Abstract
CD24 is a small heavily glycosylated glycosylphosphatidylinositol-linked cell surface protein, which is expressed in hematological malignancies as well as in a large variety of solid tumors. Very recently its expression in ovarian cancer has been found on RNA level by chip analysis. We evaluated CD24 protein expression by immunohistochemistry in 9 normal ovaries and 69 epithelial ovarian tumors (5 adenomas, 8 borderline tumors, and 56 carcinomas) with known follow-up data. Surface epithelium of normal ovaries as well as adenomas did not express CD24. In borderline tumors CD24 was expressed in membrane in 75% of cases, whereas cytoplasmic expression was detected in only one of nine cases. In invasive ovarian carcinomas, a membranous expression was detected in 84% and a cytoplasmic expression in 59% of cases. In univariate survival analysis of all invasive ovarian carcinomas, a highly significant association of increased cytoplasmic CD24 expression with shortened patient survival (mean 98 months versus 37 months, P = 0.0002, log rank test) was demonstrated. Other significant prognostic parameters were International Federation of Gynecology and Obstetrics (FIGO) stage, Silverberg grade, patient age, undifferentiated histological type, and metastatic disease. We did not detect a significant correlation of CD24 with these clinicopathological parameters. In multivariate analysis, only CD24 and FIGO stage were independent prognostic parameters. Our data suggest that the expression of CD24 as detected by immunohistochemistry is a new independent molecular marker for shortened survival time of patients with epithelial ovarian carcinomas.
Ovarian cancer is the leading cause of death from gynecologic malignancy in the United States and Europe, with another 23,300 new cases expected for 2002 in the United States alone. 1,2 The high mortality rate is usually ascribed to late diagnosis of this tumor, which lacks early symptoms. But even in late stages of the disease, the courses are highly variable. Clinicians and pathologists have tried to predict the biology of the tumor and thus the course of the disease in the individual patient to adjust therapy accordingly. Well established conventional prognostic markers are International Federation of Gynecology and Obstetrics (FIGO) stage, grade, patient age, and residual tumor. 3 In addition to these clinicopathological parameters, molecular markers are being sought and established for a wide variety of tumors. 4-9 To identify novel potential diagnostic or therapeutic target genes we screened electronic gene expression libraries. 10 A gene we identified as up-regulated in electronic ovarian cancer libraries is CD24. This is a small, heavily glycosylated mucin-like glycosylphosphatidylinositol (GPI)-linked cell surface protein, which is physiologically expressed in developing or regenerating tissue and also in granulocytes, pre-B-cells, keratinocytes, and renal tubular epithelium. 11-20 In neoplasia its expression has been described initially in hematological malignancies but also in a large variety of solid tumors, eg, renal cell carcinoma, small cell lung cancer, nasopharyngeal carcinoma, hepatocellular carcinoma, bladder carcinoma, glioma, breast cancer, and, recently, in ovarian cancer. 20-31 CD24 is a ligand of P-selectin, an adhesion receptor on activated endothelial cells and platelets, and thus might contribute to the metastasizing capacities of CD24-expressing tumor cells. 32-36 To date very little is known about CD24 in ovarian cancer. The aim of this study was to investigate the expression of CD24 in ovarian cancer and to evaluate its prognostic significance.
Patients and Methods
Patients
Tissue samples from 61 patients with epithelial ovarian tumors which were diagnosed at the Institute of Pathology, Charité Hospital, Berlin, between 1998 and 2001 were included in this study. Furthermore, nine cases with normal ovaries from hysterectomy specimens resected for non-ovarian disease were added. The cases were selected based on availability of tissue and were not stratified for known preoperative or pathological prognostic factors. The tumor cases encompassed 56 cases with histologically confirmed invasive ovarian carcinoma, 8 borderline tumors (LMP tumors), and 5 cystadenomas. Ages of the carcinoma patients ranged from 24 to 80 years (median 58 years). Clinicopathological characteristics of the tumor set are described in Table 1 ▶ . The stage of tumors was assessed according to the International Federation of Gynecology and Obstetrics. Tumors were graded according to the Silverberg grading system. All cases were reevaluated for grade and histological type by the same pathologist (S.H.). Histology was determined according to the criteria of the World Health Organization.
Table 1.
