Abstract
In response to iron deficiency, cyanobacteria synthesize the iron stress–induced chlorophyll binding protein IsiA. This protein protects cyanobacterial cells against iron stress. It has been proposed that the protective role of IsiA is related to a blue light–induced nonphotochemical fluorescence quenching (NPQ) mechanism. In iron-replete cyanobacterial cell cultures, strong blue light is known to induce a mechanism that dissipates excess absorbed energy in the phycobilisome, the extramembranal antenna of cyanobacteria. In this photoprotective mechanism, the soluble Orange Carotenoid Protein (OCP) plays an essential role. Here, we demonstrate that in iron-starved cells, blue light is unable to quench fluorescence in the absence of the phycobilisomes or the OCP. By contrast, the absence of IsiA does not affect the induction of fluorescence quenching or its recovery. We conclude that in cyanobacteria grown under iron starvation conditions, the blue light–induced nonphotochemical quenching involves the phycobilisome OCP–related energy dissipation mechanism and not IsiA. IsiA, however, does seem to protect the cells from the stress generated by iron starvation, initially by increasing the size of the photosystem I antenna. Subsequently, the IsiA converts the excess energy absorbed by the phycobilisomes into heat through a mechanism different from the dynamic and reversible light-induced NPQ processes.
INTRODUCTION
Excess light can be lethal for photosynthetic organisms because harmful reactive oxygen species are generated in the photochemical reaction centers when energy absorption exceeds the rate of carbon fixation. To survive, photosynthetic organisms have evolved several protective processes. One such mechanism is the dissipation of the excess absorbed energy as heat in the light-collecting pigment/protein complexes, the so-called antenna. In plants, this process involves the chlorophyll-containing light-harvesting complex (LHCII) of photosystem II (PSII) and is triggered by acidification of the thylakoid lumen under saturating light conditions (reviewed in Demmig-Adams, 1990; Horton et al., 1996; Niyogi, 1999; Müller et al., 2001). A drop in the thylakoid lumen pH activates the formation of the carotenoid zeaxanthin from violaxanthin as part of the xanthophyll cycle (Yamamoto, 1979; Gilmore and Yamamoto, 1993) and induces the protonation of PsbS, a PSII subunit that belongs to the LHC superfamily (Li et al., 2000, 2004). This process also involves conformational changes in LHCII, modifying the interaction between chlorophylls and carotenoids (Ruban et al., 1992; Pascal et al., 2005). Thermal energy dissipation is accompanied by a decrease of PSII-related fluorescence emission, known as high-energy quenching (qE), one of the nonphotochemical quenching (NPQ) processes. The qE is a dynamic, rapidly reversible process that is induced seconds after the plant is exposed to high light intensities.
Several recent studies have shown that cyanobacteria, which do not have the integral membrane chlorophyll-containing LHCII, also use a light-induced antenna-related NPQ mechanism to decrease the amount of energy funneled to the PSII reaction center (El Bissati et al., 2000; Rakhimberdieva et al., 2004; Scott et al., 2006; Wilson et al., 2006). In cyanobacteria, light is captured by a membrane extrinsic complex, the phycobilisome, which is attached to the outer surface of thylakoid membranes. These large complexes consist of phycobiliproteins with covalently bound bilin pigments and linker peptides that are required for the organization of the phycobilisomes (reviewed in MacColl, 1998; Adir, 2005). Phycobilisomes are composed of a core from which rods (usually six) radiate. The major core protein is allophycocyanin (APC), while the rods contain phycocyanin (PC) and, in some species, phycoerythrin or phycoerythrocyanin (in the distal end of the rod). The phycobilisomes are bound to the thylakoids via the core membrane linker protein Lcm, which also serves as the terminal energy acceptor. Harvested light energy is transferred from Lcm to the chlorophylls of PSII and photosystem I (PSI) (Mullineaux, 1992; Rakhimberdieva et al., 2001).
Results revealing the existence of a blue light–induced NPQ mechanism proposed to be associated with the phycobilisomes were first described in 2000 (El Bissati et al., 2000). Subsequently, spectral and kinetics data were presented suggesting that blue light–activated carotenoids induce quenching of phycobilisome fluorescence emission (Rakhimberdieva et al., 2004). Wilson et al. (2006) demonstrated that a soluble carotenoid binding protein, the Orange Carotenoid Protein (OCP), is specifically involved in a phycobilisome-related NPQ that appears to be associated with a photoprotective energy dissipation mechanism. OCP, a 35-kD protein that contains a single noncovalently bound carotenoid, is encoded by the slr1963 open reading frame in Synechocystis PCC 6803 (Holt and Krogmann, 1981; Wu and Krogmann, 1997; for review, see Kerfeld, 2004a, 2004b). Highly conserved homologs of OCP are found in the genomes of all cyanobacteria, with the exception of the Prochlorococcus strains, for which genomic data are available (Kerfeld, 2004a, 2004b).
In the absence of OCP, the NPQ induced by strong white or blue-green light in Synechocystis PCC 6803 cells is completely inhibited, and the cells are more sensitive to high light intensities (Wilson et al., 2006). The observation that the effective antenna size was smaller in the cells in the quenched state strongly supports the hypothesis that the OCP-related mechanism dissipates the excess absorbed energy, thereby decreasing the amount of energy arriving at the photochemical centers. The OCP phycobilisome–associated NPQ is not dependent on the presence of a transthylakoid ΔpH, on the excitation pressure on PSII, or on changes in the redox state of the plastoquinone pool (El Bissati et al., 2000; Scott et al., 2006; Wilson et al., 2006). Instead, OCP seems to act as a photoreceptor that responds to blue-green light and induces energy dissipation (and fluorescence quenching) through interaction with the phycobilisome core.
Under iron starvation conditions, blue light causes a large reversible quenching of Fo and Fm levels (Cadoret et al., 2004; Bailey et al., 2005; Joshua et al., 2005). It was proposed that the iron stress–induced protein IsiA was essential in this NPQ process. Two mechanisms were proposed: (1) blue light converts the IsiA protein from one form that is efficient in harvesting light energy for photosynthesis into another form that converts excess energy into heat (Cadoret et al., 2004); and (2) strong light induces a change in IsiA, increasing the affinity of IsiA for the phycobilisomes and diminishing the high fluorescence of free phycobilisomes (Joshua et al., 2005).
The expression of the IsiA protein, which belongs to the core complex family of chlorophyll binding proteins, is induced by iron starvation (Laudenbach and Straus, 1988; Burnap et al., 1993) and other stress conditions (such as salt stress, oxidative stress, and high-light stress) (Jeanjean et al., 2003; Yousef et al., 2003; Havaux et al., 2005). IsiA encircles the PSI reaction center, forming complexes consisting of a trimeric PSI and 18 IsiA molecules (Bibby et al., 2001; Boekema et al., 2001). Larger amounts of IsiA are bound to PSI during prolonged iron starvation (Yeremenko et al., 2004). IsiA increases the absorptional cross section of PSI, acting as an additional and efficient LHC for PSI (Andrizhiyevskaya et al., 2002; Melkozernov et al., 2003). IsiA aggregates, forming empty multimeric rings (without PSI), also accumulate and are very abundant in long-term iron-depleted cells (Yeremenko et al., 2004). These IsiA aggregates, in vitro, are in a strongly quenched state, suggesting that they are responsible for thermal dissipation of absorbed energy (Ihalainen et al., 2005). In cells grown under high light conditions, IsiA is also synthesized to protect the cyanobacterial cells from photodestruction (Havaux et al., 2005).
In addition to IsiA, the high-light-inducible proteins (HLIPs; also called SCPs) seem to protect the cells by heat dissipation of the absorbed energy, and mutants lacking these proteins are more sensitive to high light conditions (He et al., 2001; Havaux et al., 2003). The HLIPS that are synthesized in cyanobacterial cells grown under high-light conditions and other stress conditions (Dolganov et al., 1995; Funk and Vermaas, 1999; He et al., 2001) are chlorophyll binding single-helix polypeptides related to the LHC proteins and to the early-light-inducible proteins (Adamska, 1997, 2001).
Our recent results demonstrating the existence of the phycobilisome OCP–related NPQ mechanism in cyanobacteria grown in the presence of iron (Wilson et al., 2006) led us to consider the possibility that this mechanism could also play a role in the (blue) light-induced fluorescence quenching observed under iron starvation conditions. To date, only IsiA had been implicated in mediating this process. To test this idea, we studied light-induced fluorescence quenching in several iron-starved Synechocystis PCC 6803 mutants: a mutant without IsiA (ΔIsiA), a mutant without OCP (ΔOCP), and a mutant without phycobilisomes (PAL). Indeed, our results demonstrate that under iron starvation the blue-green light–induced NPQ mechanism is related to the OCP-mediated quenching of the phycobilisome emission and not to modifications of IsiA. Even though the presence of the IsiA protein protects the cells from stress, this protein is not involved in the light-induced NPQ mechanism.
RESULTS
Pigment Changes during Iron Starvation in Wild-Type, ΔIsiA, and ΔOCP Mutant Cells
Cyanobacteria grown under iron-deficient conditions exhibit decreased chlorophyll and PC content (Öquist, 1971, 1974a; Guikema and Sherman, 1983; Sandmann, 1985). These changes are systemic; the levels of PSI, PSII (Spiller and Terry, 1980; Guikema and Sherman, 1983), and thylakoid (Sherman and Sherman, 1983) all decrease during iron starvation, with the decrease in PSI being the most marked (Guikema and Sherman, 1983; Sandmann, 1985). The presence of IsiA causes a blue shift in the room temperature chlorophyll a absorbance peak (680 to 673 nm), and the chlorophyll a fluorescence at 77K becomes dominated by a high emission at 685 nm (Öquist, 1974b; Burnap et al., 1993; Falk et al., 1995; Park et al., 1999). Odom et al. (1993) were the first to describe these changes in iron-starved Synechocystis PCC 6803.
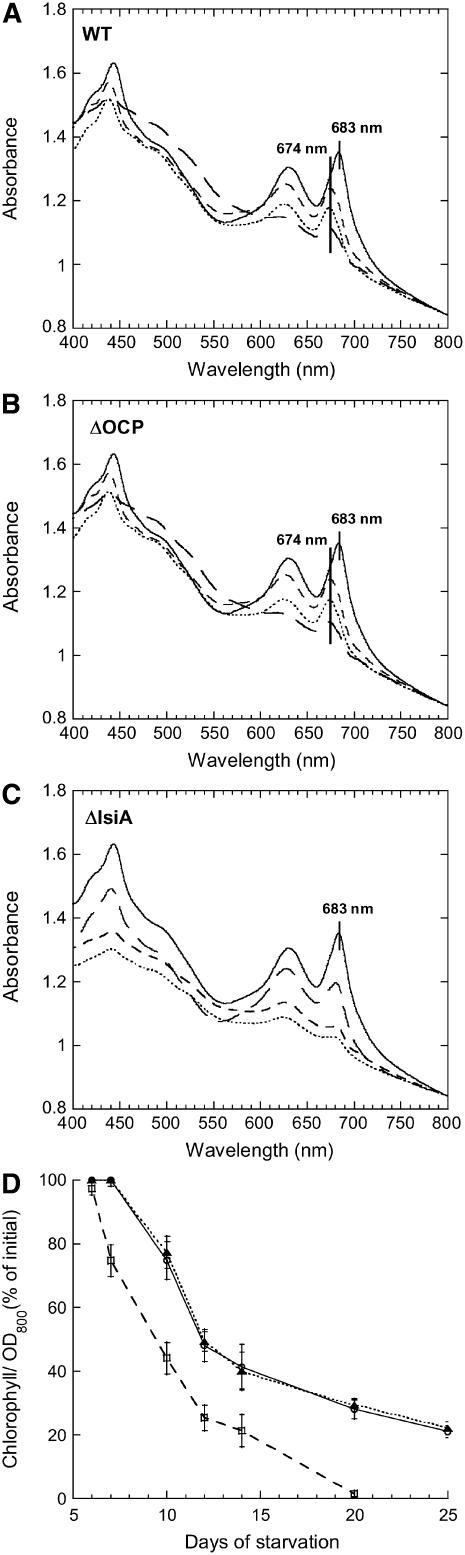
Under experimental conditions of iron starvation and low-light intensities (see Methods), during the first 6 d, the content of chlorophyll per cell and the PC/chlorophyll ratio remained similar to those in unstarved wild-type, ΔOCP, and ΔIsiA Synechocystis PCC 6803 cells (Figure 1). Subsequently, the chlorophyll content of the wild-type and ΔOCP cells decreased; the maximum of the chlorophyll absorbance peak at 683 nm down-shifted until it reached 674 nm after 10 d of starvation (Figures 1A and 1B). In ΔIsiA cells, no shift of the chlorophyll-related peak was observed, and the chlorophyll content per cell decreased faster than in wild-type cells (Figures 1C and 1D). Even though the PC content per cell also decreased, an increase of the PC/chlorophyll ratio was observed in ΔIsiA cells (Figure 1C), while in wild-type and ΔOCP cells, the PC/chlorophyll ratio remained almost constant (Figures 1A and 1B). After 20 d of iron starvation, the ΔIsiA contained almost no chlorophyll and the cells were dead (Figure 1D). By contrast, wild-type and ΔOCP cells containing a low content of chlorophyll and a high content of carotenoids continued to survive and were still alive after 50 d of iron starvation (see Supplemental Figure 4 online).
