI] [PubMed] [Google Scholar]
- 31.Schnitzer JE, Oh P, Jacobson BS, Dvorak AM: Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca2+-ATPase, and inositol triphosphate receptor. Proc Natl Acad Sci USA 1995, 92:1759-1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corley-Mastick C, Brady MJ, Saltiel AR: Insulin stimulates the tyrosine phosphorylation of caveolin. J Cell Biol 1995, 129:1523-1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robbins SM, Quintrell NA, Bishop MJ: Myryistoylation and differential palmitoylation of the HCK protein tyrosine kinases govern their attachment to membranes and association with caveolae. Mol Cell Biol 1995, 15:3507-3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, Lisanti MP: Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 2001, 276:38121-38138 [DOI] [PubMed] [Google Scholar]
- 35.Razani B, Lisanti MP: Caveolin-deficient mice: insights into caveolar function and human disease. J Clin Invest 2001, 108:1553-1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurzchalia T, Dupree P, Parton RG, Kellner R, Virta H, Lehnert M, Simons K: VIP 21, A 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol 1992, 118:1003-1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietzen DJ, Hastings WR, Lublin DM: Caveolin is palmitoylated on multiple cysteine residues: palmitoylation is not necessary for localization of caveolin to caveolae. J Biol Chem 1995, 270:6838-6842 [DOI] [PubMed] [Google Scholar]
- 38.Schlegel A, Schwab R, Scherer PE, Lisanti MP: A role for the caveolin scaffolding domain in mediating the membrane attachment of caveolin-1. The caveolin scaffolding domain is both necessary and sufficient for membrane binding in vitro. J Biol Chem 1999, 274:22660-22667 [DOI] [PubMed] [Google Scholar]
- 39.Schlegel A, Lisanti MP: A molecular dissection of caveolin-1 membrane attachment and oligomerization. Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem 2000, 275:21605-21617 [DOI] [PubMed] [Google Scholar]
- 40.Song KS, Li S, Okamoto T, Quilliam L, Sargiacomo M, Lisanti MP: Copurification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent free purification of caveolae membranes. J Biol Chem 1996, 271:9690-9697 [DOI] [PubMed] [Google Scholar]
- 41.Laxman R, Currie JL, Kurman RJ, Dudzinski M, Griffin CA: Cytogenetic profile of uterine sarcomas. Cancer 1993, 71:1283-1288 [DOI] [PubMed] [Google Scholar]
- 42.Xiu ZF: A preliminary cytogenetic study of uterine leiomyoma. Chung Hua Fu Chan Ko Tsa Chih 1993, 28:91-93 [PubMed] [Google Scholar]
- 43.Achille A, Biasi MO, Zamboni G, Bogina G, Magalini AR, Pederzoli P, Perucho M, Scarpa A: Chromosome 7q allelic losses in pancreatic carcinoma. Cancer Res 1996, 56:3808-3813 [PubMed] [Google Scholar]
- 44.Bieche I, Champeme MH, Matifas F, Hacene K, Callahan R, Lidereau R: Loss of heterozygosity on chromosome 7q and aggressive primary breast cancer. Lancet 1992, 339:139-143 [DOI] [PubMed] [Google Scholar]
- 45.Huang H, Qian C, Jenkins RB, Smith DI: Fish mapping of YAC clones at human chromosomal band 7q31.2: identification of YACS spanning FRA7G within the common region of LOH in breast and prostate cancer. Genes Chromosom Cancer 1998, 21:152-159 [DOI] [PubMed] [Google Scholar]
- 46.Zenklusen JC, Thompson JC, Troncoso P, Kagan J, Conti CJ: Loss of heterozygosity in human primary prostate carcinomas: a possible tumor suppressor gene at 7q31.1. Cancer Res 1994, 54:6370-6373 [PubMed] [Google Scholar]
- 47.Zenklusen JC, Bieche I, Lidereau R, Conti CJ: (C-A)n microsatellite repeat D7S522 is the most commonly deleted region in human primary breast cancer. Proc Natl Acad Sci USA 1994, 91:12155-12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Champeme MH, Bieche I, Beuzelin M, Lidereau R: Loss of heterozygosity on 7q31 occurs early during breast tumorigenesis. Genes Chromosom Cancer 1995, 12:304-306 [DOI] [PubMed] [Google Scholar]
- 49.Takahashi S, Shan AL, Ritland SR, Delacey KA, Bostwick BG, Lieber MM, Thibadeau SN, Jenkins RB: Frequent loss of heterozygosity at 7q31.1 in primary prostate cancer is associated with tumor aggressiveness and progression. Cancer Res 1995, 55:4114-4119 [PubMed] [Google Scholar]
- 50.Jenkins R, Takahashi S, DeLacey K, Bergstralh E, Lieber M: Prognostic significance of allelic imbalance of chromosome arms 7q, 8p, 16q, and 18q in stage T3N0M0 prostate cancer. Genes Chromosom Cancer 1998, 21:131-143 [PubMed] [Google Scholar]
- 51.Zenklusen JC, Thompson JC, Klein-Szanto AJ, Conti CJ: Frequent loss of heterozygosity in human primary squamous cell and colon carcinomas at 7q31.1: evidence for a broad range tumor suppressor gene. Cancer Res 1995, 55:1347-1350 [PubMed] [Google Scholar]
