Abstract
The discovery of the genes for nephrin and podocin, which are mutated in two types of congenital nephrotic syndrome, was pivotal in establishing the podocyte as the central component of the glomerular filtration barrier. In vivo the proteins have been localized to the podocyte slit diaphragm, and there is recent evidence for interaction between the two via the adapter molecule CD2AP. We describe in a human podocyte cell line, the subcellular distribution of nephrin, podocins, and CD2AP and their functional interaction with the cytoskeleton. In addition to membrane expression, nephrin and podocin were detected intracellularly in a filamentous pattern. Double immunolabeling and depolymerization studies showed that nephrin and podocin partially co-localize with actin, most strikingly seen protruding from the tips of actin filaments, and are dependent on intact actin polymers for their intracellular distribution. Treatment of differentiated podocytes with puromycin aminonucleoside, an agent that causes foot process effacement in vivo, disrupted actin and nephrin simultaneously, with loss of cell surface localization. We demonstrate an intimate relationship between nephrin podocin and filamentous actin, and reason that disruption of nephrin/podocin could be a final common pathway leading to foot process effacement in proteinuric diseases.
Selective permeability to serum proteins is a hallmark of renal glomerular function, the precise mechanism of which has remained poorly understood for decades. The glomerular filtration barrier comprises three layers: a single cell layer of highly fenestrated capillary endothelial cells, the glomerular basement membrane, and a layer of glomerular epithelial cells, known as podocytes. Podocytes are highly specialized, terminally differentiated cells, with cell bodies, major processes, and foot processes interlinked by slit diaphragms. 1 The breakdown of this filtration barrier results in proteinuria in diseases as diverse as diabetes, human immunodeficiency virus-associated nephropathy, and the nephrotic syndromes. Idiopathic nephrotic syndrome, in both childhood and adulthood, has remained an unexplained disease process, despite many decades of research. Undoubtedly these are a heterogeneous group of conditions, but there is a common pathological observation of foot process effacement. The discovery of the molecular basis of effacement could therefore be a major step in establishing targeted therapy.
A breakthrough at the molecular level came in 1998 with the discovery of nephrin, the gene mutated in Finnish type congenital nephrotic syndrome. 2 This was pivotal in establishing the podocyte as the central component of the glomerular filtration barrier. In vivo nephrin protein has been localized to the podocyte slit diaphragm, 3 which is the still unresolved structure connecting adjacent foot processes, thus establishing the first podocyte-specific protein at this unique structure, as well as emphasizing its role in maintaining glomerular permselectivity. Shortly after this came the discovery of the gene mutated in early onset steroid-resistant nephrotic syndrome, termed NPHS2, encoding the novel protein, podocin, expressed solely in the podocyte. 4 Central to understanding the filtration process will be to define the functional properties of nephrin and podocin.
In podocyte foot process effacement there is presumed loss of the specialized slit diaphragm architecture. 5 If, functionally, the mature foot process and slit diaphragm were to be formed and exist in synchrony, the mechanism underlying this would intuitively form the basis for a final common disease pathway.
The only known interactor of nephrin to date is CD2-associated protein (CD2AP), 6 a molecule hitherto known as a T-cell adaptor molecule linking the actin cytoskeleton to T-cell contacts. 7 Podocin is structurally related to the stomatin family of proteins, 4 which are transmembrane proteins, involved in cytoskeletal scaffolding. 8 More recently, it has been proposed that nephrin and podocin interact via an attachment to CD2AP, in lipid rafts, 9 and that nephrin and podocin co-operatively signal to the intracellular compartment. 10 It is becoming increasingly clear that the podocyte cytoskeleton is a common pathway in foot process disruption, so the hypothesis is that disruption of the nephrin/podocin link to the actin cytoskeleton will influence not just the slit diaphragm, but foot process integrity as a whole.
We provide functional evidence for such a mechanism. Using a novel conditionally immortalized human podocyte cell line, 11 we show that both nephrin and podocin are expressed both at the cell surface and intracellularly in a cytoskeletal pattern. Double immunolabeling revealed only partial co-localization of both with actin stress fibers (AFs), with strongest co-localization at the cell membrane. We also demonstrated co-localization of nephrin and podocin. Disruption of AFs using cytochalasin resulted in co-disruption of actin, nephrin, podocin, and CD2AP to the cytoplasm, and loss of cell surface nephrin and podocin. This demonstrated a dependence of intracellular and membrane nephrin and podocin localization on an intact actin cytoskeleton. To examine this further, we showed that puromycin, a podocyte-specific toxin used in vivo to induce foot process effacement and proteinuria, 12 caused a granular redistribution of nephrin, podocin, and AFs in a manner exactly similar to cytochalasin disruption. Thus we submit evidence for an intimate relationship between the actin cytoskeleton, CD2AP, podocin and nephrin, with functional evidence that disruption of this pathway is the mechanism for at least one type of experimental proteinuria.
