Abstract
Mitogen-activated protein kinase (MAPK) signaling plays a central role in transducing extracellular stimuli into intracellular responses, but its role in mediating plant responses to herbivore attack remains largely unexplored. When Manduca sexta larvae attack their host plant, Nicotiana attenuata, the plant's wound response is reconfigured at transcriptional, phytohormonal, and defensive levels due to the introduction of oral secretions (OS) into wounds during feeding. We show that OS dramatically amplify wound-induced MAPK activity and that fatty acid–amino acid conjugates in M. sexta OS are the elicitors. Virus-induced gene silencing of salicylic acid–induced protein kinase (SIPK) and wound-induced protein kinase revealed their importance in mediating wound and OS-elicited hormonal responses and transcriptional regulation of defense-related genes. We found that after applying OS to wounds created in one portion of a leaf, SIPK is activated in both wounded and specific unwounded regions of the leaf but not in phylotactically connected adjacent leaves. We propose that M. sexta attack elicits a mobile signal that travels to nonwounded regions of the attacked leaf where it activates MAPK signaling and, thus, downstream responses; subsequently, a different signal is transported by the vascular system to systemic leaves to initiate defense responses without activating MAPKs in systemic leaves.
INTRODUCTION
Over time, plants have acquired sophisticated defense mechanisms to cope with herbivory. Plants react to herbivore attack with finely tuned transcriptional changes (Hui et al., 2003; Reymond et al., 2004), elevated phytohormone biosyntheses (Reymond and Farmer, 1998), and, finally, the production of direct and indirect defense compounds (Kessler and Baldwin, 2001; Zavala et al., 2004). In Nicotiana attenuata, the application of Manduca sexta's oral secretions (OS) and regurgitants to mechanical wounds elicits (1) jasmonic acid (JA) and ethylene bursts that are greater than those elicited by mechanical wounding (Kahl et al., 2000); (2) high levels of trypsin proteinase inhibitor (TPI), an important direct defense compound (Halitschke and Baldwin, 2003; Zavala et al., 2004); and (3) the release of volatile organic compounds (VOCs), which function as indirect defenses by attracting predators to feeding herbivores (Kessler and Baldwin, 2001).
Several lines of evidence point to the importance of fatty acid–amino acid conjugates (FACs) in herbivore OS in eliciting herbivory-specific responses (Alborn et al., 1997, 2003). Removing FACs from OS by ion-exchange chromatography abolished N. attenuata's herbivory-specific responses (i.e., cis-α-bergamotene emission, JA bursts, and extensive OS-specific transcript accumulation). Moreover, adding synthetic FACs back to FAC-free OS restored all of the OS-elicited responses, and treating wounds with aqueous FAC solutions mimicked the effects of OS (Alborn et al., 1997; Halitschke et al., 2001, 2003). These facts demonstrate that when a plant perceives FACs, it activates its antiherbivore defenses. Ethylene and, more importantly, JA have long been recognized as the main signaling molecules mediating a plant's defense system against herbivores (Creelman and Mullet, 1997; Reymond and Farmer, 1998; Halitschke and Baldwin, 2003). However, the early steps in a plant's response to herbivore attack, namely, how it senses herbivory-specific elicitors and uses signal transduction and regulatory networks to regulate defenses, are poorly understood.
The mitogen-activated protein kinase (MAPK) cascade is a conserved pathway involved in modulating a large number of cellular responses in all eukaryotes (Herskowitz, 1995; Chang and Karin, 2001; MAPK Group, 2002). MAPKs are activated by the dual phosphorylation of Thr and Tyr residues in a TXY motif located in the activation loop between subdomains VII and VIII by their upstream MAPK kinases. Subsequently, MAPKs phosphorylate their substrates, which are mainly transcription factors and which in turn trigger downstream reactions (Hill and Treisman, 1995; Karin and Hunter, 1995; Hazzalin and Mahadevan, 2002). In plants, MAPKs also play essential signaling roles, mediating responses to various stress stimuli (reviewed in Hirt, 1997; Romeis, 2001; Zhang and Klessig, 2001). A growing body of evidence has revealed that in plants, MAPKs also regulate transcription factors at both transcription and protein activity levels (Kim and Zhang, 2004; Andreasson et al., 2005; Menke et al., 2005; Yap et al., 2005), and thus potentially mediate downstream stress-related responses.
Salicylic acid–induced protein kinase (SIPK) and wound-induced protein kinase (WIPK), two tobacco (Nicotiana tabacum) MAPKs and their homologs in other plant species, are rapidly activated after various challenges, such as ozone (Samuel et al., 2000; Ahlfors et al., 2004), temperature (Jonak et al., 1996; Link et al., 2002; Sangwan et al., 2002), pathogens (Romeis et al., 1999; Asai et al., 2002; Jin et al., 2003), and osmotic stress (Droillard et al., 2000; Mikolajczyk et al., 2000). Both SIPK and WIPK are activated after wounding. Using transgenic cultivated tobacco, Seo et al. (1995, 1999) demonstrated that WIPK regulates wound-induced JA biosynthesis and is systemically activated after stems are cut. Similarly, in wounded tomato (Solanum lycopersicum) plants, a 48-kD MAPK is activated both locally and systemically (Stratmann and Ryan, 1997). MMK4, the homolog of WIPK in alfalfa (Medicago sativa), is also quickly activated after wounding (Bogre et al., 1997). Biochemical analysis has demonstrated that SIPK and MPK4 are highly activated by wounding (Zhang and Klessig, 1998a; Ichimura et al., 2000). Although MAPKs are clearly involved in the wound response, it is unknown how they are involved in responses elicited by herbivore attack, particularly in plant species such as N. attenuata, where the wound response is known to be reconfigured during herbivore attack.
Here, we demonstrate that in N. attenuata, applying M. sexta OS to puncture wounds in leaves dramatically amplifies the wound-induced increase in SIPK activity and WIPK transcripts and that FACs in M. sexta OS are the responsible elicitors. We show that both SIPK and WIPK are upstream signaling components regulating wound- and OS-elicited JA, salicylic acid (SA), JA-Ile/JA-Leu conjugate, and ethylene biosynthesis. Transcriptional analyses revealed that SIPK and WIPK mediate the wounding- and OS-elicited accumulation of many defense-related genes, including three MAPKs and four calcium-dependent protein kinases (CDPKs); moreover, they even regulate each other's transcript accumulation, highlighting the complicated transcriptional crosstalk that occurs among protein kinases. After OS elicitation, a mobile signal quickly moves to particular undamaged regions of the elicited leaf and activates MAPK signaling and downstream responses. In contrast with the signaling observed within attacked leaves, a distinct signal travels to systemic distal leaves and activates defense-related responses without activating MAPKs.
RESULTS
Herbivory Activates MAPKs in N. attenuata
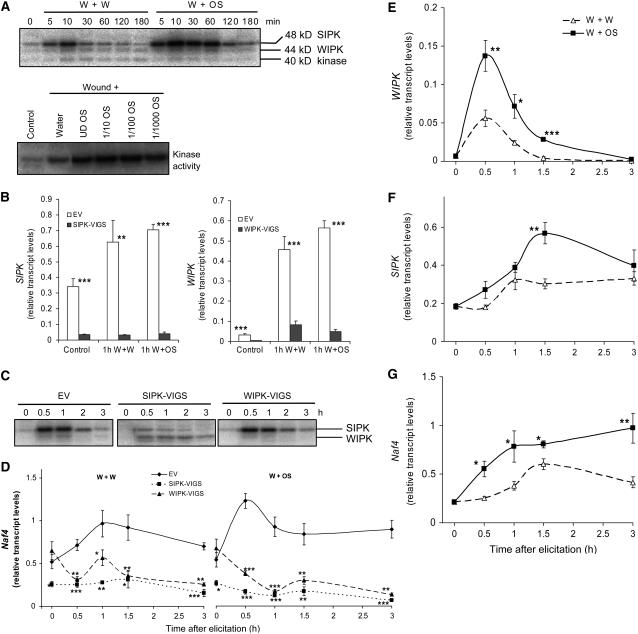
MAPKs are rapidly activated after mechanical wounding (Bogre et al., 1997; Zhang and Klessig, 1998a). To determine if MAPKs are involved in activating defense responses to herbivore attack, N. attenuata leaves were wounded with a pattern wheel; immediately thereafter puncture wounds were treated with water (W+W) or fivefold-diluted M. sexta OS (W+OS), and MAPK activity was analyzed by an in-gel kinase assay using myelin basic protein as a substrate (Figure 1A, top panel). After mechanical wounding, a 48-kD protein kinase was activated that reached a maximum at 10 min and rapidly declined to a basal level within 1 h; in comparison, leaves wounded with W+OS dramatically increased the activity of 48-kD kinase within 10 min and sustained elevated levels for 2 h. The in-gel activity assay also showed that a 44-kD kinase, although characterized by significantly less activity than the 48-kD protein kinase, was also activated, but this activity was not enhanced by the W+OS treatment. An unidentified 40-kD protein kinase having rather low levels of in-gel kinase activity was also activated 10 min after both W+W and W+OS treatments. Notably, W+W-treated plants showed higher activity levels of 40-kD protein kinase for as long as 2 h than did W+OS-treated plants. Schittko et al. (2000) demonstrated that applying highly diluted M. sexta OS (up to 1000-fold) to N. attenuata elicits the same level of JA burst as using undiluted OS. We also tested the ability of diluted OS to activate MAPKs (Figure 1A, bottom panel). Consistent with the JA analysis (Schittko et al., 2000), the in-gel kinase activity assay showed that even 1000-fold–diluted OS significantly increased the levels of SIPK activity above those of the W+W treatment.
Figure 1.
Applying M. sexta OS to N. attenuata Leaves Activates MAPKs.
(A) Top panel: N. attenuata leaves were wounded with a pattern wheel; 20 μL of water (W+W) or M. sexta OS (1/5-diluted) (W+OS) was applied to the wounds, and leaves from four replicate plants were harvested at the indicated times. Bottom panel: 20 μL of water, undiluted OS (UD OS), 1/10-, 1/100-, and 1/1000-diluted OS was applied to wounds, and leaves from four replicate plants were harvested after 30 min. Kinase activity was analyzed by an in-gel kinase assay using myelin basic protein (MBP) as the substrate.
(B) N. attenuata plants were infiltrated with Agrobacterium carrying pTV00 EV or constructs harboring a fragment of SIPK or WIPK to generate EV, SIPK-VIGS, and WIPK-VIGS plants, respectively. Mean (+se) levels of SIPK and WIPK transcripts in SIPK-VIGS and WIPK-VIGS plants were measured with q-PCR using five replicate untreated (control) and 1 h W+W- and W+OS-treated samples.
(C) EV, SIPK-VIGS, and WIPK-VIGS plants were treated with W+OS and collected at indicated times; five replicate samples were pooled and MAPK activity was detected with an in-gel kinase assay.
(D) Mean transcript levels (±se) of Naf4 in EV, SIPK-VIGS, and WIPK-VIGS plants after W+W and W+OS treatments as measured with q-PCR. Asterisks represent significantly different transcript levels between EV and VIGS plants at the indicated times (n = 5, two-way analysis of variance [ANOVA], Fisher's protected least-square difference (PLSD); *, P < 0.05; **, P < 0.01; ***, P < 0.001).
(E) to (G) Mean transcript levels (±se) of WIPK, SIPK, and Naf4 after W+W and W+OS treatments as measured with q-PCR in wild-type N. attenuata. Asterisks represent significant differences between transcript levels in samples treated with W+OS and W+W at the indicated times (n = 5, unpaired t test; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
Since the SIPK and WIPK from cultivated tobacco (Seo et al., 1995; Zhang and Klessig, 1998a) are also 48- and 44-kD kinases, we silenced transcript levels of N. attenuata's SIPK and WIPK by virus-induced gene silencing (VIGS) to confirm their identities (Ratcliff et al., 2001; Saedler and Baldwin, 2004). Agrobacterium tumefaciens was transformed with pTV-SIPK and pTV-WIPK constructs carrying cDNA fragments of N. attenuata's SIPK and WIPK. Although these two fragments share a certain homology, the longest perfect match is 16 nucleotides long. Given that a 22- or 23-nucleotide perfect match is the minimum size capable of initiating RNA interference (RNAi) (Thomas et al., 2001; Xu et al., 2006), neither construct had a cosilencing effect on the other's transcripts. Young N. attenuata leaves were inoculated with these construct-transformed Agrobacterium, generating SIPK-silenced (SIPK-VIGS) and WIPK-silenced (WIPK-VIGS) plants, respectively; plants inoculated with Agrobacterium, which carried empty vector (EV) pTV00, were used for comparison. No morphological differences associated with SIPK or WIPK silencing were observed. Eighteen days after inoculation, transcript levels of SIPK and WIPK were reduced by ∼90 and 80%, respectively (Figure 1B). An in-gel kinase assay was performed to examine activity levels in various kinase-silenced plants (Figure 1C). Compared with EV plants, SIPK-VIGS plants had greatly reduced 48-kD protein kinase levels; the 44-kD kinase bands apparent in EV plants were not detected in WIPK-VIGS plants, indicating that the 48- and 44-kD protein kinases are SIPK and WIPK, respectively. Repeated kinase assays showed the same results (data not shown). Notably, levels of WIPK activity were higher in SIPK-silenced plants than in EV plants. This increase was likely a result of decreased competition between SIPK and WIPK for their common upstream kinase, MEK2 (Yang et al., 2001), which was also demonstrated in cultivated tobacco plants: after ozone exposure, SIPK-silenced plants had higher levels of WIPK activity than did wild-type plants, a difference that could be attributed to decreased competition for the binding to MEK2 (Samuel and Ellis, 2002).
A recent study of the cultivated tobacco Ntf4 gene, a MAPK gene having high homology to SIPK, indicated that Ntf4 is also involved in stress responses (Ren et al., 2006). The VIGS construct used for silencing SIPK contained a 35-nucleotide perfect match with Naf4, the homolog of Ntf4 in N. attenuata. Reportedly, a match as small as 22 or 23 nucleotides can trigger RNAi (Thomas et al., 2001; Xu et al., 2006). We determined whether levels of Naf4 transcripts in SIPK-VIGS plants differed from those in EV plants (Figure 1D). Even in untreated SIPK-VIGS plants, transcript levels of Naf4 were 50% lower than in EV plants, and even after both treatments, Naf4 transcript levels remained several times lower than those in EV plants. This could have resulted from the cosilencing of Naf4 by the pTV-SIPK construct. Strikingly, although levels of Naf4 transcripts in untreated WIPK-VIGS plants did not differ from those in untreated EV plants, levels of Naf4 transcripts in WIPK-VIGS plants were lower than those in EV plants in both treatments (Figure 1D). It is unlikely that silencing WIPK cosilenced Naf4, given that pTV-WIPK and Naf4 sequences have no matches longer than 20 nucleotides (Thomas et al., 2001; Xu et al., 2006).
Wounding is known to elevate WIPK transcript levels in cultivated tobacco (Seo et al., 1995; Zhang and Klessig, 1998a). In N. attenuata, 0.5 h after wounding, WIPK transcript levels were at their highest, and when OS were added to wounds, this wound-induced maximum increased 1.5-fold (P = 0.01, unpaired t test) (Figure 1E). Quantitative RT-PCR (q-PCR) measurement also showed that 1.5 h after W+W treatment, SIPK transcript levels were slightly elevated (P = 0.011, unpaired t test) compared with those in untreated plants. W+OS increased SIPK transcript levels twofold after 1.5 h (P = 0.0018, unpaired t test); afterward, these levels gradually decreased to those found in W+W-treated plants (Figure 1F). We also measured transcript levels of Naf4 after W+W and W+OS treatments (Figure 1G). Both treatments enhanced the levels of Naf4 transcripts: 1.5 h after W+W treatment, a significant increase in the transcript levels of Naf4 was detected (P = 0.0083, unpaired t test), and treatment with W+OS elicited even higher levels, which lasted 3 h. These data demonstrate that SIPK, WIPK, and Naf4 are involved in this plant–herbivore interaction.
