Abstract
Whey acidic protein (WAP)-transforming growth factor (TGF)-α transgenic mice acquire both cancerous and noncancerous mammary lesions. For this study, we evaluated the effect of mouse strain background on the incidence, latency, and histotype of two noncancerous lesions, hyperplastic alveolar nodules (analogous to typical hyperplasias in women), and macrocysts. These lesions display characteristics of fibrocystic changes observed in breasts of women, and in both mice and humans are associated with an uncertain risk of progression to neoplasia. Virgin transgenic mice of the (C57BL/6J;SJL)F2 background developed very few hyperplastic alveolar nodules and no macrocysts. In contrast, when the WAP-TGF-α transgene was carried on the FVB/N strain, congenic virgin transgenic mice acquired both lesion types with ∼100% penetrance. In the (FVB;C57BL/6J)F1 background, hyperplastic alveolar nodule incidence was reduced to approximately the nontransgenic mouse level, and macrocyst latency was increased dramatically. Crossing into C57BL/6 resulted in elimination of the macrocyst phenotype. Finally, FVB strain transgenic mammary epithelium transplanted into nontransgenic recipients of the FVB/N or (FVB;C57BL/6J)F1 backgrounds displayed macrocyst latency characteristic of the recipient, and not donor, mouse strain. Quantitative real-time polymerase chain reaction analysis demonstrated that, despite the difference in macrocyst incidence between (FVB;C57BL/6J)F1 and C57BL/6 virgin transgenic mice (81% versus 0%), the level of TGF-α expression was not different. FVB strain transgenic mice expressed only twofold more TGF-α than the other backgrounds. These findings indicate that C57BL/6J modifier alleles inhibit mammary lesion incidence and macrocyst latency in a semidominant manner, and that suppression of lesion development can involve host factors that are independent of mammary epithelial genotype.
Genetic variation in the human population significantly influences susceptibility to disease. For breast cancer, inherited genetic alterations can increase the incidence of disease, as exemplified by the effect of mutant null alleles at the tumor suppressor loci BRCA1 and BRCA2. However, depending on the cohort evaluated, only 28 to 85% of individuals with either mutation manifest disease, indicating that other modifier genes, as well as the environment, must influence disease penetrance. 1-4 In addition to affecting disease penetrance, modifier genes also may act by affecting breast tumor latency, multiplicity, and/or progression. In the outbred human population, it is difficult to evaluate the relative contributions of modifier genes in breast carcinogenesis because of the independent segregation of multiple alleles at multiple modifier loci. In contrast, inbred mice permit assessment of disease phenotype in the presence of only a single allele at each modifier locus. This genetic uniformity is advantageous, in one context, because it allows for precise characterization of lesion progression in individuals of identical genotype. This advantage also applies to the study of mammary carcinogenesis in genetically engineered mice, in which the effect of oncogene or growth factor overexpression or loss of tumor suppressor gene expression can be investigated in a uniform host population. In a second context, this genetic uniformity is a disadvantage, because extrapolation from models generated in only a single inbred or hybrid mouse strain to the much more genetically variable human population will provide only a partial view of how breast carcinogenesis is influenced by modifier genes. To address this limitation, we examined the effect of genetic strain background on mammary lesions that develop in transgenic mice in which the whey acidic protein (WAP) gene regulatory elements direct expression of transforming growth factor (TGF)-α), a factor also associated with breast cancer etiology. We report that strain background has a dramatic influence on the penetrance, latency, and histotype of mammary lesions.
Materials and Methods
Mice
Genotype designations used in this report are abbreviated as follows: C57BL/6J, B6; FVB/N, FVB; (FVB/NxC57BL/6J)F1, (FVB;B6)F1; (C57BL/6JxSJL)F2, (B6;SJL)F2. WAP-TGF-α line 3573-2 transgenic mice, designated TgN(WapTgfa)215Bri, have been described. 5 These mice initially were generated in the (B6;SJL)F2 background, and for this study were crossed into the FVB (Taconic) strain background for at least 10 generations. Two additional WAP-TGF-α transgenic mouse lines, 1177-5 and 1177-15, were generated by microinjecting transgene DNA into FVB strain eggs. FVB congenic WAP-TGF-α line 3573-2 and FVB line 1177-15 mice also were crossed once to B6 mice to produce (FVB;B6)F1transgenic mice. All mice were housed in AAALAC-accredited facilities and handled in accordance with the Guide for the Care and Use of Laboratory Animals. All studies were approved by the School of Veterinary Medicine Animal Care and Use Committee.
Histopathology and Whole-Mount Preparation
Tissues were fixed in 10% neutral buffered formalin at room temperature for 18 to 24 hours, or in 4% paraformaldehyde at 4°C for 4 hours, dehydrated in ethanols, embedded in paraffin, sectioned (6 μm), and stained with hematoxylin and eosin (H&E). For mammary whole-mount preparations, isolated glands were flattened between glass microscope slides and immersed in acetone immediately after collection or after 1 hour of fixation in 10% neutral buffered formalin or 1 hour of fixation in 1:3 glacial acetic acid:absolute ethanol. Glands then were defatted by three to four changes of acetone (24 hours each), rehydrated through graded alcohols, and stained with hematoxylin for 1 to 2 hours or Carmine stain overnight. Specimens were destained in graded ethanols or 2% HCl in 70% ethanol. Samples were stored in methyl salicylate or glycerol.
