Abstract
Salivary gland cancers comprise a heterogeneous group of neoplasms whose biological and clinical characteristics differ considerably from those of mucosal squamous cell carcinomas of the head and neck. One of the most common subtypes, adenoid cystic carcinoma (ACC), is notable for its myoepithelial differentiation, proclivity for hematogenous spread, and slow but progressive clinical course. The molecular alterations that underlie its development and progression are poorly characterized. Here we used oligonucleotide microarray analysis to survey the expression of 8920 different human genes in 15 ACCs, one ACC cell line, and five normal major salivary glands. We observed expression of genes indicative of myoepithelial differentiation, as expected, including those whose protein products are components of basement membranes and extracellular matrix. Other genes that were highly ranked for their expression in ACC were those encoding the transcription factors SOX4 and AP-2γ, the latter of which also was overexpressed in ACC relative to 175 other carcinomas from 10 anatomical sites that we had previously profiled. Additional genes, which were highly expressed in ACC compared to the other carcinomas, included casein kinase 1, epsilon and frizzled-7, both members of the Wnt/β-catenin signaling pathway. Our study documents for the first time the diverse spectrum of genes overexpressed in ACC and highlights gene products and pathways that in the future might be exploited as therapeutic targets for this cancer, which up until now, has shown limited response to chemotherapeutic approaches.
Unlike mucosal squamous cell carcinomas of the head and neck, carcinomas of the salivary glands consist of a diverse histopathological spectrum of neoplasms. Adenoid cystic carcinoma (ACC) is one of the most common subtypes of salivary gland cancer, and in several series is the most frequent malignant tumor of the submandibular and minor salivary glands. 1,2 Histopathologically, ACC often forms both true lumens as well as pseudolumens in varying proportions. It characteristically shows myoepithelial differentiation and produces in large amounts specific proteins that comprise basement membranes and extracellular matrix. 3 The neoplasm has a proclivity for invading nerves, but it infrequently spreads via the lymphatic system. ACC has a protracted clinical course with local recurrences, hematogenous metastases, and poor response to classical chemotherapeutic approaches. After surgery and radiation therapy for patients with ACC, the disease-specific survival at 15 years is ∼40%. 4
The constellation of genes that are critical for the development and progression of ACC are not known. Furthermore, genes whose expression in ACC is altered relative to those in normal salivary glands and other carcinomas have not yet been compiled. In this study, we used large-scale microarray analysis in an effort to characterize the expression profiles in this salivary gland malignancy, and to specifically identify those genes differentially expressed between ACC and normal salivary gland epithelium. Importantly, we have also compared the compendium of expressed genes in ACC with that for 175 other carcinomas representing the 10 most common types of fatal carcinoma in the United States to determine which genes are uniquely expressed in ACC, and which specific pathways might be exploited for novel therapeutic approaches in this malignancy.
Materials and Methods
Tissue Specimens, cRNA Synthesis, Oligonucleotide Array Analysis
The use of human tissue samples from the University of Virginia was approved by the UVA Human Investigation Committee, whereas those from M.D. Anderson Cancer Center were approved by the M.D. Anderson Institutional Review Board. The histological distinction of ACC from other salivary gland tumors was made using established criteria, 5 whereas the grade of ACC was determined using the criteria of Szanto and colleagues 6 in which grade I tumors had no solid component, grade II ACC had <30% solid areas, and grade III cancers contained >30% solid components. Hematoxylin and eosin (H&E)-stained frozen sections of portions of normal submandibular gland from two patients and normal parotid gland from three patients were examined to assess the relative amount of normal epithelium versus stroma and lymphocytes; frozen tissue blocks were then trimmed to enrich for normal salivary gland epithelium, while stroma and lymphocytes were avoided as much as possible. Frozen tumor samples from 15 ACCs that consisted predominantly of neoplastic cells were also selected. It is estimated that trimmed frozen tissues consisted of at least 75% neoplastic cells. Of the 15 patients with ACC, the age range was 26 to 74 years (median, 48 years). Ten patients were men and five were women. Five ACCs arose from a major salivary gland, and 10 arose in minor salivary glands. Seven ACCs were grade I, five were grade II, and three were grade III.
Frozen specimens were stored at −80°C before processing for microarray analysis and uniformly provided high-quality RNA. In duplicate analysis, one normal submandibular gland sample and one ACC specimen were each divided and processed independently. Several milligrams of each sample were sharply dissected and homogenized with a rotary homogenizer in RNeasy lysis buffer (Qiagen, Valencia, CA). RNA was prepared using the RNeasy Mini Kit (Qiagen). Labeled cRNA was then prepared and hybridized to Hu95a Affymetrix oligonucleotide GeneChips (Affymetrix, Santa Clara, CA) as described previously 7 and references cited therein.
Cell Culture
ACC3 cells, procured from a human ACC, 8 were cultured in RPMI 1640 with 10% fetal calf serum, 1% glutamine, 50 μg/ml streptomycin, and 50 IU/ml penicillin. RNA was extracted and prepared for microarray analysis as above.