Figure 1.
Changes in Absorption Spectra and Chlorophyll Content Induced by Iron Starvation in Wild-Type, ΔOCP, and ΔIsiA Cells.
(A) and (B) Absorption spectra of wild-type (A) and ΔOCP (B) cells grown in iron-containing medium (solid line) or in iron-depleted medium for 12 (small dashed line), 14 (dotted line), or 20 (large dashed line) d.
(C) Absorption spectra of ΔIsiA cells grown in iron-containing medium (solid line) or in iron-free medium for 7 (large dashed line), 12 (small dashed line), or 14 (dotted line) d. The spectra were normalized at OD800.
(D) Decrease of chlorophyll content in iron-starved wild-type (circles), ΔOCP (triangles), and ΔIsiA (squares) cells. The results are the average of seven independent experiments. Error bars show the maximum and minimum chlorophyll/OD800 values for each point. 100% of chlorophyll/OD800 = 7.5, corresponding to ∼5.8 μg chlorophyll/mL for a culture at OD800 = 0.78.
Fluorescence Changes during Iron Starvation in Wild-Type and ΔIsiA Cells
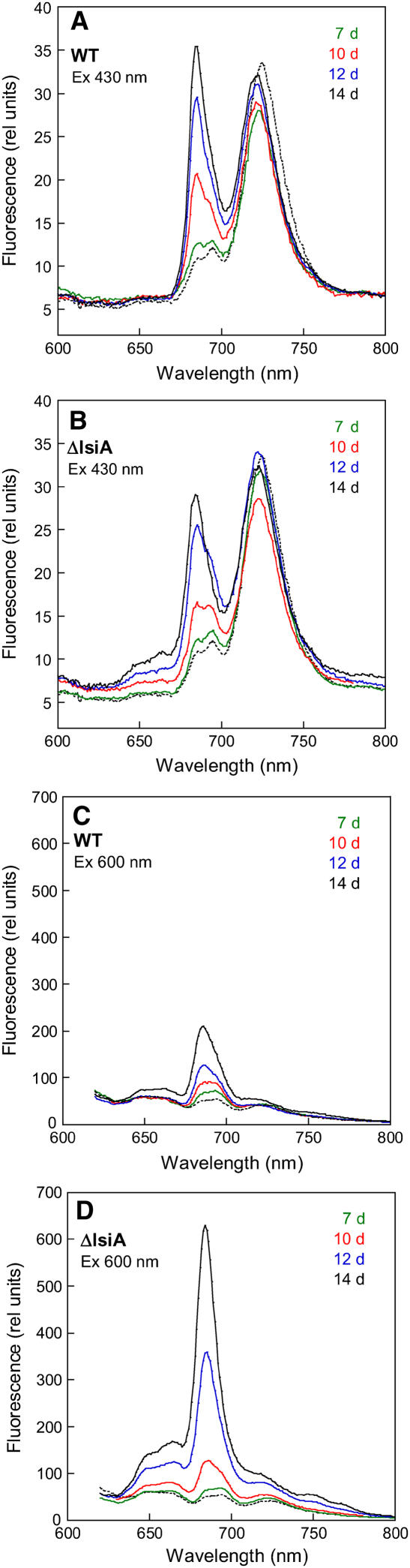
Figure 2 shows the 77K fluorescence emission spectra of wild-type and ΔIsiA cells grown in complete medium or medium lacking iron for 7, 10, 12, and 14 d. Fluorescence excitation experiments using 430-nm light, which is preferentially absorbed by chlorophyll, and 600-nm light, which is preferentially absorbed by the phycobilisomes, were used to monitor the changes in the photosynthetic apparatus induced by iron starvation. When wild-type and ΔIsiA cells grown in iron-containing medium were excited at 430 nm, the 77K fluorescence spectra showed bands at 685 and 695 nm (related to the CP43 and the CP47 chlorophyll antenna of PSII, respectively; Van Dorssen et al., 1987; Siefermann-Harms, 1988), and a large PSI chlorophyll-related band at 725 nm (Figures 2A and 2B). In the early phases of iron starvation, wild-type and ΔIsiA cells showed an increase in the ratio of PSII fluorescence (685- and 695-nm peaks) to PSI fluorescence, suggesting an increased PSII/PSI ratio (Figures 2A and 2B). This is in accordance with the observation that the content of PSI, which contains three 4Fe-4S centers, decreases faster than that of PSII, which contains only three iron sulfur centers (Guikema and Sherman, 1983; Sandmann, 1985; Falk et al., 1995; Ivanov et al., 2000).
Figure 2.
Changes in 77K Fluorescence Emission Spectra Induced by Iron Starvation in Wild-Type and ΔIsiA Mutant Cells.
The 77K fluorescence spectra of wild-type ([A] and [C]) and ΔIsiA ([B] and [D]) cells grown in iron-containing medium (dotted line) or in iron-lacking medium for 7 (green), 10 (red), 12 (blue), and 14 (black) d. The excitation wavelength was 430 ([A] and [B]) or 600 nm ([C] and [D]). Each spectrum shown is the mean of four spectra. The 77K fluorescence spectra were normalized to the fluorescence emitted at 800 nm. The figure shows a representative iron starvation experiment; the experiments consistently showed similar fluorescence changes and kinetics. The cells were at 3 μg chlorophyll/mL.
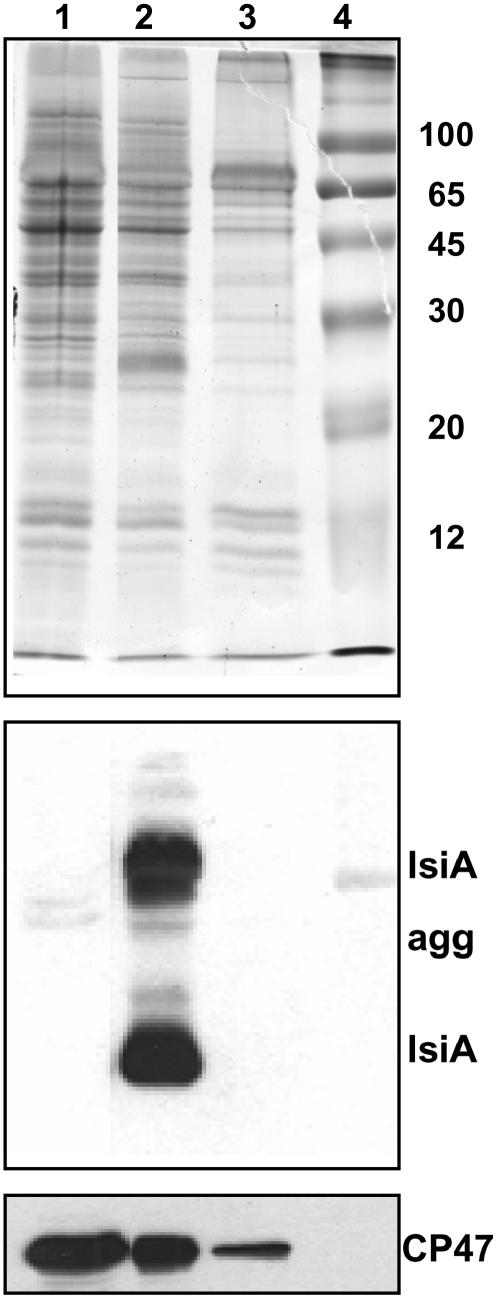
After prolonged (10 to 14 d) iron starvation, both strains showed a more rapid increase in the emission at 685 nm relative to that at 695 nm (Figures 2A and 2B). This was particularly marked in wild-type cells in which the 685-nm fluorescence emission reflects the presence of PSI-less IsiA aggregates (Yeremenko et al., 2004). PSI-IsiA supercomplexes have a very small 686-nm emission that makes a negligible contribution to this band (Andrizhiyevskaya et al., 2002; Yeremenko et al., 2004). The presence of IsiA and IsiA aggregates was confirmed by protein gel blot experiments (Figure 3). IsiA was present in 10 d iron-starved wild-type cells, but it was absent in wild-type cells grown in the presence of iron and in iron-starved ΔIsiA cells. The amount of CP47 (a measure of PSII) was increased in cells under iron-starved conditions relative to cells grown in the presence of iron (Figure 3). In iron-starved ΔIsiA cells, the amount of CP47 was even slightly higher than in wild-type cells. These results were in accordance with the increased PSII/PSI ratio deduced from fluorescence spectra and variable fluorescence measurements (see below). Dühring et al. (2006), using Blue-Native PAGE gels, has also shown that in ΔIsiA iron-starved cells there is a higher concentration of PSII dimer and monomer than in wild-type iron-starved cells.
Figure 3.
Immunodetection of IsiA and CP47 in Membranes Isolated from Nonstarved and Starved Synechocystis Wild-Type and Mutant Cells.
Coomassie blue–stained gel electrophoresis and immunoblot detection of IsiA and CP47 in the membrane fractions isolated from 10 d iron-starved ΔIsiA (lane 1) and wild-type (lane 2) cells and nonstarved wild-type cells (lane 3). The IsiA antibody also reacts with IsiA aggregates (IsiA agg). Lane 4, molecular mass markers (in kilodaltons). Each sample contained 1 μg of chlorophyll.
In iron-starved ΔIsiA cells, the increase of the 685-nm emission is evidently not associated with IsiA; instead, the source of this emission may be the phycobilisome terminal emitter, the Lcm, in disconnected phycobilisomes (see Supplemental Figure 1 online). In long-term iron-starved wild-type cells, a contribution of the emission from Lcm of uncoupled phycobilisomes may also be present.
In cyanobacteria, the fluorescence spectrum generated at 77K by 600-nm excitation contains emission bands related to PC (650 nm), APC (660 nm), PSII (685 and 695 nm), and PSI (725 nm) (Figures 2C and 2D). The peak at 695 nm derives from the chlorophyll a emission of the PSII antenna CP47 and the reaction center. The emission at 685 nm is principally related to the phycobilisome terminal emitter, but fluorescence emission from the CP43 PSII antenna also contributes to this peak. When empty (without PSI) IsiA complexes are present, their fluorescence emission significantly contributes to the 685-nm peak. In the fluorescence spectra, the first manifestation of iron starvation in wild-type and ΔIsiA cells was an increase in the ratio of PSII fluorescence (685- and 695-nm peaks) to PSI fluorescence (Figures 2C and 2D). Extended iron starvation resulted in a large increase of the 685-nm peak. This increase was faster and more pronounced in ΔIsiA cells than in wild-type cells (Figures 2C and 2D). The origin of this increase was different in each strain. In ΔIsiA cells, this increase can only be related to the phycobilisome terminal emitter, reflecting an accumulation of functionally disconnected, high fluorescent phycobilisomes. In wild-type cells, the increase could be attributed to IsiA emission (Yeremenko et al., 2004) due to energy transfer from the phycobilisomes to IsiA complexes, as suggested by Joshua et al. (2005), and to a small population of uncoupled phycobilisomes in long-term iron-starved cells.