Quantification of Hyperplastic Alveolar Nodules (HANs)
Whole mount preparations of mammary glands were examined under a dissecting microscope and the number of HANs per gland was counted. HANs appear as darkly stained, variably sized collections of alveolar-like structures. The number of discrete HANs was recorded for each gland. If more than 100 HANs were present (in which case these lesions often became confluent), total HAN number was recorded as ≥100. Statistical comparisons of HAN incidence between mouse strains of virgin females were performed using Mann-Whitney rank sum test.
Identification of Macrocysts
WAP-TGF-α females were evaluated weekly for the presence of grossly visible cysts of ≥1 cm (which were identified easily by their fluid-filled character). In addition, light microscopic analysis was performed on H&E-stained sections of mammary glands. Statistical comparisons of macrocyst incidence between virgin females of the FVB versus the (FVB;B6)F1 mouse strains were performed using Mann-Whitney rank sum test. Statistical comparisons of mean age at sacrifice (because of macrocyst development) between FVB versus (FVB;B6)F1 mouse strains, and between parous versus virgin females, were performed using unpaired two-tailed student’s t-test with significance at P ≤ 0.05.
Quantification of TGF-α Expression Levels in the Mammary Gland
Real-time reverse transcriptase-polymerase chain reaction (PCR) was used to measure expression levels of TGF-α in mammary glands of transgenic mice. Mammary glands were frozen in liquid nitrogen immediately after collection and stored at −80°C until use. Samples were thawed, and total RNA was extracted 6 using the Qiagen RNeasy Mini Kit (Qiagen Inc., Valencia, CA). First strand complementary DNA (cDNA) was synthesized as described. 6 Briefly, 0.25 μg of total RNA and 600 U of M-MLV RT were incubated for 2 hours at 42°C after priming with 1.6 μg of pd(N)6 random hexamers in a total reaction volume of 2 μl. Expression of mouse endocytokeratin 18 (mCK18) also was measured to indicate the extent of epithelialization of mammary fat pads, and this value was used to normalize TGF-α expression to mammary epithelial cell number in each mouse. mCK18 primers were described previously. 6 TGF-α forward and reverse primer sequences used for PCR were 5′-gtcaggctctggagaacagc-3′ and 5′-aggagatctgcatgctcaca-3′, respectively. Real-time PCR products were detected using SYBR Green I dye (enhanced fluorescence with binding to dsDNA; Applied Biosystems, Foster City, CA) and the LightCycler (a rapid thermal cycler equipped with fluorimeter optics; Roche, Indianapolis, IN). TGF-α and mCK18 values for each sample were averages of triplicate PCR reactions (except two samples that were averages of duplicate TGF-α values, and two samples that were averages of duplicate mCK18 values). Each sample value was determined by comparison to the average of duplicated standard curves generated for each PCR run. Mammary glands from three to five diestrual transgenic mice between 4 and 5.5 months of age were evaluated per group. Transcript levels in nontransgenic mice (n = 6) of different strains were combined for analysis. Data were analyzed using student’s t-test with significance at P ≤ 0.05. To compare the extent of epithelialization among transgenic mice of different strain backgrounds, we also measured expression of the housekeeping gene L19. 6 L19 expression levels were determined as described above and analyzed using Student’s t-test with significance at P ≤ 0.05. Comparative epithelial content was determined by dividing the mCK18 value by the L19 value for each sample. Data were analyzed using student’s t-test with significance at P ≤ 0.05.
Mammary Epithelial Cell Transplantation
Mammary epithelial cell transplants were performed as described by Moser and colleagues. 7 Briefly, mammary glands were isolated from young adult FVB females carrying WAP-TGF-α and a marker transgene encoding human cytokeratin 18 (hCK18), which also was generated in FVB mice. 8 Glands were pooled and treated enzymatically with collagenase 9 to produce a single cell suspension of mammary epithelial cells. Viability was assessed by trypan blue exclusion. Viable cells (5 × 105) were injected bilaterally into the no. 3 (distal cranial) or no. 4 (proximal caudal) mammary glands of tribromethanol (Avertin; Aldrich, Milwaukee, WI)-anesthetized nontransgenic recipient mice using a 26-gauge needle. Each recipient mouse received transplanted cells in two to four separate glands. Recipient mice were 3 weeks old and had undergone surgical excision of endogenous mammary epithelium immediately before transplantation by removal of the mammary fat pad in the region of the nipple. 10 After puberty, recipient mice were housed with males and all females experienced at least one full-term pregnancy. Animals were evaluated weekly for the presence of macrocysts ≥1 cm, and age at sacrifice because of the presence of cysts was recorded. To confirm the presence of donor mammary epithelium, tissue sections of recipient mammary glands were deparaffinated, incubated overnight with monoclonal antibody against hCK18 (InnoGenex, San Ramon, CA), followed by an anti-mouse link antibody, peroxidase label (InnoGenex), and diaminobenzidine. This allowed detection of transgene-encoded hCK18 present in donor cells with minimal cross reaction with mCK18. Student’s t-test was used to compare the age at sacrifice because of enlarged cysts between transplant recipients of FVB versus (FVB;B6)F1 backgrounds.