Data Analysis
Scanned image files were inspected visually for artifacts and analyzed with GeneChip 3.1 (Affymetrix). Each GeneChip was scaled to an average hybridization intensity of 200, which corresponds to ∼3 to 5 transcripts per cell. 9 Differential expression of genes in normal salivary and ACC specimens was estimated using a hybrid metric 10,11 based on equally weighted contributions from the difference of hybridization intensities, the quotient of hybridization intensities, and the result of an unpaired t-test between expression levels in tumor and normal tissues. The genes were scored with respect to each of the three metrics, and then ranked according to the sum of the three scores. Analysis of variance was used to determine whether there were differences in gene expression according to grade of ACC (I, II, or III) or site (major or minor salivary glands). Genes that were differentially expressed in ACC relative to the 10 most common types of fatal carcinoma previously analyzed were determined using recently described molecular classification methods. 7 For each gene, a Wilcoxon rank score was successfully calculated for samples in each carcinoma class with the highest mean expression versus samples from all of the other carcinoma classes (implemented in Matlab v6.0). The genes with the lowest P values in each carcinoma class were then ranked based on their predictive accuracy for discriminating one carcinoma class versus all other carcinoma classes by leave-out-one cross-validation using a support vector machine classifier. 7 Expression of the ACC classifier genes was compared in ACC samples and normal salivary gland samples using an unpaired t-test to identify those genes with elevated expression in ACC.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
The ACC3 cell line and frozen tissue specimens from five normal major salivary glands and five ACCs were used for RT-PCR analysis. Three of the ACCs had undergone gene expression profiling, while two had not. H&E-stained frozen sections of the ACCs and normal salivary glands were microdissected to obtain target epithelial populations of ∼1000 cells each with at least 95% purity for tumor and, also, at least 95% purity for normal gland using a Leica AS LMD (laser microdissection system) (Leica Microsystems Inc., Bannockburn, IL). Each microdissected sample was subjected to RNA isolation by guanidine isothiocyanate buffer extraction, phenol/chloroform extraction, and alcohol precipitation (Micro RNA isolation kit; Stratagene, La Jolla, CA). The RNA samples were treated with 20 U of RNase-free DNase (Stratagene) for 1 hour at 37°C, followed by phenol/chloroform extraction and alcohol precipitation. First strand cDNA was synthesized in 20-μl reactions using random hexamer primers and Superscript II (Life Technologies, Inc., Gaithersburg, MD). Reactions were incubated on ice for 5 minutes, then at ambient temperature for 5 minutes, then at 42°C for 2 hours. Ten μL of the cDNA solutions were placed in 50-μL PCR containing 2 U of Taq polymerase. Sox 4 primers (5′-GCGGCGGGAGCAGCAAC-3′, 5′-GGAGCCGCAGCTCTTTTTC-3′, 92-bp product) were at 1 μmol/L final concentration and β2-microglobulin primers (5′-ATTCACCCCCACTGAAAAAG-3′, 5′-TCCATGATGCTGCTTACATG-3′, 106-bp product) were at 0.1 μmol/L final concentration. Cycling conditions were: 40 cycles of 94°C for 30 seconds, 55°C for 60 seconds, and 72°C for 30 seconds. PCR products were visualized by agarose gel electrophoresis, ethidium bromide staining, and UV light illumination. Relative RNA concentration was determined by image capture and analysis on an AlphaImager workstation (α Innotech, San Leandro, CA).
Immunohistochemistry
Deparaffinized zinc formalin-fixed, paraffin-embedded tissue sections of normal parotid and submandibular glands, ACC3, and three ACCs that were analyzed for global gene expression were immunostained using antibodies to keratin 17, cyclin D1, collagen IV, laminin, and β-catenin. An independent set of 10 ACCs whose transcripts were not profiled was also immunostained with antibodies to cyclin D1, collagen IV, laminin, and β-catenin to assess the frequency of protein expression in these tumors. Slides were placed in citrate buffer and heated in a microwave oven for 20 minutes before staining for keratin 17, cyclin D1, and β-catenin. Predigestion with protease was used for anti-collagen IV, while trypsin was used for anti-laminin. The primary antibodies selected included a mouse monoclonal antibody to keratin 17 (clone Ks17.E3, 1:50 dilution; Research Diagnostics, Flanders, NJ), a rabbit polyclonal antibody to cyclin D1 (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), a mouse monoclonal antibody to collagen IV (clone CIV22, 1:50 dilution; DAKO, Glostrup, Denmark), a mouse monoclonal antibody to β-catenin (clone 14, 1:200 dilution; BD Biosciences, San Diego, CA), and a mouse monoclonal antibody to laminin (clone LAM-89; 1:50 dilution; Novocastra, Newcastle, UK). After incubation with the primary antibody and the addition of the biotinylated secondary antibody, avidin-biotin immunoperoxidase was applied. Diaminobenzidine was used as the chromogen. Sections were then counterstained with hematoxylin. For the antibodies to keratin 17, cyclin D1, and laminin, immunoreactivity was scored semiquantitatively as negative, 1+ (<1% cells positive), 2+ (1 to 10% cells), 3+ (11 to 25% cells), or 4+ (>25% cells).
Results
Profiles of Gene Expression Distinguish Samples of Normal Salivary Glands and ACC
The expression levels of genes in normal and ACC tissue samples and one ACC-derived cell line (ACC3) were determined by hybridization of RNA to oligonucleotide microarrays comprising 12,533 probe sets representing 8920 unique human genes (listed by RefSeq numbers and Unigene ID). In total we hybridized 23 RNA samples from five normal major salivary glands, 15 ACCs, and one ACC cell line (ACC3). One of the normal salivary gland samples and one ACC sample were divided into approximately equal proportions, processed, and hybridized in duplicate to assess reproducibility of the experiments. Genes that showed the largest variation across the samples were selected to group them based on their similarities in expression levels. 12 With a SD cut-off of 500, 1038 genes were clustered. Using this approach, a dendrogram was constructed, and showed that the samples of normal salivary gland in duplicate were highly correlated, as were the duplicate samples of one ACC (Figure 1) ▶ . Within the dendrogram, the normal submandibular and parotid glands clustered near each other but separately, whereas the 15 ACCs clustered into three groups. No overt differences were seen for patient age, sex, or site of tumor origin between the three clustered groups of tumors. All three grade III ACCs clustered in the largest group of tumors, whereas all ACCs in the smallest group (n = 3) were grade I; three other grade I ACCs were in the largest clustered group, however, and one other was in the third group. Analysis of the ACC3 cell line revealed that it clustered with the largest group of ACCs from patient samples.
Figure 1.
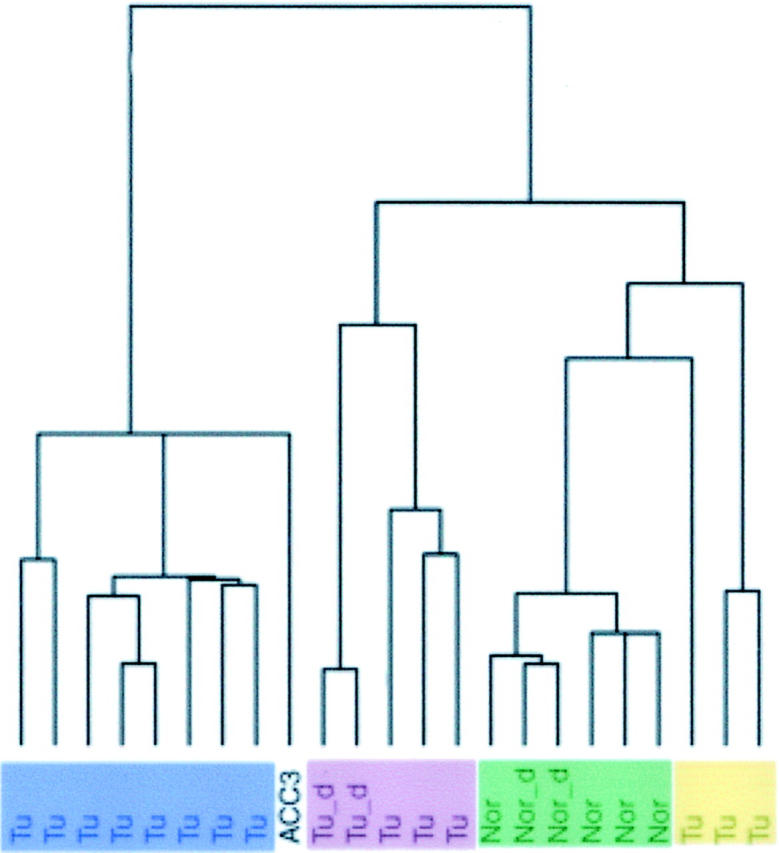
Dendrogram of samples showing overall similarity in gene expression profiles across all samples. Tu, each human ACC sample; Tu_d, duplicate ACC sample; ACC3, cell line; Nor, normal human major salivary gland; Nor_d, duplicate normal salivary gland sample.