Materials and Methods
Primary Culture of Podocytes
A human conditionally immortalized podocyte cell line was generated as described. 11 Briefly, a human nephrectomy specimen without glomerular pathology was obtained and glomeruli isolated as previously described, 13 and cultured in 25-cm2 flasks (BD Falcon, NJ, USA) in RPMI 1640 medium with added penicillin, streptomycin, insulin, transferrin, selenite (Sigma-Aldrich, Dorset, UK), and 10% fetal calf serum. Epithelial cell outgrowths appeared and grew to confluence at 10 to 14 days, and the cells were passaged at this point, and transfected with the immortalizing temperature-sensitive antigen.
Additional cell lines were generated using the same method, from nephrectomy specimens from a child with a heterozygous R>Q exchange in position 138 of podocin and a child with a compound heterozygous nephrin mutation, one in exon 14, and the other in the promoter region.
Retroviral Construct and Virus Infection
The retroviral construct consisted of a SV40 large T-antigen gene containing both the tsA58 and the U19 mutations. Cultures of primary human podocytes were infected with retrovirus-containing supernatants from the packaging cell line (PA317). Infection, selection, and continuous culture were performed at 33°C. Cells derived from a single cell clone were used for all of the experiments described.
Induction of Differentiation
Subsequently cells were grown on type I collagen-coated flasks layered with glass coverslips for purposes of immunostaining. Cells were then plated onto the flasks and grown either at the permissive temperature of 33°C (in 5% CO2), to promote cell propagation as a cobblestoned phenotype, or at the nonpermissive temperature of 37°C (in 5% CO2) to inactivate the SV40 T antigen, and allow the cells to differentiate.
Antibodies
A panel of monoclonal mouse anti-nephrin IgG1 antibodies and polyclonal rabbit anti-nephrin antibodies were used. For this study, we used monoclonal antibodies raised against whole recombinant protein (whole extracellular domain) produced in human embryonic kidney 293 cells. The intracellular portion was produced in Escherichia coli. The extracellular epitopes were mapped using a set of recombinant proteins from the same cell line, and the antibodies in this study mapped to epitopes on immunoglobulin motifs 2 (designated 41F2 and 43C7), motif 8 (49H12), the fibronectin type III domain (48E11), the intracellular domain (7C1), and also polyclonal antibody K2737 (raised against the intracellular domain), all of which gave a similar staining pattern. These antibodies have previously been reported in in vivo studies on nephrin expression in human kidney, 14 and also in this human cell line. 11 Rabbit polyclonal anti-CD2AP antibody was a kind gift of Dr A. Shaw, St. Louis, MO. Rabbit polyclonal anti-podocin antibody was raised as described. 9 Texas Red-conjugated phalloidin (Molecular Probes, Eugene, Oregon) was used for actin filament (AF) labeling.
The secondary antibodies that were used were: fluorescein isothiocyanate-conjugated anti-mouse IgG, anti-rabbit IgG (Jackson ImmunoResearch, Philadelphia, PA), and rhodamine-conjugated anti-mouse IgG (Jackson ImmunoResearch). Controls used were rabbit or mouse serum (as appropriate) for polyclonal primary antibodies or mouse IgG1 for monoclonal anti-nephrin antibody. Fluorescein isothiocyanate, and rhodamine-conjugated secondary antibody alone, were used in all experiments as additional controls.
Immunostaining
The immunolabeling was done as previously described. 13 Briefly, coverslips were fixed with 2% paraformaldehyde, 4% sucrose in phosphate-buffered saline (PBS) for 10 minutes, then permeabilized with 0.3% Triton X-100 (Sigma) in PBS for 10 minutes. Nonspecific binding sites were blocked with 4% fetal calf serum and 0.1% Tween 20 (Sigma) in PBS for 30 minutes. Primary and secondary antibodies were applied at the appropriate dilutions according to standard techniques, and the coverslips were mounted on glass slides with 15% Mowiol (Calbiochem, La Jolla, CA) and 50% glycerol in PBS. Double staining was achieved by incubating with primary antibody and fluorescein isothiocyanate-conjugated secondary antibody as above, then incubating further with Texas Red-preconjugated phalloidin for 20 minutes at room temperature. Further washes and mounting were as above. Standard images were obtained using a Leica photomicroscope, attached to a Spot 2 slider digital camera (Diagnostic Instruments Inc.) and processed with Adobe Photoshop 5.0 software. Images of nephrin fluorescein isothiocyanate and actin tetramethyl-rhodamine isothiocyanate were obtained by confocal laser-scanning microscopy using a Leica TCS NT confocal system attached to a Leica DM RBE microscope and equipped with an ArKr laser (488 nm and 568 nm excitation) (Leica Microsystems, Heidelberg, Germany). An oil-immersion objective lenses ×63, numerical aperture 1.32 was used and imaging parameters selected to optimize confocal resolution.
