Abstract
Background
The use of strategies to aid performance when undertaking neuropsychological tasks is dependent on intact fronto-striatal circuitry, and growing evidence suggests impaired spontaneous use of strategies in patients with obsessive–compulsive disorder (OCD). However, studies to date have not examined the effects of strategy training on task performance in OCD or in trichotillomania (compulsive hair-pulling, a condition that has been argued to share overlap with OCD in terms of phenomenology and co-morbidity).
Method
The ability to generate novel visuospatial sequences using a computer interface was examined before and after undertaking optimal strategy training in 20 OCD patients, 17 trichotillomania patients, and 20 controls (matched for age, education, and IQ).
Results
OCD patients failed to improve ability to generate novel sequences above baseline despite successfully completing strategy training to the same extent as other groups. In contrast, performance of trichotillomania patients improved significantly after training to the same extent as controls. Groups did not differ on memory span, trial-by-trial action monitoring, or ability to generate novel visuospatial sequences prior to strategy training.
Conclusions
Strategy implementation deficits, suggestive of cognitive inflexibility and fronto-striatal dysfunction, appear integral to the neurocognitive profile of OCD but not trichotillomania. Future research should investigate cognitive flexibility in obsessive–compulsive spectrum disorders using a variety of paradigms, and clarify the contribution of specific neural structures and transmitter systems to deficits reported.
INTRODUCTION
Obsessive–compulsive disorder (OCD) is characterized by intrusive, troubling thoughts that are perceived as the product of one's own mind (obsessions) and/or repetitive behaviours or mental rituals (compulsions) according to DSM-IV criteria (APA, 1994). Trichotillomania is characterized by debilitating and repetitive hair-pulling (APA, 1994). There is continuing debate about whether trichotillomania might be considered an OCD subtype (Tynes et al. 1990), or an obsessive–compulsive spectrum (OCS) disorder (Stein et al. 1995; Jaisoorya et al. 2003). Although repetitive behaviours are common to the diagnostic criteria for both conditions, only OCD is characterized by compulsions performed according to rigid rules that suggest problems with cognitive flexibility (Chamberlain et al. 2005, in press). Furthermore, previous studies employing the attentional set-shifting task from the Cambridge Neuropsychological Test Automated Battery (CANTAB, www.camcog.com) have identified cognitive inflexibility in OCD but not trichotillomania patients (Veale et al. 1996; Watkins et al. 2005; Chamberlain et al. in press).
The coordination of cognitive abilities to optimize performance, or implementation of strategy, can be considered to be a function of the central executive, and is dependent on integrity of fronto-striatal circuitry (Owen et al. 1990; Shallice & Burgess, 1991; Iddon et al. 1998). Studies have reported that patients with OCD are less likely than controls to use spontaneous strategies to aid performance when undertaking some neurocognitive tasks, especially those involving memory (Savage et al. 1999, 2000; Deckersbach et al. 2000; Cabrera et al. 2001). However, the ability to optimize task performance via strategy training has not been examined in OCD or trichotillomania, yet this may provide insights into the nature of impaired strategy in OCD, and whether these two conditions share overlapping or distinct cognitive profiles. The aim of this study was to compare strategy implementation in OCD, trichotillomania, and healthy controls using a computerized visuospatial sequence generation previously shown to be sensitive to strategy implementation deficits in schizophrenia (Iddon et al. 1998). It was predicted that only the OCD group would show impaired strategy implementation after training, consistent with cognitive inflexibility being characteristic of OCD but not trichotillomania.
METHOD AND MATERIALS
Participants
Twenty OCD patients, 17 trichotillomania patients, and 20 controls took part in the study. Clinical diagnoses were made by a consultant psychiatrist (N.F.), using extended clinical interview supplemented with the Mini-International Neuropsychiatric Inventory (MINI; Sheehan et al. 1998). Depressive mood was quantified with the Montgomery–Asberg Depression Rating Scale (MADRS; Montgomery & Asberg, 1979). Patients were excluded in the event of any DSM-IV Axis-I co-morbidities, including significant levels of depression (MADRS >16). Controls were also screened with extended clinical interview and MINI, and were excluded in the event of any current Axis-I disorders or significant past history of neurological or psychiatric illness. Within the OCD group, only patients with archetypal washing/checking symptoms without hoarding were included, and disease severity was assessed with the Yale–Brown Obsessive Compulsive Scale (YBOCS; Goodman et al. 1989). At the time of participation, 16 out of 20 OCD patients were receiving serotonin-selective reuptake inhibitors (SSRIs), and the remainder were un-medicated (free from psychotropic medications for at least 6 months). In the trichotillomania group, disease severity was assessed with the Massachusetts General Hospital Hairpulling Scale (MGH; Keuthen et al. 1995), and all were un-medicated (free from psychotropic medications for at least 6 months). We were careful to exclude trichotillomania from the OCD group and vice versa. The study was approved by Local Research Ethics Committee, and volunteers gave informed written consent prior to participation.