Differentially Expressed Genes in ACC and Normal Salivary Glands
As normal major salivary glands and ACCs could be distinguished by their gene expression profile, we used a metric method to rank genes with the largest and most uniform differences in expression between the two sets of tissue samples (Table 1) ▶ . For the 30 genes with the highest ranked differential over-expression in ACL, the fold level ranged from 3.5 to 78 (mean, 13.9; median, 9.2).
Table 1.
List of Highest Ranked Overexpressed Genes in ACC Compared with Normal Salivary Glands
| Affy ID | Symbol | Annotation* | RefSeq number | Unigene ID | P value† | Fold change |
|---|---|---|---|---|---|---|
| 33131_at | SOX4 | SRY (sex determining region Y)-box 4 | NM_003107 | Hs.83484 | 6.92E-07 | 14.9 |
| 40567_at | none | X01703:Human gene for alpha-tubulin (bα 1)/cds = (213 GenBank = X01703 | X01703 (ACC#) | None | 6.94E-07 | 14.8 |
| 38111_at | CSPG2 | Chondroitin sulfate proteoglycan 2 (versican) | NM_004385 | Hs.81800 | 1.72E-06 | 29.5 |
| 33878_at | FLJ13612 | Hypothetical protein FLJ13612 | NM_025202 | Hs.24391 | 1.53E-07 | 9.1 |
| 40454_at | FAT | FAT tumor suppressor homolog 1 (Drosophila) | NM_005245 | Hs.166994 | 6.90E-08 | 6.9 |
| 37678_at | NMA | Putative transmembrane protein | NM_012342 | Hs.78776 | 2.28E-07 | 11.7 |
| 41531_at | TM4SF1 | Transmembrane 4 superfamily member 1 | None | Hs.351316 | 2.19E-07 | 6.3 |
| 2020_at | CCND1 | Cyclin D1 (PRAD1: parathyroid adenomatosis 1) | NM_053056 | Hs.82932 | 9.27E-07 | 9.7 |
| 581_at | LAMB1 | Laminin β 1 | NM_002291 | Hs.82124 | 5.88E-06 | 77.7 |
| 32832_at | MAEA | Macrophage erythroblast attacher | NM_005882 | Hs.20815 | 1.45E-07 | 7.8 |
| 34301_r_at | KRT17 | Keratin 17 | NM_000422 | Hs.2785 | 7.16E-06 | 13.8 |
| 38086_at | IGSF3 | Immunoglobulin superfamily, member 3 | NM_001542 | Hs.81234 | 4.96E-08 | 28.3 |
| 34320_at | PTRF | Polymerase I and transcript release factor | None | Hs.29759 | 8.82E-08 | 5.3 |
| 33178_at | JAG1 | Jagged 1 (Alagille syndrome) | NM_000214 | Hs.91143 | 2.79E-07 | 14.9 |
| 38126_at | BGN | Biglycan | NM_001711 | Hs.821 | 1.40E-07 | 4.8 |
| 34677_f_at | None | AJ012755:Homo sapiens mRNA for TL132/cds = (1241 GenBank = AJ012755 | AJ012755 (ACC#) | None | 2.60E-07 | 9 |
| 38750_at | NOTCH3 | Notch homolog 3 (Drosophila) | NM_000435 | Hs.8546 | 3.03E-07 | 5.9 |
| 40303_at | TFAP2C | Transcription factor AP-2 γ(activating enhancer binding protein 2 gamma) | NM_003222 | Hs.61796 | 1.82E-08 | 5.2 |
| 32749_s_at | FLNA | Filamin A, α(actin-binding protein 280) | NM_001456 | Hs.195464 | 2.20E-07 | 4.6 |
| 32623_at | GABBR1 | γ-Aminobutyric acid (GABA) B receptor, 1 | NM_001470 | Hs.167017 | 2.15E-06 | 14.2 |
| 38692_at | NAB1 | NGFI-A binding protein 1 (EGR1 binding protein 1) | NM_005966 | Hs.107474 | 5.14E-11 | 9.3 |
| 40339_at | GABRP | γ-Aminobutyric acid (GABA) A receptor, π | NM_014211 | Hs.70725 | 2.72E-05 | 42.7 |
| 36186_at | RNPS1 | RNA binding protein S1, serine-rich domain | NM_006711 | Hs.75104 | 1.13E-08 | 4.5 |
| 39333_at | COL4A1 | Collagen, type IV, alpha 1 | NM_001845 | Hs.119129 | 7.38E-06 | 9.4 |
| 38233_at | HOMER-3 | Homer, neuronal immediate early gene, 3 | NM_004838 | Hs.166146 | 2.35E-08 | 8 |
| 36224_g_at | SFPQ | Splicing factor proline/glutamine rich (polypyrimidine tract-binding protein associated) | NM_005066 | Hs.180610 | 9.44E-07 | 5.3 |
| 34296_at | None | ESTs | None | Hs.355863 | 6.54E-07 | 19.7 |
| 34304_s_at | SAT | Spermidine/spermine N1-acetyltransferase | NM_002970 | Hs.28491 | 1.06E-09 | 3.5 |
| 32154_at | TFAP2A | Transcription factor AP-2 α (activating enhancer binding protein 2 α) | NM_003220 | Hs.334334 | 1.42E-07 | 3.8 |
| 37333_at | DNMT1 | DNA (cytosine-5-)-methyltransferase 1 | NM_001379 | Hs.77462 | 3.96E-08 | 17.6 |
*Genes 1, 7, 9, 10, 11, 12, 14, 19, 20, 21, 23, 26, 27, and 30 were overexpressed >3 fold in the ACC3 cell line compared with normal major salivary glands.
†Calculated by unpaired t-test.