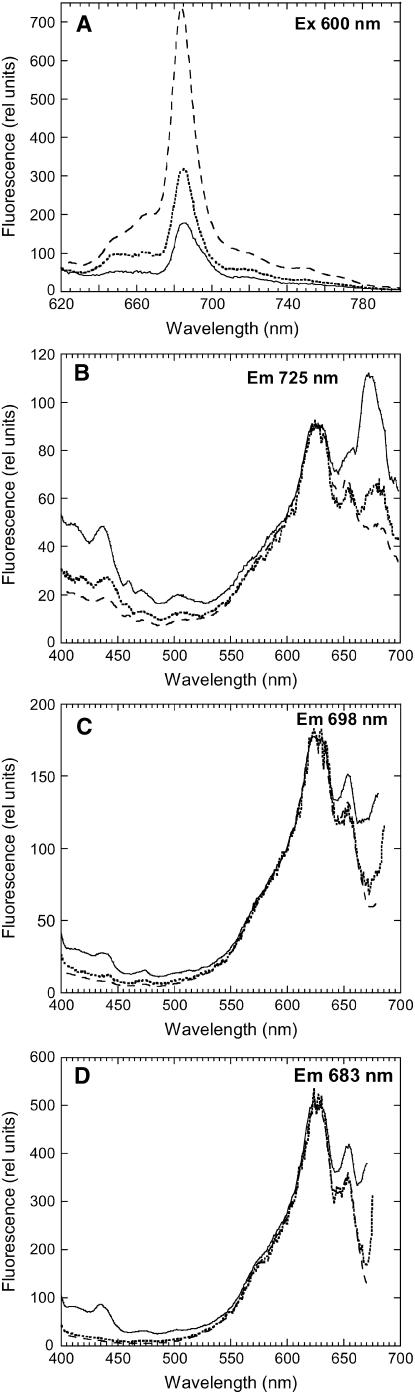
This suggestion was confirmed by comparison of 77K excitation fluorescence spectra of the emissions at 725 (PSI emission), 698 (PSII emission), and 683 nm (IsiA aggregates and phycobilisome emission) in iron-starved wild-type and iron-starved ΔIsiA cells. Isolated phycobilisomes also have a relatively high emission at 698 and 725 nm (see Supplemental Figure 2 online). In iron-starved wild-type cells, the spectra showed higher contributions from chlorophyll a (peaks at around 435 and 680 nm) and smaller contributions from components at 570, 620 (from PC), and 650 nm (from APC) than in iron-starved ΔIsiA cells (Figure 4). Moreover, the relative contribution of PC to the spectra increased with the increase of the 685-nm emission peak in iron-starved cells. These results indicated that in ΔIsiA cells, a large quantity of functionally disconnected phycobilisomes was present. The spectra also strongly suggest that in iron-starved wild-type cells, most of the phycobilisomes transferred the absorbed energy not only to the photosystems but also to IsiA complexes (see Supplemental Figure 2 online).
Figure 4.
The 77K Excitation Fluorescence Spectra of Iron-Starved Wild-Type and ΔIsiA Cells.
The 77K fluorescence emission spectra (Ex 600 nm) of 11 d iron-starved wild-type cells (solid line) and 11 d (dotted line) and 15 d (dashed line) iron-starved ΔIsiA cells (A). The 77K fluorescence excitation spectra for an emission at 725 (B), 698 (C), and 683 nm (D) of 11 d iron-starved wild-type cells (solid line) and 11 d (dotted line) and 15 d (dashed line) iron-starved ΔIsiA cells. The cells were at 2 μg chlorophyll/mL.
Phycobilisome-associated membrane fractions (MPs) from nonstarved and 11 or 15 d iron-starved wild-type and ΔIsiA cells were prepared to elucidate if the functionally disconnected phycobilisomes remained associated with the thylakoids. The cells were broken in a phosphate/citrate buffer. After discarding unbroken cells, the MPs were obtained by centrifugation. In all cases, the supernatant was nearly colorless, indicating that the bulk of the phycobilisomes remained attached to the thylakoids. This was confirmed by absorption spectra (see Supplemental Figure 3 online). The PC/chlorophyll ratio was higher in ΔIsiA MP compared with that of MP from wild-type cells. Fifteen days iron-starved ΔIsiA cells and MPs showed high and similar fluorescence at 685 nm. The magnitude of this fluorescence suggested that already in whole cells a large quantity of phycobilisomes (perhaps nearly all) were functionally disconnected. The rest of the MP preparations produced a larger emission at 685 nm than whole cells, suggesting a compromised connection between the phycobilisomes and the thylakoids in vitro (Figure 5). Nevertheless, as in whole cells, ΔIsiA MPs presented a larger 685-nm emission than wild-type MPs (Figures 5B and 5D). Thus, we conclude that in ΔIsiA iron-starved cells, all the phycobilisomes were attached to the thylakoids as in wild-type cells, but a larger number were functionally disconnected; they did not transfer energy to any chlorophyll complex.
Figure 5.
The 77K Fluorescence Emission Spectra of Iron-Starved Wild-Type and ΔIsiA MP Fractions.
The 77K fluorescence spectra of nonstarved (dashed line), 12 d iron-starved wild-type (solid line; [A]), and iron-starved ΔIsiA (dotted line; [A]) cells, 15 d iron-starved wild-type cells (solid line; [C]), and iron-starved ΔIsiA (dotted line; [C]) cells and of the corresponding MP fractions ([B], wild-type; [D], ΔIsiA). The 77K fluorescence emission spectra were normalized to the fluorescence emitted at 800 nm. The cells were at 2 μg chlorophyll/mL.
Fluorescence Quenching in Iron-Starved Wild-Type and ΔIsiA Cells
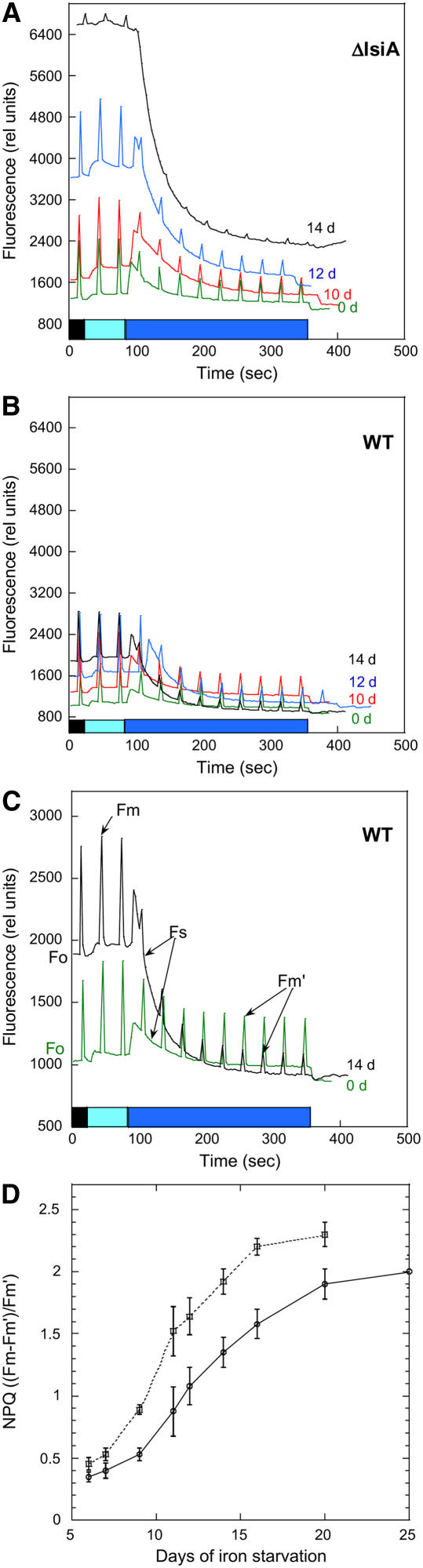
The induction of the blue light–induced fluorescence quenching was monitored using a pulse-amplitude modulated fluorimeter (PAM). In cyanobacteria, the fluorescence detected by a PAM fluorometer is emitted from chlorophyll and phycobiliproteins (Campbell et al., 1998). In the PAM fluorometer, the measuring light has a maximum of excitation at 650 nm, and the fluorescence is detected at wavelengths beyond 700 nm. In cyanobacteria, which lack chlorophyll b, most of the measuring light is absorbed by the phycobilisomes. Thus, Fo, the minimal fluorescence level in dark-adapted cells, varies according to the cellular phycobiliprotein concentration (e.g., very low in mutants without phycobiliproteins) (Campbell et al., 1998; El Bissati and Kirilovsky, 2001). The Fo level also depends on the coupling of phycobilisomes: energetically coupled phycobilisomes show low-yield fluorescence emission, while uncoupled phycobilisomes show high-yield fluorescence emission. Figures 6A to 6C compare the room temperature fluorescence traces measured with a PAM fluorometer after 0, 7, 10, 12, and 14 d of iron starvation from wild-type and ΔIsiA cells. Dark-adapted cells were successively illuminated by dim and strong blue-green light. The cells under dim blue-green light, which preferentially excites PSI, showed a high level of fluorescence characteristic of State 1, which is induced by oxidation of the plastoquinone pool upon illumination of dark-adapted cells. Subsequently, exposure of cells to strong blue-green light induced the quenching of all levels of fluorescence (Fm′, Fs, and Fo) in both wild-type and ΔIsiA cells. The fluorescence quenching increased as iron starvation was prolonged. Figure 6D shows the increase of NPQ [(Fm − Fm′)/Fm′] values during iron starvation in wild-type and ΔIsiA cells. This increase was faster and more marked in ΔIsiA cells.
Figure 6.
Blue-Green Light–Induced Fluorescence Quenching in Iron-Starved Wild-Type and ΔisiA Cells.
(A) to (C) The 0 d (green), 7 d (data not shown; similar to 0 d), 10 d (red), 12 d (blue), and 14 d (black) iron-starved ΔIsiA (A) and wild-type ([B] and [C]) cells (at 3 μg chlorophyll/mL) were dark-adapted and then illuminated successively with low-intensity blue-green light (400 to 550 nm, 80 μmol photons m−2 s−1) and high-intensity blue-green light (740 μmol photons m−2 s−1). Saturating pulses were applied to measure maximal fluorescence levels. Fm, maximal fluorescence under low intensities of blue light; Fm′, maximal fluorescence under high intensities of blue light; Fs, steady state fluorescence; Fo, minimal fluorescence (see [C]). In (C), the changes of fluorescence traces in 7 and 14 d iron-starved wild-type cells are shown with a different scale than (A) and (B) to clarify the differences in fluorescence quenching.
(D) Increase of NPQ [(Fm − Fm′)/Fm′] during iron starvation of wild-type (circles and solid line) and ΔIsiA (squares and dotted line) cells. The graph is the average of four independent experiments. Error bars show the maximum and minimum NPQ values for each point.
We also observed that after 6 to 7 d of iron starvation, differences in Fo values and Fv/Fo or Fv/Fm ratios appeared. First, in both strains at equimolar chlorophyll concentrations, Fo and Fv increased in parallel, and the ratios Fv/Fo and Fv/Fm remained high (even slightly higher than in nonstarved cells) (Figure 6, Table 1), suggesting that the increase of Fo and Fv was due to an increase in the PSII/PSI ratio, in agreement with the 77K fluorescence spectra and the observation that the per cell PSI content decreased faster than PSII content. With prolonged iron starvation, Fv began to decrease while Fo continued to increase, indicating a loss of active PSII (Figure 6, Table 1). In ΔIsiA cells, this process was faster. After 14 d of Fe starvation, Fv was very small, while it was still relatively large in wild-type cells (Figure 6, Table 1). Wild-type cells were still alive after 48 d of iron starvation and had partially recovered the lost Fv, while ΔIsiA cells died after 18 d of iron starvation (see Supplemental Figure 4 online).
Table 1.
Changes in Fluorescence Levels during Iron Starvation in Wild-Type and ΔIsiA Cells
| 0 d Wild- Type/ΔIsiA | 7 d Wild- Type/ΔIsiA | 10 d Wild- Type/ΔIsiA | 12 d Wild- Type/ΔIsiA | 14 d Wild- Type/ΔIsiA | 19 d Wild- Type/ΔIsiA | |
|---|---|---|---|---|---|---|
| Fo (% ± 10%) | 100/100 | 100/105 | 125/160 | 154/350 | 180/642 | 260/– |
| Fv (% ± 10%) | 100/100 | 100/147 | 140/187 | 148/183 | 120/27 | 30/– |
| Fv/Fo (±0.05) | 0.78/0.78 | 0.76/1.1 | 0.80/0.95 | 0.74/0.4 | 0.5/0.035 | 0.09/– |
| Fv/Fm (±0.05) | 0.44/0.44 | 0.43/0.50 | 0.47/0.47 | 0.43/0.3 | 0.33/0.03 | 0.085/– |
Each result is the average of four independent iron starvation experiments.