- 52.Zenklusen JC, Weitzel JN, Ball HG, Conti CJ: Allelic loss at 7q31.1 in human primary ovarian carcinomas suggests the existence of a tumor suppressor gene. Oncogene 1995, 11:359-363 [PubMed] [Google Scholar]
- 53.Koike M, Takeuchi S, Yokota J, Park S, Hatta Y, Miller CW, Tsuruoka N, Koeffler HP: Frequent loss of heterozygosity in the region of the D7S523 locus in advanced ovarian cancer. Genes Chromosom Cancer 1997, 19:1-5 [DOI] [PubMed] [Google Scholar]
- 54.Matsuura K, Shiga K, Yokoyama J, Saijo S, Miyagi T, Takasaka T: Loss of heterozygosity of chromosome 9p21 and 7q31 is correlated with high incidence of recurrent tumor in head and neck squamous cell carcinoma. Anticancer Res 1998, 18:453-458 [PubMed] [Google Scholar]
- 55.Wang XL, Uzawa K, Miyakawa A, Shiiba M, Watanabe T, Sato T, Miya T, Yokoe H, Tanzawa H: Localization of a tumour-suppressor gene associated with human oral cancer on 7q31.1. Int J Cancer 1998, 75:671-674 [DOI] [PubMed] [Google Scholar]
- 56.Lin JC, Scherer SW, Tougas L, Traverso G, Tsui LC, Andrulis IL, Jothy S, Park M: Detailed deletion mapping with a refined physical map of 7q31 localizes a putative tumor suppressor gene for breast cancer in the region of MET. Oncogene 1996, 13:2001-2008 [PubMed] [Google Scholar]
- 57.Kerr J, Leary JA, Hurst T, Shih YC, Antalis TM, Friedlander M, Crawford E, Khoo SK, Ward B, Chenevix-Trench G: Allelic loss on chromosome 7q in ovarian adenocarcinomas: two critical regions and a rearrangement of the PLANH1 locus. Oncogene 1996, 13:1815-1818 [PubMed] [Google Scholar]
- 58.Engelman JA, Zhang XL, Galbiati F, Lisanti MP: Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, -2, and -3). Cav-1 and Cav-2 genes map to a known tumor suppressor locus (6-A2/7q31). FEBS Lett 1998, 429:330-336 [DOI] [PubMed] [Google Scholar]
- 59.Engelman JA, Zhang XL, Lisanti MP: Genes encoding human caveolin-1 and -2 are co-localized to the D7S522 locus (7q31.1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett 1998, 436:403-410 [DOI] [PubMed] [Google Scholar]
- 60.Koleske AJ, Baltimore D, Lisanti MP: Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA 1995, 92:1381-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engelman JA, Wycoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP: Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem 1997, 272:16374-16381 [DOI] [PubMed] [Google Scholar]
- 62.Razani B, Altschuler Y, Zhu L, Pestell RG, Mostov KE, Lisanti MP: Caveolin-1 expression is down-regulated in cells transformed by the human papilloma virus in a p53-dependent manner. Replacement of caveolin-1 expression suppresses HPV-mediated cell transformation. Biochemistry 2000, 39:13916-13924 [DOI] [PubMed] [Google Scholar]
- 63.Park DS, Razani B, Lasorella A, Schreiber-Agus N, Pestell RG, Iavarone A, Lisanti MP: Evidence that Myc isoforms transcriptionally repress caveolin-1 gene expression via an INR-dependent mechanism. Biochemistry 2001, 40:3354-3362 [DOI] [PubMed] [Google Scholar]
- 64.Fiucci G, Ravid D, Reich R, Liscovitch M: Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene 2002, 21:2365-2375 [DOI] [PubMed] [Google Scholar]
- 65.Fra AM, Mastroianni N, Mancini M, Pasqualetto E, Sitia R: Human caveolin-1 and caveolin-2 are closely linked genes colocalized with WI-5336 in a region of 7q31 frequently deleted in tumors. Genomics 1999, 56:355-356 [DOI] [PubMed] [Google Scholar]
- 66.Bender FC, Reymond MA, Bron C, Quest AF: Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res 2000, 60:5870-5878 [PubMed] [Google Scholar]
- 67.Bagnoli M, Tomassetti A, Figini M, Flati S, Dolo V, Canevari S, Miotti S: Downmodulation of caveolin-1 expression in human ovarian carcinoma is directly related to alpha-folate receptor overexpression. Oncogene 2000, 19:4754-4763 [DOI] [PubMed] [Google Scholar]
- 68.Morgan MB, Stevens GL, Tannenbaum M, Salup R: Expression of the caveolins in dermal vascular tumors. J Cutan Pathol 2001, 28:24-28 [DOI] [PubMed] [Google Scholar]
- 69.Wiechen K, Sers C, Agoulnik A, Arlt K, Dietel M, Schlag PM, Schneider U: Down-regulation of caveolin-1, a candidate tumor suppressor gene, in sarcomas. Am J Pathol 2001, 158:833-839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, Arlt K, Zhumabayeva B, Siebert PD, Dietel M, Schafer R, Sers C: Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol 2001, 159:1635-1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pflug BR, Reiter RE, Nelson JB: Caveolin expression is decreased following androgen deprivation in human prostate cancer cell lines. Prostate 1999, 40:269-273 [DOI] [PubMed] [Google Scholar]
- 72.Cui J, Rohr LR, Swanson G, Speights VO, Maxwell T, Brothman AR: Hypermethylation of the caveolin-1 gene promoter in prostate cancer. Prostate 2001, 46:249-256 [DOI] [PubMed] [Google Scholar]