According to our ranking metric, the most significantly overexpressed gene in ACC was Sox4, which encodes a transcription factor. We also observed overexpression of other genes encoding transcription factors among the 30 most highly ranked genes, such as AP-2α and γ, and the NGFI-A binding protein 1. Functionally, a group of genes encoding extracellular matrix proteins and basement membrane components such as versican, biglycan, laminin-β1, and type IV collagen-α1 were also identified. Indeed, the highest fold differences between ACC and normal major salivary glands were seen for laminin (78-fold) and versican (29-fold). Overexpressed genes in ACC encoding cytoskeletal or associated proteins included keratin 17, α-tubulin (bα1), and filamin A. Genes encoding membrane proteins were observed at elevated levels in ACC, and included macrophage erythroblast attacher, FAT tumor suppressor, transmembrane 4 superfamily member 1, Notch 3, Homer neuronal immediate early gene 3, GABA A and B receptors, and immunoglobulin superfamily member 3.
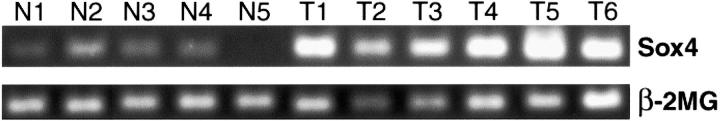
Overexpression of Sox4 transcripts in ACC relative to normal parotid and submandibular glands was validated by RT-PCR using laser-microdissected cells (Figure 2) ▶ . This was observed for each of the five ACCs as well as for ACC3.
Figure 2.
RT-PCR showing amplification of Sox4 transcripts (top row) in laser microdissected cells from frozen sections of five separate normal major salivary glands (N1 to N5) and five different human ACCs (T1 to T5). T6, ACC3 cell line. Amplification of b-2-microglobulin transcripts (bottom row).
We also sought to compare specifically overexpressed transcripts to protein expression using immunohistochemistry and antibodies to keratin 17, cyclin D1, collagen IV, and laminin. For keratin 17, two ACCs and ACC3 showed 3+ immunoreactivity, one ACC was 2+, while the normal major salivary glands had 2+ staining. The three ACCs whose transcripts were profiled showed 2+ to 3+ immunopositivity for cyclin D1, whereas the epithelial cells from the normal salivary glands stained 1+ (<1% positive cells) (Figure 3) ▶ . The 10 ACCs not profiled showed 2+ to 4+ immunoreactivity for cyclin D1. Immunostaining with anti-collagen IV for the three ACCs that were profiled revealed very thick bands of positivity around nests, ducts, and the inner lining of pseudolumens as well as some conspicuous (often intense) immunoreactivity within the hyaline material of the pseudolumens (Figure 3) ▶ . Each of the additional 10 ACCs also showed strong immunopositivity for collagen IV in the same distribution. Collagen IV was inconspicuous or, at most, appeared as a very thin line around normal salivary ducts and, less often, around acini. Similarly, staining for laminin was inconspicuous or sometimes focal around normal salivary ducts and acini. In the three ACCs whose transcripts were profiled, there was 4+ immunoreactivity in tumor cells as well as staining immediately surrounding nests, lining pseudocystic spaces, and sometimes within hyaline material (Figure 3) ▶ . Seven of the nine additional ACCs also showed 4+ immunopositivity. Approximately 10% of the ACC3 cells were immunopositive for laminin.
Figure 3.
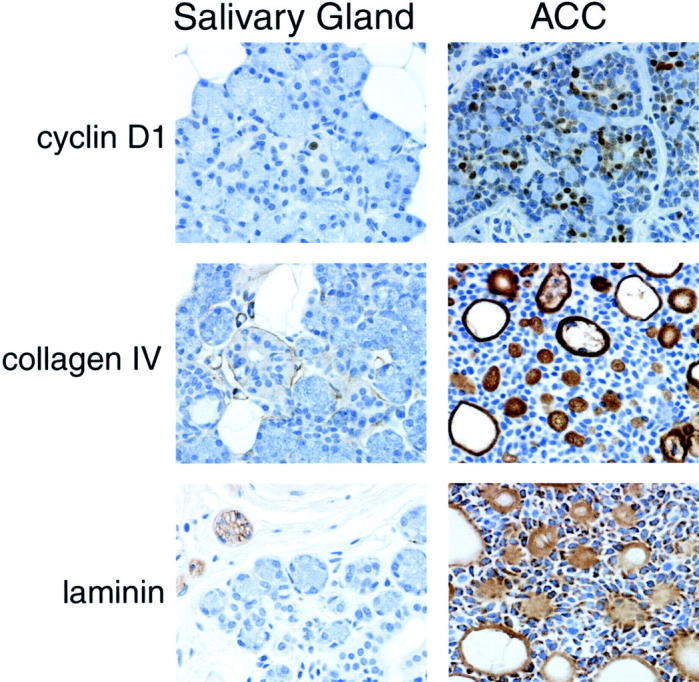
Immunohistochemical staining of normal major salivary gland (left) and ACC (right) showing differential immunoreactivity for cyclin D1, collagen IV, and laminin. Original magnifications, ×400.
Some differences in gene expression in ACC were found relative to tumor grade and anatomical site of occurrence. Approximately 100 genes showed differences in expression according to tumor grade (P < 0.01), while ∼40 had variable differences relative to tumor location in major or minor salivary glands (P < 0.01). (These lists of genes will be made available on request.)
Approximately 60% of the 100 genes that showed the highest levels of expression in the 15 ACCs relative to the normal salivary glands also showed high levels of expression in the ACC3 cell line. Among these genes were Sox4, transmembrane 4 superfamily member 1, keratin 17, jagged 1, filamin A, GABA-B receptor 1, and NGFI-A binding protein 1.
We also identified many genes that were significantly down-regulated in ACC relative to normal salivary glands (Table 2) ▶ . Of the most highly ranked down-regulated genes were those encoding secretory proteins such as histatin 3, amylase, fucosyltransferase 6, carbonic anhydrase VI, lactoperoxidase, statherin, and salivary proline-rich proteins.
Table 2.