SIPK and WIPK Regulate Transcript Levels of Three WRKY Transcription Factors
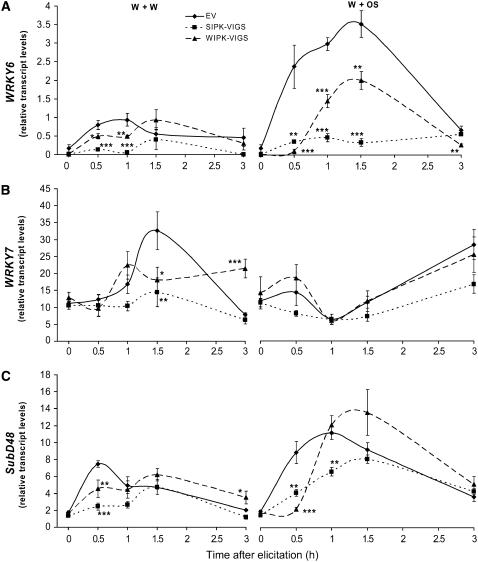
WRKYs are an important family of transcription factors in plants that modulate both developmental and defense responses (Eulgem et al., 2000; Ulker and Somssich, 2004). MAPKs are known to be upstream regulators modulating the transcript accumulation of WRKY transcription factors (Kim and Zhang, 2004). In N. attenuata, we have identified WRKY6 as playing a central role in orchestrating FAC- and OS-elicited responses (N. Qu and I.T. Baldwin, unpublished data). Here, we determine whether OS-elicited MAPKs regulate the transcript levels of WRKY6 and of two additional N. attenuata WRKYs: WRKY7 and SubD48 (Figures 2A to 2C). In EV plants, OS elicitation increased WRKY6 and SubD48 transcript levels, which peaked after 1.5 h, but tended to suppress the wounding-induced increases in WRKY7 transcripts. W+OS-treated SIPK-VIGS and WIPK-VIGS plants had significantly attenuated WRKY6 and SubD48 levels. Transcript levels of WRKY6, which are known to be FAC-elicited (Qu and I.T. Baldwin, unpublished data), were greatly reduced in SIPK-VIGS and WIPK-VIGS plants; after 1.5 h, their levels were 10 and 50%, respectively, of the levels in EV plants. Similar reductions were found when these plants were treated with W+W, but the responses were smaller than with OS elicitation. Interestingly, in WIPK-VIGS plants, transcript levels tended to rebound after the initial suppression of the elicited responses, eventually attaining values higher (among WRKY7 and SubD48 in W+W-treated plants) than those found in EV plants (Figure 2).
Figure 2.
SIPK and WIPK Regulate Transcript Accumulation of WRKY Genes.
N. attenuata plants were inoculated with Agrobacterium carrying pTV00 EV or constructs harboring a fragment of SIPK or WIPK to generate EV, SIPK-VIGS, and WIPK-VIGS plants, respectively. Leaves were wounded with a pattern wheel; 20 μL of either water (W+W) or M. sexta OS and regurgitants (W+OS) was applied to the wounds and harvested at the indicated times. Mean levels (±se) from five replicate plants of WRKY6 (A), WRKY7 (B), and SubD48 (C) transcripts in EV, SIPK-VIGS, and WIPK-VIGS plants after W+W and W+OS treatments were measured with q-PCR. Asterisks represent significantly different transcript levels between EV and SIPK-VIGS or WIPK-VIGS plants after the indicated times (n = 5, two-way ANOVA, Fisher's PLSD; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
SIPK and WIPK Mediate Transcript Accumulation of Three MAPKs and Four CDPKs
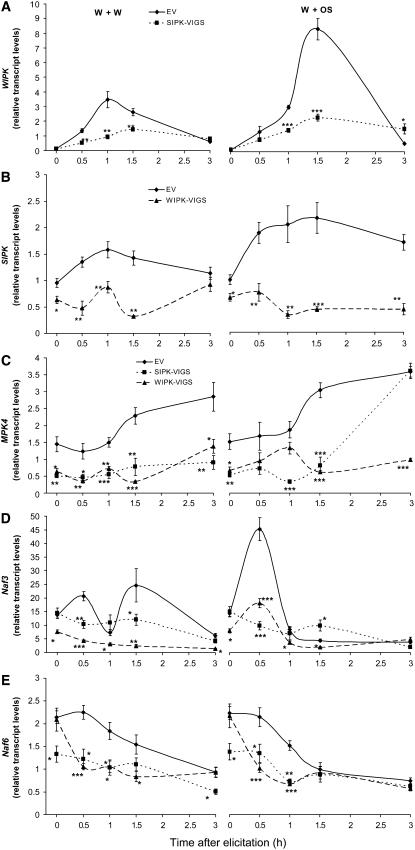
Liu et al. (2003) have elegantly demonstrated that SIPK mediates pathogen-elicited increases in WIPK transcript levels in cultivated tobacco. To determine if OS-elicited WIPK transcript levels are also mediated by SIPK, we measured transcript levels of WIPK in SIPK-silenced plants (Figure 3A). Untreated SIPK-VIGS plants had similar WIPK transcript levels compared with EV plants, and transcript levels in EV plants increased almost 20 and 80 times 1.5 h after W+W and W+OS treatments, respectively. In SIPK-VIGS plants, WIPK transcript levels were only ∼50 and 25% of those in EV plants after W+W and W+OS treatments. We conclude that OS-elicited activation of SIPK enhances levels of WIPK transcript in N. attenuata. SIPK transcript levels in WIPK-VIGS plants were significantly reduced compared with EV plants, even when untreated; both treatments slightly increased SIPK transcript levels in EV plants but not in WIPK-VIGS plants (Figure 3B).
Figure 3.
SIPK and WIPK Regulate Transcript Accumulation of MAPKs.
N. attenuata plants were inoculated with Agrobacterium carrying pTV00 EV or constructs harboring a fragment of SIPK or WIPK to generate EV, SIPK-VIGS, and WIPK-VIGS plants, respectively. Leaves were wounded with a pattern wheel; 20 μL of either water (W+W) or M. sexta OS (W+OS) was applied to the wounds. Individual leaves from five replicate plants were harvested at the indicated times. Asterisks represent significantly different transcript levels between EV and VIGS plants at the indicated times ([A] and [B], unpaired t test; [C] to [E], two-way ANOVA, Fisher's PLSD; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
(A) Mean WIPK transcript levels (±se) in EV and SIPK-VIGS plants after W+W and W+OS treatments as measured with q-PCR.
(B) Mean transcript levels (±se) of SIPK in EV and WIPK-VIGS plants after W+W and W+OS treatments as measured with q-PCR.
(C) to (E) Mean transcript levels (±se) of MPK4, Naf3, and Naf6 in EV, SIPK-VIGS, and WIPK-VIGS plants after W+W and W+OS treatments, respectively, as measured with q-PCR.
To determine if other MAPKs are also transcriptionally regulated by SIPK and WIPK, transcript levels of N. attenuata MPK4, a homolog of Arabidopsis thaliana MPK4, were measured with q-PCR (Figure 3C). Levels of MPK4 transcripts were about three times as high in untreated EV plants as in untreated SIPK- and WIPK-VIGS plants. Both W+W and W+OS treatments increased MPK4 transcript levels in EV plants but not in SIPK- and WIPK-VIGS plants, except that 3 h after OS elicitation, transcript levels in SIPK-VIGS rebounded to those in EV plants.
Two N. attenuata MAPKs, Naf3 and Naf6, which are orthologous to Ntf3 and Ntf6 of cultivated tobacco, were cloned and their transcript levels measured (Figures 3D and 3E). In EV plants, Naf3 levels increased significantly 0.5 h after W+W treatment (P = 0.010, unpaired t test) and then quickly declined before rebounding by 1.5 h. OS treatment dramatically amplified the wound-induced 0.5 h peak in Naf3 transcript levels, which rapidly declined. When untreated, EV and SIPK-VIGS plants showed similar levels of Naf3 transcript, while levels in WIPK-VIGS plants were significantly reduced. After W+W treatment, lower levels of Naf3 transcripts were detected in SIPK-VIGS and WIPK-VIGS plants compared with EV plants, except after 1 h when Naf3 levels in EV plants transiently declined. W+OS-treated WIPK-VIGS plants had lower levels of Naf3 transcripts than did EV plants, except after 3 h; levels in SIPK-VIGS plants were lower than in EV plants only at 0.5 h and significantly higher at 1.5 h.
In contrast with the response in Naf3 transcripts, Naf6 transcripts were not elevated. W+W and W+OS treatments gradually decreased Naf6 transcript levels in EV plants. When untreated, SIPK-VIGS plants had significantly lower levels of Naf6 transcripts, but WIPK-VIGS and EV plants had the same. Following W+W treatment, SIPK-VIGS and WIPK-VIGS plants had lower levels of Naf6 transcripts than did EV plants before 3 h, when SIPK-VIGS plants still had low levels, but levels in WIPK-VIGS plants returned to those found in EV plants. W+OS treatment significantly reduced levels in both SIPK-VIGS and WIPK-VIGS plants for up to 1 h.
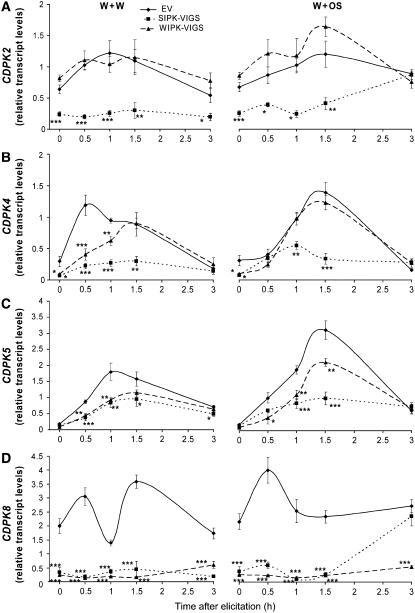
In addition to MAPKs, CDPKs are also known to be involved in plant development and defense reactions (Romeis et al., 2000, 2001; Ivashuta et al., 2005; Yoon et al., 2006). CDPKs are largely plant-specific, calcium-dependent, and calmodulin-independent protein kinases (reviewed in Cheng et al., 2002). We cloned CDPK2, CDPK4, CDPK5, and CDPK8 from N. attenuata and measured the transcript accumulation profiles of these genes in EV, SIPK-VIGS, and WIPK-VIGS plants.
While CDPK2 transcript levels were strongly suppressed in SIPK-VIGS plants (to 40% of those in EV plants), levels in WIPK-VIGS plants did not differ significantly from those in EV controls (Figure 4A). After W+W treatment, CDPK2 transcripts in SIPK-VIGS plants remained low throughout the 3-h time course, but in OS-treated plants, levels returned to those found in EV plants by 3 h.
Figure 4.
SIPK and WIPK Mediate Transcript Accumulation of CDPKs.
N. attenuata plants were inoculated with Agrobacterium carrying pTV00 EV or constructs harboring a fragment of SIPK or WIPK to generate EV, SIPK-VIGS, and WIPK-VIGS plants, respectively. Leaves were wounded with a pattern wheel; 20 μL of either water (W+W) or M. sexta OS (W+OS) was applied to the wounds. Individual leaves from five replicate plants were harvested at the indicated times. Mean (±se) transcript levels in five replicate plants of CDPK2 (A), CDPK4 (B), CDPK5 (C), and CDPK8 (D) in EV, SIPK-VIGS, and WIPK-VIGS plants after W+W and W+OS treatments were measured with q-PCR. Asterisks represent significantly different transcript levels between EV and SIPK-VIGS or WIPK-VIGS plants after the indicated times (two-way ANOVA, Fisher's PLSD; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
In EV plants, OS elicitation increased levels of CDPK4 transcripts; these reached maximum levels after 1.5 h, whereas in W+W-treated plants, levels of CDPK4 transcripts peaked as early as 0.5 h and after 1.5 h attained similar levels to those in OS-elicited plants (Figure 4B). Compared with EV plants, untreated SIPK-VIGS and WIPK-VIGS plants showed significantly lower levels of CDPK4 transcripts. CDPK4 transcript levels were lower in SIPK-VIGS plants than in EV plants for up to 3 h after both treatments. WIPK-VIGS plants, by contrast, showed a different profile: following W+W treatment, their levels of CDPK4 transcripts were lower than those of EV plants before 1.5 h, but no difference was observed thereafter; however, in W+OS-treated WIPK-VIGS and in EV plants, levels of CDPK4 transcripts were similar as early as 0.5 h.
We also measured the transcript accumulation profiles of CDPK5 (Figure 4C). After both treatments, EV plants dramatically increased the levels of CDPK5 transcripts; W+OS treatment in particular led to an almost 20-fold increase after 1.5 h, while W+W treatment resulted in an almost 10-fold increase after 1 h. When untreated, both SIPK-VIGS and WIPK-VIGS plants showed similar levels of CDPK5 transcripts compared with those in EV plants. However, after either elicitation, levels of CDPK5 transcripts in both SIPK-VIGS and WIPK-VIGS plants were significantly lower than in EV plants for up to 3 h. After this time, CDPK5 transcript levels were similar in all plants, except that W+W-treated SIPK-VIGS plants still showed slightly but significantly lower levels of CDPK5 transcripts.
Similarly, substantially lower levels of CDPK8 transcripts before and after either treatment were detected in SIPK-VIGS and WIPK-VIGS plants, compared with transcript levels in EV plants (Figure 4D). Following W+W treatment, EV plants had increased CDPK8 transcript levels after 0.5 and 1.5 h, whereas after W+OS treatment, levels of transcripts significantly increased only after 0.5 h (P = 0.0092, unpaired t test) and then quickly declined, as was seen in Naf3 transcripts following the same treatment (Figure 3D). CDPK8 transcript levels in SIPK-VIGS and WIPK-VIGS plants were significantly suppressed throughout the time course, except that 3 h after W+OS treatment, CDPK8 transcript levels in SIPK-VIGS plants rebounded to those in EV plants.
In summary, both SIPK and WIPK regulate the transcript levels of MPK4, Naf3, Naf6, CDPK4, CDPK5, and CDPK8. The transcript level of CDPK2 is mediated by SIPK but not WIPK. Furthermore, SIPK and WIPK regulate each other's transcript levels.
Silencing SIPK and WIPK Attenuates OS-Elicited JA, SA, JA-Ile/JA-Leu, and Ethylene Biosynthesis
JA, SA, JA-Ile, and ethylene are known to regulate a large number of genes related to plant defense responses (Dong, 1998; Reymond and Farmer, 1998; Farmer et al., 2003; Kang et al., 2006). Several studies have described an association between MAPK activation and stress-related phytohormone biosynthesis (Seo et al., 1995, 1999; Yang et al., 2001; Kim et al., 2003; Kim and Zhang, 2004). To determine whether wounding- and OS-activated MAPKs mediate the biosyntheses of wounding- and OS-elicited phytohormones, we measured the accumulation of JA, SA, JA-Ile/JA-Leu, and ethylene in EV and SIPK-VIGS and WIPK-VIGS plants.
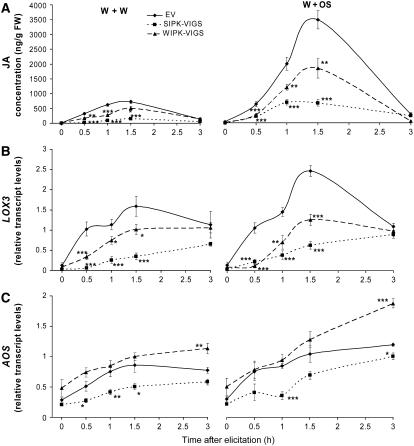
W+OS treatment elicited higher JA levels than did W+W, and these were remarkably reduced in SIPK-VIGS and WIPK-VIGS plants compared with EV plants. After either treatment in SIPK-VIGS plants, JA levels in particular were reduced by almost 80%; by contrast, JA levels in WIPK-VIGS plants were reduced by only ∼40% (Figure 5A). In N. attenuata, LIPOXYGENASE3 (LOX3) supplies the fatty acid hydroperoxides required for JA biosynthesis. The patterns of LOX3 transcript accumulation were largely consistent with those of JA accumulation: levels of LOX3 transcript were dramatically lower in both SIPK-VIGS and WIPK-VIGS plants compared with EV plants, with SIPK-VIGS plants showing the greater reductions (Figure 5B). Transcripts of Allene Oxide Synthase (AOS), which is involved in a later step in JA synthesis, responded more gradually to elicitation. SIPK-VIGS plants had significantly lower levels of AOS transcripts than did EV plants, but levels in WIPK-VIGS plants were the same as those in EV plants or higher at 3 h (Figure 5C).
