Abstract
Cognitive functions dependent on the prefrontal cortex, such as the ability to suppress behavior (response inhibition) and to learn from complex feedback (probabilistic learning), play critical roles in activities of daily life. To what extent do different neurochemical systems modulate these two cognitive functions? Here, using stop-signal and probabilistic learning tasks, we show a double dissociation for the involvement of noradrenaline and serotonin in human cognition. In healthy volunteers, inhibition of central noradrenaline reuptake improved response inhibition but had no effect on probabilistic learning, whereas inhibition of central serotonin reuptake impaired probabilistic learning with no effect on response inhibition.
Ascending monoamine projections play important neuromodulatory roles in high-level cognition through actions upon the prefrontal cortex (PFC), a major brain structure with considerable functional heterogeneity in humans (1). Dysfunction in these neurochemical systems is implicated in the etiology and psychopathology of psychiatric illnesses associated with cognitive deficits and PFC abnormalities, including depression, attention deficit–hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), and drug addiction (2-7). Dopamine regulates executive functions dependent on the dorsolateral PFC, including working memory and attentional set-shifting, but the role of noradrenaline (NA) and serotonin [5-hydroxytryptamine (5-HT)] in cognition is less well characterized (8). The orbitofrontal cortex (OFC) is involved in emotion-cognition interactions, and 5-HT drugs modulate response to feedback and decision-making within this region (9-15). 5-HT and NA have both been implicated in response inhibition (16, 17), a function that has been linked to the right inferior frontal gyrus (RIFG) (18).
We investigated the differential involvement of NA and 5-HT transmitter systems in these processes in humans, using the selective NA reuptake inhibitor (SNRI) atomoxetine and the selective 5-HT reuptake inhibitor (SSRI) citalopram. These agents are among the most selective inhibitors for brain NA and 5-HT reuptake transporters available for human use, according to in vitro and in vivo findings (19-21). Microdialysis studies in experimental animals have shown that acute systemic administration of atomoxetine rapidly increases PFC NA but not 5-HT and that the administration of citalopram rapidly increases PFC 5-HT but not NA (19, 22). As such, these agents represent useful neurochemical tools for investigating the differential involvement of NA and 5-HT in human cognition.
Response inhibition, the ability to exert high-level inhibitory control over motor responses so as to suppress unwanted actions, can be assessed with the stop-signal procedure (6, 23). In this procedure, volunteers are required to make rapid motor responses on Go trials but to inhibit responses if an auditory stop-signal occurs. By the infrequent nature of Stop trials, motor responses are made “prepotent.” Response inhibition can be quantified by the stop-signal reaction time (SSRT), an estimate of the time taken to inhibit the prepotent motor response (18, 23). Probabilistic learning refers to the ability to develop cognitive associations between stimuli and outcomes on the basis of punishing and rewarding feedback, and to modify these associations as appropriate (12). On probabilistic learning tasks, volunteers are required to select which of two stimuli they believe to be correct over a series of trials. After each choice, the computer provides punishing or rewarding feedback that is “degraded” (i.e., misleading on a subset of trials) (12).
The aim of the present study was to delineate the precise differential contribution of NA and 5-HT neurochemical systems to response inhibition and probabilistic learning. Sixty healthy male participants were recruited from the local community on the basis of being free from medical or psychiatric disorders according to assessment by a psychiatrist (mean age 25.7 ± SD 4.7 years, range 20 to 35) (24). Participants received single clinically relevant oral doses of atomoxetine (60 mg), citalopram (30 mg), or placebo in a double-blind parallel-groups design (24). Groups were matched for demographic characteristics (table S1). After spending 1.5 hours in a quiet waiting area to ensure drug absorption, volunteers completed the stop-signal and probabilistic learning tasks (Fig. 1).