List of Highest Ranked Overexpressed Genes in Normal Major Salivary Glands
| Affy ID | Symbol | Annotation | RefSeq number | Unigene ID | P value* | Fold change |
|---|---|---|---|---|---|---|
| 35051_at | CA6 | Carbonic anhydrase VI | NM_001215 | Hs.100322 | 3.36E-08 | 23.7 |
| 32006_r_at | PROL3 | Proline rich 3 | NM_006685 | Hs.2207 | 1.08E-07 | 18 |
| 39680_at | STATH | Statherin | NM_003154 | Hs.37048 | 2.90E-09 | 11.7 |
| 36680_at | AMY2B | Amylase, alpha 2B; pancreatic | NM_020978 | Hs.335493 | 9.29E-13 | 10.4 |
| 36290_s_at | FUT6 | Fucosyltransferase 6 (alpha (1,3) fucosyltransferase) | NM_000150 | Hs.32956 | 1.21E-07 | 11.0 |
| 41148_at | HTN3 | Histatin 3 | NM_000200 | Hs.177888 | 7.71E-11 | 8 |
| 37006_at | None | Homo sapiens, clone MGC:24130 IMAGE:4692359, mRNA, complete cds | None | Hs.76325 | 1.57E-08 | 9.4 |
| 35691_r_at | NFIX | Nuclear factor I/X (CCAAT-binding transcription factor) | NM_002501 | Hs.35841 | 4.00E-04 | 18.0 |
| 31977_at | GUCY2D | Guanylate cyclase 2D, membrane (retina-specific) | NM_000180 | Hs.1974 | 5.87E-07 | 11.0 |
| 34161_at | LPO | Lactoperoxidase | None | Hs.234742 | 4.62E-06 | 26.8 |
| 31446_s_at | PBI | Protein homologous to salivary proline-rich protein P-B | NM_012390 | Hs.166099 | 2.98E-06 | 11.9 |
| 41846_at | CRX | Cone-rod homeobox | NM_000554 | Hs.249186 | 4.44E-06 | 8.3 |
| 31635_g_at | PRH1 | Proline-rich protein HaeIII subfamily 1 | NM_006250 | Hs.278469 | 7.48E-08 | 5.9 |
| 33613_at | none | Homo sapiens SNC73 protein (SNC73) mRNA, complete cds | None | Hs.293441 | 4.17E-06 | 10 |
| 34823_at | DPP4 | Dipeptidylpeptidase IV (CD26, adenosine deaminase-complexing protein 2) | NM_001935 | Hs.44926 | 4.28E-06 | 35.0 |
| 41770_at | MAOA | Monoamine oxidase A | NM_000240 | Hs.183109 | 6.96E-08 | 7.1 |
| 41832_s_at | JTB | Jumping translocation breakpoint | NM_006694 | Hs.6396 | 1.22E-11 | 5.2 |
| 39756_g_at | XBP1 | X-box binding protein 1 | NM_005080 | Hs.149923 | 4.56E-06 | 9.8 |
| 31459_i_at | IGLα | Immunoglobulin λ locus | None | Hs.181125 | 7.38E-06 | 11.3 |
| 34213_at | KIAA0869 | KIAA0869 protein | None | Hs.21543 | 1.54E-06 | 10.4 |
| 40456_at | LOC64116 | Up-regulated by BCG-CWS | NM_022154 | Hs.284205 | 3.89E-06 | 12.5 |
| 41144_g_at | CALM1 | Calmodulin 1 (phosphorylase kinase, δ) | NM_006888 | Hs.177656 | 7.40E-07 | 6.5 |
| 39755_at | XBP1 | X-box-binding protein 1 | NM_005080 | Hs.149923 | 1.96E-06 | 6.7 |
| 41388_at | MEIS2 | Meis1, myeloid ecotropic viral integration site 1 homolog 2 (mouse) | NM_020149 | Hs.104105 | 3.65E-07 | 11.4 |
| 33274_f_at | IGLJ3 | Immunoglobulin lambda joining-3 | None | Hs.336946 | 4.15E-06 | 6.6 |
| 33501_r_at | none | Homo sapiens SNC73 protein (SNC73) mRNA, complete cds | None | Hs.293441 | 3.73E-06 | 6.4 |
| 38966_at | GPSN2 | Glycoprotein, synaptic 2 | NM_004868 | Hs.306122 | 8.81E-07 | 5.1 |
| 33442_at | KIAA0367 | KIAA0367 protein | None | Hs.23311 | 1.50E-09 | 47.5 |
| 34674_at | S100A1 | S100 calcium-binding protein A1 | NM_006271 | Hs.292707 | 9.88E-06 | 17.6 |
*Calculated by unpaired t-test
Identification of Genes Uniquely Overexpressed in ACC Relative to Other Common Carcinomas
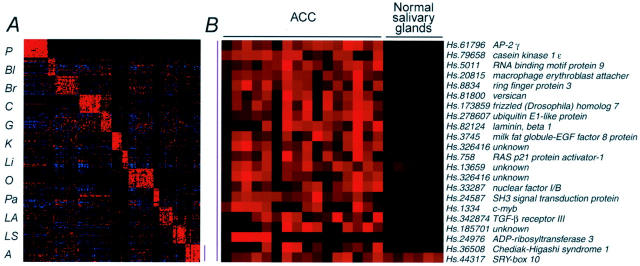
To identify genes whose overexpression may be characteristic of ACC relative to other more common carcinomas, we used a recently described molecular classification method that seeks classifier genes that can predict the anatomical origin or tumor type of a blinded tumor sample by cross-validation or independent testing. 7 Genes expressed in ACC were compared with those from 175 other carcinomas from 10 different anatomical sites (lung, breast, prostate, bladder/ureter, liver, kidney, esophagus and stomach, colorectum, ovary, and pancreas). 7 Using this approach, we identified 21 genes that could accurately classify 13 of the 15 ACC samples with high confidence (Figure 4A) ▶ . We have previously shown that such classifier genes typically represent features of the tissues from which the tumors arise, as well as features of the carcinomas from these tissues. Thus, we compared the expression of the 21 classifier genes in ACC with the normal major salivary glands (Figure 4B) ▶ . The differential expression of these genes was generally highly significant (t-test; 8.4 × 10−3 to 1.8 × 10−8 with the exception for Sox10), implying that most of these classifiers represent genes expressed at high levels in ACC rather than the normal salivary glands from which they arose. Four of these genes (AP-2γ, macrophage erythroblast attacher, versican, and laminin-β1) were among the most highly ranked genes in ACC compared with normal major salivary glands. Known genes that were the most specific for ACC relative to the other carcinomas included versican, TGF-β type III receptor, and c-myb. Two other highly expressed genes included casein kinase 1, epsilon and frizzled-7, members of the Wnt/β-catenin signal transduction pathway. Immunostaining for β-catenin in 13 ACCs (3 whose transcripts were profiled and 10 others) revealed variable immunopositivity limited to the cytoplasmic membrane; no nuclear or cytoplasmic immunoreactivity was observed.
Figure 4.