Results
Characterization of WAP-TGF-α Mammary Lesions in Different Strains
(B6;SJL)F2 Background
The WAP gene regulatory element directs mammary epithelial-specific transgene expression that is present at low levels in adult virgin females, and is up-regulated highly during pregnancy and lactation. 11 WAP-TGF-α-induced mammary lesions in the (B6;SJL)F2 background have been described. 5,12 Virgin transgenic mice did not develop lesions. WAP-TGF-α transgene expression during mid to late pregnancy resulted in acceleration of mammary alveolar proliferation and differentiation, so that by day 18 of pregnancy (parturition occurs at day 20) the transgenic mouse gland resembled a day 1 lactating nontransgenic mouse gland. After parturition and removal of pups, mammary involution was delayed in transgenic versus nontransgenic female mice. Some alveolar structures failed to involute and persisted within both cranial and caudal mammary fat pads in equivalent numbers. These persistent alveolar structures, or HANs, increased in number with successive pregnancies and often became confluent. 5,12 Finally, at a mean age of 9.5 months in 3573-2 line mice, non-virgin WAP-TGF-α transgenic female mice required sacrifice because of the development of mammary neoplasms. Large mammary cysts were observed only infrequently in multiparous females (EPS, unpublished observations). These findings, including the lack of HAN development in virgin female mice and the lack of macrocystic disease in virgin or parous animals, were reproduced in two additional WAP-TGF-α transgenic mouse lineages in the (B6;SJL)F2 background. 5
FVB Strain: Virgin Mice
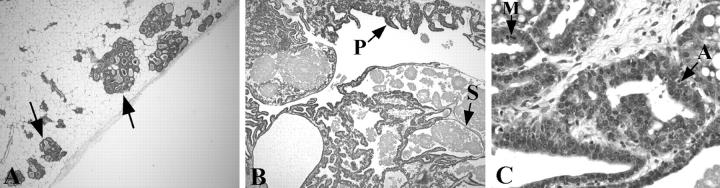
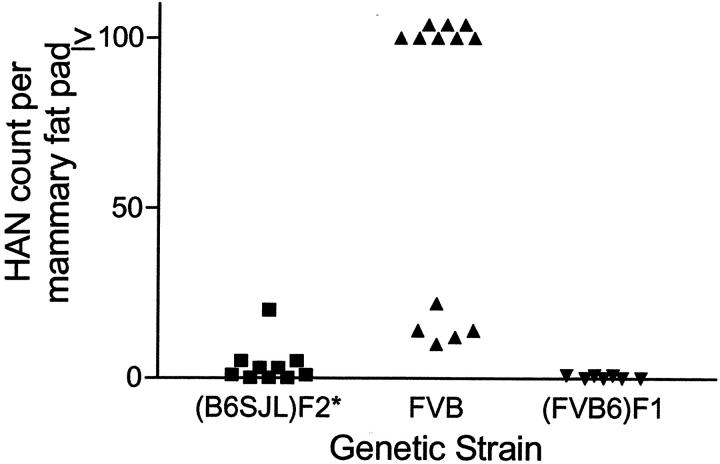
To examine the effect of strain background on lesion development, we crossed line 3573-2 WAP-TGF-α mice into the FVB strain. We also reinjected the WAP-TGF-α transgene into FVB mouse eggs to produce two new lineages. This allowed us to compare three WAP-TGF-α lineages in the FVB background with the three lineages originally generated in the (B6;SJL)F2 background. Remarkably, the susceptible population of mice as well as the character of resulting lesions changed dramatically. The most striking finding was the development of lesions in virgin mice. Virgin WAP-TGF-α line 3573-2 females in the FVB strain displayed grossly identifiable cystic mammary lesions with 100% incidence, and with a mean latency of 6.5 months (Table 1) ▶ . These lesions developed almost exclusively in the cranial fat pads. The high incidence and short latency of cysts observed in two additional mouse lineages in the FVB strain (Table 1) ▶ indicates that this phenotype is not a transgene integration site-associated phenomenon. In addition, mammary alveolar lesions including HANs (Figure 1A) ▶ were observed in virgin line 3573-2 transgenic mice in the FVB strain, in contrast to findings in (B6;SJL)F2 transgenic mice of this line reported earlier. 5 The number of HANs was increased dramatically in virgin FVB females versus (B6;SJL)F2 strain females (P = 0.002; Figure 2 ▶ ). Histologically, mammary glands removed from WAP-TGF-α virgin females in the FVB strain revealed complex alveolar and ductal lesions. Some areas resembled mid-to-late pregnancy with lactating epithelium. Macrocysts were composed of multiple smaller cystic structures lined by either simple epithelium or papillary epithelium (Figure 1B) ▶ . Papillary epithelium frequently contained cells with secretory vesicles suggesting a differentiated state. More complex epithelial lesions were interspersed throughout the macrocyst. These regions typically contained epithelial cells in multiple layers that exhibited anisonucleosis, mitotic figures, and apoptosis (Figure 1C) ▶ . Importantly, these mammary lesions observed in mice are reminiscent of fibrocystic-like lesions identified in human breast. 13
Table 1.