In ΔIsiA cells, the large increase of Fo can be explained by a rapid increase of a population of energetically uncoupled phycobilisomes detected in 77K fluorescence spectra. In wild-type cells, the increase of Fo could be related not only to an increase of fluorescence emitted by the uncoupled phycobilisome population but also to fluorescence emitted by IsiA complexes. In wild-type cells, the presence of IsiA protects the cell (previously shown in Park et al., 1999; Sandström et al., 2001; Havaux et al., 2005) from the stress generated by iron starvation (Havaux et al., 2005; Latifi et al., 2005). Alone, in ΔIsiA cells, the blue light–induced NPQ mechanism seems to be insufficient protection from this specific stress.
When the iron-starved, quenched, wild-type, and ΔIsiA cells were exposed to dim blue-green light in the presence of chloramphenicol, an inhibitor of protein synthesis, they recovered their maximal level of Fm′ (Figure 7). These results confirmed that the blue-green light–induced fluorescence quenching (in both iron-starved strains) was not related to photoinhibition/D1 damage. Similar effects were seen in comparable experiments in wild-type cells grown in the presence of iron (El Bissati et al., 2000; Wilson et al., 2006).
Figure 7.
Blue Light–Induced Quenching in Iron-Starved Wild-Type and ΔIsiA Cells Is Reversible without Protein Synthesis.
Measurements of fluorescence yield by a PAM fluorometer in iron-starved wild-type ([A] and [C]) and ΔIsiA ([B] and [D]) cells for 7 ([A] and [B]) and 14 d ([C] and [D]) at 3 μg chlorophyll/mL illuminated successively with low-intensity blue-green light (400 to 550 nm; 80 μmol photons m−2 s−1) and high-intensity blue-green light (740 μmol photons m−2 s−1) and then again with dim blue-green light. Chloramphenicol was present during all experiments. The figure shows a representative experiment.
Phycobilisome or Chlorophyll Emission Quenching?
As stated above, a decrease in the fluorescence levels observed in a PAM fluorometer could be a result of (1) a diminution of the phycobilisome emission, (2) a decrease in the chlorophyll antenna emission, or (3) a decrease of energy transfer from the phycobilisomes to PSII. We have already demonstrated that in Synechocystis PCC 6803 grown in iron-containing medium, the blue-green light–induced fluorescence decrease observed in the PAM fluorometer is due to the quenching of the phycobilisome fluorescence emission and a concomitant decrease in the energy transfer from the phycobilisomes to the photosystems (Wilson et al., 2006).
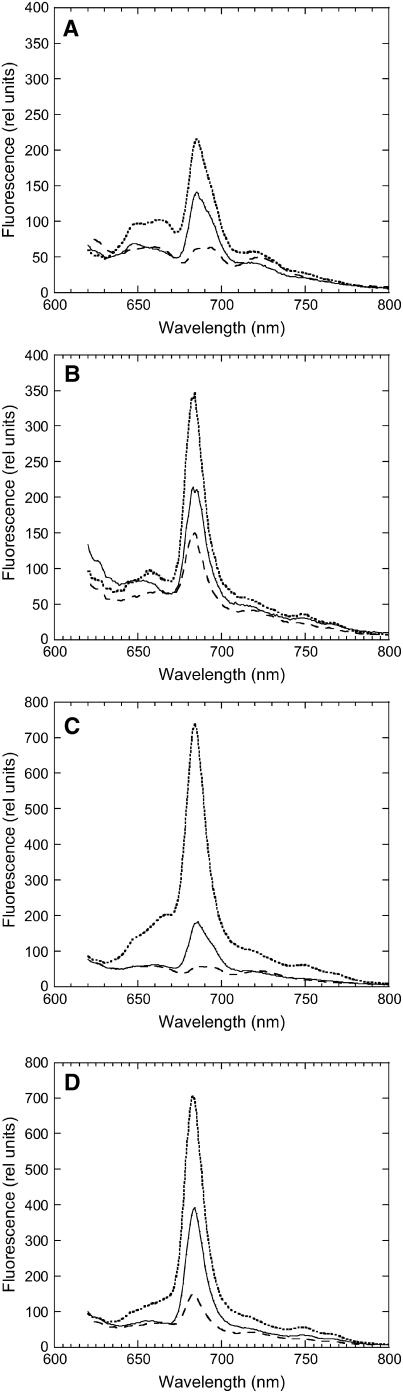
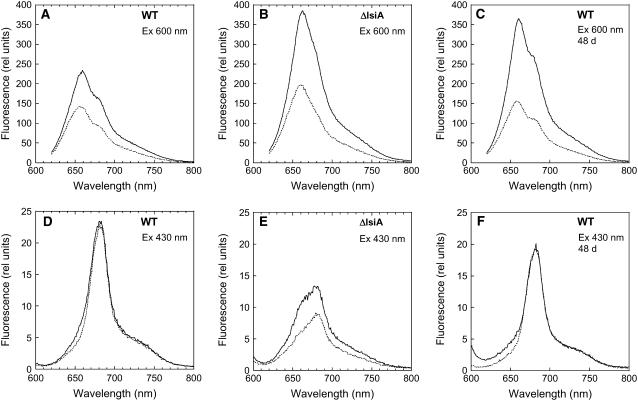
Room temperature fluorescence spectra were used to elucidate the origin of the fluorescence quenching in iron-starved cells. When cells iron-starved for 12 d were excited at 600 nm (light principally absorbed by phycobilisomes), the peak at 660 nm (phycobilisome-related) was more pronounced in iron-starved ΔIsiA cells than in iron-starved wild-type cells (Figures 8A and 8B). The large 660-nm fluorescence in ΔIsiA cells is most probably the result of disconnected phycobilisomes (see also Figure 2D). In iron-starved wild-type cells, an increase of the shoulder at 680 nm (chlorophyll related) compared with nonstarved cells was observed (see Supplemental Figure 5 online). This was attributed to the presence of IsiA and to energy transfer from the phycobilisomes to IsiA. In iron-starved wild-type and ΔIsiA cells that were illuminated with strong blue-green light for 5 min (quenched cells), a very large decrease of the 660-nm band was observed (Figures 8A and 8B). Figure 8C shows that in wild-type cells iron-starved for an extended period (48 d), the 660-nm fluorescence emission increased and the fluorescence quenching was larger.
Figure 8.
Room Temperature Fluorescence Spectra of Iron-Starved Unquenched and Quenched Cells.
Room temperature fluorescence spectra of dark-adapted (solid line) 12 d iron-starved wild-type cells ([A] and [D]), 48 d iron-starved wild-type cells ([C] and [F]), and 12 d iron-starved ΔIsiA cells ([B] and [E]) and after 5 min of high-intensity blue-green light illumination (740 μmol photons m−2 s−1) (dotted line) at 3 μg chlorophyll/mL. Excitation was performed at 600 nm ([A] to [C]) and at 430 nm ([D] to [F]).
When iron-starved wild-type cells were excited at 430 nm (light principally absorbed by chlorophyll), a large band at 680 nm was observed, corresponding to the accumulation of IsiA complexes. This chlorophyll-related band was not decreased after illumination of iron-starved wild-type cells with strong blue light (Figures 8D, 12 d, and 8F, 48 d). In iron-starved ΔIsiA cells, even when the cells were excited at 430 nm, a relatively large emission at 660 nm (a phycobilisome related band) was observed (Figure 8E; see Supplemental Figure 5 online). A decrease of this emission was induced by strong blue-green light illumination. In conclusion, room temperature fluorescence spectra of unquenched and quenched iron-starved wild-type and ΔIsiA cells strongly suggested that under iron starvation conditions the blue-green-induced fluorescence quenching was attributable to a decrease of phycobilisome fluorescence emission and a concomitant decrease in the energy transfer from the phycobilisome to the photosystems and to IsiA (in the wild type).
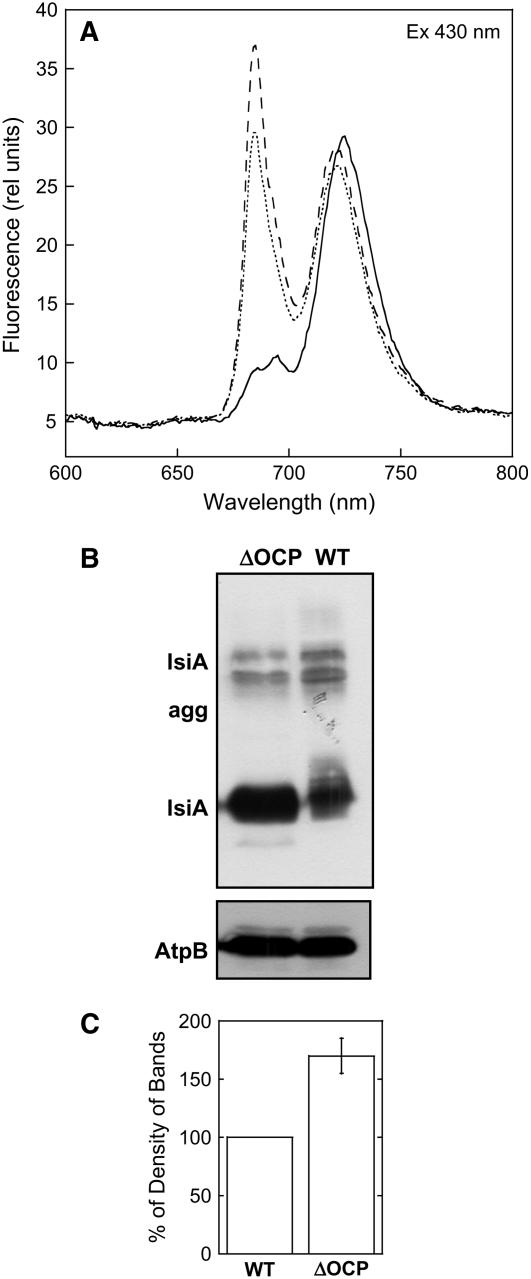
Figure 12.
IsiA Presence in Iron-Starved ΔOCP Cells.
(A) The 77K fluorescence emission spectra of iron-containing (solid line) and 14 d iron-starved (dotted line) wild-type cells and of 14 d iron-starved ΔOCP cells (dashed line). Excitation was done at 430 nm. The cells were at 3 μg chlorophyll/mL.
(B) Immunoblot detection of IsiA of the membrane fraction isolated from 13 d iron-starved ΔOCP (lane 1) and wild-type (lane 2) cells. An antibody against the subunit AtpB of the ATP synthase was used as internal standard (bottom panel). Each sample contained 2 μg of chlorophyll.
(C) Comparative densitometry of IsiA levels in iron-starved wild-type and ΔOCP thylakoids. The results represent the average of three independent experiments. Each error bar shows the maximum and minimum density of band values for each point.