Procedures
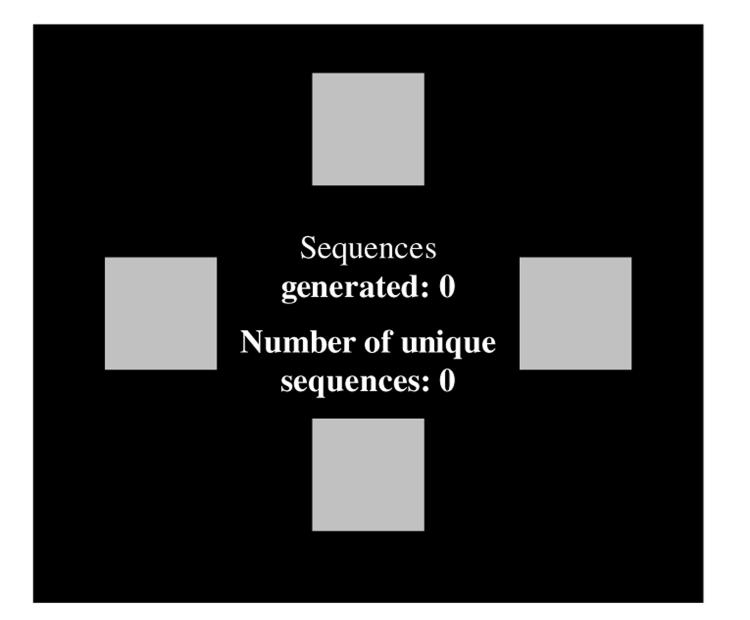
Neurocognitive testing was conducted by an experienced neuropsychologist in a quiet office. The four-stage visuospatial sequence task was administered on a touch-screen computer with task instructions read from a standard instruction set, to ensure consistency of administration. There were no time limits on task completion, but test duration was typically 15–20 min. The interface comprised four red blocks arranged symmetrically on screen that could be selected in turn to generate ‘sequences of four’ (see Fig. 1). A given sequence could start and end at any of the four blocks, but every sequence had to include all of the blocks. Thus, there were 24 possible different ‘sequences of four’. Task stages were: (1) practice using the interface, (2) novel visuospatial sequence generation, (3) strategy training, (4) novel visuospatial sequence generation. Each stage involved 24 sequence attempts.
Fig. 1.
The task interface. Sequences of four are generated by clicking the blocks in turn. For example, one sequence would be top→bottom→left→right. Continuous feedback (centre) is provided for all stages except the initial practice stage.
On stage 1, an example ‘sequence of four’ block touches was demonstrated by the researcher, and volunteers were asked to practice making sequences in any order they desired until the stage ended, with repetitions of sequences allowed. On stages 2 and 4, volunteers were asked to generate as many unique/novel sequences of four as possible, and to avoid making sequence repetitions. On the intervening stage 3, training on an effective strategy was undertaken. This was accomplished by asking volunteers to try to generate six different sequences always starting at the same position (fixed and indicated by the computer). After six attempts, the starting position rotated clockwise, and six more sequences were attempted, and so on until all four starting positions had been used. This method of breaking the task down into sub-goals has been argued to represent the most effective strategy for maximizing novel sequence generation ability. The reader is referred to Iddon et al. (1998) for a more detailed task description.
Task variables
Task variables recorded for different stages included: number of novel sequences generated, strategy scores, and number of consecutive perseverations. Strategy scores represented the number of times five or more successive sequences were initiated from the same starting position (maximum score 4). Consecutive perseverations were defined as immediate repetitions of identical sequences (i.e. one immediately following the other), thereby indexing trial-by-trial action monitoring on stages where repeat sequences were to be avoided. Baseline memory span was quantified by the number of novel sequences generated prior to the first sequence repetition (memory failure) on the initial novel sequence generation stage (stage 2).