Effect of Genetic Background on Incidence of Macrocysts in Virgin WAP-TGF-α Mice
| Lineage | Genetic background | Macrocyst incidence | Macrocyst latency mean age ± SD (months) |
|---|---|---|---|
| 3573-2 | FVB* | 48/48 (100%) | 6.5 ± 2.1 |
| 3573-2 | (FVB;B6)F1 | 25/31 (81%) | 18.6 ± 4.0 |
| 3573-2 | B6† | 0/16 (0%)‡ | — |
| 1177-15§ | FVB | 16/16 (100%) | 4.6 ± 1.3 |
| 1177-15§ | (FVB;B6)F1 | 18/19 (95%) | 10.3 ± 1.9 |
| 1177-05§ | FVB | 14/16 (88%)¶ | 5.8 ± 1.4 |
| Nontransgenic | FVB | 0/60 (0%)∥ | — |
*Congenic genotype of ≥ 10 generations from the (B6;SJL)F2 into the FVB strain.
†Congenic genotype of ≥ 6 generations from the FVB into the B6 strain.
‡Tissues collected from mice 18.4 to 23.9 months of age that died of other causes (n = 11, mean = 21.0) or still are living (n = 5, mean = 20.2).
§Generated by reinjection of the WAP-TGF-α transgene into FVB mouse eggs.
¶Two animals that did not acquire mammary cysts developed nonmacrocystic mammary tumors.
∥Three animals acquired nonmacrocystic mammary epithelial tumors between 17.9 and 22.5 months of age.
Figure 1.
Photomicrograph of isolated mammary glands obtained from FVB strain WAP-TGF-α transgenic mice. A: HANs are variable in size (arrows) and can become confluent. B: Macrocyst composed of several microcysts that exhibit single cell layers of papillary (P) and simple (S) epithelium. C: Interspersed between microcysts are focal proliferative lesions in which mammary epithelium forms several layers and contains pleomorphic nuclei undergoing mitosis (M) and apoptosis (A). Original magnifications: ×40 (A, B); ×200 (C).
Figure 2.
Effect of mouse strain on HAN number in virgin line 3573-2 WAP-TGF-α transgenic mice. All mice were at least 10 months old when examined. In the (B6;SJL)F2 background, HANs were infrequent. When crossed into the FVB strain, virgin females acquired HANs, often reaching confluence. One additional cross into the B6 strain [yielding (FVB;B6)F1 mice] reduced the incidence of HANs to near 0%. *, HAN counts in (B6;SJL)F2 taken from Sandgren and colleagues. 5
FVB Strain: Parous Mice
Because the WAP promoter is up-regulated during mid-to-late pregnancy, we expected animals undergoing at least one pregnancy to have a shortened latency of large cyst development. In fact, this was not observed in FVB strain line 3573-2 transgenic mice, as the mean age to onset of large cyst development for both virgin and parous mice was 6.5 months (Figure 3) ▶ .
Figure 3.
Box and whiskers plot depicting age at sacrifice because of macrocyst development for WAP-TGF-α virgin and parous animals of the FVB and (FVB;B6)F1 mouse strains. In addition, mammary epithelial cells isolated from FVB WAP-TGF-α transgenic females (that also carried a marker gene, hCK18) were transplanted into recipient mice of the FVB and (FVB;B6)F1 strains, and these recipients subsequently were subjected to at least one full-term pregnancy. Age at macrocyst development for recipients is indicated as “transplt parous.” The box extends from the 25th percentile to the 75th percentile (includes 50% of data points). The median age at sacrifice is depicted by the horizontal line within each box. The whiskers represent the range of data. Statistical differences between means across strains and parity were determined using Student’s t-test with significance at a P value ≤0.05. Letters above data columns that are similar indicate no statistical difference between groups; different letters represent statistical differences. Numbers in parentheses above the x axis represent the number of animals evaluated in each group. In addition, 77 recipient mice of the FVB background received normal mammary epithelial cells carrying the marker transgene using this method (WC Kisseberth and EP Sandgren, manuscript in preparation). Only two developed solid tumors and one developed a macrocyst with latencies of 19 to 22 months.
(FVB;B6)F1 Background: Virgin Mice
Both the incidence and latency of mammary lesions in virgin WAP-TGF-α females were altered by one generation of crossing of FVB line 3573-2 mice into the B6 background. By a mean age of 18.6 months, only 25 of 31 virgin (FVB;B6)F1 transgenic females of this line developed macrocysts (Table 1) ▶ . Mean latency of macrocyst development in virgin transgenic mice of the 3573-2 and 1177-15 lines was delayed dramatically in the (FVB;B6)F1 versus FVB background (P < 0.0001). Suppression of HAN development in mice <15 months of age also was observed (P = 0.0009; Figure 2 ▶ ).
(FVB;B6)F1 Background: Parous Mice
Pregnancy increased the incidence of macrocyst development to 100% in WAP-TGF-α line 3573-2 mice in the (FVB;B6)F1 background, and these mice exhibited a decrease in mean latency to macrocyst development compared to virgin (FVB;B6)F1 animals (P < 0.0001; Figure 3 ▶ ). However, latency to macrocyst development in parous (FVB;B6)F1 background WAP-TGF-α mice remained significantly longer than in parous FVB transgenic mice (P < 0.0001; Figure 3 ▶ ).