Clinicopathological Parameters of Patients with Different Epithelial Ovarian Tumors or Normal Ovaries
| Parameter | Number of cases |
|---|---|
| Total number | 78 |
| Histology | |
| Normal ovaries | 9 |
| Cystadenomas | 5 |
| Borderline tumors | 8 |
| Invasive carcinomas | 56 |
| Serous | 29 |
| Mucinous | 5 |
| Endometrial | 7 |
| Clear cell | 2 |
| Transitional | 1 |
| Undifferentiated | 12 |
| pT stage | |
| pT1 | 11 |
| pT2 | 7 |
| pT3 | 38 |
| FIGO stage | |
| I | 10 |
| II | 6 |
| III | 36 |
| IV | 4 |
| Silverberg grade | |
| G1 | 11 |
| G2 | 23 |
| G3 | 22 |
Immunohistochemistry
Formalin-fixed paraffin embedded tissue was freshly cut (4 μm). The sections were mounted on Superfrost slides (Menzel Gläser, Braunschweig, Germany), dewaxed with xylene, and gradually hydrated. Antigen retrieval was achieved by pressure cooking in 0.01 mol/L citrate buffer for 5 minutes. The primary CD24 antibody (Ab-2, clone 24C02, Neomarkers, Fremont, CA) was diluted 1:100 using a background reducing dilution buffer from DAKO (Hamburg, Germany). No other blocking agents were used. The primary antibody was incubated at room temperature for 2 hours. Detection took place by the conventional labeled streptavidin-biotin (LSAB-kit, DAKO) method with alkaline phosphatase as the reporting enzyme according to the manufacturer’s instructions. Fast Red (Sigma-Aldrich, Munich, Germany) served as chromogen. Afterward the slides were briefly counterstained with hematoxylin and aqueously mounted.
For evaluation of the staining, two pathologists, who were unaware of patient outcome, independently examined the slides. The membranous and the cytoplasmic staining intensity of CD24 were evaluated separately and scored semiquantitatively as CD24 negative, weak, moderate, or strong positive. For Kaplan-Meier analysis, patients with tumors that showed a weak, moderate or strong CD24 immunoreactivity were combined to a CD24 positive group and survival times were compared to patients with tumors negative for CD24.
Statistical Analysis
For statistical evaluation the SPSS software v. 10.0 was used. We used Fisher’s exact test to assess the statistical significance of the correlation between expression of CD24 and clinicopathological parameters. For survival analysis, we analyzed only the 56 patients with invasive ovarian carcinoma by Kaplan-Meier analysis. Log rank test was used to compare different survival curves. Multivariate survival analysis was performed on all parameters that were found to be significant on univariate analysis using the Cox regression model. P values <0.05 were considered significant.
Results
CD24 Immunostaining in Different Ovarian Lesions
Normal ovarian surface epithelium (9 cases) had no immunoreactivity for CD24 (Figure 1B ▶ , right), while normal salpingeal mucosa showed a membranous staining of the luminal cell surface with no cytoplasmic staining at all (Figure 1A ▶ , left). Borderline tumors stained similar to salpingeal epithelium with a moderate to strong membranous staining in 6 of 8 tumors (Figure 1C) ▶ , while only one borderline tumor showed a weak cytoplasmic staining.
Figure 1.
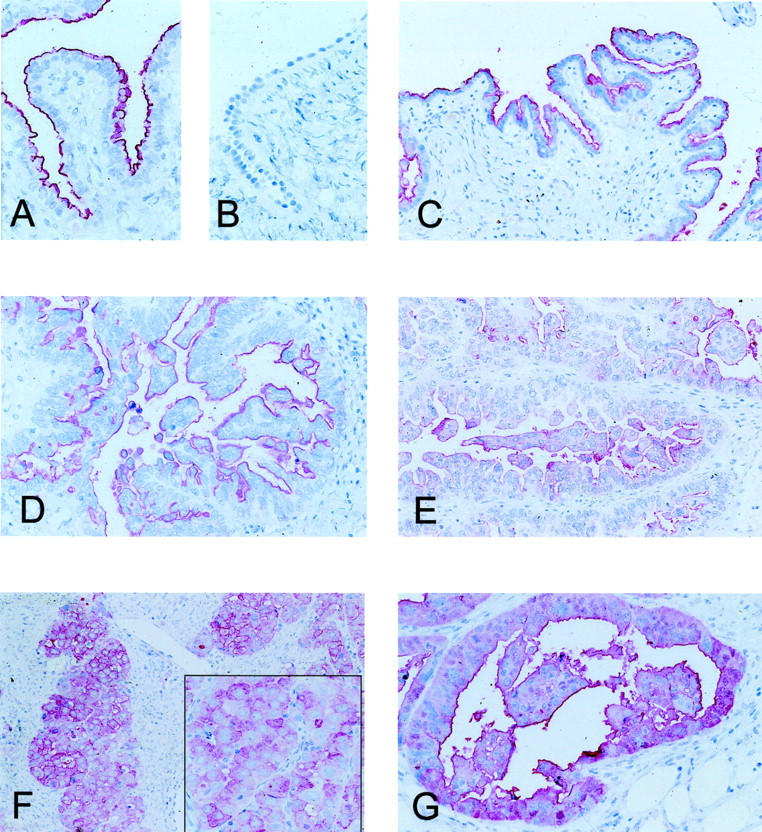
CD24 Immunohistochemistry. A: Physiological salpingeal mucosa with a strong luminally polarized membranous staining. B: Ovarian surface epithelium without any signal. C and D: Membranous staining without cytoplasmic reactivity in a borderline tumor of the ovary (C) and a serous carcinoma (D). E: Serous ovarian carcinoma with a weak cytoplasmic staining. F and G: Two invasive ovarian carcinomas with strong cytoplasmic staining, which is in part circumferentially accentuated.