Protein Extraction and Western Blotting
Cell proteins were extracted by addition of modified RIPA buffer (Sigma Chemical) containing 50 mmol/L Tris-HCL, 150 mmol/L NaCl, 1% Triton X-100, 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L phenylmethyl sulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mmol/L Na vanadate, at 4°C. The suspension was centrifuged at 14,000 × g, and the supernatant containing cellular protein was collected. For Western blotting an 8% sodium dodecyl sulfate-polyacrylamide gel was run under standard conditions, loading 25 μg of total protein in each lane. The gel was placed in transfer buffer for 15 minutes and set up for transfer to a polyvinylidene fluoride membrane at 200 mA for 1 hour. The membrane was rinsed in Tris-buffered saline followed by blocking buffer (5% milk powder) for 5 minutes. The membrane was then immersed in blocking buffer for 1 hour, followed by incubation with primary antibody at 1:1000 dilution. After rinsing in wash buffer, horseradish peroxidase-conjugated anti-mouse secondary antibody (Amersham Biotech, UK) was used for 1 hour at 1:2500 dilution. After final washing the membrane was developed using ECL chemiluminescence reagent (Amersham Biotech, UK).
Depolymerization of Actin and Microtubules
Cells were grown for 14 days at 37°C on collagen I-coated coverslips, until differentiated. Medium was changed and they were then treated for 12 hours at 37°C with colcemid or for 1 hour at 37°C with cytochalasin D (Sigma Chemical), for microtubule or AF depolymerization, respectively. For overnight recovery experiments, treated coverslips were washed twice in PBS and returned to standard medium at 37°C for 24 hours. Coverslips were immunostained as before.
Exposure of Cells to Puromycin Aminonucleoside
Cells were allowed to differentiate for 14 days at 37°C on collagen-coated coverslips. Puromycin aminonucleoside (PAN) (Sigma Chemical) was added to the medium at a concentration of 100 μg/ml for 24 to 48 hours (equates approximately to a dose of 1 mg/100 g in vivo, which is sufficient to induce proteinuria in the rat model 15 ), and cells were fixed and permeabilized for immunostaining as described.
Results
Generation of Differentiated Human Podocytes
At the permissive temperature of 33°C the cells grew in a typical (of epithelial cells) cobblestone morphology. Shifting the cells to 37°C resulted in arrest of proliferation, and throughout a period of 7 to 14 days the cell bodies enlarged in an irregular shape, with the formation of processes both short and more rounded, and also long, spindle-like projections. The podocyte-specific markers WT-1, 16 synaptopodin, 17 and nephrin 2 were expressed as described. 11 For all experiments, except where stated, cells were used in this differentiated form.
The Subcellular Localization of Nephrin in Differentiated Podocytes
Nephrin is a newly described transmembrane protein located at podocyte slit pores. 2 In cobblestoned cells we observed an indistinct cytoplasmic stain, mainly in a perinuclear distribution. In differentiated cells, we observed strong nephrin expression at the cell periphery, as would be expected, but also, unexpectedly, demonstrated filamentous intracytoplasmic expression (Figure 1A) ▶ . The cell membrane expression was evident equally all around the cell periphery, and not just at the cell-cell junctions, as seen for the known slit diaphragm proteins in this cell line, 11 and in the described murine cell line. 13 This was the first clue that the function of nephrin may not be restricted to positional cell junction integrity. We also observed consistent nuclear staining, above the level of control antibody, although the significance of this is at present unclear. It is notable that antibodies against other components of the slit diaphragm, eg, p-cadherin and ZO-1, 18 also give a nuclear stain above the level of controls.
Figure 1.
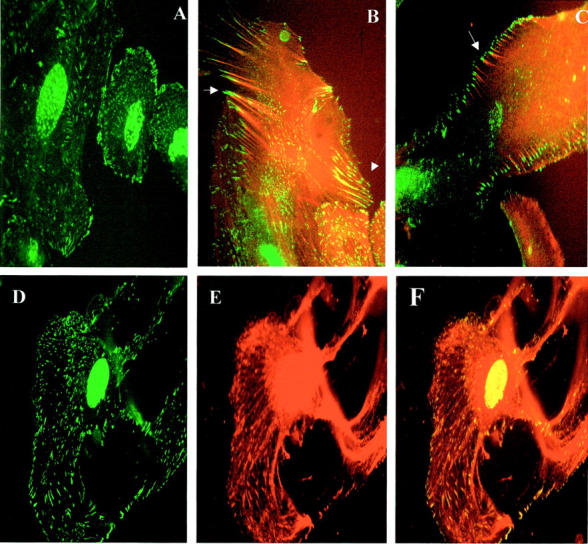
A–C: Subcellular distribution of nephrin and actin—double immunolabeling. A: Nephrin (green) expressed at cell surface and intracytoplasmically. B: Nephrin (green) double staining with AFs (red), showing nephrin cell membrane localization, and co-localization along AF extending into processes (dashed arrow), and mature expression at tips of AF (solid arrow). Cytoplasmic nephrin is seen to be distinct from AF. C: Nephrin (green) double staining with AF (red), showing mature peripheral nephrin expression at tips of AF (arrow). D–F: Nephrin co-localization with podocin. D: Nephrin cytoplasmic and cell surface distribution. E: Podocin, showing same distribution pattern as nephrin, with a more complete, filamentous appearance. F: Merged image, showing co-localization of nephrin with podocin appearing as yellow, with the rest of the podocin filaments remaining as red.