Strain Specificity of Transgene Expression
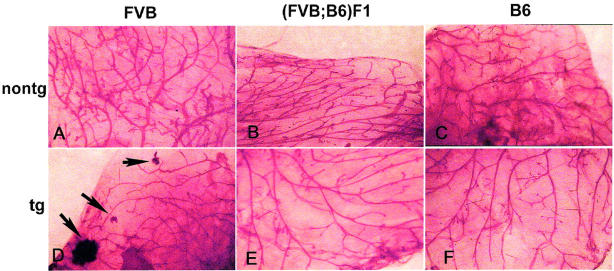
To determine whether the level of TGF-α expression in mammary gland correlated with lesion incidence or latency, we measured TGF-α mRNA in 4- to 5.5-month-old virgin female mice in diestrus using quantitative real-time PCR analysis (Figure 4) ▶ . For this analysis, TGF-α values were divided by mouse endocytokeratin 18 (mCK18) values from the same sample to normalize TGF-α expression to a per epithelial cell basis. Both B6 and (FVB;B6)F1 transgenic mice displayed similar levels of transgene expression, ∼20-fold higher than background detected in nontransgenic mice. FVB strain transgenic mice expressed approximately twice as much TGF-α as the B6 and (FVB;B6)F1 transgenic mice. The relative epithelial content in transgenic mouse mammary gland did not differ among strains as determined by dividing mCK18 values by L19 values from the same sample: relevant ratios were 0.77 ± 0.33 for FVB, 0.78 ± 0.17 for (FVB;B6)F1, and 0.88 ± 0.23 for B6 (mean ± SEM). P values for all comparisons were >0.75. However, TGF-α expression in mammary epithelium did reduce the mCK18/L19 ratio relative to nontransgenic virgin mice (P = 0.04; Figure 5 ▶ ). In addition, microscopic (Figure 6) ▶ and whole mount evaluations (Figure 7) ▶ were performed on mammary fat pads collected from the same individuals to determine whether changes in the amount of epithelium could be detected visually. In mammary glands from three of four transgenic FVB mice, some HAN development was evident, but otherwise glands appeared similar.
Figure 4.
Bar graph depicting relative TGF-α transgene expression (mean + SD) as measured using real-time PCR in mammary glands of virgin transgenic mice of three strain backgrounds versus virgin nontransgenic (nontg) mice. Mammary glands from three to six diestrual mice between 4 and 5.5 months of age were evaluated per group. Statistical differences between means across backgrounds and transgene status were determined using Student’s t-test with significance at a P value ≤0.05. Letters above data columns that are similar indicate no statistical difference between groups; different letters represent statistical differences.
Figure 5.
Bar graph depicting relative expression of the endogenous mCK18 gene, a marker for epithelium, as measured using real-time PCR in mammary glands of virgin WAP-TGF-α transgenic mice versus virgin nontransgenic mice (mean ± SD; n = 11 for transgenic and 5 for nontransgenic). Statistical difference between groups was determined using Student’s t-test with significance at a P value ≤0.05. Although transgenic values were not different from one another (see text), expression of TGF-α in any strain background reduced the ratio of mCK18/L19 in virgin mouse mammary glands (P = 0.04).
Figure 6.

Representative H&E-stained microscopic sections of mammary fat pads from nontransgenic (A–C) and WAP-TGF-α transgenic (D–F) mice of the FVB, (FVB;B6)F1, and B6 mouse strains. Note that mammary fat pads appear similar except the FVB WAP-TGF-α gland that exhibits multifocal HANs. Mammary fat pads collected from the same mice were evaluated in Figures 4, 5, 6, and 7 ▶ ▶ ▶ . Scale bar, 1.0 mm.
Figure 7.
Representative whole mounts of mammary fat pads from nontransgenic (A–C) and WAP-TGF-α transgenic (D–F) mice of the FVB, (FVB;B6)F1, and B6 mouse strains. Note that mammary fat pads appear similar except that the FVB WAP-TGF-α gland contains two small and one large HANs. Mammary fat pads collected from the same mice were evaluated in Figures 4, 5, 6, and 7 ▶ ▶ ▶ . Original magnifications, ×10.
Recipient Strain Specificity of Lesion Latency in Transplanted FVB strain WAP-TGF-α Mammary Epithelium
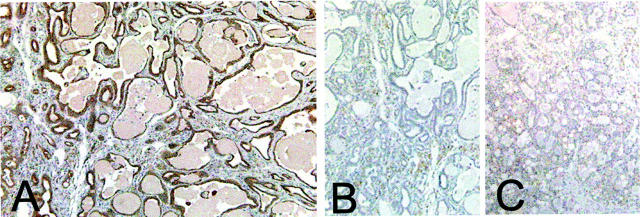
Mammary epithelial cells isolated from FVB strain WAP-TGF-α transgenic mice were transplanted into nontransgenic recipients of either the FVB or (FVB;B6)F1 backgrounds. Our goal was to determine whether the relatively short latency to macrocyst development observed in the FVB strain could be prolonged by transplantation into a recipient of the (FVB;B6)F1 background. Recipients went through one or more pregnancies after transplantation. Presence of donor epithelium in all recipient fat pads was confirmed by immunohistochemical detection of the hCK18 marker (Figure 8) ▶ . Each recipient mouse developed at least one cyst regardless of genetic strain. However, mean latency was prolonged significantly when recipient mice were of the (FVB;B6)F1 genetic background compared to FVB strain (P = 0.0005; Figure 3 ▶ ). Interestingly, the ages at cyst development in both transplant recipients and unmanipulated parous transgenic animals of the same genetic background were not different statistically (Figure 3) ▶ . Thus, for (FVB;B6)F1 recipients, the influence of this host environment substantially delays the development of macrocysts in FVB strain mammary epithelium.