Figure 5.
SIPK and WIPK Mediate Levels of JA and Transcript Accumulation of JA Biosynthetic Genes.
N. attenuata plants were inoculated with Agrobacterium carrying pTV00 EV or constructs harboring a fragment of SIPK or WIPK to generate EV, SIPK-VIGS, and WIPK-VIGS plants, respectively. Leaves were wounded with a pattern wheel; 20 μL of either water (W+W) or M. sexta OS (W+OS) was applied to the wounds. Individual leaves from five replicate plants were harvested at the indicated times. Asterisks represent significantly different levels between EV and SIPK-VIGS or WIPK-VIGS plants after the indicated times (two-way ANOVA, Fisher's PLSD; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
(A) Mean (±se) JA concentrations were measured using HPLC–tandem mass spectrometry (MS/MS). FW, fresh weight.
(B) and (C) Mean transcript levels (±se) of LOX3 and AOS as measured with q-PCR.
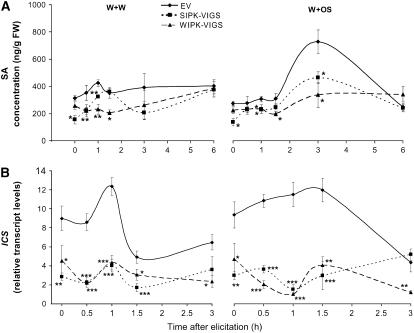
Seo et al. (1999) demonstrated that WIPK of cultivated tobacco is involved in wound-induced SA accumulation. To determine if this is also the case in N. attenuata, we measured SA levels in SIPK-VIGS, WIPK-VIGS, and EV plants. When untreated, SIPK-VIGS plants showed significantly lower levels of SA than did EV plants, whereas WIPK-VIGS plants and EV plants had similar levels (Figure 6A). One hour after W+W treatment, EV plants had slightly increased SA levels, but these quickly decreased to basal levels. Both SIPK- and WIPK-VIGS plants had lower levels of SA than did EV plants up to 1 h; after that, levels of SA in both plants gradually reached the same as those in EV plants. W+OS elicitation dramatically increased SA levels by the 3 h harvest in EV plants, but this increase was suppressed in SIPK- and WIPK-VIGS plants. Notably, W+W and W+OS treatments didn't elicit significantly different SA levels in EV plants for up to 1.5 h (P > 0.12, unpaired t test). Isochorismate synthase (ICS) is one of the key enzymes involved in SA biosynthesis. Following either treatment, levels of ICS transcripts were lower in SIPK- and WIPK-VIGS plants compared with those of EV plants, except at the 3 h harvest, when levels of ICS transcripts in SIPK-VIGS plants rebounded to those in EV plants (Figure 6B).
Figure 6.
SIPK and WIPK Mediate Levels of SA and Transcript Levels of ICS.
N. attenuata plants were inoculated with Agrobacterium carrying pTV00 EV or constructs harboring a fragment of SIPK or WIPK to generate EV, SIPK-VIGS, and WIPK-VIGS plants, respectively. Leaves were wounded with a pattern wheel; 20 μL of either water (W+W) or M. sexta OS (W+OS) was applied to the wounds. Individual leaves from five replicate plants were harvested at the indicated times after elicitation. Asterisks represent significantly different levels between EV and SIPK-VIGS or WIPK-VIGS plants at the indicated times (two-way ANOVA, Fisher's PLSD; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
(A) Mean (±se) SA concentrations were measured using HPLC-MS/MS.
(B) Mean transcript levels (±se) of ICS in EV, SIPK-VIGS, and WIPK-VIGS plants after W+W and W+OS treatments were measured with q-PCR.
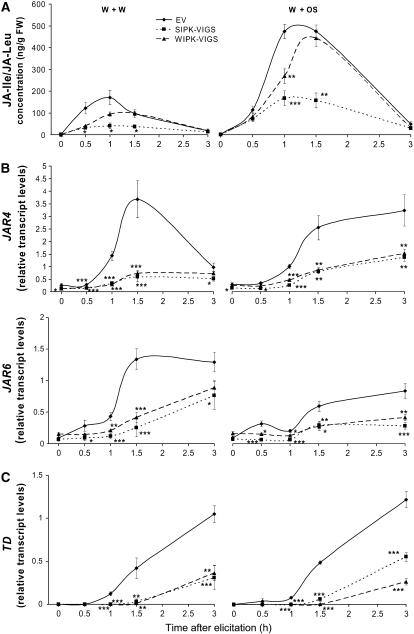
JA-Ile is emerging as an important signal compound activating defense responses to herbivores (Staswick and Tiryaki, 2004; Kang et al., 2006). The elicited dynamics in JA-Ile/JA-Leu levels (Figure 7A) closely followed those of JA (Figure 6A), with the exception that JA-Ile/JA-Leu levels were not as reduced as the JA levels in WIPK-VIGS plants. In N. attenuata, JASMONATE RESISTANT4 (JAR4) and JAR6 are enzymes conjugating JA, Ile, and Leu to form JA-Ile and JA-Leu (Kang et al., 2006; Wang et al., 2007). q-PCR analyses revealed that transcripts of both genes were strongly elicited by either treatment in EV plants; SIPK-VIGS and WIPK-VIGS plants had significantly lower levels of JAR4 and JAR6 transcripts than did EV plants (Figure 7B). Although higher levels of JA-Ile/JA-Leu were found in WIPK-VIGS plants than in SIPK-VIGS plants after either elicitation, both had the same levels of JAR4 and JAR6 transcripts. A THREONINE DEAMINASE (TD) gene in N. attenuata also plays important roles in JA-Ile biosynthesis, converting Thr to Ile at the wound site and thus supplying the amino acid for this conjugation reaction (Kang et al., 2006). After either treatment, TD transcripts were dramatically increased in EV plants, and these increases were suppressed in SIPK-VIGS and WIPK-VIGS plants (Figure 7C). These data suggest that both SIPK and WIPK regulate the levels of JAR4, JAR6, and TD transcripts.
Figure 7.
SIPK and WIPK Mediate Levels of JA-Ile/JA-Leu and Transcript Levels of Genes Involved in JA-Ile/JA-Leu Biosynthesis.
N. attenuata plants were inoculated with Agrobacterium carrying pTV00 EV or constructs harboring a fragment of SIPK or WIPK to generate EV, SIPK-VIGS, and WIPK-VIGS plants, respectively. Leaves were wounded with a pattern wheel; 20 μL of either water (W+W) or M. sexta OS (W+OS) was applied to the wounds. Individual leaves from five replicate plants were harvested at the indicated times after elicitation. Asterisks represent significantly different transcript levels between EV and SIPK-VIGS or WIPK-VIGS plants at the indicated times (two-way ANOVA, Fisher's PLSD; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
(A) Mean (±se) JA-Ile/JA-Leu concentrations were measured using HPLC-MS/MS.
(B) and (C) Mean transcript levels (±se) of JAR4, JAR6, and TD in EV, SIPK-VIGS, and WIPK-VIGS plants after W+W and W+OS treatments as measured with q-PCR.
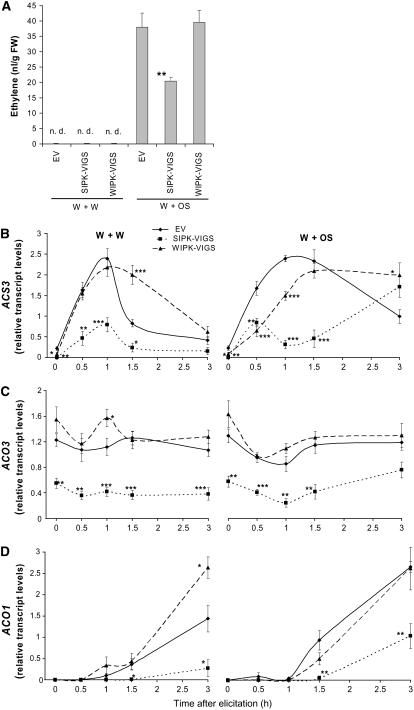
M. sexta herbivory and OS elicitation, but not wounding, increase ethylene biosynthesis and emissions in N. attenuata (Kahl et al., 2000). To determine if OS-elicited ethylene production correlates with the activation of MAPKs, we analyzed ethylene production in SIPK-VIGS, WIPK-VIGS, and EV plants. Leaves cut from petioles and subsequently treated with W+W or W+OS were sealed in 250-mL flasks for 5 h; the accumulated ethylene was measured with a photoacoustic laser spectrometer (Figure 8A). No detectable amount of ethylene was observed in any plant treated with W+W. After W+OS treatment, both EV and WIPK-VIGS plants accumulated similar levels of ethylene, but SIPK-VIGS plants only accumulated about half as much, indicating that SIPK, but not WIPK, plays an important role in regulating ethylene biosynthesis after herbivory. In cultivated tobacco, elegant gain-of-function analyses have demonstrated that SIPK/WIPK activation mediates transcript accumulations of ACC SYNTHASE (ACS) and ACC Oxidase (ACO) genes (Kim et al., 2003). In EV plants, both treatments strongly increased levels of ACS3 to a similar degree; although OS treatment amplified the wound response for ACO1 transcripts, ACO3 transcripts did not respond (Figures 8B to 8D). When untreated, both WIPK-VIGS and SIPK-VIGS plants had lower levels of ACS3 transcripts (Figure 8B), and SIPK-VIGS plants had lower ACO3 transcripts (Figure 8C) than did EV plants. After both treatments, SIPK-VIGS plants had significantly suppressed transcript levels of both ACO genes for the duration of the time course, and for the ACS3 transcript, suppressed levels were found at all harvests with the exception of the 3 h harvest, when levels returned to those in EV plants. By contrast, WIPK-VIGS plants had only transiently lower ACS3 transcript levels after OS elicitation (Figure 8B); all other ethylene biosynthetic transcripts were at levels comparable to (for W+OS elicitations) or higher than (for W+W elicitations) those found in EV plants. For example, ACO1 transcript levels in WIPK-VIGS plants were 1.8 times higher 3 h after wounding than those in EV plants (P = 0.024, unpaired t test), but OS elicitation abolished the difference between EV and WIPK-VIGS plants (Figure 8D). These data indicate that both SIPK and WIPK positively regulate OS-elicited JA, SA, and JA-Ile; moreover, SIPK positively regulates ethylene biosynthesis.
Figure 8.
SIPK but Not WIPK Mediates Ethylene Biosynthesis.
N. attenuata plants were infiltrated with Agrobacterium carrying pTV00 EV or constructs harboring a fragment of SIPK or WIPK to generate EV, SIPK-VIGS, and WIPK-VIGS plants, respectively. Asterisks represent significant differences between EV and SIPK-VIGS or WIPK-VIGS plants (two-way ANOVA, Fisher's PLSD; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
(A) Mean (±se) ethylene accumulated. Leaves were wounded with a pattern wheel; 20 μL of water (W+W) or M. sexta OS (W+OS) was applied to the wounds, and leaf samples were collected in 250-mL flasks. After 5 h, ethylene produced in four replicates was measured with a photoacoustic laser spectrometer. n.d., not detected.
(B) to (D) Leaves were wounded with a pattern wheel; 20 μL of water (W+W) or M. sexta OS (W+OS) was applied to the wounds. Individual leaf samples from five replicate plants were harvested at indicated times. Average transcript levels (±se) of ACS3, ACO3, and ACO1 in EV, SIPK-VIGS, and WIPK-VIGS plants after W+W and W+OS treatments were measured with q-PCR.
JA and Ethylene Signaling Do Not Mediate OS-Elicited MAPK Activity
Research with cell suspension cultures and in-gel kinase assays has shown that treatment with methyl jasmonate (MeJA) or ethylene activates kinases (Kumar and Klessig, 2000; Ouaked et al., 2003). To determine whether this is also the case with intact plants, we analyzed kinase activity and WIPK transcript accumulation in N. attenuata plants that had been treated with MeJA and ethephon; these readily diffuse into plants to release JA and ethylene, respectively. Up to 1 h after treatment, slightly increased levels of WIPK transcripts and SIPK activity were detected in MeJA- and ethephon-treated plants; however, these levels were not higher than those in lanolin- and MES-treated plants, their respective controls. These increased levels are likely to have resulted from the plants being touched when these chemicals were applied. Even after 6 h, MeJA and ethephon treatments didn't elevate SIPK or WIPK activity or WIPK transcript levels more than their respective controls (see Supplemental Figure 1A online), although applying MeJA dramatically elicited the JA-responsive gene TPI, and ethephon effectively released a large quantity of ethylene as measured by a photoacoustic spectrometer (see Supplemental Figures 1A and 2 online). MAPK activity was also analyzed in transgenic plants impaired in their JA and ethylene signaling. We used an antisense-LOX3 line (asLOX3) that elicited only 50% of the JA produced by W+OS elicitation in wild-type N. attenuata plants (Halitschke and Baldwin, 2003), and a COI1-silenced line (irCOI1), whose ability to perceive JA was remarkably reduced (Paschold et al., 2007). To study the impact of ethylene production and perception on MAPK activation, we used an ACO-silenced line (irACO) of N. attenuata, which produced substantially less ethylene after OS elicitation than wild-type plants did, and a line overexpressing a mutant Arabidopsis ETR1 gene, whose ethylene perception was highly attenuated (ETR1) (von Dahl et al., 2007). After these transgenic plants were challenged with W+OS, in-gel kinase activity assays revealed that their levels of MAPK activity were the same as those of wild-type plants (see Supplemental Figure 1B online). All these data suggest that JA and ethylene signaling do not mediate OS-elicited MAPK activity levels in N. attenuata plants.
Silencing SIPK and WIPK Leads to Decreased Levels of TPI Activity and Phe Ammonia-Lyase mRNA
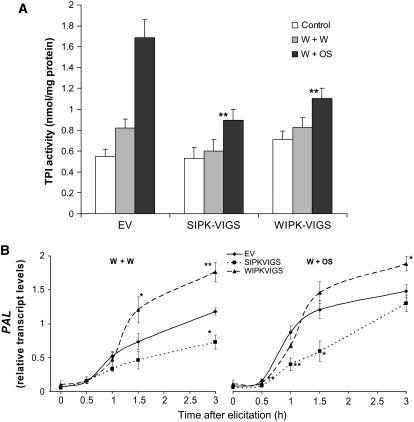
TPI is an antidigestive protein that is strongly OS-elicited in N. attenuata and functions as a direct defense by slowing M. sexta growth and increasing its mortality (Ryan, 1990; Glawe et al., 2003; Zavala et al., 2004). Knowing that JA and ethylene strongly modulate TPI levels in numerous Solanaceae taxa (O'Donnell et al., 1996; Koiwa et al., 1997; Halitschke and Baldwin, 2003) and that silencing MAPK expression attenuates OS-elicited JA and ethylene led us to hypothesize that TPIs would be impaired in SIPK- and WIPK-VIGS plants. W+OS-treated EV plants significantly increased TPI levels compared with W+W and untreated EV plants. As predicted, W+OS-treated SIPK-VIGS and WIPK-VIGS plants showed only ∼50% of the TPI activity found in W+OS-treated EV plants, but no significant differences were found among any control or W+W-treated plants (Figure 9A).
Figure 9.
SIPK and WIPK Mediate Levels of TPI Activity and Transcript Levels of PAL.
N. attenuata plants were inoculated with Agrobacterium carrying pTV00 EV or constructs harboring a fragment of SIPK or WIPK to generate EV, SIPK-VIGS, and WIPK-VIGS plants, respectively. Asterisks represent significant differences between EV and SIPK-VIGS or WIPK-VIGS plants after the specific treatments (n = 5, two-way ANOVA, Fisher's PLSD; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
(A) Mean (+se) TPI activity in EV, SIPK-VIGS, and WIPK-VIGS plants. Leaves were wounded with a pattern wheel; 20 μL of either water (W+W) or M. sexta OS (W+OS) was applied to the wounds, and leaves from five replicate plants were harvested individually 3 d after treatments. Untreated plants served as controls.