A: Tumor-specific genes for class prediction of ACC. Using ∼20 genes/class, 13 of 15 ACCs could be easily classified with high confidence, having gene expression profiles different from those of 175 examples from 10 other anatomical sites. P, prostate; Bl, bladder/ureter; Br, breast; C, colorectum; G, stomach/esophagus; K, kidney; Li, liver; O, ovary; Pa, pancreas; LA, lung adenocarcinoma; LS, lung, squamous cell carcinoma; A, ACC. B: List of classifier genes for adenoid cystic carcinoma.
The two outlier ACCs that we could not readily classify by leave-out-one cross-validation had profiles similar to pulmonary squamous cell carcinomas, although they lacked histological evidence of squamous differentiation. These two ACCs had relatively low levels of expressed transcripts for AP-2γ, versican, frizzled-7, and laminin-β1, genes that showed important differential expression for the other ACCs compared with the cancers from the 10 other anatomical sites.
Discussion
Salivary gland neoplasms consist of a diverse category of tumors with a spectrum of histological appearances, various degrees of differentiation, and differing clinical behaviors. ACC is one of several salivary gland neoplasms that characteristically shows dual myoepithelial and ductal epithelial differentiation. 13 More than many others, these tumors frequently secrete abundant extracellular matrix proteins (such as chondroitin sulfate) and basement membrane components. This is accurately reflected in our immunohistochemical analyses by the presence of the myoepithelial/basal keratin 17, 14 laminin, and collagen IV. Previous immunohistochemical studies have also documented the presence of laminin and type IV collagen in ACC. 3,15-19 We found highly overexpressed transcripts for versican, biglycan, laminin-β1, and collagen IV α1 in ACC relative to normal major salivary glands.
The most significantly overexpressed gene in ACC relative to the normal major salivary glands was SOX4, which encodes a transcription factor expressed in a wide variety of tissues in mice, and has functional importance in heart, brain, the reproductive system, and B-cell development. 20-23 The SOX gene family members have a highly conserved HMG-domain responsible for sequence-specific DNA binding. 24 In serial analysis of gene expression, SOX4 was overexpressed in normal mammary epithelium relative to ductal carcinoma in situ and invasive carcinoma of the breast. 25 Some breast cancer cell lines, however, have been shown to highly express SOX4, which is increased by progestins. 26 Although elevated expression of SOX4 was not unique to ACC, the genes that it transcribes and its role in tumor development or progression requires additional study.
The genes encoding transcription factors AP-2α and AP-2γ were also overexpressed in ACC. The AP-2 family members have been reported to regulate the expression of genes required for murine development of several tissues such as neural crest and skin. 27,28 Genes regulated by AP-2 are important in numerous biological functions and include, among others, estrogen receptor, keratinocyte-specific genes, and c-kit. 29-32 KIT protein has been found to be overexpressed in many ACCs. 33 Interestingly, versican 34 and biglycan 35 have binding sites for AP-2 suggesting a direct correlation between expression levels of these genes and AP-2. Both AP-2α and AP-2γ are reported to be overexpressed in breast carcinoma. 36 Although we noted that AP-2γ was modestly expressed in our breast cancers, there was on average a threefold increase in expression in ACC compared with the mammary tumors.
Genes encoding transmembrane proteins highly expressed in ACC included FAT tumor suppressor and transmembrane 4 superfamily member 1. The former contains 34 cadherin repeats, is expressed in many types of epithelium, and encodes a putative tumor suppressor protein. 37-39 FAT tumor suppressor has been shown to be down-regulated in metastatic prostate cancer. 40 The importance of its overexpression in ACC, therefore, is interesting and the functional consequences of its overexpression require additional study. Transmembrane 4 superfamily member 1, also known as tumor-associated antigen L6, encodes a cell surface protein implicated in cell growth. It is highly expressed in several carcinomas including those of the lung, breast, ovary, and colon, 41 and has been suggested as a target for monoclonal antibody therapy.
Genes encoding secreted proteins highly expressed in normal parotid and submandibular glands relative to ACC are those indicative of acinar cell differentiation. These genes such as amylase, carbonic anhydrase VI, and salivary proline-rich proteins, among others, would be predicted to be expressed in salivary gland neoplasms having acinar differentiation such as acinic cell carcinoma.
Approximately 60% of the 100 genes overexpressed in ACC relative to normal major salivary glands were also overexpressed in the ACC3 cell line. These genes included Sox 4, keratin 17, transmembrane 4 superfamily member 1, and laminin. ACC3 has been shown to secrete large quantities of basement membrane proteins including laminin and type 4 collagen in vitro as well as in mice. 42,43 The overlap in overexpressed genes in human ACC and ACC3 would suggest that ACC3 is a valid model system for the study of molecular alterations that might be important in human ACC carcinogenesis.
Thirteen of 15 ACCs had profiles distinctly different from 175 other carcinomas from 10 anatomical sites, enabling their classification by cross-validation with high confidence. Both versican and AP-2γ were among the top ranked classifier genes in ACC. The former was either absent or lowly expressed in carcinomas other than ACC, whereas AP-2γ was consistently expressed at modest levels among many of the 175 other tumors, but showed the highest statistical difference in ACC relative to the other tumors and the normal major salivary glands.
Two of the most highly ranked classifier genes in ACC included components of the Wnt/β-catenin signal transduction pathway. Frizzled-7, expressed normally in various adult and fetal tissues, encodes a receptor for WNT proteins and may down-regulate the function of APC and therefore promote β-catenin signaling. 44 The protein product of casein kinase 1, ε, 45 expressed in multiple human cell lines, complexes with axin and perhaps other components of the Wnt system; its overexpression stabilizes β-catenin, thereby promoting the transcription of β-catenin-dependent genes. 46 Using immunohistochemistry, we noted variable cytoplasmic membrane immunopositivity for β-catenin in ACC, but no nuclear or cytoplasmic staining. This finding would suggest that the Wnt/β-catenin pathway is not overtly dysregulated in ACC, as it is, for example, in colorectal carcinomas in which a pathway member is typically mutated leading to translocation of β-catenin to the nucleus.
Another classifier gene in ACC, nuclear factor I/B, encodes a transcription factor that is important in tissue-specific gene expression during growth and differentiation. 47,48 Nuclear factor I/B, located at 9p24, has been found to be involved in a hybrid transcript with HMGIC in two pleomorphic adenomas of salivary gland, 49 perhaps extending the importance of this gene in ACC. In that regard, it is of note that ∼30% of ACCs have cytogenetic evidence of translocations involving 9p13-23. 50 Whether nuclear factor I/B is a target of this translocation or is dysregulated by other means in ACC is currently unknown. In addition, c-myb, located at 6q22, was also overexpressd in ACC. Interestingly, 6q is usually the translocation partner with 9p in ACC.
