Abstract
The COP9 signalosome (CSN) is an evolutionarily conserved multisubunit protein complex that regulates a variety of biological processes. Among its eight subunits, CSN5 and CSN6 contain a characteristic MPN (for Mpr1p and Pad1p N-terminal) domain and, in Arabidopsis thaliana, are each encoded by two genes: CSN5A, CSN5B and CSN6A, CSN6B, respectively. We characterized both MPN subunits using a series of single and double mutants within each gene family. Our results indicate that although CSN6A and CSN6B retain mostly redundant functions, CSN5A and CSN5B play unequal roles in the regulation of plant development. Complete depletion of either of the two MPN members results in CSN instability and the decay of various CSN components, along with the complete loss of CUL1, CUL3, and CUL4 derubylation. Furthermore, we demonstrate that CSN interacts with CUL3, in addition to CUL1 and CUL4, and that the lack of CSN activity differentially affects the stability of those three cullins. Interestingly, we also show that optimal CUL3 activity is required to maintain the cellular pool of CSN5, through a posttranscriptional mechanism. Our data suggest the existence of reciprocal regulation between CUL3 and CSN5 accumulation. This study thus completes the genetic analysis of all CSN subunits and confirms the structural interdependence between PCI and MPN subunits in functional CSN complex formation.
INTRODUCTION
The COP9 signalosome (CSN) is a nucleus-enriched multisubunit protein complex conserved throughout evolution (Wei et al., 1994a, 1998; Chamovitz et al., 1996; Seeger et al., 1998; Freilich et al., 1999; Mundt et al., 1999; Busch et al., 2003). Genetic and molecular analyses indicate that the CSN is involved in the regulation of a variety of signaling and developmental processes, including embryogenesis, cell cycle, circadian rhythms, DNA repair, and plant responses to light and hormones (reviewed in Wei and Deng, 2003; Richardson and Zundel, 2005). The CSN interacts with a large number of proteins, including several components or regulators of the ubiquitin–proteasome system (Smalle and Vierstra, 2004). Among the CSN's eight subunits, six contain a PCI domain (for Proteosome, COP9, Initiation factor 3) and two (CSN5 and CSN6) contain a MPN/MOV34 domain (for Mpr1p and Pad1p N-terminal) (Glickman et al., 1998; Hofmann and Bucher, 1998; Wei et al., 1998).
A known biochemical activity of the CSN is the removal of the ubiquitin-like protein RUB1/NEDD8 from the cullin subunit of the Cullin-RING ligase (CRL) family of E3 complexes (Cope and Deshaies, 2003; Wei and Deng, 2003; Harari-Steinberg and Chamovitz, 2004). To date, it has been shown that the CSN interacts with and promotes RUB1/NEDD8 deconjugation from the Cullin1-containing SCF (for SKP1-CUL1/CDC53-F-box protein), Cullin3-containing BCR (for BTB/POZ domain-CUL3-RING), and Cullin4-containing E3 ubiquitin ligases (Lyapina et al., 2001; Schwechheimer et al., 2001; Liu et al., 2002, 2005; Feng et al., 2003; Groisman et al., 2003; Higa et al., 2003; Pintard et al., 2003; Wang et al., 2003). CSN also deconjugates RUB1/NEDD8 from CUL2, a subunit of the VCB (for Von Hippel Lindau-Elongin B-Elongin C) E3 complex (Yang et al., 2002). Like the ubiquitin pathway, the RUB1/NEDD8 conjugation pathway is catalyzed by an enzymatic cascade and is essential in Schizosaccharomyces pombe, Arabidopsis thaliana, Caenorhabditis elegans, Drosophila, and mouse (Jones and Candido, 2000; Osaka et al., 2000; Tateishi et al., 2001; Ou et al., 2002; Bostick et al., 2004). In plants, rubylation is also important for several physiological processes, including auxin and ethylene responses (del Pozo et al., 2002; Gray et al., 2002; Dharmasiri et al., 2003; Bostick et al., 2004; Larsen and Cancel, 2004). Conjugation of RUB1/NEDD8 to CUL1 increases the activity of the SCF complexes in vitro and in vivo, probably by facilitating substrate polyubiquitination and E2 recruitment to the E3 ligases (Kawakami et al., 2001; Pan et al., 2004). In addition, it was demonstrated recently that the increased availability of the F-box subunit SKP2, and its substrate, promotes neddylation and the assembly of SCFSKP2 complexes (Bornstein et al., 2006). At the same time, CSN-mediated cullin derubylation is essential for in vivo organized E3 functions (Wolf et al., 2003). In fact, CSN derubylation and its associated UBP12-mediated deubiquitination activities (Zhou et al., 2003) promote CRL E3 function by counteracting the instability of the E3 components caused by CRL autoubiquitination activity (He et al., 2005; Wee et al., 2005; Cope and Deshaies, 2006; Wu et al., 2006). Together, these findings demonstrate that cycles of rubylation/derubylation are essential for maintaining an optimal pool of active Cullin-RING ligases and explain the mechanism underlying substrate accumulation in csn mutants (Lykke-Andersen et al., 2003; Cope and Deshaies, 2006; Denti et al., 2006).
The CSN derubylation activity is located within the JAMM (for JAB1/MPN/MOV34) or MPN+ motif of CSN5 (Cope et al., 2002), which is embedded within its MPN domain. Interestingly, this motif is absent in the other MPN domain subunit of CSN (CSN6), whose function remains unknown.
The CSN was initially discovered during genetic screens for constitutive photomorphogenic development in darkness, which led to the identification of 10 COP/DET/FUS loci (reviewed in Wei and Deng, 2003). Six of the nine molecularly characterized COP/DET/FUS loci encode the six PCI subunits of the COP9 complex, whereas the MPN/MOV34 subunits CSN5 and CSN6 are not represented by any of those genes. In Arabidopsis, both MPN subunits are encoded by two highly homologous genes: CSN5A and CSN5B (Kwok et al., 1998) and CSN6A and CSN6B (Peng et al., 2001a), respectively. In the case of CSN5, we have shown previously that CSN5A and CSN5B incorporate into distinct CSN (CSNCSN5A and CSNCSN5B) complexes in vivo (Gusmaroli et al., 2004). The initial characterizations of mutant plants defective in one (csn5a-1 and csn5a-2) or the other (csn5b-1) CSN5 subunit have been reported (Gusmaroli et al., 2004; Dohmann et al., 2005). Both studies showed that the two CSN5 isoforms play unequal roles in plant development, with CSN5A exerting a more prominent one.
In plants and mammals, loss of a PCI subunit normally triggers the cellular depletion of the entire CSN (Kwok et al., 1998; Serino et al., 1999; Peng et al., 2001a, 2001b; Wang et al., 2002; Lykke-Andersen et al., 2003; Yan et al., 2003). In the case of the MPN subunit CSN5, however, nonidentical effects have been observed in different organisms. Whereas in mammals (Tomoda et al., 2004; Denti et al., 2006) and fission yeast (Mundt et al., 2002), CSN5 is necessary for CSN stability, in Drosophila csn5Null mutants, CSN4 and CSN7 still form a large protein complex similar to that of the wild type (Oron et al., 2002). In Arabidopsis, the characterization of a double csn5 mutant line (csn5a-2 cns5b-1) led to the conclusion that loss of CSN5 results in the formation of a stable CSN form lacking CSN5 only (Dohmann et al., 2005). However, the csn5a-2 allele used in that study was a partial loss-of-function allele, still producing reduced levels of a functional CSN5A protein.
Here, we have undertaken a systematic characterization of each member of the CSN5 and CSN6 gene families, using a series of single and double null mutants within each gene family. Together, our results reveal the specific role of each CSN5 and CSN6 family member in CSN complex assembly and stability as well as their contributions to the derubylation activity and CSN functions in plant development. In addition, we demonstrate that CSN interacts with CUL3-based E3 ubiquitin ligases both in vitro and in vivo and that CSN derubylation activity is essential to maintain the cellular pool of CUL3. Finally, we provide genetic and biochemical evidence to show that CSN5 levels, in turn, can be modulated by CUL3, revealing a reciprocal regulation between CSN5 and CUL3 accumulation.
RESULTS
Generation and Characterization of csn5a and csn5b Single and Double Mutants Reveal That CSN5A and CSN5B Are Differentially Expressed throughout Plant Development
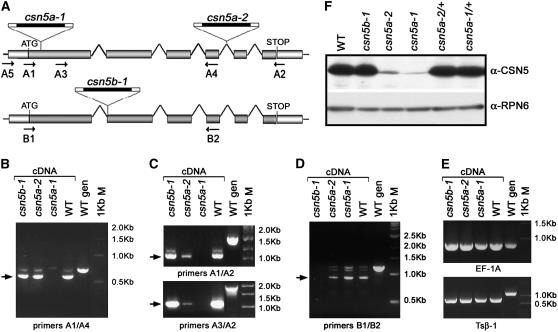
To investigate whether the complete loss of CSN5 activity would lead to the destabilization of the entire complex, we conducted a detailed characterization of csn5a and csn5b single and double mutants. We used two CSN5A T-DNA insertion lines and one CSN5B T-DNA insertion line (see Methods for details) (Gusmaroli et al., 2004; Dohmann et al., 2005). To determine whether the two T-DNA insertions in CSN5A give rise to reduction or complete loss of function, RT-PCR analyses were performed on RNA extracted from each single mutant line (Figure 1). The positions of the gene-specific primers used to selectively amplify the CSN5A and CSN5B transcripts are shown in Figure 1A. The complete absence of the CSN5A transcript in the csn5a-1 mutant (Figures 1B and 1C, third lanes) demonstrates that the T-DNA insertion in the first exon of CSN5A gives rise to a null mutation. On the other hand, we could amplify the CSN5A transcript in the csn5a-2 homozygous line using different sets of CSN5A gene-specific primers (Figures 1B and 1C, second lanes). We detected wild-type levels of CSN5A transcript using sets of primers located 5′ of the T-DNA insertion (Figure 1B, second lane), whereas the level of full-length CSN5A transcript was reduced considerably but still detectable using different sets of primers spanning the entire CSN5A coding region (Figure 1C, second lane). Together, these results demonstrate that, in the csn5a-2 homozygous line, the intron containing the T-DNA insertion can be correctly spliced out, at low efficiency, producing full-length CSN5A transcript. The removal of the T-DNA during pre-mRNA processing has been observed previously in other T-DNA homozygous lines when the T-DNA is inserted within an intron (our unpublished data). In conclusion, the RT-PCR analyses demonstrated that the csn5a-2 mutant still retains reduced levels of wild-type CSN5A transcript and therefore should be considered a CSN5A reduction-of-function line and not a null csn5a mutant. On the other hand, the absence of CSN5B transcript in csn5b-1 seedlings demonstrated that the T-DNA insertion in CSN5B gives rise to a null mutation (Figure 1D, first lane).
Figure 1.
Expression Analyses of csn5 Single Mutants.
(A) Structures of the Arabidopsis CSN5A (At1g22920) and CSN5B (At1g71230) loci and graphical representation of the T-DNA insertion mutants used in this study. Arrows indicate the positions and orientations of the gene-specific primers used for the RT-PCR analyses.
(B) to (E) RT-PCR analyses of the wild type and csn5b-1, csn5a-2, and csn5a-1 homozygous mutants. Different sets of gene-specific primers were used to selectively amplify the CSN5A 5′ (B) and full-length (C) transcripts or the CSN5B transcript (D). The eEF-1A (E) (top panel) and Tsβ1 (E) (bottom panel) transcripts were used as positive controls for the RT-PCR analyses. Genomic DNA was used as a positive control for the PCR. Arrows indicate the PCR products corresponding to mature CSN5A and CSN5B transcripts. The csn5a-2 mutants still express low levels of full-length CSN5A mRNA.