Western blotting was also used to detect nephrin protein signal, and demonstrated a distinct band, as previously detected in glomerular extracts 19 and cultured podocytes 11 at 180 kd.
Nephrin Co-Localizes with AFs at the Tips of Cell Processes
Double labeling of nephrin and actin revealed partial co-localization of nephrin with AFs, with predominantly separate expression of the two molecules in the cytoplasm, and co-localization particularly at the tips of cell processes and at cell surfaces in forming processes (Figure 1B) ▶ . Detailed examination of cells as they matured revealed a consistent pattern, whereby growing cell processes displayed nephrin along the AFs extending into those processes (Figure 1B) ▶ , and mature processes displayed nephrin purely at the very tips of AFs, extending to just beyond the point at which AFs end, at the cell surface (Figure 1C) ▶ . This was demonstrated by the finding that nephrin at the tips of AF remained green, whereas nephrin localizing intracellularly with AF merged to yellow.
Podocin Is Expressed in a Filamentous Pattern in the Cytoplasm, and Co-Localizes with Nephrin
We observed distribution of podocin in a filamentous pattern extending to the cell surface, analogous to the pattern of nephrin expression described above (Figure 1, D and E) ▶ . This was confirmed by co-localization of the two molecules, in the cytoplasm and at the cell surface, with nephrin seen in a punctate linear expression, and podocin being seen in a more complete linear distribution. The pattern of co-localization was of both cytoplasmic and cell surface nephrin completely co-localizing with podocin, with the rest of filamentous podocin in the cytoplasm remaining distinct (Figure 1F) ▶ .
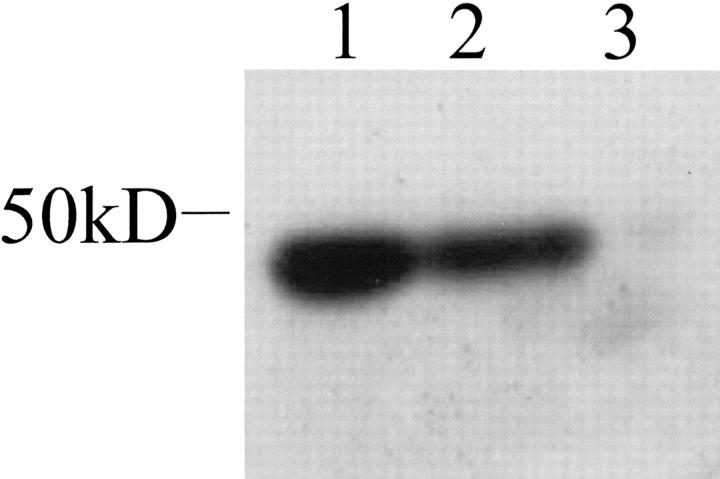
Podocin was also detected in cultured podocytes using the same antibody, by Western blotting (Figure 2) ▶ . Podocin was detected in differentiated cells at the predicted size of 42 kd, and was not detected in undifferentiated cells. For additional specificity we examined cultured conditionally immortalized podocytes from a congenital nephrotic syndrome patient with a podocin mutation (with no podocin expression on glomerular immunofluorescence), and from a congenital nephrotic syndrome patient with a nephrin mutation (reduced levels of nephrin on IF). On Western blotting there was no podocin band from the podocin mutant cells, and a 42-kd band from the nephrin mutant cells.
Figure 2.
Western blotting for podocin on differentiated podocytes from three different human cell lines. Protein band seen at the expected size of 42 kd in two of three lanes. Lane 1, normal podocytes; lane 2, nephrin mutation cell line; lane 3, podocin mutation cell line.
Depolymerization of Actin Filaments (AFs) and Microtubules Indicates Nephrin and Podocin Distribution Is Dependent on Intact AFs
Addition of colcemid, which depolymerizes microtubules (MT), to differentiated cells, resulted in loss of major processes, leading to disk-shaped cells. No filamentous MTs were seen on immunostaining (Figure 3, A and B) ▶ , and AF bundles were maintained (Figure 3, D and E) ▶ . Filamentous nephrin distribution was also preserved, except for a markedly disrupted pattern in the perinuclear area (Figure 3C) ▶ . This indicated firstly that MTs support the shape of major processes (as opposed to foot processes), whereas cell body shape is primarily supported by AFs (see next paragraph), and that nephrin transport from the Golgi apparatus is likely to be primarily dependent on MTs.
Figure 3.