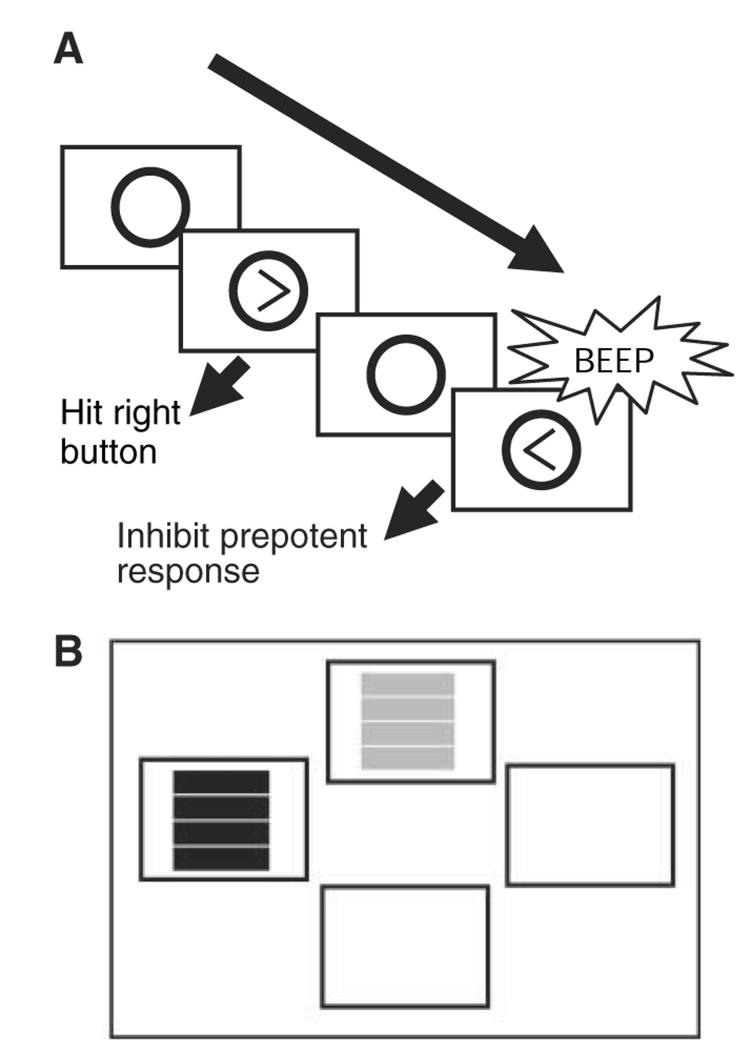
Fig. 1.
(A) On the computerized stop-signal task, subjects respond rapidly to left- or right-facing arrows on screen with corresponding motor responses, and they attempt to inhibit responses when an auditory stop-signal sounds. Over the course of the task, the time between stimulus onset and occurrence of the stop-signal is varied by means of a tracking algorithm. This permits calculation of the SSRT, which reflects an estimate of the time taken to internally suppress prepotent motor responses [for further details of calculation, see (18, 23)]. The average response time for Go trials is also recorded. (B) On the probabilistic learning task, volunteers make a two-alternative forced choice between two stimuli (one red, one green) on each trial. The “correct” stimulus (always the first stimulus touched) receives an 8:2 ratio of positive:negative feedback, and the opposite ratio is given for the “incorrect” stimulus. Feedback is provided in the form of “CORRECT” or “INCORRECT” appearing on screen after each choice. Ability to acquire the stimulus-reward association on the basis of this degraded feedback is assessed by the number of errors made before reaching criterion, defined as eight consecutive correct responses to the maximally rewarded stimulus. After 40 trials (stage 1), the contingencies reverse for the subsequent 40 trials (stage 2) (i.e., if “red” was previously correct, then “green” becomes correct). Ability to reverse the previously acquired stimulus-reward association is assessed by the number of perseverative errors to the previously maximally rewarded stimulus. Ability to acquire the new stimulus-reward association is again assessed by the number of errors made before reaching criterion. The detrimental effect of misleading negative feedback on learning is assessed by means of an overall “feedback sensitivity” score. This is defined as the overall likelihood that the volunteer inappropriately switched to choose the incorrect stimulus after misleadingly being informed that his or her correct response on the previous trial was not correct.