Figure 8.
Photomicrographs of mammary epithelial cell transplants. Mammary epithelium was isolated from mammary fat pads of bitransgenic mice carrying WAP-TGF-α and a marker transgene encoding hCK18 (details in Materials and Methods). A: Immunohistochemical demonstration of the hCK18 marker in transplanted epithelium incubated with anti-hCK18 antibody. B: Adjacent histological section incubated with a nonspecific primary antibody demonstrating the lack of hCK18 staining. C: Transplanted mouse mammary epithelium lacking the hCK18 transgene incubated with anti-hCK18 antibody, demonstrating lack of cross-reaction of the antibody with unmarked mouse epithelium. Original magnifications, ×100.
Discussion
In mice, as in humans, genotype can modify the phenotype associated with expression of cancer-related genes. 12 For inbred mouse strains and their hybrids, numerous reports document strain-related differences in physiology and disease predisposition. 13 In addition, different strain backgrounds display varying susceptibility to specific cancers and/or sensitivities to x-irradiation or chemical carcinogens. 14,15 The importance of the strain background used in an experimental model is particularly relevant to genetically engineered mice. 16-21 In transgenic animals, modifier genes can influence not only the downstream cellular and tissue responses to transgene expression, but also alter the temporal pattern and level of transgene expression by way of their influence on gene regulatory sequences contained within the transgene construct. Our objective for this study was to compare the development of mammary lesions in transgenic mice overexpressing TGF-α when the transgene was carried on different mouse strain backgrounds. We chose to evaluate FVB and B6 strains because of their preeminence in the study of mammary cancer. We chose to evaluate TGF-α transgenic mice because of the strong association between TGF-α overexpression and breast cancer in both humans and rodents. In women, TGF-α is the most common EGF-like growth factor found in primary breast tumors: 30 to 100% of tumors express TGF-α mRNA and/or protein. 22-26 In addition, overexpression of TGF-α in the mammary gland of transgenic mice or rats is oncogenic. 5,27-31 Our findings suggest the existence of modifier loci with distinct alleles in FVB and B6 strains that affect incidence, latency, multiplicity, and histotype of mammary lesions in WAP-TGF-α transgenic mice. In addition, these modifier alleles appear to exert some of their effects via the host environment rather than within the mammary epithelial cell itself, as demonstrated by the delay in latency of macrocyst development after transplantation of FVB transgenic mammary epithelium into (FVB;B6)F1 recipients.
Of particular interest is the prevalence of a novel lesion histotype, the macrocyst, in FVB strain mice. The incidence of macrocyst development was 100% in FVB stain line 3573-2 WAP-TGF-α transgenic mice. In contrast, this lesion was observed rarely or never in three WAP-TGF-α lineages in the (B6;SJL)F2 background 5 or in B6 background 3573-2 transgenic mice. In two lines of (FVB;B6)F1 transgenic mice (including 3573-2), the incidence was reduced and/or the latency greatly increased. The development of macrocysts in FVB strain transgenic mice, including virgins, could be explained either by initiation of transgene expression in a novel mammary cell type in this strain, or by a genetic influence on the character of lesion progression. The complex microscopic composition of macrocysts is reminiscent of fibrocystic lesions observed commonly in women. These transgenic mice may prove a useful model to study the neoplastic potential of these lesions, as well as the influence of modifier alleles on the development of certain lesion histotypes. This information would be of special importance because, in women, lesions of the fibrocystic complex have an uncertain risk for progression to neoplasia.