Statistical analysis
Repeated-measures analysis of variance (ANOVA) was used to examine effects of strategy training on novel sequence generation ability. Other measures were examined using one-way ANOVA. Simple effects were calculated when significant interactions were found. Least Significant Difference (LSD) tests were performed on single measure scores as appropriate. Correlation analyses were undertaken between task indices and clinical measures using Pearson's r.
RESULTS
Demographic and clinical characteristics
Demographic and clinical characteristics are shown in Table 1, where it can be seen that groups were matched for age, education, and IQ (Nelson, 1982). However, groups differed overall in terms of MADRS scores, due to OCD cases scoring higher than other groups (p<0·05). Trichotillomania and controls did not differ on MADRS (p>0·10).
Table 1.
Demographic and clinical characteristics
| Variable | OCD (n=20) (M : F=4 : 16) Mean (s.d.) |
Trichotillomania (n=17) (M : F=2 : 15) Mean (s.d.) |
Controls (n=20) (M : F=4 : 16) Mean (s.d.) |
F(2, 54) |
|---|---|---|---|---|
| Age (yr) | 35·30 (14·10) | 36·10 (12·60) | 31·40 (8·10) | 0·79 |
| Education score (max. 4) | 2·80 (1·00) | 2·80 (0·80) | 3·10 (0·80) | 0·47 |
| NART IQ | 115·70 (5·90) | 117·70 (7·50) | 117·90 (5·50) | 0·73 |
| MADRS | 6·90 (4·40) | 4·20 (3·60) | 3·10 (4·40) | 8·44** |
| YBOCS | 20·40 (4·10) | — | — | |
| MGH | — | 16·40 (4·70) | — |
NART, National Adult Reading Test; MADRS, Montgomery–Asberg Depression Rating Scale; YBOCS, Yale–Brown Obsessive Compulsive Scale ; MGH, Massachusetts General Hospital Hairpulling Scale. Significant group differences overall:
p<0·01.
Task performance
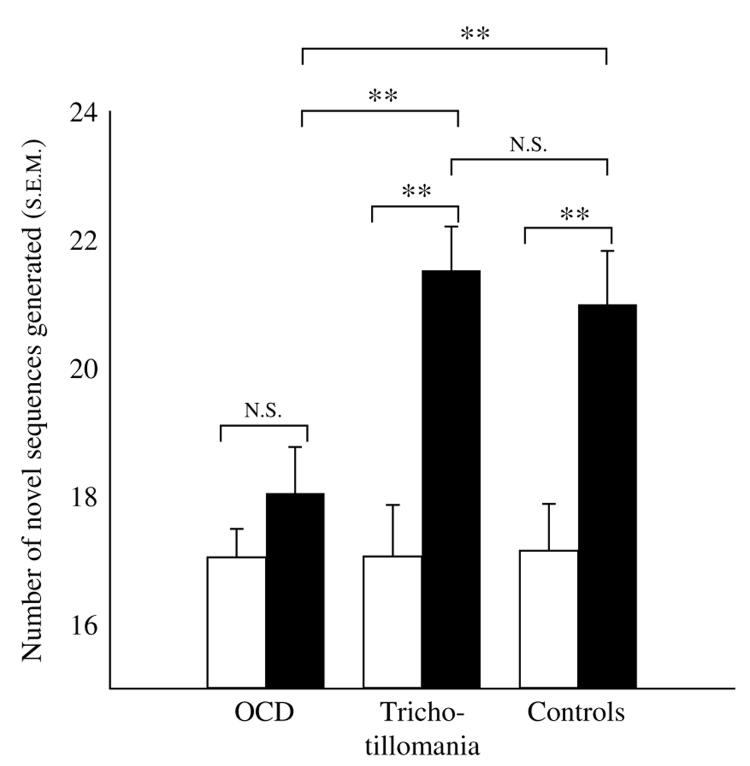
Performance indices for all task stages are shown in Table 2. There were no significant group differences on any task measures for the practice stage, initial novel sequence generation stage, or strategy training stage itself (all p>0·10). With regard to the number of novel sequences generated for the two novel sequence generation stages (stages 2 and 4), there was a significant effect of strategy training [F(1, 54) =46·384, p<0·01] and a significant strategy training by group interaction [F(2, 54)=5·561, p<0·01]. This was attributable to OCD patients generating fewer novel sequences in comparison to both other groups after training (p<0·01), whereas performance of trichotillomania patients did not differ from controls. As shown in Fig. 2, trichotillomania patients and controls significantly increased the number of novel sequences generated through training (p<0·01) but OCD patients did not (p>0·10). As anticipated given these findings, OCD patients demonstrated lower strategy scores compared to other groups after training (p<0·01).