A moderate to strong membranous CD24 staining was also observed in 84% of the invasive carcinomas (Figure 1, D–G) ▶ . The membranous staining was significantly increased in ovarian carcinomas and borderline tumors compared to normal ovaries and cystadenomas (P < 0.0001; Table 2 ▶ ). In contrast to the benign lesions an additional cytoplasmic staining was detected in 59% of the invasive carcinomas. This difference was found to be statistically significant (P = 0.04, Fisher’s exact test; Table 2 ▶ ). The correlations between membranous or cytoplasmic CD24 staining and clinicopathological parameters were investigated in univariate analyses. We found no significant association of cytoplasmic CD24 staining intensity and tumor type, grade, patient age, or FIGO stage. Similarly, no correlations between membranous CD24 immunoreactivity and these clinicopathological parameters were obtained (Table 3) ▶ .
Table 2.
Cytoplasmic and Membranous Expression of CD24 in Normal Ovaries and Benign and Malignant Ovarian Tumors
| CD24 | Invasive carcinomas (n = 56; 100%) | Borderline tumors (n = 8; 100%) | Cystadenomas (n = 5; 100%) | Normal ovaries (n = 9; 100%) |
|---|---|---|---|---|
| Cytoplasmic | ||||
| Negative | 23 (41.1%) | 7 (87.5%) | 5 (100%) | 9 (100%) |
| Weak | 12 (21.4%) | 1 (12.5%) | 0 (0%) | 0 (0%) |
| Moderate | 12 (21.4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Strong | 9 (16.1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Membranous | ||||
| Negative | 5 (8.9%) | 2 (25%) | 5 (100%) | 9 (100%) |
| Weak | 4 (7.1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Moderate | 32 (57.1%) | 4 (50%) | 0 (0%) | 0 (0%) |
| Strong | 15 (26.8%) | 2 (25%) | 0 (0%) | 0 (0%) |
Table 3.
Relationship Between Cytoplasmic CD24 Expression and Various Clinicopathological Factors in All Patients with Invasive Ovarian Carcinoma
| Characteristic | All cases | Cytoplasmic CD24-negative | Cytoplasmic CD24-positive | Significance |
|---|---|---|---|---|
| All carcinomas | 56 (100%) | 23 (41.1%) | 33 (58.9%) | |
| Age at surgery (years) | 0.422* | |||
| ≤60 | 30 (100%) | 14 (46.7%) | 16 (53.3%) | |
| >60 | 26 (100%) | 9 (34.6%) | 17 (65.4%) | |
| Histological grade (Silverberg) | 0.184† | |||
| G1 | 11 (100%) | 7 (63.6%) | 4 (36.4%) | |
| G2 | 23 (100%) | 7 (30.4%) | 16 (69.6%) | |
| G3 | 22 (100%) | 9 (40.9%) | 13 (59.1%) | |
| Histological type | 0.385† | |||
| Serous | 29 (100%) | 14 (48.3%) | 15 (51.7%) | |
| Undifferentiated | 12 (100%) | 3 (25%) | 9 (75%) | |
| Non-serous | 15 (100%) | 6 (40%) | 9 (60%) | |
| pT | 0.934† | |||
| pT1 | 11 (100%) | 5 (45.5%) | 6 (54.5%) | |
| pT2 | 7 (100%) | 3 (42.9%) | 4 (57.1%) | |
| pT3 | 38 (100%) | 15 (39.5%) | 23 (60.5%) | |
| pN | 0.725* | |||
| pN0 | 14 (100%) | 7 (50%) | 7 (50%) | |
| pN1 | 17 (100%) | 7 (41.2%) | 10 (58.8%) | |
| pM | 0.636* | |||
| pMX | 52 (100%) | 22 (42.3%) | 30 (57.7%) | |
| pM1 | 4 (100%) | 1 (25%) | 3 (75%) | |
| FIGO stage | 0.823† | |||
| I | 10 (100%) | 5 (50%) | 5 (50%) | |
| II | 6 (100%) | 2 (33.3%) | 4 (66.7%) | |
| III | 36 (100%) | 15 (41.7%) | 21 (58.3%) | |
| IV | 4 (100%) | 1 (25%) | 3 (75%) |
*Fisher’s test.