Modifier loci could influence mammary lesion pathogenesis in WAP-TGF-α transgenic mice in several ways. First, modifier genes may influence cellular and tissue responses to TGF-α, with B6 alleles tending to reduce disease severity and abolish the development of macrocysts in a semidominant manner. Second, modifier loci could increase the amount of mammary epithelium. In this study, several FVB strain WAP-TGF-α transgenic animals displayed HANs, yet quantitative reverse transcriptase-PCR analysis of mCK18 levels normalized to the housekeeping gene L19 indicated that, over the whole gland, mammary epithelial content was similar among WAP-TGF-α mice of different strain backgrounds. Furthermore, except for the presence of some HANs in FVB strain transgenic mice, virgin mice of each background at 4 to 5 months of age exhibited similar mammary histology, arguing that phenotypic differences do not reflect only a change in epithelial content. However, the mCK18/L19 ratio was reduced (P = 0.04) in pooled transgenic versus nontransgenic mammary glands, indicating that TGF-α may affect expression of mCK18 or L19 irrespective of strain background. Third, modifier genes in the FVB strain could increase the basal level of expression of the endogenous WAP gene in virgin mouse mammary epithelial cells, thereby up-regulating TGF-α transgene expression as a secondary effect. In this scenario, the FVB-associated pattern of transgene expression would be inhibited by B6 strain alleles. Indeed, WAP-TGF-α mice of either (FVB;B6)F1 background or B6 background expressed approximately twofold less TGF-α than transgenic mice in the FVB strain. It is uncertain whether a twofold decrease in TGF-α expression alone could result in a 12-month increase in macrocyst latency (6.5 versus 18.6 months; Table 1 ▶ ) or the near complete suppression of HAN development (Figure 2) ▶ . However, more striking is the finding that lesion incidence was dramatically different in virgin WAP-TGF-α transgenic mice in the (FVB;B6)F1 versus B6 backgrounds (81% versus 0%), despite the equivalent level of transgene expression in these strain backgrounds. These observations implicate an interaction between TGF-α and mouse genetic background, rather than only the level of transgene expression, as a principal determinant of macrocyst development. Candidate strain characteristics that may account for these observations include differences in 1) TGF-α processing or turnover; 2) the fraction of epithelium present as alveoli; 3) number and/or persistence of terminal endbuds; 4) epithelial content in aging mice; 5) mammary stromal characteristics; and 6) endocrine function.
Our findings point to a critical need to assess mouse strain background effects when characterizing the lesion potential of single genetic alterations, or when comparing phenotypes among transgenic lineages carrying different transgene constructs. This conclusion also is supported by observations of transgenic mice expressing ras or neu in the mammary gland, in which tumor pathogenesis varied among different mouse backgrounds. 32,33 Most importantly, determining those characteristics of transgene-induced lesion pathogenesis that are different between mouse strains provides a mechanism to map and eventually identify tumor modifier genes in mice, a process with important relevance to the understanding of breast disorders in humans.
Acknowledgments
We thank Dorothy Nesbit of the UW–Madison Environmental Health Science Center Molecular Biology Services Core for technical assistance with the LightCycler, Kristin Wentworth for immunohistochemistry, Elizabeth Scheef for assistance with tissue collection, Dr. William Kisseberth for communicating data before publication, and Dr. Amy Moser for comments on the text.
Footnotes
Address reprint requests to Dr. Eric P. Sandgren, Department of Pathobiological Sciences, School of Veterinary Medicine, University of Wisconsin-Madison, 2015 Linden Dr., Madison, Wisconsin 53706. E-mail: sandgren@svm.vetmed.wisc.edu.
Supported in part by National Institutes of Health grants K01 RR00145 (to T. A. R.-H.), R01-CA64843 (to E. P. S.) and ES09090.
References
- 1.Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, Paterson C, Ozcelik H, Goss P, Allingham-Hawkins D, Hamel N, Di Prospero L, Contiga V, Serruya C, Klein M, Moslehi R, Honeyford J, Liede A, Glendon G, Brunet JS, Narod S: Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 1999, 91:1241-1247 [DOI] [PubMed] [Google Scholar]
- 2.Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J, Sobol H, Teare MD, Struewing J, Arason A, Scherneck S, Peto J, Rebbeck TR, Tonin P, Neuhausen S, Barkardottir R, Eyfjord J, Lynch H, Ponder BA, Gayther SA, Zelada-Hedman M, : the Breast Cancer Linkage Consortium: Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet 1998, 62:676-689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber BL: Update on breast cancer susceptibility genes. Recent Results Cancer Res 1998, 152:49-59 [DOI] [PubMed] [Google Scholar]
- 4.Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmern MM, Brody LC, Tucker MA: The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997, 336:1401-140 [DOI] [PubMed] [Google Scholar]
- 5.Sandgren EP, Schroeder JA, Qui TH, Palmiter RD, Brinster RL, Lee DC: Inhibition of mammary gland involution is associated with transforming growth factor alpha but not c-myc-induced tumorigenesis in transgenic mice. Cancer Res 1995, 55:3915-3927 [PubMed] [Google Scholar]