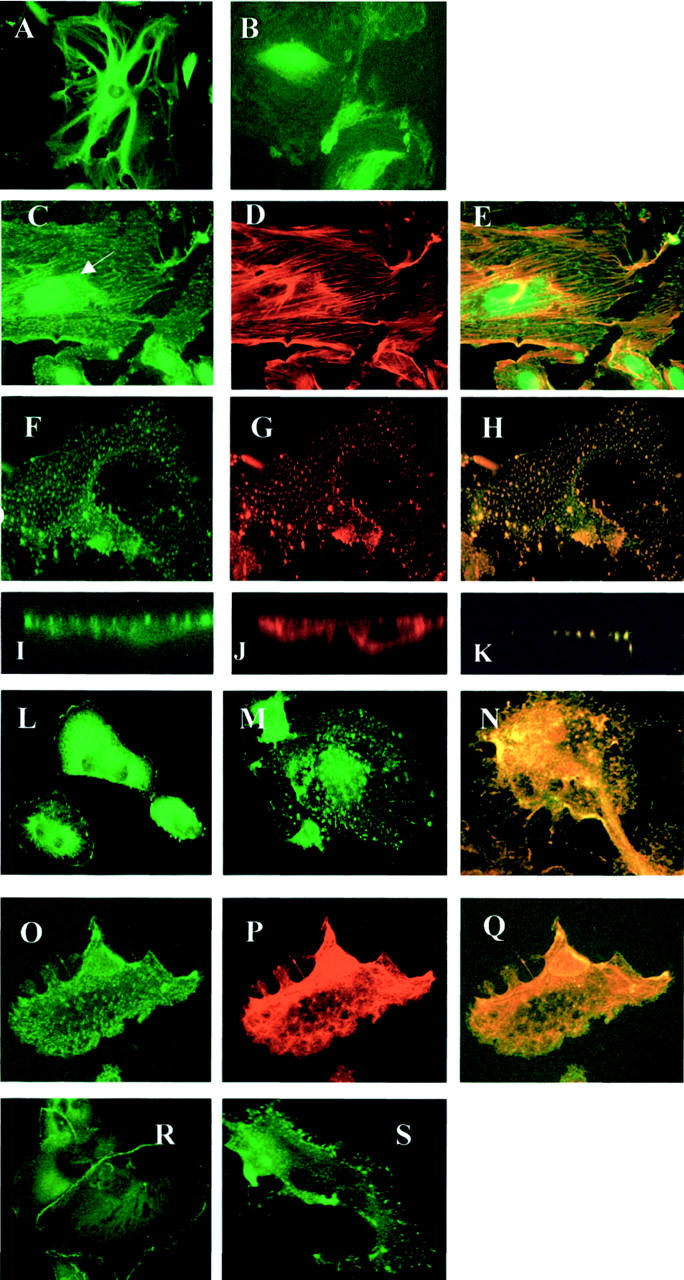
Depolymerization experiments. A–E: Microtubule depolymerization. Microtubule expression in untreated cells (A) and cells treated with colcemid (B). Colcemid-treated cells stained for nephrin (C), actin (D), and merged images (E), showing maintenance of filamentous nephrin and actin staining in cytoplasm, and markedly disrupted nephrin patterning in perinuclear region (arrow). F–S: Actin depolymerization using cytochalasin. Differentiated podocytes, confocal imaging, showing plane sections at the same level. Immunostaining for nephrin (F), actin (G), and merged image (H) shows granular redistribution of both, which co-localizes in the cell cytoplasm. I–K: Cross-sectional confocal images, showing nephrin (I) and actin (J) at cell surface in untreated cell, and actin depolymerized cell (K) double-stained for actin and nephrin, with both colors merged in intracellular granules. L–N: CD2AP cell distribution, cell surface distribution in normal cells (L), changing to granular cytoplasmic location after cytochalasin (M). N: Co-localization of disrupted granular CD2AP (green) with actin (red). O–Q: Podocin disruption by cytochalasin. Granular cytoplasmic redistribution (O), corresponding to nephrin redistribution (P), and demonstrating co-localization on the merged image (Q). ZO-1 cell junction distribution in normal cells (R), and disruption to submembranous location after cytochalasin treatment (S). Original magnifications, × 40 (A–E); ×63 (O to Q).
Addition of cytochalasin D, which is known to depolymerize AFs, led to a shrunken cell body, although with pre-existing major processes intact. Immunostaining for actin showed degeneration of the AF bundles, with globular relocation within the cytoplasm (Figure 3G) ▶ . There was concomitant disruption of nephrin distribution, to the same localization as depolymerized actin, with loss of cell membrane nephrin expression (Figure 3H) ▶ . Washing off cytochalasin and allowing overnight recovery of the cells in standard medium led to reconstitution of filamentous actin and cell surface nephrin (data not shown). The apparent cellular internalization of nephrin was also confirmed by staining nonpermeabilized cells before and after depolymerization with an extracellular nephrin antibody. Nephrin was seen on the cell surface before treatment, diminished almost completely after treatment, and reappeared after overnight recovery (data not shown).