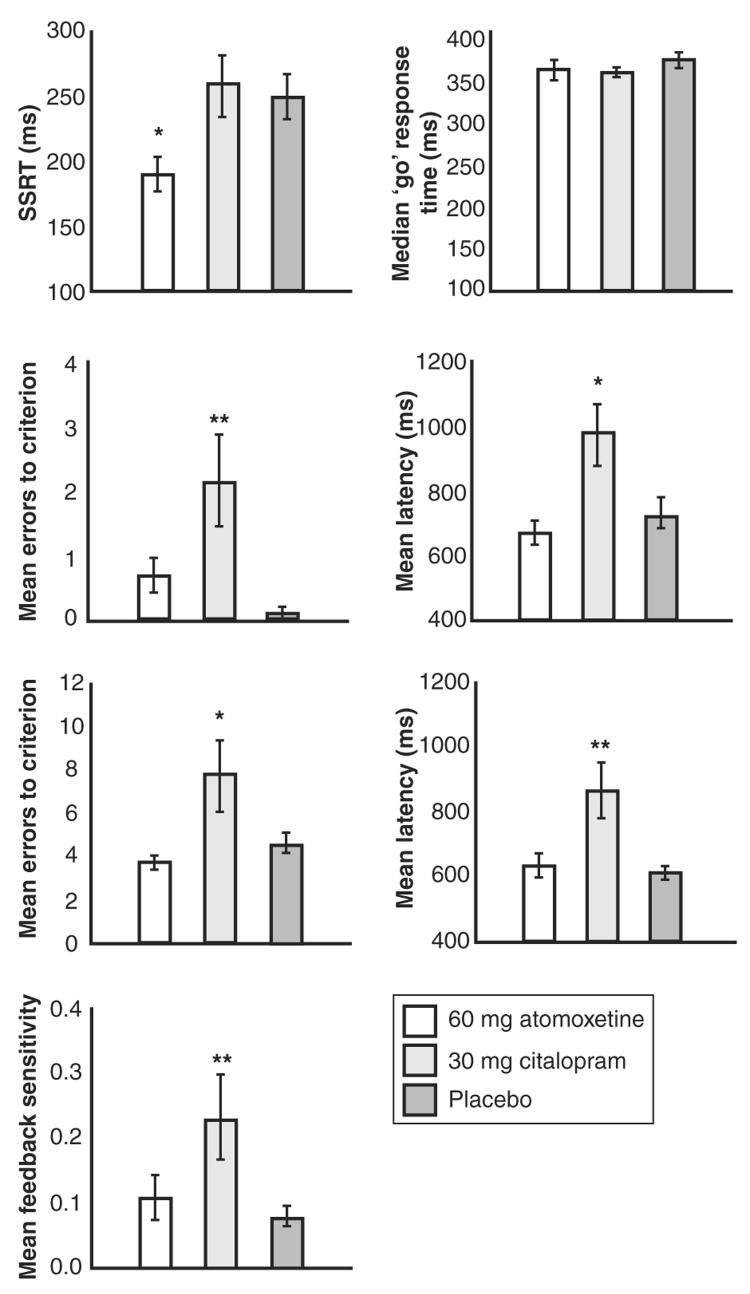
The results from the two tasks are shown in Fig. 2. The citalopram-treated group did not differ from controls in terms of response inhibition, but the atomoxetine-treated group showed shorter SSRTs (i.e., superior response inhibition) relative to both of the other groups. On the probabilistic learning task, the performance of the citalopram-treated volunteers was impaired on several measures, whereas the performance of the atomoxetine-treated group did not differ from that of the placebo group. The citalopram-treated group made increased numbers of errors before achieving learning criterion, were slower to respond, and were more likely to shift responding away from the correct stimulus after receiving misleading feedback.
Fig. 2.