†χ2 test.
CD24 Expression and Patient Survival
In univariate survival analyses, cumulative survival curves were calculated according to the Kaplan-Meier method. Differences in the survival were assessed with the log-rank test. First, we analyzed established prognostic predictors of patient survival to confirm the representativity of our tumor collective. Kaplan-Meier analysis demonstrated a significant impact of well known clinicopathological prognostic parameters such as tumor grade (P = 0.007), FIGO stage (P = 0.001), metastatic disease (P = 0.0004), patient age (P = 0.015), and undifferentiated histological type (P = 0.02) on patient survival (Figure 2, C and D ▶ ; Table 4 ▶ ).
Figure 2.
Univariate survival analysis of CD24 and other clinicopathological parameters in all 56 invasive ovarian carcinomas. Cytoplasmic CD24 expression is a prognostic factor for poor survival (P = 0.0002, A), while membranous expression of CD24 has no influence on patient survival (B). Other prognostic parameters are FIGO stage (P = 0.001, C) and Silverberg grade (P = 0.007, D).
Table 4.
Univariate Survival Analysis (Kaplan-Meier): Survival Times of All Patients with Invasive Ovarian Carcinomas According to Clinicopathological Factors and Cytoplasmic or Membranous CD24 Expression
| Characteristic | No. of cases | Mean survival time (months ± SE) | Median survival time (months ± SE) | Log rank |
|---|---|---|---|---|
| Cytoplasmic CD24 expression | 0.0002 | |||
| Negative | 23 | 97.8 ± 11.8 | n.r. | |
| Positive | 33 | 36.5 ± 6.3 | 30.1 ± 3.3 | |
| Membranous CD24 expression | 0.94 | |||
| Negative | 5 | 66.0 ± 22.5 | n.r. | |
| Positive | 51 | 65.8 ± 8.9 | 52.5 ± 13.1 | |
| Age at surgery (y) | 0.015 | |||
| ≤60 | 30 | 82.8 ± 11.2 | n.r. | |
| >60 | 26 | 39.3 ± 7.2 | 32.7 ± 4.3 | |
| Histological grade (Silverberg) | 0.007 | |||
| G1 | 11 | 109.9 ± 10.3 | n.r. | |
| G2 | 23 | 36.1 ± 5.8 | 34.6 ± 7.9 | |
| G3 | 22 | 35.1 ± 5.1 | 37.9 ± 5.0 | |
| Histological type | 0.02 | |||
| Serous | 29 | 66.3 ± 10.5 | 52.5 ± 12.1 | |
| Non-serous | 15 | 69.2 ± 8.9 | n.r. | |
| Undifferentiated | 12 | 20.2 ± 4.2 | 17.8 ± 8.4 | |
| pT | 0.08 | |||
| pT1 | 11 | 99.6 ± 8.5 | n.r. | |
| pT2 | 7 | 50.3 ± 11.6 | 52.5 ± 22.9 | |
| pT3 | 38 | 56.8 ± 10.7 | 37.9 ± 4.3 | |
| pN | 0.19 | |||
| pN0 | 14 | 66.8 ± 9.0 | n.r. | |
| pN1 | 17 | 29.7 ± 2.5 | n.r. | |
| pM | 0.0004 | |||
| pMX | 52 | 69.6 ± 8.7 | 52.5 ± 13.7 | |
| pM1 | 4 | 9.4 ± 5.2 | 0.8 ± 5.3 | |
| FIGO stage | 0.001 | |||
| I | 10 | 98.5 ± 9.5 | n.r. | |
| II | 6 | 47.6 ± 12.5 | 52.5 ± 39.1 | |
| III | 36 | 62.4 ± 11.4 | 37.9 ± 8.1 | |
| IV | 4 | 9.4 ± 5.2 | 0.8 ± 5.3 |
n.r., not reached.