Table 2.
Visuospatial task measures
| Variable | OCD (n=20) Mean (s.d.) |
Trichotillomania (n=17) Mean (s.d.) |
Controls (n=20) Mean (s.d.) |
F(2, 54) |
|---|---|---|---|---|
| Stage 1 (practice stage) | ||||
| Novel sequences generated (max. 24) | 15·00 (2·50) | 15·50 (3·70) | 13·50 (4·70) | 1·47 |
| Consecutive perseverations | 1·00 (2·50) | 1·40 (2·60) | 3·20 (6·30) | 2·04 |
| Stage 2 (novel sequence generation) | ||||
| Baseline span score | 8·70 (2·80) | 10·60 (6·10) | 8·40 (5·50) | 0·93 |
| Novel sequences generated (max. 24) | 17·10 (2·00) | 17·10 (3·30) | 17·20 (3·30) | 0·00 |
| Strategy use (max. 4) | 0·20 (0·90) | 0·40 (0·70) | 0·60 (1·20) | 0·79 |
| Consecutive perseverations | 0·30 (0·60) | 0·20 (90·60) | 0·20 (0·40) | 0·16 |
| Stage 3 (strategy training) | ||||
| Novel sequences generated (max. 24) | 19·50 (2·10) | 20·00 (2·20) | 20·70 (2·90) | 1·09 |
| Stage 4 (novel sequence generation) | ||||
| Novel sequences generated (max. 24) | 18·10 (3·20) | 21·50 (2·80) | 21·00 (3·70) | 5·09** |
| Strategy use (max. 4) | 1·10 (1·60) | 2·90 (1·40) | 2·70 (1·70) | 6·74** |
| Consecutive perseverations | 0·90 (1·00) | 0·30 (0·80) | 0·40 (0·70) | 2·65 |
Significant group differences overall:
p<0·01.
Fig. 2.
Comparison of novel sequence generation performance before (□) and after (■) strategy training in OCD, trichotillomania, and controls (** p<0·01). Group performances did not differ at baseline.
Correlation analyses
Measures of disease severity (YBOCS for OCD, MGH for trichotillomania) did not correlate with any task measures (all p>0·10). MADRS scores and IQ did not correlate significantly with any task measures (all p>0·10).
DISCUSSION
OCD patients showed profound impairments in strategy implementation, despite intact memory span, trial-by-trial action monitoring, and ability to generate novel visuospatial sequences prior to undertaking strategy training. In contrast to this deficit in OCD, trichotillomania patients demonstrated intact strategy implementation, consistent with cognitive inflexibility being limited to OCD only (Chamberlain et al. in press). OCD and trichotillomania are associated with repetitive behaviours, but only OCD includes ‘compulsive’ symptomatology suggestive of inflexibility, including rituals that must be performed according to strict rules, and difficulty shifting attentional focus away from inappropriate intrusive thoughts or aspects of the environment (Chamberlain et al. in press). Furthermore, previous work has identified cognitive inflexibility in OCD but not trichotillomania on an attentional set-shifting test from the CANTAB battery (Chamberlain et al. in press).
It is interesting to compare the present findings to those reported previously for other disorders. Using a task that examined only novel sequence generation ability in the absence of strategy training (equivalent to stage 2 in the current task), patients with frontal lobe lesions and patients with Parkinson's disease were found to show a baseline deficit in novel sequence generation ability (Owen et al. 1995). In another study, this time employing a conceptually identical task to that used by the present study, patients with schizophrenia demonstrated impaired strategy implementation but also made increased numbers of consecutive perseverative errors across multiple stages of the task (inappropriate immediate repetitions of identical sequences) (Iddon et al. 1998). OCD findings are dissociable from these other conditions, as patients were intact at baseline sequence generation and did not make increased numbers of consecutive perseverative errors. From the point of view of neuropathology, while neural involvement in frontal lesions and Parkinson's disease can be highly focal, there is no established focal site of pathology as such in OCD. Rather, abnormalities exist in a distributed set of neural structures including the anterior cingulate cortex, orbitofrontal cortex, and basal ganglia (especially caudate). Indeed, it has been proposed that OCD neurobiology can be conceptualized in terms of dysfunctional ‘habit forming’ circuitry in which there is insufficient high-level inhibitory control over learnt patterns of behaviour (Graybiel & Rauch, 2000; Chamberlain et al. 2005). Our findings are consistent with this suggestion, as OCD patients were cognitively inflexible and failed to modulate task performance in order to implement the strategy. With regard to prefrontal involvement, neuroimaging evidence to date implicates orbitofrontal cortex abnormalities in OCD but dorsolateral prefrontal cortex abnormalities in schizophrenia (Whitney et al. 2004). The finding that OCD patients, unlike schizophrenia patients, did not make increased numbers of consecutive perseverative errors does imply different neural involvement between these conditions, but without further research this issue cannot be clarified further. From a neuropsychological point of view, increased numbers of consecutive perseverative errors suggest chronic problems with monitoring behaviour on a trial-by-trial basis in schizophrenia (Iddon et al. 1998), whereas normal numbers of such errors in OCD suggests that the strategy implementation deficit is more directly attributable to higher-level cognitive inflexibility.
