Abstract
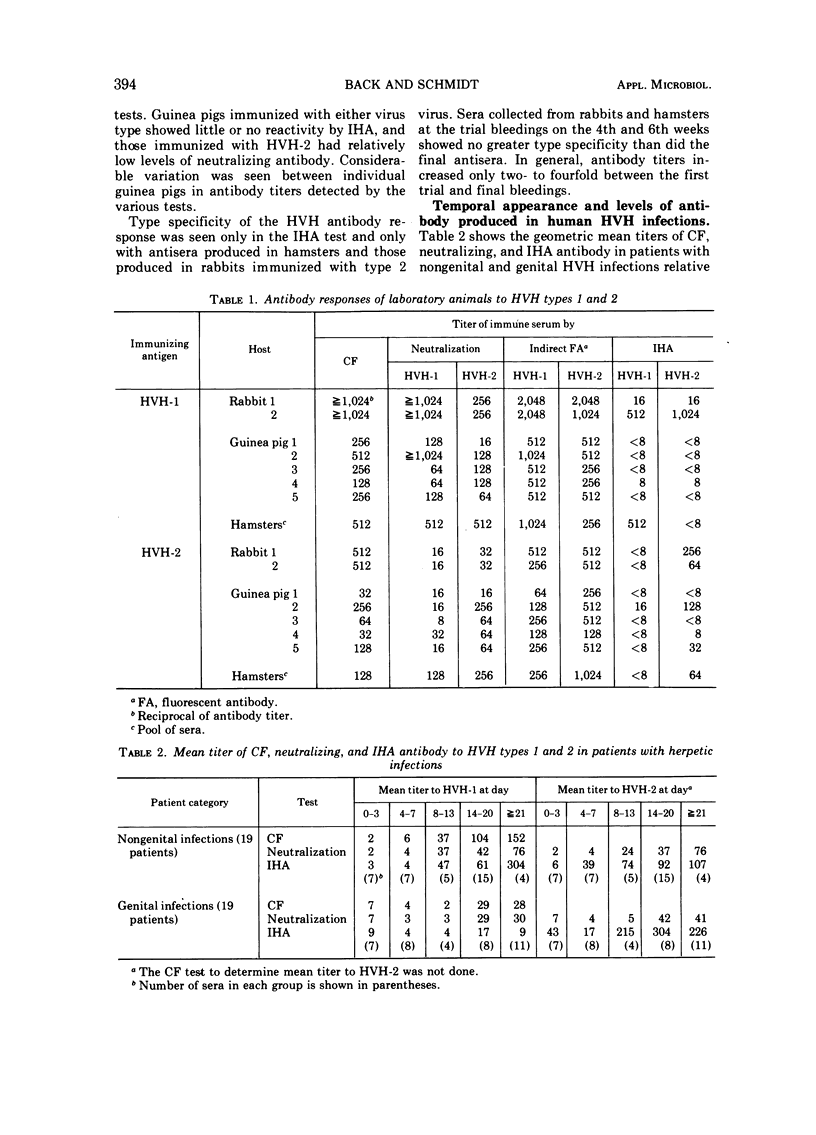
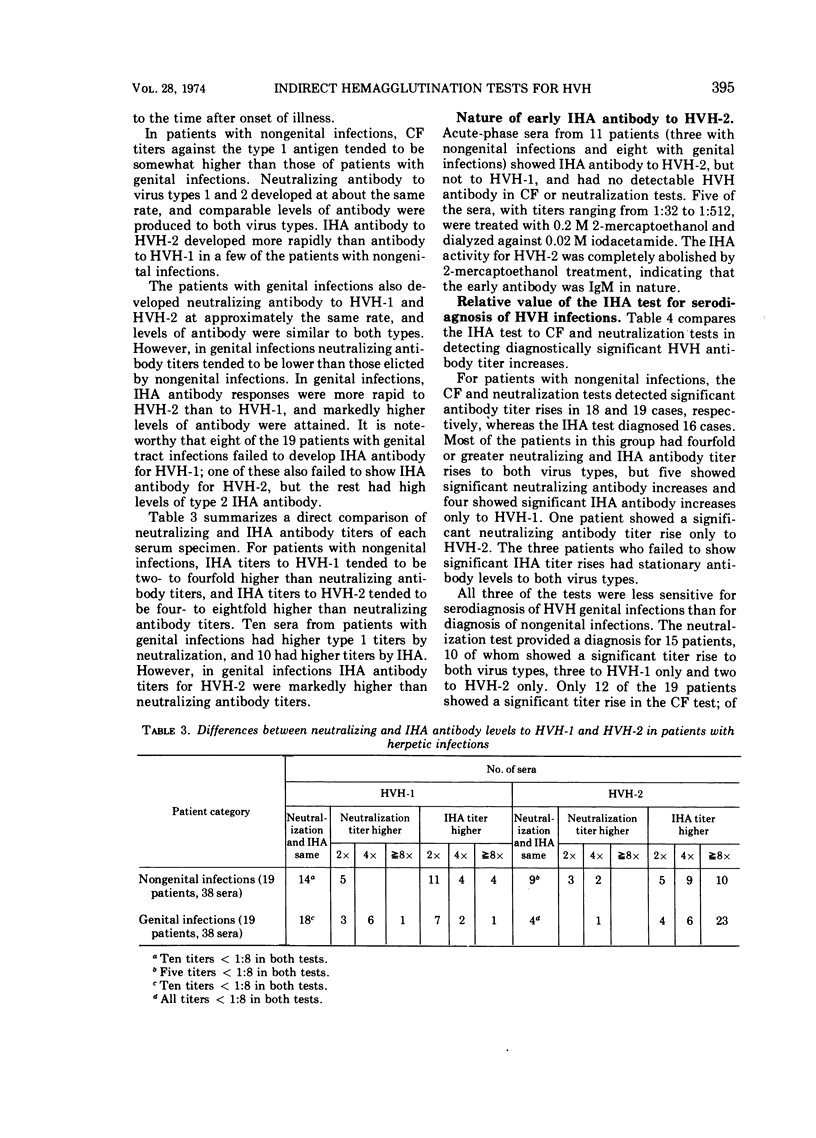
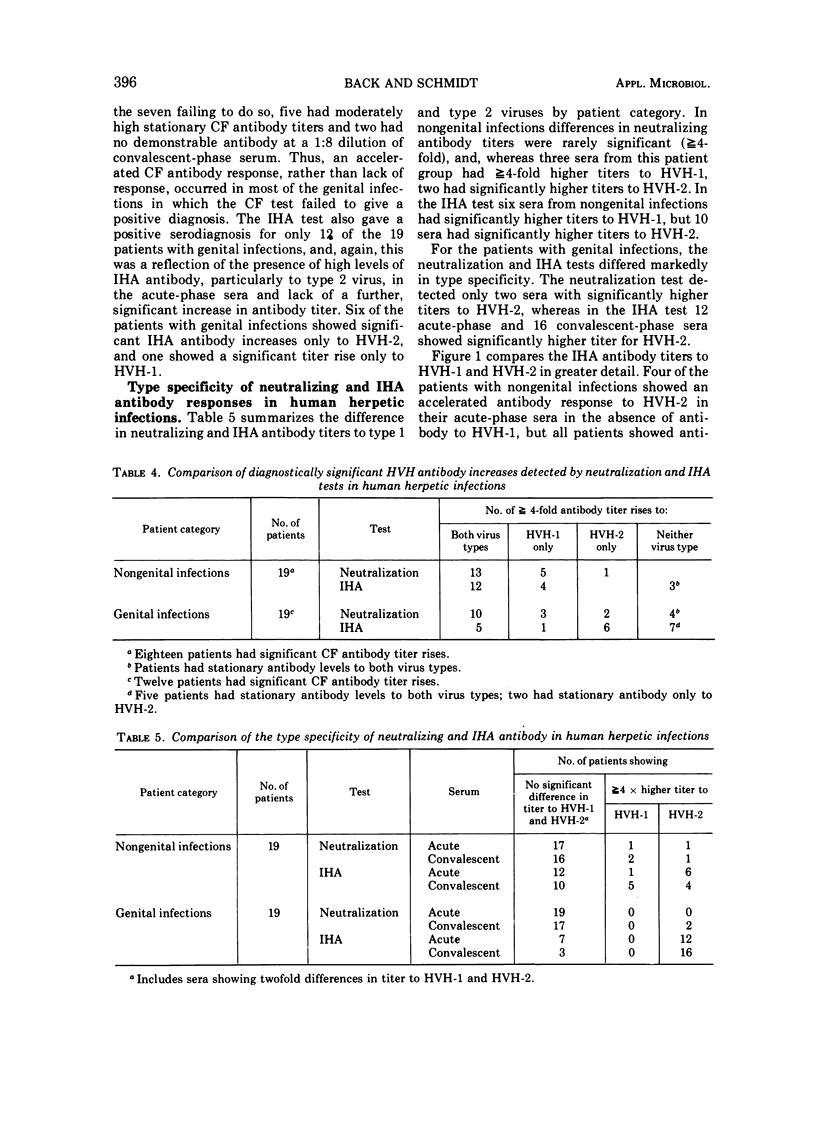
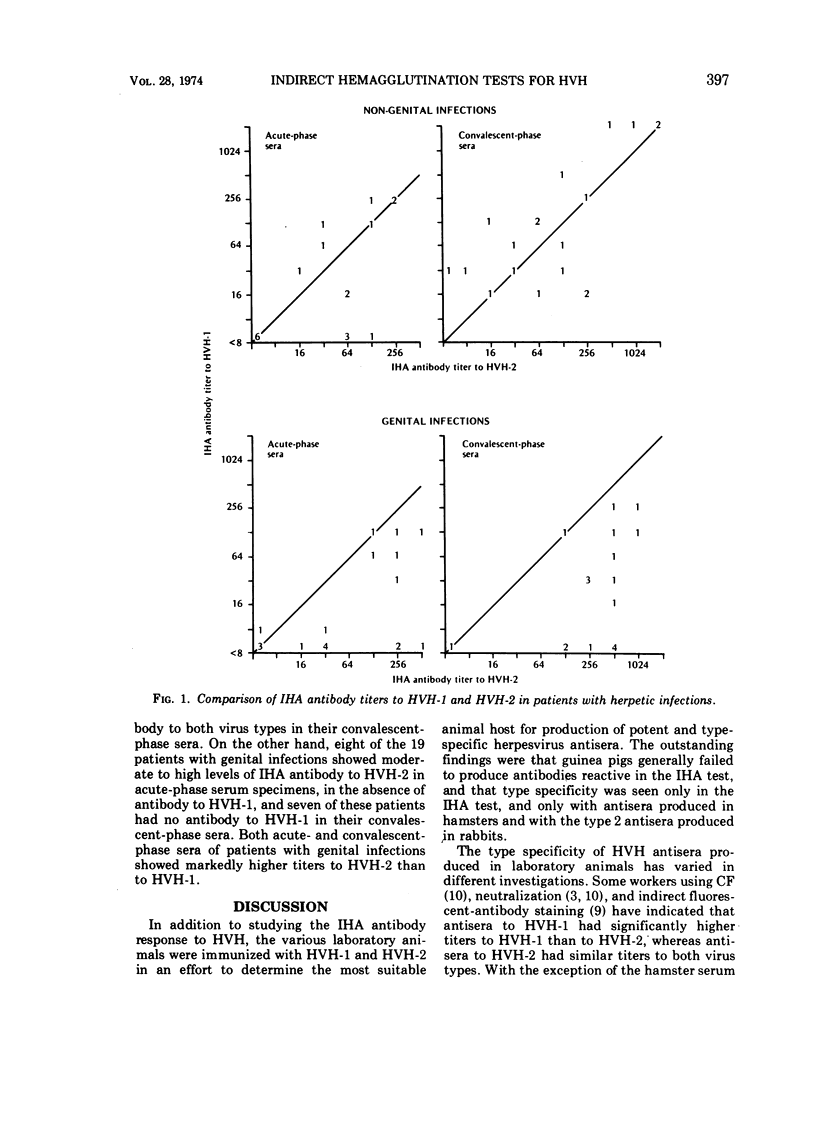
Indirect hemagglutinating (IHA) antibody responses to Herpesvirus hominis types 1 and 2 (HVH-1 and HVH-2) were compared to complement-fixing and neutralizing antibody responses in immunized laboratory animals (rabbits, guinea pigs, and hamsters) and in natural infections of man. With the immunized animals, type specificity was seen only in the IHA test and only with antisera produced in hamsters and in the rabbits immunized with HVH-2. In human nongenital infections (considered to be caused predominately by HVH-1), IHA and neutralizing antibodies developed at about the same rate and reached approximately the same levels for HVH-1 and HVH-2. IHA titers tended to be higher than neutralizing antibody titers for both virus types. In genital infections (considered to be caused predominately by HVH-2), there was a rapid IHA antibody response to HVH-2, and the early HVH-2 antibody demonstrable by IHA, but not by neutralization tests, was found to be immunoglobulin M in nature. In genital infections, IHA titers for HVH-2 were markedly higher than neutralization titers, but there was no pronounced