The overall survival of all patients with ovarian carcinoma was compared according to cytoplasmic or membranous CD24 expression. Both expression patterns were analyzed separately. The mean survival time for patients with tumors without cytoplasmic CD24 staining was 98 months compared to 37 months for patients with tumors with cytoplasmic CD24 expression (P = 0.0002, Figure 2A ▶ ; Table 4 ▶ ). In contrast, the intensity of membranous CD24 staining was not predictive of patient survival (P = 0.94, Figure 2B ▶ ; Table 4 ▶ ).
Multivariate Survival Analysis
A multivariate progression analysis based on the Cox proportional hazard model was performed to test the independent value of each parameter predicting overall survival. Cytoplasmic expression of CD24 as well as other clinicopathological parameters that were significant in univariate analysis (age, FIGO stage, grade, histological type) were included in multivariate analysis (Table 5) ▶ . Cytoplasmic expression of CD24 was found to be an independent prognostic factor for poor overall survival (relative risk (RR) 7.77, 95% CI 2.3–26.8, P = 0.001). Of the other parameters, only FIGO stage was demonstrated as an independent prognostic factor (P = 0.003) for overall survival, while undifferentiated histological type was of borderline significance (P = 0.051).
Table 5.
Multivariate Survival Analysis (Cox Regression Model)
| Characteristic | β | Standard error | Wald | df | RR | 95% CI of RR | P |
|---|---|---|---|---|---|---|---|
| Cytoplasmic CD24 | 0.001 | ||||||
| Negative | 1.00 | ||||||
| Positive | 2.050 | 0.632 | 10.5 | 1 | 7.77 | 2.3–26.8 | 0.001 |
| FIGO stage | 11.9 | 2 | 0.003 | ||||
| I | 1.00 | ||||||
| II–III | 1.603 | 1.041 | 2.4 | 1 | 4.97 | 0.64–38.1 | 0.124 |
| IV | 3.781 | 1.234 | 9.4 | 1 | 43.85 | 3.90–492.3 | 0.002 |
| Histological diagnosis | 0.051 | ||||||
| Serous or non-serous | 1.00 | ||||||
| Undifferentiated | 1.001 | 0.513 | 3.8 | 1 | 2.72 | 0.995–7.44 | 0.051 |
Discussion
In this study we describe the expression of CD24 protein as detected by immunohistochemistry in benign and malignant epithelial ovarian tumors. We confirm that CD24 protein is expressed in a subset of invasive ovarian tumors, as predicted by our analysis of electronic gene expression libraries and as recently found by others on RNA level using chip-based transcript profiling. 31 CD24 expression has been investigated in several human tissues. It was described as a B-cell marker expressed during development or in B cell neoplasia. 11-13,21,22 Physiologically, CD24 is also expressed in the developing brain and pancreas, regenerating muscle, keratinocytes and renal tubules. 14-20 In non-hematological malignancies, CD24 expression has been detected in renal cell carcinoma, small cell lung cancer, nasopharyngeal carcinoma, hepatocellular carcinoma, bladder cancer, breast cancer, and glioma. 20,23-30 As it is so widely expressed, CD24 expression cannot be used as a specific marker for ovarian carcinomas.
In our study, we found no CD24 staining of normal ovarian surface epithelium. Normal salpingeal mucosa cells showed a membranous staining of the luminal border of which might be expected of a membrane bound cell surface molecule, although the biological function of CD24 in this organ is still unknown. In ovarian tumors we observed another quality of staining. In addition to the polarized membranous staining which was quite ubiquitous, a variable degree of cytoplasmic staining emerged. So far we can only speculate if this cytoplasmic expression of CD24 reflects an overproduction of the protein or a disturbance of protein distribution or degradation within the cell. Cytoplasmic CD24 expression was observed only in invasive ovarian carcinomas and was not found in normal ovaries and ovarian adenomas. In eight borderline tumors only a single weakly positive case was detected. Moreover, we found that cytoplasmic CD24 expression was a strong and independent predictor of short overall survival as evidenced by Kaplan-Meier curves and multivariate Cox proportional hazards regression analysis. This leads to the question of the underlying biological mechanism in this tumor.