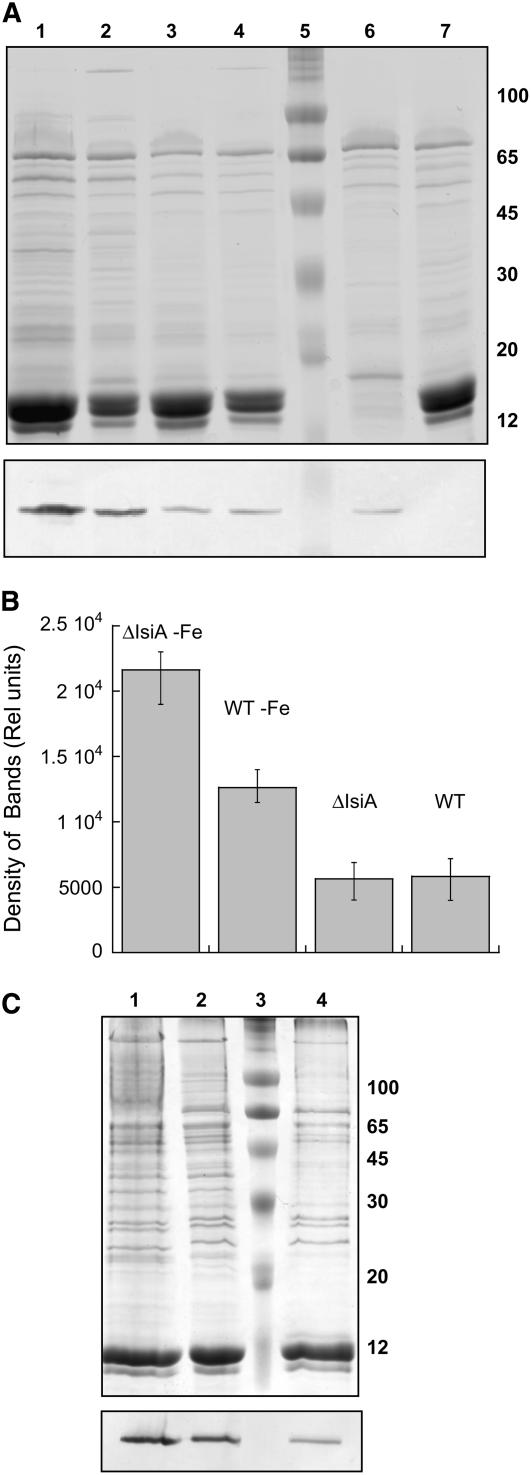
To elucidate if there was a relationship between the larger NPQ in iron-starved cells relative to nonstarved cells and to the quantity of OCP present, protein gel blot analyses were undertaken. Total cellular proteins or MP proteins were separated by SDS-PAGE, and the OCP was detected by an anti-OCP antibody (Figure 9). The comparison was done on a per chlorophyll basis because all the fluorescence experiments were performed at the same chlorophyll concentration. The antibody reacts with a 35-kD polypeptide absent in the ΔOCP mutant. The immunoreaction was more pronounced in iron-starved cells and their MP fractions than in non-iron-starved cells and their MP fractions (per chlorophyll). Moreover, there was more OCP in ΔIsiA iron-starved cells and MP fractions (per chlorophyll) than in wild-type iron-starved cells and MP fractions (Figure 9).
Figure 9.
OCP Detection in Whole Cells and MP Fractions from Nonstarved and Starved Wild Type and Mutants.
(A) Coomassie blue–stained gel electrophoresis and immunoblot detection (bottom panel) of OCP in 12 d iron-starved ΔIsiA (lane 1) and wild-type (lane 2) cells and nonstarved ΔIsiA (lane 3), wild-type (lane 4), PAL (lane 6), and ΔOCP (lane 7) cells. Lane 5 shows molecular mass markers. Each lane contained 1.5 μg of chlorophyll.
(B) Comparative densitometry of OCP bands in nonstarved and iron-starved wild-type and ΔIsiA whole cells. The results represent the average of four independent experiments. Error bars show the maximum and minimum density of band values for each point.
(C) Coomassie blue–stained gel electrophoresis and immunoblot detection (bottom panel) of the OCP in MP fractions isolated from 12 d iron-starved ΔIsiA (lane 1) and wild-type (lane 2) cells and nonstarved wild-type cells (lane 4). Lane 3 shows molecular mass markers. Each lane contained 1 μg of chlorophyll.
Mutant Strains Lacking Phycobilisomes or OCP
To further characterize the light-induced NPQ generated under iron starvation conditions, two additional mutants were studied: one lacking phycobilisomes (PAL mutant, ΔapcAB, ΔapcE, and PC−) (Ajlani and Vernotte, 1998) and a second lacking the OCP, the protein essential for the induction of the phycobilisome-related NPQ under normal growth conditions (ΔOCP mutant) (Wilson et al., 2006).
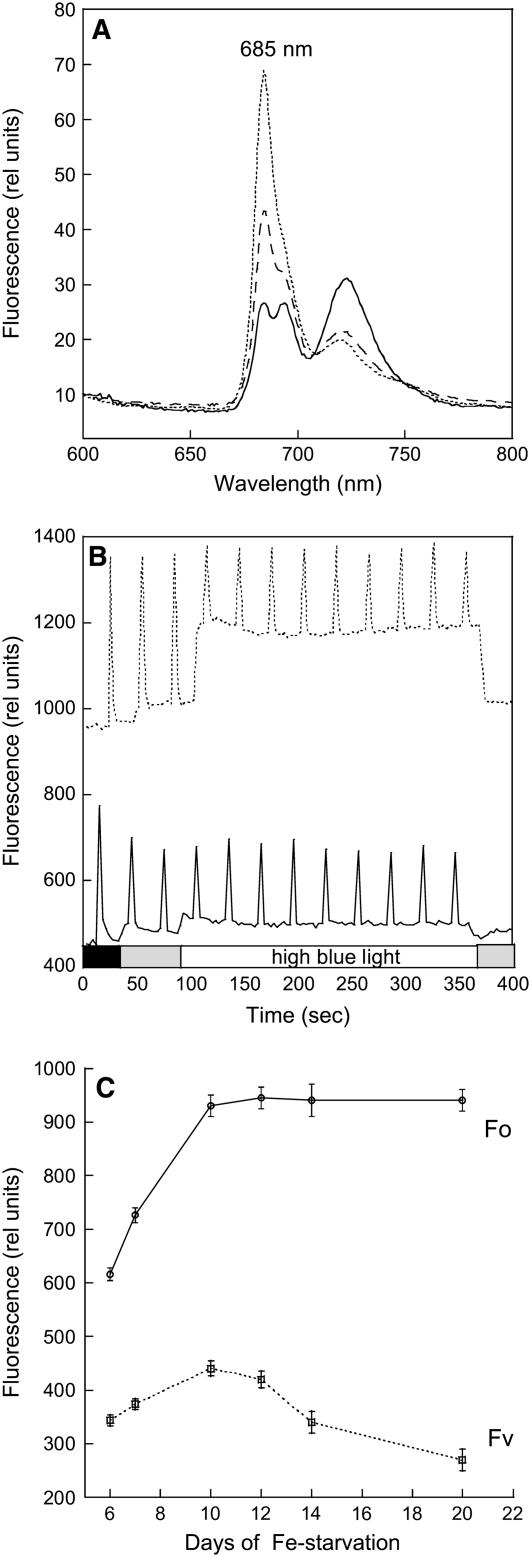
In the PAL mutant, the PSII/PSI ratio is higher than in wild-type cells (Ajlani and Vernotte, 1998). This is reflected in the 77K fluorescence emission spectra obtained by 430-nm excitation: the 685- and 695-nm fluorescence emission bands are larger in PAL than in wild-type cells (cf. Figures 10A and 2A). After only 7 d of starvation, an increase of the 685-nm emission was observed; after 10 d of iron starvation, a sharp peak at 685 nm, characteristic of IsiA, dominates the 77K fluorescence spectra (Figure 10A).
Figure 10.
Fluorescence Changes in Iron-Starved PAL Cells.
(A) The 77K fluorescence spectra of iron-containing (solid line) and iron-starved PAL cells for 7 (dashed line) and 10 d (dotted line). The excitation wavelength was 430 nm.
(B) Dark-adapted iron-containing (solid line) and 12 d iron-starved PAL (dotted line) cells were illuminated successively with low-intensity blue-green light (400 to 550 nm; 80 μmol photons m−2 s−1) and high-intensity blue-green light (740 μmol photons m−2 s−1).
(C) Changes in Fo and Fv in PAL cells during iron starvation. Fluorescence yield changes were detected in a PAM fluorometer. The cells were at 2 μg chlorophyll/mL. The results represent the average of three independent experiments. Error bars show the maximum and minimum chlorophyll/OD800 values for each point.
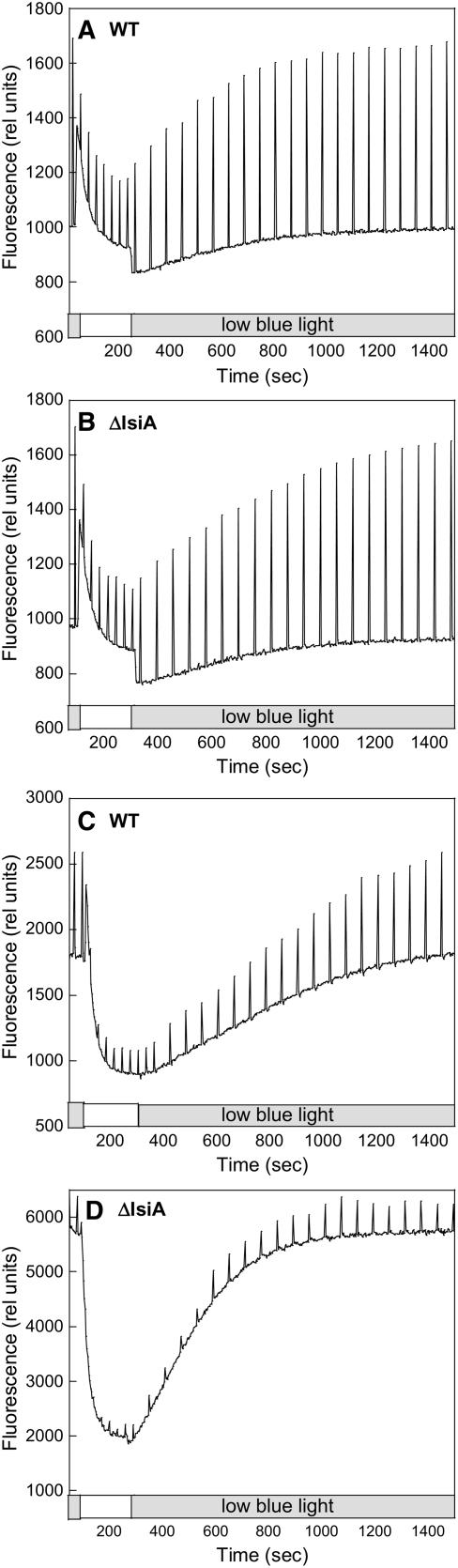
In PAL cells grown in iron-containing medium, high intensities of blue-green light were unable to induce any fluorescence quenching (Wilson et al., 2006; Figure 10B). Figure 10B shows that this is also the case with PAL cells grown under iron starvation conditions. Since OCP is present in the PAL mutant (Figure 9), the lack of fluorescence quenching is due to the lack of phycobilisomes.
As with iron-starved wild-type cells, PAL cells showed changes in chlorophyll content and Fo and Fv values during iron starvation. During the first days of iron starvation, the chlorophyll content decreased to 60% of that of nonstarved cells and then it remained constant. During this time, Fv increased 1.5 times compared with nonstarved PAL cells, suggesting a larger number of active PSII centers on a chlorophyll basis (Figures 10B and 10C). The increase of Fo was higher (2.2 times), suggesting that part of the Fo increase was due to fluorescence emitted by IsiA complexes. As the duration of iron starvation increased, Fo remained almost constant and Fv slowly decreased to a slightly slower value than that in nonstarved cells (Figure 10C). The cells were still alive after 1 month of iron starvation.
The chlorophyll content and the PC/chlorophyll ratio of ΔOCP cells under iron starvation conditions were similar to that of wild-type cells (Figures 1A and 1B). Likewise, Fo and Fv values were similar in wild-type and ΔOCP cells grown in iron-containing medium. However, in general, the Fo level was higher in the wild-type than in ΔOCP iron-starved cells, while the Fv value was larger in ΔOCP than in wild-type cells (Figures 11A and 11B; see Supplemental Figure 4 online).
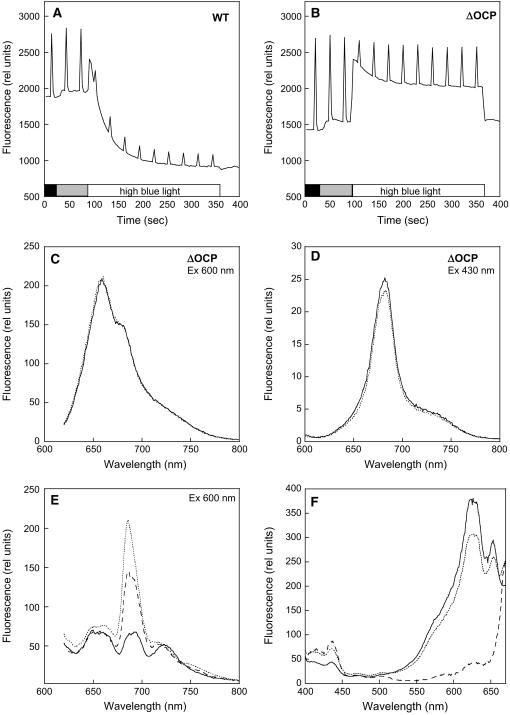
Figure 11.