(B) Mean transcript levels (±se) of PAL in EV, SIPK-VIGS, and WIPK-VIGS plants after W+W and W+OS treatments as measured with q-PCR. Leaves were wounded with a pattern wheel; 20 μL of either water (W+W) or M. sexta OS (W+OS) was applied to the wounds.
Phe ammonia-lyase (PAL) is another gene important for plant defense (Bennett and Wallsgrove, 1994). We investigated PAL transcript levels in EV, SIPK-VIGS, and WIPK-VIGS plants (Figure 9B) and found that they were suppressed in SIPK-VIGS plants but not in WIPK-VIGS plants. In SIPK-VIGS plants, suppression lasted for the duration of the experiment, except in OS-elicited plants, and levels of PAL transcripts returned to those of EV plants by 3 h. WIPK-VIGS plants had significantly higher levels of PAL transcripts than did EV plants 3 h after either elicitation, suggesting that WIPK negatively regulates PAL transcript levels.
FACs in M. sexta OS Are Elicitors That Activate Herbivory-Specific MAPK Signaling
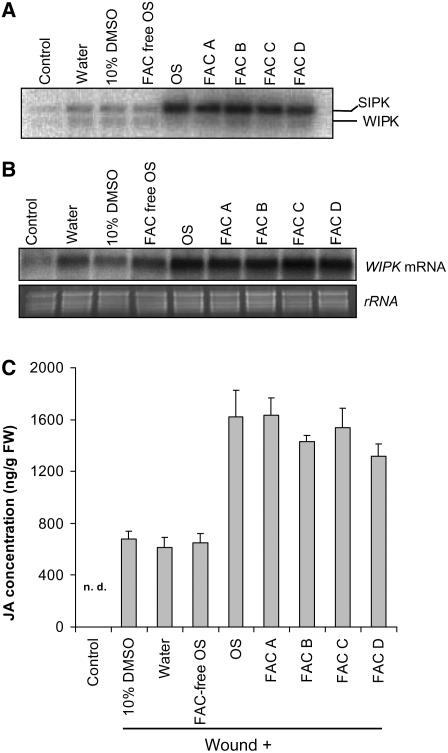
Microarray and biochemical analyses indicated that the FACs in M. sexta OS are responsible for eliciting OS-specific JA, VOCs, and transcriptional responses in N. attenuata (Halitschke et al., 2001, 2003). To determine if FACs activate MAPKs in N. attenuata, synthetic FACs were applied to wounded N. attenuata leaves. N-linolenoyl-l-Gln (FAC A), N-linolenoyl-l-Glu (FAC B), N-linoleoyl-l-Gln (FAC C), and N-linoleoyl-l-Glu (FAC D), the four most abundant FACs in OS that are found at concentrations of 0.6 to 1.2 mM in M. sexta OS, potently elicit herbivory-specific responses in N. attenuata (Halitschke et al., 2001). In-gel kinase activity assays demonstrated that all FACs at concentrations of 0.2 mM, which are similar to the concentrations of FACs in the fivefold-diluted OS we used for W+OS treatments, elicited levels of SIPK activity that were several times those elicited by W+W or 10% DMSO, the solvent for the FAC treatments (Figure 10A). Furthermore, removing FACs by ion-exchange chromatography made the OS no more able than water to elicit SIPK. The activity assay also revealed that all FACs were similarly able to activate SIPK. Consistent with the finding that W+OS does not elicit higher levels of WIPK activity compared with W+W, plants treated with wounding and various FACs showed levels of WIPK activity similar to those in plants treated with wounding and OS, 10% DMSO, FAC-free OS, or water (Figure 10A).
Figure 10.
FACs in M. sexta OS Elicit MAPK Activity and JA Burst.
N. attenuata leaves were wounded with a pattern wheel, and 20 μL of the following solutions was immediately applied to the puncture wounds: water, 10% DMSO, FAC-free OS, OS, and FAC A, B, C, and D dissolved in 10% DMSO. Untreated and unwounded plants were used as controls.
(A) Four replicate samples were harvested 30 min after each treatment and pooled; an in-gel kinase assay was performed to test each treatment's ability to activate MAPKs.
(B) Total RNA was extracted from samples 1 h after elicitation; WIPK transcript accumulation was determined by RNA gel blotting from four pooled replicates.
(C) Mean (+se) JA concentrations in five replicate leaf samples 1 h after elicitation were measured by HPLC-MS/MS. n.d., not detected.
Consistent with the activity assay, RNA gel blotting analysis revealed that levels of WIPK transcript were highly amplified when different FACs were applied to puncture wounds compared with when both 10% DMSO, the solvent for FACs, and the pure water treatments were used (Figure 10B). Similar levels of WIPK transcripts were found when leaves were treated with FAC-free OS and with W+W. RNA gel blotting also demonstrated that FAC-treated and W+OS-treated samples accumulated comparable levels of WIPK transcripts. Furthermore, we measured the JA bursts elicited by different FAC treatments. One hour after elicitation, all plants treated with OS or with different FACs had accumulated similarly elevated levels of JA (P = 0.69, ANOVA), levels that were ∼1.5 times higher than those in water-, 10% DMSO-, and FAC-free-OS–treated plants (Figure 10C). These data strongly support the idea that FACs are important elicitors in M. sexta OS; FACs are recognized by N. attenuata and in turn trigger downstream responses by activating MAPKs.
OS Elicitation of MAPKs at the Wound Site and in Systemic Tissues within a Leaf
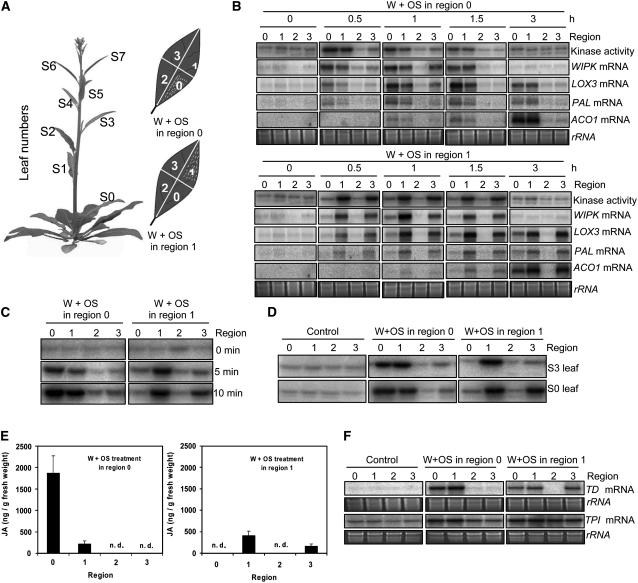
M. sexta larvae typically spend hours feeding at the same location before moving on to other regions of the leaf on which they were oviposited (for the first two instars) or to other leaves (in later instars) (McFadden, 1968; Kessler and Baldwin, 2002b). Herbivory triggers defense responses in adjoining attacked tissues and in unattacked leaves sharing vascular connections with the attacked leaves (Schittko and Baldwin, 2003; Orians, 2005). Yet, how the responses are conveyed to nonattacked regions within an attacked leaf has not been well studied (Schittko et al., 2000). We divided fully expanded S0 leaves from bolting plants into four regions, along each leaf's midrib vein and perpendicularly at the middle of each midrib vein; the four regions were designated 0, 1, 2, and 3 (Figure 11A). In four replicated plants, either regions 0 or 1 were treated with W+OS, and all regions were harvested at different times.
Figure 11.
Spatial Distribution of OS-Elicited Responses within Single Leaves Growing at Different Nodes.
Experiments were conducted on leaves growing at either S0 or S3 nodes from 40-d-old bolting wild-type N. attenuata plants. Four replicate leaves from different plants were used for each treatment.
(A) Numbering of the leaves at different phyllotaxic positions (nodes) on bolting plants and illustration of treatments at different leaf regions. Wounds are illustrated with dotted lines; each leaf was wounded with a pattern wheel, and 10 μL M. sexta OS (W+OS) was applied to either region 0 or 1. Leaves were harvested in four sections at the specified times.
(B) Spatial distribution of W+OS-elicited responses in S0 leaves. Total protein and RNA were extracted from S0 leaves. Kinase activity was determined by an in-gel kinase assay using MBP as the substrate. Transcript levels of WIPK, LOX3, PAL, and ACO1 were examined with RNA gel blotting.
(C) Spatial distribution of W+OS-elicited SIPK activity in S0 leaves shortly after elicitation. S0 leaves were treated with W+OS; after 5 and 10 min, samples were collected. Kinase activity was analyzed using an in-gel assay.
(D) Spatial distribution of W+OS-elicited SIPK activity in S0 and S3 leaves. Both S0 and S3 leaves were treated with W+OS and harvested after 30 min. The spatial distribution of kinase activity was analyzed by an in-gel kinase assay.
(E) Mean (+se) JA concentrations in four replicate S0 leaf samples collected 1 h after W+OS treatment. n.d., not detected.
(F) In W+OS-treated S0 leaves, the accumulation of TD and TPI transcripts after 3 h and 12 h, respectively, was determined by RNA gel blotting.
Remarkably, W+OS application in regions 0 and 1 elicited SIPK activity in distinct regions of the leaf (Figure 11B). When region 0 was elicited, high levels of SIPK activity were observed in treated region 0; slightly lower levels were detected in region 1; SIPKs in region 3 were also activated, although levels were much lower than those in regions 0 and 1; in region 2, no elevation of SIPK activity was observed. Over time, SIPK activity levels in all regions waned, and region 2 remained unelicited throughout. Eliciting region 1 produced a distinct distribution of kinase activity: SIPK activity levels increased greatly in region 1 but only slightly in region 0. However, SIPK activity levels in region 3 also highly increased, reaching those of region 1. Similarly, region 2 did not increase SIPK activity levels when region 1 was treated with OS. These findings suggest that certain mobile signals move to various regions of the herbivore-attacked leaf and elicit SIPK activity.
The duration of the response was also influenced by the region that was elicited. Elicited kinase levels lasted longer when leaves were treated in region 1. Even in leaves that had been elicited 1.5 h earlier in region 1, SIPK activity levels were higher than in leaves elicited in region 0 (Figure 11B, kinase activity panels). These results suggest that there might be less negative SIPK regulator activity at the tip than at the base of a leaf. The pattern of WIPK transcript accumulation followed the pattern of SIPK activity: regions 0 and 1 showed greatly elevated WIPK transcript levels when leaves were elicited in 0, and regions 1 and 3 showed highly elevated WIPK transcript levels when region 1 was elicited (Figure 11B), confirming the idea that WIPK transcript accumulation is regulated by SIPK. Moreover, in undamaged regions of the herbivore-attacked leaf, WIPK is also likely involved in herbivore resistance.
To examine how fast the mobile signals move across the leaf, we analyzed the spatial distribution of SIPK activity shortly after elicitation in S0 leaves (Figure 11C). When leaves were treated in region 0, region 1 showed elevated SIPK activity levels as soon as 5 min afterwards. Similarly, when leaves were treated in region 1, region 3 also showed increased levels of SIPK activity after only 5 min. After 10 min, SIPK activity levels in leaves treated in either region 0 or 1 were similar to those harvested after 30 min.
We compared SIPK activity levels in leaves at different developmental stages (Figure 11D). When OS were applied to region 0, after 30 min both younger (S3) and older (S0) leaves had elevated SIPK activity levels in both regions 0 and 1. Notably, eliciting region 1 in older leaves rapidly activated SIPK activity levels in 1 and 3. Region 3 of younger leaves showed only slightly enhanced SIPK activity levels after 30 min, suggesting that the maturation of the vascular system that comes with age allows the OS-elicited responses to be propagated more vigorously within an elicited leaf.
JA, SA, and ethylene are known to regulate the transcript levels of defense genes in many plant species (Dong, 1998; Reymond and Farmer, 1998). The data obtained from SIPK-VIGS and WIPK-VIGS plants reveal a correlation between OS-elicited MAPK activation and the accumulation of phytohormones. Thus, RNA gel blotting was performed to examine the spatial patterns of OS-elicited transcript accumulation for LOX3, PAL, and ACO1, genes important in JA, SA, and ethylene biosynthesis, respectively (Figure 11B). After OS were applied, the transcript accumulation patterns of LOX3, PAL, and ACO1 resembled those of kinase activation, albeit with different kinetics. Elicitation of region 0 quickly increased LOX3 and PAL transcript levels. As with the kinase activity, the levels of both genes were highest in elicited region 0 and adjacent region 1; region 3 only showed transiently enhanced levels of both transcripts. By contrast, the elicitation of region 1 resulted in similar transcript levels of induced LOX3 and PAL in regions 1 and 3 but only slightly enhanced levels in region 0, a pattern that resembled the distribution of SIPK activity. Neither treatment affected LOX3 or PAL transcript levels in region 2 at any time. Long-lasting kinase activity obtained by applying OS to region 1 prolonged the levels of LOX3 and PAL transcripts as well. ACO1 showed a slower kinetic than the other two genes, which reached maximum levels at 3 h; nevertheless, the pattern of its transcript accumulation in different regions resembled that of SIPK activity.
To investigate if the spatial pattern of JA levels also matched the distribution of LOX3 accumulation, JA levels produced in different regions were examined 1 h after treating either region 0 or 1 with W+OS, which is when wild-type plants accumulate the most JA (Figure 11E). Leaves in which region 0 had been elicited showed elevated JA levels in regions 0 and 1; remarkably, region 0 showed almost 7 times more JA than adjacent region 1, though such a dramatic difference did not characterize levels of SIPK activity and LOX3 transcripts in these two regions. When leaves were treated in region 1, JA was detected only in regions 1 and 3, which was also consistent with the spatial distribution of SIPK activity and LOX3 transcript levels. Similarly, region 3 had ∼1.5 times less JA than region 1. However, JA levels in OS-treated region 1 were 3.5 times lower than in OS-treated region 0. JA levels in regions 2 and 3 in leaves with OS-treated region 0 and in regions 0 and 2 in leaves with OS-treated region 1 were not detectable.
TD and TPI are two genes involved in plants' direct defenses against herbivores (Zavala et al., 2004; Chen et al., 2005; Kang et al., 2006), and both are regulated at least in part by JA (Hermsmeier et al., 2001; Glawe et al., 2003). After regions 0 and 1 were elicited, transcript levels of TD and TPI genes were analyzed by RNA gel blotting in samples harvested after 3 and 12 h, respectively, which is when transcript levels are at their highest (Kang et al., 2006; Wu et al., 2006) (Figure 11F). The spatial distribution of TD transcript levels 3 h after elicitation largely resembled that of JA, except that although JA was not detectable in region 0 of leaves treated in region 1, the level of TD transcripts in region 0 was comparable to TD transcript levels in both regions 1 and 3. When leaves were treated in region 0, high levels of TPI were observed in both regions 0 and 1, while regions 2 and 3 showed comparatively lower levels, albeit higher than those in noninduced samples. All regions in leaves elicited in region 1 had similar levels of TPI transcripts, and these levels were comparable to levels in regions 0 and 1 when leaves were elicited in region 0.
Systemic TPI Elicitation Doesn't Require SIPK Activation in Systemic Leaves
M. sexta attack increases levels of TPI transcripts and activity in N. attenuata. This response is not limited to attacked leaves but spreads throughout the plant (van Dam et al., 2001; Zavala et al., 2004). In tomato, wounding has been found to activate MAPKs both locally and systemically (Stratmann and Ryan, 1997); wounding tobacco leaves with carborundum quickly increases levels of WIPK transcript in systemic leaves; cutting tobacco stem activates WIPK systemically as well (Seo et al., 1999). To investigate if MAPK signaling is involved in systemic TPI induction in N. attenuata, leaves at node +1 from plants at the rosette stage were elicited with W+OS. Kinase activity was assayed in both treated local and untreated systemic leaves at node −1. In contrast with the high SIPK activity levels in elicited leaves, no elevation of SIPK activity was detected in systemic leaves (Figure 12A). RNA gel blotting also revealed that no systemic induction of WIPK took place even after 96 h, although both local and systemic samples had strongly elevated levels of TPI transcripts (Figure 12B). To investigate whether applying OS to wounds suppresses systemic MAPK responses elicited by wounding and whether systemic leaves other than −1 leaves have any elevated MAPK activity, we treated N. attenuata plants with W+W and W+OS and examined kinase activity and WIPK transcript accumulation in +1 (local) and −1 and +2 (systemic) leaves (see Supplemental Figure 3 online). Up to 1 h, neither activation of SIPK and WIPK nor elevation of WIPK transcript levels was detected in systemic leaves. These data suggest that the systemic induction of TPI doesn't require the activation of SIPK in systemic leaves.