Our study has elucidated those genes that are overexpressed in ACC relative to normal major salivary glands. We have also identified certain genes that are selectively overexpressed in ACC relative to 10 other types of carcinoma. Among the overexpressed genes are several that encode transcription factors, extracellular matrix components, and signal transduction pathway members. The information derived from such global transcription profiling offers clues as to which regulatory pathways should be first targeted for further study regarding their effects on tumor growth and behavior. The identification of genes overexpressed in ACC may suggest new therapeutic targets for this carcinoma, which up until now, has primarily been attacked by the surgical approach, and which when it becomes metastatic cannot be treated with complete success.
Footnotes
Supported in part by a grant to CAM from the National Organization for Rare Disorders (New Fairfield, CT).
References
- 1.Spiro RH: Salivary neoplasms, overview of a 35 year experience with 2,807 patients. Head Neck Surg 1986, 8:177-184 [DOI] [PubMed] [Google Scholar]
- 2.Vander Poorten VL, Balm AJ, Hilgers FJ, Tan IB, Loftus-Coll BM, Keus RB, Hart AA: Prognostic factors for long term results of the treatment of patients with malignant submandibular gland tumors. Cancer 1999, 85:2255-2264 [DOI] [PubMed] [Google Scholar]
- 3.Cheng J, Saku T, Okabe H, Furthmayr H: Basement membranes in adenoid cystic carcinoma. An immunohistochemical study. Cancer 1992, 69:2631-2640 [DOI] [PubMed] [Google Scholar]
- 4.Fordice J, Kershaw C, el-Naggar A, Goepfert H: Adenoid cystic carcinoma of the head and neck. Predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg 1999, 125:149-152 [DOI] [PubMed] [Google Scholar]
- 5.Ellis GL, Auclair PL: Tumors of the salivary glands. Atlas of Tumor Pathology. 1996:pp 203-216 Armed Forces Institute of Pathology, Washington DC
- 6.Szanto PA, Luna MA, Tortoledo E, White RA: Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer 1984, 54:1062-1069 [DOI] [PubMed] [Google Scholar]
- 7.Su AI, Welsh JB, Sapinoso LM, Kern SG, Dimitrov P, Lapp H, Schultz PG, Powell SM, Moskaluk CA, Frierson HF, Jr, Hampton GM: Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res 2001, 61:7388-7393 [PubMed] [Google Scholar]
- 8.He RG, Zhang XS, Zhou XJ, Wang Z, Zhang XL, Qiu WL, Han YS, Zhang RX: The establishment of cell lines of adenoid cystic carcinoma of human salivary glands (ACC2, ACC3) and a study of morphology. West Chin J Stomatol 1988, 6:1-4 [Google Scholar]
- 9.Wodicka L, Dong H, Mittmann M, Ho MH, Lockhart DJ: Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat Biotechnol 1997, 15:1359-1367 [DOI] [PubMed] [Google Scholar]
- 10.Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, Frierson HF, Jr, Hampton GM: Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res 2001, 61:5974-5978 [PubMed] [Google Scholar]
- 11.Welsh JB, Zarrinkar PP, Sapinoso LM, Kern SG, Behling CA, Monk BJ, Lockhart DJ, Burger RA, Hampton GM: Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc Natl Acad Sci USA 2001, 98:1176-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisen MB, Spellman PT, Brown PO, Botstein D: Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 1998, 95:14863-14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Therkildsen MH, Mandel U, Christensen M, Dabelsteen E: Thomsen-Friedenreich (T) antigen as a marker of myoepithelial and basal cells in the parotid gland, pleomorphic adenomas and adenoid cystic carcinomas. An immunohistochemical comparison between T and sialosyl-T antigens, alpha-smooth muscle actin and cytokeratin 14. APMIS 1995, 103:558-567 [DOI] [PubMed] [Google Scholar]
- 14.Troyanowsky SM, Guelstein VI, Tchypysheva TA, Krutovskikh VA, Bannikov GA: Patterns of expression of keratin 17 in human epithelia: dependency on cell position. J Cell Sci 1989, 93:419-426 [DOI] [PubMed] [Google Scholar]
- 15.Sobue M, Takeuchi J, Niwa M, Yasui C, Nakagaki S, Nagusaka T, Fukatsu T, Saga S, Nakashima N: Establishment of a cell line producing basement membrane components from an adenoid cystic carcinoma of the human salivary gland. Virchows Arch 1989, 57:203-208 [DOI] [PubMed] [Google Scholar]
- 16.Azumi N, Battifora H: The cellular composition of adenoid cystic carcinoma. An immunohistochemical study. Cancer 1987, 60:1589-1598 [DOI] [PubMed] [Google Scholar]
- 17.Toida M, Takeuchi J, Hara K, Sobue M, Tsukidate K, Goto K, Nakashima N: Histochemical studies of intercellular components of salivary gland tumors with special reference to glycosaminoglycans, laminin and vascular elements. Virchows Arch 1984, 403:15-26 [DOI] [PubMed] [Google Scholar]
- 18.Toida M, Takeuchi J, Sobue M, Tsukidate K, Akao S, Fukatsu T, Nakashima N: Histochemical studies on pseudocysts in adenoid cystic carcinoma of the human salivary gland. Histochem J 1985, 17:913-924 [DOI] [PubMed] [Google Scholar]
- 19.Caselitz J, Schulze I, Seifert G: Adenoid cystic carcinoma of the salivary glands: an immunohistochemical study. J Oral Pathol 1986, 15:308-318 [DOI] [PubMed] [Google Scholar]
- 20.Cheung M, Abu-Elmagd M, Clevers H, Scotting PJ: Roles of Sox4 in central nervous system development. Brain Res Mol Brain Res 2000, 79:180-191 [DOI] [PubMed] [Google Scholar]
- 21.Hunt SM, Clarke CL: Expression and hormonal regulation of the Sox4 gene in mouse female reproductive tissues. Biol Reprod 1999, 61:476-481 [DOI] [PubMed] [Google Scholar]
- 22.Van de Wetering M, Oosterwegel M, van Norren K, Clevers H: Sox4, an Sry-like HMG box protein, is a transcriptional activator in lymphocytes. EMBO J 1993, 12:3847-3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schilham MW, Oosterwegel MA, Moerer P, Ya J, de Boer PA, van de Wetering M, Verbeek S, Lamers WH, Kruisbeek AM, Cumano A, Clevers H: Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox4. Nature 1996, 380:711-714 [DOI] [PubMed] [Google Scholar]