- 6.Schroeder MD, Rose-Hellekant TA, Sandgren EP, Schuler LA: Dysregulation of mammary Stats 1, 3 and 5 and PRL receptors by overexpression of TGFα. Mol Cell Endocrinol 2001, 175:173-183 [DOI] [PubMed] [Google Scholar]
- 7.Moser AR, Mattes EM, Dove WF, Lindstrom MJ, Haag JD, Gould MN: APCmin, a mutation in the murine Apc gene, predisposes to mammary carcinomas and focal alveolar hyperplasias. Proc Natl Acad Sci USA 1993, 90:8977-8981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neznanov N, Thorey IS, Cecena G, Oshima RG: Transcriptional insulation of the human keratin 18 gene in transgenic mice. Mol Cell Biol 1993, 13:2214-2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang R, Haag JD, Gould MN: Site of expression and biological function of rat mammary carcinoma suppressor gene. Carcinogenesis 1991, 11:1765-1770 [DOI] [PubMed] [Google Scholar]
- 10.DeOme KB, Faulkin LJ, JR, Bern HA, Blair PB: Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res 1959, 78:515-520 [PubMed] [Google Scholar]
- 11.Robinson GW, McKnight RA, Smith GH, Hennighausen L: Mammary epithelial cells undergo secretory differentiation in cycling virgins, but require pregnancy for the establishment of terminal differentiation. Development 1995, 121:2079-2090 [DOI] [PubMed] [Google Scholar]
- 12.Rose-Hellekant TA, Sandgren EP: Transforming growth factor alpha-and c-myc-induced mammary carcinogenesis in transgenic mice. Oncogene 2000, 19:1092-1096 [DOI] [PubMed] [Google Scholar]
- 13.Rosen PP, Oberman HA: Tumors of the mammary gland. Atlas of Tumor Pathology, third series, fascicle 7. 1993:pp 136-139 Armed Forces Institute of Pathology, Washington, DC
- 14.Wild CP, Kleihues P: Etiology of cancer in humans and animals. Exp Toxicol Pathol 1996, 48:95-100 [DOI] [PubMed] [Google Scholar]
- 15.Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, Fisher EM: Genealogies of inbred mouse strains. Nat Genet 2000, 24:23-25 [DOI] [PubMed] [Google Scholar]
- 16.Balmain A, Nagase H: Cancer resistance genes in mice: models for the study of tumour modifiers. Trends Genet 1998, 14:139-144 [DOI] [PubMed] [Google Scholar]
- 17.Wolf CR, Henderson CJ: Use of transgenic animals in understanding molecular mechanisms of toxicity. J Pharm Pharmacol 1998, 50:567-574 [DOI] [PubMed] [Google Scholar]
- 18.Doetschman T: Interpretation of phenotype in genetically engineered mice. Lab Anim Sci 1999, 49:137-143 [PubMed] [Google Scholar]
- 19.Macleod KF, Jacks T: Insights into cancer from transgenic mouse models. J Pathol 1999, 187:43-60 [DOI] [PubMed] [Google Scholar]
- 20.Phillips TJ, Hen R, Crabbe JC: Complications associated with genetic background effects in research using knockout mice. Psychopharmacology 1999, 147:5-7 [DOI] [PubMed] [Google Scholar]
- 21.Gerlai R: Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci 1996, 19:177-181 [DOI] [PubMed] [Google Scholar]
- 22.Ethier SP: Growth factor synthesis and human breast cancer progression. J Natl Cancer Inst 1995, 87:964-973 [DOI] [PubMed] [Google Scholar]
- 23.Rudland PS, Fernig DG, Smith JA: Growth factors and their receptors in neoplastic mammary glands. Biomed Pharmacother 1995, 49:389-399 [DOI] [PubMed] [Google Scholar]
- 24.Artagaveytia N, Le Penven S, Falette N, Lucero R, Garofalo EG, Saez S: Epidermal growth factor and transforming growth factor alpha mRNA expression in human breast cancer biopsies; analysis in relation to estradiol, progesterone and EGF receptor content. Steroid Biochem Mol Biol 1997, 60:221-228 [DOI] [PubMed] [Google Scholar]
- 25.Pilichowska M, Kimura N, Fujiwara H, Nagura H: Immunohistochemical study of TGF-alpha, TGF-beta1, EGFR, and IGF-1 expression in human breast carcinoma. Mod Pathol 1997, 10:969-975 [PubMed] [Google Scholar]
- 26.de Jong JS, van Diest PJ, van der Valk P, Baak JP: Expression of growth factors, growth inhibiting factors, and their receptors in invasive breast cancer. I: an inventory in search of autocrine and paracrine loops. J Pathol 1998, 184:44-52 [DOI] [PubMed] [Google Scholar]
- 27.Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC: Overexpression of TGFα in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell 1990, 61:1121-1135 [DOI] [PubMed] [Google Scholar]
- 28.Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, Merlino GT: TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell 1990, 61:1137-1146 [DOI] [PubMed] [Google Scholar]
- 29.Halter SA, Dempsey P, Matsui Y, Stokes MK, Graves-Deal R, Hogan BL, Coffey RJ: Distinctive patterns of hyperplasia in transgenic mice with mouse mammary tumor virus transforming growth factor-alpha. Characterization of mammary gland and skin proliferations. Am J Pathol 1992, 140:1131-1146 [PMC free article] [PubMed] [Google Scholar]
- 30.Matsui Y, Halter SA, Holt JT, Hogan BLM, Coffey RJ: Development of mammary hyperplasia and neoplasia in MMTV-TGFα transgenic mice. Cell 1990, 61:1147-1155 [DOI] [PubMed] [Google Scholar]
- 31.Davies BR, Platt-Higgins AM, Schmidt G, Rudland PS: Development of hyperplasias, preneoplasias, and mammary tumors in MMTV-c-erbB-2 and MMTV-TGFα transgenic rats. Am J Pathol 1999, 55:303-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen LL, Gurnami M, Catino JJ, Tyler RD: In wap-ras transgenic mice, tumor phenotype but not cyclophosphamide-sensitivity is affected by genetic background. Anticancer Res 1995, 15:385-392 [PubMed] [Google Scholar]
- 33.Rowse GJ, Ritland SR, Gendler SJ: Genetic modulation of neu proto-oncogene-induced mammary tumorigenesis. Cancer Res 1998, 58:2675-2679 [PubMed] [Google Scholar]