Figure 12.
TPI Transcript Accumulation in Systemic Leaves Doesn't Require Activating SIPK in Systemic Leaves.
Leaves at node +1 from rosette-stage N. attenuata were wounded with a pattern wheel; 20 μL of M. sexta OS (OS) was applied to the wounds. Treated leaves (+1, local) and systemic untreated leaves (−1) were harvested at indicated times. Four replicate leaves were pooled after harvesting.
(A) Kinase activity assay in both local and systemic leaves after elicitation.
(B) Transcript accumulation analyses of WIPK and TPI in local and systemic leaves by RNA gel blotting.
DISCUSSION
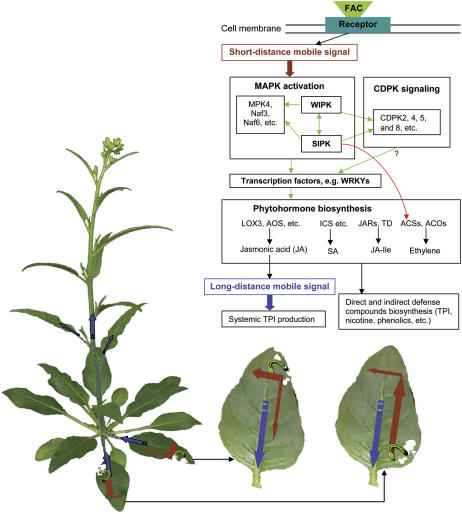
JA and ethylene are known to play a central role in mediating a plant's responses to herbivore attack, which involves reconfiguring a plant's wound response (reviewed in Reymond and Farmer, 1998; Kessler and Baldwin, 2002a). How plants perceive herbivory and along which signaling pathways the messages are transformed into cellular responses are poorly understood. Here, we demonstrate that applying M. sexta OS to wounds in N. attenuata leaves rapidly and markedly elicits MAPKs more than wounding does. By removing FACs from OS and applying synthetic FACs, we demonstrate that FACs elicit these responses. Silencing SIPK and WIPK by VIGS revealed that both kinases regulate the transcript levels not only of WRKY transcription factors, MAPKs, and CDPKs but also of JA and ethylene, which highlights their central role in the OS-elicited, hormonally mediated, signal transduction network. By applying JA and ethylene and using transgenic plants impaired in the production and perception of JA and ethylene, we show that neither OS-elicited JA nor ethylene production contribute to OS-elicited MAPK activation.
OS elicitation activates MAPKs and other defense responses not only in the damaged regions of a leaf but also in particular nondamaged regions. The spatial distribution of these responses depends on the developmental stages of the leaves and on the locations of herbivore damage, both of which implicate vascular maturation in the signaling. In addition to these responses in the attacked leaf, enhanced levels of TPI transcripts were found in unelicited leaves on the same plant. However, this between-leaf systemic signaling of defense responses occurs without MAPK activation in systemic leaves and suggests the existence of a mobile signal other than the one activating responses within attacked leaves.
Herbivore-Specific Elicitors, FACs, and Plant Perception of Herbivory
Volicitin, a hydroxylated FAC, has been reported to bind to Zea mays cell membranes with high affinity (Truitt et al., 2004), suggesting that a ligand-receptor binding mechanism may be critical for herbivory recognition. Moreover, FACs vary in their ability to elicit VOCs in Z. mays seedlings: 18:2-Glu (FAC D) were least active, while 18:3-Gln (FAC A) and 18:3-Glu (FAC B) were significantly more active (Alborn et al., 2003). By contrast, applying similar concentrations of different FACs to N. attenuata resulted in similar levels of SIPK activity and similar levels of WIPK transcripts and JA production. Assuming that FAC-specific receptors exist in the N. attenuata cell membrane, two possible scenarios may account for these results: (1) there is only one receptor gene; this gene encodes a FAC receptor protein, and different FACs bind to it with similar affinities, which in turn activates downstream MAPKs and herbivore-specific genes; or (2) several genes encode different receptors, which bind to specific individual FACs; these receptors likely are similarly abundant and share the same downstream signaling networks.
Although no FAC-specific receptors have yet been identified, some evidence suggests that ligand–receptor interactions play important roles in plant resistance to pathogens. In wild-type Arabidopsis protoplast, flagellin binds to receptor FLS2 and initiates downstream responses through MEKK1, MKK4/MKK5, and MPK3/MPK6 signaling cascades. In the fls2 mutant, these responses are absent, indicating the critical role of flagellin perception by receptor FLS2 (Asai et al., 2002; Zipfel et al., 2004). The N gene, a putative receptor involved in cultivated tobacco's resistance to the Tobacco mosaic virus (TMV), is structurally similar to the toll and the interleukin-1 receptors (Whitham et al., 1994). Tobacco carrying the N gene activated WIPK and elevated levels of WIPK transcripts and proteins after TMV infection; by contrast, tobacco without the N gene neither activated WIPK nor altered levels of WIPK mRNA or protein (Zhang and Klessig, 1998b). Identifying FAC binding proteins and FAC-specific receptors will clarify how plants perceive herbivores on the molecular level and enrich our understanding of this ubiquitous interaction.
SIPK and WIPK Regulate Transcript Levels of Defense-Related Genes in N. attenuata
Both the range and scope of genes found to be regulated by SIPK and WIPK, ranging from genes involved in JA and ethylene biosyntheses to transcription factors, MAPKs, and several CDPKs, were surprising. The regulation of WRKY transcription factors is likely to contribute significantly to the large scope of transcriptional responses resulting from silencing SIPK. SIPK is known to phosphorylate WRKY1 in tobacco (Menke et al., 2005), and the activation of SIPK and WIPK is known to lead to higher transcript levels of several WRKYs (Kim and Zhang, 2004). We propose that SIPK and WIPK regulate transcript levels of WRKY transcription factors and may even directly phosphorylate particular WRKYs and thereby mediate the transcript levels of defense-related genes, including transcription factors.
WRKYs themselves can bind to the promoters of WRKY genes and modulate their transcription (Eulgem et al., 1999; Turck et al., 2004). A recent study of yeast suggests that MAPKs may physically associate with promoters and thus influence the transcription of certain genes (Pokholok et al., 2006). SIPK and WIPK may also directly associate with the promoters of transcription factors, phytohormone biosynthesis genes, and thus modulate their transcript accumulations.
CDPKs have been shown to be involved in development and stress responses (Cheng et al., 2002; Ludwig et al., 2004). CDPK2 mediates JA and ethylene biosynthesis and also biotic stress responses (Romeis et al., 2001; Ludwig et al., 2005). Expression analysis showed that CDPK4 is involved in plant stress reactions (Zhang et al., 2005); our q-PCR analysis also suggested that CDPK2, 4, 5, and 8 are involved in herbivore resistance in N. attenuata. In Arabidopsis and cultivated tobacco, MPK4 was also shown to be involved in various stress responses and the regulation of phytohormone biosynthesis (Ichimura et al., 2000; Petersen et al., 2000; Droillard et al., 2004; Gomi et al., 2005). The homolog of Naf6 in cultivated tobacco has been shown to be involved in pathogen resistance (Liu et al., 2004), and our results suggest that MPK4, Naf6, and Naf3 are involved in responses to herbivore attack. It is possible that in both SIPK- and WIPK-VIGS plants, the decreased transcript levels of these genes reduced their respective kinase activity and thereby influenced downstream targets.
Using q-PCR, we found that after both wild-type and EV N. attenuata plants were treated with either W+W or W+OS, SIPK transcript levels were elevated (Figures 1F and 3B). By contrast, SIPK transcript levels in cultivated tobacco were unchanged after wounding as analyzed by RNA gel blotting (Zhang and Klessig, 1998a). Differences in the sensitivity of the detection techniques used (q-PCR and RNA gel blotting) or species-specific differences are likely to account for the differences between these two studies.
SIPK and WIPK were found to influence the accumulation of each other's transcripts. An important study has demonstrated that SIPK positively regulates the accumulation of WIPK and also of its protein after TMV infection (Liu et al., 2003). In N. attenuata, after either W+W or W+OS treatment, SIPK activation increased levels of WIPK transcripts. However, compared with those in wild-type control plants, levels of WIPK transcripts in SIPK-silenced rice (Oryza sativa) and Arabidopsis were higher and unchanged after plants were inoculated with fungal elicitors and pathogens, respectively (Menke et al., 2004; Lieberherr et al., 2005). These data reflect the treatment- or species-specific effects of SIPK regulation on WIPK transcript levels. Although silencing WIPK was shown to reduce SIPK transcript levels, SIPK activity was not affected according to the in-gel kinase activity assay, suggesting its independence from transcriptional regulation. Moreover, silencing WIPK also reduces levels of Naf4 after W+W and W+OS treatments. Since pTV-WIPK and Naf4 sequences have no matches longer than 20 nucleotides, it is not likely that silencing WIPK also cosilenced Naf4 (Thomas et al., 2001; Xu et al., 2006). Our transcriptional analyses suggest that the transcriptional interactions among different MAPKs, even CDPKs, form a complicated crosstalking network, and the interactions that occur at the level of protein activity may be even more complicated.
In SIPK-VIGS plants, we detected highly decreased transcript levels of Naf4, a closely related homolog of SIPK (Ren et al., 2006). The VIGS construct pTV-SIPK, which shares a 35-nucleotide perfect match with Naf4, may have cosilenced Naf4. Alternatively, Naf4 may be transcriptionally regulated by SIPK; that is, silencing SIPK may reduce levels of Naf4 transcripts, as we demonstrate happens between SIPK and WIPK and among other kinases. Naf4 may have contributed at least partly to the phenotypes we observed in SIPK-VIGS plants. Both proteins have >90% identity, which suggests a short history of divergence; whether they have similar functions remains unknown. In cultivated tobacco, where long-lasting Naf4 activity causes hypersensitive-like responses, such as cell death, a comparable phenotype was obtained when SIPK was constitutively activated (Zhang and Liu, 2001; Ren et al., 2006), suggesting that both kinases may have similar functions. Using gene-specific VIGS constructs or transforming plants with specific RNAi constructs to individually silence Naf4 and SIPK will elucidate the nature, redundant or distinct, of these genes' functions.
The transcript levels of three genes directly related to resistance to herbivores in N. attenuata, TPI, PAL, and TD, were found to be regulated by SIPK and WIPK. As an antidigestive protein, TPI plays an important role in N. attenuata's resistance to herbivory (Glawe et al., 2003; Zavala et al., 2004). PAL is important in the biosyntheses of phenolic compounds that are implicated in resistance to abiotic and biotic stresses (Bennett and Wallsgrove, 1994). TD has been shown to play an antinutritive defense role by decreasing the level of Thr in M. sexta's midgut and causing nutritional deficiency (Chen et al., 2005). Both SIPK and WIPK positively regulate transcript levels of TPI and TD; by contrast, levels of PAL transcripts are regulated positively by SIPK but negatively by WIPK, demonstrating overlapping yet distinct functions of SIPK and WIPK. Studies have suggested that PAL is activated by CDPKs (Allwood et al., 1999; Cheng et al., 2001); whether OS-elicited CDPK activity or even MAPK activity is involved in activating PAL in addition to regulating its transcriptional levels is unknown. The ecological significance of SIPK and WIPK in relation to herbivore resistance in N. attenuata needs to be studied further.
Activation of SIPK and WIPK and Accumulation of Phytohormones
The OS elicitation of SIPK and WIPK regulates levels of JA, SA, JA-Ile, and ethylene in N. attenuata. Both SIPK-VIGS and WIPK-VIGS plants had highly reduced levels of JA after either wounding or herbivory, strongly suggesting that most elevated JA levels are due to either wounding- or OS-elicited SIPK and WIPK activation. Other kinases (e.g., CDPKs) are probably involved in regulating JA levels, as shown by the activation of CDPK2, which also elevates JA levels (Ludwig et al., 2005). In cultivated tobacco, genetic analyses of WIPK indicated that WIPK is located upstream of JA biosynthesis (Seo et al., 1995, 1999). Conversely, applying dexamethasone to transgenic tobacco plants carrying a dexamethasone-inducible promoter-active mutant MEK2DD construct highly elevated SIPK and WIPK activity levels; however, levels of JA remained the same (Kim et al., 2003). In addition to the MAPKs, several lines of evidence suggest the importance of calcium in regulating JA biosynthesis. After wounding or herbivore attack, leaves quickly increase cytosolic calcium concentrations and depolarize cell membranes (Fisahn et al., 2004; Maffei et al., 2004); leaves and cell suspension cultures to which calcium channel blockers were applied lowered their elevated levels of JA after heat and oligogalacturonide treatment, respectively (Fisahn et al., 2004; Moscatiello et al., 2006). In humans, calcium is necessary for the activity of LOX and phospholipase A2, which are enzymes involved in the production of leukotrienes and lipoxins. Although no indisputable evidence exists, it is likely that calcium signaling partly modulates JA metabolism (Creelman and Mullet, 1997). Wounding or herbivory probably triggers not only MAPKs but calcium and other signaling pathways, and this likelihood may also be reflected in the possible involvement of CDPK2 in wounding/osmotic stress responses (Ludwig et al., 2005).
The transcript levels of genes involved in JA biosynthesis change after W+OS and W+W treatments, yet W+OS leads to a more dramatic increase in JA production than does W+W treatment. Moreover, the OS-elicited JA burst comes well before the OS-elicited increase in LOX3 and AOS transcripts in wild-type N. attenuata plants (Ziegler et al., 2001; Halitschke and Baldwin, 2003). This implies that posttranslational processes are important for the JA burst. Consistent with this view, the much higher activity levels of SIPK (and likely other kinases) found in W+OS-treated plants than in W+W-treated plants might result in better phosphorylation of enzymes involved in JA biosynthesis and, thus, higher levels of enzyme activity.
After wounding, WIPK-silenced cultivated tobacco plants produce higher levels of SA than do wild-type plants (Seo et al., 1995). The opposite happened in N. attenuata: after W+OS treatment, both SIPK-VIGS and WIPK-VIGS plants had lower levels of SA than did EV plants. Although the interaction between the SA- and JA-signaling pathways appears to be positive and negative, it is primarily negative (reviewed in Kunkel and Brooks, 2002). The lower levels of JA in SIPK- and WIPK-VIGS plants did not correlate with higher levels of SA. Furthermore, N. attenuata plants had substantially higher levels of JA and SA after W+OS treatment compared with those treated with W+W, suggesting that herbivory-elicited JA and SA may interact synergistically. The responses of N. attenuata and cultivated tobacco in wound- and probably also OS-induced SA production are likely species-specific.
Evidence has emerged that the conjugation reaction between JA and Ile, catalyzed by JAR, plays an important role in activating plant defenses (Staswick and Tiryaki, 2004; Kang et al., 2006; Wang et al., 2007). After wounding and OS elicitation, dramatically lower levels of JA-Ile/JA-Leu in SIPK-VIGS plants were detected compared with EV plants for up to 3 h. WIPK-VIGS plants, on the other hand, showed decreased levels of JA-Ile/JA-Leu only up to 1.5 h, although levels of one of the substrates for JARs, JA, were still ∼50% lower than levels in EV plants. The decreased levels of JA-Ile/JA-Leu in SIPK- and WIPK-VIGS plants may have resulted from the decreased levels of JA, one of the substrates for conjugating JA-Ile/JA-Leu. It is also likely that the lower transcript levels of JARs and TD in SIPK- and WIPK-VIGS plants also contributed to their JA-Ile/JA-Leu phenotype.