CD2AP and Podocin Are Disrupted in the Same Pattern as Nephrin
CD2AP is a T-cell scaffolding protein found to associate with nephrin in podocytes. 6 We showed CD2AP in a diffuse distribution in the cell cytoplasm, and prominent cell surface expression, and demonstrated CD2AP disruption after treatment with cytochalasin, which co-localized with depolymerized actin, as shown with nephrin (Figure 3; L to N) ▶ .
Podocin was also seen to undergo a granular internalization on actin depolymerization, with nephrin co-localization (Figure 3; O to Q) ▶ , showing that nephrin and podocin cytoplasmic and cell surface distribution was dependent on an actin cytoskeleton. For comparison with other known slit diaphragm proteins, we also examined the effect of cytochalasin on ZO-1 distribution, and demonstrated a breakdown of cell surface distribution, although with the redistribution being just below the plasma membrane (Figure 3, R and S) ▶ .
PAN Causes Nephrin and Actin Redistribution
Treatment of differentiated cells with PAN resulted in a marked alteration in nephrin patterning, with a granular appearance consistent with that described by Kawachi and colleagues 20 in vivo in the rat experimental model. Cell surface expression of nephrin disappeared. Actin filaments were also lost, with a granular cytoplasmic pattern of actin distribution. Double immunolabeling confirmed that actin and nephrin were indeed relocalized to the same place, after 24 hours of treatment, in a manner exactly similar to the pattern seen with AF depolymerization (Figure 4; A to C) ▶ . After 48 hours and beyond, however, the granular disruption of both actin and nephrin became more pronounced, and co-expression of actin and nephrin was lost (Figure 4; D to F) ▶ .
Figure 4.
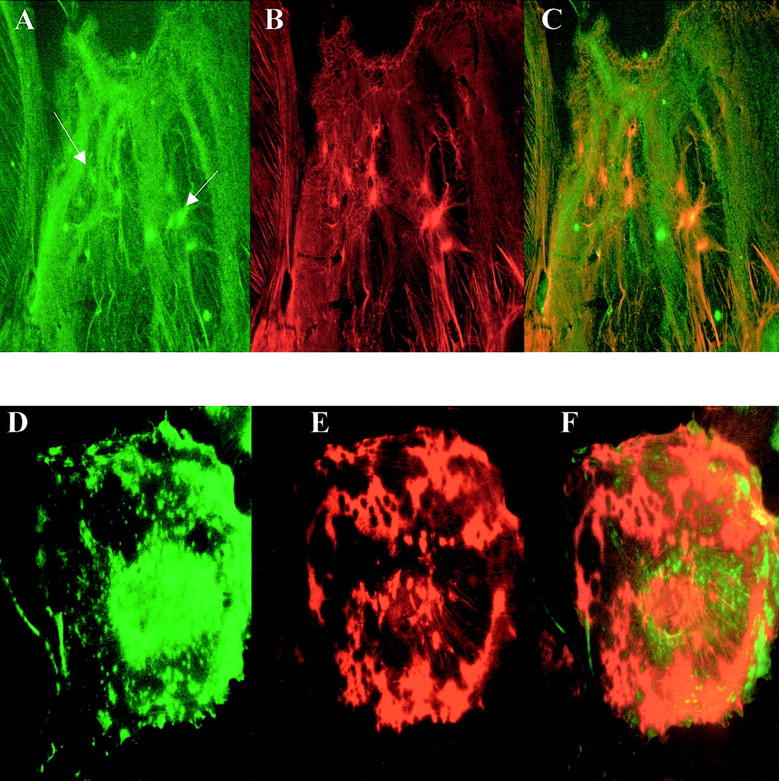
PAN treatment of cells. Nephrin in (A) 24-hour-treated cells shows a granular relocation to the cytoplasm (arrows), and loss of cell surface expression. Double labeling for actin in the treated cells (B) shows a similar redistribution, which co-localized on the merged image (C). Partial maintenance of the filamentous pattern of both nephrin and AF can be seen at this time point. Prolonged treatment (72 hours) resulted in more profound nephrin (D) and actin (E) disruption, with considerable loss of the cytoplasmic co-localization (F). Original magnifications, ×40.
Discussion
In this study we present details of nephrin and podocin interacting with the cell cytoskeleton, particularly with AFs, and show that in mature podocytes, PAN disrupts both actin and nephrin in a co-dependent manner.
In vivo, nephrin has been localized to the podocyte slit diaphragm, with a suggestion of intracellular expression. 21 The question of nephrin’s relationship with the intracellular compartment is particularly pertinent in understanding its potential role in foot process effacement, 2 a unifying pathological feature of proteinuric diseases. We have recently described the in vitro expression and distribution of nephrin and podocin in this cell line. 11 An important clue from these cells to a novel function for nephrin was the lack of dependence in vitro on cell-cell contacts for its described distribution. The known slit diaphragm proteins α-catenin, β-catenin, γ-catenin, ZO-1, and p-cadherin are expressed in these podocytes almost exclusively at cell-cell contacts, whereas this was not the case for nephrin, with some of the strongest expression seen at the tips of cell processes, away from cell junctions. Interpretation of these data should take into account the possibility that the distribution of nephrin in cultured podocytes may not replicate the fully mature in vivo phenotype. It may be that the slit diaphragm is not accurately replicated in cell culture, and/or that nephrin is in a greater state of intracellular dynamic flux in cultured cells. Our experience with the cells is that they express other markers of maturity (eg, cell cycling, synaptopodin), 11 and therefore we feel the mechanistic observations of nephrin’s interactions with the actin cytoskeleton described here are to a great extent applicable to the in vivo situation.