There is in vitro evidence suggesting a pro-metastatic role of CD24 in human tumor cells as CD24 can function as a ligand to P-selectin. 32-34 Physiologically P-selectin is expressed by activated endothelial cells and platelets and plays an important role in marginal adhesion and migration of cells under shear forces in the bloodstream. Its primary ligand is P-selectin glycoprotein ligand-1 (PSGL-1), which is expressed by neutrophils. It is conceivable that CD24-expressing tumor cells can spread more easily due to their capacity to form thrombi with activated platelets or to adhere to endothelia in the bloodstream, which has been shown for CD24 expressing breast cancer cells. 35 Still, it remains unclear whether this P-selectin-dependent mechanism of hematogenous tumor metastasis is the basis for the shortened survival we observed in ovarian cancers with cytoplasmic CD24 expression. In ovarian carcinomas, hematogenous metastasis is usually an infrequent and late event. In our study, only four cases had known metastases at the time of diagnosis. Three of these cases were positive for CD24. It would be interesting to investigate the correlation between CD24 expression and metastasis for a larger number of metastatic ovarian carcinomas. Peritoneal mesothelial cells do not express P-selectin, so the initial metastatic spread in the peritoneal cavity cannot be based on CD24-P-selectin interaction. However, in ovarian cancer the tumor-mesothelium interaction might be mediated via a yet unknown CD24 ligand. Possibly, CD24 does not only implicate a higher metastatic potential (via selectins) but also confers an increased invasiveness of a tumor as observed in glioma by another yet unexplained mechanism. 26
So far, there are few reports of a prognostic significance of CD24 expression in human tumors. In acute lymphatic leukemia a reduction of CD24 expression appeared to predict a better prognosis. 13 To our knowledge, this study demonstrates for the first time a highly significant prognostic value of CD24 expression in a solid non-hematological tumor. Further confirming studies are needed to verify our results to establish CD24 as a molecular prognostic marker in ovarian cancer. This might aid the clinician to select an appropriate therapy for the individual patient, eg, favoring a more aggressive regimen in tumors with a strong cytoplasmic CD24 expression.
Another aspect of our findings is of importance, that is that the expression of CD24 in ovarian cancer, which is in part membrane bound, might bear therapeutic options if targeted with therapeutic antibodies. Transplantation associated B-cell proliferative syndrome had been treated with intravenous administration of CD21- and CD24-specific antibodies by Fischer et al 37-39 : in 16 of 26 cases a remission could be achieved, and the treatment was relatively well tolerated. As ovarian cancer is restricted to the peritoneal cavity until late stages, a therapeutic intraperitoneal application of CD24 antibodies might be considered.
In summary, we found that CD24 is commonly expressed in ovarian cancer and that immunohistochemically determined CD24 expression is a strong and independent molecular marker of prognosis in this disease. The biological basis and significance of this phenomenon is yet unclear and warrants further studies.
Footnotes
Address reprint requests to Prof. Dr. Steffen Hauptmann, Institute of Pathology, Charité Hospital, Campus Mitte, Schumannstrasse 20/21, D-10117 Berlin, Germany. E-mail: steffen.hauptmann@charite.de.
G.K. and C.D. contributed equally to this work.
References
- 1.Jemal A, Thomas A, Murray T, Thun M: Cancer statistics, 2002. CA Cancer J Clin 2002, 52:23-47 [DOI] [PubMed] [Google Scholar]
- 2.Pisani P, Parkin DM, Bray F, Ferley J: Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999, 83:18-29 [DOI] [PubMed] [Google Scholar]
- 3.Trope C: Prognostic factors in ovarian cancer. Cancer Treat Res 1998, 95:287-352 [DOI] [PubMed] [Google Scholar]
- 4.Masciullo V, Ferrandina G, Pucci B, Fanfani F, Lovergine S, Palazzo J, Zannoni G, Mancuso S, Scambia G, Giordano A: P27/Kip1 Expression is associated with clinical outcome in advanced epithelial ovarian cancer: multivariate analysis. Clin Cancer Res 2000, 6:4816-4822 [PubMed] [Google Scholar]