Atomoxetine enhances response inhibition on the stop-signal task, whereas citalopram impairs performance on the probabilistic learning task. *P < 0.05 difference versus controls; **P < 0.01 difference versus controls. Error bars show SEM. In the stop-signal task, groups differed significantly on SSRTs (F2,57 = 4.377, P = 0.017) but not on median Go response times (F2,57 = 0.780, P = 0.463). The atomoxetine-treated group showed significantly shorter SSRTs relative to the citalopram-treated group (P = 0.013) and the placebo group (P = 0.014), whereas the citalopram-treated group did not differ from the placebo group (P = 0.973). In the probabilistic learning task, groups in stage 1 (graphs, second row) differed overall on number of errors made before attaining criterion (F2,57 = 5.549, P = 0.006) and on response latency (F2,57 = 5.588, P = 0.006). The citalopram-treated group made more errors before reaching criterion than did the atomoxetine-treated group (P = 0.012) and the placebo group (P = 0.002) and displayed longer mean response latencies than did the atomoxetine-treated group (P = 0.003) and the placebo group (P = 0.012). The atomoxetine-treated group did not differ from the placebo group on these measures (P = 0.379; P = 0.616). In stage 2, groups did not differ significantly in terms of perseverative errors made to the previously maximally rewarded stimulus (mean errors ± SD: atomoxetine, 2.95 ± 1.67; citalopram, 4.00 ± 6.54; placebo, 3.15 ± 1.73; F2,57 = 0.390, P = 0.679). Groups differed overall (graphs, third row) on number of errors made before attaining criterion (F2,57 = 5.019, P = 0.010) and on response latency (F2,57 = 7.981, P = 0.001). The citalopram-treated group made more errors before reaching criterion than did the atomoxetine-treated group (P = 0.004) and the placebo group (P = 0.020) and displayed longer mean response latencies than did the atomoxetine-treated group (P = 0.001) and the placebo group (P = 0.001). The atomoxetine-treated group did not differ from the placebo group on these measures (P = 0.557; P = 0.885). Groups differed on feedback sensitivity scores (F2,57 = 4.109, P = 0.022). The citalopram-treated group showed greater feedback sensitivity than did the atomoxetine-treated group (P = 0.037) and the placebo group (P = 0.009). The atomoxetine-treated group did not differ from the placebo group on this measure (P = 0.554).
These findings show that response inhibition and probabilistic learning are separable cognitive functions that are differentially modulated by ascending monoamine systems. Response inhibition was enhanced by inhibition of central NA reuptake but was unaffected by inhibition of central 5-HT reuptake. Conversely, probabilistic learning was impaired by inhibition of central 5-HT reuptake but was unaffected by inhibition of central NA reuptake. We excluded a nonspecific influence of atomoxetine and citalopram on attentional function or arousal. There were no significant effects of either drug on subjective rating scales for factors of alertness, contentedness, or calmness (24). There were also no effects of drug on a sensitive background test of sustained attention (table S2) or on the median Go reaction time on the stop-signal procedure. As a double dissociation was observed, these data strongly support the proposal that NA and 5-HT play distinct roles in the control of response inhibition and probabilistic learning. These results have important implications for our understanding of coupling between neurochemical systems and PFC processing.
Response inhibition is critically dependent on the PFC. ADHD patients show impaired response inhibition alongside abnormalities in the RIFG, according to structural and functional neuroimaging investigations (6, 25). Further, patients with lesions of the right PFC show impaired response inhibition that correlates with the degree of volume loss (18). The finding that atomoxetine improved response inhibition in these healthy volunteers implicates ascending NA systems in its control. Inhibition of 5-HT reuptake had no effect on response inhibition, consistent with previous work showing that depletion of central 5-HT likewise had no effect on response inhibition in healthy volunteers (26) and contradicting the simple hypothesis of 5-HT involvement in behavioral inhibition (16).
Our findings are important in relation to current treatment algorithms for ADHD, in which problems with response inhibition have been argued to represent a core cognitive deficit (6). Although the evidence to date does not support the utility of 5-HT drugs in mitigating core cognitive symptoms of this disorder, atomoxetine and psychostimulant medications such as methylphenidate are known to be effective and to act via mechanisms involving NA and/or dopamine (27, 28). Atomoxetine augments PFC NA and may also alter PFC dopamine levels via actions on NA reuptake transporters (19, 29). However, bilateral infusion of the α2-adrenoceptor blocker yohimbine into monkey PFC has been shown to impair response inhibition (30), whereas in humans, yohimbine and desipramine (a nonselective NA reuptake inhibitor) impair and improve response inhibition, respectively (17, 31). Administration of l-dioxyphenylalanine (l-DOPA), acting predominantly on dopaminergic mechanisms, has no effect on response inhibition in children with ADHD (17) and has limited efficacy in treating clinical symptoms (17, 27). Therefore, the most parsimonious explanation of the beneficial effects of atomoxetine is that it enhances stopping selectively via actions on NA uptake. Despite the traditional association between impulse control disorders and abnormal 5-HT transmission (16, 32), NA drugs may be more suited to ameliorating impaired response inhibition as a therapeutic target (28).