(F) Detection of CSN5 proteins from the wild type and null homozygous csn5b-1 and weak (csn5a-2) or null (csn5a-1) homozygous and heterozygous csn5a mutants with anti (α)-CSN5 polyclonal antibodies, which recognize both CSN5A and CSN5B isoforms. Equal protein loading was confirmed using α-RPN6 antibody. The csn5a-2 mutants express low levels of CSN5A protein in addition to traces of CSN5B.
Protein gel blot analyses of the single csn5 mutants show that CSN5A is expressed predominantly (Figure 1F, second lane), whereas CSN5B is barely detectable (Figure 1F, fourth lane), in Arabidopsis seedlings and throughout plant development (data not shown). We did not observe a detectable dose effect of CSN5 function in heterozygous csn5a plants (csn5a-2/+ and csn5a-1/+), either at the biochemical (Figure 1F, last two lanes) or the morphological (data not shown) level. The comparison of CSN5 level between csn5a-2 and csn5a-1 mutants (Figure 1F, third and fourth lanes) indicates that csn5a-2 expresses reduced levels of an intact CSN5A subunit (in addition to the CSN5B protein), as anticipated by the presence of low levels of full-length CSN5A transcript in the csn5a-2 genetic background.
CSN5A and CSN5B Play Unequal Roles in Regulating Photomorphogenesis, Root Elongation, Auxin Response, and Vegetative and Reproductive Growth
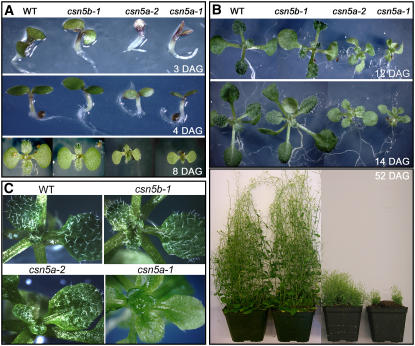
During the initial days after germination (DAG) in white light, the csn5a-1 and csn5a-2 seedlings exhibit a phenotype reminiscent of the fusca-type phenotype of the cop/det/fus mutants, characterized by purple cotyledons. However, both csn5a single mutants are not lethal at the seedling stage, and at a few DAG the purple cotyledons turn light green before the production of true leaves (Figure 2A). The detailed characterization of the single csn5 mutants at representative stages revealed that reduction or loss of CSN5A results in shorter hypocotyls in the dark, shorter roots, smaller flowers, a decreased number of root hairs in response to jasmonic acid treatment, and altered light and auxin responses (see Supplemental Figure 1 online).
Figure 2.
Mutations in CSN5A but Not CSN5B Result in Multifaceted Developmental Defects at Vegetative and Reproductive Stages.
(A) and (B) Phenotypes of light-grown wild-type and csn5 single mutant plants at different DAG as indicated. At 14 DAG, the plantlet were transferred to soil and grown in long-day conditions. At each time point, photographs were taken at the same magnification.
(C) Close-up images of wild type, csn5b-1, csn5a-2, and csn5a-1 new leaves at 14 DAG. For comparison, at each stage the photographs were taken at the same magnification.
At the vegetative stage, the csn5a plantlets are characterized by small, light green, and curly rosettes (Figure 2B, 12 and 14 DAG), which are almost completely depleted of trichomes, in contrast with wild-type and csn5b-1 plants (Figure 2C). At the reproductive stage, csn5a-1 and csn5a-2 mutants exhibit severe developmental defects that result in dwarf stature and the loss of apical dominance (Figure 2B, 52 DAG).
It is worth noting that whereas csn5a-1 and csn5a-2 are virtually indistinguishable from each other in the initial few DAG (Figure 2A), starting from 8 DAG and throughout the vegetative stage, the phenotype of csn5a-2 is less severe than that of csn5a-1, in terms of both rosette size (Figure 2B) and trichome density (Figure 2C). This difference becomes particularly evident at the reproductive phase (Figure 2B, 52 DAG) and could be explained by the residual activity of CSN5A in csn5a-2 mutants, as shown below. By contrast, csn5b-1 mutants are virtually indistinguishable from wild-type plants at all developmental stages (Figures 2A and 2B).
Genetic and Biochemical Analyses of Two Different Double Mutant Lines Indicate That csn5a-2 csn5b-1 Still Retains Partial CSN5A Function
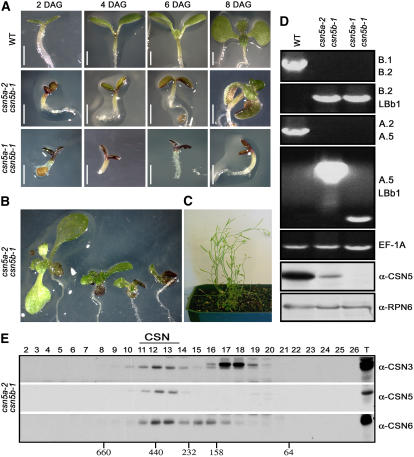
As a necessary step in the characterization of the contribution of CSN5 to overall CSN function, we generated csn5a-1 csn5b-1 and csn5a-2 csn5b-1 double mutant lines. Because of low levels of wild-type CSN5A transcript (Figure 1C, second lane) and protein (Figure 1F, third lane) produced by the csn5a-2 allele, we characterized both csn5a-1 csn5b-1 and csn5a-2 csn5b-1 double mutants. As shown in Figure 3A, during the first 2 to 3 DAG, both csn5a-1 csn5b-1 and csn5a-2 csn5b-1 double mutant lines display the typical photomorphogenic phenotype of the fusca mutants. At these time points, both lines are virtually indistinguishable from each other. However, at 6 to 7 DAG, the csn5a-2 csn5b-1 double mutants uniformly start to form the first pair of true leaves (Figure 3A) and eventually develop into impaired small plantlets, with dark red cotyledons and asymmetrical leaves (Figure 3B). Similar defects also have been described for other CSN reduction-of-function lines generated by CSN6A antisense and cosuppression approaches (Peng et al., 2001a). Interestingly, none of the csn5a-2 csn5b-1 double mutants arrest at the seedling stage, and even though severely compromised, csn5a-2 csn5b-1 plantlets survive to a mature stage (Figure 3C). It is worth noting that the phenotype of the csn5a-2 csn5b-1 double mutants shown in Figure 3 has been homogeneously observed in 100% of the segregating csn5a-2 csn5b-1 F2 seedlings derived from several independent crosses between the csn5a-2 and csn5b-1 homozygous parental lines. On the other hand, csn5a-1 csn5b-1 double null homozygous mutants invariably die at the seedling stage (Figure 3A), do not express detectable CSN5 proteins (Figure 3D, third lane), and are virtually identical to the null alleles of the cop/det/fus mutants.
Figure 3.
Comparison of the Developmental Defects of Two csn5 Double Mutants, Which Are Severely (csn5a-2 csn5b-1) or Completely (csn5a-1 csn5b-1) Defective in CSN5 Activity.
(A) Phenotypes of light-grown wild-type and csn5 double mutant seedlings at different developmental stages.
(B) Phenotype of csn5a-2 csn5b-1 homozygous mutants at 14 DAG.
(C) Phenotype of 9-week-old csn5a-2 csn5b-1 double mutants, grown in long-day conditions.
(D) First five panels, PCR-based genotype analyses of wild-type, csn5a-2 csn5b-1, and csn5a-1 csn5b-1 double mutant lines. The positions of the CSN5A and CSN5B gene-specific primers are indicated in Figure 1A. Primers B1/B2 and B2/LBb1 were used to selectively amplify the CSN5B wild-type and csn5b-1 T-DNA insertion alleles, respectively. Primers A2/A5 were used to selectively amplify the CSN5A wild-type allele. Primers A5/LBb1 were used to selectively amplify the csn5a-2 (lane 2) and csn5a-1 (lane 3) T-DNA insertion alleles. The genomic sequence of EF-1A was used as a positive control for the PCR. Last two panels, protein blot analyses of wild-type and csn5 double mutant lines, as indicated. Equal amounts of total protein extracts were subjected to SDS-PAGE and immunoblot analyses with anti (α)-CSN5 antibody. The α-RPN6 antibody was used as a loading control.
(E) Immunoblot analyses of Superose 6 gel filtration fractions obtained from 13-d-old light-grown csn5a-2 csn5b-1 seedlings. Column fractions were subjected to SDS-PAGE and immunoblot analyses with α-CSN3, α-CSN5, and α-CSN6 polyclonal antibodies. Fraction numbers are indicated. Lane T contains the total unfractionated extracts. The csn5a-2 csn5b-1 double mutant possesses reduced levels of an intact COP9 complex containing a functional CSN5A subunit.
The discrepancy between the severe pleiotropic phenotype of csn5a-2 csn5b-1 mutants and the lethality of the csn5a-1 csn5b-1 seedlings can be explained by the residual CSN5A activity in the csn5a-2 csn5b-1 background. In fact, these double mutants express reduced levels of an intact CSN5A protein (Figure 3D, second lane), which elutes almost exclusively in high molecular mass fractions and cofractionates with other CSN subunits in csn5a-2 csn5b-1 seedling extracts (Figure 3E). In agreement, we observed a residual derubylation activity in csn5a-2 csn5b-1 seedlings, as shown below in Figure 7B.
Figure 7.
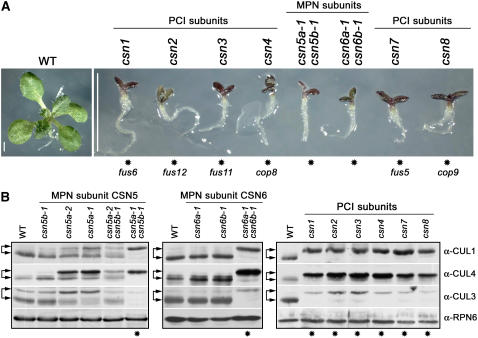
The Essential Role of the CSN in Cullin Derubylation as Well as the Maintenance of the Cellular Pools of CUL3 and CUL4.
(A) Phenotypes of 13-d-old white light–grown wild-type (left) and the set of eight csn complete loss-of-function mutant (right) seedlings, corresponding to each CSN subunit. The six PCI subunit mutants shown correspond to cop/det/fus mutants identified by genetic screens (see Methods), as indicated below the asterisks.
(B) Protein blot analyses of the wild type and csn5 single and double mutants (left panel), the wild type and csn6 single and double mutants (middle panel), or the wild type and the six PCI subunit mutants shown in (A) (right panel), as indicated. Equal amounts of total proteins extracted from 13-d-old white light–grown seedlings were subjected to SDS-PAGE and immunoblot analyses with anti (α)-CUL1, α-CUL4, and α-CUL3 polyclonal antibodies. α-CUL3 antibody recognizes both CUL3A and CUL3B isoforms. The α-RPN6 antibody was used as a loading control. Top arrows indicate rubylated CUL1, CUL4, and CUL3, respectively, and bottom arrows indicate the un-rubylated forms. The asterisks indicate the complete loss-of-function mutants.