- 5.Gotlieb WH, Goldberg I, Weisz B, Davidson B, Novikov I, Kopolovic J, Ben-Baruch G: Topoisomerase II immunostaining as a prognostic marker for survival in ovarian cancer. Gynecol Oncol 2001, 82:99-104 [DOI] [PubMed] [Google Scholar]
- 6.Shen GH, Ghazizadeh M, Kawanami O, Shimizu H, Araki T, Sugisaki Y: Prognostic significance of vascular endothelial growth factor expression in human ovarian cancer. Br J Cancer 2000, 83:196-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmalfeldt B, Prechtel D, Härting K, Späthe K, Rutke S, Konik E, Fridman R, Berger U, Schmitt M, Kuhn W, Lengyel E: Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res 2001, 7:2396-2404 [PubMed] [Google Scholar]
- 8.Hata K, Fujiwaki R, Nakayama K, Miyazaki K: Expression of the endostatin gene in epithelial ovarian cancer. Clin Cancer Res 2001, 7:2405-2409 [PubMed] [Google Scholar]
- 9.Luo LY, Katsaros D, Scorilas A, Fracchioli S, Pincinno R, Rigault de la Longrais IA, Howarth DJC, Diamandis EP: Prognostic value of human kallikrein 10 expression in epithelial ovarian cancer. Clin Cancer Res 2001, 7:2372-2379 [PubMed] [Google Scholar]
- 10.Schmitt AO, Specht T, Beckmann G, Dahl E, Pilarsky CP, Hinzmann B, Rosenthal A: Exhaustive mining of EST libraries for genes differentially expressed in normal and tumor tissues. Nucleic Acids Res 1999, 27:4251-4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirucello SJ, LeBien TW: The human B cell-associated antigen CD24 is a single chain sialoglycoprotein. J Immunol 1986, 136:3779-3784 [PubMed] [Google Scholar]
- 12.Fischer GF, Majdic O, Gadd S, Knapp W: Signal transduction in lymphocytic and myeloid cells via CD24, a new member of phosphoinositol-anchored membrane molecules. J Immunol 1990, 144:638-641 [PubMed] [Google Scholar]
- 13.Lavabre-Bertrand T, Duperray C, Brunet C, Poncelet P, Exbrayat C, Bourquard P, Lavabre-Bertrand C, Brochier J, Navarro M, Janossy G: Quantification of CD24 and CD45 antigens in parallel allows a precise determination of B-cell maturation stages: relevance for the study of B-cell neoplasias. Leukemia 1994, 8:402-408 [PubMed] [Google Scholar]
- 14.Akashi T, Shirasawa T, Hirokawa K: Gene expression of CD24 core polypeptide molecule in normal rat tissues and human tumor cell lines. Virchows Arch 1994, 425:399-406 [DOI] [PubMed] [Google Scholar]
- 15.Poncet C, Frances V, Gristina R, Scheiner C, Pellissier JF, Figarella-Branger D: CD24, a glycosylphosphatidylinositol-anchored molecules is transiently expressed during the development of human central nervous system and is a marker of human neural cell lineage tumors. Acta Neuropathol (Berl) 1996, 91:400-408 [DOI] [PubMed] [Google Scholar]
- 16.Cram DS, McIntosh A, Oxbrow L, Johnston AM, DeAizpurua HJ: Differential mRNA display analysis of two related but functionally distinct rat insulinoma (RIN) cell lines: identification of CD24 and its expression in the developing pancreas. Differentiation 1999, 64:237-246 [DOI] [PubMed] [Google Scholar]
- 17.Shirasawa T, Akashi T, Sakamoto K, Takahashi H, Maruyama N, Hirokawa K: Gene expression of CD24 core peptide molecule in developing brain and developing non-neural tissues. Dev Dyn 1993, 198:1-13 [DOI] [PubMed] [Google Scholar]
- 18.Figarella-Branger D, Moreau H, Pellissier JF, Bianco N, Rougon G: CD24, a signal-transducing molecule expressed on human B lymphocytes, is a marker for human regenerating muscle. Acta Neuropathol (Berl) 1993, 86:275-284 [DOI] [PubMed] [Google Scholar]
- 19.Redondo P, Garcia-Foncillas J, Okroujnov I, de Felipe I, Quintanilla E: CD24 expression on human keratinocytes. Exp Dermatol 1998, 7:175-178 [DOI] [PubMed] [Google Scholar]
- 20.Droz D, Zachar D, Charbit L, Gogusev J, Chrétien Y, Iris L: Expression of the human nephron differentiation molecules in renal cell carcinoma. Am J Pathol 1990, 137:895-905 [PMC free article] [PubMed] [Google Scholar]
- 21.Pirrucello SJ, Lang MS: Differential expression of CD24-related epitopes in mycosis fungoides/Sezary syndrome: a potential marker for circulating Sezary cells. Blood 1990, 76:2343-2347 [PubMed] [Google Scholar]