No Blue Light–Induced NPQ in Iron-Stressed ΔOCP Cells.
(A) and (B) Fluorescence changes in iron-starved ΔOCP cells. Dark-adapted 14 d iron-starved wild-type (A) and ΔOCP (B) cells were illuminated successively with low-intensity blue-green light (400 to 550 nm; 80 μmol photons m−2 s−1) and high-intensity blue-green light (740 μmol photons m−2 s−1). Fluorescence yield changes were detected in a PAM fluorometer. The cells were at 3 μg chlorophyll/mL.
(C) and (D) Room temperature fluorescence spectra of ΔOCP cells adapted to low light intensities of blue-green light (solid line) and after 5 min of high intensities of blue-green light illumination (dotted line). Excitation was done at 600 nm (C) and at 430 nm (D).
(E) The 77K fluorescence emission spectra of iron-containing (solid line), 14 d iron-starved (dotted line) wild-type cells, and 14 d iron-starved ΔOCP cells (dashed line). Excitation was done at 600 nm.
(F) The 77K fluorescence excitation spectra of 14 d iron-starved wild-type (solid line) and ΔOCP (dotted line) and PAL cells (dashed line). Emission was monitored at 685 nm. The cells were at 3 μg chlorophyll/mL.
When the cells were excited at 600 nm, the emission peak at 685 nm was consistently smaller in ΔOCP relative to wild-type cells, suggesting a smaller quantity of uncoupled phycobilisomes in the ΔOCP iron-starved cells (Figure 11E). This was confirmed by comparison of 77K excitation fluorescence spectra of the emission at 685 nm in iron-starved wild-type and iron-starved ΔOCP cells. In iron-starved ΔOCP cells, the spectrum showed higher contributions from chlorophyll a (peaks at ∼435 nm) and smaller contributions from components at 570, 620 (from PC), and 650 nm (from APC) than in iron-starved wild-type cells (Figure 11F). In iron-starved PAL cells, as expected, the major contribution to the excitation spectrum came from chlorophyll a (435 nm) (Figure 11F).
In general, fluorescence emission spectra at 77K using 430-nm excitation showed a larger 685-nm peak, suggesting a larger population of IsiA complexes in ΔOCP than in wild-type cells (Figure 12A). This was confirmed by protein gel blot experiments (Figure 12B). The iron-starved ΔOCP cells contained ∼1.4 to 1.6 times more IsiA than iron-starved wild-type cells.
Figures 11A and 11B show the fluorescence traces in 14 d iron-starved wild-type and ΔOCP cells. In the absence of the OCP, blue-green light did not induce any fluorescence quenching (Figure 11B). Room temperature fluorescence spectra confirmed the absence of blue-green light–induced fluorescence quenching (Figures 11C and 11D). Figure 11D clearly demonstrates that blue light did not induce any quenching of chlorophyll fluorescence, which in the iron-starved ΔOCP cells is principally related to IsiA aggregates. In conclusion, under low light growth conditions, the presence of IsiA was sufficient to protect the ΔOCP cells, despite the absence of the blue light–induced NPQ mechanism.
DISCUSSION
When cyanobacteria grow under iron-limited conditions, the expression of IsiA, encoded by the isiA gene (Laudenbach and Straus, 1988), is synthesized in abundance. IsiA, which is a chlorophyll binding protein closely related to CP43, acts as an LHC of PSI under iron-deficient conditions (Bibby et al., 2001; Boekema et al., 2001). On the basis of results with a ΔIsiA of Synechococcus elongatus 7942 and a mutant of Synechococcus elongatus 7942 overproducing IsiA, a protective function of IsiA against photoinduced damage was suggested with IsiA acting as a dissipator of energy (Park et al., 1999; Sandström et al., 2001). Subsequent experiments using isolated IsiA complexes showed that these complexes are indeed efficient energy dissipators (Ihalainen et al., 2005).
It was proposed that this protection was associated with a strong blue (or white) light-induced NPQ mechanism: strong light could induce conformational changes of IsiA that transform IsiA complexes into energy dissipators (Cadoret et al., 2004) or modify the affinity of IsiA for phycobilisomes (Joshua et al., 2005). Our results clearly demonstrate that IsiA is not involved in a light-induced NPQ mechanism. In iron-starved cells (just as in iron-containing cells), the blue light–induced fluorescence quenching is associated with the phycobilisomes and with the OCP and not with IsiA. In the ΔIsiA mutant, a large reversible fluorescence quenching was always induced by blue light. By contrast, in mutants lacking phycobilisomes (PAL mutant) or OCP (ΔOCP mutant), blue light was unable to induce fluorescence quenching, even after very long periods of iron starvation. In addition, in iron-starved wild-type and ΔIsiA cells, fluorescence emission spectra showed that no chlorophyll-related fluorescence quenching was induced, while a large decrease of the fluorescence emitted by phycobilisomes and of the energy transfer from phycobilisomes to chlorophyll complexes was detected.
Blue light–induced quenching was observed in wild-type and ΔIsiA iron-containing cells (El Bissati et al., 2000; Wilson et al., 2006) but was more marked in iron-starved cells (Cadoret et al., 2004; this article). Moreover, during iron starvation, the increase in fluorescence quenching was faster in ΔIsiA cells than in wild-type cells. In iron-starved ΔIsiA cells, a rapid increase of the 685-nm peak in 77K fluorescence emission spectra, of the 660-nm peak in room temperature fluorescence spectra, and of the Fo level indicated a very fast increase of a population of functionally disconnected highly fluorescent phycobilisomes. In wild-type cells, the number of disconnected phycobilisomes also increased, but more slowly than in ΔIsiA cells. Thus, our results clearly reveal a relationship between the increase of fluorescence quenching and the number of functionally disconnected phycobilisomes. Cells containing a higher number of disconnected phycobilisomes showed a higher level of thermal energy dissipation (NPQ), thereby protecting themselves by diminishing the energy arriving at the photosystems and the thylakoids. The increased NPQ is associated with a higher concentration of the OCP: a higher concentration of the OCP was observed in iron-starved cells compared with nonstarved cells. The abundance of OCP is even greater in iron-starved ΔIsiA cells. The transcription of the slr1963 gene is known to be increased under other stress conditions: high white light illumination (Hihara et al., 2001), UV-B light (I. Vass, personal communication), and saline stress (Fulda et al., 2006). It is possible that other stresses also upregulate the expression of this gene. It may be that the OCP-related NPQ mechanism plays a significant protective role under a range of stress conditions.
In ΔOCP cells under prolonged iron starvation, blue light was unable to induce any fluorescence quenching even in the presence of functionally disconnected phycobilisomes and empty IsiA complexes. Thus, the OCP is essential for the NPQ occurring under iron starvation conditions. Interestingly, in iron-starved ΔOCP cells, the increase in the amount of uncoupled phycobilisomes and the decrease of Fv were slower than in wild-type cells. This appears to be the result of a higher concentration of IsiA. Increased IsiA concentration could be explained by the fact that ΔOCP cells, being more sensitive to light, are permanently under a greater oxidative stress than wild-type cells. It has already been demonstrated that oxidative stress induces isiA transcript accumulation (Jeanjean et al., 2003; Yousef et al., 2003). Singh et al. (2005) have shown that ΔIsiA cells were slightly more resistant to the presence of H2O2 than wild-type cells. They proposed that this higher resistance is related to the greater transcription induction of a gene cluster, including a gene encoding a peroxiredoxin that is involved in the detoxification of peroxide. Thus, under stress conditions, ΔOCP cells can synthesize not only IsiA but also other proteins (e.g., HLIPs, catalases, and peroxidases) to try to compensate for the lack of the phycobilisome-related NPQ mechanism. This could explain why ΔOCP cells were slightly more resistant to iron starvation than wild-type cells.
By comparing the effect of iron starvation in ΔIsiA cells to that in ΔOCP cells, we conclude that even though the phycobilisome-related NPQ mechanism is an important photoprotective process under high light conditions, under iron starvation (under low light conditions), the IsiA-related mechanism provides more effective protection of the cells. Under low light growth conditions, the phycobilisome-related NPQ mechanism is not induced. We have already shown that this mechanism is only induced by high intensities of blue or white light (Wilson et al., 2006). Thus, even if the capacity of induction of the phycobilisome-related NPQ mechanism is increased during iron starvation, this will not protect the cells from the iron starvation stress. However, in the iron-starved cells that are more sensitive to photoinhibition, the synthesis of OCP and the capacity of quenching are increased, providing greater photoprotective capacity under high light.
The blue light–induced fluorescence quenching is clearly not associated with the binding of free phycobilisomes to IsiA or to conformational changes in the IsiA protein that might cause the protein to become a more efficient thermal energy dissipator. Since the absence of IsiA renders Synechocystis cells more sensitive to the stress generated by iron starvation and high light (Park et al., 1999; Havaux et al., 2005; Latifi et al., 2005; this article) but does not inhibit the NPQ process, IsiA protects the cells via other mechanism(s). Combining our observations with results presented in the literature, we posit that during the first days of iron starvation, IsiA protects the cells by acting as the LHC of PSI, increasing the cross section of the PSI antenna (Andrizhiyevskaya et al., 2002; Melkozernov et al., 2003). In this way, a higher activity of PSI is maintained and a balance between PSII and PSI activities, decreasing the excitation pressure on PSII and, hence, the oxidative stress. During prolonged iron starvation, empty IsiA complexes may receive the energy coming from phycobilisomes and convert it into heat, thereby decreasing the energy arriving at the PSII reactions centers and at the thylakoids. Isolated IsiA aggregates, which accumulate upon chronic iron starvation, show very short fluorescence lifetimes, indicating that they are good energy dissipators (Ihalainen et al., 2005).
We observed that the increase of the population of highly fluorescent phycobilisomes was inversely proportional to the concentration of empty IsiA complexes (very high in ΔIsiA cells and lower in wild-type cells), suggesting that, indeed, the phycobilisomes transferred the absorbed energy to the IsiA complexes. This was supported by the fluorescence emission and excitation spectra of wild-type cells. The peak at 685 nm observed in fluorescence emission spectra (77K) with excitation at 600 nm was largely related to IsiA emission, indicating energy transfer from phycobilisomes to IsiA. Park et al. (1999) and Sandström et al. (2001) have already reported that the PSII antenna size was inversely proportional to the concentration of IsiA: the more IsiA present, the smaller the antenna size. Thus, IsiA complexes could protect PSII by competing for the energy coming from the phycobilisomes, diminishing the effective antenna size.
We suggest that the energy absorbed by the phycobilisomes that is not used for photochemistry could also be responsible for oxidative damage (e.g., by peroxidation of lipids). For example, Havaux et al. (2005) have already demonstrated that in ΔIsiA mutants, there is more lipid peroxidation and higher concentrations of reactive oxygen species are produced than in wild-type cells. This can be caused by a higher production of oxygen radicals in the chlorophyll antenna but also by production of carbon-centered radicals in other proteins of the membrane that by reacting with oxygen will make peroxyl radicals. Our results demonstrate that a mutant lacking phycobilisomes (PAL) seemed to be less affected by iron starvation conditions. In PAL cells, IsiA was accumulated and the PSII/PSI ratio increased, indicating a faster degradation of PSI complexes relative to PSII. However, a high concentration of active PSII complexes remained after a long period of iron starvation. These results support the idea that the presence of functionally disconnected phycobilisomes is deleterious to the cell.
Conclusions
The recently described light-induced photoprotective phycobilisome OCP–mediated NPQ mechanism also occurs under iron starvation conditions, where it is solely responsible for the light-induced fluorescence quenching observed in iron-starved cyanobacteria. In our working model for this process, blue-green light absorbed by the carotenoid of the OCP induces changes in the carotenoid and/or the protein that facilitates the interaction between the OCP and the phycobilisome core and renders the OCP capable of absorbing the energy arriving from the phycobilisomes and dissipates it as heat. Under iron starvation conditions, conditions in which the phycobilisomes become disconnected from the photosystems, energy dissipation in phycobilisomes increases (increased NPQ) to better protect the cells by diminishing the energy arriving to the thylakoids.