Characterization of csn6a and csn6b Single and Double Mutants Reveals That CSN6A and CSN6B Are Differentially Expressed throughout Plant Development
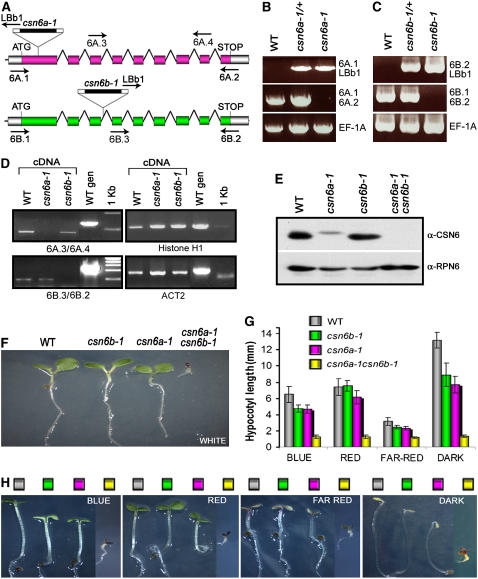
Arabidopsis CSN6 is encoded by two conserved genes, CSN6A and CSN6B (Peng et al., 2001a). The two genes encode proteins that are 87% identical to each other and 47% identical to their human counterpart. To evaluate their respective functional roles, T-DNA insertion mutants in CSN6A and CSN6B loci were identified (Figures 4A to 4C) (see Methods). RT-PCR analyses confirmed that the T-DNA insertions in both CSN6 loci result in null mutations (Figure 4D). We refer to the corresponding mutants as csn6a-1 and csn6b-1. Protein gel blot analyses with polyclonal antibody raised against a conserved CSN6A polypeptide suggest that CSN6A is expressed predominantly (Figure 4E, third lane), whereas CSN6B accumulates at a lower level (Figure 4E, second lane) both in seedlings (Figure 4E) and in mature plants (data not shown). These data are consistent with the expression profiles of the two CSN6 transcripts obtained from Genevestigator (http://www.genevestigator.ethz.ch), which collected hundreds of microarray expression data sets. In fact, in all organs and conditions reported to date, CSN6A is expressed consistently at a higher level than CSN6B.
Figure 4.
The MPN Subunits CSN6A and CSN6B of CSN Are Essential and Functionally Redundant.
(A) Structures of Arabidopsis CSN6A (At5g56280) and CSN6B (At4g26430) loci and graphical representation of the corresponding T-DNA insertion lines used in this study. Arrows represent the positions and orientations of the gene-specific primers and the left border T-DNA primer (LBb1) used for the PCR-based genotype (6A.1, 6A.2, 6B.1, and 6B.2) and RT-PCR (6A.3, 6A.4, 6B.2, and 6B.3) analyses.
(B) and (C) PCR-based genotype analyses of Arabidopsis wild type and csn6a-1 (B) or wild type and csn6b-1 (C) heterozygous and homozygous mutants. The genomic sequence of the EF-1A locus was used as a positive control for the PCR.
(D) RT-PCR analyses of the wild type and csn6a-1 and csn6b-1 mutants. Primers 6A.3/6A.4 and 6B.2/6B.3 were used to selectively amplify the CSN6A (top left panel) or CSN6B (bottom left panel) mature transcripts, as indicated. Sets of primers specific for histone H1 (top right panel) and ACT2 (bottom right panel) were used as positive controls for cDNA calibration.
(E) Detection of CSN6 proteins from the wild type and single and double null homozygous mutants by SDS-PAGE and immunoblot analyses with anti (α)-CSN6 polyclonal antibodies, which recognize both CSN6A and CSN6B subunits. Equal protein loading was confirmed using α-RPN6 antibody.
(F) Phenotypes of 5-d-old wild-type, csn6b-1, csn6a-1, and csn6a-1 csn6b-1 seedlings grown in continuous white light.
(G) and (H) Hypocotyl elongation (G) and phenotypes (H) of wild-type and csn6 mutant seedlings grown for 5 d in darkness or different light qualities, as indicated (see Methods). Values shown in (G) represent means of 25 seedlings ± sd. The colored boxes shown in (H) correspond to the genotypes indicated in (G).
CSN6A and CSN6B Subunits Are Functionally Redundant and Essential for Plant Development
As shown in Figures 4G and 4H, both csn6 mutants display a mild partial photomorphogenic phenotype in the dark and in blue light, characterized by shorter hypocotyls with respect to wild-type plants grown under the same conditions. In the dark, loss of CSN6A seems to affect hypocotyl elongation slightly more than loss of CSN6B; however, the absence of a characteristic fusca-type phenotype in the single null lines clearly indicates that both genes act in a largely redundant manner in the regulation of photomorphogenesis. Despite a mild phenotype in the dark and in blue light, csn6a-1 and csn6b-1 do not display any obvious morphological defects in white light (Figure 4F) and after the seedling stage (data not shown).
We further investigated the role of CSN6 in plant development by generating csn6a-1 csn6b-1 double null mutant plants. In the dark and under all light conditions tested, loss of function for both CSN6 proteins results in lethality at the seedling stage and in a photomorphogenic phenotype indistinguishable from the fusca-type phenotype of the null cop/det/fus mutants described previously for the other CSN subunits (Figures 4F to 4H).
Loss of Either CSN5 or CSN6 Results in a Comparable Destabilization of Other CSN Components
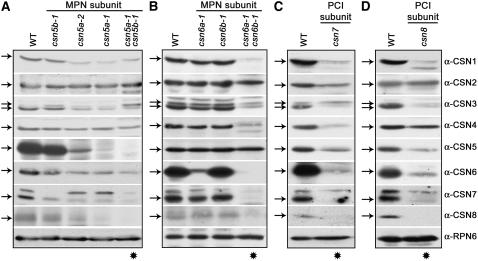
In plants and mammals, it has been shown that several CSN subunits are unstable in an unbound form (Wei and Deng, 2003). Therefore, a dramatic reduction in their accumulation generally reflects the absence of an intact CSN complex and has been commonly used as a marker of CSN ablation. To define what happens to the Arabidopsis CSN complex in the absence of one of its MPN subunits, we compared the stability of all CSN subunits in the single and double csn5 and csn6 mutants. We also included in our analyses two known PCI subunit mutants, csn7 (fus5-1) (Karniol et al., 1999) and csn8 (cop9-1) (Wei et al., 1994a), for comparison.
As shown in Figure 5A (third and fourth lanes), in csn5a single mutants, the cellular pools of CSN1, CSN3, CSN5, CSN6, CSN7, and CSN8 are reduced drastically compared with those in wild-type and csn5b-1 seedlings, whereas no significant changes are observed for CSN2 and CSN4. On the other hand, loss of function of CSN5B does not trigger any detectable changes in the cellular pools of other CSN subunits, including CSN5 (Figure 5A, second lane). Together, the results presented in Figure 5A suggest that when CSN5A or CSN5B is reduced or lost, their corresponding complexes (CSNCSN5A or CSNCSN5B) become unstable. Because CSNCSN5A is much more abundant, this will affect more dramatically the cellular pools of other CSN subunits than the loss of CSNCSN5B. This is different from what is observed for csn6a-1 and csn6b-1 single mutants (Figure 5B). In fact, lack of one or the other member of the CSN6 family does not affect the stability of the other CSN subunits (Figure 5B, second and third lanes).
Figure 5.
Loss of Function of CSN5, CSN6, CSN7, or CSN8 Triggers the Destabilization of Several Other CSN Subunits.
Protein blot analyses of CSN subunits in the wild type and csn5 single and double mutants (A), the wild type and csn6 single and double null mutants (B), the wild type and the csn7 (fus5-1) mutant (C), or the wild type and the csn8 (cop9-1) mutant (D). Equal amounts of total protein extracts from 13-d-old light-grown seedlings were subjected to SDS-PAGE and immunoblot analyses with anti (α)-CSN1, α-CSN2, α-CSN3, α-CSN4, α-CSN5, α-CSN6, α-CSN7, and α-CSN8 polyclonal antibodies. The α-RPN6 antibody was used as a loading control. The lanes indicated by asterisks correspond to complete loss-of-function mutants.
Complete loss of function of CSN5, in the csn5a-1 csn5b-1 double null mutant (Figure 5A, last lane), results in the significant reduction of CSN1, CSN6, CSN7, and CSN8 and in a very slight reduction of CSN4. In the case of CSN3, the complete loss of function of CSN5 results in the accumulation of a larger and possibly modified CSN3 form, whereas the normal (smaller) CSN3 form is present predominantly in wild-type and csn5b-1 extracts. The only subunit that does not appear to be affected is CSN2. Effects comparable to that caused by CSN5 depletion on CSN subunit stability have also been observed in the case of csn6a-1 csn6b-1 double null mutants (Figure 5B, last lane). In fact, complete loss of function of CSN6 (csn6a-1 csn6b-1) results in the significant reduction of CSN1, CSN3, CSN4, CSN7, and CSN8. The only subunits not affected are CSN2 and CSN5.
Analogous effects on the stability of other CSN components have also been observed in the absence of the PCI subunits CSN7 and CSN8. In fact, complete loss of function of CSN7 (Figure 5C, last lane) results in CSN1, CSN3, CSN4, CSN6, and CSN8 instability, accompanied by a mild reduction of CSN5. Likewise, complete loss of function of CSN8 (Figure 5D, last lane) triggers the instability of CSN1, CSN3, CSN5, CSN6, and CSN7. Once again, the only subunit that does not seem do be significantly affected is CSN2 and, in the case of the csn8 mutant, also CSN4. Together, these results confirm that whereas most CSN subunits are unstable in the absence of an intact CSN, CSN2 and CSN4 cannot be reliably used as markers of CSN ablation, because their stability is differentially affected in different csn null mutants.
The MPN Subunits CSN5 and CSN6 Are Essential for the Structural Integrity of the CSN Holocomplex
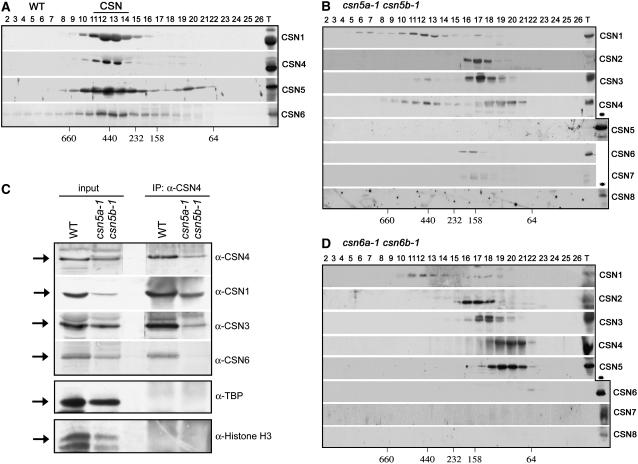
In wild-type Arabidopsis seedling extracts, CSN subunits cofractionate in high molecular mass fractions in gel filtration chromatography (Figure 6A). The elution peak is centered between 450 and 550 kD and corresponds to the CSN complex. Besides the CSN holocomplex, some CSN subunits can be found in smaller molecular mass forms (ranging from 50 to 300 kD) in several organisms, including mammals (Tomoda et al., 2002; Fukumoto et al., 2005), Drosophila (Oron et al., 2002), fission yeast (Zhou et al., 2001; Mundt et al., 2002), and Arabidopsis (Wei and Deng, 2003).
Figure 6.
Both MPN Subunits CSN5 and CSN6 Are Essential for Maintaining the Structural Integrity of the CSN Complex.
(A), (B), and (D) Immunoblot analyses of Superose 6 gel filtration chromatography fractions obtained from 13-d-old light-grown wild-type (A), CSN5 complete loss-of-function mutant (csn5a-1 csn5b-1) (B), or CSN6 complete loss-of-function mutant (csn6a-1 csn6b-1) (D) seedlings. Column fractions were subjected to SDS-PAGE and immunoblot analyses with anti (α)-CSN1, α-CSN4, α-CSN5, and α-CSN6 (A) or α-CSN1, α-CSN2, α-CSN3, α-CSN4, α-CSN5, α-CSN6, α-CSN7, and α-CSN8 ([B] and [D]) polyclonal antibodies. Fraction numbers are indicated. Lane T contains the total unfractionated extracts. The asterisks indicate total unfractionated wild-type extract. Loss of function of CSN5 (B) or CSN6 (D) in csn5a-1 csn5b-1 or csn6a-1 csn6b-1 double null backgrounds results in the disassembly of CSN subunits into CSN-independent subcomplex forms.
(C) CSN4 associates with CSN1 and CSN3 in csn5a-1 csn5b-1 double null mutant seedlings. Total soluble protein extracts (input) from 13-d-old wild-type and csn5a-1 csn5b-1 double mutant seedlings were incubated with CSN4 antibody coupled to protein A-agarose (see Methods). The immunoprecipitates (IP) were then separated by SDS-PAGE and immunoblotted with antibodies against CSN1, CSN3, CSN4, CSN6, TBP, and histone H3, as indicated.