Strategy implementation can be considered to be a high-level cognitive function that is dependent on fronto-striatal circuitry (Alexander et al. 1986; Owen et al. 1990, 1995; Shallice & Burgess, 1991; Iddon et al. 1998; Savage et al. 2001). Impaired strategy implementation in OCD but not trichotillomania is consistent with neuroimaging findings to date. The anterior cingulate and orbitofrontal cortices are frequently reported to be structurally and functionally abnormal in OCD (Chamberlain et al. 2005), whereas there is little evidence for involvement of these regions in trichotillomania (Swedo et al. 1991; Grachev, 1997; O'Sullivan et al. 1997; Stein et al. 1997). However, it should be noted that our current understanding of trichotillomania neuropathology is limited by the relative paucity of studies. The anterior cingulate cortex is thought to play an important role in strategy implementation and/or in assessing the effects of strategy on performance (Botvinick et al. 1999; Carter et al. 1999), and recent evidence suggests that the orbitofrontal cortex plays an important but previously unappreciated role in the mobilization of behavioural strategies in novel or ambiguous circumstances (Savage et al. 2001).
OCD patients demonstrated higher depressive mood scores than other groups in this study. We feel it unlikely that this was responsible for the strategy deficit, as mood scores did not correlate with task indices, mean mood scores were beneath cut-off for depression in full remission, and we were careful to exclude volunteers who were clinically depressed. The majority of patients in the OCD group were medicated on SSRIs, suggesting that cognitive inflexibility persists despite pharmacological treatment. This may have ramifications for ability of patients to successfully undertake and apply psychological treatments to improve quality of life. Future research work should clarify the contribution of anterior cingulate and orbitofrontal cortices to strategy deficits in OCD, and the involvement of specific neurotransmitter systems. Wider application of neurocognitive tests designed to tap cognitive flexibility and inhibition processes (Chamberlain et al. 2005) may help elucidate the relationship between OCD and other putatively related disorders.
ACKNOWLEDGEMENTS
S.R.C. is on the Cambridge MB/PhD programme, funded by a Medical Research Council (MRC) research studentship. This research work was funded by the Wellcome Trust (programme grant 019407) and the MRC (centre grant G0001354). The authors are grateful to the volunteers who took part, to Henry Chase for programming, and to Ulrich Muller for advice.
Footnotes
DECLARATION OF INTEREST
A.D.B., T.W.R., and B.J.S. consult for Cambridge Cognition.