The empty IsiA complexes that accumulate during iron starvation could be responsible for a permanent form of thermal energy dissipation. However, this IsiA-based mechanism is not modulated by light. Ihalainen et al. (2005) have shown that the quenched state of the IsiA complexes was independent of the quality or intensity of light, at least in vitro. Under our experimental conditions, cells lacking IsiA were more sensitive to iron starvation than cells unable to activate blue light–induced NPQ. Thus, under iron starvation and low light conditions, IsiA complexes provide better protection than the phycobilisome OCP–related NPQ mechanism. The absence of IsiA and HLIPs, like the absence of the OCP, renders Synechocystis PCC 6803 cells more sensitive to high light intensities. In wild-type cells, IsiA and HLIPs are present only under prolonged high light conditions, whereas the OCP is always present, suggesting that the IsiA- and HLIP-related mechanisms appear when the OCP-related NPQ mechanism is insufficient to protect the cell. A comparative study of the role and effectiveness in photoprotection of these proteins under high light conditions will increase our understanding of the different NPQ mechanisms existing in cyanobacteria.
METHODS
Culture Conditions
Wild-type and mutant Synechocystis PCC 6803 cells were grown photoautotrophically in a modified BG11 medium described by Herdman et al. (1973) containing twice the concentration of sodium nitrate. Cells were shaken in a rotary shaker (120 rpm) at 30°C and illuminated by fluorescent white lamps giving a total intensity of ∼30 to 40 μmol photons m−2 s−1 under a CO2-enriched atmosphere. The ΔOCP, ΔIsiA, and PAL mutants were grown in the presence of 25 μg/mL spectinomycin and 10 μg/mL streptinomycin. The cells were maintained in the logarithmic phase of growth and were collected at OD800 = 0.6 to 0.8. The construction of the ΔOCP and ΔIsiA mutants in which the slr1963 gene and the isiA gene are interrupted by a spectinomycin/streptinomycin resistance cassette was described by Wilson et al. (2006). Construction of the PAL mutant, lacking PC, APC, and the phycobilisome linker protein Lcm, was described by Ajlani and Vernotte (1998).
Iron Starvation
Wild-type and mutant Synechocystis PCC 6803 cells were collected at the logarithmic phase of growth, precipitated, resuspended (OD800 = 0.6) in modified BG11 medium (Herdman et al., 1973) lacking Fe, and grown under low light (30 to 40 μmol photons m−2 s−1). During the first week, the cells were diluted each day to maintain the same cell concentration (OD800 ranging from 0.6 in the morning to 1.2 to 0.9 the next morning). When the chlorophyll concentration per cell decreased, the cells were diluted once every 2 d, then once every 3 d, and finally undiluted. The PAL mutant was diluted only every 3 d consistently. When the cell concentration was maintained at less than OD800 = 0.5, the phenotype of protractedly iron-starved cells was reached faster (in 6 to 7 d) (data not shown). This facilitated study of the different stages of iron starvation.
Fluorescence Measurements
The yield of chlorophyll fluorescence was monitored in a modulated fluorometer (PAM; Walz) adapted to a Hansatech oxygen electrode as previously described (El Bissati et al., 2000). All NPQ induction and recovery experiments were performed in a stirred cuvette of 1 cm diameter (32°C) at a chlorophyll concentration of 3 μg chlorophyll/mL (with the exception of the PAL mutant, 2 μg chlorophyll/mL) and in the presence of chloramphenicol (30 μg/mL) to inhibit protein synthesis. Recovery was realized under 50 to 80 μmol photons m−2 s−1 of blue-green light. Fluorescence quenching was induced by a blue-green light (400 to 550 nm) at 740 μmol photons m−2 s−1 of light intensity. Nomenclature used is as follows: Fo, minimal fluorescence level, the fluorescence emitted by open reaction centers in dark-adapted cells; Fm, the maximal fluorescence level in samples adapted to dim blue-green light; Fm′, maximum fluorescence under strong blue-green illumination, corresponding to the fluorescence emitted by the maximum concentration of closed reaction centers; and Fs, the steady state fluorescence level. The Fo level was determined by illuminating dark-adapted cells with a low intensity of red-modulated light (pulses of 1 μs, 1.6 kHZ, and 0.024 μmol photons m−2 s−1). For PAL measurements, the measuring light was 2.5 times more intense. Saturating pulses (2000 μmol photons m−2 s−1, 1 s) were applied to measure Fm and Fm′ levels. Application of such pulses that transiently close all the PSII centers serves to distinguish between photochemical quenching (qp) and NPQ.
Fluorescence emission and excitation spectra at room temperature and at 77K were done in a CARY Eclipse fluorescence spectrophotometer fluorometer (Varian). All samples were at a concentration of 2 to 3 μg chlorophyll/mL. For 77K spectra, samples in nuclear magnetic resonance tubes (5-mm ratio) were quickly frozen by immersion in a mixture of ice, CO2, and ethanol and then in liquid nitrogen.
MP, MP-Free, and Phycobilisome Preparations
Cells (at a 1 mg chlorophyll/mL concentration) were resuspended in a 0.7 M K-phosphate/0.3 M Na-citrate, pH 6.8, buffer to obtain MP or in a 20 mM MES, pH 6.8, buffer to obtain MP-free (M) and broken in a mini-bead-beater in the presence of glass beads. The M and MP fractions were collected by centrifugation and frozen at –80°C until used for gel electrophoresis. For fluorescence spectra, the MP fraction was immediately used without freezing. The PC/chlorophyll absorption ratio was similar in MP fractions and in whole cells.
Entire phycobilisomes were isolated as described by Ajlani et al. (1995). Cells were broken in a buffer containing 0.75 M K-phosphate, pH 7.5, and treated with Triton 2% during 1 h. The nonsolubilized material was separated by centrifugation, and the supernatant was applied to a sucrose gradient and centrifuged. The blue band (in the interface between 1.5 M sucrose and 0.75 M sucrose) contained intact phycobilisomes used in fluorescence spectra.
Gel Electrophoresis and Protein Gel Blot Analysis
Total cell protein and MP and M fractions were analyzed by SDS-PAGE on a 12% polyacrylamide/2 M urea (Figures 9A and 12B) or on a 12% polyacrilamide/6M urea (Figures 3 and 9C) in a TRIS/MES system (Kashino et al., 2001). The IsiA protein was detected by a polyclonal antibody against the IsiA protein (Dühring et al., 2006), and the OCP protein was detected by a polyclonal antibody against OCP. Anti-OCP polyclonal rabbit antisera was made (CoVance) using recombinant Synechocystis PCC6803 OCP (1.4 mg/mL in 20 mM Tris, pH 8.0, and 30% sucrose). Anti-OCP was purified from the sera using Affi-Gel 15 (Bio-Rad) following the manufacturer's instructions. Binding of OCP antibody was monitored by an alkaline phosphatase colorimetric reaction. The blots were scanned and the density of bands was measured using the Image Mastertotal Lab v1.11 software (Amersham Pharmacia). Binding of IsiA, CP47, and ATPase antibodies was visualized with a goat anti-rabbit IgG-peroxidase conjugate and SuperSignal West Pico as chemiluminescent substrate (Pierce).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NP_441508 (slr1963) and NP_441268 (isiA; sll0247).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The 77K Emission Fluorescence Spectra of Iron-Starved ΔIsiA Cells and Isolated Phycobilisomes.
Supplemental Figure 2. The 77K Emission and Excitation Fluorescence Spectra of Nonstarved Wild-Type Cells, the MP Fraction, and Isolated Phycobilisomes.
Supplemental Figure 3. Absorption Spectra of Wild-Type and ΔIsiA Cells and MP Fractions Corresponding to the Samples Used in Figure 5.
Supplemental Figure 4. Blue-Green Light–Induced Fluorescence Quenching in Long-Term, Iron-Starved, Wild-Type, ΔOCP, and ΔIsiA Cells.
Supplemental Figure 5. Comparison of Room Temperature Fluorescence Spectra of Nonstarved and Iron-Starved Wild-Type and ΔIsiA Cells.
Supplementary Material
Acknowledgments
We thank Ghada Ajlani for the PAL mutant and Shiho Tanaka for assistance in the preparation of the anti-OCP antibody. We also thank A. William Rutherford for stimulating discussions and critical reading of this manuscript and Anja Liszkay-Krieger for her critical reading of the manuscript. The research was partially supported by European Union network INTRO2.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Diana Kirilovsky (diana.kirilovsky@cea.fr).
Online version contains Web-only data.
References
- Adamska, I. (1997). Elips: Light-induced stress proteins. Physiol. Plant 100 794–805. [Google Scholar]
- Adamska, I. (2001). The Elip family of stress proteins in the thylakoids membranes of pro- and eukaryota. In Advances in Photosynthesis and Respiration-Regulation of Photosynthesis, Vol. 11, E.M. Aro and B. Andersson, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 487–505.