In this study we have demonstrated that cytoskeletal integrity is pivotal to accurate nephrin and podocin membrane localization, and secondly have addressed the question of whether nephrin itself is required for the specialized foot process architecture formed in the mature podocyte. Because of the discovered link between nephrin and CD2AP, an actin-associated molecule, 7 we have examined the localization of actin with nephrin. We demonstrated nephrin only partially co-localized with actin, with the strongest co-expression at the cell peripheries, particularly protruding from the tips of AFs, at the presumed sites of foot processes. Intracytoplasmic co-expression was incomplete, most notably in the perinuclear region. Observation of cell processes in differing stages of maturation was revealing. In incompletely formed processes, nephrin co-localized at several points along the extending peripheral AFs, although not yet having reached the tips of these filaments. In fully formed processes, nephrin was exclusively at the very tip of each AF, and in these cells there was much less cytoplasmic nephrin expression. This indicated a process whereby mature nephrin is expressed at the very tips of cell processes, and by implication in the podocyte foot processes, possibly transported to this localization from the cytoplasm along AFs, and finally supported in place at the ends of these fibers. Our data supports CD2AP as a scaffolding molecule, linking nephrin to actin in the foot processes, 9 but not in the cytoplasmic compartment. These data also illustrate the uniqueness of this cell line in its ability to constitutively express nephrin. Previous studies in murine conditionally immortalized podocytes 13 have not consistently demonstrated nephrin expression on IF, and the pattern of expression is weak and diffuse. 22 This has also been the case with human embryonic kidney (HEK) cells (without constitutive nephrin expression) transfected with a full-length nephrin construct. 22
The discovery that another novel podocyte protein, podocin, is disrupted in another type of congenital nephrotic syndrome 4 provides potentially another piece of the molecular jigsaw linking the slit-diaphragm with the cytoskeleton. There is now biochemical evidence of interaction of podocin, nephrin, and CD2AP, with localization of podocin at the slit diaphragm shown by immunogold electron microscopy. 9 Our data indicate that this is indeed the case, with podocin being distributed in exactly the same manner as nephrin, co-localizing throughout the cytoplasm, and at the cell peripheries. This supports a mechanism whereby nephrin, podocin, and CD2AP are functionally linked to the cell cytoskeleton at the cell periphery, which was then explored by selectively disrupting cytoskeletal components.
We examined the effect of depolymerizing the main components of the cell cytoskeleton, namely AFs and microtubules. Microtubules are known to form the main structural component of the podocyte major processes, whereas foot processes are supported by AFs. 1 After AF depolymerization, nephrin and podocin followed exactly the same pattern of disruption as actin. Cell membrane localization of both molecules was lost, indicating that AF support is required for nephrin and podocin at the cell periphery.
Depolymerization of microtubules yielded disk-shaped cells, with attenuation of major processes. Filamentous expression of nephrin, however, was preserved in the cell periphery, as well as its cell surface distribution. In the immediate perinuclear area we saw a marked disruption of coherent nephrin patterning, indicating a dependence on microtubule integrity for the trafficking of nephrin from the Golgi apparatus. This correlated with the lack of actin co-localization with nephrin in this area.
Using this cell line, we were in a position to test the hypothesis that nephrin is altered in experimental proteinuric states, and to further explore the link with the cytoskeleton. PAN is thought to be a podocyte-specific toxin, 23 which induces proteinuria when infused into an experimental rat model, in a manner said to be akin to human MCNS. 12 An intriguing question, taking into account our data on nephrin/actin localization, is whether the podocyte damage because of PAN involves nephrin, either directly, or indirectly via actin. Using PAN on cultured podocytes, we found that PAN induces a cytoplasmic granular rearrangement of nephrin distribution that, in early stages of disruption, correlated exactly with cytoplasmic actin redistribution, mimicking the actin depolymerization effect seen with cytochalasin. Cell membrane localization of nephrin was lost on both PAN and cytochalasin-treated podocytes. This reinforces the interdependence of actin and nephrin at the cell surface.
Patients with homozygous nephrin mutations display foot process effacement, 24 and the nephrin knockout mouse has poorly developed podocyte foot processes. 25 We believe that our data provide functional support for these observations, in that nephrin interacts with the actin cytoskeleton in mature foot processes. Far less is known about either the distribution or potential role of podocin, or indeed its behavior in acquired and experimental glomerular disease. Our data suggests it has a functional synergy with nephrin and the actin cytoskeleton, which needs to be explored further. Interestingly in our podocin mutant cell line, which does not express podocin on Western blot (Figure 2) ▶ or IF, nephrin and actin distribution appears intact (unpublished data).