ACS phosphorylation by SIPK has been demonstrated in Arabidopsis to be a key step in ethylene biosynthesis (Kim et al., 2003; Liu and Zhang, 2004). Herbivore-elicited ethylene production also likely depends on the phosphorylation of ACS enzymes by SIPK; however, since substantially lower levels of ACS3, ACO1, and ACO3 were detected in SIPK-VIGS plants, the transcript accumulation of these genes may also contribute to the regulation of ethylene biosynthesis. Compared with the very low levels of JA and JA-Ile, ethylene levels in SIPK-VIGS plants were only reduced by 50%, which is similar to ethylene levels produced in Arabidopsis SIPK mutants after flagellin treatment, suggesting that there may be other signaling pathways leading to ethylene biosynthesis (e.g., CDPKs) (Tatsuki and Mori, 2001; Liu and Zhang, 2004; Ludwig et al., 2005). The remarkable difference between ethylene production in N. attenuata after wounding and after OS elicitation may also result from the substantially different abilities of these two forms of elicitation to activate kinases. Compared with W+OS, wounding alone is much less able to activate SIPK and probably certain CDPKs involved in ethylene biosynthesis as well. Moreover, both flagellin and W+OS treatments activate SIPK and in turn trigger ethylene biosynthesis, confirming the hypothesis that various stress stimuli converge onto MAPK pathways (Zhang and Klessig, 2001).
Genetic and biochemical studies have indicated that MAPKs are located upstream of JA and ethylene production (Seo et al., 1999; Kim et al., 2003; Liu and Zhang, 2004). Our loss-of-function analysis provides additional evidence. Our experience with intact plants rather than cell suspension cultures leads us to posit that neither JA nor ethylene has any feedback effect on MAPKs, as these compounds are located downstream of MAPKs. We propose that the differences observed in suspension cells and intact plants are due to the specificity of MAPK signaling in different tissues, although these differences may also reflect the easily disturbed physiology of cell suspension culture, for example, an easily changed cytosolic pH (Tena and Renaudin, 1998).
Functions of SIPK and WIPK
Both transcriptional analyses and phytohormone levels point to overlapping functions of SIPK and WIPK. Silencing both kinases reduces JA and JA-Ile accumulation after W+OS treatment, a reduction that is also reflected in their transcript levels of phytohormone biosynthetic genes. In addition, both SIPK and WIPK modulate the transcript accumulation of WRKY transcription factors; more strikingly, they both influence the transcript accumulation of several other MAPKs and CDPKs, although with different specificity and at different levels. It is likely that SIPK and WIPK share common substrates, including transcription factors, and thus both mediate the transcript levels of the same target genes. Consistent with this idea, using a protein-microarray approach, Feilner et al. (2005) demonstrated that SIPK and WIPK share many common substrates. Intriguingly, SIPK and WIPK transcriptionally regulate each other, suggesting that one (or a few) transcription factor(s) involved in the regulation of transcript levels of both SIPK and WIPK is (are) their common substrate(s).
Transcriptional analyses also revealed different functions of SIPK and WIPK. Although in most cases SIPK and WIPK positively regulate the transcript levels of many defense-related genes, WIPK was found to negatively regulate a few genes, namely, AOS, ACO1, and PAL. The substantially decreased levels of the phytohormones JA, JA-Ile, and ethylene in SIPK- and WIPK-silenced plants led us to hypothesize that both kinases may have many more phosphorylation targets in phytohormone biosynthetic pathways than the known ACS in ethylene synthesis. Identifying the substrates for SIPK and WIPK and the ability of these two kinases to directly phosphorylate phytohormone biosynthetic enzymes will help us to better understand how SIPK and WIPK regulate phytohormone biosynthesis.
Mobile Signals and Defense Response Activation in Systemic Tissues and Leaves
In this study, we demonstrate that applying OS to a specific region on a single leaf effected a specific distribution of MAPK activity and, subsequently, of defense-related gene transcripts. Kinetic data showed that even after only 5 min the kinase activity had spread to adjacent regions in fully developed leaves, indicating that certain signals were being quickly transmitted. This mobile signal may be elicited by FACs and located downstream of FAC receptors and upstream of MAPKs; after FACs bind to receptors, it may be quickly activated or released and transported to adjacent regions. To our knowledge, neither the nature of this signal nor the mechanism of transport has been studied. In lima bean (Phaseolus lunatus), Spodoptera littoralis feeding rapidly and strongly depolarizes membrane potential throughout the entire attacked leaf (Maffei et al., 2004). In animal neurons, the changes of membrane potential are known to be critical for sending signals long distances; however, the study of plant electrophysiology is still in its infancy (Brenner et al., 2006). Given how rapidly MAPKs are activated in undamaged regions, it may be that the signal is carried by electricity or rapidly propagated hydraulic signals; another hypothesis has rather small molecules, such as FACs or FAC-elicited signal compounds, entering the xylem vessel through wounds and being carried to specific regions of the leaf (Malone et al., 1994). Whether electric signaling or xylem transport is involved in activating MAPKs in undamaged regions needs further investigation.
Clearly, plants regulate the direction and extent of signal transport within a leaf. In fully developed leaves, when region 0 was treated with W+OS, SIPK was quickly and markedly activated in both regions 0 and 1, and a slight increase of activity was also observed in region 3. The application of W+OS to 1 led to substantially increased kinase activity in regions 1 and 3 as well as to marginally elevated activity levels in region 0. No sample from region 2 showed any elevated SIPK activity. The specific spatial distribution of MAPK activation and subsequent accumulation of defense-related genes may directly influence how the mobile signal molecules are transported or moved. The significant difference in MAPK activity levels among regions 1 and 3 and regions 3 and 0, after regions 0 and 1 were treated with OS, suggests that the transport of the mobile signal molecules is also controlled quantitatively. Using leaves at different developmental stages, we showed that applying W+OS to a specific region in a single leaf results in a pattern of activity distribution that is also developmentally regulated: in younger sink leaves, applying W+OS to region 1 activates SIPK; however, adjacent region 3 showed low activity levels that contrasted with high activity levels in older source leaves. Studies have shown that during the development of a leaf, there is a gradient in maturation from the base to the tip that allows veins to load or unload sugar (reviewed in Turgeon, 1989); in not fully developed leaves, some parts of a leaf still import sugar, but other parts export sugar. This suggests that leaves at different developmental stages develop different transport systems. Whether this holds true for the transport of the signal eliciting MAPK activity in adjacent regions in the herbivore-attacked leaf needs to be studied further.
The spatial distribution of accumulated JA in these four regions after W+OS treatment in either region 0 or region 1 largely resembles the distribution of LOX3. Remarkably, when region 0 was treated with OS, JA was highly induced and levels were much higher than those in region 1, or those in regions 1 and 3, which were elicited when leaves were treated with W+OS in region 1. The complex interplay of mechanisms modulating JA biosynthesis includes MAPK activation, JA biosynthetic gene transcript accumulation, and even the particular region of a leaf attacked by herbivores. Both TD and TPI are known to be involved in eliciting and producing direct defenses (Zavala et al., 2004; Chen et al., 2005; Kang et al., 2006) and are largely regulated by JA. Intriguingly, the spatial distribution of TPI transcripts doesn't match the distribution of JA levels: TPI transcripts were elevated in all regions of the leaf whenever OS was applied to the base or the tip of the leaf. Perhaps other mobile signals are activated by JA and move to all parts of the herbivore-attacked leaf before finally eliciting TPI.
The patterns of spatial distribution among MAPK activity, JA levels, and direct-defense gene transcript accumulations within the damaged leaf suggest that various intercellular signal cascades are involved: different mobile signals induce or activate different downstream elements on signal transduction pathways, and the directions and extent of their movements are probably tightly regulated by certain transport systems. Finally, complex interactions among the signaling pathways may also account for the spatial regulation of these defense-related responses. Our results demonstrate that the responses depend on both the ages of the attacked leaves and the position of the herbivore on the leaves. However, the ecological significance of these different responses has not been established.
Herbivory has long been known to enhance proteinase inhibitor (PI) activity in intact systemic leaves (Green and Ryan, 1972), although the mechanism of this long-distance signal transduction is still not known. Several studies have found that systemic leaves orthostichous to the wounded leaf accumulate markedly higher levels of PI transcripts and activity than do nonorthostichous leaves, suggesting that the signal is transported by the vascular system (Jones et al., 1993; Orians et al., 2000; Schittko and Baldwin, 2003; Orians, 2005). The involvement of electric and hydraulic signals in mediating the systemic responses has also been proposed (Alarcon and Malone, 1994; Stankovic and Davies, 1997). More importantly, an elegant genetic study strongly argues that JA plays important roles in mediating systemic PI response: either JA or a JA-elicited mobile signal is responsible for systemic PI induction (Li et al., 2002).
In tomato, wounding activates MAPK both locally and systemically (Stratmann and Ryan, 1997). Infecting cultivated tobacco plants with the tobacco mosaic virus leads to the systemic accumulation of WIPK mRNA and in turn the protein (Zhang and Klessig, 1998b). Cutting cultivated tobacco stems also activates WIPK activity in systemic leaves (Seo et al., 1999). These studies implicate MAPKs in systemic signaling. However, N. attenuata plants treated with either W+W or W+OS didn't show systemic SIPK activity or WIPK transcript accumulation, suggesting that different plant species have specific mechanisms for modulating systemic MAPK signaling; such mechanisms are not conserved even in closely related species, such as N. attenuata, cultivated tobacco, and tomato. Whether these discrepancies are due to differences among treatments or the morphology of the experimental plants still needs to be investigated. It is very likely that the mobile signal that activates SIPK in undamaged regions on a single leaf and the signal that moves to distal undamaged leaves and induces TPI are distinct molecules, since the former is located upstream of SIPK but the latter clearly is not.
In conclusion, in this study, we examine temporally and spatially how plants deploy defense reactions that are modulated by SIPK and WIPK in response to herbivore attack and summarize the results in a model (Figure 13). Plants recognize FAC components in herbivores' OS and quickly activate SIPK and WIPK. Both kinases in turn regulate a broad array of gene transcript levels and likely even modify specific enzyme activity by phosphorylation, for example, ACS; together with the subsequently elevated levels of JA, ethylene, and SA, SIPK and WIPK further modulate the transcript accumulation of other defense-related genes. Spatially, when an herbivore chews on a leaf, a quickly elicited mobile signal conveys the attack alert message to particular unattacked regions of the leaf, thus activating MAPKs and the transcript accumulation of defense-related genes. Then, a signal compound, either JA itself or something downstream of JA, transmits the attack alert message to distal leaves without activating SIPK and WIPK in those leaves. Identifying substrates for SIPK and WIPK and understanding how they interact with other components of the signaling pathway will shed light on the complicated signaling pathways that have evolved during the long history of the plant-herbivore arms race.
Figure 13.
Model Summarizing How OS-Elicited Responses Activate Defenses in Local and Systemic Leaves of N. attenuata.
After attack from M. sexta larvae, FACs in the larvae's OS bind to hypothetical receptors in the cell membranes and activate a short-distance mobile signal that enhances SIPK and WIPK activity in both wounded regions and, in particular, nonwounded adjacent regions in the leaf. Afterwards, activating SIPK and WIPK leads to the transcriptional regulation of other MAPKs, CDPKs, and transcription factors, such as WRKYs. Through WRKY and other transcription factors, both kinases subsequently enhance transcript levels of genes involved in JA, SA, JA-Ile, and ethylene biosynthesis, which in turn enhance levels of JA, SA, JA-Ile, and ethylene. SIPK may also directly phosphorylate some of these genes' protein products (for example, ACS) and thus enhance their activity. A long-distance mobile signal, such as JA or a JA-elicited substance, moves through the vascular system to distal leaves and enhances both local and systemic levels of TPI activity. Green arrows represent regulation at transcriptional levels; red arrows represent direct phosphorylation; arrows in brown and blue represent short- and long-distance mobile signals, respectively.
METHODS
Plant Growth and Sample Treatments
Nicotiana attenuata (Solanaceae) seeds were from a line maintained in our laboratory that was originally collected in Utah; seeds were germinated on agar with Gamborg B5 media (Duchefa). N. attenuata plants of the same genotype and generation as the wild-type plants were transformed with a construct carrying an antisense LOX3 gene to obtain asLOX3 plants (Halitschke and Baldwin, 2003); constructs carrying fragments in an inverted repeat orientation of COI1, which mediates JA perception (Paschold et al., 2007), and ACO, which mediates ethylene production (von Dahl et al., 2007), were transformed into N. attenuata to form irCOI1 and irACO lines, respectively. The ETR1 line was prepared by expressing the Arabidopsis thaliana mutant ethylene receptor gene ETR1 to inhibit ethylene perception (von Dahl et al., 2007). All transformed plants were homozygous for a single transgene insertion, diploid as determined by flow cytometry, and used in the T2-T3 generation.
All plants except those used for VIGS were grown in the greenhouse in 1-liter individual pots at 26 to 28°C under 16 h of light supplied by Philips Sun-T Agro 400- or 600-W sodium lights. All treatments were performed on +1 leaves, unless otherwise noted. For W+W treatments, leaves were wounded with a pattern wheel, and 20 μL of water was rubbed onto each leaf; for W+OS treatments, 20 μL of Manduca sexta OS (one-fifth diluted, unless otherwise noted) was rubbed into wounds. MeJA was dissolved in heat-liquefied lanolin at a concentration of 7.5 μg/μL; 20 μL of the resulting lanolin paste was applied to leaves, and pure lanolin was applied as a control. Ethephon was dissolved in 5 mM MES, pH 5.5, at 5 μg/μL, and 20 μL was applied to each leaf; MES buffer solution was used as a control. FAC A, FAC B, FAC C, and FAC D were synthesized in-house (Halitschke et al., 2001). Each FAC was dissolved in DMSO at a concentration of 2 mM and then diluted in water to 0.2 mM. FAC-free OS were prepared by passing OS four times through spin columns filled with Amberlite IRA-400 resin (Sigma-Aldrich) (Halitschke et al., 2001). Twenty microliters of each test solution was applied to each leaf. After specific times, leaves were excised, immediately frozen in liquid nitrogen, and stored at −80°C until use.
Molecular Cloning
Genes were cloned by PCR, using cDNA obtained from 1 h W+OS-treated wild-type N. attenuata leaves as the template. Products were gel-purified and cloned into pGEM-T easy vector (Promega) and sequenced.
RNA Gel Blotting and q-PCR
Total RNA was extracted from leaves using TRIZOL reagent (Invitrogen) following the manufacturer's instructions. Ten micrograms of RNA from pooled leaf tissue from four replicated leaf samples was separated on 1.2% formaldehyde-agarose gels and transferred to GeneScreen Plus Hybridization Transfer membranes (Perkin-Elmer Life and Analytical Sciences). Ten nanograms of DNA probes was labeled with [α-32P]dCTP (Perkin-Elmer Life and Analytical Sciences) using a random primer labeling kit (Amersham Biosciences). Membranes were prehybridized with ULTRAhyb hybridization buffer (Ambion) for 1 h and hybridized with individual probes overnight; after washing, imaging was conducted on an FLA-3000 phosphor imager system (Fuji Photo Film). Probes were PCR-amplified using plasmids carrying respective cDNA as templates, and the primers listed in Supplemental Table 1 online were used.
For q-PCR analysis, five replicated biological samples were used. To minimize errors generated by the preparation of cDNA, all total RNA samples were diluted to 0.5 μg/μL; in 96-well PCR plates and using the same master enzyme mix, 2 μL of each diluted RNA sample was reverse-transcribed using oligo(dT) and Superscript II reverse transcriptase (Invitrogen) in a total volume of 20 μL. cDNA samples were further diluted with water to 40 μL. q-PCR was performed on an ABI PRISM 7700 sequence detection system (Applied Biosystems) using qPCR Core kits (Eurogentec). For each analysis, a linear standard curve, threshold cycle number versus log (designated transcript level), was constructed using a series dilutions of a specific cDNA standard; the levels of the transcript in all unknown samples were determined according to the standard curve. An N. attenuata Sulfite Reductase (ECI), which is a housekeeping gene involved in plant sulfur metabolism and has been shown to have constant levels of transcript by both RNA gel blotting and q-PCR after W+W and W+OS treatments (B. Bubner and J. Wu, unpublished data), was used as an internal standard for normalizing cDNA concentration variations. The primers, Taqman probe sequences used for Taqman q-PCR, and primer sequences for SYBR Green–based q-PCR are provided in Supplemental Tables 2 and 3 online, following the PCR conditions recommended by the manufacturer.