difference in neutralizing the IHA antibody titers for HVH-1. Several patients with genital infections fialed to develop IHA antibody for HVH-1. The IHA test possessed no greater sensitivity than did complement fixation or neutralization tests for serodiagnosis of HVH infections. Despite the fact that a number of patients with genital infections produced IHA antibody only for HVH-2, the test was no more effective than the neutralization test in providing a type-specific serodiagnosis of infection, due largely to the fact that the rapid IHA antibody response to HVH-2 prevented demonstration of a further, significant antibody titer increase in a number of cases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein M. T., Stewart J. A. Indirect hemagglutination test for detection of antibodies to Cytomegalovirus. Appl Microbiol. 1971 Jan;21(1):84–89. doi: 10.1128/am.21.1.84-89.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein M. T., Stewart J. A. Method for typing antisera to Herpesvirus hominis by indirect hemagglutination inhibition. Appl Microbiol. 1971 Apr;21(4):680–684. doi: 10.1128/am.21.4.680-684.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle W. R., Nahmias A. J., Harwell R. W., Pauls F. P. Association of antigenic type of Herpesvirus hominis with site of viral recovery. J Immunol. 1967 Nov;99(5):974–980. [PubMed] [Google Scholar]
- Fuccillo D. A., Moder F. L., Catalano L. W., Jr, Vincent M. M., Sever J. L. Herpesvirus hominis types I and II: a specific microindirect hemagglutination test. Proc Soc Exp Biol Med. 1970 Mar;133(3):735–739. doi: 10.3181/00379727-133-34554. [DOI] [PubMed] [Google Scholar]
- Fuccillo D. A., Moder F., Traub R. G., Hensen S., Sever J. L. Micro indirect hemagglutination test for Cytomegalovirus. Appl Microbiol. 1971 Jan;21(1):104–107. doi: 10.1128/am.21.1.104-107.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A. M., Lauter C. B., Nolan D. C., Shippey M. J. Passive hemagglutinating antibodies in cerebrospinal fluids in herpesvirus hominis encephalitis. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1460–1466. doi: 10.3181/00379727-140-36696. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Chiang W. T., Del Buono I., Duffey A. Typing of Herpesvirus hominis strains by a direct immunofluorescent technique. Proc Soc Exp Biol Med. 1969 Oct;132(1):386–390. doi: 10.3181/00379727-132-34221. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Dowdle W. R., Naib Z. M., Josey W. E., Luce C. F. Genital infection with Herpesvirus hominis types 1 and 2 in children. Pediatrics. 1968 Oct;42(4):659–666. [PubMed] [Google Scholar]