To investigate the fate of the CSN holocomplex in the complete absence of both CSN5 subunits, we analyzed the gel filtration profile of csn5a-1 csn5b-1 double null mutants. In agreement with the severe reduction of several CSN subunits (Figure 5A, last lane), longer exposure times were required to detect their corresponding signals after gel filtration chromatography. Protein blot analyses with antibodies against each CSN subunit confirmed the absence of a CSN5-free large CSN complex in csn5a-1 csn5b-1 double null seedlings (Figure 6B). In fact, CSN2, CSN3, CSN4, CSN6, and traces of CSN7 exclusively or mostly fractionate in low molecular mass fractions, ranging from 250 to 60 kD. Only the residual CSN1 subunit (whose accumulation is reduced dramatically), traces of CSN3, and the first peak of CSN4 elute in high molecular mass fractions that do not contain CSN2, CSN5, CSN6, CSN7, and CSN8, possibly as a result of an association with unknown proteins or self-aggregation. An identical situation has been observed in the case of csn8 (cop9) null mutants. In fact, in csn8 mutants, CSN1 and CSN4 (plus traces of CSN3) still elute in high molecular mass fraction that do not contain any other CSN subunits (our unpublished data). To test whether in csn5a-1 csn5b-1 double mutants CSN1, CSN3, and CSN4 can still associate in the formation of a partial CSN complex, we performed coimmunoprecipitation analyses using total protein extracts obtained from the csn5a-1 csn5b-1 seedlings. As shown in Figure 6C, CSN4 can efficiently pull down CSN1 and CSN3, but not the negative control proteins, from the csn5a-1 csn5b-1 extract, indicating that CSN1, CSN3, and CSN4 associate with each other in the absence of the other CSN subunits.
In the case of csn6a-1 csn6b-1 double null mutants (Figure 6D), whereas only residual CSN1 elutes in high molecular mass fractions, CSN2, CSN3, CSN4, and CSN5 fractionate exclusively in low molecular mass fractions, ranging from 250 to 60 kD. In the case of CSN7 and CSN8, we have been unable to detect their corresponding signals, in agreement with a dramatic reduction in their accumulation levels in this mutant (Figure 5B, last lane).
All CSN Complete Loss-of-Function Mutants Share a Similar Impairment in Cullin Derubylation
The six PCI subunit mutants identified by genetic screens for constitutive photomorphogenic phenotype are virtually indistinguishable from the csn5 and csn6 complete loss-of-function double mutant lines described in this study (Figure 7A). On this basis, we investigated whether their identical phenotype reflected an identical impairment in cullin derubylation, the major known biochemical function of CSN. We were also interested in establishing the functional contribution of each CSN5 and CSN6 family member to the overall CSN biochemical activity. To this end, we compared the rubylated/derubylated ratio of CUL1, CUL3, and CUL4 in the single and double csn mutants. As shown in Figure 7B, in the case of the CSN5 gene family (left panel), CSN5A and CSN5B differentially contribute to the derubylation activity observed in wild-type plants. In fact, whereas loss of CSN5B has no effects (Figure 7B, left panel, second lane), reduction or loss of CSN5A results in significantly increased levels of rubylated CUL1, CUL3, and CUL4 in seedlings (Figure 7B, left panel, third and fourth lanes). In the case of the CSN6 gene family, however, loss of either CSN6A or CSN6B does not affect the derubylation of cullins (Figure 7B, middle panel, second and third lanes).
As expected, in csn5a-2 csn5b-1 seedlings, the residual activity of CSNCSN5A results in compromised, but not abolished, CUL1, CUL3, and CUL4 derubylation (Figure 7B, left panel, fifth lane). This correlates with the severe but not lethal phenotype of csn5a-2 csn5b-1 double mutants, which can survive to the adult stage.
The total depletion of CSN5 (csn5a-1 csn5b-1) (Figure 7B, left panel, last lane) or CSN6 (csn6a-1 csn6b-1) (Figure 7B, middle panel, last lane), as well as each one of the six PCI subunits of the CSN (Figure 7B, right panel), results in complete loss of derubylation activity and the accumulation of CUL1, CUL3, and CUL4 exclusively in the rubylated forms. These data confirm that CSN possesses the only activity able to deconjugate RUB/NEDD8 from cullins in Arabidopsis seedlings.
The Cellular Levels of CUL1, CUL3, and CUL4 Are Differentially Affected by CSN Depletion
It is interesting that in seedling extracts, the total cellular level of CUL1 remains almost unchanged and loss of a PCI or an MPN subunit only induces a redistribution of CUL1 from the unmodified to the modified form (Figure 7B). On the contrary, in the case of CUL3, the lack of CSN activity results in a drastic decrease in the total cellular pool of this cullin. In fact, in csn complete loss-of-function mutants (Figure 7B, lanes indicated by asterisks), whereas unmodified CUL3 is completely absent, RUB-CUL3 is barely detectable. Interestingly, we observed an opposite effect of CSN on the stability of CUL4. In fact, the absence of CSN activity triggers a significant increase in the total cellular level of CUL4 (Figure 7B, lanes indicated by asterisks) compared with the level of CUL4 in the wild type.
Arabidopsis CUL3-Based E3 Ubiquitin Ligases Associate with CSN and the Proteasome in Vivo
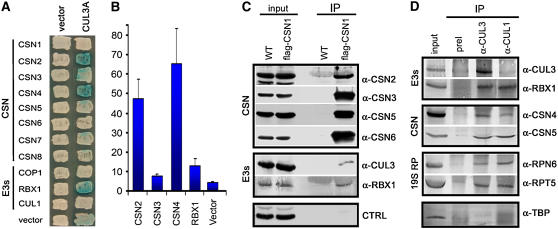
In Arabidopsis, it has been shown that CSN associates with and regulates the activity of several SCF complexes (Schwechheimer et al., 2001; Wang et al., 2003; Feng et al., 2003). Similarly, Arabidopsis CSN interacts with a CUL4-RBX1-CDD E3 ubiquitin ligase in vivo (Chen et al., 2006). Recently, it was shown that Arabidopsis CUL3 associates directly with RBX1 and BTB proteins to form multisubunit E3 ubiquitin ligases (Figueroa et al., 2005; Gingerich et al., 2005; Weber et al., 2005). However, an in vivo biochemical interaction between CSN and CUL3-RBX1–based E3 complexes has not been reported. To test whether CSN interacts physically with CUL3-based E3 ubiquitin ligases, we first used a yeast two-hybrid assay (Figure 8A). Positive interactions in the plate assay (Figure 8A) were tested again with a liquid β-galactosidase activity assay (Figure 8B). As shown in Figures 8A and 8B, CUL3A interacts with CSN2 and CSN4. In addition, CUL3 binds to RBX1 (the conserved catalytic RING subunit common to all classes of cullin-based E3s) but not to CUL1 or COP1 (components of other classes of E3s ligases). These results are consistent with the previously reported in vitro interaction between CSN2 and CUL3A (Serino et al., 2003).
Figure 8.
The in Vivo Association of CSN, CUL3-Based E3 Ligases, and the 26 Proteasome.
(A) In vitro interaction between Arabidopsis CUL3A, RBX1, and CSN subunits by yeast two-hybrid assay. Blue colonies indicate LacZ production and thus protein interaction.
(B) Colonies that tested positive for interactions in (A) were subjected to liquid quantitative assays to verify the strength of the binding. Numbers shown on the y axis are means of two experiments. For each experiment, the numbers on the y axis were calculated as mean values of at least six independent transformants. Error bars represent sd.
(C) CSN specifically associates with the CUL3A-RBX1 E3 module in vivo. Total soluble protein extracts (input) from wild-type and FLAG-CSN1 transgenic seedlings were incubated with monoclonal anti-FLAG–conjugated agarose (α-FLAG; see Methods), and the immunoprecipitates (IP) were separated by SDS-PAGE and immunoblotted with antibodies against CSN2, CSN3, CSN5, CSN6, CUL3, and RBX1. The control represents a protein band recognized by α-CSN2 antibody.
(D) CUL3 specifically associates with CSN and the proteasome in vivo. Total soluble protein extracts (input) from wild-type seedlings were incubated with CUL3 preimmune serum (preI) or with CUL3 and CUL1 antibodies coupled to protein A-agarose (see Methods), as indicated. The immunoprecipitates (IP) were then separated by SDS-PAGE and immunoblotted with antibodies against CUL3, RBX1, CSN4, CSN5, RPT5, RPN6, and TBP.
The specific in vivo association between CSN and BTB-CUL3-RBX1 (BCR) E3 ubiquitin ligases was then investigated by immunoprecipitation in a stable transgenic line expressing a FLAG-tagged version of CSN1 (FLAG-CSN1) (Wang et al., 2002). As shown in Figure 8C, FLAG-CSN1 can efficiently pull down the CSN complex and consistently coimmunoprecipitate CUL3 and RBX1. We further immunoprecipitated CUL3 and, as a positive control, CUL1 from wild-type seedling protein extracts. As predicted, CUL3, like CUL1, was capable of interacting specifically with RBX1 and coimmunoprecipitating several CSN subunits, clearly demonstrating that CSN and BCR E3 ubiquitin ligases can associate with each other in vivo.
It has been suggested that the E3 ligases could make physical contact with the proteasome as part of their function in the protein degradation process (Peng et al., 2003). Based on this consideration, we tested whether CUL3 was able to coimmunoprecipitate representative subunits of the 26S proteasome in vivo. As shown in Figure 8D, CUL3, like CUL1, could efficiently pull down a lid subunit (RPN6) and a base subunit (RPT5) of the 19S regulatory particles, indicating a strong physical association between CUL1- and CUL3-based E3 ligase complexes, CSN, and the proteasome.
Mutations in CUL3A or CUL3B Suppress the Pleiotropic Phenotype of csn5a Mutants
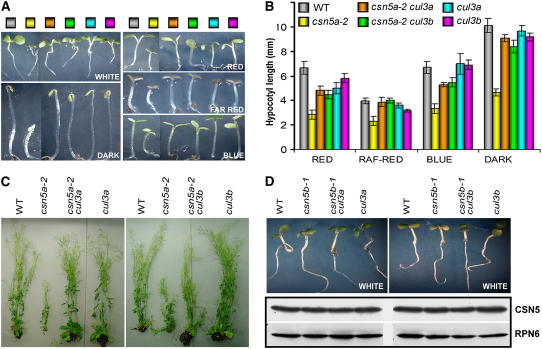
Arabidopsis has two largely redundant CUL3 genes (CUL3A and CUL3B), which together are essential for embryo development (Figueroa et al., 2005; Gingerich et al., 2005; Tohmann et al., 2005). However, when grown under normal conditions, the single cul3a and cul3b mutants are virtually indistinguishable from their wild-type siblings throughout their entire life cycles (Figueroa et al., 2005). To gain new insight into possible developmental pathways regulated by CUL3, we crossed the single cul3a and cul3b mutants with the csn5a mutant, which exhibits impaired CUL3 derubylation. Considering possible further analyses, we used the weak csn5a-2 mutant, which is not lethal when combined with csn5b. We generated two double homozygous mutant lines: csn5a-2 cul3a and csn5a-2 cul3b. As a control, we crossed the csn5b-1 mutant with cul3a and cul3b, generating two double homozygous mutant lines, csn5b-1 cul3a and csn5b-1 cul3b. For each cross, to eliminate or reduce possible effects attributable to the variability between different parental lines, which might differ significantly in terms of allelic composition at any given locus, we reisolated all relevant single and double homozygous lines (including the wild type) from the same segregating F2 population for comparative analysis.