Multiple tiers of evidence implicate the PFC (particularly the OFC) in affective processing, in the establishment of stimulus-outcome contingencies, and in the deployment of this information to guide behavior (12, 33-36). Consistent with a neuromodulatory role for 5-HT in probabilistic learning, acute administration of citalopram exerted deleterious effects on probabilistic learning in healthy volunteers. Similar impairments in feedback learning have been demonstrated in depression and in studies of 5-HT depletion in healthy volunteers (10, 12, 15, 37, 38). One possible explanation for the deleterious effects of citalopram reported here is that presynaptic autoreceptor feedback effects may have induced a temporary reduction in 5-HT function after acute citalopram dosing (39, 40). An alternative account is that the relationship between 5-HT neurotransmission and probabilistic learning operates according to an “inverted-U” function, whereby either underactivity or overactivity of a neurotransmitter system can impair cognition (8, 41). As such, citalopram may have caused supraoptimal PFC 5-HT availability. The effects of citalopram on probabilistic learning may differ in psychiatric disorders associated with functionally abnormal 5-HT systems (such as OCD and depression), and contrasting the effects of acute and chronic SSRI treatment would help to further elucidate the neuropsychological mechanisms by which SSRIs exert their beneficial treatment effects.
This study has provided theoretically important evidence, in normal subjects, for the modulation of distinct cognitive functions after acute selective NA and 5-HT reuptake blockade. The findings also have clinical implications in the context of the treatment by atomoxetine of response inhibition deficits manifested in ADHD, and in understanding the effects of SSRI treatment on the cognitive sequelae of OCD and depression.
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/311/5762/861/DC1
Materials and Methods
Tables S1 and S2
References
References and Notes
- 1.Robbins TW. Prog. Brain Res. 2000;126:469. doi: 10.1016/S0079-6123(00)26030-5. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain SR, Sahakian BJ. Curr. Psychiatry Rep. 2004;6:451. doi: 10.1007/s11920-004-0010-3. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain SR, Blackwell AD, Fineberg N, Robbins T, Sahakian B. Psychol. Med. 2006;36:91. doi: 10.1017/S0033291705006124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamberlain SR, Blackwell AD, Fineberg N, Robbins TW, Sahakian BJ. Neurosci. Biobehav. Rev. 2005;29:399. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Am. J. Psychiatry. in press. [Google Scholar]
- 6.Aron AR, Poldrack RA. Biol. Psychiatry. 2005;57:1285. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Drug Alcohol Depend. 2005;79:273. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Cools R, Robbins TW. Philos. Trans. R. Soc. London Ser. A. 2004;362:2871. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- 9.Murphy FC, et al. Psychol. Med. 1999;29:1307. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 10.Murphy FC, Michael A, Robbins TW, Sahakian BJ. Psychol. Med. 2003;33:455. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- 11.Clark L, Cools R, Robbins TW. Brain Cogn. 2004;55:41. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 12.Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Science. 2004;304:878. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 13.Clarke HF, et al. J. Neurosci. 2005;25:532. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fellows LK, Farah MJ. Brain. 2003;126:1830. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 15.Evers EA, et al. Neuropsychopharmacology. 2005;30:1138. doi: 10.1038/sj.npp.1300663. [DOI] [PubMed] [Google Scholar]
- 16.Soubrié P. Behav. Brain Res. 1986;9:319. doi: 10.1016/0166-4328(90)90067-o. [DOI] [PubMed] [Google Scholar]
- 17.Overtoom CC, et al. Behav. Brain Res. 2003;145:7. doi: 10.1016/s0166-4328(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 18.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Nat. Neurosci. 2003;6:115. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 19.Bymaster FP, et al. Neuropsychopharmacology. 2002;27:699. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 20.Wong DT, Threlkeld PG, Best KL, Bymaster FP. J. Pharmacol. Exp. Ther. 1982;222:61. [PubMed] [Google Scholar]
- 21.Spinks D, Spinks G. Curr. Med. Chem. 2002;9:799. doi: 10.2174/0929867024606795. [DOI] [PubMed] [Google Scholar]
- 22.Bymaster FP, et al. Psychopharmacology. 2002;160:353. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- 23.Logan GD, Cowan WB, Davis KA. J. Exp. Psychol. Hum. Percept. Perform. 1984;10:276. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 24. See supporting material on Science Online.