- Peutherer J. F. The specificity of rabbit antisera to Herpesvirus hominis and its dependence on the dose of virus inoculated. J Med Microbiol. 1970 May;3(2):267–272. doi: 10.1099/00222615-3-2-267. [DOI] [PubMed] [Google Scholar]
- SCOTT L. V., FELTON F. G., BARNEY J. A. Hemagglutination with herpes simplex virus. J Immunol. 1957 Mar;78(3):211–213. [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Comparison of various methods for preparation of viral serological antigens from infected cell cultures. Appl Microbiol. 1971 Feb;21(2):217–226. [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Woodie J. D., Ho H. H. Immunofluorescent staining in the laboratory diagnosis of varicella-zoster virus infections. J Lab Clin Med. 1965 Sep;66(3):403–412. [PubMed] [Google Scholar]
- Schneweis K. E., Nahmias A. J. Antigens of Herpes simplex virus type 1 and 2-immunodiffusion and inhibition passive hemagglutination studies. Z Immunitatsforsch Exp Klin Immunol. 1971 Jun;141(5):471–487. [PubMed] [Google Scholar]
- Walker W. S., Fishman M., Adler F. L. Detection of anti-phage antibody by passive hemagglutination. II. Early antibodies produced in vivo or in vitro. J Immunol. 1971 Oct;107(4):953–956. [PubMed] [Google Scholar]