In conclusion, the discovery of nephrin, podocin, and CD2AP, and the generation of a cell line that expresses these molecules, has opened up a window into understanding podocyte-related diseases at the molecular level. We have demonstrated in vitro a functional interrelationship between nephrin, podocin, CD2AP, and the podocyte cytoskeleton, with the implication that these proteins are intimately involved in podocyte foot process formation via AFs.
Acknowledgments
We thank Mark Jepson and the Medical Research Council (MRC) cell imaging facility, University of Bristol, for performing confocal microscopy.
Footnotes
Address reprint requests to Moin A. Saleem, Ph.D., Academic Renal Unit, University of Bristol, Southmead Hospital, Bristol, BS10 5NB, UK. E-mail: m.saleem@bristol.ac.uk.
Supported by a grant from Children Nationwide, UK (to L. N.) and the Southmead Hospital Research Fund. P.M. is supported by NIH RO-11DK57603.
References
- 1.Mundel P, Kriz W: Structure and function of podocytes: an update. Anat Embryol (Berl) 1995, 192:385-397 [DOI] [PubMed] [Google Scholar]
- 2.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1998, 1:575-582 [DOI] [PubMed] [Google Scholar]
- 3.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K: Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 1999, 96:7962-7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 2000, 24:349-354 [DOI] [PubMed] [Google Scholar]
- 5.Smoyer WE, Mundel P: Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med 1998, 76:172-183 [DOI] [PubMed] [Google Scholar]
- 6.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 1999, 286:312-315 [DOI] [PubMed] [Google Scholar]
- 7.Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA, Allen PM, Shaw AS: A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 1998, 94:667-677 [DOI] [PubMed] [Google Scholar]
- 8.Salzer U, Prohaska R: Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood 2001, 97:1141-1143 [DOI] [PubMed] [Google Scholar]
- 9.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P: Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 2001, 108:1621-1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T: Interaction with podocin facilitates nephrin signaling. J Biol Chem 2001, 276:41543-41546 [DOI] [PubMed] [Google Scholar]
- 11.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 2002, 13:630-638 [DOI] [PubMed] [Google Scholar]
- 12.Furness PN, Harris K: An evaluation of experimental models of glomerulonephritis. Int J Exp Pathol 1994, 75:9-22 [PMC free article] [PubMed] [Google Scholar]
- 13.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 1997, 236:248-258 [DOI] [PubMed] [Google Scholar]
- 14.Ruotsalainen V, Patrakka J, Tissari P, Reponen P, Hess M, Kestila M, Holmberg C, Salonen R, Heikinheimo M, Wartiovaara J, Tryggvason K, Jalanko H: Role of nephrin in cell junction formation in human nephrogenesis. Am J Pathol 2000, 157:1905-1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowenborg EK, Jaremko G, Berg UB: Glomerular function and morphology in puromycin aminonucleoside nephropathy in rats. Nephrol Dial Transplant 2000, 15:1547-1555 [DOI] [PubMed] [Google Scholar]
- 16.Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B: Nuclear localization of the protein encoded by the Wilms’ tumor gene WT1 in embryonic and adult tissues. Development 1993, 119:1329-1341 [DOI] [PubMed] [Google Scholar]
- 17.Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W: Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 1997, 139:193-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiser J, Kriz W, Kretzler M, Mundel P: The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 2000, 11:1-8 [DOI] [PubMed] [Google Scholar]
- 19.Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR: Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 1999, 56:1481-1491 [DOI] [PubMed] [Google Scholar]
- 20.Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F: Cloning of rat nephrin: expression in developing glomeruli and in proteinuric states. Kidney Int 2000, 57:1949-1961 [DOI] [PubMed] [Google Scholar]
- 21.Holthofer H, Ahola H, Solin ML, Wang S, Palmen T, Luimula P, Miettinen A, Kerjaschki D: Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol 1999, 155:1681-1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan K, Khoshnoodi J, Ruotsalainen V, Tryggvason K: N-linked glycosylation is critical for the plasma membrane localization of nephrin. J Am Soc Nephrol 2002, 13:1385-1389 [DOI] [PubMed] [Google Scholar]
- 23.Fishman JA, Karnovsky MJ: Effects of the aminonucleoside of puromycin on glomerular epithelial cells in vitro. Am J Pathol 1985, 118:398-407 [PMC free article] [PubMed] [Google Scholar]
- 24.Patrakka J, Kestila M, Wartiovaara J, Ruotsalainen V, Tissari P, Lenkkeri U, Mannikko M, Visapaa I, Holmberg C, Rapola J, Tryggvason K, Jalanko H: Congenital nephrotic syndrome (NPHS1): features resulting from different mutations in Finnish patients. Kidney Int 2000, 58:972-980 [DOI] [PubMed] [Google Scholar]
- 25.Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 2001, 10:1-8 [DOI] [PubMed] [Google Scholar]