- 25.Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Am. J. Psychiatry. 2005;162:1067. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 26.Clark L, et al. Psychopharmacology. 2005;182:570. doi: 10.1007/s00213-005-0104-6. [DOI] [PubMed] [Google Scholar]
- 27.Biederman J, Spencer T, Wilens T. Int. J. Neuropsychopharmacol. 2004;7:77. doi: 10.1017/S1461145703003973. [DOI] [PubMed] [Google Scholar]
- 28.Arnsten AF, Li BM. Biol. Psychiatry. 2005;57:1377. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Liprando LA, Miner LH, Blakely RD, Lewis DA, Sesack SR. Synapse. 2004;52:233. doi: 10.1002/syn.20023. [DOI] [PubMed] [Google Scholar]
- 30.Ma CL, Qi XL, Peng JY, Li BM. Neuroreport. 2003;14:1013. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- 31.Swann AC, Birnbaum D, Jagar AA, Dougherty DM, Moeller FG. Biol. Psychiatry. 2005;57:1209. doi: 10.1016/j.biopsych.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Hollander E, Rosen J. J. Psychopharmacol. 2000;14:S39. doi: 10.1177/02698811000142S106. [DOI] [PubMed] [Google Scholar]
- 33.Elliott R, Dolan RJ, Frith CD. Cereb. Cortex. 2000;10:308. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- 34.Cox SM, Andrade A, Johnsrude IS. J. Neurosci. 2005;25:2733. doi: 10.1523/JNEUROSCI.3360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoenbaum G, Roesch M. Neuron. 2005;47:633. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neuroimage. 2005;26:609. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Park SB, et al. Neuropharmacology. 1994;33:575. doi: 10.1016/0028-3908(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 38.Rogers RD, et al. Psychopharmacology. 1999;146:482. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- 39.Chaput Y, de Montigny C, Blier P. Naunyn Schmiedebergs Arch. Pharmacol. 1986;333:342. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- 40.El Mansari M, Blier P. J. Psychiatry Neurosci. 2005;30:268. [PMC free article] [PubMed] [Google Scholar]
- 41.Zahrt J, Taylor JR, Mathew RG, Arnsten AF. J. Neurosci. 1997;17:8528. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Supported by Wellcome Trust programme grant 076274/Z/04/Z (T.W.R., B. J. Everitt, A. C. Roberts, and B.J.S.), a Medical Research Council (MRC) pathfinder grant (U.M.), and an MRC priority studentship (S.R.C.). The Behavioural and Clinical Neuroscience Institute is supported by a joint award from the MRC and the Wellcome Trust. B.J.S. holds the Donders Chair at Utrecht University. We thank the nurses and administrative staff at the Wellcome Trust Clinical Research Facility (Addenbrooke's Hospital, Cambridge), the Pharmacy Production Unit (Sheffield Teaching Hospitals NHS Foundation Trust), and all participants. This study was approved by Local Research Ethics Committee (Cambridge 04/038) and by the Medicines and Healthcare Products Regulatory Agency (MHRA), London. All volunteers gave informed written consent before participation. A.D.B., T.W.R., and B.J.S. consult for Cambridge Cognition. T.W.R. consults for and U.M. has received speaker honoraria from Eli Lilly.