Surprisingly, loss of either CUL3A or CUL3B results in the suppression of the pleiotropic phenotype of csn5a-2 mutants (Figures 9A to 9C). In fact, whereas the csn5a-2 mutant displayed the characteristic phenotype shown in Figure 2, the double csn5a-2 cul3a and csn5a-2 cul3b mutants are virtually indistinguishable from wild-type siblings in the dark and in all light conditions, both at seedling (Figures 9A and 9B) and mature (Figure 9C) stages. On the other hand, we could not detect any genetic interaction between CSN5B and CUL3A or CUL3B. In fact, the single csn5b-1, cul3a, and cul3b mutants and the double csn5b-1 cul3a and csn5b-1 cul3b mutants did not show any observable defects throughout their life cycle (Figure 9D; data not shown).
Figure 9.
CUL3A or CUL3B Loss of Function Results in the Suppression of the Pleiotropic Developmental Defects of the csn5a-2 Mutant.
(A) and (B) Genetic interaction between CSN5 and CUL3. Phenotypes (A) and hypocotyl elongation (B) of 5-d-old wild-type, csn5a-2, cul3a, and cul3b single and double mutant seedlings grown in darkness or in different light qualities, as indicated (see Methods). Values shown represent means of 25 seedlings ± sd. The colored boxes shown in (A) correspond to the genotypes indicated in (B).
(C) Loss of CUL3A or CUL3B suppresses csn5a developmental defects at all stages. Phenotypes of 8-week-old wild-type, csn5a-2, and cul3a single and double mutant plants (left) or wild-type, csn5a-2, and cul3b single and double mutant plants (right), as indicated, grown in long-day conditions.
(D) Top panels, phenotypes of 4-d-old wild-type, csn5b-1, and cul3a single and double mutant plants (left) or wild-type, csn5b-1, and cul3b single and double mutant plants (right) grown for 4 d in white light. Bottom panels, detection of CSN5 proteins from wild-type, csn5b-1, cul3a, and cul3b single and double mutant plants, as indicated. Total protein extracts from 6-d-old white light–grown seedlings were subjected to SDS-PAGE and immunoblot analyses with anti (α)-CSN5 polyclonal antibodies. Equal protein loading was confirmed using α-RPN6 antibody.
Loss of Either CUL3A or CUL3B Function Results in the Accumulation of Higher Levels of CSN5 Proteins
As shown in Figure 9D, protein gel blot analyses of double csn5b-1 cul3a and csn5b-1 cul3b mutants indicate that loss of CUL3A or CUL3B does not affect CSN5A expression. On the contrary, in the csn5a-2 cul3a and csn5a-2 cul3b backgrounds, loss of function of CUL3A or CUL3B results in a significant increase in the total amount of CSN5 with respect to the CSN5 level observed in the csn5a-2 single mutant (Figure 10A, lanes indicated by asterisks). Using an image-analyses software (http://rsb.info.nih.gov/ij/), we estimated that loss of cul3a or cul3b results in an increase of approximately fivefold to sixfold in the cellular pool of CSN5.
Figure 10.
Loss of CUL3A or CUL3B Activity Results in a Significant Increase in the Cellular Pool of CSN5.
(A) Protein blot analyses of the wild type and csn5 and cul3 single and double mutants, as indicated. Equal amounts of total proteins from 6-d-old white light–grown seedlings were subjected to immunoblot analyses with anti (α)-CSN5, α-CUL1, α-CUL4, and α-CUL3 polyclonal antibodies. The α-RPN6 antibody was used as a loading control. Loss of either CUL3A or CUL3B results in CSN5 accumulation.
(B) and (C) Semiquantitative RT-PCR analyses of the wild type and csn5 and cul3 single and double mutants, as indicated. The positions of the gene-specific primers used to selectively amplify the CSN5A (B) or CSN5B (C) transcripts are shown in Figure 1. Primers A1/A2 selectively amplify the full-length CSN5A cDNA. A set of primers specific for ACT2 was used for sample calibration. Genomic DNA obtained from wild-type seedlings was used as a positive control for PCR. The asterisks indicate the relevant genotypes for comparison.
Protein gel blot analyses of seedling extracts with antibody against CUL3 confirmed that the loss of one (cul3a) or the other (cul3b) functional CUL3 loci triggers a measurable reduction in the cellular pool of CUL3, confirming that both genes contribute to the maintenance of optimal CUL3 levels, even though mutation in CUL3A seems to affect the pool slightly more at this examined developmental stage (Figure 10A, middle panel, last two lanes).
Not surprisingly, the increase in the cellular pool of CSN5 in the csn5a-2 cul3a and csn5a-2 cul3b double mutants resulted in improved derubylation of all three cullins (Figure 10A, middle panel). In fact, the ratios of rubylated/un-rubylated CUL1, CUL3, and CUL4 are lower in the csn5a-2 cul3a and csn5a-2 cul3b mutants with respect to csn5a-2 (Figure 10A, middle panel, lanes indicated by asterisks), particularly in the case of CUL1 and CUL4. Thus, the increased accumulation of CSN5 is responsible for ameliorating the csn5a-2 deficiency in cullin derubylation, suppressing the csn5a-2 developmental defects at all stages.
Loss of CUL3A or CUL3B Does Not Significantly Affect CSN5A and CSN5B Expression at the Transcriptional Level
To distinguish whether transcriptional or posttranscriptional mechanisms were responsible for the observed changes in CSN5 abundance, we performed semiquantitative RT-PCR analyses on RNA extracted from wild-type and single and double csn5 and cul3 lines (Figures 10B and 10C). In the case of CSN5A transcript, csn5a-1 null mutants were used as negative controls for the RT-PCR analysis (Figure 10B, fourth lane). We first used a set of CSN5A-specific primers spanning the region 5′ to the T-DNA insertion (Figure 10B, top panel). We could not detect any major change in the total level of CSN5A transcript between csn5a-2 and csn5a-2 cul3a or csn5a-2 cul3b (lanes indicated by asterisks). Subsequently, we used a set of primers spanning the entire CSN5A coding sequence (Figure 10B, bottom panel). As shown in Figure 1, the transcript of the csn5a-2 allele (which is transcribed at wild-type levels) is correctly spliced into a full-length CSN5A mRNA at low efficiency. Therefore, in the csn5a-2 background, the full-length CSN5A transcript is detectable only after 35 cycles of PCR amplification (Figure 10B, bottom panel, third lane). Once again, however, we observed no significant difference in CSN5A expression levels between the csn5a-2 mutant and the csn5a-2 cul3a or csn5a-2 cul3b double mutant lines (Figure 10B, bottom panel, lanes indicated by asterisks). As shown in Figure 10C, similar results were obtained in the case of the CSN5B transcript (lanes indicated by asterisks). A semiquantitative RT-PCR analysis (at 28 cycles or less) confirmed the absence of significant changes in CSN5B expression and a very minor change (∼50%) in CSN5A transcript between the single csn5a-2 and the double csn5a-2 cul3a or csn5a-2cul3b mutants. Even though we cannot completely exclude the possibility that subtle changes (below our detectable threshold) in CSN5 mRNA expression might, at least in part, contribute to CSN5 accumulation, the results shown in Figure 10 suggest that CUL3 affects CSN5 abundance most likely at the posttranscriptional level.
DISCUSSION
CSN5 and CSN6 Subunits Are Essential for Plant Viability and Development but with Distinct Functional Diversification between Their Isoforms
In Arabidopsis, the two CSN MPN subunits, CSN5 and CSN6, have escaped the genetic screenings that led to the identification of the six PCI subunits. Here, we have undertaken a reverse genetic approach to systematically identify and characterize single and double null mutants in each of those two-gene families. Our results demonstrate that, despite some degree of divergence in their coding sequences, CSN6A and CSN6B proteins are functionally equivalent in the regulation of plant development at all stages (Figure 4). In fact, both in white light and long-day conditions (16 h of light/8 h of dark), the two CSN6 subunits are completely redundant and the expression of only one of the two genes is sufficient for full function. The CSN5 gene family is somewhat different. In fact, CSN5A and CSN5B have largely diverged in their ability to regulate plant development. In agreement with their different expression levels (Figure 1F), we have shown that lack of CSN5B does not cause any obvious defects in all conditions tested, whereas loss of CSN5A triggers pleiotropic defects in multiple developmental processes at all stages (Figure 2; see Supplemental Figure 1 online). Remarkably, the pleiotropic phenotypes of csn5a-1 and csn5a-2 are reminiscent of the severe developmental defects caused by the overexpression of catalytically inactive CSN5A derivatives (Gusmaroli et al., 2004).
Our early and current studies suggest that many aspects of the csn5a mutant phenotypes are caused by a partial (csn5a-2) or complete (csn5a-1) loss of CSN5A-mediated cullin derubylation, likely resulting in a certain level of impairment in the ubiquitination activity of multiple E3 ubiquitin ligases, such as SCFTIR1 (Gray et al., 1999), SCFCOI1 (Xu et al., 2002), or CUL4-RBX1-E3 complexes (Bernhardt et al., 2006; Chen et al., 2006), all of which interact with CSN in the regulation of hormone- or light-mediated responses (Schwechheimer et al., 2001; Feng et al., 2003; Chen et al., 2006).
Nevertheless, the multifaceted developmental defects of csn5a mutants are less severe than the fusca-type phenotype of csn5a-1 csn5b-1 double null mutants (Figure 3). Indeed, the residual derubylation activity observed in csn5a null mutants (Figure 7B, left panel, fourth lane) reveals a minor role for low-abundance CSN5B (and its CSNCSN5B complex) in cullin derubylation. This finding implies that CSN5A is the predominantly functional subunit, whereas CSN5B contributes to CSN5 function to a lesser extent, suggesting that the two CSN5 genes might have experienced a dissimilar reduction of functions with respect to the ancestor gene. Finally, we showed that the complete loss of function of CSN5 or CSN6 results in severe abnormalities, which lead to postembryonic lethality at the seedling stage (csn5a-1 csn5b-1 [Figure 3A] and csn6a-1 csn6b-1 [Figure 4F]). These findings demonstrate that, despite the fact that only CSN5 is catalytically active in cullin derubylation, both MPN subunits are essential for plant viability and development.
Arabidopsis MPN Subunits Are Essential for CSN Assembly or Stability
It was reported recently that, in Arabidopsis, a lethal csn5 double mutant line (csn5a-2 csn5b-1) retains a CSN complex lacking only its subunit 5 (Dohmann et al., 2005). However, we showed here that the csn5a-2 allele used in that study is not a null allele (Figures 1B and 1C, second lanes, and 1F, third lane). In fact, the csn5a-2 csn5b-1 double mutants are not lethal at the seedling stage (Figures 3A to 3C) and possess reduced levels of an intact CSN complex containing a functional CSN5A subunit (Figure 3E).
To unambiguously determine the contributions of CSN5 and CSN6 to the structure and function of the CSN complex, we generated csn5 and csn6 double null mutant lines. We demonstrated that the complete depletion of CSN5 or CSN6 results in the loss of the CSN holocomplex and the redistribution of some CSN subunits into subcomplex forms (Figures 6B and 6D), accompanied by the instability of several subunits, including CSN1, CSN3, CSN6, CSN7, and CSN8 (Figures 5A and 5B, last lanes). Therefore, in Arabidopsis, the CSN complex is unstable in the absence of one of its MPN subunits, even though residual CSN1, CSN3, and CSN4 still associate with each other in a complex (Figures 6B and 6C). This association of CSN1, CSN3, and CSN4 in the csn5 double null mutants likely has an altered stoichiometry or includes other proteins.
Together, our data are in agreement with previous findings that CSN1 (FUS6), CSN6, and CSN8 (COP9) are unstable as free forms and that their accumulation depends upon the formation of an intact complex (Staub et al., 1996; Peng et al., 2001a, 2001b; Wang et al., 2002). Similarly, it has been demonstrated that a reduction of CSN5 in CSN5 antisense/cosuppression plants leads to a reduction in the level of the entire CSN (Schwechheimer et al., 2001). Null csn1 (Wang et al., 2002), csn5, and csn6 mutants display slightly different gel filtration patterns for CSN4 and CSN7, which suggests that, although all three subunits are required for the structural integrity of the CSN holocomplex, their specific roles in complex assembly are not identical.