- Adir, N. (2005). Elucidation of the molecular structures of components of the phycobilisome: Reconstructing a giant. Photosynth. Res. 85 15–32. [DOI] [PubMed] [Google Scholar]
- Ajlani, G., and Vernotte, C. (1998). Construction and characterization of a phycobiliprotein-less mutant of Synechocystis sp. PCC 6803. Plant Mol. Biol. 37 577–580. [DOI] [PubMed] [Google Scholar]
- Ajlani, G., Vernotte, C., DiMagno, L., and Haselkorn, R. (1995). Phycobilisome core mutants of Synechocystis PCC 6803. Biochim. Biophys. Acta 1231 189–196. [Google Scholar]
- Andrizhiyevskaya, E.G., Schwabe, T.M., Germano, M., D'Haene, S., Kruip, J., van Grondelle, R., and Dekker, J.P. (2002). Spectroscopic properties of PSI-IsiA supercomplexes of the cyanobacterium Synechococcus PCC 7942. Biochim. Biophys. Acta 1556 265–272. [DOI] [PubMed] [Google Scholar]
- Bailey, S., Mann, N., Robinson, C., and Scanlan, D.J. (2005). The occurrence of rapidly reversible non-photochemical quenching of chlorophyll a fluorescence in cyanobacteria. FEBS Lett. 579 275–280. [DOI] [PubMed] [Google Scholar]
- Bibby, T.S., Nield, J., and Barber, J. (2001). Iron deficiency induces the formation of an antenna ring around trimeric Photosystem I in cyanobacteria. Nature 412 743–745. [DOI] [PubMed] [Google Scholar]
- Boekema, E.J., Hifney, A., Yakushevska, A.E., Piotrowski, M., Keegstra, W., Berry, S., Michel, K.P., Pistorius, E.K., and Kruip, J. (2001). A giant chlorophyll-protein complex induced by iron-deficiency in cyanobacteria. Nature 412 745–748. [DOI] [PubMed] [Google Scholar]
- Burnap, R., Troyan, T., and Sherman, L. (1993). The highly abundant chlorophyll-protein of iron-deficient Synechococcus sp PCC 7942 (CP43') is encoded by the isiA gene. Plant Physiol. 103 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoret, J.-C., Demoulière, R., Lavaud, J., van Gorkom, H., Houmard, J., and Etienne, A.-L. (2004). Dissipation of excess energy triggered by blue light in cyanobacteria with CP43' (isiA). Biochim. Biophys. Acta 1659 100–104. [DOI] [PubMed] [Google Scholar]
- Campbell, D., Hurry, V., Clarke, A., Gustafsson, P., and Öquist, G. (1998). Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. 62 667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams, B. (1990). Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta 1020 1–24. [Google Scholar]
- Dolganov, N.A., Bhaya, D., and Grossman, A.R. (1995). Cyanobacterial protein with similarity to the chlorophyll a/b-binding proteins of higher plants: Evolution and regulation. Proc. Natl. Acad. Sci. USA 92 636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dühring, U., Axmann, I., Hess, W., and Wilde, A. (2006). An internal antisense RNA regulates expression of the photosynthesis gene isiA. Proc. Natl. Acad. Sci. USA 103 7054–7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bissati, K., Delphin, E., Murata, N., Etienne, A.-L., and Kirilovsky, D. (2000). Photosystem II fluorescence quenching in the cyanobacterium Synechocystis PCC 6803: Involvement of two different mechanisms. Biochim. Biophys. Acta 1457 229–242. [DOI] [PubMed] [Google Scholar]
- El Bissati, K., and Kirilovsky, D. (2001). Regulation of psbA and psaE expression by light quality in Synechocystis species PCC 6803. A redox control mechanism. Plant Physiol. 125 1988–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk, S., Samson, G., Bruce, D., Huner, N.P.A., and Laudenbach, D.E. (1995). Functional analysis of the iron-stress induced CP43' polypeptide of PSII in the cyanobacterium Synechococcus sp. PC 7942. Photosynth. Res. 45 51–60. [DOI] [PubMed] [Google Scholar]
- Fulda, S., Mikkat, S., Huang, F., Huckauf, J., Marin, K., Norling, B., and Hagemann, M. (2006). Proteome analysis of salt stress response in the cyanobacterium Synechocystis sp. PCC 6803. Proteomics 6 2733–2745. [DOI] [PubMed] [Google Scholar]
- Funk, C., and Vermaas, W. (1999). A cyanobacterial gene family coding for single helix proteins resembling part of the light-harvesting proteins from higher plants. Biochemistry 38 9397–9404. [DOI] [PubMed] [Google Scholar]
- Gilmore, A., and Yamamoto, H. (1993). Linear models relating xanthophylls and lumen acidity to non-photochemical fluorescence quenching. Evidence that antheraxanthin explains zeaxanthin-independent quenching. Photosynth. Res. 35 67–68. [DOI] [PubMed] [Google Scholar]
- Guikema, J.A., and Sherman, L.A. (1983). Organization and function of chlorophyll in membranes of cyanobacteria during iron starvation. Plant Physiol. 73 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux, M., Guedeney, G., Hagemann, M., Yeremenko, N., Matthijs, H., and Jeanjean, R. (2005). The chlorophyll-binding protein IsiA is inducible by high light and protects the cyanobacterium Synechocystis PCC6803 from photooxidative stress. FEBS Lett. 579 2289–2293. [DOI] [PubMed] [Google Scholar]
- Havaux, M., Guedeney, G., He, Q., and Grossman, A. (2003). Elimination of high-light-inducible polypeptides related to eukaryotic chlorophyll a/b-binding proteins results in aberrant photoacclimation in Synechocystis PCC6803. Biochim. Biophys. Acta 1557 21–33. [DOI] [PubMed] [Google Scholar]
- He, Q., Dolganov, N., Björkman, O., and Grossman, A.R. (2001). The high light-inducible polypeptides in Synechocystis PCC 6803. Expression and function in high light. J. Biol. Chem. 276 306–314. [DOI] [PubMed] [Google Scholar]
- Herdman, M., Delaney, S.F., and Carr, N.G. (1973). A new medium for the isolation and growth of auxotrophic mutants of the blue-green alga Anacystis nidulans. J. Gen. Microbiol. 79 233–237. [Google Scholar]
- Hihara, Y., Kamei, A., Kanehisa, M., Kaplan, A., and Ikeuchi, M. (2001). DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13 793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, T.K., and Krogmann, D.W. (1981). A carotenoid protein from cyanobacteria. Biochim. Biophys. Acta 637 408–414. [Google Scholar]
- Horton, P., Ruban, A.V., and Walters, R.G. (1996). Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 655–684. [DOI] [PubMed] [Google Scholar]
- Ihalainen, J., D'Haene, S., Yeremenko, N., van Roon, H., Arteni, A., Boekema, E., van Grondelle, R., Matthijs, H., and Dekker, J. (2005). Aggregates of the chlorophyll-binding protein IsiA (CP43') dissipate energy in cyanobacteria. Biochemistry 44 10846–10853. [DOI] [PubMed] [Google Scholar]
- Ivanov, A.G., Park, Y.-I., Miskiewicz, E., Raven, J.A., Huner, N.P.A., and Öquist, G. (2000). Iron stress restricts photosynthetic intersystem electron transport in Synechococcus sp PCC 7942. FEBS Lett. 485 173–177. [DOI] [PubMed] [Google Scholar]
- Jeanjean, R., Zuther, E., Yeremenko, N., Havaux, M., Matthijs, H., and Hagemann, M. (2003). A photosystem I psaFJ-null mutant of the cyanobacterium Synechocystis 6803 expresses the isiAB operon under iron replete conditions. FEBS Lett. 549 52–56. [DOI] [PubMed] [Google Scholar]
- Joshua, S., Bailey, S., Mann, N., and Mullineaux, C. (2005). Involvement of phycobilisome diffusion in energy quenching in cyanobacteria. Plant Physiol. 138 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashino, Y., Koike, K., and Satoh, K. (2001). An improved SDS-PAGE system for the analysis of membrane protein complexes. Electrophoresis 22 1004–1007. [DOI] [PubMed] [Google Scholar]
- Kerfeld, C.A. (2004. a). Structure and function of the water-soluble carotenoid-binding proteins in cyanobacteria. Photosynth. Res. 81 215–225. [DOI] [PubMed] [Google Scholar]
- Kerfeld, C.A. (2004. b). Water-soluble carotenoid proteins of cyanobacteria. Arch. Biochem. Biophys. 430 2–9. [DOI] [PubMed] [Google Scholar]
- Latifi, A., Jeanjean, R., Lemeille, S., Havaux, M., and Zhang, C.-C. (2005). Iron starvation leads to oxidative stress in Anabaena sp strain PCC 7120. J. Bacteriol. 187 6596–6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenbach, D., and Straus, N. (1988). Characterization of a cyanobacterial iron stress-induced gene similar to psbC. J. Bacteriol. 170 5018–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X.-P., Björkman, O., Shih, C., Grossman, A.R., Rosenquist, M., Jansson, S., and Niyogi, K.K. (2000). A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403 391–395. [DOI] [PubMed] [Google Scholar]
- Li, X.-P., Gilmore, A.M., Caffarri, S., Bassi, R., Golan, T., Kramer, D., and Niyogi, K.K. (2004). Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 279 22866–22874. [DOI] [PubMed] [Google Scholar]
- MacColl, R. (1998). Cyanobacterial phycobilisomes. J. Struct. Biol. 124 311–334. [DOI] [PubMed] [Google Scholar]
- Melkozernov, A.N., Bibby, T.S., Lin, S., Barber, J., and Blankenship, R.E. (2003). Time-resolved absorption and emission show that the CP43' antenna ring of iron-stressed Synechocystis sp PCC 6803 is efficiently coupled to the photosystem I reaction center core. Biochemistry 42 3893–3903. [DOI] [PubMed] [Google Scholar]
- Müller, P., Li, X.-P., and Niyogi, K. (2001). Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux, C.K. (1992). Excitation energy transfer from phycobilisomes to Photosystem I in a cyanobacterium. Biochim. Biophys. Acta 1100 285–292. [Google Scholar]
- Niyogi, K. (1999). Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50 333–359. [DOI] [PubMed] [Google Scholar]
- Odom, W.R., Hodges, R., Chitnis, P.R., and Guikema, J.A. (1993). Characterization of Synechocystis sp PCC 6803, in iron-supplied and iron deficient media. Plant Mol. Biol. 23 1255–1264. [DOI] [PubMed] [Google Scholar]
- Öquist, G. (1971). Changes in pigment composition and photosynthesis induced by iron-deficiency in the blue-green alga Anacystis nidulans. Physiol. Plant 25 188–191. [Google Scholar]
- Öquist, G. (1974. a). Iron deficiency in the blue-green alga Anacystis nidulans: Changes in pigmentation and photosynthesis. Physiol. Plant 30 30–37. [Google Scholar]
- Öquist, G. (1974. b). Iron deficiency in the blue-green alga Anacystis nidulans: Fluorescence and absorption spectra recorded at 77K. Physiol. Plant 31 55–58. [Google Scholar]
- Park, Y., Sandström, P., Gustafsson, P., and Öquist, G. (1999). Expression of the isiA gene is essential for the survival of the cyanobacterium Synechococcus sp PCC 7942 by protecting photosystem II from excess light under iron limitation. Mol. Microbiol. 32 123–129. [DOI] [PubMed] [Google Scholar]
- Pascal, A., Liu, Z., Broess, K., van Oort, B., van Amerongen, H., Wang, C., Horton, P., Robert, B., Chang, W., and Ruban, A. (2005). Molecular basis of photoprotection and control of photosynthetic light harvesting. Nature 436 134–137. [DOI] [PubMed] [Google Scholar]
- Rakhimberdieva, M., Boichenko, V.A., Karapetyan, N., and Stadnichuk, I. (2001). Interaction of phycobilisomes with Photosystem II dimers and Photosystem I monomers and trimers in the cyanobacterium Spirulina platensis. Biochemistry 40 15780–15788. [DOI] [PubMed] [Google Scholar]
- Rakhimberdieva, M., Stadnichuk, I., Elanskaya, I., and Karapetyan, N. (2004). Carotenoid-induced quenching of the phycobilisome fluorescence in photosystem II-deficient mutant of Synechocystis sp. FEBS Lett. 574 85–88. [DOI] [PubMed] [Google Scholar]
- Ruban, A.V., Ress, D., Pascal, A.A., and Horton, P. (1992). Mechanism of ΔpH-dependent dissipation of absorbed excitation energy by photosynthetic membranes. II. The relationship between LHCII aggregation and QE in isolated thylakoids. Biochim. Biophys. Acta 1102 39–44. [Google Scholar]
- Sandmann, G. (1985). Consequences of iron deficiency on photosynthetic and respiratory electron transport in blue-green algae. Photosynth. Res. 6 261–271. [DOI] [PubMed] [Google Scholar]
- Sandström, S., Park, Y., Öquist, G., and Gustafsson, P. (2001). CP43' the isiA gene product, functions as an excitation energy dissipator in the cyanobacterium Synechococcus sp PCC 7942. Photochem. Photobiol. 74 431–437. [DOI] [PubMed] [Google Scholar]
- Scott, M., McCollum, S., Vasil'ev, C., Crozier, C., Espie, G., Krol, M., Huner, N., and Bruce, D. (2006). Mechanism of the down regulation of photosynthesis by blue light in the cyanobacterium Synechocystis sp PCC 6803. Biochemistry 45 8952–8958. [DOI] [PubMed] [Google Scholar]
- Sherman, D.M., and Sherman, L.A. (1983). Effect of iron deficiency and iron restoration on ultrastucture of Anacystis nidulans. J. Bacteriol. 156 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefermann-Harms, D. (1988). Fluorescence properties of isolated chlorophyll-protein complexes. In Application of Chlorophyll Fluorescence, H.K. Lichtenthaler, ed (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 45–54.
- Singh, A., Li, H., Bono, L., and Sherman, L. (2005). Novel adaptative responses revealed by transcription profiling of a Synechocystis sp PCC 6803 ΔisiA mutant in the presence and absence of hydrogen peroxide. Photosynth. Res. 84 65–70. [DOI] [PubMed] [Google Scholar]
- Spiller, S., and Terry, N. (1980). Limiting factors in photosynthesis. Iron stress diminishes photochemical capacity by reducing the number of photosynthetic units. Plant Physiol. 65 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dorssen, R.J., Plijter, J.J., Decker, J., den Ouden, A., Amesz, J., and van Gorkom, H.J. (1987). Spectroscopic properties of chloroplast grana membranes and of the core of photosystem II. Biochim. Biophys. Acta 890 134–143. [Google Scholar]
- Wilson, A., Ajlani, G., Verbavatz, J.-M., Vass, I., Kerfeld, C., and Kirilovsky, D. (2006). A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell 18 992–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y.P., and Krogmann, D.W. (1997). The orange carotenoid protein of Synechocystis PCC 6803. Biochim. Biophys. Acta 1322 1–7. [DOI] [PubMed] [Google Scholar]
- Yamamoto, H. (1979). Biochemistry of the violaxanthin cycle in higher plants. Pure Appl. Chem. 51 639–648. [Google Scholar]
- Yeremenko, N., Kouril, R., Ihalainem, J., D'Haene, S., van Oosterwijk, N., Andrizhiyevkaya, E., Keegstra, W., Dekker, H., Hagemann, M., Boekema, E., Matthijs, H., and Dekker, J. (2004). Supramolecular organization and dual function of the IsiA chlorophyll-binding protein in cyanobacteria. Biochemistry 43 10308–10313. [DOI] [PubMed] [Google Scholar]
- Yousef, N., Pistorius, E.K., and Michel, K.-P. (2003). Comparative analysis of idiA and isiA transcription under iron starvation and oxidative stress in Synechococcus elongatus PCC 7942 wild-type and selected mutants. Arch. Microbiol. 180 471–483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.