Protein Extraction
Leaf tissue pooled from four replicate leaves was crushed in liquid nitrogen, and 250 μL extraction buffer was used for every 100 mg of tissue (100 mM HEPES, pH 7.5, 5 mM EDTA, 5 mM EGTA, 10 mM Na3VO4, 10 mM NaF, 50 mM β-glycerolphosphate, 1 mM phenylmethylsulfonyl floride, 10% glycerol, and complete proteinase inhibitor cocktail tablets [Roche]). Leaf tissue was then completely suspended by vortexing. After being centrifuged at 4°C for 20 min, supernatant was transferred to a fresh tube. Protein concentration was measured using a Bio-Rad protein assay kit with BSA as a standard.
In-Gel Kinase Activity Assay
Kinase activity assay was performed according to Zhang and Klessig (1997) using MBP as a substrate. In brief, protein samples containing 10 μg of total protein were separated on 10% SDS-polyacrylamide gels embedded with 0.2 mg/mL MBP in the separation gel. After electrophoresis, gels were washed three times for 30 min each in washing buffer (25 mM Tris, pH 7.5, 0.5 mM DTT, 0.1 mM Na3VO4, 5 mM NaF, 0.5 mg/mL BSA, and 0.1% Triton X-100 [v/v]) at room temperature. Then, kinases in the gel were renatured in 25 mM Tris, pH 7.5, 0.5 mM DTT, 0.1 mM Na3VO4, and 5 mM NaF at 4°C overnight, with three changes of buffer. At room temperature, the gels were then incubated in reaction buffer (25 mM Tris, pH 7.5, 2 mM EGTA, 12 mM MgCl2, 1 mM DTT, and 0.1 mM Na3VO4) with 0.2 mM ATP plus 1.6 μCi/mL γ-32P-ATP (3000 Ci/mmol) for 1 h. The reactions were stopped by a fixation solution (5% trichloroacetic acid and 1% sodium pyrophosphate [w/v]). After 6 h of washing with five changes, the gels were dried on a gel dryer (Bio-Rad), and images were obtained on an FLA-3000 phosphor imager system.
TPI Activity Analysis
The TPI activities were quantified using a radial diffusion assay protocol described by van Dam et al. (2001).
Analysis of JA and JA-Ile/JA-Leu Accumulation
Five replicated leaf samples were used. Approximately 300 mg of each tissue sample was collected in FastPrep tubes containing 0.9 g of FastPrep matrix, flash-frozen in liquid nitrogen, and stored at −80°C until use. One milliliter of ethyl acetate spiked with 200 ng of 13C2-JA, D4-SA, and para-coumaric acid, used as the internal standards for JA, SA, and JA-Ile/JA-Leu, respectively, was added to each sample and then homogenized on a FastPrep homogenizer (Thermo Electron). After centrifugation at maximum speed for 10 min at 4°C, supernatants were transferred to fresh 2-mL Eppendorf tubes. Each pellet was reextracted with 0.5 mL of ethyl acetate and centrifuged; supernatants were combined and then evaporated to dryness on a vacuum concentrator (Eppendorf). The residue was resuspended in 0.5 mL of 70% methanol (v/v) and centrifuged to clarify phases. The supernatants were pipetted to glass vials and then analyzed by HPLC-MS/MS.
Measurements were conducted on a 1200L liquid chromatography–mass spectrometry system (Varian). At a flow rate of 0.1 mL/min, 15 μL of each sample was injected onto a Pursuit C8 column (3 μm, 150 × 2 mm) (Varian). A mobile phase composed of solvent A (0.05% formic acid) and solvent B (0.05% formic acid in methanol) was used in a gradient mode for the separation. A negative electrospray ionization mode was used for detection. An ion with a specific mass-to-charge ratio generated from each endogenous phytohormone or internal standard (the parent ion) was selected and fragmented to obtain its daughter ions; a specific daughter ion was used for generating the corresponding compound's chromatogram. The parent ions, daughter ions, and collision energies used in these analyses are listed in Supplemental Table 4 online. Each phytohormone was quantified by comparing its peak area with the peak area of its respective internal standard. As JA-Leu and JA-Ile have the same molecular weight and retention time, these two compounds could not be separated with the method we developed, which is described in detail by Wang et al. (2007).
Measuring Ethylene Accumulation
Four replicated measurements were used to quantify ethylene production in N. attenuata. Three leaves were treated either with W+W or W+OS and immediately afterward sealed in a three-neck 250-mL round bottom flask with a round bottom and kept in the greenhouse for 5 h. The headspace was flashed into a photoacoustic laser spectrometer with hydrocarbon-free clean air (Invivo), and the ethylene concentration was quantified by comparing ethylene peak areas with peak areas generated by a standard ethylene gas.
VIGS
A VIGS system based on tobacco rattle virus was used (Ratcliff et al., 2001). Fragments of both SIPK and WIPK were amplified by PCR using plasmids carrying SIPK and WIPK cDNA sequences and the following primers: for SIPK, SIPK1-31 (5′-GCGGCGGTCGACGAGCCACGGTGGCAGGTTC-3′) and SIPK2-32 (5′-GCGGCGGGATCCGTATCCATAAGCTCATACGC-3′); for WIPK, WIPK1-32 (5′-GCGGCGGTCGACGAGCTGAATGAGATGGTTGC-3′) and WIPK2-31 (5′-GCGGCGGGATCCTCTATGAAGAACATTCGCG-3′). PCR products were digested with BamHI and SalI, gel purified, and then cloned into the binary pTV00 vector, which was digested with same restriction enzymes to form pTV-SIPK and pTV-WIPK constructs. The fragments of SIPK and WIPK used for the preparation of pTV-SIPK and pTV-WIPK are shown in Supplemental Table 5 online. Agrobacterium tumefaciens strain GV3101 carrying either construct was coinoculated with Agrobacterium carrying pBINTRA6 into young N. attenuata plants following VIGS procedures optimized for N. attenuata (Saedler and Baldwin, 2004).
Accession Numbers
Sequence data from this article can be found in GenBank/EMBL database under accession numbers DQ991135, DQ991136, EF121304, EF121305, EF121306, EF121307, EF121308, EF121309, EF121310, EF121311, EF152550, EF152551, and DQ768747.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. OS-Elicited SIPK and WIPK Activity Is Not Mediated by JA and Ethylene.
Supplemental Figure 2. Ethephon Application to Leaves Generates Ethylene.
Supplemental Figure 3. SIPK Activity and WIPK Transcript Levels Do Not Change in Systemic Leaves of N. attenuata after Wounding or Applying M. sexta OS to Wounds.
Supplemental Table 1. Primers Used for Preparation of RNA Gel Blotting Probes.
Supplemental Table 2. Primers and Probes Used for Taqman-Based q-PCR.
Supplemental Table 3. Primers Used for SYBR Green–Based q-PCR.
Supplemental Table 4. Parent Ions, Daughter Ions, and Collision Energy Used for JA, SA, and JA-Ile/JA-Leu Quantification by HPLC-MS/MS.
Supplemental Table 5. Sequences of Partial SIPK and WIPK Used for the Preparation of the Virus-Induced Gene Silencing Constructs pTV-SIPK and pTV-WIPK.
Supplementary Material
Acknowledgments
We thank Albrecht Berg and Eva Rothe for their help with the phytohormone analysis, Klaus Gase and Antje Wissgott for the preparation of VIGS constructs, Susan Kutschbach for transforming the plants, Caroline von Dahl and Anja Paschold for screening and characterizing transformed lines, and Emily Wheeler for editorial assistance.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Ian T. Baldwin (baldwin@ice.mpg.de).
Online version contains Web-only data.
References
- Ahlfors, R., Macioszek, V., Rudd, J., Brosche, M., Schlichting, R., Scheel, D., and Kangasjarvi, J. (2004). Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 40 512–522. [DOI] [PubMed] [Google Scholar]
- Alarcon, J.J., and Malone, M. (1994). Substantial hydraulic signals are triggered by leaf-biting insects in tomato. J. Exp. Bot. 45 953–957. [Google Scholar]
- Alborn, H.T., Brennan, M.M., and Tumlinson, J.H. (2003). Differential activity and degradation of plant volatile elicitors in regurgitant of tobacco hornworm (Manduca sexta) larvae. J. Chem. Ecol. 29 1357–1372. [DOI] [PubMed] [Google Scholar]
- Alborn, T., Turlings, T.C.J., Jones, T.H., Stenhagen, G., Loughrin, J.H., and Tumlinson, J.H. (1997). An elicitor of plant volatiles from beet armyworm oral secretion. Science 276 945–949. [Google Scholar]
- Allwood, E.G., Davies, D.R., Gerrish, C., Ellis, B.E., and Bolwell, G.P. (1999). Phosphorylation of phenylalanine ammonia-lyase: Evidence for a novel protein kinase and identification of the phosphorylated residue. FEBS Lett. 457 47–52. [DOI] [PubMed] [Google Scholar]
- Andreasson, E., et al. (2005). The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J. 24 2579–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, T., Tena, G., Plotnikova, J., Willmann, M.R., Chiu, W.L., Gomez-Gomez, L., Boller, T., Ausubel, F.M., and Sheen, J. (2002). MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415 977–983. [DOI] [PubMed] [Google Scholar]
- Bennett, R.N., and Wallsgrove, R.M. (1994). Secondary metabolites in plant defense-mechanisms. New Phytol. 127 617–633. [DOI] [PubMed] [Google Scholar]
- Bogre, L., Ligterink, W., Meskiene, I., Barker, P.J., Heberle-Bors, E., Huskisson, N.S., and Hirt, H. (1997). Wounding induces the rapid and transient activation of a specific MAP kinase pathway. Plant Cell 9 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, E.D., Stahlberg, R., Mancuso, S., Vivanco, J., Baluska, F., and Van Volkenburgh, E. (2006). Plant neurobiology: An integrated view of plant signaling. Trends Plant Sci. 11 413–419. [DOI] [PubMed] [Google Scholar]
- Chang, L., and Karin, M. (2001). Mammalian MAP kinase signalling cascades. Nature 410 37–40. [DOI] [PubMed] [Google Scholar]
- Chen, H., Wilkerson, C.G., Kuchar, J.A., Phinney, B.S., and Howe, G.A. (2005). Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc. Natl. Acad. Sci. USA 102 19237–19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, S.H., Sheen, J., Gerrish, C., and Bolwell, G.P. (2001). Molecular identification of phenylalanine ammonia-lyase as a substrate of a specific constitutively active Arabidopsis CDPK expressed in maize protoplasts. FEBS Lett. 503 185–188. [DOI] [PubMed] [Google Scholar]
- Cheng, S.H., Willmann, M.R., Chen, H.C., and Sheen, J. (2002). Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 129 469–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman, R.A., and Mullet, J.E. (1997). Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 355–381. [DOI] [PubMed] [Google Scholar]
- Dong, X. (1998). SA, JA, ethylene, and disease resistance in plants. Curr. Opin. Plant Biol. 1 316–323. [DOI] [PubMed] [Google Scholar]
- Droillard, M.J., Boudsocq, M., Barbier-Brygoo, H., and Lauriere, C. (2004). Involvement of MPK4 in osmotic stress response pathways in cell suspensions and plantlets of Arabidopsis thaliana: Activation by hypoosmolarity and negative role in hyperosmolarity tolerance. FEBS Lett. 574 42–48. [DOI] [PubMed] [Google Scholar]
- Droillard, M.J., Thibivilliers, S., Cazale, A.C., Barbier-Brygoo, H., and Lauriere, C. (2000). Protein kinases induced by osmotic stresses and elicitor molecules in tobacco cell suspensions: Two crossroad MAP kinases and one osmoregulation-specific protein kinase. FEBS Lett. 474 217–222. [DOI] [PubMed] [Google Scholar]
- Eulgem, T., Rushton, P.J., Robatzek, S., and Somssich, I.E. (2000). The WRKY superfamily of plant transcription factors. Trends Plant Sci. 5 199–206. [DOI] [PubMed] [Google Scholar]
- Eulgem, T., Rushton, P.J., Schmelzer, E., Hahlbrock, K., and Somssich, I.E. (1999). Early nuclear events in plant defence signalling: Rapid gene activation by WRKY transcription factors. EMBO J. 18 4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, E.E., Almeras, E., and Krishnamurthy, V. (2003). Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr. Opin. Plant Biol. 6 372–378. [DOI] [PubMed] [Google Scholar]
- Feilner, T., et al. (2005). High throughput identification of potential Arabidopsis mitogen-activated protein kinases substrates. Mol. Cell. Proteomics 4 1558–1568. [DOI] [PubMed] [Google Scholar]
- Fisahn, J., Herde, O., Willmitzer, L., and Pena-Cortes, H. (2004). Analysis of the transient increase in cytosolic Ca2+ during the action potential of higher plants with high temporal resolution: Requirement of Ca2+ transients for induction of jasmonic acid biosynthesis and PINII gene expression. Plant Cell Physiol. 45 456–459. [DOI] [PubMed] [Google Scholar]
- Glawe, G.A., Zavala, J.A., Kessler, A., Van Dam, N.M., and Baldwin, I.T. (2003). Ecological costs and benefits correlated with trypsin protease inhibitor production in Nicotiana attenuata. Ecology 84 79–90. [Google Scholar]
- Gomi, K., Ogawa, D., Katou, S., Kamada, H., Nakajima, N., Saji, H., Soyano, T., Sasabe, M., Machida, Y., Mitsuhara, I., Ohashi, Y., and Seo, S. (2005). A mitogen-activated protein kinase NtMPK4 activated by SIPKK is required for jasmonic acid signaling and involved in ozone tolerance via stomatal movement in tobacco. Plant Cell Physiol. 46 1902–1914. [DOI] [PubMed] [Google Scholar]
- Green, T.R., and Ryan, C.A. (1972). Wound-induced proteinase inhibitor in plant leaves - Possible defense mechanism against insects. Science 175 776–777. [DOI] [PubMed] [Google Scholar]
- Halitschke, R., and Baldwin, I.T. (2003). Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J. 36 794–807. [DOI] [PubMed] [Google Scholar]
- Halitschke, R., Gase, K., Hui, D., Schmidt, D.D., and Baldwin, I.T. (2003). Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiol. 131 1894–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke, R., Schittko, U., Pohnert, G., Boland, W., and Baldwin, I.T. (2001). Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 125 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazzalin, C.A., and Mahadevan, L.C. (2002). MAPK-regulated transcription: A continuously variable gene switch? Nat. Rev. Mol. Cell Biol. 3 30–40. [DOI] [PubMed] [Google Scholar]
- Hermsmeier, D., Schittko, U., and Baldwin, I.T. (2001). Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol. 125 683–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz, I. (1995). MAP kinase pathways in yeast: For mating and more. Cell 80 187–197. [DOI] [PubMed] [Google Scholar]
- Hill, C.S., and Treisman, R. (1995). Transcriptional regulation by extracellular signals: Mechanisms and specificity. Cell 80 199–211. [DOI] [PubMed] [Google Scholar]
- Hirt, H. (1997). Multiple roles of MAP kinases in plant signal transduction. Trends Plant Sci. 2 11–15. [Google Scholar]
- Hui, D., Iqbal, J., Lehmann, K., Gase, K., Saluz, H.P., and Baldwin, I.T. (2003). Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. V. Microarray analysis and further characterization of large-scale changes in herbivore-induced mRNAs. Plant Physiol. 131 1877–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura, K., Mizoguchi, T., Yoshida, R., Yuasa, T., and Shinozaki, K. (2000). Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 24 655–665. [DOI] [PubMed] [Google Scholar]
- Ivashuta, S., Liu, J., Lohar, D.P., Haridas, S., Bucciarelli, B., Vandenbosch, K.A., Vance, C.P., Harrison, M.J., and Gantt, J.S. (2005). RNA interference identifies a calcium-dependent protein kinase Involved in Medicago truncatula root development. Plant Cell 17 2911–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, H., Liu, Y., Yang, K.Y., Kim, C.Y., Baker, B., and Zhang, S. (2003). Function of a mitogen-activated protein kinase pathway in N gene-mediated resistance in tobacco. Plant J. 33 719–731. [DOI] [PubMed] [Google Scholar]
- Jonak, C., Kiegerl, S., Ligterink, W., Barker, P.J., Huskisson, N.S., and Hirt, H. (1996). Stress signaling in plants: A mitogen-activated protein kinase pathway is activated by cold and drought. Proc. Natl. Acad. Sci. USA 93 11274–11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C.G., Hopper, R.F., Coleman, J.S., and Krischik, V.A. (1993). Control of systemically induced herbivore resistance by plant vascular architecture. Oecologia 93 452–456. [DOI] [PubMed] [Google Scholar]