REFERENCES
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Cabrera AR, McNally RJ, Savage CR. Missing the forest for the trees? Deficient memory for linguistic gist in obsessive-compulsive disorder. Psychological Medicine. 2001;31:1089–1094. doi: 10.1017/s0033291701004354. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews of Neuroscience. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg N, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neuroscience and Biobehavioral Reviews. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg N, Blackwell AD, Robbins T, Sahakian B. Motor inhibition and cognitive flexibility in OCD and trichotillomania. American Journal of Psychiatry. doi: 10.1176/ajp.2006.163.7.1282. in press. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Otto MW, Savage CR, Baer L, Jenike MA. The relationship between semantic organization and memory in obsessive-compulsive disorder. Psychotherapy and Psychosomatics. 2000;69:101–107. doi: 10.1159/000012373. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Grachev ID. MRI-based morphometric topographic parcellation of human neocortex in trichotillomania. Psychiatry and Clinical Neuroscience. 1997;51:315–321. doi: 10.1111/j.1440-1819.1997.tb03205.x. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Iddon JL, McKenna PJ, Sahakian BJ, Robbins TW. Impaired generation and use of strategy in schizophrenia: evidence from visuospatial and verbal tasks. Psychological Medicine. 1998;28:1049–1062. doi: 10.1017/s0033291798006758. [DOI] [PubMed] [Google Scholar]
- Jaisoorya TS, Reddy YC, Srinath S. The relationship of obsessive-compulsive disorder to putative spectrum disorders: results from an Indian study. Comprehensive Psychiatry. 2003;44:317–323. doi: 10.1016/S0010-440X(03)00084-1. [DOI] [PubMed] [Google Scholar]
- Keuthen NJ, O'Sullivan RL, Ricciardi JN, Shera D, Savage CR, Borgmann AS, Jenike MA, Baer L. The Massachusetts General Hospital (MGH) Hairpulling Scale: 1. Development and factor analyses. Psychotherapy and Psychosomatics. 1995;64:141–145. doi: 10.1159/000289003. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National Adult Reading Test (NART): Test Manual. Windsor: NFER-Nelson; 1982. [Google Scholar]
- O'Sullivan RL, Rauch SL, Breiter HC, Grachev ID, Baer L, Kennedy DN, Keuthen NJ, Savage CR, Manzo PA, Caviness VS, Jenike MA. Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biological Psychiatry. 1997;42:39–45. doi: 10.1016/S0006-3223(96)00297-1. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Hodges JR, Summers BA, Polkey CE, Robbins T. Dopamine-dependent frontostriatal planning deficits in early Parkinson's disease. Neuropsychology. 1995;9:126–140. [Google Scholar]
- Savage CR, Baer L, Keuthen NJ, Brown HD, Rauch SL, Jenike MA. Organizational strategies mediate nonverbal memory impairment in obsessive-compulsive disorder. Biological Psychiatry. 1999;45:905–916. doi: 10.1016/s0006-3223(98)00278-9. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, Wagner AD, Schacter DL, Alpert NM, Fischman AJ, Rauch SL. Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: evidence from PET. Brain. 2001;124:219–231. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Wilhelm S, Rauch SL, Baer L, Reid T, Jenike MA. Strategic processing and episodic memory impairment in obsessive compulsive disorder. Neuropsychology. 2000;14:141–151. doi: 10.1037//0894-4105.14.1.141. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl. 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Stein DJ, Coetzer R, Lee M, Davids B, Bouwer C. Magnetic resonance brain imaging in women with obsessive-compulsive disorder and trichotillomania. Psychiatry Research. 1997;74:177–182. doi: 10.1016/s0925-4927(97)00010-3. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Simeon D, Cohen LJ, Hollander E. Trichotillomania and obsessive-compulsive disorder. Journal of Clinical Psychiatry. 1995;56(Suppl. 4):28–34. doi: 10.1176/ajp.150.7.1131-a. discussion 35. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Rapoport JL, Leonard HL, Schapiro MB, Rapoport SI, Grady CL. Regional cerebral glucose metabolism of women with trichotillomania. Archives of General Psychiatry. 1991;48:828–833. doi: 10.1001/archpsyc.1991.01810330052008. [DOI] [PubMed] [Google Scholar]
- Tynes LL, White K, Steketee GS. Toward a new nosology of obsessive compulsive disorder. Comprehensive Psychiatry. 1990;31:465–480. doi: 10.1016/0010-440x(90)90033-o. [DOI] [PubMed] [Google Scholar]
- Veale DM, Sahakian BJ, Owen AM, Marks IM. Specific cognitive deficits in tests sensitive to frontal lobe dys-function in obsessive-compulsive disorder. Psychological Medicine. 1996;26:1261–1269. doi: 10.1017/s0033291700035984. [DOI] [PubMed] [Google Scholar]
- Watkins LH, Sahakian B, Robertson M, Veale DM, Rogers R, Pickard KM, Aitken M, Robbins T. Executive function in Tourette's syndrome and obsessive-compulsive disorder. Psychological Medicine. 2005;35:571–582. doi: 10.1017/s0033291704003691. [DOI] [PubMed] [Google Scholar]
- Whitney KA, Fastenau PS, Evans JD, Lysaker PH. Comparative neuropsychological function in obsessive-compulsive disorder and schizophrenia with and without obsessive-compulsive symptoms. Schizophrenia Research. 2004;69:75–83. doi: 10.1016/j.schres.2003.08.013. [DOI] [PubMed] [Google Scholar]