Because the gel filtration profiles of several CSN subcomplexes do not overlap completely (Figures 6B and 6D), it is possible that other proteins associate with some of the CSN subunits. In fact, the existence of small complexes containing only one subunit of CSN, in association with other unknown proteins, has been described for mammalian CSN5, CSN7, and CSN8 (Fukumoto et al., 2005). In addition, several reports demonstrated that specific CSN subunits, particularly CSN5, could bind to interacting proteins in a CSN-independent manner (Wei and Deng, 2003).
The Complete Collection of csn Mutants Highlights That CSN Regulates All Major Classes of Known Cullin-Based E3 Uqibuitin Ligases
With this study, we concluded the collection and analysis of complete loss-of-function mutants in each one of the eight CSN subunits and established the specific contribution of each CSN5 and CSN6 family member to the structure and function of the complex as a whole. Our biochemical analyses demonstrated that PCI and MPN subunits are structurally interdependent during the formation of the COP9 complex, explaining why all csn loss-of-function mutants share an identical phenotype (Figure 7A). Our data confirm that CSN function is essential for cullin derubylation and to sustain the optimal activity of CUL1-, CUL3-, and CUL4-based E3 complexes and suggest that the lethality of csn mutants is triggered by the pleiotropic deregulation of multiple ubiquitin-mediated pathways.
At this point, it would be interesting to establish what additional CSN functions might contribute to the lethal phenotype of csn loss-of-function mutants. In fact, several lines of evidence in Arabidopsis (Wang et al., 2002) and yeast (Zhou et al., 2001; Mundt et al., 2002) have demonstrated that there are other essential functions of CSN independent from cullin derubylation. However, because the loss of each CSN subunit results in the loss of the entire complex, this important issue still remains unanswered.
CSN5 and CUL3 Interact in Vivo and Reciprocally Regulate Each Other's Abundance
In this study, we have demonstrated that CSN interacts both in vitro and in vivo with CUL3-RBX1–based E3 ubiquitin ligases (Figure 8). We have also shown that CUL3-RBX1–based E3 complexes associate with the proteasome 19S regulatory particle in vivo, consistent with their function in substrate polyubiquitination and proteasome-mediated degradation (Figure 8D).
Interestingly, the complete lack of CSN activity results in a drastic depletion of CUL3 in seedlings (Figure 7B, lanes indicated by asterisks). In agreement with our findings, a recent report showed that rubylated CUL1 and CUL3 are destabilized in csn5Null and csn5D148N Drosophila mutant larvae, demonstrating that CSN activity is required to prevent the degradation of rubylated CUL3 (Wu et al., 2005). These results suggest that CSN protects CUL1 and CUL3 from degradation only when it possesses a reliable derubylation activity and highlight the idea that RUB1 might function as a degradation signal for cullins. However, our results showed that, in Arabidopsis seedlings, only rubylated CUL3 is unstable whereas rubylated CUL1 is relatively stable. Notably, CUL1 appears to be unstable in the flower tissue of an Arabidopsis csn1 partial mutant (Wang et al., 2002), suggesting that the stability of rubylated cullins may be regulated in a tissue-specific manner. Moreover, the observation that loss of function of CSN has an opposite effect on the stability of CUL4 emphasizes that cullin stability is regulated by specific mechanisms and is not simply triggered by RUB/NEDD8 modification.
In the process of investigating possible physiological responses regulated by CUL3 during plant development, we revealed a genetic interaction between CSN5 and CUL3 (Figures 9A to 9C). We have shown that a suboptimal dose of active CUL3 (attributable to either cul3a or cul3b loss of function) causes CSN5 upregulation, most likely through a posttranscriptional mechanism (Figure 10, lanes indicated by asterisks). Therefore, whereas CSN activity is essential to positively regulate CUL3 function by protecting CUL3 from degradation, a robust CUL3 activity is required to negatively regulate CSN5 accumulation.
Because CSN5A is expressed at very high levels in wild-type plants and in the single cul3a and cul3b mutants, the effect of CUL3 reduction on CSN5 expression becomes evident only when CSN5A is reduced dramatically (e.g., in csn5a-2 cul3a and csn5a-2 cul3b backgrounds). Interestingly, CSN5A and CSN5B possess a certain degree of divergence in their coding sequences (Kwok et al., 1998) and incorporate into distinct CSN complexes (Gusmaroli et al., 2004), which are present at very different levels throughout plant development. Therefore, it is plausible to speculate that, in normal growth conditions, the activity of both cullins is required to differentially regulate CSN5A and CSN5B expression. Even though we cannot rule out a possible indirect effect on the control of CSN5 mRNA translation, an intriguing possibility is that CUL3, as a component of E3 ubiquitin ligases, controls CSN5 stability by targeting this subunit for degradation.
METHODS
Plant Materials and Growth Conditions
The wild-type Arabidopsis thaliana plants used in this study are the Columbia-0 and Landsberg erecta ecotypes. The CSN5 and CSN6 T-DNA insertion lines SALK_063436 (csn5a-1), SALK_027705 (csn5a-2), SALK_007134 (csn5b-1), SALK_146926 (csn6a-1), and SALK_036965 (csn6b-1) were identified in the SIGnAL database (Alonso et al., 2003). The csn5a-1 and csn5a-2 T-DNA insertion mutants have lost the resistance markers associated with their corresponding T-DNA insertions. Both lines were crossed to wild-type plants before further investigations. The phenotypes of csn5a-2 and csn5a-1 homozygous mutants cosegregate in 100% of cases with the homozygous T-DNA insertion at the CSN5A locus. In the case of csn5b-1, heterozygous T1 plants carrying the csn5b-1 T-DNA insertion allele show a segregation ratio of 3:1 kanamycin-resistant:kanamycin-sensitive in T2 progeny, indicative of a single T-DNA insertion (Gusmaroli et al., 2004). The T-DNA insertion lines in CUL3A (cul3a mutant) and CUL3B (cul3b mutant) have been described previously (Figueroa et al., 2005). The cop/det/fus mutants used in this study were fus6-1 (Wang et al., 2002), fus12-U228 (Serino et al., 2003), fus11-U203 (Peng et al., 2001b), cop8-1 (Wei et al., 1994b), fus5-1 (Karniol et al., 1999), and cop9-1 (Wei et al., 1994a).
Arabidopsis seedlings were surface-sterilized, cold-treated at 4°C for 3 to 5 d, and then germinated on solid Murashige and Skoog medium with Gamborg's vitamins (Sigma-Aldrich) supplemented with 1% sucrose (GM) in continuous white light (150 μmol·m−2·s−1) at 22°C. To obtain adult plants, 8- to 16-d-old seedlings were transferred to soil and grown under long-day conditions (16 h of light/8 h of dark) in a controlled-environment chamber at 22°C.
Light and Hormone Treatments
For the hypocotyl elongation experiments shown in Figure 4 and Supplemental Figure 1 online, 4-d cold-treated seeds were exposed to white light for 12 h (150 μmol·m−2·s−1) and then transferred to continuous darkness or white (150 μmol·m−2·s−1), far-red (4 μmol·m−2·s−1), red (80 μmol·m−2·s−1), or blue (11 μmol·m−2·s−1) light. For the other analyses shown in Supplemental Figure 1 online, growth conditions were as follows. For root length measurements, 4-d cold-treated seeds were germinated in continuous white light (150 μmol·m−2·s−1) on GM vertical plates and root length was measured after 5 d. For auxin and jasmonic acid treatments, 4-d cold-treated seeds were germinated in continuous white light (150 μmol·m−2·s−1) on vertical Murashige and Skoog plates for 4 d and then transferred to vertical GM plates supplemented with 2,4-D as indicated or with 2 μM jasmonic acid. Root length and number of lateral roots were measured 4 d after transfer to the auxin-containing medium. Root hairs were photographed at 4 d after transfer to jasmonic acid–containing medium. In all cases, seedlings were grown in controlled chambers at 22°C.
PCR-Based Genotyping
DNA of the Arabidopsis T-DNA lines and their progeny was extracted and screened for T-DNA insertions at the CSN5A, CSN5B, CSN6A, CSN6B, CUL3A, and CUL3B loci. In each case, sets of forward and reverse gene-specific primers, in combination with the T-DNA left border–specific primer (Alonso et al., 2003), were used to selectively amplify the wild-type or T-DNA insertion alleles at the locus of interest. In the case of csn5a-1, csn5a-2, csn5b-1, csn6a-1, and csn6b-1 mutants isolated in this study, the identity of PCR fragments was confirmed by sequencing. All crosses using csn5a-1, csn5a-2, csn5b-1, csn6a-1, csn6b-1, cul3a, and cul3b alleles were confirmed in F1 by PCR-based genotyping. In all cases, the genotypes of the segregating F2 single and double homozygous mutants (csn5a-1, csn5a-2, csn5b-1, csn6a-1, csn6b-1, cul3a, cul3b, csn5a-1 csn5b-1, csn5a-2 csn5b-1, csn6a-1 csn6b-1, csn5a-2 cul3a, csn5a-2 cul3b, csn5b-1 cul3a, and csn5b-1 cul3b) and their progeny were confirmed by PCR-based genotyping.
RT-PCR Analyses
Total RNA was extracted from the wild type, the single csn5a-1, csn5a-2, csn5b-1, csn6a-1, csn6b-1, cul3a, and cul3b mutants, and the double csn5a-2 cul3a and csn5a-2 cul3b homozygous mutant lines using the RNeasy Plant Mini Kit (Qiagen). For each genotype, 100 mg of fresh tissue obtained from 10-d-old light-grown seedlings was used as starting material. For semiquantitative RT-PCR analyses, before retrotranscription, total RNAs were treated with DNase I (Roche) to eliminate possible traces of contaminant DNA. The DNase I treatments and RT-PCR were performed as described previously (Gusmaroli et al., 2004). Sets of primers specific for eEF-1A (gene identifier 837307) and Tsβ1 (gene identifier 835571) were used as positive controls for RT-PCR. For semiquantitative RT-PCR analyses, the cDNAs were first calibrated using ACT2 (gene identifier 821411). Different sets of gene-specific primers were used to selectively amplify the cDNA corresponding to each particular mRNA transcript. RT-PCR was performed at 25, 28, 35, and 38 cycles.
Protein Extraction, Immunoblot Analyses, and Gel Filtration Chromatography
Protein extractions and immunoblot analyses were performed as described previously (Gusmaroli et al., 2004). The protein concentration in the supernatants was determined by Bradford assay (Bio-Rad). For gel filtration analyses, 300 μg of total proteins was fractionated onto a Superose 6 (HR10/30) protein liquid chromatography column (Amersham Biosciences) and fractions were concentrated using Strataclean resin (Stratagene), as described previously (Gusmaroli et al., 2004). In all cases in which equal loading of the samples was required, the same samples or the blots were probed with α-RPN6 to confirm equal loading. The antibodies used in this study are as follows: CSN1 (Staub et al., 1996), CSN2 (Serino et al., 2003), CSN3 (Peng et al., 2001a), CSN4 (Serino et al., 1999), CSN5 (Kwok et al., 1998), CSN6 (Peng et al., 2001b), CSN7 (Karniol et al., 1999), CSN8 (Wei et al., 1994a), RPN6 and RPT5 (Kwok et al., 1999), TBP (Schwechheimer et al., 2001), RBX1 (Schwechheimer et al., 2002), CUL1 (Wang et al., 2002), CUL3 (Figueroa et al., 2005), CUL4 (Chen et al., 2006), and histone H3 (Upstate).