- 24.Laudet V, Stehelin D, Clevers H: Ancestry and diversity of the HMG box superfamily. Nucleic Acids Res 1993, 21:2493-2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter DA, Krop IE, Nasser S, Sgroi D, Kaelin CM, Marks JR, Riggins G, Polyak K: A SAGE (serial analysis of gene expression) view of breast tumor progression. Cancer Res 2001, 61:5697-5702 [PubMed] [Google Scholar]
- 26.Graham JD, Hunt SMN, Tran N, Clarke CL: Regulation of the expression and activity by progestins of a member of the SOX gene family of transcriptional modulators. J Mol Endocrinol 1999, 22:295-304 [DOI] [PubMed] [Google Scholar]
- 27.Hilger-Eversheim K, Moser M, Schorle H, Buettner R: Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene 2000, 260:1-12 [DOI] [PubMed] [Google Scholar]
- 28.McPherson LA, Baichwal VR, Weigel RJ: Identification of ERF-1 as a member of the AP2 transcription factor family. Proc Natl Acad Sci USA 1997, 94:4342-4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leask A, Byrne C, Fuchs E: Transcription factor AP2 and its role in epidermal-specific gene expression. Proc Natl Acad Sci USA 1991, 88:7948-7952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha S, Degenstein L, Copenhaver C, Fuchs E: Defining the regulatory factors required for epidermal gene expression. Mol Cell Biol 2000, 20:2543-2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuur ER, McPherson LA, Yang GP, Weigel RJ: Genomic structure of the promoters of the human estrogen receptor-alpha gene demonstrate changes in chromatin structure induced by AP-2 gamma. J Biol Chem 2001, 276:15519-15526 [DOI] [PubMed] [Google Scholar]
- 32.Huang S, Jean D, Luca M, Tainsky MA, Bar-Eli M: Loss of AP-2 results in downregulation of c-KIT and enhancement of melanoma tumorigenicity and metastasis. EMBO J 1998, 17:4358-4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holst VA, Marshall CE, Moskaluk CA, Frierson HF, Jr: KIT protein expression and analysis of c-kit gene mutation in adenoid cystic carcinoma. Mod Pathol 1999, 12:956-960 [PubMed] [Google Scholar]
- 34.Naso MF, Zimmermann DR, Iozzo RV: Characterization of the complete genomic structure of the human versican gene and functional analysis of its promoter. J Biol Chem 1994, 269:32999-33008 [PubMed] [Google Scholar]
- 35.Ungefroren H, Krull NB: Transcriptional regulation of the human biglycan gene. J Biol Chem 1996, 271:15787-15795 [DOI] [PubMed] [Google Scholar]
- 36.Turner BC, Zhang J, Gumbs AA, Maher MG, Kaplan L, Carter D, Glazer PM, Hurst HC, Haffty BG, Williams T: Expression of AP-2 transcription factors in human breast cancer correlates with the regulation of multiple growth factor signalling pathways. Cancer Res 1998, 58:5466-5472 [PubMed] [Google Scholar]
- 37.Bryant PJ, Huettner B, Held LI, Jr, Ryerse J, Szidonya J: Mutations at the fat locus interfere with cell proliferation control and epithelial morphogenesis in Drosophila. Dev Biol 1988, 129:541-554 [DOI] [PubMed] [Google Scholar]
- 38.Mahoney PA, Weber U, Onofrechuk P, Biessmann H, Bryant PJ, Goodman CS: The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell 1991, 67:853-868 [DOI] [PubMed] [Google Scholar]
- 39.Dunne J, Hanby AM, Poulsom R, Jones TA, Sheer D, Chin WG, Da SM, Zhao Q, Beverley PCL, Owens MJ: Molecular cloning and tissue expression of FAT, the homologue of the Drosophila fat gene that is located on chromosome 4q34–q35 and encodes a putative adhesion molecule. Genomics 1995, 30:207-223 [DOI] [PubMed] [Google Scholar]
- 40.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM: Delineation of prognostic biomarkers in prostate cancer. Nature 2001, 412:822-826 [DOI] [PubMed] [Google Scholar]
- 41.Marken JS, Schieven GL, Hellstrom I, Hellstrom KE, Aruffo A: Cloning and expression of the tumor-associated antigen L6. Proc Natl Acad Sci USA 1992, 89:3503-3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng J, Irie T, Munakata R, Kimura S, Nakamura H, He R-G, Liu A-R, Saku T: Biosynthesis of basement membrane molecules by salivary adenoid cystic carcinoma cells: an immunofluorescence and confocal microscopic study. Virchows Arch 1995, 426:577-586 [DOI] [PubMed] [Google Scholar]
- 43.Irie T, Cheng J, Kimura S, Munakata R, Taira S, Saku T: Intercellular transport of basement membrane-type heparan sulphate proteoglycan in adenoid cystic carcinoma cells of salivary gland origin: an immunoelectron microscopic study. Virchows Arch 1998, 433:41-48 [DOI] [PubMed] [Google Scholar]
- 44.Sagara N, Toda G, Hirai M, Terada M, Katoh M: Molecular cloning, differential expression, and chromosomal localization of human frizzled-1, frizzled-2, and frizzled-7. Biochem Biophys Res Commun 1998, 252:117-122 [DOI] [PubMed] [Google Scholar]
- 45.Fish KJ, Cegielska A, Getman ME, Landes GM, Virshup DM: Isolation and characterization of human casein kinase I epsilon (CKI), a novel member of the CKI gene family. J Biol Chem 1995, 270:14875-14883 [DOI] [PubMed] [Google Scholar]
- 46.Sakanaka C, Sun TQ, Williams LT: New steps in the Wnt/beta-catenin signal transduction pathway. Recent Prog Horm Res 2000, 55:225-236 [PubMed] [Google Scholar]
- 47.Chaudhry AZ, Lyons GE, Gronostajski RM: Expression patterns of the four nuclear factor I genes during mouse embryogenesis indicate a potential role in development. Dev Dyn 1997, 208:313-325 [DOI] [PubMed] [Google Scholar]
- 48.Gronostajski RM: Roles of the NFI/CTF gene family in transcription and development. Gene 2000, 249:31-45 [DOI] [PubMed] [Google Scholar]
- 49.Geurts JM, Schoenmakers EF, Roijer E, Astrom AK, Stenman G, van de Ven WJ: Identification of NFIB as recurrent translocation partner gene of HMGIC in pleomorphic adenomas. Oncogene 1998, 16:865-872 [DOI] [PubMed] [Google Scholar]
- 50.Nordkvist A, Mark J, Gustafsson H, Bang G, Stenman G: Non-random chromosome rearrangements in adenoid cystic carcinoma of the salivary glands. Genes Chromosom Cancer 1994, 10:115-121 [DOI] [PubMed] [Google Scholar]