- 22.Raife TJ, Lager DJ, Kemp JD, Dick FR: Expression of CD24 (BA-1) predicts monocytic lineage in acute myeloid leukemia. Am J Clin Pathol 1994, 101:296-299 [DOI] [PubMed] [Google Scholar]
- 23.Jackson D, Waibel R, Weber E, Bell J, Stahel RA: CD24, a signal-transducing molecule expressed on human B cells, is a major surface antigen on small cell lung carcinomas. Cancer Res 1992, 52:5264-5270 [PubMed] [Google Scholar]
- 24.Karran L, Jones M, Morley G, van Noorden S, Smith P, Lampert I, Griffin BE: Expression of a B-cell marker, CD24, on nasopharyngeal carcinoma cells. Int J Cancer 1995, 60:562-566 [DOI] [PubMed] [Google Scholar]
- 25.Huang LR, Hsu HC: Cloning and expression of CD24 gene in human hepatocellular carcinoma: a potential early tumor marker gene correlates with p53 mutation and tumor differentiation. Cancer Res 1995, 55:4717-4721 [PubMed] [Google Scholar]
- 26.Senner V, Sturm A, Baur I, Schrell UH, Distel L, Paulus W: CD24 promotes invasion of glioma cells in vivo. J Neuropathol Exp Neurol 1999, 58:795-802 [DOI] [PubMed] [Google Scholar]
- 27.Gromova I, Gromov P, Celis JE: Identification of true differentially expressed mRNAs in a pair of human bladder transitional cell carcinomas using an improved differential display procedure. Electrophoresis 1999, 20:241-248 [DOI] [PubMed] [Google Scholar]
- 28.Fogel M, Friederichs J, Zeller Y, Husar M, Smirnov A, Roitman L, Altevogt P, Sthoeger ZM: CD24 is a marker for human breast carcinoma. Cancer Lett 1999, 143:87-94 [DOI] [PubMed] [Google Scholar]
- 29.Yang GP, Ross DT, Kuang WW, Brown PO, Weigel RJ: Combining SSH and cDNA microarrays for rapid identification of differentially expressed genes. Nucleic Acids Res 1999, 27:1517-1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Vadgama JV: Identification and characterization of amino acid starvation-induced CD24 gene in MCF-7 in human breast cancer cells. Int J Oncol 2000, 16:1049-1054 [DOI] [PubMed] [Google Scholar]
- 31.Welsh JB, Zarrinkar PP, Sapinoso LM, Kern SG, Behling CA, Monk BJ, Lockhart DJ, Burger RA, Hampton GM: Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc Natl Acad Sci USA 2001, 98:1176-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sammar M, Aigner S, Hubbe M, Schirrmacher V, Schachner M, Vestweber D, Altevogt P: Heat-stable antigen (CD24) as ligand for mouse P-selectin. Int Immunol 1994, 6:1027-1036 [DOI] [PubMed] [Google Scholar]
- 33.Aigner S, Ruppert M, Hubbe M, Sammar M, Sthoeger Z, Butcher EC, Vestweber D, Altevogt P: Heat stable antigen (mouse CD24) supports myeloid cell binding to endothelial and platelet P-selectin. Int Immunol 1995, 7:1557-1565 [DOI] [PubMed] [Google Scholar]
- 34.Aigner S, Sthoeger ZM, Fogel M, Weber E, Zarn J, Ruppert M, Zeller Y, Vestweber D, Stahel R, Sammar M, Altevogt P: CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood 1997, 89:3385-3395 [PubMed] [Google Scholar]
- 35.Aigner S, Ramos CL, Hafezi-Moghadam A, Lawrence MB, Friederichs J, Altevogt P, Ley K: CD24 mediates rolling of breast carcinoma cells on P-selectin. EMBO J 1998, 12:1241-1251 [DOI] [PubMed] [Google Scholar]
- 36.Friederichs J, Zeller Y, Hafezi-Moghadam A, Grone HJ, Ley K, Altevogt P: The CD24/P-selectin binding pathway initiates lung arrest of human A125 adenocarcinoma cells. Cancer Res 2000, 60:6714-6722 [PubMed] [Google Scholar]
- 37.Fischer A, Blanche S, Le Bidois J, Bordigoni P, Garnier JL, Niaudet P, Morinet F, Le Deist F, Fischer AM, Griscelli C: Anti-B-cell monoclonal antibodies in the treatment of severe B-cell lymphoproliferative syndrome following bone marrow and organ transplantation. N Engl J Med 1991, 324:1451-1456 [DOI] [PubMed] [Google Scholar]
- 38.Benkerrou M, Jais JP, Leblond V, Durandy A, Sutton L, Bordigoni P, Garnier JL, Le Bidois J, Le Deist F, Blanche S, Fischer A: Anti-B-cell monoclonal antibody treatment of severe post-transplant B-lymphoproliferative disorder: prognostic factors and long-term outcome. Blood 1998, 92:3137-3147 [PubMed] [Google Scholar]
- 39.Garnier JL, Stevenson G, Blanc-Brunat N, Touraine JL, Milpied N, Leblond V, Blay JY: Treatment of post-transplant lymphomas with anti-B-cell monoclonal antibodies. Recent Results Cancer Res 2002, 159:113-122 [DOI] [PubMed] [Google Scholar]