- Kahl, J., Siemens, D.H., Aerts, R.J., Gabler, R., Kuhnemann, F., Preston, C.A., and Baldwin, I.T. (2000). Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta 210 336–342. [DOI] [PubMed] [Google Scholar]
- Kang, J., Wang, L., Giri, A., and Baldwin, I.T. (2006). Silencing threonine deaminase and the JAR1 homologue in Nicotiana attenuata impairs JA-isoleucine-mediated defenses against the specialist herbivore, Manduca sexta. Plant Cell 18 3303–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin, M., and Hunter, T. (1995). Transcriptional control by protein phosphorylation: Signal transmission from the cell surface to the nucleus. Curr. Biol. 5 747–757. [DOI] [PubMed] [Google Scholar]
- Kessler, A., and Baldwin, I.T. (2001). Defensive function of herbivore-induced plant volatile emissions in nature. Science 291 2141–2144. [DOI] [PubMed] [Google Scholar]
- Kessler, A., and Baldwin, I.T. (2002. a). Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 53 299–328. [DOI] [PubMed] [Google Scholar]
- Kessler, A., and Baldwin, I.T. (2002. b). Manduca quinquemaculata's optimization of intra-plant oviposition to predation, food quality, and thermal constraints. Ecology 83 2346–2354. [Google Scholar]
- Kim, C.Y., Liu, Y., Thorne, E.T., Yang, H., Fukushige, H., Gassmann, W., Hildebrand, D., Sharp, R.E., and Zhang, S. (2003). Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell 15 2707–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C.Y., and Zhang, S. (2004). Activation of a mitogen-activated protein kinase cascade induces WRKY family of transcription factors and defense genes in tobacco. Plant J. 38 142–151. [DOI] [PubMed] [Google Scholar]
- Koiwa, H., Bressan, R.A., and Hasegawa, P.M. (1997). Regulation of protease inhibitors and plant defense. Trends Plant Sci. 2 379–384. [Google Scholar]
- Kumar, D., and Klessig, D.F. (2000). Differential induction of tobacco MAP kinases by the defense signals nitric oxide, salicylic acid, ethylene, and jasmonic acid. Mol. Plant Microbe Interact. 13 347–351. [DOI] [PubMed] [Google Scholar]
- Kunkel, B.N., and Brooks, D.M. (2002). Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 5 325–331. [DOI] [PubMed] [Google Scholar]
- Li, L., Li, C., Lee, G.I., and Howe, G.A. (2002). Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc. Natl. Acad. Sci. USA 99 6416–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberherr, D., Thao, N.P., Nakashima, A., Umemura, K., Kawasaki, T., and Shimamoto, K. (2005). A sphingolipid elicitor-inducible mitogen-activated protein kinase is regulated by the small GTPase OsRac1 and heterotrimeric G-protein in rice. Plant Physiol. 138 1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link, V., Sinha, A.K., Vashista, P., Hofmann, M.G., Proels, R.K., Ehness, R., and Roitsch, T. (2002). A heat-activated MAP kinase in tomato: A possible regulator of the heat stress response. FEBS Lett. 531 179–183. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Jin, H., Yang, K.Y., Kim, C.Y., Baker, B., and Zhang, S. (2003). Interaction between two mitogen-activated protein kinases during tobacco defense signaling. Plant J. 34 149–160. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Schiff, M., and Dinesh-Kumar, S.P. (2004). Involvement of MEK1 MAPKK, NTF6 MAPK, WRKY/MYB transcription factors, COI1 and CTR1 in N-mediated resistance to tobacco mosaic virus. Plant J. 38 800–809. [DOI] [PubMed] [Google Scholar]
- Liu, Y., and Zhang, S. (2004). Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16 3386–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig, A.A., Romeis, T., and Jones, J.D. (2004). CDPK-mediated signalling pathways: specificity and cross-talk. J. Exp. Bot. 55 181–188. [DOI] [PubMed] [Google Scholar]
- Ludwig, A.A., Saitoh, H., Felix, G., Freymark, G., Miersch, O., Wasternack, C., Boller, T., Jones, J.D., and Romeis, T. (2005). Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc. Natl. Acad. Sci. USA 102 10736–10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei, M., Bossi, S., Spiteller, D., Mithofer, A., and Boland, W. (2004). Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol. 134 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone, M., Alarcon, J.J., and Palumbo, L. (1994). An hydraulic interpretation of rapid, long-distance wound signaling in the tomato. Planta 193 181–185. [Google Scholar]
- MAPK Group (2002). Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 7 301–308. [DOI] [PubMed] [Google Scholar]
- McFadden, M.W. (1968). Observations on feeding and movement of tobacco hornworm larvae. J. Econ. Entomol. 61: 352–356.
- Menke, F.L., Kang, H.G., Chen, Z., Park, J.M., Kumar, D., and Klessig, D.F. (2005). Tobacco transcription factor WRKY1 is phosphorylated by the MAP kinase SIPK and mediates HR-like cell death in tobacco. Mol. Plant Microbe Interact. 18 1027–1034. [DOI] [PubMed] [Google Scholar]
- Menke, F.L., van Pelt, J.A., Pieterse, C.M., and Klessig, D.F. (2004). Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell 16 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk, M., Awotunde, O.S., Muszynska, G., Klessig, D.F., and Dobrowolska, G. (2000). Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell 12 165–178. [PMC free article] [PubMed] [Google Scholar]
- Moscatiello, R., Mariani, P., Sanders, D., and Maathuis, F.J. (2006). Transcriptional analysis of calcium-dependent and calcium-independent signalling pathways induced by oligogalacturonides. J. Exp. Bot. 57 2847–2865. [DOI] [PubMed] [Google Scholar]
- O'Donnell, P.J., Calvert, C., Atzorn, R., Wasternack, C., Leyser, H.M.O., and Bowles, D.J. (1996). Ethylene as a signal mediating the wound response of tomato plants. Science 274 1914–1917. [DOI] [PubMed] [Google Scholar]
- Orians, C. (2005). Herbivores, vascular pathways, and systemic induction: Facts and artifacts. J. Chem. Ecol. 31 2231–2242. [DOI] [PubMed] [Google Scholar]
- Orians, C.M., Pomerleau, J., and Ricco, R. (2000). Vascular architecture generates fine scale variation in systemic induction of proteinase inhibitors in tomato. J. Chem. Ecol. 26 471–485. [Google Scholar]
- Ouaked, F., Rozhon, W., Lecourieux, D., and Hirt, H. (2003). A MAPK pathway mediates ethylene signaling in plants. EMBO J. 22 1282–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschold, A., Halitschke, R., and Baldwin, I.T. (2007). Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata reveals the role of herbivore movement in avoiding defenses. Plant J., in press. [DOI] [PubMed]
- Petersen, M., et al. (2000). Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 103 1111–1120. [DOI] [PubMed] [Google Scholar]
- Pokholok, D.K., Zeitlinger, J., Hannett, N.M., Reynolds, D.B., and Young, R.A. (2006). Activated signal transduction kinases frequently occupy target genes. Science 313 533–536. [DOI] [PubMed] [Google Scholar]
- Ratcliff, F., Martin-Hernandez, A.M., and Baulcombe, D.C. (2001). Technical advance. Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J. 25 237–245. [DOI] [PubMed] [Google Scholar]
- Ren, D., Yang, K.Y., Li, G.J., Liu, Y., and Zhang, S. (2006). Activation of Ntf4, a tobacco MAPK, during plant defense response and its involvement in hypersensitive response-like cell death. Plant Physiol. 141 1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond, P., Bodenhausen, N., Van Poecke, R.M., Krishnamurthy, V., Dicke, M., and Farmer, E.E. (2004). A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell 16 3132–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond, P., and Farmer, E.E. (1998). Jasmonate and salicylate as global signals for defense gene expression. Curr. Opin. Plant Biol. 1 404–411. [DOI] [PubMed] [Google Scholar]
- Romeis, T. (2001). Protein kinases in the plant defence response. Curr. Opin. Plant Biol. 4 407–414. [DOI] [PubMed] [Google Scholar]
- Romeis, T., Ludwig, A.A., Martin, R., and Jones, J.D. (2001). Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J. 20 5556–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis, T., Piedras, P., and Jones, J.D. (2000). Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell 12 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis, T., Piedras, P., Zhang, S., Klessig, D.F., Hirt, H., and Jones, J.D. (1999). Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: Convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, C.A. (1990). Protease inhibitors in plants - Genes for improving defenses against insects and pathogens. Annu. Rev. Phytopathol. 28 425–449. [Google Scholar]
- Saedler, R., and Baldwin, I.T. (2004). Virus-induced gene silencing of jasmonate-induced direct defences, nicotine and trypsin proteinase-inhibitors in Nicotiana attenuata. J. Exp. Bot. 55 151–157. [DOI] [PubMed] [Google Scholar]
- Samuel, M.A., and Ellis, B.E. (2002). Double jeopardy: Both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. Plant Cell 14 2059–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, M.A., Miles, G.P., and Ellis, B.E. (2000). Ozone treatment rapidly activates MAP kinase signalling in plants. Plant J. 22 367–376. [DOI] [PubMed] [Google Scholar]
- Sangwan, V., Orvar, B.L., Beyerly, J., Hirt, H., and Dhindsa, R.S. (2002). Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J. 31 629–638. [DOI] [PubMed] [Google Scholar]
- Schittko, U., and Baldwin, I.T. (2003). Constraints to herbivore-induced systemic responses: Bidirectional signaling along orthostichies in Nicotiana attenuata. J. Chem. Ecol. 29 763–770. [DOI] [PubMed] [Google Scholar]
- Schittko, U., Preston, C.A., and Baldwin, I.T. (2000). Eating the evidence? Manduca sexta larvae can not disrupt specific jasmonate induction in Nicotiana attenuata by rapid consumption. Planta 210 343–346. [DOI] [PubMed] [Google Scholar]
- Seo, S., Okamoto, M., Seto, H., Ishizuka, K., Sano, H., and Ohashi, Y. (1995). Tobacco MAP kinase: A possible mediator in wound signal transduction pathways. Science 270 1988–1992. [DOI] [PubMed] [Google Scholar]
- Seo, S., Sano, H., and Ohashi, Y. (1999). Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic, B., and Davies, E. (1997). Intercellular communication in plants: Electrical stimulation of proteinase inhibitor gene expression in tomato. Planta 202 402–406. [Google Scholar]
- Staswick, P.E., and Tiryaki, I. (2004). The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16 2117–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann, J.W., and Ryan, C.A. (1997). Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc. Natl. Acad. Sci. USA 94 11085–11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuki, M., and Mori, H. (2001). Phosphorylation of tomato 1-aminocyclopropane-1-carboxylic acid synthase, LE-ACS2, at the C-terminal region. J. Biol. Chem. 276 28051–28057. [DOI] [PubMed] [Google Scholar]
- Tena, G., and Renaudin, J.P. (1998). Cytosolic acidification but not auxin at physiological concentration is an activator of MAP kinases in tobacco cells. Plant J. 16 173–182. [DOI] [PubMed] [Google Scholar]
- Thomas, C.L., Jones, L., Baulcombe, D.C., and Maule, A.J. (2001). Size constraints for targeting post-transcriptional gene silencing and for RNA-directed methylation in Nicotiana benthamiana using a potato virus X vector. Plant J. 25 417–425. [DOI] [PubMed] [Google Scholar]
- Truitt, C.L., Wei, H.X., and Pare, P.W. (2004). A plasma membrane protein from Zea mays binds with the herbivore elicitor volicitin. Plant Cell 16 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck, F., Zhou, A., and Somssich, I.E. (2004). Stimulus-dependent, promoter-specific binding of transcription factor WRKY1 to its native promoter and the defense-related gene PcPR1-1 in parsley. Plant Cell 16 2573–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon, R. (1989). The sink-source transition in leaves. Annu. Rev. Plant Physiol. 40 119–138. [Google Scholar]
- Ulker, B., and Somssich, I.E. (2004). WRKY transcription factors: From DNA binding towards biological function. Curr. Opin. Plant Biol. 7 491–498. [DOI] [PubMed] [Google Scholar]
- van Dam, N.M., Horn, M., Mares, M., and Baldwin, I.T. (2001). Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J. Chem. Ecol. 27 547–568. [DOI] [PubMed] [Google Scholar]
- von Dahl, C.C., Winz, R., Halitschke, R., Gase, K., Kühnemann, F., and Baldwin, I.T. (2007). Tuning the herbivore-induced ethylene burst: The role of transcript accumulation and ethylene perception in Nicotiana attenuata. Plant J., in press. [DOI] [PubMed]
- Wang, L., Halitschke, R., Kang, J., Berg, A., Harnish, F., and Baldwin, I.T. (2007). Independently silencing two members of JAR family impairs trypsin proteinase inhibitor's activities but not nicotine accumulations. Planta, in press. [DOI] [PubMed]
- Whitham, S., Dinesh-Kumar, S.P., Choi, D., Hehl, R., Corr, C., and Baker, B. (1994). The product of the tobacco mosaic virus resistance gene N: Similarity to toll and the interleukin-1 receptor. Cell 78 1101–1115. [DOI] [PubMed] [Google Scholar]
- Wu, J., Hettenhausen, C., and Baldwin, I.T. (2006). Evolution of proteinase inhibitor defenses in North American allopolyploid species of Nicotiana. Planta 224 750–760. [DOI] [PubMed] [Google Scholar]
- Xu, P., Zhang, Y., Kang, L., Roossinck, M.J., and Mysore, K.S. (2006). Computational estimation and experimental verification of off-target silencing during posttranscriptional gene silencing in plants. Plant Physiol. 142 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K.Y., Liu, Y., and Zhang, S. (2001). Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. USA 98 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, Y.K., Kodama, Y., Waller, F., Chung, K.M., Ueda, H., Nakamura, K., Oldsen, M., Yoda, H., Yamaguchi, Y., and Sano, H. (2005). Activation of a novel transcription factor through phosphorylation by WIPK, a wound-induced mitogen-activated protein kinase in tobacco plants. Plant Physiol. 139 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, G.M., Dowd, P.E., Gilroy, S., and McCubbin, A.G. (2006). Calcium-dependent protein kinase isoforms in petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell 18 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala, J.A., Patankar, A.G., Gase, K., Hui, D., and Baldwin, I.T. (2004). Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses. Plant Physiol. 134 1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M., Liang, S., and Lu, Y.T. (2005). Cloning and functional characterization of NtCPK4, a new tobacco calcium-dependent protein kinase. Biochim. Biophys. Acta 1729 174–185. [DOI] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1997). Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell 9 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998. a). The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proc. Natl. Acad. Sci. USA 95 7225–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998. b). Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 95 7433–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (2001). MAPK cascades in plant defense signaling. Trends Plant Sci. 6 520–527. [DOI] [PubMed] [Google Scholar]
- Zhang, S., and Liu, Y. (2001). Activation of salicylic acid-induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco. Plant Cell 13 1877–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, J., Keinanen, M., and Baldwin, I.T. (2001). Herbivore-induced allene oxide synthase transcripts and jasmonic acid in Nicotiana attenuata. Phytochemistry 58 729–738. [DOI] [PubMed] [Google Scholar]
- Zipfel, C., Robatzek, S., Navarro, L., Oakeley, E.J., Jones, J.D., Felix, G., and Boller, T. (2004). Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428 764–767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.