Yeast Two-Hybrid Assay
The full-length cDNA clones of RBX1 and COP1 were translationally fused to the transcription activation domain of pJG4-5 (Origene Technologies). The two-hybrid constructs of the CSN and CUL1 subunits fused to the pJG4-5 activation domain and the CUL3A construct fused to the pEG202 LexA binding domain have been described previously (Schwechheimer et al., 2001; Serino et al., 2003). The CUL3A-LexA fusion construct was transformed into yeast strain EGY48 (Invitrogen). Activation domain fusion constructs were transformed into yeast strain L40 (Invitrogen), which contains a β-galactosidase reporter gene. The plate and liquid assays were performed as described previously (Serino et al., 1999).
In Vivo Coimmunoprecipitation Analyses
For pull-down assays, Arabidopsis tissues were ground in liquid nitrogen and subsequently homogenized in cold immunoprecipitation buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5% glycerol, 0.05% Nonidet P-40, 2.5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1× complete cocktail of protease inhibitors). The total protein extracts were centrifuged twice at 13,000g for 15 min at 4°C, and the supernatants were filtered through 0.22-μm filters for use in immunoprecipitation assays. For coimmunoprecipitation, 1.4 mg of total proteins was incubated for 4 h at 4°C with 30 μL of monoclonal anti-FLAG antibody immobilized onto agarose beads (Sigma-Aldrich) or with 30 μL of polyclonal CUL1, CUL3, and CSN4 antibodies coupled to protein A-agarose beads (Sigma-Aldrich). The antigene-antibody-protein A-agarose conjugates were washed three times at 4°C for 15 min in immunoprecipitation buffer and centrifuged at 4°C for 5 min at 3000 rpm between washes. The immunoprecipitates were subsequently released by boiling for 5 min in 2× SDS sample buffer. Protein blot analyses were performed as described previously (Gusmaroli et al., 2004).
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: At3g18780 (ACT2); At3g61140 (CSN1); At2g26990 (CSN2); At5g14250 (CSN3); At5g42970 (CSN4); At1g22920 (CSN5A); At1g71230 (CSN5B); At5g56280 (CSN6A); At4g26430 (CSN6B); At1g02090 (CSN7); At4g14110 (CSN8); At4g02570 (CUL1); At1g26230 (CUL3A); At1g69670 (CUL3B); At5g46210 (CUL4); At5g60390 (EF-1A); At1g06760 (histone H1); At3g42830 (RBX1); At1g29150 (RPN6); At3g05530 (RPT5); At3g13445 (TBP); and At5g54810 (TSB1).
Supplemental Data
The following material is available in the online version of this article.
Supplemental Figure 1. Characterization of the Single csn5 Mutants in Different Growth Conditions at Representative Developmental Stages.
Supplementary Material
Acknowledgments
We are grateful to Ning Wei and Sinead Drea for critical reading of the manuscript. We also thank the Salk Institute Genomic Analyses Laboratory for generating the sequence-indexed Arabidopsis T-DNA insertion mutants and the Nottingham and Ohio Arabidopsis stock centers for providing seeds of the corresponding lines. This research was supported by National Science Foundation 2010 Grant MCB-0519970 and by National Institutes of Health Grant GM-47850 to X.W.D. G.G. was initially supported by a fellowship from the Universita' degli Studi di Milano.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Xing Wang Deng (xingwang.deng@yale.edu).
Online version contains Web-only data.
References
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Bernhardt, A., Lechner, E., Hano, P., Schade, V., Dieterle, M., Anders, M., Dubin, M.J., Benvenuto, G., Bowler, C., Genschik, P., and Hellmann, H. (2006). CUL4 associates with DDB1 and DET1 and its downregulation affects diverse aspects of development in Arabidopsis thaliana. Plant J. 47 591–603. [DOI] [PubMed] [Google Scholar]
- Bornstein, G., Ganoth, D., and Hershko, A. (2006). Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate. Proc. Natl. Acad. Sci. USA 103 11515–11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostick, M., Lochhead, S.R., Honda, A., Palmer, S., and Callis, J. (2004). Related to Ubiquitin 1 and 2 are redundant and essential and regulate vegetative growth, auxin signaling, and ethylene production in Arabidopsis. Plant Cell 16 2418–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch, S., Eckert, S.E., Krappmann, S., and Braus, G.H. (2003). The COP9 signalosome is an essential regulator of development in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 49 717–730. [DOI] [PubMed] [Google Scholar]
- Chamovitz, D.A., Wei, N., Osterlund, M.T., von Arnim, A.G., and Staub, J.M. (1996). The COP9 complex, a novel multisubunit nuclear regulator involved in light control of a plant developmental switch. Cell 86 115–121. [DOI] [PubMed] [Google Scholar]
- Chen, H., Shen, Y., Tang, X., Yu, L., Wang, J., Guo, L., Zhang, Y., Feng, S., Strickland, E., Zheng, N., and Deng, X.W. (2006). Arabidopsis CULLIN4 forms an E3 ubiquitin ligase with RBX1 and the CDD complex in mediating light control of development. Plant Cell 18 1991–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope, G.A., and Deshaies, R.J. (2003). COP9 signalosome: A multifunctional regulator of SCF and other cullin-based ubiquitin ligases. Cell 114 663–671. [DOI] [PubMed] [Google Scholar]
- Cope, G.A., and Deshaies, R.J. (2006). Targeted silencing of Jab1/Csn5 in human cells downregulates SCF activity through reduction of F-box protein levels. BMC Biochem. 7 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope, G.A., Suh, G.S., Aravind, L., Schwarz, S.E., Zipursky, S.L., Koonin, E.V., and Deshaies, R.J. (2002). Role of predicted metalloprotease motif of Jab1/CSN5 in cleavage of Nedd8 from Cul1. Science 298 606–611. [DOI] [PubMed] [Google Scholar]
- del Pozo, J.C., Dharmasiri, S., Hellmann, H., Walker, L., Gray, W.M., and Estelle, M. (2002). AXR1–ECR1-dependent conjugation of RUB1 to the Arabidopsis Cullin AtCUL1 is required for auxin response. Plant Cell 14 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denti, S., Fernandez Sanchez, M.E., Lars, R., and Bianchi, E. (2006). The COP9 signalosome regulates Skp2 levels and proliferation of human cells. J. Biol. Chem. 281 32188–32196. [DOI] [PubMed] [Google Scholar]
- Dharmasiri, S., Dharmasiri, N., Hellmann, H., and Estelle, M. (2003). The RUB/Nedd8 conjugation pathway is required for early development in Arabidopsis. EMBO J. 22 1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmann, E.M., Kuhnle, C., and Schwechheimer, C. (2005). Loss of the CONSTITUTIVE PHOTOMORPHOGENIC9 signalosome subunit 5 is sufficient to cause the cop/det/fus mutant phenotype in Arabidopsis. Plant Cell 17 1967–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, S., Ma, L., Wang, X., Xie, D., Dinesh-Kumar, S.P., Wei, N., and Deng, X.W. (2003). The COP9 signalosome interacts physically with SCFCOI1 and modulates jasmonate responses. Plant Cell 15 1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa, P., Gusmaroli, G., Serino, G., Habashi, J., Ma, L., Shen, Y., Feng, S., Bostick, M., Callis, J., Hellmann, H., and Deng, X.W. (2005). Arabidopsis has two redundant Cullin3 proteins that are essential for embryo development and that interact with RBX1 and BTB proteins to form multisubunit E3 ubiquitin ligase complexes in vivo. Plant Cell 17 1180–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freilich, S., Oron, E., Kapp, Y., Nevo-Caspi, Y., Orgad, S., Segal, D., and Chamovitz, D.A. (1999). The COP9 signalosome is essential for development of Drosophila melanogaster. Curr. Biol. 9 1187–1190. [DOI] [PubMed] [Google Scholar]
- Fukumoto, A., Tomoda, K., Kubota, M., Kato, J.Y., and Yoneda-Kato, N. (2005). Small Jab1-containing subcomplex is regulated in an anchorage- and cell cycle-dependent manner, which is abrogated by ras transformation. FEBS Lett. 579 1047–1054. [DOI] [PubMed] [Google Scholar]
- Gingerich, D.J., Gagne, J.M., Salter, D.W., Hellmann, H., Estelle, M., Ma, L., and Vierstra, R. (2005). Cullins 3a and 3b assemble with members of the broad complex/tramtrack/bric-a-brac (BTB) protein family to form essential ubiquitin-protein ligases (E3s) in Arabidopsis. J. Biol. Chem. 280 18810–18821. [DOI] [PubMed] [Google Scholar]
- Glickman, M.H., Rubin, D., Coux, O., Wefes, I., Pfeifer, G., Cjeka, Z., Baumeister, W., Fried, V.A., and Finley, D. (1998). A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell 94 615–623. [DOI] [PubMed] [Google Scholar]
- Gray, W.M., del Pozo, J.C., Walker, L., Hobbie, L., Risseeuw, E., Banks, T., Crosby, W.L., Yang, M., Ma, H., and Estelle, M. (1999). Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev. 13 1678–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, W.M., Hellmann, H., Dharmasiri, S., and Estelle, M. (2002). Role of the Arabidopsis RING-H2 protein RBX1 in RUB modification and SCF function. Plant Cell 14 2137–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman, R., Polanowska, J., Kuraoka, I., Sawada, J., Saijo, M., Drapkin, R., Kisselev, A.F., Tanaka, K., and Nakatani, Y. (2003). The ubiquitin ligase activity of the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell 133 357–367. [DOI] [PubMed] [Google Scholar]
- Gusmaroli, G., Feng, S., and Deng, X.W. (2004). Arabidopsis CSN5A and CSN5B subunits are present in distinct COP9 signalosome complexes, and mutations in their JAMM domains exhibit differential dominant negative effects on development. Plant Cell 16 2984–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari-Steinberg, O., and Chamovitz, D.A. (2004). The COP9 signalosome. Mediating between kinase signaling and protein degradation. Curr. Protein Pept. Sci. 5 185–189. [DOI] [PubMed] [Google Scholar]
- He, Q., Cheng, P., He, Q., and Liu, Y. (2005). The COP9 signalosome regulates the Neurospora circadian clock by controlling the stability of the SCFFWD-1 complex. Genes Dev. 19 1518–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa, L.A.A., Mihaylov, I.S., Banks, D.P., Zhneg, J., and Zhang, H. (2003). Radiation-mediated proteolyses of CDT1 by CUL4–ROC1 and CSN complexes constitutes a new checkpoint. Nat. Cell Biol. 5 1008–1015. [DOI] [PubMed] [Google Scholar]
- Hofmann, K., and Bucher, P. (1998). The PCI domain: A common theme in three multiprotein complexes. Trends Biochem. Sci. 23 204–205. [DOI] [PubMed] [Google Scholar]
- Jones, D., and Candido, E.P. (2000). The NED-8 conjugating system in Caenorhabditis elegans is required for embryogenesis and terminal differentiation of the hypodermis. Dev. Biol. 226 152–165. [DOI] [PubMed] [Google Scholar]
- Karniol, B., Malec, P., and Chamovitz, D.A. (1999). Arabidopsis FUSCA5 encodes a novel phosphoprotein that is a component of the COP9 complex. Plant Cell 11 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami, T., Chiba, T., Suzuki, T., Iwai, K., Yamanaka, K., Minato, N., Suzuki, H., Shimbara, N., Hidaka, Y., Osaka, F., Omata, M., and Tanaka, K. (2001). NEDD8 recruits E2-ubiquitin to SCF E3 ligase. EMBO J. 20 4003–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok, S.F., Solano, R., Tsuge, T., Chamovitz, D.A., Ecker, J.R., Matsui, M., and Deng, X.W. (1998). Arabidopsis homologs of a c-Jun coactivator are present both in monomeric form and in the COP9 complex, and their abundance is differentially affected by the pleiotropic cop/det/fus mutations. Plant Cell 10 1779–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok, S.F., Staub, J.M., and Deng, X.W. (1999). Characterization of two subunits of Arabidopsis 19S proteasome regulatory complex and its possible interaction with the COP9 complex. J. Mol. Biol. 285 85–95. [DOI] [PubMed] [Google Scholar]
- Larsen, P.B., and Cancel, J.D. (2004). A recessive mutation in the RUB1-conjugating enzyme, RCE1, reveals a requirement for RUB modification for control of ethylene biosynthesis and proper induction of basic chitinase and PDF1.2 in Arabidopsis. Plant J. 38 626–638. [DOI] [PubMed] [Google Scholar]
- Liu, C., Poitelea, M., Watson, A., Yoshida, S.H., Shimoda, C., Holmberg, C., Nielsen, O., and Carr, A.M. (2005). Transactivation of Schizosaccharomyces pombe cdt2(+) stimulates a Pcu4-Ddb1-CSN ubiquitin ligase. EMBO J. 24 3940–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.-C., Schiff, M., Serino, G., Deng, X.W., and Dinesh-Kumar, S.P. (2002). Role of SCF ubiquitin-ligase and the COP9 signalosome in the N gene-mediated resistance response to tobacco mosaic virus. Plant Cell 14 1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyapina, S., Cope, G., Shevchenko, A., Serino, G., Tsuge, T., Zhou, C., Wolf, D.A., Wei, N., Shevchenko, A., and Deshaies, R.J. (2001). Promotion of NEDD8–CUL1 conjugate cleavage by the COP9 signalosome. Science 292 1382–1385. [DOI] [PubMed] [Google Scholar]
- Lykke-Andersen, K., Schaefer, L., Menon, S., Deng, X.W., Boone Miller, J., and Wei, N. (2003). Disruption of the COP9 signalosome Csn2 subunit in mice causes deficient cell proliferation, accumulation of p53 and cyclin E, and early embryonic death. Mol. Cell. Biol. 23 6790–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt, K.E., Liu, C., and Carr, A.M. (2002). Deletion mutants in COP9/signalosome subunits in fission yeast Schizosaccharomyces pombe display distinct phenotypes. Mol. Biol. Cell 13 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt, K.E., Porte, J., Murray, J.M., Brikos, C., Christensen, P.U., Caspari, T., Hagan, I.M., Millar, J.B., Simanis, V., Hofmann, K., and Carr, A.M. (1999). The COP9/signalosome complex is conserved in fission yeast and has a role in S phase. Curr. Biol. 9 1427–1430. [DOI] [PubMed] [Google Scholar]
- Oron, E., Mannervik, M., Rencus, S., Harari-Steinberg, O., Neuman-Silberberg, S., Segal, D., and Chamovitz, D.A. (2002). COP9 signalosome subunits 4 and 5 regulate multiple pleiotropic pathways in Drosophila melanogaster. Development 129 4399–4409. [DOI] [PubMed] [Google Scholar]
- Osaka, F., Saeki, M., Katayama, S., Aida, N., Toh-e, A., Kominami, K., Toda, T., Suzuki, T., Chiba, T., Tanaka, K., and Kato, S. (2000). Covalent modifier NEDD8 is essential for SCF ubiquitin-ligase in fission yeast. EMBO J. 19 3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, C.Y., Lin, Y.F., Chen, Y.J., and Chien, C.T. (2002). Distinct protein degradation mechanisms mediated by Cul1 and Cul3 controlling Ci stability in Drosophila eye development. Genes Dev. 16 2403–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Z., Kentsis, A., Dias, D.C., Yamoah, K., and Wu, K. (2004). Nedd8 on cullin: Building an expressway to protein destruction. Oncogene 23 1985–1997. [DOI] [PubMed] [Google Scholar]
- Peng, Z., Serino, G., and Deng, X.W. (2001. a). Molecular characterization of subunit 6 of the COP9 signalosome and its role in multifaceted developmental processes in Arabidopsis. Plant Cell 13 2393–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Z., Serino, G., and Deng, X.W. (2001. b). A role of Arabidopsis COP9 complex in multifaceted developmental processes revealed by the characterization of its subunit 3. Development 128 4277–4288. [DOI] [PubMed] [Google Scholar]
- Peng, Z., Shen, Y., Feng, S., Wang, X., Chitteti, B.N., Vierstra, R.D., and Deng, X.W. (2003). Evidence for a physical association of the COP9 signalosome, the proteasome, and specific SCF E3 ligases in vivo. Curr. Biol. 13 504–505. [DOI] [PubMed] [Google Scholar]
- Pintard, L., Kurz, T., Glaser, S., Willis, J.H., Peter, M., and Bowerman, B. (2003). Neddylation and deneddylation of CUL3 is required to target MEI-1/Katanin for degradation at the meiosis-to-mitosis transition in C. elegans. Curr. Biol. 13 911–921. [DOI] [PubMed] [Google Scholar]
- Richardson, K.S., and Zundel, W. (2005). The emerging role of the COP9 signalosome in cancer. Mol. Cancer Res. 3 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer, C., Serino, G., Callis, J., Crosby, W.L., Lyapina, S., Deshaies, R.J., Gray, W.M., Estelle, M., and Deng, X.W. (2001). Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 292 1379–1382. [DOI] [PubMed] [Google Scholar]
- Schwechheimer, C., Serino, G., and Deng, X.W. (2002). Multiple ubiquitin ligase-mediated processes require COP9 signalosome and AXR1 function. Plant Cell 14 2553–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger, M., Kraft, R., Ferrell, K., Bech-Otschir, D., Dumdey, R., Schade, R., Gordon, C., Naumann, M., and Dubiel, W. (1998). A novel protein complex involved in signal transduction possessing similarities to 26S proteasome subunits. FASEB J. 12 469–478. [PubMed] [Google Scholar]
- Serino, G., Su, H., Peng, Z., Tsuge, T., Wei, N., Gu, H., and Deng, X.W. (2003). Characterization of the last subunit of the COP9 signalosome: Implications for the overall structure and origin of the complex. Plant Cell 15 580–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino, G., Tsuge, T., Kwok, S., Matsui, M., Wei, N., and Deng, X.W. (1999). Arabidopsis cop8 and fus4 mutations define the same gene that encodes subunit 4 of the COP9 signalosome. Plant Cell 11 1967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle, J., and Vierstra, R.D. (2004). The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 55 555–590. [DOI] [PubMed] [Google Scholar]
- Staub, J.M., Wei, N., and Deng, X.W. (1996). Evidence for FUS6 as a component of the nuclear-localized COP9 complex in Arabidopsis. Plant Cell 8 2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi, K., Omata, M., Tanaka, K., and Chiba, T. (2001). The Nedd8 system is essential for cell cycle progression and morphogenetic pathway in mice. J. Cell Biol. 155 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohmann, A., Brukhin, V., Dieterle, M., Gheyeselinck, J., Vantard, M., Grossniklaus, U., and Genschik, P. (2005). Arabidopsis CUL3A and CUL3B genes are essential for normal embryogenesis. Plant J. 43 437–448. [DOI] [PubMed] [Google Scholar]
- Tomoda, K., Kubota, Y., Arata, Y., Mori, S., Maeda, M., Tanaka, T., Yoshida, M., Yoneda-Kato, N., and Kato, J.Y. (2002). The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J. Biol. Chem. 277 2302–2310. [DOI] [PubMed] [Google Scholar]
- Tomoda, K., Yoneda-Kato, N., Fukumoto, A., Yamanaka, S., and Kato, J.Y. (2004). Multiple functions of Jab1 are required for early embryonic development and growth potential in mice. J. Biol. Chem. 279 43013–43018. [DOI] [PubMed] [Google Scholar]
- Wang, X., Feng, S., Nakayama, N., Crosby, W.L., Irish, V., Deng, X.W., and Wei, N. (2003). The COP9 signalosome interacts with SCFUFO and participates in Arabidopsis flower development. Plant Cell 15 1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Kang, D., Feng, S., Serino, G., Schwechheimer, C., and Wei, N. (2002). CSN1 N-terminal-dependent activity is required for Arabidopsis development but not for Rub1/Nedd8 deconjugation of cullins: a structure-function study of CSN1 subunit of the COP9 signalosome. Mol. Biol. Cell 13 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, H., Bernhardt, A., Dieterle, M., Hano, P., Mutlu, A., Estelle, M., Genschik, P., and Hellmann, H. (2005). Arabidopsis AtCUL3a and AtCUL3b form complexes with members of the BTB/POZ-MATH protein family. Plant Physiol. 137 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee, S., Geyer, R.K., Takashi, T., and Wolf, D.A. (2005). CSN facilitates Cullin-RING ubiquitin ligase function by counteracting autocatalytic adapter instability. Nat. Cell Biol. 7 387–391. [DOI] [PubMed] [Google Scholar]
- Wei, N., Chamovitz, D.A., and Deng, X.W. (1994. a). Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development. Cell 78 117–124. [DOI] [PubMed] [Google Scholar]
- Wei, N., and Deng, X.W. (2003). The COP9 signalosome. Annu. Rev. Cell Dev. Biol. 19 261–286. [DOI] [PubMed] [Google Scholar]
- Wei, N., Kwok, S.E., von Arnim, A.G., and Deng, X.W. (1994. b). Arabidopsis COP8, COP10 and COP11 are involved in repression of photomorphogenic development in darkness. Plant Cell 6 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, N., Tsuge, T., Serino, G., Dohmae, N., Takio, K., Matsui, M., and Deng, X.W. (1998). The COP9 complex is conserved between plants and mammals and is related to the 26S proteasome regulatory complex. Curr. Biol. 8 919–922. [DOI] [PubMed] [Google Scholar]
- Wolf, D.A., Zhou, C., and Wee, S. (2003). The COP9 signalosome: An assembly and maintenance platform for cullin ubiquitin ligases? Nat. Cell Biol. 5 1029–1033. [DOI] [PubMed] [Google Scholar]
- Wu, J.T., Chan, Y.R., and Chien, C.T. (2006). Protection of cullin-RING E3 ligases by CSN-UBP12. Trends Cell Biol. 16 362–369. [DOI] [PubMed] [Google Scholar]
- Wu, J.T., Lin, H.-C., Hu, Y.-C., and Chien, C.-T. (2005). Neddylation and deneddylation regulate Cul1 and Cul3 protein accumulation. Nat. Cell Biol. 7 1014–1020. [DOI] [PubMed] [Google Scholar]
- Xu, L., Liu, F., Lechner, E., Genschik, P., Crosby, W.L., Ma, H., Peng, W., Huang, D., and Xie, D. (2002). The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14 1919–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, J., Walz, K., Nakamura, H., Carattini-Rivera, S., Zhao, Q., Vogel, H., Wei, H., Justice, M.J., Bradley, A., and Lupski, J.R. (2003). COP9 signalosome subunit 3 is essential for maintenance of cell proliferation in the mouse embryonic epiblast. Mol. Cell. Biol. 23 6798–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., Menon, S., Lykke-Andersen, K., Tsuge, T., Xiao, D., Wang, X., Rodriguez-Suarez, R.J., Zhang, H., and Wei, N. (2002). The COP9 signalosome inhibits p27(Kip1) degradation and impedes G1-S phase progression via de-neddylation of SCF Cul1. Curr. Biol. 12 667–672. [DOI] [PubMed] [Google Scholar]
- Zhou, C., Seibert, V., Geyer, R., Rhee, E., Lyapina, S., Cope, G., Deshaies, R., and Wolf, D.A. (2001). The fission yeast COP9/signalosome is involved in cullin modification by ubiquitin-related Ned8p. BMC Biochem. 2 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C., Wee, S., Rhee, E., Naumann, M., Dubiel, W., and Wolf, D.A. (2003). Fission yeast COP9/signalosome suppresses cullin activity through recruitment of the deubiquitylating enzyme Ubp12p. Mol. Cell 11 927–938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.